Abstract
The screening of frailty can trigger interventions aiming to delay disability in older people. Whereas the prevalence, the consequences, and the factors associated with frailty are well described, little is known about the duration of the state of frailty. This study aimed to estimate the time spent in the state of frailty in men and women using the Sullivan method. Data used were the age- and sex-specific prevalence of frailty found in SIPAF study (“Système d’Information sur la Perte d’Autonomie Fonctionnelle de la personne âgée”) and statistics of mortality from the Human Mortality Database. The SIPAF study included 2350 individuals aged 70 and over and living in France. Participants were interviewed at home by trained nurses. Frailty was defined as impairment in three domains or more among nutrition, energy, physical activity, strength, and mobility. People requiring assistance in basic activities of daily living were considered in a separate category. Mean age of the study sample was 83.3 ± 7.5 years, with 59.4% of women. Overall, the prevalence of pre-frailty, frailty, and dependency was 39.1, 17.0, and 15.4%, respectively. Life expectancy at age 70 was 18.3 years for women, of which 7.4 years (95% CI 6.9–7.9) were pre-frail, 3.4 years (95% CI 3.0–3.8) frail, and 2.4 (95% CI 2.1–2.7) with disability. In contrast, LE for men at 70 was 14.8 years, of which pre-frail, frail, and disabled years were 6.0 years (95% CI 5.5–6.5), 1.2 years (95% CI 1.0–1.5), and 1.2 (95% CI 1.0–1.5), respectively. In conclusion, frailty is a transition state that is relatively limited in time compared to pre-frailty that may represent a larger time window for prevention.
Keywords: Pre-frailty, Frailty, Disability, Health expectancy, Sullivan method
Introduction
Frailty is defined as an ageing-related state, resulting from a decrease in physiological reserves of multiple systems, which increases vulnerability to stressors (Clegg et al. 2013). Based on this theoretical basis, multiple operational definitions of frailty have emerged, the most famous being the frailty phenotype (Fried et al. 2001) and the Frailty Index (FI) (Rockwood et al. 2005). The former is based on a set of five criteria exploring physical strength, physical activity, nutrition, mobility, and energy, while the latter is a count of up to 70 deficits including the presence and severity of current diseases, ability in activities of daily living, and physical signs from clinical and neurologic exams (Cesari et al. 2014). Whatever the definition of frailty, it is a predictor of adverse health outcomes, such as falls, institutionalisation, and mortality (Shamliyan et al. 2013).
As such, frailty has become a major issue in the prevention of functional decline and disability in aged populations (Bergman et al. 2007). The range of possible interventions is large and includes improvements in the management of chronic conditions, increased physical activity, and nutritional skills (Morley et al. 2013). The great majority of the experts now agree on the relevance of detecting frailty as early as possible in order to implement corrective and/or preventive actions to delay disability in the frail old population (Rodriguez-Manas et al. 2013).
In this context, times issues are essential. How large is the window for action, i.e. the period of time during which frailty can be detected and corrective/preventive actions implemented? The few longitudinal studies with repeated measures of frailty over time do not answer this question, either because they describe the gradual evolution of old people over time with FI derived from repeated geriatric evaluations (Marshall et al. 2015; Mitnitski et al. 2007; Yang and Lee 2010), or because time intervals between phenotypic frailty measures are too long, at best 18 months (Dapp et al. 2014; Gill et al. 2006; Lee et al. 2014; Shardell et al. 2012; Xue et al. 2008).
In the absence of longitudinal data about frailty with very close intervals between measures that would allow the estimation of the duration of frailty, Romero-Ortuno et al. suggested to calculate life expectancy in the state of frailty by using the Sullivan method (Romero-Ortuno et al. 2014). Health expectancy is the number of remaining years, at a particular age, which an individual can expect to live in a healthy or unhealthy state (Robine et al. 2013). Although longitudinal information is theoretically required to estimate the incidence of health states and thus calculate health expectancies, the Sullivan method is of particular practical interest as it is usable in cross-sectional designs. Data required are the sex- and age-specific prevalence of each health state in the population and mortality information (Jagger et al. 2014). The Sullivan method has been used to analyse health priorities and notably to estimate disability-free life expectancy (Manton et al. 1993). Of note, the Sullivan method does not require assumption of a linear progression from frailty to functional disability.
Applying the Sullivan method to the data from the Survey of Health, Ageing and Retirement in Europe (SHARE), life expectancy in frailty at age 70 years was estimated to be 1.8 years on average for women and 0.7 year for men (Romero-Ortuno et al. 2014). To our knowledge, this first calculation of life expectancy in the state of frailty has not been replicated in other settings.
Using the Sullivan method, this study aimed to calculate the time spent in each frailty state (robust, pre-frail, frail, and dependent) in men and women included in the SIPAF study, an observational study including a large proportion of very old people in France.
Methods
Study design and sample
This work is part of a cross-sectional study carried out to characterise health and functional independence among people aged 70 and older (SIPAF study, French acronym for “Système d’Information sur la Perte d’Autonomie Fonctionnelle de la personne âgée”). Subjects were selected at random among participants in a supplementary pension fund, AG2R La Mondiale (Paris, France). The French pension system is composed of three levels: the basic pension, supplementary pension, and private pension. The system is based on solidarity between generations; active employees contribute to pay the pensions of their elders. In return, they are eligible for an old-age pension. The supplementary pension is the second level of the pension system. Like the basic pension, it is mandatory and operates on the principle of “pay-as-you-go” (Herr et al. 2015).
The selection of a random sample to be surveyed was centralised and performed by the actuary of AG2R La Mondiale. Using information about the geographic area of residence, the sampling method was designed to ensure the inclusion of participants from all regions of France excluding overseas territories, in rural as well as in urban areas. The sample selection was stratified by age group in order to include a larger proportion of oldest old aged 90 years and over than expected with a simple random drawing. Recruitment took place across France from 2008 to 2010, in 21 survey areas that were representative of all regions and sizes of cities in mainland France. Participants were interviewed at home by trained nurses. In 16.6% of the cases, a close relative was present to confirm or complete the answers of the participants. Information about non-participants included age, gender, and department of residence. Tertiles of departmental statistics were used in the analysis of participation: density of population, proportion of people aged 65 years and over, and median income in the population (www.insee.fr). The research protocol was approved by an independent ethics committee (permission no. 060316).
Data collection
The interviews followed a standard procedure, starting with a questionnaire and ending by physical measurements.
Information was collected about self-rating of health, and chronic diseases. Chronic diseases were identified by reported diagnosis or treatment of 14 diseases, including asthma, allergies, diabetes, cataract, high blood pressure, heart attack, stroke/cerebral haemorrhage, chronic bronchitis/emphysema, rheumatoid arthritis/osteoarthritis, osteoporosis, gastric or duodenal ulcer, malignant tumour, migraines/frequent headaches, and chronic anxiety/depression. The number of drugs prescribed was determined from the prescriptions that the participants had at home. Polypharmacy was defined as five medications or more and excessive polypharmacy as 10 medications or more.
The geriatric assessment included measured height and weight and a question about unintentional weight loss (of 10% of body weight during the past 6 months), difficulty to walk up and down stairs, and difficulty to lift a bag weighing 5 kg. Cognitive impairment was defined as a Mini-Mental State Examination score of 26 or less (Folstein et al. 1975). The evaluation of activity limitations examined five activities of daily living (ADL) included in the Katz index (Katz et al. 1963), i.e. bathing, dressing, toileting, transferring, continence, and feeding, as well as instrumental activities of daily living (IADL) such as food preparation, the ability to use a telephone, housekeeping, shopping, and the ability to manage one’s finances (Lawton and Brody 1969). Dependency was defined as the need of assistance with at least one ADL. Emotional status was assessed with the 15-item geriatric depression scale (GDS-15) (Yesavage et al. 1982).
The level of physical activity was assessed with the International Physical Activity Questionnaire (IPAQ), and three levels of activity were distinguished (low, moderate, and high) according to time spent walking and doing moderate (for instance, carrying light loads, leisure bicycle ride, tennis) and vigorous activities (for instance, carrying heavy loads, digging, lifting a pack of six bottles, or speed bicycle) during the past 7 days (Hurtig-Wennlof et al. 2010). The GDS-15 provided information about the renunciation of activities participants used to do (“Have you dropped many of your activities and interests”). Variables dealing with social support dealt with emotional features (lack of support when needing to talk, when needing an advice, or when needing affection) and practical features (lack of support when bedridden, when needing to be accompanied to a medical appointment, or when needing help to prepare meals). People were also invited to report elements of their environment that limit their activity (open question).
In addition to health variables, socio-demographic information was collected: age, gender, marital status, and level of occupation during midlife. Three levels of occupation were defined: low (blue-collar workers), intermediate (intermediate white-collar workers, employees, shopkeepers), and high (high-level white-collar workers). When possible, we assigned to housewives and people who had never worked the level of occupation of the spouse (4.2% of the study sample).
Frailty definition
Frailty was defined according to the construct derived from the Cardiovascular Health Study (CHS) (Fried et al. 2001), in order to differentiate people in different categories of phenotypic frailty. Due to variations in health assessment between the SIPAF study and the CHS, some components of our operational measurement of frailty differ from the original definition. In particular, measures of grip strength and slow walking speed were replaced by self-reported variables, as previously done in other epidemiological settings (Castrejon-Perez et al. 2012; Theou et al. 2013). The five frailty components were defined as follows:
Nutrition: unintentional weight loss or body mass index ≤18.5 kg/m2;
Energy: positive answer to the question “Do you feel weak now?” or negative answer to the question “Do you have a lot of energy?”;
Physical activity: low level of activity according to the IPAQ questionnaire;
Physical strength: difficulty lifting a bag weighing 5 kg;
Mobility: difficulty walking up and down stairs.
Frail persons were identified as having three or more of the five components. Persons having one or two of the five components were considered pre-frail. Because frailty is a precursor and etiologic factor in disability (Bergman et al. 2007; Cesari et al. 2014), persons requiring assistance with at least one ADL were considered in an additional category. In cases where people met both the criteria for frailty and dependency, precedence was given to dependency.
Statistical analysis
Life expectancy in each state of frailty was calculated using the Sullivan method. Information about the prevalence of frailty came from the interviews that were conducted between 2008 and 2010 in the SIPAF study, whereas information about mortality in France in 2009 was taken from the Human Mortality Database (Human Mortality Database). Health expectancies were calculated using an abridged life table (5-year intervals, from 70 to 74 years to 95 years and over), and standard errors of health expectancies were calculated according to the guidelines available on the Eurohex website (Jagger et al. 2014). The variance of mortality rates was ignored as it is classically negligible compared to the variance of the prevalence rates.
Analyses were performed using Stata® software (version 13.0) and Microsoft Excel®.
Results
Participation and characteristics of the study sample
A total of 2350 people agreed to participate in the study (participation rate 18.9%). The main reasons for non-participation were the lack of interest in the study (28.3% of the non-participants), followed by a self-reported state of frailty (10.8%), and the refusal of a close relative (7.3%). At the departmental level, participation was better in low-populated areas and in departments where the population was ageing or had a lower standard of living. At the individual level, participation was higher among males and younger persons. The socioeconomic profile of the participants was comparable to national statistics, with about 12% of executives and higher intellectual professions within the national workforce in 1999 as in the SIPAF study (www.insee.fr).
The study sample consisted of 1395 women (59.4%) and 955 men (40.6%), of mean age 83.3 ± 7.5 years. Among the 2286 participants with data on all five dimensions of frailty (i.e. no missing) or with sufficient information to classify them in the frail category (i.e. those having deficits in at least three criteria for frailty), 654 (28.9%) were robust, 893 (39.1%) were pre-frail, 388 (17.0%) were frail, and the remaining 351 (15.4%) were considered dependent because they needed help with at least one ADL. Notice that 84.0% of dependent people also met the criteria for frailty, in line with our hypothesis that frailty often precedes disability.
Table 1 describes the characteristics of the subjects according to their frailty state. More than three quarters of the frail individuals reported a lack of energy, difficulty lifting a bag weighing 5 kg, or a low level of physical activity. Lack of energy and low level of physical activity already affected 58.9 and 36.5% of the pre-frail individuals, respectively. As expected, there was a gradient between poor health and increasing frailty in different domains: self-perceived health, cognition, mood, functional abilities, chronic diseases, and polypharmacy. Social support also diminished with increasing frailty. When asked about elements of their environment that limit their activity, participants often mentioned inappropriate bath facility and stairs.
Table 1.
Characteristics of the subjects included in the SIPAF study according to their frailty state
| Variables | Robust N = 654 | Pre-frail N = 893 | Frail N = 388 | Dependent N = 351 |
|---|---|---|---|---|
| Socio-demographic information | ||||
| Women (%) | 298 (45.6) | 514 (57.6) | 293 (75.5) | 250 (71.2) |
| Age (mean ± SD) | 79.5 ± 6.4 | 82.5 ± 6.9 | 86.3 ± 7.0 | 88.5 ± 6.8 |
| Married or living as a couple (%) | 359 (55.0) | 401 (45.0) | 107 (27.7) | 106 (30.2) |
| Level of former job (%) | ||||
| High | 106 (16.2) | 116 (13.0) | 33 (8.5) | 22 (6.3) |
| Intermediate | 319 (48.4) | 423 (47.5) | 187 (48.2) | 155 (44.3) |
| Low | 229 (35.0) | 351 (39.4) | 168 (43.3) | 173 (49.4) |
| Frailty variables | ||||
| Unintentional weight loss or low BMI (%) | 0 | 72 (8.1) | 84 (21.7) | 53 (15.5) |
| Lack of energy (%) | 0 | 526 (58.9) | 321 (82.7) | 270 (79.0) |
| Low level of physical activity (%) | 0 | 326 (36.5) | 301 (78.8) | 289 (83.3) |
| Difficulty lifting a bag weighing 5 kg (%) | 0 | 202 (22.6) | 324 (83.9) | 284 (82.8) |
| Difficulty walking up- and downstairs (%) | 0 | 128 (14.3) | 286 (74.5) | 264 (76.3) |
| Other health and social variables | ||||
| Self-rating of health (%) | ||||
| Good | 473 (72.4) | 402 (45.2) | 97 (25.0) | 88 (25.2) |
| Fair | 171 (26.2) | 407 (45.8) | 208 (53.6) | 160 (45.9) |
| Poor | 9 (1.4) | 80 (9.0) | 83 (21.4) | 101 (28.4) |
| Need of help in IADL | 42 (6.4) | 209 (23.4) | 255 (65.7) | 337 (96.3) |
| Cognitive impairment (MMSE score of 26 or less) (%) | 49 (7.6) | 103 (11.7) | 71 (18.8) | 96 (30.2) |
| Depression (geriatric depression scale 15 items) (%) | ||||
| No (<5) | 643 (98.3) | 732 (82.0) | 248 (63.9) | 203 (59.0) |
| Probable (5–9) | 11 (1.7) | 151 (16.9) | 121 (31.2) | 128 (37.2) |
| Severe (10 or greater) | 0 | 10 (1.1) | 19 (4.9) | 13 (3.8) |
| Number of comorbidities (%) | ||||
| 0–1 | 417 (64.2) | 412 (46.4) | 145 (37.6) | 125 (35.8) |
| 2–3 | 135 (20.8) | 203 (22.9) | 110 (28.5) | 90 (25.8) |
| 4 or more | 98 (15.1) | 273 (30.7) | 131 (33.9) | 134 (38.4) |
| Number of drugs per day (%) | ||||
| 0–4 drugs | 331 (53.1) | 251 (28.8) | 79 (21.2) | 65 (19.3) |
| Polypharmacy (5–9 drugs) | 265 (42.5) | 523 (60.1) | 209 (56.0) | 174 (51.6) |
| Excessive polypharmacy (≥10 drugs) | 27 (4.3) | 97 (11.1) | 85 (22.8) | 98 (29.1) |
| Lack of social support (%) | ||||
| Emotional | 75 (11.5) | 162 (18.3) | 86 (22.3) | 61 (17.6) |
| Practical | 170 (26.0) | 327 (36.9) | 161 (41.8) | 101 (29.4) |
| Renunciation of a number of activities (%) | 147 (22.5) | 421 (47.4) | 270 (70.3) | 267 (79.2) |
| Elements of the environment that limit the activity or create disability (%) | 26 (4.0) | 118 (13.4) | 101 (26.7) | 123 (37.4) |
BMI body mass index, IADL instrumental activities of daily living, MMSE Mini-Mental State Examination
Time spent in each frailty state
Table 2 displays health expectancies by 5-year age intervals with 95% CI. On average, life expectancy at age 70 was 18.3 years for women and 14.8 years for men, including 87% of life expectancy without dependency for women and 92% for men. The expected duration of frailty was 3.4 years (95% CI 3.0–3.8) for women and 1.2 years (95% CI 1.0–1.5) for men. Life expectancy in pre-frailty was 7.4 years (95% CI 6.9–7.9) for women and 6.0 years (95% CI 5.5–6.5) for men. Women were likely to spend 2.2 years more frail and 1.4 years more pre-frail than men. Pre-frailty lasted longer than frailty in men of every age and in women before age 90 years (up to five times the duration of frailty in men aged 70–74 years). Although men had shorter life expectancy than women, they were expected to live longer in a robust state compared to women.
Table 2.
Life expectancy by frailty state in women and men by 5-year age group in the SIPAF study
| Gender | Age at start of the interval | N | Total life expectancy | Robust | Pre-frail | Frail | Dependent | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Life expectancy in years with 95% CI | % of total life expectancy | Life expectancy in years with 95% CI | % of total life expectancy | Life expectancy in years with 95% CI | % of total life expectancy | Life expectancy in years with 95% CI | % of total life expectancy | ||||
| Women | 70 | 197 | 18.34 | 5.12 [4.63–5.60] | 27.9 | 7.41 [6.89–7.94] | 40.4 | 3.40 [3.02–3.79] | 18.5 | 2.41 [2.11–2.71] | 13.1 |
| 75 | 261 | 14.27 | 3.24 [2.87–3.61] | 22.7 | 5.59 [5.16–6.02] | 39.2 | 3.02 [2.68–3.36] | 21.2 | 2.42 [2.12–2.71] | 17.0 | |
| 80 | 299 | 10.53 | 1.64 [1.37–1.91] | 15.6 | 4.00 [3.65–4.35] | 38.0 | 2.62 [2.32–2.93] | 24.9 | 2.27 [1.99–2.54] | 21.6 | |
| 85 | 288 | 7.35 | 0.74 [0.56–0.93] | 10.1 | 2.53 [2.25–2.82] | 34.4 | 2.00 [1.73–2.26] | 27.2 | 2.08 [1.82–2.34] | 28.3 | |
| 90 | 209 | 5.04 | 0.28 [0.15–0.40] | 5.6 | 1.42 [1.17–1.67] | 28.2 | 1.49 [1.24–1.74] | 29.6 | 1.85 [1.59–2.12] | 36.7 | |
| 95 | 141 | 3.34 | 0.15 [0.03–0.27] | 4.5 | 0.60 [0.28–0.82] | 18.0 | 1.12 [0.86–1.39] | 33.5 | 1.47 [1.19–1.75] | 44.0 | |
| Men | 70 | 178 | 14.76 | 6.35 [5.85–6.86] | 43.0 | 5.97 [5.47–6.47] | 40.4 | 1.21 [0.97–1.46] | 8.2 | 1.23 [0.98–1.48] | 8.3 |
| 75 | 227 | 11.35 | 4.16 [3.75–4.57] | 36.7 | 4.73 [4.32–5.15] | 41.7 | 1.25 [1.00–1.50] | 11.0 | 1.21 [0.96–1.45] | 10.7 | |
| 80 | 221 | 8.33 | 2.69 [2.35–3.03] | 32.3 | 3.50 [3.13–3.86] | 42.0 | 1.06 [0.82–1.29] | 12.7 | 1.08 [0.84–1.31] | 13.0 | |
| 85 | 167 | 5.80 | 1.40 [1.11–1.69] | 24.1 | 2.33 [2.00–2.66] | 40.2 | 1.09 [0.83–1.35] | 18.8 | 0.98 [0.74–1.22] | 16.9 | |
| 90 | 111 | 4.00 | 0.81 [0.55–1.06] | 20.3 | 1.62 [1.30–1.93] | 40.5 | 0.57 [0.35–0.79] | 14.3 | 1.00 [0.72–1.27] | 25.0 | |
| 95 | 51 | 2.73 | 0.63 [0.30–0.95] | 23.1 | 0.91 [0.55–1.28] | 33.3 | 0.51 [0.21–0.82] | 18.7 | 0.68 [0.35–1.02] | 24.9 | |
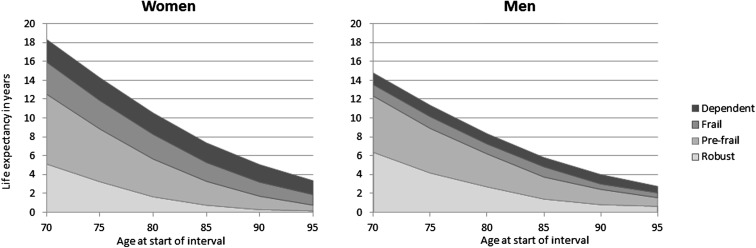
The relative importance of each state over time is presented graphically in Fig. 1. Of note, this figure illustrates how years spent dependent and frail concentrate at the end of life, whereas periods of pre-frailty and robustness are larger in younger ages, for both women and men.
Fig. 1.
Life expectancy by frailty state in women and men by 5-year age group in the SIPAF study
Discussion
Using the Sullivan method, this study combined cross-sectional health data with period life tables to estimate age- and sex-specific life expectancy in each state of frailty. At age 70 years, women were expected to live 3.4 years in frailty (95% CI 3.0–3.8) and men 1.2 year (95% CI 1.0–1.5). Before that, they were expected to stay pre-frail much longer, 7.4 years (95% CI 6.9–7.9) for women and 6.0 years (95% CI 5.5–6.5) for men.
Applying the Sullivan method to the data from the SHARE study, Romero-Ortuno et al. estimated life expectancy in frailty at age 70 to 1.8 years for women and 0.7 year for men on average (Romero-Ortuno et al. 2014). Although higher, our estimates still stand within the range of values found across the different European countries included in the SHARE study, i.e. 0.4–5.5 years for women and 0.1–1.8 years for men. Furthermore, the ratio of the duration of frailty between women and men was comparable between the two studies: 2.6 in SHARE and 2.8 in SIPAF. In accordance with Romero-Ortuno et al., we distinguished disabled people from frail people. However, we defined disability as the need of help in at least one ADL, whereas Romero-Ortuno et al. identified disabled people as those reporting severe activity limitation. Our definition of disability may be more objective but also more stringent, explaining why we found lower life expectancies in disability compared to the SHARE study (2.4 vs. 5.7 years for women and 1.2 vs. 3.6 years for men at age 70). Furthermore, our results concerning life expectancy without dependency were consistent with those from previous studies in France, which reported that life expectancy at age 65 without severe disability represented 86–91% of total life expectancy for men and 80–86% for women (Cambois et al. 2008).
Our estimate of the time spent in the pre-frailty state was about 40% of total life expectancy for both women and men, which is largely higher than previous estimates from the SHARE study where life expectancy in the pre-frail state represented 22% of total life expectancy at age 70 years for women and 13% for men. The Sullivan method is sensitive to the definition of health states, and our results should be interpreted with regard to the definition of frailty we used. Differences in the assessment of frailty may explain why we had a higher estimate of the years spent in pre-frail state in our study sample, at the cost of the estimated years lived robust. The SIPAF study assessed the same five dimensions as the SHARE study but involved trained nurses and used different tools to estimate the prevalence of each criterion. Notably, the wording of the questions we used to asses exhaustion may explain why this criterion was highly prevalent (almost half of the study sample). Furthermore, the use of self-reported difficulties climbing stairs or carrying a heavy bag instead of performance measurement may overestimate the prevalence of deficits in the strength and mobility criteria in the SIPAF study.
We confirm the sex differences in health expectancy observed in the SHARE study. Frailty is known to be more prevalent in women than in men; in the meta-analysis by Shamliyan et al. (2013), the pooled prevalence of the frailty phenotype was 13% in women and 7% in men. The fact that women live longer whereas they bear a larger burden of health deficits than men is designated as the “male–female health-survival paradox” (Oksuzyan et al. 2010), and explanatory factors involve social, behavioural, and biological factors, whose influence may vary throughout life (Alvarado et al. 2008).
From a public health perspective, frailty is a useful outcome to target comprehensive assessment and geriatric interventions. Our results highlight the potential interest of pre-frailty in terms of prevention. First, the pre-frailty state offers a larger time window for action compared to the frailty state. Second, our results indicate that a number of pre-frail subjects are already characterised by polypharmacy, lack of energy, or depressed mood. In addition, a significant proportion of both robust and pre-frail subjects reported lack of social support and renunciation of some activities. Furthermore, previous studies stressed that prevention may be more effective at the early stages of frailty (Gill et al. 2002; Puts et al. 2005).
Main strengths of this study are its large assessment of geriatric health problems performed by trained nurses and the recruitment of a large number of people aged 90 years and over (n = 512). In addition, participants were randomly selected among the 2,100,000 recipients of a supplementary pension fund with a sampling method designed to ensure the representativeness of the study sample with regard to regions of France. Nevertheless, we cannot exclude a participation bias due to the low participation, where participants were more likely to be healthy than persons who refused to participate in the study. Our comparative approach to the results of the SHARE study was limited by the fact that we did not use the algorithm developed to determine the frailty phenotype in men and women in the SHARE study (Romero-Ortuno et al. 2010). This algorithm uses coefficients which give different weights to the five dimensions of frailty. Because they were determined using close but different questions and in a younger population (mean age 64 years, i.e. approximately 20 years lower compared to the SIPAF study), the use of these coefficients was inappropriate in the SIPAF study. The Sullivan method is recommended for its relative accuracy compared to longitudinal measures. If the transition rates between health states are stable or gradually change over time, the Sullivan method provides fair estimates of the life expectancy in the various health states (Mathers and Robine 1997). Errors could have affected the results if the transition rates between the frailty states had suddenly changed before the SIPAF survey (in the sense of an overestimation if the rates had increased and in the sense of underestimation if the rates had decreased). However, nothing in the literature suggests, in France as well as globally, a sudden change in the transition rates between the frailty states. A limitation of the Sullivan method is that it does not describe transitions between states and hence does not allow the identification of risk factors for functional decline.
In conclusion, this work provides elements to answer a fundamental question in ageing research, which is whether the extension of life expectancy is made up of healthy life expectancy or not. Although women have a longer life expectancy, they also spend more time in a frail state compared to men. Our results also show that the state of pre-frailty last longer than the state of frailty itself, thereby providing an important opportunity to identify those at risk of subsequent frailty and disability for targeted preventative interventions.
Acknowledgements
This work was supported by AG2R La Mondiale (data collection) and Université Versailles St-Quentin-en-Yvelines (data analyses).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63:1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm-issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambois E, Clavel A, Romieu I, Robine JM. Trends in disability-free life expectancy at age 65 in France: consistent and diverging patterns according to the underlying disability measure. Eur J Ageing. 2008;5:287–298. doi: 10.1007/s10433-008-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrejon-Perez RC, Borges-Yanez SA, Gutierrez-Robledo LM, Avila-Funes JA. Oral health conditions and frailty in Mexican community-dwelling elderly: a cross sectional analysis. BMC Public Health. 2012;12:773. doi: 10.1186/1471-2458-12-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapp U, Minder CE, Anders J, Golgert S, von Renteln-Kruse W. Long-term prediction of changes in health status, frailty, nursing care and mortality in community-dwelling senior citizens-results from the Longitudinal Urban Cohort Ageing Study (LUCAS) BMC Geriatr. 2014;14:141. doi: 10.1186/1471-2318-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- Herr M, Robine JM, Aegerter P, Arvieu JJ, Ankri J. Contribution of socioeconomic position over life to frailty differences in old age: comparison of lifre-course models in a French sample of 2350 old people. Ann Epidemiol. 2015;25:674–680. doi: 10.1016/j.annepidem.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Human Mortality Database. http://www.mortality.org/. Accessed Aug 2015
- Hurtig-Wennlof A, Hagstromer M, Olsson LA. The International Physical Activity Questionnaire modified for the elderly: aspects of validity and feasibility. Public Health Nutr. 2010;13:1847–1854. doi: 10.1017/S1368980010000157. [DOI] [PubMed] [Google Scholar]
- Jagger C, Van Oyen H, Robine JM (2014) Health expectancy calculation by the Sullivan method: a practical guide, 4th edn. http://reves.site.ined.fr/fichier/s_rubrique/20182/sullivan.guide.pre.final.oct2014.en.pdf. Accessed Aug 2015
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontolog. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15:281–286. doi: 10.1016/j.jamda.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Manton K, Stallard E, Liu K (1993) Frailty and forecasts of active life expectancy in the United States. In: Manton KG, Singer BH, Suzman RM (eds) Forecasting the health of elderly populations. Springer series in statistics (statistics in the health sciences). Springer, New York, pp 159–181
- Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. 2015;69:316–321. doi: 10.1136/jech-2014-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Robine JM. How good is Sullivan’s method for monitoring changes in population health expectancies? J Epidemiol Community Health. 1997;51:80–86. doi: 10.1136/jech.51.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski A, Song X, Rockwood K. Improvement and decline in health status from late middle age: modeling age-related changes in deficit accumulation. Exp Gerontol. 2007;42:1109–1115. doi: 10.1016/j.exger.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Morley JE, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A, Brønnum-Hansen H, Jeune B. Gender gap in health expectancy. Eur J Ageing. 2010;7:213–218. doi: 10.1007/s10433-010-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts MTE, Lips P, Deeg DJH. Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning. J Clin Epidemiol. 2005;58:1188–1198. doi: 10.1016/j.jclinepi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Robine JM, Cambois E, Nusselder W, Jeune B, Oyen HV, Jagger C, Team JE. The joint action on healthy life years (JA: EHLEIS) Arch Public Health. 2013 doi: 10.1186/0778-7367-71-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manas L, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the survey of health, ageing and retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ortuno R, Fouweather T, Jagger C. Cross-national disparities in sex differences in life expectancy with and without frailty. Age Ageing. 2014;43:222–228. doi: 10.1093/ageing/aft115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Shardell M, et al. Serum 25-hydroxyvitamin D, transitions between frailty states, and mortality in older adults: the Invecchiare in Chianti Study. J Am Geriatr Soc. 2012;60:256–264. doi: 10.1111/j.1532-5415.2011.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the women’s health and aging study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol Ser B Psychol Sci Soc Sci. 2010;65B:246–255. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]