Abstract
The current study aimed to assess the binding potential of herbal lead molecules against the prioritized molecular targets of chikungunya virus (CHIKV) and dengue virus (DENV) by computational virtual screening and suggests a novel therapeutic intervention. Based on the metabolic pathway analysis and virulent functions, the non-structural and envelop proteins present in CHIKV and DENV were identified as putative drug targets. The structures of the protein not available in their native forms were computationally predicted by homology modeling. The lead compounds from 43 herbal sources were screened and their drug likeliness and pharmacokinetics properties were computationally predicted. The binding potential of selected phytoligands against the prioritized drug targets were analyzed by molecular docking studies. This study revealed that Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one) and Chymopain (disodium;4,5-dihydroxybenzene-1,3-disulfonate), natural flavonols present in Carica papaya and Gossypetin (3, 5, 7, 8, 3′, 4′-hexahydroxyflavone), a natural flavonoid available in Hibiscus sabdariffa were demonstrated promising good binding potential with minimum binding energy (kcal/mol) and maximum stabilizing interactions to the putative drug targets of CHIKV and DENV. The selected lead molecules demonstrated ideal drug likeliness, ADMET (adsorption, distribution, excretion, metabolism and toxicity) features required for the drug development. The molecular docking studies suggested that the presence of these compounds probably responsible for the antiviral properties of Carica papaya, which was traditionally known as therapeutic remedy for dengue viral infections. This study provides profound insight for the experimental validation of the applied approach and industrial scale-up of the suggested herbal lead molecules as promising lead candidates against CHIKV and DENV infections.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1303-2) contains supplementary material, which is available to authorized users.
Keywords: Chinkungunya virus (CHIKV), Dengue virus (DENV), Computational screening, Kaempferol, Chymopain, Gossypetin, Promising lead candidates
Introduction
Dengue virus (DENV) and chikungunya virus (CHIKV) are the most widespread flaviviruses that re-emerged throughout recent decades and contributed high-level global public health threats (Al-Tawfiq and Memish 2018; Carrillo-Hernández et al. 2018). Chikungunya and dengue, both the co-infections are transmitted by Aedes mosquito with the same geographical areas of infection (Perera-Lecoin et al. 2016). Dengue virus was first reported in China during AD 265–420 while Chikugunya virus was first reported in Tanganyika, Africa, in 1952 (Rashad et al. 2014; Dayaraj 2014). There are four serotypes of dengue viruses identified which included DENV-1, DENV-2, DENV-3, and DENV-4, where the infection of any of these serotypes showed a wide symptoms ranging from mild to severe dengue (SD) fever with various complications such as bleeding and plasma leakage (Soe et al. 2018). The co-infections transmitted through enzootic cycle such as sylvatic cycle and urban cycle. Sylvatic cycle circulates between forest residing mosquito and non-human primates; whereas urban cycle circulates between human-to-mosquito and mosquito-to-human (Powers 2015).
The World Health Organization (WHO) has recently reported the presence of DENV in more than 125 countries, and more than 50% of the society is at greater risk of DENV. Approximately 2.5 billion people from more than 110 countries are at threat of the infections caused by these viruses every year due to the lack of efficient therapies and treatments (Soe et al. 2018). Around 50–100 million cases were estimated to occur in more than 100 countries. The survey carried out in 2016 reported approximately 12,255 and 27,879 cases of chikungunya and dengue, respectively. Millions of the people were suffered from CHIKV infection across the world. Among them, India was one of the worst affected countries with over 1.4 million reported cases (Luis et al. 2016).
CHIKV and DENV are single-stranded positive sense RNA viruses of approximately 11 kb of genome size (Perera-Lecoin et al. 2016). CHIKV has two terminal open reading frames (ORFs). N-terminal codes for four non-structural proteins (NSP1-NSP4). 3′-terminal codes for five structural proteins [caspid protein, envelop proteins (E1, E2, E3) and RNA protein (6K)] and 5′- terminal codes for four non-structural proteins (NSP1, NSP2, NSP3 and NSP4) (Rashad et al. 2014). Whereas DENV genome contains a cap-like structure at 5′-terminal, however, lacks the 3′-polyadenylate tail. 5′-terminal open reading frame (ORF) codes for large number of poly-proteins and 3′-terminal codes for un-translated regions (UTRs) (Bruno 2011).
Till date, there are no reports of licensed vaccine available for dengue and chikugunya. One of the recent studies revealed that certain protein biomarkers were identified in understanding the severity of dengue viral infection by immune-associated protein microarray (Soe et al. 2018). Furthermore, modern drug discovery approaches depend on computational biology and comparative genomics and proteomics which play a vital role in finding probable drug targets and novel lead molecules towards several molecular targets (Abu Bakar and Ng 2018; Tan et al. 2018). The metabolic pathways have become an essential ensemble in the field of computational biology. Previous studies have described that most of the viral pathogens utilize toll-like and RIG-I-like receptor signaling pathways. These pathogens consist of unique motifs which are identified in the surface of the cells and endosome-associated toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) (Paymen et al. 2009; Kawai and Akira 2008). Based on this knowledge, it can be assumed that surface protein plays a vital role in the interaction of the virus to the host and probably considered as putative molecular targets. The selected targets have diverse range of functions: CHIKV non-structural proteins take part in cleaving and cellular protein inhibition. CHIKV envelop proteins helps in viral attachment. In the case of DENV, non-structural protein plays an important role in replication of the viral genomes, and envelop and caspid proteins take part in virion budding and replication.
The medicinal plants are known to possess several innumerable bioactive compounds and depicted importance in tackling invasive viral infections which include DENV and CHIKV. The formulations of bioactive compounds from several herbal sources have previously demonstrated to possess good antiviral activities (Powers and Setzer 2016; Vázquez-Calvo et al. 2017; Gómez-Calderón et al. 2017; Dhama et al. 2018). Although the extract of papaya (Carica papaya) is known to have antiviral activities against DENV, major compounds present in papaya extract and their therapeutic potential is yet to be explored. Further, to the best of our knowledge, the antiviral potential of compounds present in the herbal sources commonly found in India such as Hibiscus sabdariffa are not yet elucidated. Thus, screening of lead molecules from these herbal sources against the putative molecular targets provides crucial ideas for the development of future antiviral agents against DENV and CHIKV. Hence, the present study aimed to screen several natural molecules from herbal sources that can potentially bind to prioritized drug targets of both DENV and CHIKV, and predict the probable inhibitory mechanism of these lead molecules by computational screening.
Materials and methods
Metabolic pathway analysis using KEGG
The metabolic pathway analysis was carried out by database search in Kyoto Encyclopedia of Genes and Genomes (KEGG). The metabolic pathways of host systems of DENV and CHIKV were retrieved from KEGG pathway database. Most of the virus families follow the RIG-I-like and Toll like pathways, and play a major role in triggering anti immune response in the host (Yoneyama and Fujita 2009; Zhong et al. 2008). The target genes such as MITA (Mediator of IFN regulatory transcription factor 3 activation), NLRX1 (NLR Family Member X1) (Moore et al. 2008), TRAF3 (Bowie 2010), IPS-1(interferon-β promotor stimulator-1) were selected based on network analysis and functional roles by detailed pathway analysis.
Prediction of probable drug targets
Through extensive analysis of the metabolic pathways, the target genes which take part in triggering anti-immune response in the host cell were identified. Thus, the protein targets which perform the resembling functions were retrieved from UniProt-KB. The non-structural proteins such as NSP2 and NSP3, and envelop proteins such as E1 and E2 were selected as the probable drug targets of CHIKV. Similarly, the non-structural proteins such as NS1 and NS5, and envelop protein ENVP were selected as the putative drug targets of DENV.
Molecular modeling of target proteins
The three-dimensional structures of the probable drug targets which are not available in their native form were modeled by homology modeling by Modeller 9v18 (Fiser and Sali 2003). Thus, the three-dimensional structures of CHIKV non-structural protein 3 (NSP3), CHIKV envelop protein 1 (ENVP1), CHIKV envelop protein 2 (ENVP2), DENV non-structural protein 1(NS1), DENV non-structural protein 5(NS5) and DENV envelope protein (Protein E) were computationally predicted. The amino acid sequences of these proteins were retrieved from Uniprot-KB. The best template structures were obtained by BLAST search based on the alignºy ranges from 30 to 98%, query coverage ranges from 60 to 100% and E-value ranges from 0.00 to 8e-132. The description of the best templates selected for homology modeling of the protein targets are shown in Supplementary material, Table 1. The template structures were retrieved from PDB database (Meyer 1991) and were saved in .atm format. To get the best homologous template, multiple sequence alignment between target and template was performed by Clustal W (Thompson et al. 2002) and the file was saved in PIR format. The alignment file was saved in .aln format. The script file was prepared in .py format and homology modeling was carried out by Modeller 9v18 (Fisher and Sali 2003). The secondary structures of the predicted models were visualized by PyMOL (Seeliger and de Groot 2010).
Model refinement and validation
The hypothetical proteins were refined and validated by various bioinformatics tools. The stereochemical validity of the models was predicted by ProCheck (Ramachandran plot) (Laskowski et al. 1993). The quality factors of modeled proteins were analyzed by ProSA (Wiederstein and Sippl 2007) in the form of z score. The quality check of modeled proteins by assessing the average residues qualifying the 3D–1D score was predicted by Verity 3D. The quality factor of the modeled proteins by the output plot of error function was predicted by ERRAT (Colovos and Yeates 1993). The models were further refined and assessed by the forced field such as GROMOS, ANOLEA and QMEAN available in SWISSMODEL server (Torsten et al. 2003). The hypothetical models were energy minimized by Modrefiner (Xu and Zhang 2011).
Selection and screening of lead compounds
Based on the medicinal importance of the plants, herbal leads were selected by exclusive literature analysis. 107 compounds from 43 medicinal plants were selected. The major phytochemicals present in Carica papaya, Hibiscus sabdariffa, Catharanthus roseus, Rauvolfia serpentina, Berberis, Celastrus paniculata, Ailanthus, Plumbago indica, Diospyros montana, Allium sativum, Azadirachta indica, Acacia catech, Coleus forskohlii, Thevetia, Heliotropium indicum, Thevetia, Cephalotaxus, Nothapodytes nimmoniana, Digitalis purpurea, Papaver somniferum, Strychnos nux-vomica, Datura stramonium, Camellia sinensis, Achyranthes aspera, Bacopa monnieri, Phoenix dactylifera, Calotropis gigantea, Baccharis trimera, Cassia auriculata, Capparis spinosa, Heliotropium indicum, Imperata cylindrica, Jasminum officinale, Jatropha curcas, Madhuca longifolia, Michelia formosana, Phyllanthus emblica, Piper betle, Piper longum, Piper nigram, Myristica fragrans and Cuminum cyminum(cumin) were selected in the present study. The three-dimensional structures of the major phytochemical compounds present in these plants were retrieved from PubChem database. The coordinate files of selected molecules were retrieved in the form of SDF/MOL format and converted to PDB format by Open Babel (O’Boyle et al. 2011).
Prediction of drug likeliness and ADMET for selected compounds
The selected lead compounds were screened for ideal drug-likeliness, ADME and toxicity properties by PreADMET server (Veber et al. 2002). Lipinski rule of five (Lipinski et al. 2001), CMC (Comprehensive Medicinal Chemistry) rule (Ajay et al. 1998), MDDR (MDL Drug Data Report)-like rule (Frimurer et al. 2000), WDI (World drug index)-like rule (Wagener et al. 2000) and Lead-like rule were used as filters for the prediction of the drug-likeliness properties of the compounds. The ADME prediction was carried out for various statistical models available in PreADMET such as blood brain barrier (BBB) (Ajay et al. 1999), Human intestinal absorption (HIA), caco2 cell permeability (Yazdanian et al. 1998) and MDCK cell permeability (Irvine et al. 1999). The statistical models for toxicity in algae models (Ames and Gold 2000), Ames test (Mortelmans and Zeiger 2000), carcinogenicity in mouse and rat models, daphina toxicity, hERG inhibition, fish toxicity (Medaka and Minnow), TA100_10RLI test, TA100_NA, TA1535_10RLI and TA1535_NA were used to predict the toxicity of the selected lead molecules.
Molecular docking studies of probable dug targets with the selected leads
The molecules filtered by drug likeliness and pharmacokinetic prediction which qualified the features required for ideal lead molecules were selected. The three selected ligands were docked against all the selected protein targets of CHIKV and DENV by AutoDock Vina (Trott and Olson 2010). The selected receptors were energy minimized by ModRefiner before the docking studies. The target protein structures were loaded into the file and the polar hydrogen atoms were added. The probable binding pockets present in the selected receptors were predicted by Q-SiteFinder (Laurie and Jackson 2005). The ligand structure was loaded into the program and the root atoms, number of torsion, rotatable and non rotatable bonds were assigned to the ligand and the file was saved in .pdbqt format. The grid box was assigned for target structures by setting the 3D coordinates for the binding pocket. The x, y, and z coordinates were specified in the configuration file. The total grid point per map for each of the selected receptor was assigned by fixing the values for number of points in x, y, and z-dimensions (size_x, size_y, size_z). Similarly, the parameters values for center grid box of each receptor (center_x, center_y, center_z) were assigned as per the standard protocol (Trott and Olson 2010). The exhaustiveness was set as eight. The docking was performed through command prompt. The output (log) files of nine best confirmations were generated. The best docked conformations were ranked according to minimum binding energy (kcal/mol), docking score, cluster RMSD, number of hydrogen bond formed and the residues present at the close proximity of 1. 0 Å VWD scaling factors. Out of which the first confirmations were selected as the best predicted model.
Similarly, the three-dimensional structures of the two putative targets of CHIKV (non-structural protein 2; PDB ID: 3TRK) and DENV (Caspid Protein–Protein C; PDB: 1R6R) which are available in their native form were retrieved from PDB database. The binding pockets present in the selected receptors were predicted by Q-SiteFinder. The grid box was assigned to the binding pockets of each receptor and docking was performed by Lamarckian Genetic algorithm as per the standard protocol. Each selected targets from CHIKV and DENV were docked against three selected ligands such Kaempferol (Carica papaya), Chymopain (Carica papaya) and Gossypetin (Hibiscus sabdariffa) by AutoDock Vina. Out of various conformations of best binding poses, the first conformation was selected. The interaction between target and ligand was within VDW scaling factor 1.00 which was selected based on the binding energy (kcal/mol), hydrogen bonding, cluster RMSD, number of interacting residues of the first and best docked pose. The binding potential of phytoligands was thus predicted.
Results and discussion
Pathway analysis
The protein targets were selected by the analyzing the interaction between the host (Homo sapiens) and pathogen by KEGG pathway searches. It was found that most of the virus families follow the RIG-I-like and Toll-like pathways (Kawai and Akira 2008). Studies reported that the replication of the CHIKV could be through Toll-like receptors (TLR3), which is responsible for the production of antiviral response and interferons. Innate immune responses play a vital role in clearing the CHIKV infection in cells (Li et al. 2012). Thus, through metabolic pathway analysis, the proteins which take part in triggering innate immune response and helps in immune cell invasion were selected as the protein targets. MITA (Mediator of IFN regulatory transcription factor 3 activation), NLRX1 (NLR Family Member X1), TRAF3 and IPS-1 (Fig. 1) genes were selected from the RIG-I-like pathway based on the functional role. Previous studies also suggested that membrane proteins play a major role in trigging anti-immune response in virus family and can be explored as molecular targets (Yoneyama and Fujita 2009). MITA is a transmembrane protein, which is an important adapter protein to mediate the induction of type 1 interferon. This protein acts as a facilitator for innate immune signaling (Zhong et al. 2008). NLRX1 is a protein coding gene which regulates antiviral immunity of mitochondria (Moore et al. 2008). IPS-1 (interferon-β promotor stimulator-1) is mitochondrial signaling protein which produces innate response against RNA viruses (Fredericksen 2008). TRAF3 is a TNF receptor-associated factor which takes part in signal transduction, which is an important factor for the activation of the immune response (Bowie 2010). Hence, considering all these key properties of the proteins, these protein coding genes were selected as putative molecular target for computational drug screening.
Fig. 1.
RIG-I-like receptor signaling pathway. The RIG-I-like pathway was analyzed to predict the probable targets taking part in immune system of the viral cells. MITA, NLRX1, IPS-1, and TRAF3 are the target protein genes selected based on their functions.
(Source: KEGG pathway database)
Prediction of probable drug targets
The protein targets were selected through extensive analysis of RIG-I-like pathway, which revealed that the membrane protein play a major role in immune response of viral cells (Yoneyama and Fujita 2009). The genes which perform similar functions were selected from pathway analysis, and proteins which play resembling functions in the CHIKV and DENV were selected as the target proteins. In the present study, eight proteins were selected as the probable drug targets out of which four targets for DENV and four targets for CHIKV. The three-dimensional crystal structures of the proteins were retrieved from PDB database (Meyer 1991) (Table 1). Based on membrane functions, the protein targets selected were CHIKV non-structural proteins 2 (PDB: 3TRK) which cleave and release the four mature proteins and perform cellular protein inhibition (Xie et al. 2015), CHIKV non-structural proteins 3 which promote the viral nucleocapsid releases in cytoplasm after endosome and viral membrane fusion (Malet et al. 2009), CHIKV envelop protein 2 (CHIKV E2) is responsible for viral attachment to target host cell, by binding to the cell receptor (Khan et al. 2002). A DENV non-structural protein 1 is involved in the immune evasion, pathogenesis and viral replication (Puerta-Guardo et al. 2016). A DENV non-structural protein 5 takes part in the replication of the viral genome and functions as the capping of genomes in the cytoplasm (Ashour et al. 2009). DENV caspid protein (PDB: 1R6R) plays a role in virus budding by binding to membrane and gathering the viral RNA. DENV envelope protein plays an important role in virion budding in the endoplasmic reticulum (Ma et al. 2004).
Table 1.
The probable drug targets identified based on the metabolic pathway analysis for CHIKV and DENV
| Virus | Target protein gene | Functions | Length of the sequence | PDB_ID/Hypothetical model |
|---|---|---|---|---|
| CHIKV | Non-structural protein 2 (NSP2) | Cleaves and releases the four mature proteins and cellular protein inhibition | 798 | 3TRK |
| CHIKV | Non-structural protein 3 (NSP3) | Binding protein plays a major role in RNA synthesis | 530 | Theoretical model |
| CHIKV | Envelop protein 1 (ENVP1) | Oxidoreductase protein promotes viral nucleocapsid release in cytoplasm after endosome and fusion of viral membrane fusion | 439 | Theoretical model |
| CHIKV | Envelop protein 2 (ENVP2) | Helps for viral attachment to target of the host cell, cell receptor binding | 423 | Theoretical model |
| DENV | Non-structural protein 1(NS1) | Involved in immune evasion, pathogenesis and viral replication | 352 | Theoretical model |
| DENV | Non-structural protein 5 (NS5) | Replicates the viral genome and performs the capping of genomes in the cytoplasm | 899 | Theoretical model |
| DENV | Caspid protein (Protein C) | Plays a major role in virus budding by binding to membrane and gathering the viral RNA | 100 | 1R6R |
| DENV | Envelope protein (Protein E) | Play a important role in virion budding in the ER | 495 | Theoretical model |
These targets were considered as the potential targets for computational drug screening. The targets were either modeled or retrieved from PDB
Molecular modeling and assessment of protein targets
The proteins which lack the three-dimensional structure were modeled by Modelleor 9v18 (Fiser and Sali 2003) and the description is shown in Supplementary materials, Table 1. The best templates were obtained based on BLAST search against PDB database. The parameters such as total score, query coverage, percentage of identity and expected value (E value) were used for the selection of ideal templates (Supplementary materials, Table 1). The secondary structure of the modeled proteins after homology modeling is shown in Supplementary materials, Fig. 1. The quality assessment of modeled proteins was performed using various bioinformatics tools. The stereochemical validation of the theoretical models in terms of z score was obtained from ProSA (Wiederstein and Sippl 2007) are shown in Supplementary material, Fig. 2. The z score of the modeled proteins was estimated to be − 8.97, − 5.36, − 7.81, − 11.85, − 5.52, − 8.97 for CHIKV NSP3, CHIKV E1, CHIKV E2, DENV NS5, DENV NS1 and DENV ENVP, respectively. These results depicted that the z scores of the models were in the range of acceptable cut-off in comparison with the z scores expected in the experimental structures. The stereochemical stability of the theoretical models predicted by ProCheck (Ramachandran plot) is shown in Supplementary material, Fig. 3. The prediction suggested that the modeled proteins showed good stereochemical stability and validity. Ramachandran plot of the modeled protein CHIKV E1 demonstrated that 88.2% residues are located in most favored region, 11.2% residues are present in additionally allowed region and 0.6% residues are located in disallowed region. Similarly, Ramachandran plot of the hypothetical model of CHIKV E2 demonstrated 86.3, 12.3 and 0.7% of residues present in most favored, additionally allowed and disallowed regions, respectively. CHIKV NSP3 demonstrated 94.8, 4.5, and 0.7% of residues present in most favored, additionally allowed, and generously allowed region, respectively. The 3D model of DENV NS1 demonstrated 88.6, 10.1, 0.7 and 0.7% in most favored, additionally allowed, generously allowed and disallowed region, respectively. The theoretical model of DENV NS5 demonstrated 90.4, 8.4, 0.5 and 1.0% residues in most favored, additionally allowed, generously allowed and disallowed region, respectively. The model of DENV ENVP protein demonstrated 94.8, 4.5, and 0.7% in most favored, additionally allowed, and generously allowed region, respectively. Thus, it is clear that the theoretical models of the targets showed that more than 90% of the residues located in favored region with high stereochemical validity. The overall quality factors of modeled proteins were further validated using ERRAT which demonstrated 93.987, 94.881, 91.548, 76.552, 93.625 and 91.549 quality factor for CHIKV E1, CHIKV E2, CHIKV NSP3, DENV NS1, DENV NS5 and DENV ENVP, respectively, as shown in Supplementary material, Fig. 4. Further, the model evaluation and quality check performed for the theoretical models by GROMOS, ANOLEA and QMEAN are shown in Supplementary Table, Fig. 5. From the computational validation exercises, it is clear that most of the structures qualify the assessment criteria required for a quality model. Hence, these modeled structures might have similar accuracy in comparison with experimentally solved structures and probably considered to be the putative drug targets.
Computational screening of phytoligands
Among 107 herbal-based molecules selected, 42 compounds qualified the drug-likeliness prediction that was predicted by the statistical filters such as Lipinski’s rule of five, MDDR-like rule, lead-like rule, and CMC-like rule (Supplementary material, Table 2). Further screening suggested that 27 compounds qualified for ADME and selected for toxicity prediction. The predicted ADME results suggested that most of the molecules predicted to have good skin and Caco2 permeability, good human intestinal absorption and other features (Supplementary material, Table 3). The lead compounds were predicted to be positive for mouse or rat carcinogenicity models. The ADEMET predictions of the selected molecules are shown in Supplementary material, Table 4. The molecules which qualified various criteria required for toxicity, such as algae test, mutagenicity assay, carcino mouse or rat models, were selected as the lead compounds for the study.
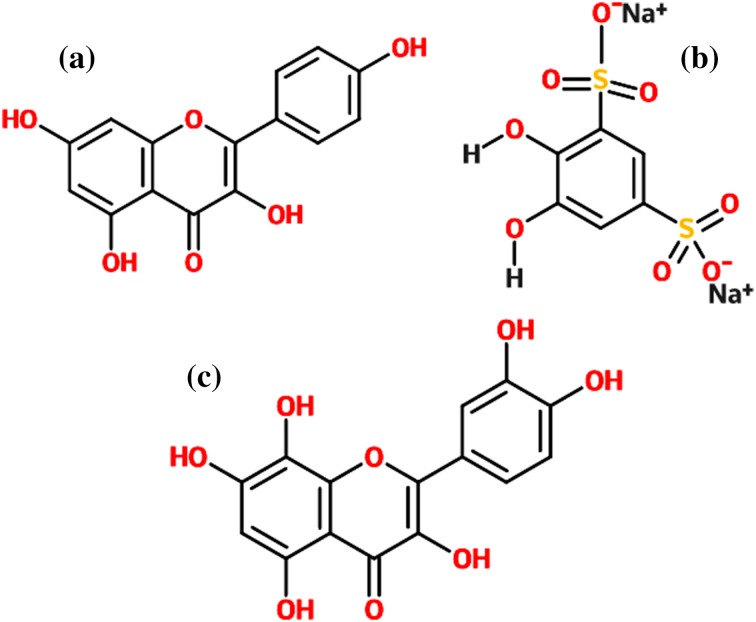
The computational screening suggested that Papain, Carpaine, Kaempferol, Chitinase, Glutaminol, Cysteine, and Chymopain were selected as the possible ligands present in Carica papaya with best drug likeliness and ADMET properties. Gossypetin and Anthocyanin were selected were the probable ligands present in Hibiscus sabdariffa. Atropine and hyoscyamine are selected as the possible compounds from Datura stramonium. Out of 27 molecules screened, 3 compounds, namely, Kaempferol, Chymopain and Gossypetin, were found to be ideal lead molecules in terms of carcinogenicity in mice or rat models and minimum scoring function for algae model. Hence, these lead molecules were considered as the probable ligands. Chymopain was predicted to be positive for carcinogenicity in mouse and rat models, and hence considered as a lead compound. Since the drug discovery process is the long-term process, computer-aided screening provides an efficient way in screening and scrutinizing the compounds with ideal drug likeliness and pharmacokinetics features, major parameters required for drug discovery and development processes.
Study of the binding potential of phytoligands against probable drug targets
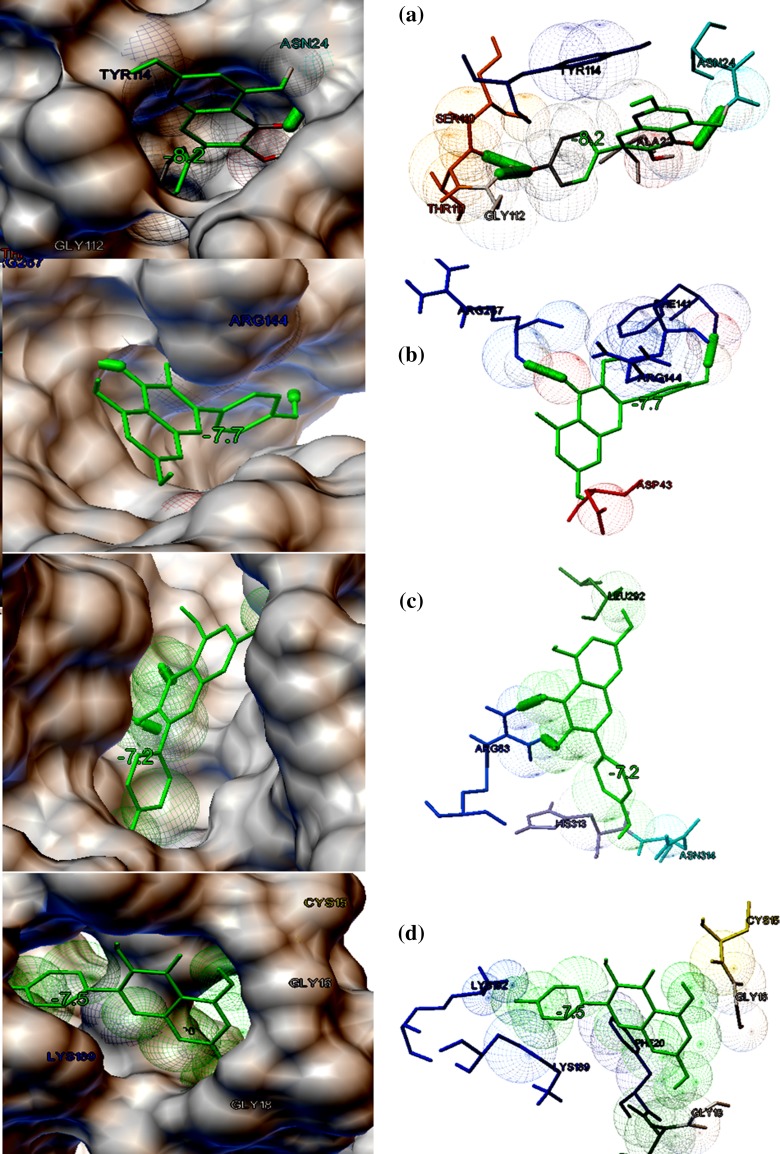
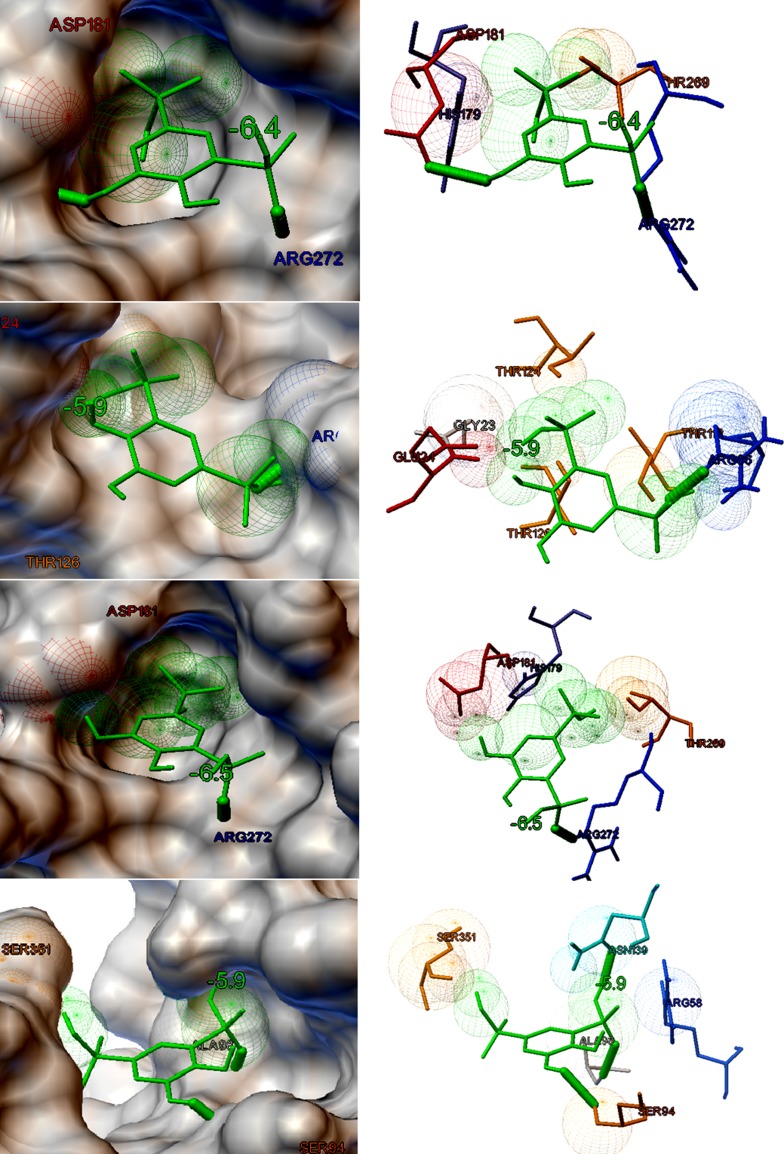
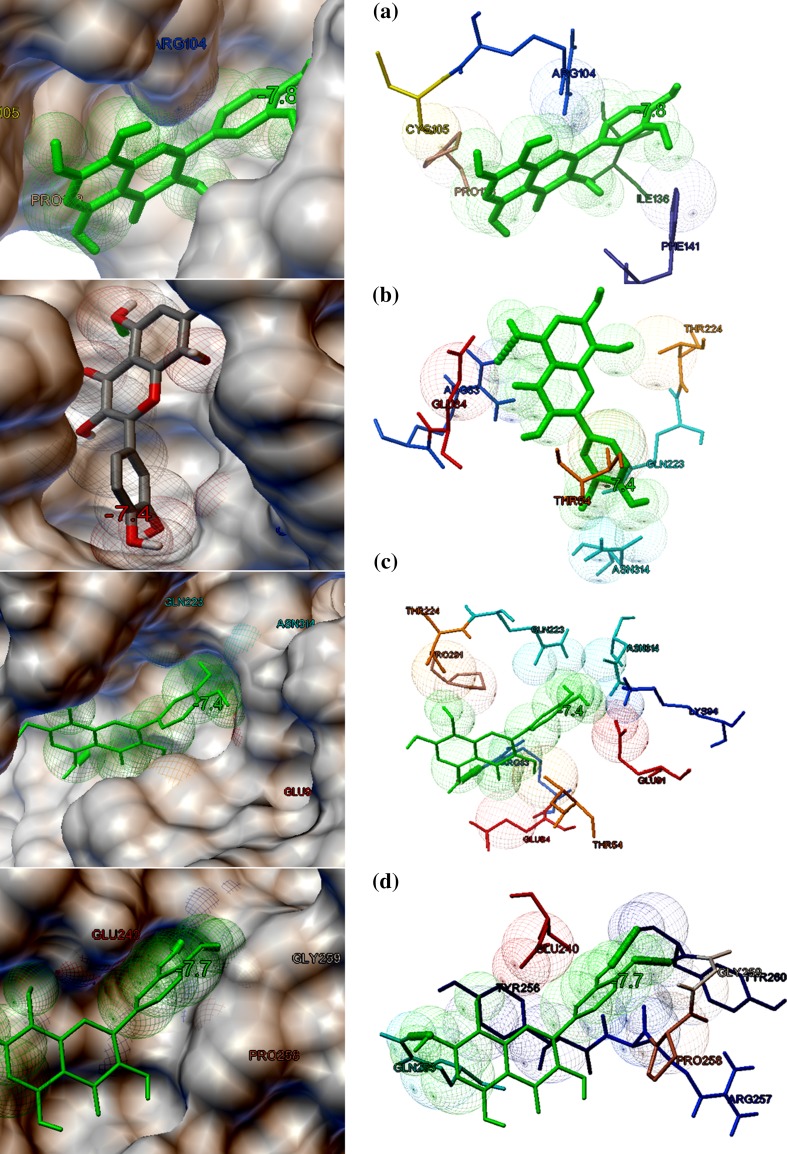
The eight target proteins were selected as the probable targets for the study based on their functional properties. The selected protein targets were docked against three lead compounds such as Kaempferol and Chymopain present in Carica papaya and Gossypetin present in Hibiscus sabdariffa (Fig. 2). The best docked conformations were analyzed based on their interaction, binding energies (kcal/mol), cluster RMS values, number of hydrogen bonds taking part in the interaction and number amino acid residues present at the binding site. The binding potential of three ligands screened from 107 compounds towards the selected drug targets by molecular docking studies are shown in Table 2. Docking studies suggested that Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one), a natural flavonol, present in Carica papaya was predicted to possess a good interaction with choline-binding protein of CHICKV known as chikungunya non-structural protein 3(NSP3) which is shown in Fig. 3a. Kaempferol binds with NSP3 with the binding energy of −8.2 kcal/mol. The interaction is stabilized by three hydrogen bonds and Ala22, Asn24, Ser110, Thr111, Gly112 and Tyr114 were the residues present at the binding site (Fig. 3a). Kaempferol also showed good binding potential to chikungunya envelop protein E2 with the binding energy of − 7.7 kcal/mol. The interaction between CHIKV E2 and Kaempferol is stabilized by two hydrogen bonds and Asp43, Phe141, Arg144 and Arg267 were identified to be the interacting residues present at the binding site (Fig. 3b). Kaempferol showed good binding affinity towards DENV envelop protein with the binding energy of − 7.2 kcal/mol and Arg83, Leu292, His313 and Asn314 were identified to be the major binding residues at the binding pocket (Fig. 3c). Similarly, Kaempferol demonstrated good binding to dengue non-structural protein 1(NS1) with the binding energy of − 7.5 kcal/mol. There were no hydrogen bonds formed and Ile19, Cys15, Gly16, Gly18, Phe20, Lys92 and Lys189 were identified to be the amino acid residues present at the binding cavity (Fig. 3d). Chymopain (disodium; 4, 5-dihydroxybenzene-1, 3-disulfonate) was another selected lead molecule probably present in Carica papaya which showed good binding potential against chikungunya non-structural protein 3(NSP3) with the binding energy of − 6.4 kcal/mol. The interaction was stabilized by two hydrogen bonds and His179, Asp181, Thr269 and Arg272 were found to be the interacting residues at the binding site (Fig. 4a). Chymopain also showed the good interactions with chikungunya envelop protein 2 with the binding energy of − 5.9 kcal/mol and the interaction was stabilized by a hydrogen bond. Gly23, Glu24, Arg86, Thr112, Thr124 and Thr126 were identified to be the interacting residues found at the binding site (Fig. 4b). Chymopain showed interaction with dengue envelop protein with the binding energy of – 6.5 kcal/mol and the interaction was stabilized by a hydrogen bond. His179, Asp181, Thr269 and Arg272 were identified to be the interacting residues present at the binding cavity (Fig. 4c). Similarly, Chymopain showed good interaction with dengue non-structural protein 1 with the binding energy of − 5.9 kcal/mol and the interaction was stabilized by three hydrogen bonds. Arg58, Ser94, Ala98, Asn139 and Ser351 were identified to be the interacting residues present at the binding pocket (Fig. 4d). Further, the molecular docking studies suggested that Gossypetin (3, 5, 7, 8, 3′, 4′-hexahydroxyflavone), a natural flavonoid available in Hibiscus sabdariffa, was selected as the lead molecule that showed good binding potential to chikungunya non-structural protein 2 (CHIKV NSP2) with binding energy of − 7.8 kcal/mol (Fig. 5a) and the interaction is stabilized by a hydrogen bond. Arg104, Cys105, Pro133, Ile136 and Phe141 were identified to be the interacting residues at the binding pocket. Gossypetin also showed good interactions with other selected target proteins. The best docked complexes between Gossypetin and CHIKV NSP3, CHIKV E2 and DENV NS1 were stabilized by the binding energies of − 7.4, − 7.4 and − 7.7 kcal/mol, respectively (Fig. 5b–d). The interacting residues present in the binding pocket and number of hydrogen bonds formed are shown in Table 2.
Fig. 2.
The two-dimensional structures of the best three lead molecules a Kaempferol b Chymopain c Gossypetin. The structure is drawn by the structure drawing tool eMolecules (https://www.emolecules.com)
Table 2.
The binding potential of selected herbal-based lead molecules towards the probable drug targets of DENV and CHIKV predicted by molecular docking studies by AutoDock Vina
| Ligand | Source plant | Protein targets | Cluster RMSD | Binding energy (kcal/mol) | Amino acid residues at the binding cavity | Number of hydrogen bonds and amino acid residues responsible for H bond formation |
|---|---|---|---|---|---|---|
| Kaempferol | Carica papaya | Chikungunya non-structural protein 3 (CHIKV NSP3) | 0.00 | − 8.2 | Ala22, Asn24, Thr111, Ser110, Gly112, Tyr114 | 3 (Asn24, Thr111, Gly112) |
| Chikungunya envelop protein 2 (CHIKV E2) | 0.00 | − 7.7 | Asp43, Phe141, Arg144, Arg267 | 2 (Phe141, Arg267) | ||
| Dengue envelop protein (DENV ENVP) | 0.00 | − 7.2 | Arg83 Leu292, His313, Asn314 |
2 (Arg83) | ||
| Dengue non-structural protein 1 (DENV NS1) | 0.00 | − 7.5 | Ile19, Cys15, Gly16, Gly18, Phe20, Lys92, Lys189 | Nil | ||
| Chymopain | Carica papaya | Chikungunya non-structural protein 3 (CHIKV NSP3) | 0.00 | − 6.4 | His179, Asp181, Thr269, Arg272 | 2 (Asp181, Arg272) |
| Chikungunya envelop protein 2 (CHIKV E2) | 0.00 | − 5.9 | Glu24,Gly23, Arg86, Thr112, Thr124, Thr126 | 1 (Arg86) | ||
| Dengue envelop protein (DENV ENVP) | 0.00 | − 6.5 | His179, Asp181, Thr269, Arg272 | 1 (Arg272) | ||
| Dengue non-structural protein 1 (DENV NS1) | 0.00 | − 5.9 | Arg58, Ser94, Ala98, Asn139, Ser351 | 3 (Arg58, Ser94, Ala98) | ||
| Gossypetin | Hibiscus sabdariffa | Chikungunya non-structural protein 2 (CHIKV NSP2) | 0.00 | − 7.8 | Arg104, Cys105, Pro133, Ile136, Phe141 | 1 (Ile136) |
| Chikungunya non-structural protein 3 (CHIKV NSP3) | 0.00 | − 7.4 | Arg83, Glu84,Thr54,Gln223,Thr224,Asn314 | 1 (Arg83) | ||
| Chikungunya envelop protein 2(CHIKV E2) | 0.00 | − 7.4 | Arg104, Cys105, Pro133, Ile136, Phe141 | 1 (Ile136) | ||
| Dengue non-structural protein 1 (DENV NS1) | 0.0 | − 7.7 | Glu240, Tyr256, Gln253, Gly259, Tyr260, Pro258, Arg257 | 3 (Gln 253, Gly259, Tyr260) |
Fig. 3.
The binding potential of Kaempferol present in Carica papaya against probable protein targets of DENV and CHICKV predicted by molecular docking studies. Both molecular surface display and stick figure representation of the binding pocket are shown in the figure. The binding residues and hydrogen bonds involved in the interaction are shown in figures. The thick green-colored sticks represent the hydrogen bonds stabilized in the interaction. a The best docked confirmation of CHIKV non-structural protein 3 NSP3 (choline-binding protein) interacted with Kaempferol (binding energy of − 8.2 kcal/mol). b The best docked confirmation of Kaempferol and CHICKV envelop protein E2 stabilized by the binding energy of -7.7 kcal/mol. c The best docked confirmation of Kaempferol and DENV envelop protein (binding energy of − 7.2 kcal/mol). d The best docked pose of Kaempferol and DENV non-structural protein 1(NS1) (binding energy − 7.5 kcal/mol)
Fig. 4.
The binding potential of Chymopain present in Carica papaya against probable protein targets of DENV and CHICKV predicted by molecular docking studies. Both molecular surface display and stick figure representation of the binding pocket are shown in the figure. a The best docked confirmation of Chymopain and CHIKV non-structural protein 3 NSP3 with the binding energy of − 6.4 kcal/mol. b The best docked pose of Chymopain and CHIKV envelop protein with the binding energy of − 5.9 kcal/mol. c The best docked confirmation of of Chymopain and DENV envelop protein with the binding energy of − 6.5 kcal/mol. d The best docked confirmation of Chymopain and DENV non-structural protein 1 (NS1) with the binding energy of − 5.9 kcal/mol
Fig. 5.
The binding potential of Gossypetin present in Hibiscus sabdariffa against probable protein targets of DENV and CHICKV predicted by molecular docking studies. Both molecular surface display and stick figure representation of the binding pocket are shown in the figure. a The best docked confirmation of Gossypetin and CHICKV non-structural protein 2 (NSP2) (binding energy − 7.8 kcal/mol). b The best docked confirmation of Gossypetin and CHIKV non-structural protein 3 (NSP3) stabilized by the binding energy of − 7.4 kcal/mol. c The best docked confirmation of Gossypetin and CHIKV envelop protein E2 stabilized by the binding energy of − 7.4 kcal/mol. d The best docked pose of Gossypetin and DENV non-structural protein 1 (NS1) (binding energy − 7.7 kcal/mol)
The current study based on computational modeling suggested that three selected herbal ligands demonstrated the good binding potential against the prioritized targets of dengue and chikungunya viruses. Previous studies based on computational modeling suggested that natural phytoligands can be used as potential inhibitors of viral infections such as dengue and chikungunya (Byler et al. 2016; Seyedi et al. 2016). Previous studies also suggested that plant-based bioactive compounds showed good inhibitory properties against various viral targets and found to be putative lead molecules in terms of bioactivity, safety and efficiency towards various protein targets (Ruwali et al. 2013).
From the study, it is clear that the lead compounds selected qualified the drug-likeliness and ADMET features and, therefore, can be prioritized as leads against the selected protein targets. Previous studies revealed that some non-peptide inhibitors of papain family can be used as potential inhibitors against dengue virus (Ramakrishnan et al. 2017). Thus, the current study provides the more detailed analysis of Chymopain which can probably be the putative ligand against DENV and CHIKV targets. There are reports on Kaempferol as the lead compound against various targets of DENV and CHIKV (Santhanam and Waheeta 2017); however, limited description is available on their binding potential and inhibitory activities towards the prioritized targets of DENV and CHIKV. Thus, the current study provides insight on further experimental validation and biochemical assays based on the computational prediction of the binding potential Kaempferol against DENV and CHIKV targets. Gossypetin is one of the very rarely studied molecules towards viral infection and present study provides insight on the binding potential of this lead molecule towards DENV and CHIKV targets, which also opens several future investigations. The antiviral potential of the herbal extract of Carica papaya has been well established, and the leaf and seed extracts have been used as traditional medicine against dengue fever in Southeast Asia. However, the bioactive compound responsible for the antiviral potential is yet to be established. The current study thus suggested that Kaempferol and Chymopain present in Carica papaya predicted to have potential binding towards the selected drug targets of DENV and CHIKV. The presence of these two lead molecules might be probable the reasons for the antiviral potential of Carica papaya extract. Similarly, the current study suggested that Gossypetin present in Hibiscus sabdariffa showed good binding with the selected drug targets of DENV and CHIKV. There are very limited reports available on the antiviral potential of Hibiscus extract.
The present study draws conclusion based on computational prediction and the applied aspect needed to be validated experimentally. Further, the binding potential of the lead molecules are predicted based on molecular docking studies; thus, molecular dynamic simulation studies are essential to validate the stability of the docked complexes. Further, the ligand-binding site analysis of similar targets or similar ligands can be performed to cross-verify the results and get accuracy of the prediction. In such case, the ligand structures can be overlaid in the binding pocket of the known receptor with their usual ligands and RMSD calculations of the superimposed structures can be performed to achieve better modeling. Furthermore, the drug likeliness and pharmacokinetic properties of the suggested molecules needed to be tested and validated with the purified form of the suggested lead molecules. The current study certainly provides fundamental insights for the future investigation.
Conclusion
Dengue and chikungunya have become major public health concern worldwide, till now; there are no licensed medicines available in the market. Hence, there is an emerging need for screening of new lead candidates for the inhibition of DENV and CHIKV. The current study focuses on predicting the binding potential of novel herbal-based compounds against the selected drug targets of DENV and CHIKV. The computational screening suggested that natural flavanoids such Kaempferol and Chymopain present in Carica papaya and Gossypetin present in Hibiscus sabdariffa possessed ideal drug likeliness, ADMET features and good binding potential towards the most of the selected molecular targets of DENV and CHIKV. It has been known that papaya (Carica papaya) extract has been used for the treatment of dengue; however, limited knowledge is available on the bioactive compounds and probable biochemical mechanism of the antiviral activity of papaya-based formulations. Similarly, there is sparse knowledge available on Gossypetin present in Hibiscus could act as probable lead molecules towards the targets of DENV and CHIKV. Hence, this study provides insight on the probable biochemical mechanism of the inhibitory activity of the compounds present in papaya and Hibiscus. This study certainly provides novel insight for further experimentation to validate the therapeutic potential of the selected flavanoids towards the molecular targets of DENV and CHIKV.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no potential conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1303-2) contains supplementary material, which is available to authorized users.
References
- Abu Bakar F, Ng LFP. Nonstructural proteins of alphavirus-potential targets for drug development. Viruses. 2018 doi: 10.3390/v10020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajay A, Walters P, Murcko A. Can we learn to distinguish between “drug-like” and “nondrug-like” molecules? J Med Chem. 1998;41:3314–3324. doi: 10.1021/jm970666c. [DOI] [PubMed] [Google Scholar]
- Ajay A, Bemis W, Murcko A. Blood brain barrier: designing libraries with CNS activity. J Med Chem. 1999;42:4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Memish ZA. Dengue Hemorrhagic Fever Virus in Saudi Arabia: A Review. Vector Borne Zoonotic Dis. 2018;18:75–81. doi: 10.1089/vbz.2017.2209. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gold SL. Paracelsus to parascience: The environmental cancer distraction. Mutat Res. 2000;447:3–33. doi: 10.1016/S0027-5107(99)00194-3. [DOI] [PubMed] [Google Scholar]
- Ashour J, Laurent-Rolle M, Shi PY, García-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J Virol. 2009;83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie A. TRAF3: Uncovering the Real but Restricted Role in Human. Immunity. 2010;33:293–295. doi: 10.1016/j.immuni.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Bruno C (2011) Antiviral research and development against dengue Virus, WHO Rep, pp 1–101
- Byler K, Collins J, Ogungbe I, Setzer W. Alphavirus protease inhibitors from natural sources: a homology modeling and molecular docking investigation. Comput Biol Chem. 2016;64:163–184. doi: 10.1016/j.compbiolchem.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Carrillo-Hernández MY, Ruiz-Saenz J, Villamizar LJ, Gómez-Rangel SY, Martínez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect Dis. 2018;18(1):61. doi: 10.1186/s12879-018-2976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovos C, Yeates T. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayaraj C. Current status of dengue and chikungunya in India. Seajph Jour. 2014;3:22–27. doi: 10.4103/2224-3151.206879. [DOI] [PubMed] [Google Scholar]
- Dhama K, Karthik K, Khandia R, Munjal A, Tiwari R, Rana R, Khurana SK, Khan RU, Alagawany M, Farag MR, Dadar M, Joshi SK. Medicinal and therapeutic potential of herbs and plant metabolites / extracts countering viral pathogens—current knowledge and future prospects. Curr Drug Metab. 2018 doi: 10.2174/1389200219666180129145252. [DOI] [PubMed] [Google Scholar]
- Fiser A, Sali A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- Frimurer T, Bywater R, Nærum L, Lauritsen L, Brunak S. Improving the odds in discriminating “drug-like” from “non drug-like” compounds. J Chem Inform Comput Sci. 2000;40:1315–1324. doi: 10.1021/ci0003810. [DOI] [PubMed] [Google Scholar]
- Gómez-Calderón C, Mesa-Castro C, Robledo S, Gómez S, Bolivar-Avila S, Diaz-Castillo F, Martínez-Gutierrez M. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on dengue and chikungunya virus infections. BMC Complement Altern Med. 2017;17:57. doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine D, Takahashi L, Lockhart K, Cheong J, Tolan W, Selick E, Grove R. MDCK (Madin-Darby canine kidney) cells: a tool for membrane permiability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor and RIG-1-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- Khan AH, Morita K, Parquet Md Mdel C, Hasebe F, Mathenge EG, Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83(Pt 12):3075–3084. doi: 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- Laskowski A, MacArthur W, Moss D, Thornton M. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- Laurie AT, Jackson RM. Q-SiteFinder: an energy-based method for the prediction of protein-ligand binding sites. Bioinformatics. 2005;21:1908–1916. doi: 10.1093/bioinformatics/bti315. [DOI] [PubMed] [Google Scholar]
- Li Y, Siripanyaphinyo U, Tumkosit U, Noranate N, Atchareeya A, Pan Y, Kameoka M, Kurosu T, Ikuta K, Takeda N, Ananrapreecha S. Poly (I:C), an agonist of toll-like receptor-3, inhibits replication of the Chikungunya virus in BEAS-2B cells. Virol J. 2012;9:114. doi: 10.1186/1743-422X-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C, Lombardo F, Dominy B, Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Luis K, Shaohong L, Gabriel M, Ricardo J, Soares M, Archie C, Wenbiao H, Patricia B, Francesca F, Rebecca D, Laith Y (2016) Co-distribution and co-infection of chikungunya and dengue viruses, Furuya-Kanamori. BMC Infect Dis 16–84 [DOI] [PMC free article] [PubMed]
- Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. Solution structure of dengue virus capsid protein reveals another fold. Proc Natl Acad Sci USA. 2004;101:3414–3419. doi: 10.1073/pnas.0305892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malet H, Coutard B, Jamal S, Dutartre H, Papageorgiou N, Neuvonen M, Ahola T, Forrester N, Gould EA, Lafitte D, Ferron F, Lescar J, Gorbalenya AE, de Lamballerie X, Canard B. The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol. 2009;83:6534–6545. doi: 10.1128/JVI.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. The first years of the protein data bank. Protein Sci. 1991;6:1591–1597. doi: 10.1002/pro.5560060724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Bergstralh D, Duncan A, Lei Y, Morrison E, Zimmermann G, Accavitti-Loper A, Madden J, Sun L, Ye Z, Lich D, Heise T, Chen Z, Ting P. Nlrx1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;2:29–60. doi: 10.1016/S0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paymen N, Pierre G, Ahmet C, John H. RIG-I-like receptors: Sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Perera-Lecoin M, Luplertlop N, Surasombatpattana P, Liegeois F, Hamel R, Thongrungkiat S, Vargas M, Yssel H, Misse D (2016) Dengue and chikungunya coinfection—the emergence of an underestimated threat. In: Rodriguez-Morale s AJ (ed) Current topics in chikungunya. Chap. 5, InTech, Rijeka, Croatia
- Powers A. Chikungunya virus outbreak expansion and micro evolutionary events affecting epidemiology and epidemic potential. Res Rep Trop Med. 2015;6:11–19. doi: 10.2147/RRTM.S53698. [DOI] [Google Scholar]
- Powers CN, Setzer WN. In in-silico investigation of phytochemicals as antiviral agents against dengue fever. Comb Chem High Throughput Screen. 2016;19:516–36. doi: 10.2174/1386207319666160506123715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12:e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan C, Kutumbarao N, Suhitha S, Velmurugan D. Structure-function relationship of Chikungunya nsP2 protease: a comparative study with papain. Chem Biol Drug Des. 2017;89:772–782. doi: 10.1111/cbdd.12901. [DOI] [PubMed] [Google Scholar]
- Rashad A, Mahalingam S, Keller A. Chikungunya virus: emerging targets and new opportunities for medicinal chemistry. J Med Chem. 2014;57:1147–1166. doi: 10.1021/jm400460d. [DOI] [PubMed] [Google Scholar]
- Ruwali P, Rai N, Kumar N, Gautam P. Antiviral potential of medicinal plants: an overview. Int Res J Pharm. 2013;4:8–16. doi: 10.7897/2230-8407.04603. [DOI] [Google Scholar]
- Santhanam V, Waheeta H. Towards the identification of novel phytochemical leads as macrodomain inhibitors of chikungunya virus using molecular docking approach. J Appl Pharm Sci. 2017;7:074–082. [Google Scholar]
- Seeliger D, de Groot B. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des. 2010;24:417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedi S, Shukri M, Hassandarvish P, Oo A, Shankar E, Abubakar S, Zandi K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci Rep. 2016;6:1–7. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe HJ, Yong YK, Al-Obaidi MMJ, Raju CS, Gudimella R, Manikam R, Sekaran SD. Identifying protein biomarkers in predicting disease severity of dengue virus infection using immune-related protein microarray. Medicine (Baltimore) 2018;97(5):e9713. doi: 10.1097/MD.0000000000009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WL, Lee YK, Ho YF, Yusof R, Abdul Rahman N, Karsani SA. Comparative proteomics reveals that YK51, a 4-Hydroxypandurantin-A analogue, down regulates the expression of proteins associated with dengue virus infection. PeerJ. 2018;5:e3939. doi: 10.7717/peerj.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Gibson T, Hiqquins D (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinform Chap 2:Unit 2.3. 10.1002/0471250953.bi0203s00 [DOI] [PubMed]
- Torsten S, Jurgen K, Nicolas G, Manuel P. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Calvo Á, Jiménez de Oya N, Martín-Acebes MA, Garcia-Moruno E, Saiz JC. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika virus, and Dengue virus. Front Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veber F, Johnson R, Chen HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- Wiederstein J, Sippl M. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zou J, Puttikhunt C, Yuan Z, Shi PY. Two distinct sets of NS2A molecules are responsible for dengue virus RNA synthesis and virion assembly. J Virol. 2015;89:1298–1313. doi: 10.1128/JVI.02882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhang Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J. 2011;101:2525–2534. doi: 10.1016/j.bpj.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanian M, Glynn L, Wright L, Hawi Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds”. Pharm Res. 1998;15:1490–1494. doi: 10.1023/A:1011930411574. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- Zhong B, Yang Y, Li S, Wang Y, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu H. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.