Abstract
A 35 kDa rabbit erythrocyte agglutinating lectin from the seeds of Cicer arietinum was purified and designated as CAL. The lectin was inhibited by fetuin and N-acetyl-d-galactosamine at a concentration of 20 and 50 mM respectively, but not by simple mono or oligosaccharides. CAL is active between pH 5 and 10 presented thermo stability up to 50 °C and demonstrated DNA damage inhibition at 30 µg concentration. The lectin elicited maximum mitogenic activity towards mice splenocytes at 7.5 µg ml− 1. CAL exerted an inhibitory activity on HIV-1 reverse transcriptase with IC50 of 180 µM. CAL abilities in animal bioassay resulted decreased levels of total triglyceride and creatinine. In vitro and in vivo studies revealed that CAL may constitute an important role impending biomedical applications.
Keywords: Chickpea, Lectin, Anti-HIV-1 reverse transcriptase, DNA damage
Introduction
Represented by different structures and properties, plant seeds accommodate many proteins that function as storage and/or defence (Diaz et al. 2017). Amongst these, a group of plant defence proteins originally discovered in seeds were the lectins (Rudiger and Gabius 2001). Lectins are glycan-binding proteins that have ability to agglutinate red blood cells and contain at least one non-catalytic binding domain for interaction (Peumans and Van Damme 1995). They are distributed across variety of organisms and accelerate many biological processes like recognition of carbohydrates, host–pathogen interaction, cell-targeting and cell–cell communication (Sharon and Lis 1989). Literature perusal suggests that the plant lectins find wide applications in pharmacological aspects like mitogenic (Chan et al. 2011), anti-fungal (Ye and Ng 2002), anti-cancer (Faheina-Martins et al. 2012), and anti-HIV-1 reverse transcriptase activities (Ye and Ng 2002).
Chickpea belongs to the family of Leguminosae, which is the third most important pulse of the world and India is the largest producer. Chickpea belongs to the tribe Cicerae of the genus Cicer and species arietinum. Chickpea seeds are nutritious due to the presence of proteins, carbohydrates, vitamins, minerals, and low levels of antinutritional factors in comparison with other crop legumes including lectin (Bhagyawant and Srivastava 2008). Purification and characterization of lectin from new sources/genotypes may reveal different medical applications foremost in the direction of drug transformation (Diaz et al. 2017).
CAL is a lectin isolated from the seeds of Cicer arietinum. The previous reports of chickpea lectin on various aspect take account of characterization of a clone encoding vegetative lectin from C. arietinum epicotyls (Esteban et al. 2002), production of stable transgene chickpea through expression of insecticidal lectin (Chakraborti et al. 2009) and root lectins (Agrawal et al. 2011). In addition, in silico studies on recombinant chickpea lectin (Wakankar et al. 2013), three dimensional structure determination (Sharma et al. 2015), and α-amylase inhibitory activity against potato beetle larvae (Wang et al. 2017) are also published in literature domain. Our previous studies reported crystallization and preliminary X-ray characterisation of a lectin from chickpea (Katre et al. 2005). However, this lectin has not been thoroughly explored for its biochemical and functional properties in animal models. To our knowledge, this is the first report that aims to fulfill this void.
Materials and methods
Chemicals and reagents: SP sephadex and MTT were procured from (Sigma-Aldrich, St. Louis, MO, USA). DEAE cellulose was obtained from Sisco-Research Laboratories (SRL) India. Other chemicals used in the study were of analytical grade.
Seed material: The seeds of chickpea (cv-Digvijay) were obtained from the Department of Plant Breeding, Mahatma Phule Krishi Vidyapeeth, Rahuri, Maharashtra, India.
Animals: The study was performed with male wistar rats, duly approved by the Institutional Animal Ethics (IAEC) committee of Defence Research and Development Establishment, Gwalior. Animal experiments were conducted according to Animal Welfare Division, Govt. of India regulations concerning the protection of experimental animals (No. 37/GO/Rbi/S/99/CPCSEA).
Isolation and purification of CAL
All isolation and purification steps were performed in cold room as per our previous report of Katre et al. (2005). Briefly, 100 g dry chickpea seeds were ground to a fine powder and defatted twice with chilled acetone (1:2 w/v). The defatted powder was suspended in 500 ml of 10 mM Tris–HCl, 150 mM NaCl pH 7.2 buffer. The dialysate after 80% ammonium sulfate precipitation was loaded on to DEAE-cellulose column and lectin came out as unadsorbed portion. The fractions showing positive hemagglutination were pooled together and loaded on strong cation exchanger, i.e., SP sephadex. The bound lectin was eluted with NaCl gradient. The fractions showing hemagglutination were pooled together and stored in − 20 °C until further use.
Hemagglutination assay
The hemagglutination assay was performed using trypsinised rabbit erythrocytes. Hemagglutination tests were performed in the standard microtitre plate by the twofold serial dilution method (Liener 1962). The unit of hemagglutination activity (U) termed titre was expressed as the reciprocal of the highest dilution of the lectin that showed complete agglutination. Specific activity of the lectin is defined as the titre of hemagglutination per mg of protein.
Carbohydrate inhibition assay
Hemagglutination inhibition tests were performed as described in hemagglutination assay, except that serial dilution of the sugar solution (25 µl) was pre-incubated at room temperature with 25 µl of the lectin (minimum concentration showing titre) for 15 min. 50 µl of rabbit erythrocyte suspension was added, mixed and the plates read after 1 h. The different concentrations of all the sugar (10, 20, 50, and 100 mM) were tested and that of the lectin was 1 mg ml− 1. The carbohydrate inhibition assay was performed employing various sugars (Koike et al. 1995).
Effect of temperature, pH and metal ion on CAL
The effect of temperature was analyzed by heating the lectin at 10–100 °C for 30 min. The sample was immediately cooled to terminate the incubation and examined for hemagglutination activity. Percentage of residual hemagglutination activity was calculated (Wong and Ng 2006). To measure pH stability, lyophilized lectin was dissolved in a specified pH buffer starting from pH 2 to 14 to a final concentration of 1 mg ml− 1 and left to stand at room temperature for 1 h. The buffers included sodium citrate (pH 2–4), sodium acetate (pH 5), sodium phosphate (pH 6–7), Tris–HCl (pH 8–9), sodium bicarbonate (pH 10), glycine–NaOH (pH 11–12) and KCl–NaOH (pH 13–14). In all cases, rabbit erythrocytes were used and three replicates were done for each assay and percent residual hemagglutinating activity was calculated (Nakagawa et al. 1996). The interaction of chickpea lectin with the metal ions e.g., Ca2+, Mg2+, Cu2+ and Mn2+, was studied. The effect of the addition of different concentrations of 10, 20, 30, 40 and 50 mM of each divalent cations Ca2+, Mg2+, Cu2+ and Mn2+ on hemagglutination activity of the purified lectin (1 mg ml− 1) was checked at room temperature (Kawagishi et al. 1990).
Sub-unit molecular mass determination by SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using Laemmli and Favre (1973) with 12% resolving gel and a 4% stacking gel. The gel was stained with coomassie brilliant blue and subsequently destained to visualize protein bands.
Mitogenic activity
Following laboratory safety guidelines, a 3-month old male C57BL/6 mouse was used for the experiment and spleen tissue was separated aseptically. Spleen cells were isolated by pressing the tissue through a sterilized 100-mesh sieve to generate single cell suspension. The cells (1.5 × 107 cells ml− 1) were suspended in RPMI-1640 culture medium supplemented with 10% (v/v) fetal bovine serum, 100 unit penicillin ml− 1 and 100 µg streptomycin ml− 1. Splenocytes (100 µl well− 1) were seeded into a 96-well plate and different concentrations viz. 2.5, 5.0, 7.5, 10, 12.5 and 15 µg ml− 1 of lectin were tested. The Phytohemagglutinin (PHA) at a concentration of 5 µg ml− 1 was exercised as positive control. After 72 h of incubation at 37 °C in a humidified atmosphere containing 5% CO2, 10 µl MTT reagent (5 mg ml− 1) was added to each well and cells were incubated for another 4 h, followed by the replacement of the culture media with 150 µl DMSO. The absorbance of the solution was measured at 490 nm using microplate reader (Bio-Rad iMark) and the cell inhibitory rate was calculated as follows (Mosmann 1983):
DNA damage protective effect assay
The ability of CAL to protect pUC18 plasmid against Fenton’s reagent was estimated through DNA nicking assay as described by Xu et al. (2012). The protective effect of lectin on oxidative DNA strand breakage was evaluated by way of pUC18 plasmid DNA. Briefly, reaction mixtures contained 5 µl of phosphate buffer saline (PBS, 10 mM, pH 7.4), 1 µl of plasmid DNA (0.5 µg), 10 µl of lectin and 2 µl of Fenton’s reagent (30 mM H2O2, 50 mM ascorbic acid, and 80 mM FeCl3) were incubated at 37 °C for 30 min. Then, after, 3 µl of a loading buffer was added to stop the reaction and subsequently electrophoresed on 1% agarose gel (Guha et al. 2011). The protective effect was expressed as a percentage content of supercoiled form of plasmid DNA treated with lectin in relation with untreated plasmid DNA.
Anti-HIV-1 reverse transcriptase activity
The inhibition of human immunodeficiency virus (HIV-1) reverse transcriptase activity was assayed by ELISA kit (Roche, Germany) following prescribed instructions. The reverse transcriptase (RT) is able to synthesize DNA from a template. The digoxigenin and biotin-labeled nucleotides were incorporated into the same DNA as synthesized by reverse transcriptase. A 5 ng of HIV-1-RT (recombinant) was used to perform the sandwich ELISA. The absorbance of synthesized DNA was quantified at 405 nm and was directly proportional to the RT activity. Phaseolus vulgaris lectin was used as positive control. The lectin inhibitory activity was determined as percent inhibition compared to control without the addition of lectin protein− 1 (Collins et al. 1997):
2D-gel electrophoresis
The purified chickpea lectin was introduced to 2D-gel electrophoresis following the method of O’Farrell (1975). Isoelectric focusing (IEF) was performed using immobilized pH gradient (IPG) strip of pH 3–10 (Bio-Rad, USA). For the first dimension, IPG strip was passively rehydrated overnight with 300 µg of lectin sample dissolved in rehydration buffer and the lectin was focused at 250 V for 3 h until 8000 V hours under mineral oil. After electrofocusing, the strip was equilibrated in equilibration buffer-I for 15 min, followed by equilibration buffer-II for 10 min. The strip was further subjected to 2-D separation using 12% SDS-PAGE and protein spot was visualized by Coomassie blue G-250.
Peptide mass fingerprinting
The spot from 2D-gel of purified chickpea lectin was excised and digested using 0.1% trypsin in 50 mM NH4HCO3 at 37 °C in an incubator overnight. The protein digestion was done by the method of Shevchenko et al. (2007). A saturated solution of CHCA in 1:1 ratio of deionized water–acetonitrile containing 0.1% TFA was prepared and used as matrix. The peptides were identified by MALDI-TOF/MS by BrukerMicroflex LRF-20, (Flex Control Workstation, Bremen, Germany). The obtained MS spectrum of peptides was submitted to MASCOT search tool, Bio tools version 3.1. The homology of proteins was aligned using BLAST from NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Animal management
Animal experiments were performed on 19 day weaned male wistar rats of 45–60 g in weight. The animals were maintained in polypropylene cages on dust-free and autoclaved paddy husk, fed with the standard pellet diet (Ashirwad Feeds, India). Total 24 animals were randomly allocated into 4 groups and orally fed with different lectin concentrations (50, 100 and 200 mg kg− 1 body weight) of lectin diluted in distilled water.
Body weight
The rats were weighted individually at the beginning and end of the experiment. On 23rd day of experiment before noon, rats were sacrificed by ether anesthesia. The abdomen was cut, open and the liver, kidney, small intestine, heart, lungs and spleen were dissected out, weighed and preserved in formalin solution for histological analysis. The ratio of organ to body weight was also recorded.
Evaluation of biochemical and hematological parameters
About 1.5 ml blood was collected in heparinised vials for blood parameters analysis, while rest part of blood was centrifuged at 3500 rpm for 15 min for serum analysis.
Biochemical evaluation
The serum samples were used to quantify some of the biochemical parameters. Serum total cholesterol, triacylglycerol, albumin, total protein, creatinine and urea were assessed using various kits (Erba kits, Germany) according to procedures described by Richmond (1973).
Hematological analysis
Hematological analyses were performed with blood samples using automated hematology analyzer (Sysmex XT-2000, Germany). The recorded parameters were white blood cells, lymphocytes, granulocytes, red blood cells, hemoglobin, hematocrit, and platelets.
Histopathological examination
Histopathological examinations were done with liver, kidney, small intestine, heart, lungs, and spleen as a sample. Organs were fixed in formaldehyde (10%) for 24 h subsequently dehydrated in ascending concentration of ethanol (20–90%) for interval of 1 h, cleaned in xylene, impregnated and embedded in paraffin (Drury and Wallington 1980). Sections of 5 µm were cut through a rotary microtome, stained with hematoxylin and eosin stains. Light microscopic examination from each organ in all groups was performed under Leica make light microscope and image representatives of the typical histological profiles were examined.
Statistical analyses
The values were presented as means with their standard deviation (± SD). A one-way ANOVA program was performed to test the effect of lectin on biochemical and hematological parameters of blood and sera samples. The statistical analysis was performed using the Prism-5 software. Comparisons of two serum parameter i.e., triglycerides and creatinine between control and treatment groups of rat were significant (P ≤ 0.05).
Results and discussion
Legume lectins are mainly concentrated in seeds at a higher concentration compared to other tissues. Therefore, the largest reports of plant lectins are isolated from the seeds. Initial detection of hemagglutinating activity in PBS extract of chickpea seeds gave agglutination reaction. The variations in chickpea hemagglutinating activity was observed where chickpea lectin readily agglutinated both trypsin-treated and untreated rabbit erythrocytes as well as human erythrocytes, but did not agglutinate mouse and chicken erythrocytes (Qureshi et al. 2006). In the present study, C. arietinum agglutinin is not blood group specific, but agglutinates rabbit erythrocytes that have undergone treatment with trypsin. This may be due to differences in the number of binding sites per lectin molecule or/and the structure of receptor molecules on the erythrocyte membrane. Such a variation in lectin detection in chickpea seeds can be observed most likely due to using different genotypes (Rodrigues e Lacerda and Silva do Nascimento 2017).
Purification of CAL
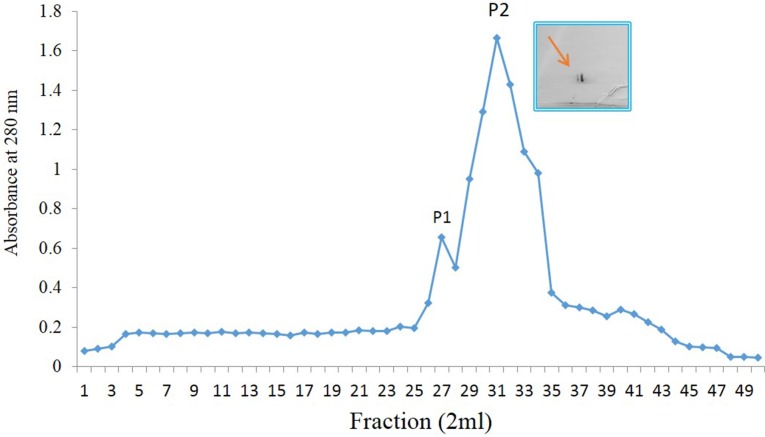
Ammonium sulfate precipitated dialysed seed extract chromatographed on DEAE-cellulose-yielded two protein peaks both showing hemagglutination activity, one in the flow through fraction and other in the elute. The minor impurity in the DEAE-cellulose unbound lectin sample was removed by subjecting SP-sephadex chromatography. The subsequent elution from the matrix was achieved with acetate buffer with NaCl step gradient of 0.1–0.5 M. To completely eluted protein of interest, 0.2 M pooled fraction showed the highest A280 and hemagglutination activity suggesting the presence of desired lectin (Fig. 1).
Fig. 1.
SP-sephadex ion-exchange chromatography of CAL. The column was eluted with NaCl of 200 mM in the same buffer and peak-2 shows the purified CAL. Inset depicts 2D-gel electrophoresis of purified CAL using 7.0 cm IPG strips
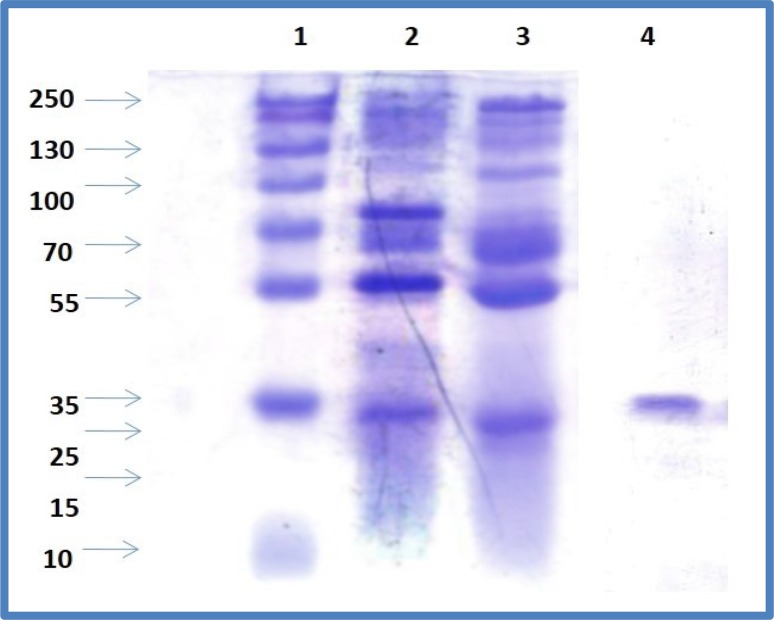
Earlier reports on chickpea lectin as described by Qureshi et al. (2006) employed ammonium sulfate fractionation and affinity chromatography on an N-acetyl-d-galactosamine-linked agarose column to purify lectin in contrast to present study. The purification chart entailing protein concentration and hemagglutinating activity values for each step are shown in Table 1. At the end of purification process, fold purification related to increased specific activity was found to be 142.83, i.e., final specific activity/initial specific activity. The electrophoretic profiles of the chickpea seed extracts, 20% ammonium sulfate fraction, 80% ammonium sulfate fraction, DEAE-cellulose unbounded protein and SP-sephadex eluted pure lectin were determined (Fig. 2).
Table 1.
Yield of Cicer arietinum L. lectin in different purification steps
| Purification steps | Protein (mg/100g) | Hemagglutination unit (HAU) | Specific activity (HAU/mg) |
|---|---|---|---|
| Crude extract | 2000 | 1024 | 51.21 |
| 0–20% ammonium sulfate fraction | 1025 | 1024 | 99.90 |
| 20–80% ammonium sulfate fraction | 580 | 2048 | 353.14 |
| DEAE-cellulose fraction | 410 | 2048 | 499.52 |
| SP-sephadex fraction | 112 | 8192 | 7314.28 |
Fig. 2.
Stages of lectin purification as revealed by SDS-PAGE. Lane-1, molecular marker; lane-2, crude extract; lane-3, 0–20% ammonium sulfate fraction; lane-4, SP-sephadex fraction
The molecular mass of the subunit as determined by SDS-PAGE was 35 kDa, which is the same in the native PAGEsuggesting that the lectin is monomeric. Our earlier report (Katre et al. 2005) identified chickpea lectin as a dimer of molecular weight 43 kDa composed of two identical subunits having molecular weight 21.5 kDa as confirmed by SDS-PAGE. These differences exist between lectins isolated from different genotypes. There exists diversity in molecular mass of leguminous lectin. Phaseolus vulgaris, also known as common bean, has 64 kDa dimeric protein consisting of two 32 kDa subunits (Chan et al. 2012), while Phaseolus lunatus L. has 128 kDa. Legume lectins can oligomerise their monomeric structure into more complex structure, forming dimmers trimers, and tetramers that are different in their native and denatured structures (Loris et al. 1998).
The previous work showed that lectins contribute tremendously in increasing our knowledge regarding protein chemistry. For understanding folding process of oligomeric protein, legume lectin serves as attractive models. Numerous legume lectins have been considered for their unfolding behaviour in the presence of denaturing agent like temperature, urea or guanidine hydrochloride. This includes chickpea lectin also. In 2013, Wakankar et al. revealed in silico studies on recombinant lectin isolated from the seeds of Cicer arietinum employing biophysical and bioinformatics tools. Thermal denaturation of rCAL caused rapid secondary structural rearrangements above 50 °C and transient exposure of hydrophobic residues at 55 °C, leading to aggregation. Their studies further revealed that thermal and chemical denaturation of rCAL is irreversible.
Sugar specificity
Based on carbohydrate-binding specificity, lectins can be classified as galactose binding, glucose binding, mannose binding and N-acetyl-d-galactosamine binding (Peumans et al. 2001). In the present study, lectin has shown affinity to fetuin and N-acetyl-d-galactosamine at a concentration of 20 and 50 mM respectively. Similar finding was observed in Chenopodium lectin, where lectin exhibited the affinity for complex glycoproteins like fetuin (Suseelan and Mitra 2001). Our current results are in agreement with earlier studies. Different taxa display numerous sugar specificities. Lectin from Bilozema bean is galactose and N-acetyl-d-galactosamine binding (Koval’chuk 2006). Lectin from French bean cultivar no. 1 is glucuronic acid binding (Chan et al. 2011). There are several other lectins, which do not exhibit specific carbohydrate binding (Sharon and Lis 1972; Salahuddin 1992). For example, a well-known Phaseolus vulgaris agglutinin showed hemagglutinin activity for complex oligosaccharide structures and not simple sugars (Kornfeld et al. 1972).
Effect of temperature, pH, and metal ion
CAL had a moderate thermostability and pH stability (Fig. 3a, b). The assay on effect of temperature demonstrated lectin stability unaffected up to 50 °C and beyond this temperature activity was abruptly lost. Majority of legume lectins display temperature stability to this temperature range. The examples includes Artocarpus hirsuta (Gurjar et al. 1998), Vigna mungo (Suseelan et al. 1997) and Cajanus cajan (Ahmad et al. 1999). The lectin showed pH stability over a wide range (pH 5–10) similar to other plant lectins. The chickpea lectin demonstrates varied pH performance in contrast to the peanut agglutinin tetramers which dissociate into dimers (Decastel et al. 1985) while wheat germ agglutinin (WGA) that dissociates into monomers (Monsigny et al. 1979) at low pH values. CAL is a metalloprotein, but does not require metal ions for its activity. A. hirsuta (Gurjar et al. 1998) and Maclura pomifera (Bausch and Poretz 1977) lectins are an example of a lectin not requiring metal ions for activity. While Con A, soybean and Dolichos biflorus lectins require Mn2+ and/or Ca2+, respectively (Goldstein and Hayes 1978; Jaffe et al. 1977; Borrebaeck et al. 1981). CAL thermostability and pH stability may prove favourable, as stable bioactive substances are more efficient during all phases of their processing vis-a-vis cloning and drug development.
Fig. 3.
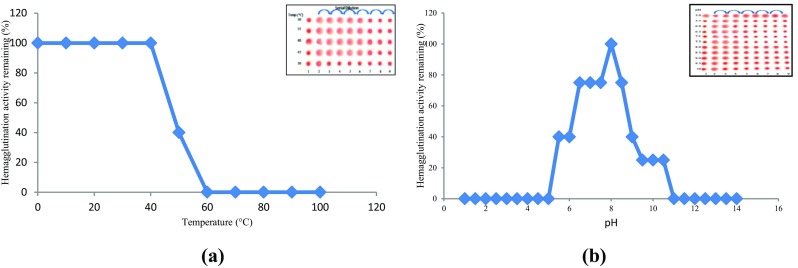
Effect of temperature (a) and pH (b) on hemagglutination activity of CAL
DNA protection assay
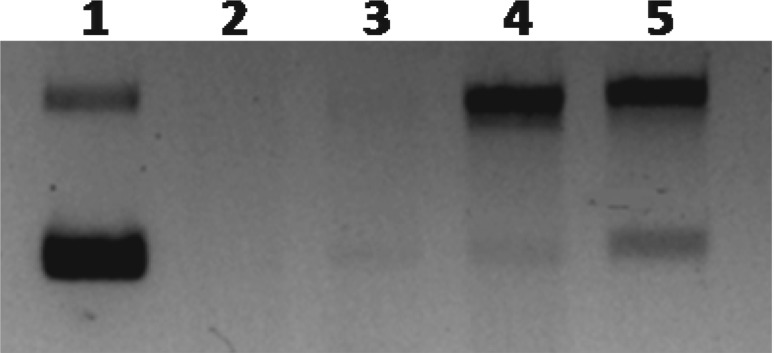
DNA protection by CAL is presented in Fig. 4. CAL displayed considerable protective activity to the bands of supercoiled and open circular plasmid DNA at 30 µg concentration. This assay was based on to protect the pUC18 plasmid DNA against damage caused by hydroxyl (·OH) radicals. The Fenton reaction can generate hydroxyl radicals that react with nitrogenous bases of DNA producing base and sugar radicals causing breakage of sugar phosphate backbone, resulting break down the DNA strands (Sonntag 1987). The scavenging effect of lectin may have the ability to reduce the Fe3+-dependent plasmid DNA nicking. The purified lectin was protecting the plasmid DNA by inhibiting the breakdown of DNA caused by Fenton’s reagent. CAL showed considerable reduction in the formation of DNA fragment and increased native form of plasmid DNA at a concentration of 30 μg. The previous studies on plant derived extracts reported to protect against free-radical mediated DNA damage (Kalita et al. 2012; Xu et al. 2012). For example, pudina extract (Mentha spicata L.) is a valued aromatic herb, used as culinary preparation throughout India exhibit DNA damaging protecting activity (Kumar and Chattopadhyay 2007). Recent studies by Verma et al. (2015) revealed that methanolic extracts of Carissa carandas leaves attributed DNA damage inhibition in vitro. This work has gathered experimental evidence on CAL for its capacity to protect organism and cell from oxidative DNA damage associated with cancer and degenerative diseases. CAL might therefore be used in the future to prevent cancer.
Fig. 4.
DNA protection assay of CAL. Lane 1, DNA (pBR322 plasmid alone); lane 2, DNA and Fenton’s reagent; lane 3, plasmid DNA, Fenton’s reagent and 10 µg lectin; lane 4, plasmid DNA, Fenton’s reagent and 20 µg lectin; and lane 5, plasmid DNA, Fenton’s reagent and 30 µg lectin
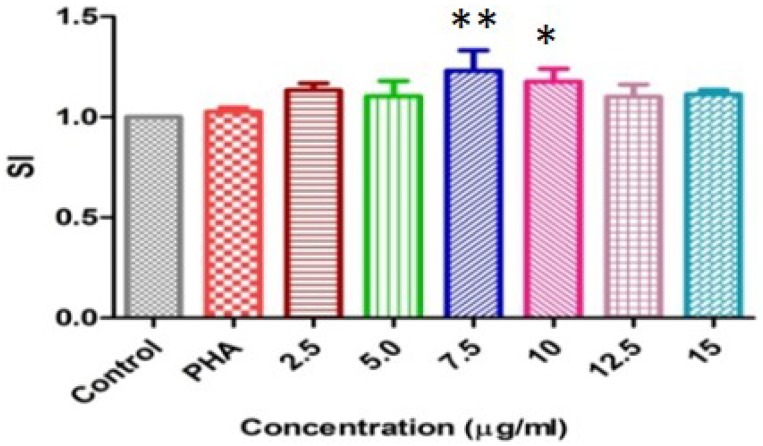
Mitogenic activity
Mitogenic activity was performed with PHA as a positive control. In vitro lectin (7.5 µg ml− 1) concentration stimulated mouse spleen cells maximum at ≤ 0.01 significant level and 10 µg ml− 1 gave at ≤ 0.05 level, while other lectin concentration did not give significant change (Fig. 5). Earlier study showed that the mitogenicity of the assayed lectins (PHA-L, Con A and PAA) indicated lectins from PHA-L and Con A possess higher potential for stimulation of cell division, showing effects at 10 µg ml− 1; on the other hand PAA showed maximum mitogenic effect at 25 µg ml− 1. Studies with Phaseolus acutifolius lectin (Villanueva et al. 2007) showed the same mitogenic capacity as the PAA. Recent studies (Chan et al. 2012) demonstrate that brown kidney bean lectin elicited mitogenic activity towards murine splenocytes. Our results are in consistency with earlier studies.
Fig. 5.
Mitogenic response of mouse splenocytes induced by CAL
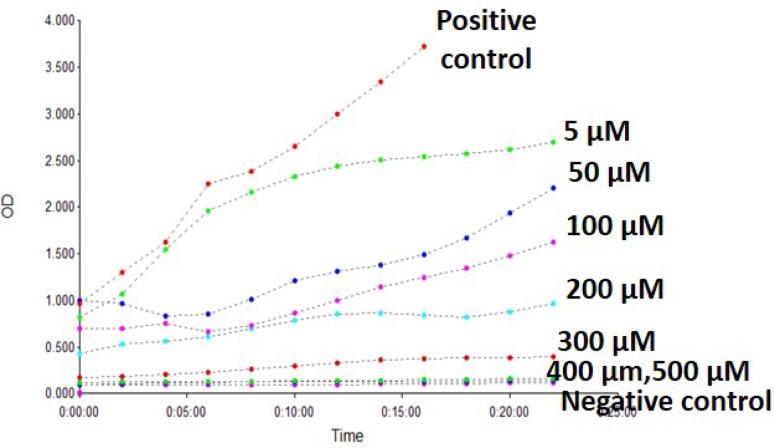
Anti-HIV-1 reverse transcriptase activity
Lectin isolates from legume seeds demonstrate important biological activities like anti-HIV activity (Boyd et al. 1997). CAL was able to inhibit HIV-1 reverse transcriptase with IC50 of 180 µM (Fig. 6). Literature domain suggests that only some of the plant lectins exhibit HIV-1 reverse transcriptase inhibitory activity. This includes lectin from Momordica charantia, mushroom Agaricus bisporus and the French bean P. Vulgaris (Wang and Ng 2001). The anti-HIV activities of lectins isolated from various sources like plants, algae, marine invertebrate animals and marine alga are reviewed (Sharon and Lis 1989). Until date, there are no reports on evaluating chickpea lectin for anti-HIV-1 activity. This needs to be further characterized for clinical applications using HIV-cell lines.
Fig. 6.
Inhibition of HIV-1 reverse transcriptase activity by CAL. Data shown here are representative of one of the three experimental repeats
2D-GE and peptide mass fingerprinting
The present study employed two-dimensional-assisted electrophoresis, which reveals nature of purified lectin is shown in the inset picture. This fractioned lectin was further digested with trypsin and resultant peptides were identified by MALDI-TOF/MS. The mass spectrometry revealed common tryptic peptide TNFGYINAAF RSSXNNEAYL FINGK in chickpea lectin. BLAST analysis corroborates 100% homology with chickpea lectin while 91% homology with Pisum sativum.
Animal management
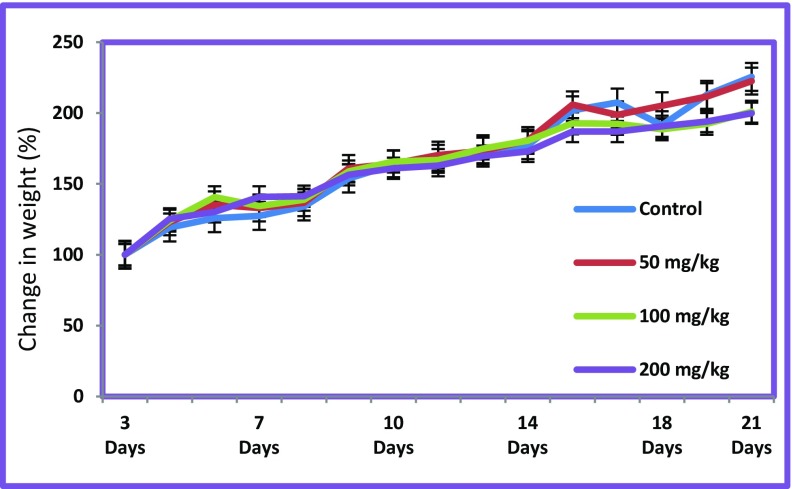
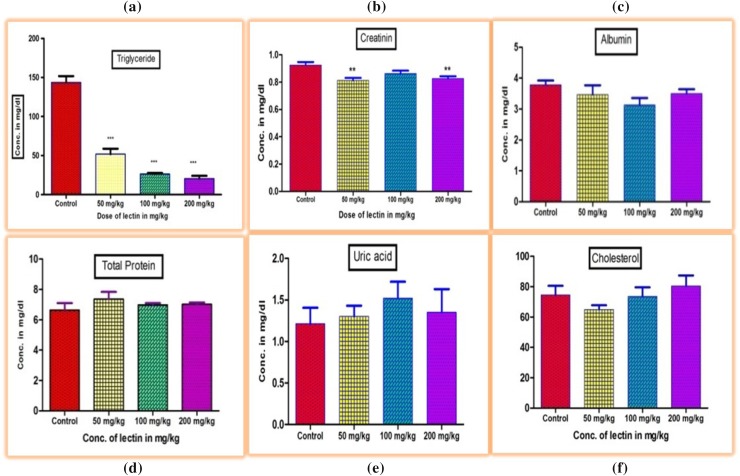
In the present study, control animals were fed with the standard pellet and distilled water, while chickpea lectin was fed to experimental rats only. The effects/changes can be attributed solely to chickpea lectin, since the feed intake was identical in all treatment group animals (Fig. 7). Our aim was to investigate the biochemical and functional changes in rats induced by chickpea lectin. The previous work on different lectin like phytohaemagglutinin (42 mg rat− 1 day− 1) and tepary bean lectin (50 mg kg− 1 of body weight) induce dose-dependent changes in animal tissue (Bardocz et al. 1995; Alatorre-Cruz et al. 2017; Coelho et al. 2017). Crude lectin contains several kinds of antinutritional factors such as trypsin inhibitor, tannins and phytic acid. (Pusztai 1991). Hence, to determine the effects of single factor such as chickpea lectin, the purified lectin was employed to exclude the effects due to other factors. The significant changes were observed under high dose of CAL. Similar studies using soybean agglutinin were carried out in rats to study effects (Zentian et al. 2003). The levels of total triglycerides observed in the animals groups fed with CAL were found significantly lower at concentrations of 50, 100 and 200 mg kg− 1 than control group (P ≤ 0.01) (Fig. 8a). The levels of total creatinine observed lower at concentration of 50 and 200 mg kg− 1 than control group (P ≤ 0.05) (Fig. 8b). Other serum parameter viz. albumin, total protein, uric acid, and total cholesterol did not show any significant changes to this lectin induction. Wheat-germ agglutinin has positive effects on rats. The dietary lectins are metabolic signals for gut modulating immune and hormone functions (Pusztai 1993). Our findings are in line with the previous research made on soybean lectin. CAL may bind to carbohydrate chains of glycoproteins and glycolipids on the small intestine membrane using its glycoprotein character. This may have resulted in morphological changes in various organs like liver, kidney, small intestine, heart, lungs and spleen.
Fig. 7.
Growth curve of normal diet rats and rats fed with CAL
Fig. 8.
Effect of CAL on serum triglyceride (a), creatinine (b), albumin (c), total protein (d), uric acid (e), and cholesterol (f) levels in rats after 21 days of treatment
Histopathological analysis
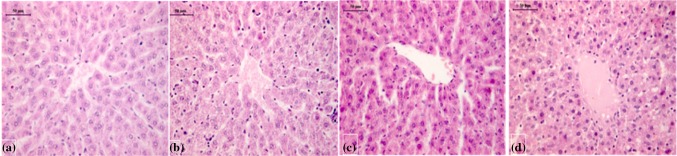
Histopathological analysis revealed only the liver of animals in the treated groups affected, while the kidney, small intestine, heart, lungs, and spleen were observed principally normal. Figure 9 shows the photomicrograph of liver sections of rats in the control and treated groups. Histopathological observations in liver of control and exposed with different doses of lectin (50, 100 and 200 mg) on 21 day posttreatment are shown in Fig. 9(a–d). Liver of control rats showed normal hepatic cord arrangement, hepatic lobes, and hepatocytes with normal hepatic parenchyma (Fig. 9a). CAL administration did not produce changes in rats’ development. However, CAL showed hepatocyte degeneration at 200 mg kg− 1of lectin administration. This is the adverse effect of CAL administration associated with hepatic cells at the higher lectin doses. Lectins are considered as a antigenic molecule (Estrada-Martinez et al. 2017; Ferriz-Martinez et al. 2015; Bardocz et al. 1995). Once they reach the digestive track, they can trigger an immune activation response and/or put pressure on the liver. It is well known that each lectin exhibits effects on cells (Sharon and Lis 2004; Estrada-Martinez et al. 2017). However, chickpea lectin did not show any effect on the other body organs even at higher dose (200 mg kg− 1). Following CAL (50 mg kg− 1 body weight) exposure, liver histology exhibited granulovacuolar degeneration of hepatocytes, perinuclear clumping of the cytoplasm, and obliteration of the chromatin material. The increased level of activated kupffer cells was also visible (Fig. 9b). The liver sections of rats exposed with 100 mg kg− 1 lectin were shown a few peri portal and intraparenchymal small aggregates of macrophages and neutrophils with disturbed histoarchitecture (Fig. 9c). However, the rat liver exposed with 200 mg kg− 1 of lectin showed hepatocyte degeneration with accumulation of oedematous fluid (Fig. 9d).
Fig. 9.
Photomicrographs of rat liver section, a control and b–d lectin dose of 50, 100 and 200 mg kg− 1 respectively
Conclusions
Cicer arietinum L. seeds (cv.Digvijay) contain potential source of lectin. CAL demonstrated biochemical and functional properties vis-a-vis cholesterol lowering potential in vivo and anti-HIV-1 activity. Moreover, it showed DNA damage protection in vitro. Pharmacological investigations are necessary to determine possible mechanism of actions to this effect. The present investigations suggest that CAL ascertain be a potential candidate for biomedical applications.
Acknowledgements
Authors are grateful to UGC, New Delhi for providing research funding (Grant No: 40–154/2011). Ajay Kumar Gautam in particular thankful to UGC for providing research fellowship. Thanks are also due to Dr. P.K. Dash, Head-Virology Division, DRDE, Gwalior for providing expertise. Providing the seed samples by Mahatma PhuleKrishiVidyapeeth, Rahuri, Maharashtra is gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest.
References
- Agrawal P, Kumar S, Jaiswal YK, Das HR, Das RH. A Mesorhizobium lipopolysaccharide (LPS) specific lectin (CRL) from the roots of nodulating host plant, Cicer arietinum. Biochimie. 2011;93:440–449. doi: 10.1016/j.biochi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Ahmad SA, Khan RHB, Ahmad A. Physicochemical characterization of Cajanus cajan lectin: effect of pH and metal ions on lectin carbohydrate interaction. Biochem Biophys Acta. 1999;1427:378–384. doi: 10.1016/S0304-4165(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Alatorre-Cruz JM, Pita-López W, López-Reyes RG, Ferriz-Martínez RA, Cervantes-Jiménez R, et al. Effects of intragastrically-administered tepary bean lectins on digestive and immune organs: preclinical evaluation. Toxicol Rep. 2017;12:56–64. doi: 10.1016/j.toxrep.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardocz P, Grant G, Ewen SWB, Duguid TJ, Brown DS, Englyst K, Pusztai A. Reversible effect of phytohaemagglutinin on the growth and metabolism of rat gastrointestinal tract. Gut. 1995;37:353–360. doi: 10.1136/gut.37.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch JN, Poretz RD. Purification and properties of the hemagglutinin from Maclura pomifera seeds. Biochemistry. 1977;16(26):5790–5794. doi: 10.1021/bi00645a023. [DOI] [PubMed] [Google Scholar]
- Bhagyawant SS, Srivastava N. Assessment of antinutritional factors and protein content in the seeds of chickpea cultivars and their divergence. J Cell Tissue Res. 2008;8(1):1333–1338. [Google Scholar]
- Borrebaeck CAK, Lonnerdal B, Etzler ME. Metal chelate affinity chromatography of the Dolichosbz florus seed lectin and its subunits. FEBS Lett. 1981;130(2):194–196. doi: 10.1016/0014-5793(81)81117-9. [DOI] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Wu RJ, Gulakowski L, Rivera MI, Laurencot CM. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti D, Sarkar A, Mondal HA, Das S. Tissue specific expression of potent insecticidal, Allium sativumleaf agglutinin (ASAL) in important pulse crop, chickpea (Cicer arietinum L.) to resist the phloem feeding Aphis craccivora. Transgenic Res. 2009;18:529–544. doi: 10.1007/s11248-009-9242-7. [DOI] [PubMed] [Google Scholar]
- Chan YS, Wong JH, Ng TB. A glucuronic acid binding leguminous lectin with mitogenic activity toward mouse splenocytes. Protein Pept Lett. 2011;18:194–202. doi: 10.2174/092986611794475110. [DOI] [PubMed] [Google Scholar]
- Chan YS, Wong JH, Fang EF, Pan W, Ng TB. Isolation of a glucosamine binding leguminous lectin with mitogenic activity towards splenocytes and anti-proliferative activity towards tumor cells. PLoS One. 2012;7(6):e38961. doi: 10.1371/journal.pone.0038961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho LC, Silva PM, Lima VL, Pontual EV, Paiva PM, Napoleão TH, Correia MT. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/1594074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RA, Ng TB, Fong WP, Wan CC, Yeung HW. A comparison of human immunodeficiency virus type 1 inhibition by partially purified aqueous extracts of Chinese medicinal herbs. Life Sci. 1997;60:PL345–351. doi: 10.1016/s0024-3205(97)00227-0. [DOI] [PubMed] [Google Scholar]
- Decastel M, Boeck HD, Goussault Y, Bruyne DCK, Loontiens FG, Frenoy JP. Effect of pH on oligomeric equilibrium and saccharide-binding properties of peanut agglutinin. Arch Biochem Biophys. 1985;240:811–819. doi: 10.1016/0003-9861(85)90090-6. [DOI] [PubMed] [Google Scholar]
- Diaz IL, Partida ANG, Moreno LV. Legume lectins: proteins with diverse applications. Int J Mol Sci. 2017;18(6):1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury RA, Wallington EA. Carleton’s histological techniques. 5. New York: Oxford University Press; 1980. p. 195. [Google Scholar]
- Esteban R, Dopico B, Mun˜oz FJ, Romo S, Labrador E. A seedling specific vegetative lectin gene is related to development in Cicer arietinum. Phys Plant. 2002;114:619–626. doi: 10.1034/j.1399-3054.2002.1140416.x. [DOI] [PubMed] [Google Scholar]
- Estrada-Martínez LA, Moreno-Celis U, Cervantes-Jiménez R, Ferriz Martínez RA, Blanco-Labra A, García-Gasca T. Plant lectins as medical tools against digestive system cancers. Int J Mol Sci. 2017;18:1403. doi: 10.3390/ijms18071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faheina-Martins GV, da Silveira AL, Cavalcanti Márcio V, Ramos BC, Moraes MO, Pessoa C, Araújom DAM. Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol In Vitro. 2012;26:1161–1169. doi: 10.1016/j.tiv.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Ferriz-Martínez R, García-García K, Torres-Arteaga I, Rodriguez-Mendez AJ, Guerrero-Carrillo MJ, et al. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol Rep. 2015;2:63–69. doi: 10.1016/j.toxrep.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein IJ, Hayes CE. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Bio Chem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Guha G, Rajkumar V, Kumar RA, Mathew L. The antioxidant and DNA protection potential of Indian tribal medicinal plants. Turk J Biol. 2011;35:233–242. [Google Scholar]
- Gurjar MM, Khan MI, Gaikwad SM. Alpha-Galactoside binding lectin from Artocarpus hirsuta: characterization of the sugar specificity and binding site. Biochem Biophys Acta. 1998;1381:256–264. doi: 10.1016/S0304-4165(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Jaffe CL, Rogozinski SE, Lis H, Sharon N. Transition metal requirements of soybean agglutinin. FEBS Lett. 1977;82(2):191–196. doi: 10.1016/0014-5793(77)80582-6. [DOI] [PubMed] [Google Scholar]
- Kalita S, Kumar G, Karthik L, Rao KBV. In vitro antioxidant and DNA damage inhibition activity of aqueous extract of Lantana camara L. (Verbenaceae) leaves. Asian Pacific J Trop Biomed. 2012;2:S1675–S1679. doi: 10.1016/S2221-1691(12)60476-6. [DOI] [Google Scholar]
- Katre UV, Gaikwad SM, Bhagyawant SS, Deshpande UD, Khan MI, Suresh CG. Crystallization and preliminary X-ray characterization of a lectin from Cicer arietinum (chickpea) Acta Crystallogr. 2005;F61:141–143. doi: 10.1107/S1744309104032166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi H, Nomura A, Mizuno T, Kimura A, Chiba S. Isolation and characterization of a lectin from Grifola frondosa fruiting bodies. Biochem Biophys Acta. 1990;1034:247–252. doi: 10.1016/0304-4165(90)90045-X. [DOI] [PubMed] [Google Scholar]
- Koike T, Beppu H, Kuzuya H, Maruta K, Shimpo K, et al. A 35 kDa mannose-binding lectin with hemagglutinating and mitogenic activities from “Kidachi Aloe” (Aloe arborescens Miller var. natalensis Berger) J Biochem. 1995;118:1205–1210. doi: 10.1093/oxfordjournals.jbchem.a125008. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Gregory WT, Kornfeld SA. Red Kidney Bean (Phaseolus vulgatis) Phytohemagglutinin. Methods Enzymol. 1972;28:344–349. doi: 10.1016/0076-6879(72)28042-9. [DOI] [Google Scholar]
- Koval’chuk NV. Dynamic of lectin activity during germination of bean seeds (Phaseolus vulgaris L.) Ukr Biokhim Zh. 2006;78:130–134. [PubMed] [Google Scholar]
- Kumar A, Chattopadhyay S. DNA damage protecting activity and antioxidant potential of pudina extract. Food Chem. 2007;100:1377–1384. doi: 10.1016/j.foodchem.2005.12.015. [DOI] [Google Scholar]
- Laemmli UK, Favre M. Gel electrophoresis of proteins. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Liener IE. Toxic factors in edible legumes and their elimination. Am J Clin Nutr. 1962;11:281–298. doi: 10.1093/ajcn/11.4.281. [DOI] [PubMed] [Google Scholar]
- Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume lectin structure. Biochem Biophys Acta Protein Struct Mol Enzymol. 1998;1383:9–36. doi: 10.1016/S0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- Monsigny M, Sene C, Obrenovitch A, Roche AC, Delmotte F, Boschetti E. Properties of succinylated wheat-germ agglutinin. Eur J Biochem. 1979;98:39–45. doi: 10.1111/j.1432-1033.1979.tb13157.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Yasokawa D, Ikeda T, Nagashima K. Purification and characterization of two lectins from calllus of Helianthus tuberosus. Biosci Biotechnol Biochem. 1996;60:259–262. doi: 10.1271/bbb.60.259. [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peumans WJ, Van Damme EJM. Lectins as plant defense proteins. Plant Physiol. 1995;109:347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peumans WJ, Van Damme EJ, Barre A, Rouge P. Classification of plant lectins in families of structurally and evolutionary related proteins. Adv Exp Med Biol. 2001;491:27–54. doi: 10.1007/978-1-4615-1267-7_3. [DOI] [PubMed] [Google Scholar]
- Pusztai A. General effect on animal cells. In: Pusztai A, editor. Plant lectins. Cambridge: Cambridge University Press; 1991. pp. 105–159. [Google Scholar]
- Pusztai A. Dietary lectins are metabolic signals foe the gut and modulate immune and hormone functions. Eur J Med Chem. 1993;47:691–699. [PubMed] [Google Scholar]
- Qureshi IA, Dash P, Srivastava PS, Koundal KR. Purification and characterization of an N-acetyl-d-galactosamine-specific lectin from seeds of chickpea (Cicer arietinum L.) Phytochem Anal. 2006;17:350–356. doi: 10.1002/pca.925. [DOI] [PubMed] [Google Scholar]
- Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- Rodrigues e Lacerda R, Silva do Nascimento E, et al. Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L.var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int J Biol Macromol. 2017;95:1072–1081. doi: 10.1016/j.ijbiomac.2016.10.097. [DOI] [PubMed] [Google Scholar]
- Rudiger H, Gabius HJ. Plant lectins: Occurrence, biochemistry, functions and applications. Glycoconjugate J. 1992;18(8):589–613. doi: 10.1023/A:1020687518999. [DOI] [PubMed] [Google Scholar]
- Salahuddin A. Legume Lectins: homologous proteins with similar structure but distinct carbohydrate binding specificity. Indian J Biochem Biophys. 1992;29:388–393. [PubMed] [Google Scholar]
- Sharma U, Katre UV, Suresh CG. Crystal structure of a plant albumin from Cicer arietinum (chickpea) possessing hemopexin fold and hemagglutination activity. Planta. 2015;241(5):1061–1073. doi: 10.1007/s00425-014-2236-6. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177:949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53–62. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havliˇs J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2007;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Sonntag VC. The chemical basis of radiation biology. London: Taylor & Francis; 1987. [Google Scholar]
- Suseelan KN, Mitra R. Purification and characterization of a hemagglutinin isolated from the leaves of chenopodium (Chenopodium amaranticolor) Indian J Biochem Biophys. 2001;38:186–192. [PubMed] [Google Scholar]
- Suseelan KN, Bhatia CR, Mitra R. Purification and characterization of two major lectins from Vigna mungo (blackgram) J Biosci. 1997;22:439–455. doi: 10.1007/BF02703190. [DOI] [Google Scholar]
- Verma K, Shrivastava D, Kumar G. Antioxidant activity and DNA damage inhibition in vitro by a methanolic extract of Carissa carandas (Apocynaceae) leaves. J Taibah Univ Sci. 2015;9:34–40. doi: 10.1016/j.jtusci.2014.07.001. [DOI] [Google Scholar]
- Villanueva AC, Ortega HC, Jafarova FA, Garfias Y, et al. Lectin from Phaseolus acutifolius var. escumite: chemical characterization, sugar specificity, and effect on human T-lymphocytes. J Agric Food Chem. 2007;55:5781–5787. doi: 10.1021/jf063644k. [DOI] [PubMed] [Google Scholar]
- Wakankar MS, Krishnasastry MV, Jaokar TM, Patel KA, Gaikwad SM. Solution and in silico studies on the recombinant lectin from Cicer arietinum seeds. Int J Biol Macromol. 2013;56:149–155. doi: 10.1016/j.ijbiomac.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Wang HX, Ng TB. Examination of lectins, polysaccharo peptide, alkaloid, coumarin and trypsin inhibitor for inhibitory activity toward immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001;67:669–672. doi: 10.1055/s-2001-17359. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen M, Zhu Y, Qian P, Zhou Y, Wei J, Shen Y, Mijiti A, Gu A, Wang Z, Zhang H, Ma H. Isolation, identification and characterization of a new type of lectin with α-amylase inhibitory activity in chickpea (Cicer arietinum L.) Protein Pept Lett. 2017;24(11):1008–1020. doi: 10.2174/0929866524666170711120501. [DOI] [PubMed] [Google Scholar]
- Wong JH, Ng TB. Isolation and characterization of a glucose/mannose-specific lectin with stimulatory effect on nitric oxide production by macrophages from the emperor banana. Int J Biochem Cell Biol. 2006;38:234–243. doi: 10.1016/j.biocel.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Xu JG, Hu QP, Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J Agric Food Chem. 2012;60:11625–11630. doi: 10.1021/jf303771s. [DOI] [PubMed] [Google Scholar]
- Ye XY, Ng TB. Isolation of a new cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci. 2002;70:1129–1138. doi: 10.1016/S0024-3205(01)01473-4. [DOI] [PubMed] [Google Scholar]
- Zentian L, Li D, Qiao S, Zhu X, Huang C. Anti-nutritional effects of a moderate dose of soybean agglutinin in the rat. Arch Anim Nutr. 2003;57:267–277. doi: 10.1080/00039420310001594414. [DOI] [PubMed] [Google Scholar]