SUMMARY
Basal nuclear factor κB (NF-κB) activation is required for hematopoietic stem cell (HSC) homeostasis in the absence of inflammation; however, the upstream mediators of basal NF-κB signaling are less well understood. Here, we describe TRAF6 as an essential regulator of HSC homeostasis through basal activation of NF-κB. Hematopoietic-specific deletion of Traf6 resulted in impaired HSC self-renewal and fitness. Gene expression, RNA splicing, and molecular analyses of Traf6-deficient hematopoietic stem/progenitor cells (HSPCs) revealed changes in adaptive immune signaling, innate immune signaling, and NF-κB signaling, indicating that signaling via TRAF6 in the absence of cytokine stimulation and/or infection is required for HSC function. In addition, we established that loss of IκB kinase beta (IKKβ)- mediated NF-κB activation is responsible for the major hematopoietic defects observed in Traf6-deficient HSPC as deletion of IKKβ similarly resulted in impaired HSC self-renewal and fitness. Taken together, TRAF6 is required for HSC homeostasis by maintaining a minimal threshold level of IKKβ/NF-κB signaling.
In Brief
Fang et al. identify TRAF6 as an essential regulator of hematopoietic stem cell (HSC) self-renewal and quiescence. TRAF6 preserves HSC homeostasis by maintaining a minimal threshold level of NF-κB signaling in the absence of inflammation.

INTRODUCTION
Hematopoiesis is a well-characterized developmental process wherein all adult blood cells are maintained by long-term hematopoietic stem cells (HSC) in the bone marrow (BM) that form mature cells of the lymphoid, myeloid, and erythroid lineages. The lineage-biased progeny of the HSCs rapidly expand, proliferate, and differentiate to respond to the immediate needs of the body until an appropriate systemic response is achieved. HSC reside in a perivascular niche where they have direct contact with quiescence- or proliferation-enforcing signals released from the BM microenvironment (Ding and Morrison, 2013; Bruns et al., 2014; Acar et al., 2015). These direct and indirect niche interactions protect HSC quiescence, which is critical for maintaining lifelong hematopoiesis. The nuclear factor κB (NF-κB) family of transcription factors is important for hematopoiesis, including development, differentiation, and homeostasis of different hematopoietic lineages (Espín-Palazón and Traver, 2016). A major function of NF-κB in hematopoietic cells is to modulate the response to cytokines, pattern-associated molecular patterns, and damage-associated molecular patterns via the tumor necrosis factor receptor (TNFR), interleukin-1 receptor (IL-1R), and toll-like receptors (TLR). NF-κB activation in HSC occurs in response to IL-1R and TLR superfamily stimulation, and in separate studies it is well established that activation of IL-1R and TLRs affects HSC function (Nagai et al., 2006; Esplin et al., 2011; Schuettpelz et al., 2014; Herman et al., 2016; Pietras et al., 2016). For example, administration of low levels of lipopolysaccharide (LPS) in mice, meant to model chronic infection, results in loss of HSC quiescence, reduced HSCs, and myeloid-biased differentiation (Esplin et al., 2011; Zhang et al., 2016a). Chronic IL1 exposure drives HSC toward precocious myeloid differentiation at the expense of self-renewal (Pietras et al., 2016). Moreover, tumor necrosis factor alpha (TNF-α) activates the Notch and NF-κB signaling pathways to establish HSC fate, indicating a requirement for inflammatory signaling in HSC generation (Espín-Palazón et al., 2014).
Upon binding to ligands, IL-1R and TLRs sequentially recruit intracellular adaptors, kinases, and effector molecules, which results in nuclear localization and DNA binding of NF-κB homo- or heterodimer transcription factors RelA, c-Rel, RelB, p50/p105, and p52/p100. The current findings support a model in which NF-κB activation occurs downstream of IL-1R/TLRs and contributes to HSC phenotypes during cytokine exposure and infection. However, impaired NF-κB activation as demonstrated by mouse genetic models (i.e., Rela, Relb, Ikbkb, Map3k7/Tak1, Tnfaip3, and Cyld) affects hematopoietic stem/progenitor cell (HSPC) function in the absence of cytokine stimulation or infection (Takaesu et al., 2012; Zhao et al., 2012; Stein and Baldwin, 2013; Nakagawa et al., 2015; Tesio et al., 2015; Zhang et al., 2015), indicating that tonic and/or stochastic NF-κB signaling is required for HSC homeostasis. Surprisingly, deletion of upstream mediators of NF-κB, such as Il1r, Tlr4, Tlr2, MyD88, or Trif results in minimal effects on HSC homeostasis (Orelio et al., 2009; Liu et al., 2015; Herman et al., 2016; Zhang et al., 2016a). Thus, despite a crucial role of inflammation- and/or cytokine- independent NF-κB signaling in HSC homeostasis, the tonic signals and upstream mediators of NF-κB in HSC have not been identified.
TNF receptor associated factor 6 (TRAF6), an E3 ubiquitin (Ub) ligase, is a signal transducer for the IL-1R and TLR superfamily, including in response to cytokines, pattern-associated molecular patterns, and damage-associated molecular patterns. In addition, TRAF6 activity is regulated through intracellular homeostatic processes, such as oxidative stress (Matsuzawa et al., 2005; Tang et al., 2013; Rezaeian et al., 2017), metabolite changes (Linares et al., 2013, 2015), and DNA damage (Hinz et al., 2010; Zhang et al., 2016b), which do not require upstream IL-1R/TLR signaling. Independent of the basis for activation, TRAF6 catalyzes the formation of Lys-63-linked polyubiquitin chains on itself and on other protein substrates, such as IκB kinase γ (IKKγ also known as NEMO), leading to a recruitment of a complex containing TAK1, TAK1 binding protein 2 (TAB2), and TAB3 (Wang et al., 2001; Kanayama et al., 2004). TAK1 activates the IKK kinase complex (IKKβ and IKKα) leading to NF-κB transcription factor activation (Deng et al., 2000; Lamothe et al., 2007). TRAF6 expression is increased in a subset of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) patients, and its over-expression results in hematopoietic defects in mouse genetic models (Starczynowski et al., 2010; Fang et al., 2012, 2017). In addition, deletion of miR-146a and TIFAB, negative regulators of TRAF6 expression and function, are commonly observed in MDS and AML (Starczynowski et al., 2010; Boldin et al., 2011; Zhao et al., 2011, 2013; Fang et al., 2014; Varney et al., 2015, 2017). Although TRAF6 overexpression is implicated in the MDS HSC defect, the physiological role of TRAF6 in HSC homeostasis and during normal hematopoiesis remains unknown. As such, we reasoned thatTRAF6 might be a critical node in the tonic activation required for maintaining a minimal threshold of NF-κB signaling in HSPC. Here, we report that TRAF6 as an essential regulator of HSC homeostasis by preserving self-renewal and quiescence through basal activation of NF-κB.
RESULTS
Deletion of Traf6 in Hematopoietic Cells Leads to Premature Lethality and Hematologic Defects
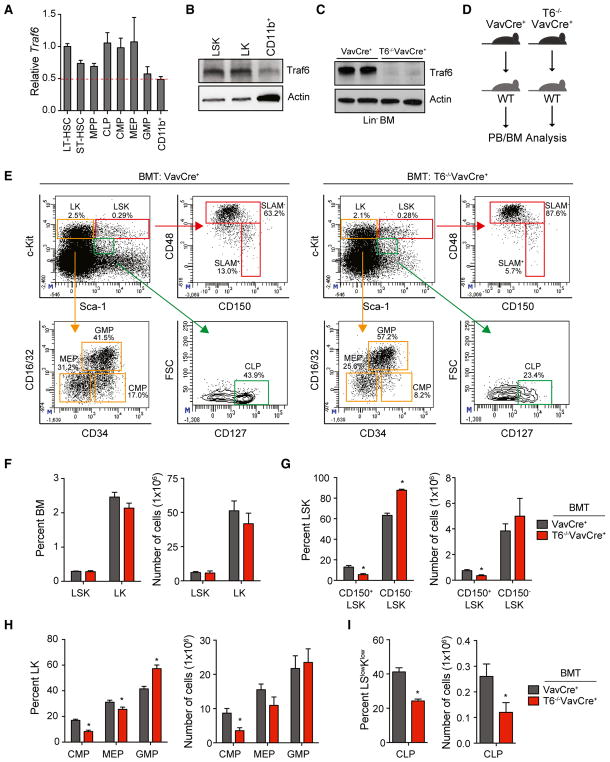
To define the role of TRAF6 in normal hematopoiesis, we first determined the expression of TRAF6 mRNA and protein in mouse BM populations by qRT-PCR and immunoblotting, respectively. Traf6 mRNA was broadly expressed in all mouse hematopoietic lineages examined (Figure 1A). Traf6 was ~2-fold higher in LT-HSC (lineage−cKit+Sca1+CD34−CD135−), common lymphoid progenitors (lineage−cKitlowSca1lowCD127+), and myeloid progenitors (lineage−cKit+Sca1+CD34+CD16/32− and lineage−cKit+Sca1−CD34−CD16/32−) as compared to CD11b+ myeloid cells (Figure 1A). Traf6 protein expression was also ~2.0-fold higher in lineage−cKit+Sca1+ (LSK) and lineage−cKit+ (LK) cells as compared to CD11b+ myeloid cells (Figure 1B). The expression of TRAF6 in HSC led us to speculate that TRAF6 might have a role in HSC function and/or homeostasis. We disrupted the Traf6 gene in the hematopoietic lineage by crossing Traf6-floxed mice with VavCre+ transgenic mice, in which a transgene encoding Cre recombinase is expressed specifically in all hematopoietic cells including LT-HSC (Traf6−/−VavCre+) (Figure S1A) (Pearce et al., 2009). Traf6 loss was confirmed in Traf6−/−VavCre+ mice by PCR of genomic DNA, by qRT-PCR to detect Traf6 mRNA, and by immunoblotting to detect loss of Traf6 protein expression (Figures 1C, S1A, and S1B). Deletion of Traf6 was restricted to hematopoietic cells, as Traf6 mRNA and protein were detectable in non-hematopoietic cells isolated from the liver, brain, and intestine of Traf6−/−VavCre+ and control mice (VavCre+) (Figures S1B and S1C). Although Traf6−/−VavCre+ were born at the expected Mendelian frequencies (Figure S2A), the deletion of Traf6 in hematopoietic cells resulted in premature lethality with a median survival of 9.5 weeks (p < 0.0001) (Figure S2B). A histological assessment of major organs revealed that all intestinal tissues from Traf6−/−VavCre+ mice appeared normal and similar to control mice (Figures S3A and S3B). However, the lungs and livers from Traf6−/−VavCre+ mice exhibited infiltration of immune cells, which may contribute to the early mortality and morbidity of the mice (Figures S3C and S3D). Deletion of Traf6 in hematopoietic cells resulted in significant effects on blood formation. Peripheral blood (PB) counts of 5- to 10-week-old Traf6−/−VavCre+ mice revealed neutrophilia (p = 0.028), lymphopenia (p < 0.0001), and anemia (p = 0.0037) (Table S1). The proportion of CD11b+ myeloid cells (p < 0.0001) was significantly increased while the proportion of CD3+ T cells (p = 0.2) and B220+ B cells (p < 0.0001) was reduced in the PB of Traf6−/−VavCre+ mice as compared to control VavCre+ mice (Figures S4A and S4B). The BM cellularity of Traf6-deleted mice was reduced by more than 50% between 5–10 weeks of age as compared to control mice, which is due to the reduced body size of Traf6−/−VavCre+ mice (Figure S4C). The proportion of myeloid cells (p = 0.2) was moderately increased, while the frequency of B cells was reduced in the BM of Traf6−/−VavCre+ mice as determined by flow cytometry (p < 0.0001) (Figure S4D).
Figure 1. Deletion of Traf6 in Hematopoietic Cells Leads to Reduced HSPC.
(A) Traf6 mRNA expression by qRT-PCR in the indicated mouse BM hematopoietic stem and progenitor cell subpopulations (n = 5 per group).
(B) Traf6 protein expression by immunoblotting in the indicated mouse BM cells.
(C) Traf6 protein expression by immunoblotting in lineage− BM (Lin− BM) cells from VavCre+ and Traf6−/−VavCre+ mice (pooled from 2 mice).
(D) Outline of non-competitive BM transplantations using VavCre+ or Traf6−/−VavCre+ mice.
(E) Representative flow cytometric analysis of HSPC subpopulations within the BM of recipient mice transplanted with the VavCre+ and Traf6−/−VavCre+ BM cells.
(F–I) Frequency (left) and number (right) of LSK or LK (F), CD150+LSK or CD150−LSK (G), CMP, GMP, or MEP (H), and CLP (I) from the BM of recipient mice transplanted with VavCre+ and Traf6−/− VavCre+ BM cells (n = 6 per group). *p < 0.05. Data are represented as mean ± SEM.
To determine whether the hematologic defects associated with deletion of Traf6 in hematopoietic cells are cell-intrinsic and transplantable, BM cells from Traf6−/−VavCre+ and control VavCre+ mice were transplanted into lethally irradiated congenic recipient mice (Figure 1D). Recipient mice reconstituted with Traf6−/−VavCre+ BM cells succumbed to hematologic defects with a median survival time of 32 weeks (p = 0.0061) (Figure S4E), indicating that Traf6-deficient hematopoietic defects are transplantable. Consistent with non-transplanted Traf6−/−VavCre+ mice, recipient mice reconstituted with Traf6−/−VavCre+ BM cells developed leukopenia (p < 0.0001), lymphopenia (p < 0.0001), and neutrophilia (p = 0.0016) (Table S2). The proportion of CD11b+ myeloid cells (p < 0.0001) were increased while the frequencies of B220+ B lymphocytes (p < 0.0001) and CD3+ T lymphocytes (p < 0.0001) were reduced in the PB of recipient mice reconstituted with Traf6−/−VavCre+ BM cells as compared to recipient mice reconstituted with control BM cells (Figure S4F). Unlike non-transplanted Traf6−/−VavCre+ mice, recipient mice reconstituted with Traf6−/−VavCre+ BM cells did not develop a hypocellular BM as compared to recipient mice reconstituted with control (VavCre+) BM cells (data not shown). Nevertheless, the proportion of myeloid cells (p < 0.05) was increased, while the frequencies of B220+ B lymphocytes (p < 0.05) were reduced in the BM of recipient mice reconstituted with Traf6−/−VavCre+ BM cells as compared to recipient mice reconstituted with control BM cells (Figure S4G).
Deletion of Traf6 Leads to Loss of HSPC
To determine whether Traf6 is required for HSPC homeostasis, BM cells were examined in Traf6−/−VavCre+ and control VavCre+ mice. The proportions of LSK and LK were comparable in the BM of Traf6−/−VavCre+ or VavCre+ mice (Figure S5). In contrast, Traf6−/−VavCre+ mice exhibited reduced proportions of CD150+LSK in the BM as compared to VavCre+ mice (Figure S5). To examine HSPC function following hematopoietic reconstitution, BM cells from Traf6−/−VavCre+ and control VavCre+ mice were transplanted into lethally irradiated congenic recipient mice (Figure 1D). Four months after transplantation, HSPC proportions were examined by flow cytometry (Figure 1E). The proportions of LSK and LK in the BM were comparable in recipient mice reconstituted with Traf6−/−VavCre+ or VavCre+ BM cells (Figure 1F). However, mice reconstituted with Traf6−/−VavCre+ BM cells had significantly reduced proportions of CD150+LSK, common myeloid progenitor (CMP), and megakaryocyte-erythroid progenitor (MEP) cells in the BM (Figures 1G and 1H). The absolute numbers of HSPC on the basis of their frequencies and BM cellularity was also calculated. We observed fewer numbers of CD150+LSK, CMP, and MEP cells in Traf6−/−VavCre+ BM (Figures 1G and 1H). Examination of HSPC proportions and absolute numbers in spleens revealed that LSK and CD150+LSK were moderately increased in the spleen of mice reconstituted with Traf6−/−VavCre+ BM as compared to control BM cells (Figures S6A and S6B). Granulocyte-monocyte progenitor (GMP) proportions and total numbers were moderately increased in the BM and spleen of mice reconstituted with Traf6−/−VavCre+ BM cells (Figures 1H and S6C). Common lymphoid progenitor (CLP) proportions and total numbers were decreased in mice reconstituted with Traf6−/−VavCre+ BM cells as compared to littermate controls, which may explain the lymphopenia in Traf6-deficient mice (Figure 1I). Collectively, Traf6 deficiency predominantly results in reduced HSC and lymphoid progenitors.
Traf6 Deletion Diminishes the Self-Renewal of HSC
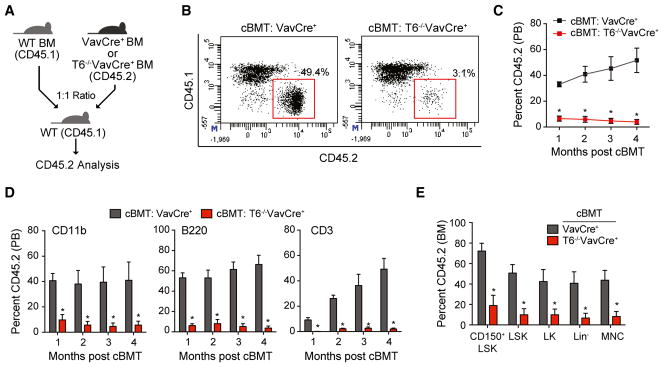
To evaluate the self-renewal potential of Traf6-deficient HSC, we performed competitive BM transplantation assays. Equal numbers of BM cells from Traf6−/−VavCre+ or VavCre+ mice (CD45.2+) were mixed with wild-type competitor cells (CD45.1+), transplanted into lethally irradiated congenic recipient mice, and then donor-derived (CD45.2+) hematopoietic reconstitution was determined by fluorescence-activated cell sorting (FACS) (Figure 2A). The hematopoietic contribution of Traf6−/−VavCre+ BM cells to peripheral blood chimerism was significantly reduced (from ~50% to <5%) compared with VavCre+ donor BM cells (Figures 2B and 2C). The reduced PB chimerism of Traf6−/−VavCre+ BM cells was a result of diminished numbers of myeloid (CD11b+) and lymphoid (CD3+ and B220+) cells as compared to mice reconstituted with control (VavCre+) BM cells, which persisted for >4 months post BM transplantations (Figure 2D). BM chimerism of LT-HSC (LSKCD150+CD48−; p = 0.003), HPC (LSK, p = 0.002; LK, p = 0.02; and Lin−, p = 0.01), and mononuclear cells was significantly reduced for Traf6−/−VavCre+ donor BM cells as compared to control donor BM cells 4 months post competitive transplantations (Figure 2E). These findings suggest that Traf6-deficient HSC are functionally defective and incapable of long-term reconstitution.
Figure 2. Traf6 Deletion Diminishes the Self-Renewal of HSC.
(A) Outline of competitive BM transplantations using VavCre+ or Traf6−/−VavCre+ mice.
(B) Representative flow cytometric analysis of donor-derived (CD45.2+) and competitor-derived (CD45.1+) PB from recipient mice after competitive transplantation using VavCre+ or Traf6−/−VavCre+ BM cells.
(C) Summary of donor-derived PB proportions at the indicated time points after competitive transplantation using VavCre+ or Traf6−/−VavCre+ BM cells (n = 7 per group).
(D) Proportion of donor-derived VavCre+ or Traf6−/−VavCre+ myeloid (CD11b+) and lymphoid (CD3+ and B220+) proportions at the indicated time following competitive transplantation (n = 7 per group).
(E) Summary of donor-derived BM HSPC proportions at 5 months after competitive transplantation using VavCre+ or Traf6−/−VavCre+ BM cells (n = 7 per group).
*p < 0.05. Data are represented as mean ± SEM.
Hematopoietic reconstitution of Traf6-deficient BM cells was significantly reduced within 4 weeks of BM transplantation, which prompted us to determine whether Traf6-deficient HSPC are defective at homing and/or migration to the BM. BM cells from Traf6−/−VavCre+ and VavCre+ mice were plated in methylcellulose to assess progenitor colony formation (“before homing”), or transplanted into lethally irradiated recipients. Sixteen hours post transplantation, BM cells were recovered from the recipients and plated in methylcellulose to determine progenitor colony formation (“after homing”) (Figure S7A). Homing of HSC to the BM was calculated based on the frequency of colony forming units recovered after BM transplantation relative to the colony forming units before BM transplantation. The hematopoietic defect associated with Traf6-deficient HSPC is not likely due to a homing deficiency as the homing of Traf6−/−VavCre+ HSPC to the BM was comparable to control (VavCre+) HSPC (Figure S7B). In parallel, migration of Traf6−/−VavCre+ and VavCre+ LSK and LK cells across a transwell toward a CXCL12 gradient was determined. As compared to VavCre+, Traf6−/−VavCre+ LSK or LK did not show a significant defect in migration (Figure S7C), thus suggesting that alterations in homing and/or migration to the BM do not account for the defects associated with Traf6-deficient HSPC.
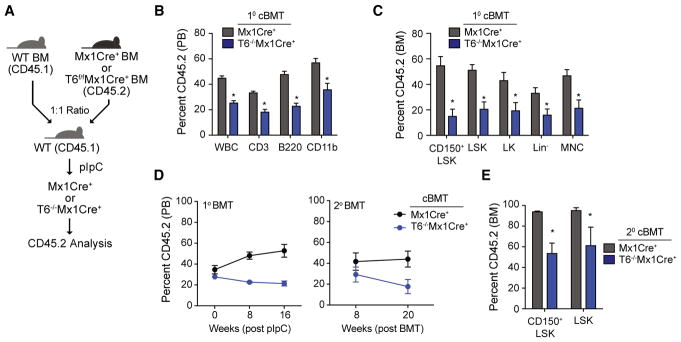
To determine whether the observed HSC phenotype in Traf6-deficient mice is cell intrinsic in older mice and unrelated to the consequences of BM transplantation, we generated conditional Traf6-deficient mice by crossing Traf6fl/fl with interferon-inducible Mx1Cre transgenic mice (Traf6f/fMx1Cre+). BM cells from Traf6f/fMx1Cre+ or Mx1Cre+ control mice (CD45.2+) together with the same number of wild-type CD45.1 competitor BM cells were transplanted into lethally irradiated CD45.1 recipient mice. One month post transplantation and prior to administration of polyinosinic-polycytidylic acid (pIpC) to induce Traf6 deletion, CD45.2+ chimerism was comparable in recipients with BM cells from Traf6−/−Mx1Cre+ mice and Mx1Cre+ mice. Subsequently, the transplanted mice were treated with pIpC injections to induce Traf6 deletion and monitored for 5 months (Figure 3A). Five months after deletion of Traf6, CD45.2+ cells were significantly reduced in the peripheral blood and BM of recipient mice (Figures 3B and 3C), which is consistent with phenotype observed in Traf6−/−VavCre+ mice. Reduced chimerism of Traf6−/−Mx1Cre+ CD45.2+ cells in the PB was observed in both myeloid (CD11b+) and lymphoid (CD3+ and B220+) populations (Figure 3B). Moreover, Traf6 deletion resulted in a significant reduction of phenotypically defined LT-HSC (LSKCD150+CD48−; p = 0.004) and HSPC (LSK, p = 0.002; LK, p = 0.03; and Lin−, p = 0.03) (Figure 3C). To define the function of LT-HSC, we examined hematopoietic contribution of Traf6−/−Mx1Cre+ mice and Mx1Cre+ LT-HSC following serial BM transplantations in lethally irradiated CD45.1 recipient mice. The hematopoietic contribution of Traf6−/−Mx1Cre+ HSC to long-term PB chimerism in secondary recipient mice was significantly reduced compared to wild-type donor Mx1Cre+ HSPC (p < 0.05) (Figure 3D). In addition, Traf6 deletion resulted in a reduction of LT-HSC (LSKCD150+; p = 0.07) and HSPC (LSK, p = 0.016) in secondary recipient mice, indicating that Traf6 is required for long-term HSC function (Figure 3E). Collectively, the data above suggests that Traf6 expression is important for HSC homeostasis, self-renewal, and progenitor differentiation.
Figure 3. Conditional Traf6 Deletion Diminishes the Long-Term Self-Renewal of HSC.
(A) Outline of competitive BM transplantations using Mx1Cre+ or Traf6f/fMx1Cre+ mice. Briefly, BM cells from Mx1Cre+ or Traf6flox/floxMx1Cre+ mice were mixed at 1:1 ratio with the competitor BM cells, followed by transplantation into lethally irradiated BoyJ mice. One month post-transplantation, pIpC was injected into the recipient mice to induce deletion of Traf6 (Traf6−/−Mx1Cre+).
(B and C) Summary of donor-derived PB (B) or BM (C) proportions at 5 months after pIpC injection in primary recipient mice using Mx1Cre+ or Traf6f/fMx1Cre+BM cells (n = 5–7 per group).
(D) Summary of donor-derived PB proportions at the indicated time points after pIpC injection following primary and secondary competitive transplantations using Mx1Cre+ or Traf6−/−Mx1Cre+ BM cells (n = 6 per group).
(E) Summary of donor-derived BM HSPC proportions at 5 months after secondary competitive transplantation using Mx1Cre+ or Traf6−/−Mx1Cre+ BM cells (n = 3 per group). *p < 0.05. Data are represented as mean ± SEM.
Traf6 Is Required for Maintaining HSC Quiescence
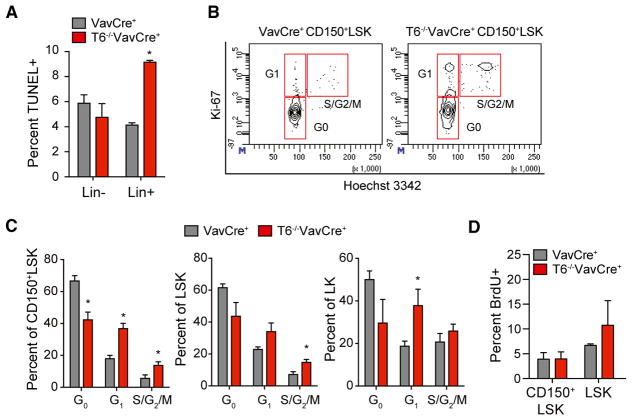
To identify the reason for the loss of HSC in Traf6-deficient mice, we examined cell survival and quiescence of HSPC, mechanisms responsible for maintaining normal HSC homeostasis. Increased cell death, as determined by TUNEL staining, was not significantly different between HSPC (Lin− cells) isolated from the BM of VavCre+ and Traf6−/−VavCre+ mice (Figure 4A). In contrast, differentiated HSPC (Lin+ cells) exhibited ~2-fold increase in TUNEL+ cells isolated from Traf6−/−VavCre+ mice as compared to Lin+ cells from VavCre+ (Figure 4A). The majority of LT-HSCs are in a quiescent cell-cycle state, which is necessary to preserve their long-term function and self-renewal capacity. Therefore, we next examined the cell-cycle status of Traf6−/−VavCre+ and VavCre+ HSPC by Ki-67 and Hoechst 33342 staining. As expected, control (VavCre+) CD150+LSK are primarily in G0 (67%) and rarely in G1 or S/G2/M of the cell cycle, an indication of cellular quiescence (Figures 4B and 4C). In contrast, the frequencies of CD150+LSK fraction in the G0 phase were reduced in Traf6−/−VavCre+ mice (43%), consistent with reduced cellular quiescence and increased proliferation (Figures 4B and 4C). Increased cell proliferation of Traf6-deficient LSK and LK was also observed (Figure 4C). BrdU incorporation was modestly increased in LSK cells (6.7% versus 10.7%; p = 0.3), but not in CD150+LSK, from Traf6−/−VavCre+ mice as compared to control VavCre+ mice (Figure 4D). These data indicate that Traf6 deficiency results in loss of cell quiescence and premature exhaustion of the HSC pool, and increased proliferation of HSPC.
Figure 4. Traf6 Is Required for Maintaining HSC Quiescence.
(A) TUNEL positive VavCre+ or Traf6−/−VavCre+ Lin- and Lin+ BM cells (n = 4 per group).
(B) Representative cell-cycle analysis of VavCre+ or Traf6−/−VavCre+ CD150+LSK cells.
(C) Proportion of VavCre+ or Traf6−/−VavCre+ CD150+LSK cells (left) or LSK cells (right) in G0, G1 and S/G2/M phase (n = 4 per group).
(D) Frequency of BrdU+ staining in VavCre+ or Traf6−/−VavCre+ CD150+LSK cells (left) or LSK cells (n = 4 per group). *p < 0.05. Data are represented as mean ± SEM.
Altered Gene Expression in Traf6-Deficient HSPC
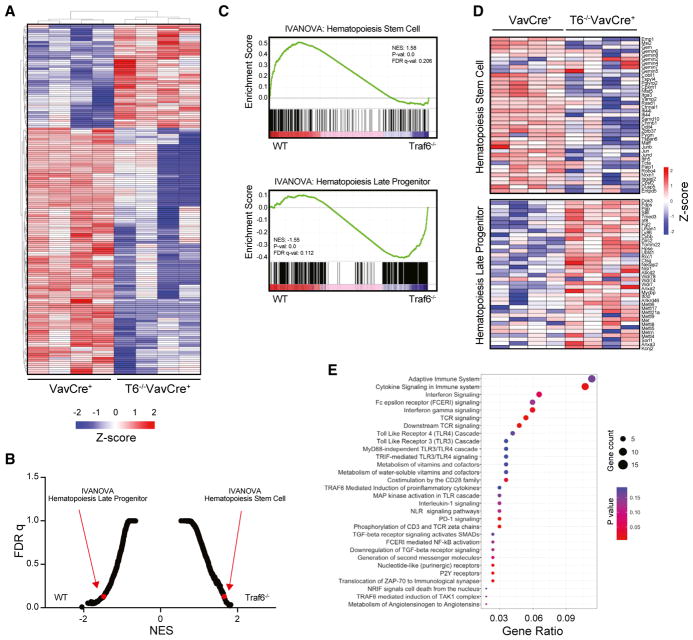
To gain mechanistic insight into the consequences of Traf6 deletion on impaired HSPC function, we performed RNA sequencing (RNA-seq) using LSK isolated from recipient mice 2 months post BM transplantation with Traf6−/−VavCre+ or VavCre+ BM cells (n = 4 per group). 776 genes were differentially expressed in Traf6−/−VavCre+ LSK compared with control (VavCre+) LSK cells (>1.5-fold; p < 0.05) (Figure 5A; Table S3). Gene set enrichment analysis (GSEA) revealed that Traf6−/−VavCre+ LSK cells had reduced expression of genes that are normally expressed by LT-HSC cells (p = 0; normalized enrichment score [NES] = 1.58), and showed increased expression of gene profiles associated with hematopoietic progenitor cells (p = 0; NES = −1.55) (Figures 5B–5D). Loss of genes that are normally expressed by LT-HSC cells and acquisition of genes associated with hematopoietic progenitors is consistent with reduced quiescence and increased proliferation of Traf6−/−VavCre+ HSC. We did not observe a gene signature associated with apoptosis in Traf6-deficient LSK, suggesting apoptosis is not the main cause resulting in diminished HSPC numbers and function. Pathway analysis networks using the Reactome Database revealed changes in adaptive immune signaling, innate immune signaling, and NF-κB signaling (Figure 5E). A loss of gene regulatory networks associated with immune signaling is particularly intriguing as it suggests that tonic signaling via TRAF6 in HSPC occurs in the absence of acute infection and correlates with diminished LT-HSC gene expression signature. Consistent with these analyses, Traf6−/−VavCre+ LSK cells had reduced expression of NF-κB target genes that are characteristically activated by inflammatory cytokines, such as TNF-α, as determined by GSEA (p = 0.0; NES = 1.72) and by qRT-PCR (Figures 6A and 6B).
Figure 5. Altered Transcriptome in Traf6-Deficient HSPC.
(A) Heatmap of all differentially expressed genes in VavCre+ and Traf6−/−VavCre+ LSK cells (1.5-fold; p < 0.05; n = 4 per group).
(B) Rank order of enriched gene signatures in VavCre+ or Traf6−/−VavCre+ LSK cells based on false discovery rate (FDR) q value (n = 4 per group).
(C) Gene Set Enrichment Analysis (GSEA) plot enriched in the VavCre+ LSK relative to Traf6−/−VavCre+ LSK (top). GSEA plot enriched in Traf6−/−VavCre+ LSK relative to VavCre+ LSK (bottom).
(D) Heatmap generated from the GSEA leading edge genes in VavCre+ or Traf6−/−VavCre+ LSK cells.
(E) Pathway analysis of differentially expressed genes in Traf6−/−VavCre+ LSK relative to VavCre+ LSK.
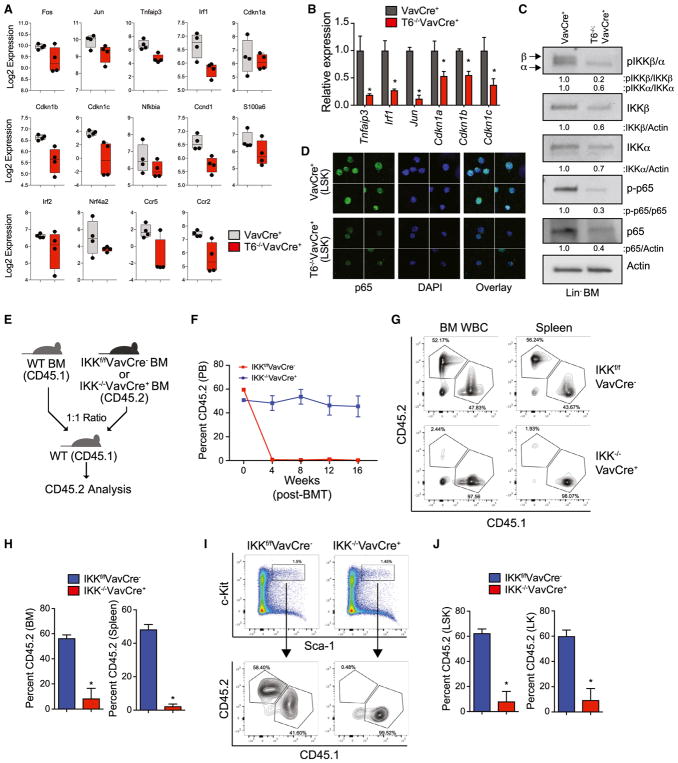
Figure 6. Reduced NF-κB Signaling and IKKβ Deficiency Leads to Loss of HSPC and Impaired Hematopoietic Reconstitution.
(A) Expression of NF-κB target genes in VavCre+ and Traf6−/−VavCre+ LSK cells as determined by RNA-seq.
(B) Expression of select NF-κB target genes measured by qRT-PCR in Traf6−/−VavCre+ relative to VavCre+ LSK cells.
(C) Immunoblot analysis of phosphorylated IKKβ, IKKα, and p65 in VavCre+ and Traf6−/−VavCre+ Lin− BM cells. Shown below is the relative expression of the indicated proteins calculated by measuring densitometry of the immunoblots.
(D) Expression and localization of p65 (green) in VavCre+ and Traf6−/−VavCre+ LSK cells as determined by indirect immunofluorescence (IF). Shown are representative images from 3 pooled mice per group.
(E) Outline of competitive BM transplantations using IKKf/fVavCre− or IKK−/−VavCre+ mice.
(F) Summary of donor-derived PB proportions at the indicated time points after competitive transplantation using IKKf/fVavCre− or IKK−/−VavCre+ BM cells (n = 5 per group). Two independent experiments were performed.
(G) Representative flow cytometric analysis of donor-derived (CD45.2+) and competitor-derived (CD45.1+) BM and spleen cells from recipient mice after competitive transplantation using IKKf/fVavCre− or IKK−/−VavCre+ BM cells.
(H) Proportion of donor-derived IKKf/fVavCre− or IKK−/−VavCre+ BM and spleen cells 8 weeks after competitive BM transplantation (n = 5 per group).
(I) Representative flow cytometric analysis of donor-derived (CD45.2+) and competitor-derived (CD45.1+) BM LSK cells from recipient mice after competitive transplantation using IKKf/fVavCre− or IKK−/−VavCre+ BM cells.
(J) Proportion of donor-derived IKKf/fVavCre− or IKK−/−VavCre+ LSK BM 8 weeks after competitive BM transplantation (n = 5 per group). *p < 0.05. Data are represented as mean ± SEM.
TRAF6 has been previously implicated in controlling alternative RNA splicing in HSPC through ubiquitination of RNA binding proteins (Fang et al., 2017). To determine whether loss of TRAF6 affects RNA isoform expression and alternative splicing, we next performed paired-end RNA-seq from poly(A)-selected Traf6−/−VavCre+ and VavCre+ LSK RNA and applied a percent spliced-in (PSI) algorithm using aligned exon-exon and exon-intron junction reads (MultiPath-PSI in the software AltAnalyze). Using a PSI difference of >10% between matched replicates, we identified 223 alternative splicing events unique to Traf6−/−VavCre+ LSK as compared to VavCre+ LSK, with an enrichment of genes essential for hematopoietic cell function (Figure S8A; Table S4). As one explanation for diminished NF-κB activation in TRAF6-deficient HSPC, we found that Traf6−/−VavCre+ LSK exhibit alternative splicing of Nfkb1, which encodes p105/p50, a member of the NF-κB transcription factor family. Traf6−/−VavCre+ LSK preferentially retain exon 25 in Nfkb1, which has previously been shown to result in expression of the cytoplasmic version of Nfkb1 (p105) and diminished NF-κB nuclear translocation and DNA-binding (Grumont et al., 1994). In contrast, control LSK cells (VavCre+) express nuclear versions of Nfkb1 (p50, p63, and p84), as evident by exclusion of exon 25 (Figures S8B and S8C). To assess NF-κB pathway activation, we examined total and phosphorylated IKKβ, IKKα, and RelA/p65 in Traf6−/−VavCre+ and control Lin− BM cells by immunoblotting, and RelA/p65 in Traf6−/−VavCre+ and control LSK cells by immunofluorescence. Phosphorylated IKKβ and p65 were reduced in Traf6−/−VavCre+ Lin− BM cells as compared with control (VavCre+) Lin− BM cells (Figure 6C). Moreover, nuclear and total p65 was reduced in Traf6−/−VavCre+ LSK cells by 45% as compared with control (VavCre+) LSK cells (Figures 6D and S9). Collectively, these findings indicate that TRAF6 is required for maintaining a minimal threshold level of IKK/NF-κB signaling in HSPC.
IKKβ Deficiency Leads to Loss of HSPC and Impaired Hematopoietic Reconstitution
HSPC are exquisitely sensitive to fluctuations in IKK/NF-κB expression, therefore re-establishing the optimal threshold of IKKβ activity in Traf6-deficient HSPC cells was not feasible (data not shown). As an alternative approach to investigate the relevance of diminished IKK/NF-κB signaling in HSPC, we determined whether IKKβ (Ikbkb) loss is sufficient to recapitulate the hematopoietic defects observed in Traf6-deficient BM. IKKβ was selected as it is immediately downstream of TRAF6 signaling and is responsible for initiating NF-κB nuclear translocation and transcriptional activation. We generated conditional IKKβ-deficient mice by crossing Ikbkb-floxed mice with VavCre+ transgenic mice (IKK−/−VavCre+). Equal numbers of whole BM cells from IKK−/−VavCre+ or IKKf/fVavCre− mice (CD45.2+) were mixed with wild-type competitor cells (CD45.1+), transplanted into lethally irradiated congenic recipient mice (Figure 6E), and then donor-derived (CD45.2+) hematopoietic reconstitution in the BM, peripheral blood, and spleen was determined by FACS. Peripheral blood chimerism of IKK−/−VavCre+ BM cells was significantly diminished within 4 weeks post transplantation and remained <1% after 16 weeks (Figure 6F). The hematopoietic contribution of IKK−/−VavCre+ BM cells to BM and spleen chimerism was also significantly reduced (from ~50% to <5%) compared with VavCre+ donor BM cells (Figures 6G and 6H). Moreover, chimerism of LSK and LK from IKK−/−VavCre+ BM cells was dramatically diminished (Figures 6I and 6J). These findings suggest that Traf6-deficient HSC are functionally defective and incapable of long-term reconstitution due in part to diminished basal activation of IKKβ and NF-κB (Figure 7).
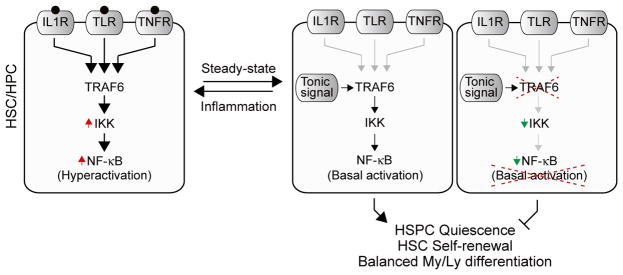
Figure 7. Model of Inflammatory and Tonic NF-κB Signaling via TRAF6 during Hematopoietic Stem and Progenitor Cell Homeostasis.
During inflammation, IL-1R, TLRs, and/or TNFRs signaling via TRAF6 results in maximal NF-κB activation in HSPC. In the absence of inflammatory signaling, tonic signals and/or stochastic activation of TRAF6 results in basal NF-κB stimulation required for HSPC homeostasis, including preserving quiescence, self-renewal, and balanced myeloid (My) and lymphoid (Ly) differentiation. In the absence of TRAF6, basal NF-κB activation is diminished, which results in loss of HSPC quiescence and self-renewal.
DISCUSSION
Our study demonstrates that TRAF6 is essential for HSC homeostasis. Hematopoietic-specific deletion of Traf6 resulted in reduced immune- and NF-κB-related gene signatures and impaired HSC function. Therefore, signaling via TRAF6 occurs in HSPC and correlates with diminished HSC function in the absence of inflammation and/or infection. We also established that loss of NF-κB signaling contributes to the major hematopoietic defects observed in Traf6-deficient HSPC; that is, IKKβ-deficient HSPC resulted in severely impaired HSC self-renewal and fitness. Our observations suggest that TRAF6 is an essential regulator of HSC homeostasis by maintaining a minimal threshold level of NF-κB signaling (Figure 7).
Although we describe a functional requirement of TRAF6 in normal hematopoiesis, a role TRAF6 in HSC function was inferred from a large-scale mRNA expression and gene mapping analysis performed to identify genes and loci that control HSC function in a panel of densely genotyped recombinant inbred mouse strains. Mapping of quantitative trait loci identified Traf6 as a trans-acting control loci associated with variations in attributes of HSC populations (Bystrykh et al., 2005). Our findings that TRAF6 dosage and activity are important for HSC homeostasis are congruent with its role in hematologic malignancies, such as MDS. Chronic TLR activation compromises HSC function and induces myeloid-biased differentiation, yet if not resolved, results in cytopenias, myeloid dysplasia, and BM failure. Canonical NF-κB signaling is not sufficient to induce MDS in mice (Beg et al., 1995; Rupec et al., 2005), indicating that other pathways downstream of TRAF6 contribute to the initiation of MDS. Utilizing a hematopoietic-specific TRAF6 overexpression mouse model, we recently reported a mechanism by which TRAF6 mediates ubiquitination of hnRNPA1, alters RNA exon usage and contributes to MDS-associated HSPC defects in part by activating Cdc42 (Fang et al., 2017). These findings along with our RNA splicing analysis in TRAF6-deficient HSPC (Figure S8) reinforce the contribution of TRAF6 to the control of hematopoietic-requisite RNA isoforms. Therefore, TRAF6 may elicit distinct and overlapping regulatory functions during normal and malignant hematopoiesis.
Through genetic loss-of-function models, TRAF6 has been linked to the function of various immune effector cells. Mice deficient in Traf6 succumb to perinatal and postnatal lethality and develop osteopetrosis due to impaired osteoclast function and defects in bone remodeling (Lomaga et al., 1999). Additional immune-related functions were revealed after deletion of TRAF6 in the myeloid and lymphoid compartments (King et al., 2006; Kobayashi et al., 2009; Han et al., 2013; Muto et al., 2013). We suspect that some of the hematologic phenotypes observed in our hematopoietic-specific Traf6-deficient mice may arise from defects in myeloid and/or lymphoid populations. However, our competitive BM transplantation data indicate that impaired HSPC numbers and function in hematopoietic-specific Traf6-deficient mice is primarily due to loss of TRAF6 in HSPC and not secondary from loss of TRAF6 in immune effector cells.
As we and others have shown, deletion of NF-κB/p65 or its kinase (IKKβ) results in aberrant HSC function (Stein and Baldwin, 2013; Zhang et al., 2015). Interestingly, deletion of upstream mediators of NF-κB, such as IL-1R, TLRs, or receptor adaptors does not result in diminished HSC homeostasis and/or function (Orelio et al., 2009; Liu et al., 2015; Herman et al., 2016; Zhang et al., 2016a), suggesting that an intermediate signaling node, such as TRAF6, is required for mediating a minimal threshold level of NF-κB activation in HSC. Thus, TRAF6 is important not only for ligand-mediated responses in immune effector cells, but also for maintaining basal NF-κB signaling in HSPC. Tonic signaling is an established survival and selection mechanism for immune cells, including B- and T-lymphocytes. Low and intermediate affinity interactions result in tonic T cell receptor signaling that is required to keep T cells responsive to antigen (Garbi et al., 2010). In addition, ligand-independent tonic signaling in B cells is essential for their survival and function. BCR signaling occurs independently of antigen, and, furthermore, these non-induced or “tonic” signals are linked to specific cellular processes operating at multiple stages of B cell development (Monroe, 2004). For example, NF-κB pathways mediate the survival of follicular B cells through tonic BCR signals that generate the NF-κB subunit p100 (Stadanlick et al., 2008).
Although our studies suggest that tonic NF-κB signaling via TRAF6 is important for the maintenance of HSC, the precise mechanism of TRAF6 activation during HSPC homeostasis remains unknown. TRAF6 activity can be regulated through intracellular homeostatic processes, such as oxidative stress (Matsuzawa et al., 2005; Tang et al., 2013; Rezaeian et al., 2017), metabolite changes (Linares et al., 2013, 2015), and DNA damage (Hinz et al., 2010; Zhang et al., 2016b), which do not require upstream IL-1R or TLR signaling. Therefore, it is possible that TRAF6 responds to oxidative, metabolic, and genotoxic mechanisms in HSC via non-receptor signaling intermediates. Alternatively, stochastic auto-activation of TRAF6 E3 ubiquitin ligase activity might stimulate downstream signaling; in this case, an upstream signal is not obligatory. In summary, we show that TRAF6 dosage is important for HSC function, and that TRAF6 maintains a minimal threshold level of NF-κB signaling necessary for hematopoietic stem cell homeostasis (Mankan et al., 2011).
EXPERIMENTAL PROCEDURES
Mice
All mice were bred, housed, and handled in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility of Cincinnati Children’s Hospital Medical Center (CCHMC) or Memorial Sloan Kettering Cancer Center (MSKCC). Animal care was in strict compliance with the institutional guidelines established by CCHMC and MSKCC, the Guide for the Care and Use of Animals, and the Association for Assessment and Accreditation of Laboratory Animal Care International. TRAF6fl/fl mice (C57BL/6) were a kind gift from Dr. Yongwon Choi (University of Pennsylvania) (Han et al., 2013). Traf6fl/fl mice were crossed with Mx1Cre (Jackson Laboratory, 003556) and VavCre mice (Jackson Laboratory, 008610) for inducible or conditional deletion of TRAF6 (Traf6fl/fl;Mx1Cre) and (Traf6fl/fl; VavCre), respectively. To delete TRAF6, Cre transgene expression in Traf6fl/fl;Mx1Cre mice was induced by pIpC. IKKβfl/fl mice were previously described and a kind gift from Dr. Albert Baldwin (University of North Carolina) (Mankan et al., 2011). To delete IKKβ in hematopoietic cells, IKKβfl/fl mice were crossed with VavCre mice. BM cells were obtained by crushing the femur, tibia, and pelvic bone, and maintained in RPMI1640 with 10% fetal bovine serum.
Alternative Splicing Analysis
To identify differentially spliced exons and introns, we performed RNA-seq from total RNA (Illumina HiSeq 2500), at an average depth of ~37 million paired-end 100 nt reads using the TruSeq RNA Library Prep Kit (Illumina) by the University of Cincinnati Genomics Sequencing Core. These subsequent FASTA files were supplied as input for genome alignment (mm10) with the software STAR to produce BAM files with read-strand predictions resulting in ~72% aligned reads on average. Alternative splicing detection and quantification was performed in the software AltAnalyze version 2.1.1 (Emig et al., 2010), using the recently developed MultiPath-PSI algorithm that applies an unbiased local splicing variation-based percent splicing analysis to all overlapping exon-exon and exon-intron aligned reads (paired-end data required for intron-retention analysis) (http://altanalyze.readthedocs.io/en/latest/Algorithms/#multipath-psi-splicing-algorithm). The default delta PSI cutoff of 0.1 (10% inclusion difference) was applied to comparison of paired Traf6−/−VavCre+ and Vav-Cre+ LSK and consistently differential splicing events selected between the two replicate experiments. Gene set enrichment analysis of the consistently detected splicing events was performed using the software ToppFun.
Statistical Analysis
RNA-seq was performed using n = 4 mice per group. Blood count analysis and organ weights were recorded as indicated in the figure legends. For secondary or competitive transplantation experiments, two donor mice were used for all recipients. Survival experiments were performed as indicated in the figure legends. The number of animals, cells, and experimental replicates can be found in the figure legends. Unless otherwise specified, results are depicted as the mean ± SEM. Statistical analyses were performed using Student’s t test (unpaired, two-tailed). For Kaplan-Meier analysis, Mantel-Cox test was used. Data were analyzed and plotted using GraphPad Prism 6 software. For correlative analyses, Spearman rank test was used.
Supplementary Material
Highlights.
TRAF6 is required for hematopoietic stem cell (HSC) homeostasis
TRAF6 regulates HSC quiescence, self-renewal, and differentiation
Deletion of TRAF6 results in loss of HSC fitness
TRAF6 maintains a minimal threshold level of IKKβ/NF-κB signaling in HSC
Acknowledgments
We thank Jeff Bailey and Victoria Summey for assistance with transplantations (Comprehensive Mouse and Cancer Core at CCHMC). TRAF6-floxed mice were kindly provided by Dr. Yongwon Choi (University of Pennsylvania). IKK2-floxed mice were kindly provided by Dr. Albert Baldwin (University of North Carolina). This work was supported by Cincinnati Children’s Hospital Research Foundation, American Society of Hematology (ASH), NIH (R35HL135787, RO1HL111103, RO1DK102759, and RO1HL114582), Gabrielle’s Angel Foundation for Cancer Research, and Edward P. Evans Foundation grants to D.T.S. This work was supported by NCI R35197594 to R.L.L. and K99 HL122503-01A1 to M.K., and work in core facilities in the Levine lab was supported by MSKCC Support Grant/Core Grant P30 CA008748. T.M. is supported by The Uehara Memorial Foundation, The Waksman Foundation of Japan, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Japan Society for the Promotion of Science.
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the data reported in this study is GEO: GSE109050.
Supplemental Information includes Supplemental Experimental Procedures, nine figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.013.
AUTHOR CONTRIBUTIONS
J.F., T.M., and D.T.S. conceived and designed the experiments and analyzed the data. M.K. and R.L.L. conceived and designed the experiments and analyzed the IKK-related data. L.C.B., K.M.H., C.S.W., K. Choi, L.S., and A.M.W. contributed to the design, execution, and analysis of the experiments. Y.C. generated mice. Y.Z. and J.A.C. provided guidance and contributed to the design, execution, and analysis of the HSC experiments. K. Chetal and N.S. performed the RNA splicing analysis. K. Chetal performed and supervised the bioinformatics analysis. D.T.S. supervised the project.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrykh L, Weersing E, Dontje B, Sutton S, Pletcher MT, Wiltshire T, Su AI, Vellenga E, Wang J, Manly KF, et al. Uncovering regulatory pathways that affect hematopoietic stem cell function using ‘genetical genomics’. Nat Genet. 2005;37:225–232. doi: 10.1038/ng1497. [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emig D, Salomonis N, Baumbach J, Lengauer T, Conklin BR, Albrecht M. AltAnalyze and DomainGraph: analyzing and visualizing exon expression data. Nucleic Acids Res. 2010;38:W755–W762. doi: 10.1093/nar/gkq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín-Palazón R, Traver D. The NF-κB family: key players during embryonic development and HSC emergence. Exp Hematol. 2016;44:519–527. doi: 10.1016/j.exphem.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Espín-Palazón R, Stachura DL, Campbell CA, García-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, Traver D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rhyasen G, Bolanos L, Rasch C, Varney M, Wunderlich M, Goyama S, Jansen G, Cloos J, Rigolino C, et al. Cytotoxic effects of bortezomib in myelodysplastic syndrome/acute myeloid leukemia depend on autophagy-mediated lysosomal degradation of TRAF6 and repression of PSMA1. Blood. 2012;120:858–867. doi: 10.1182/blood-2012-02-407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Barker B, Bolanos L, Liu X, Jerez A, Makishima H, Christie S, Chen X, Rao DS, Grimes HL, et al. Myeloid malignancies with chromosome 5q deletions acquire a dependency on an intrachromosomal NF-κB gene network. Cell Rep. 2014;8:1328–1338. doi: 10.1016/j.celrep.2014.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Bolanos LC, Choi K, Liu X, Christie S, Akunuru S, Kumar R, Wang D, Chen X, Greis KD, et al. Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia. Nat Immunol. 2017;18:236–245. doi: 10.1038/ni.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbi N, Hämmerling GJ, Probst HC, van den Broek M. Tonic T cell signalling and T cell tolerance as opposite effects of self-recognition on dendritic cells. Curr Opin Immunol. 2010;22:601–608. doi: 10.1016/j.coi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Grumont RJ, Fecondo J, Gerondakis S. Alternate RNA splicing of murine nfkb1 generates a nuclear isoform of the p50 precursor NF-kappa B1 that can function as a transactivator of NF-kappa B-regulated transcription. Mol Cell Biol. 1994;14:8460–8470. doi: 10.1128/mcb.14.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Walsh MC, Cejas PJ, Dang NN, Kim YF, Kim J, Charrier-Hisamuddin L, Chau L, Zhang Q, Bittinger K, et al. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity. 2013;38:1211–1222. doi: 10.1016/j.immuni.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AC, Monlish DA, Romine MP, Bhatt ST, Zippel S, Schuettpelz LG. Systemic TLR2 agonist exposure regulates hematopoietic stem cells via cell-autonomous and cell-non-autonomous mechanisms. Blood Cancer J. 2016;6:e437. doi: 10.1038/bcj.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- King CG, Kobayashi T, Cejas PJ, Kim T, Yoon K, Kim GK, Chiffoleau E, Hickman SP, Walsh PT, Turka LA, Choi Y. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat Med. 2006;12:1088–1092. doi: 10.1038/nm1449. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kim TS, Jacob A, Walsh MC, Kadono Y, Fuentes-Pananá E, Yoshioka T, Yoshimura A, Yamamoto M, Kaisho T, et al. TRAF6 is required for generation of the B-1a B cell compartment as well as T cell-dependent and -independent humoral immune responses. PLoS ONE. 2009;4:e4736. doi: 10.1371/journal.pone.0004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. J Biol Chem. 2007;282:4102–4112. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Duran A, Reina-Campos M, Aza-Blanc P, Campos A, Moscat J, Diaz-Meco MT. Amino acid activation of mTORC1 by a PB1-domain-driven kinase complex cascade. Cell Rep. 2015;12:1339–1352. doi: 10.1016/j.celrep.2015.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang Y, Ding Y, Baez I, Payne KJ, Borghesi L. Cutting edge: hematopoietic stem cell expansion and common lymphoid progenitor depletion require hematopoietic-derived, cell-autonomous TLR4 in a model of chronic endotoxin. J Immunol. 2015;195:2524–2528. doi: 10.4049/jimmunol.1501231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankan AK, Canli O, Schwitalla S, Ziegler P, Tschopp J, Korn T, Greten FR. TNF-alpha-dependent loss of IKKbeta-deficient myeloid progenitors triggers a cytokine loop culminating in granulocytosis. Proc Natl Acad Sci USA. 2011;108:6567–6572. doi: 10.1073/pnas.1018331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Monroe JG. Ligand-independent tonic signaling in B-cell receptor function. Curr Opin Immunol. 2004;16:288–295. doi: 10.1016/j.coi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Muto G, Kotani H, Kondo T, Morita R, Tsuruta S, Kobayashi T, Luche H, Fehling HJ, Walsh M, Choi Y, Yoshimura A. TRAF6 is essential for maintenance of regulatory T cells that suppress Th2 type autoimmunity. PLoS ONE. 2013;8:e74639. doi: 10.1371/journal.pone.0074639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa MM, Thummar K, Mandelbaum J, Pasqualucci L, Rathinam CV. Lack of the ubiquitin-editing enzyme A20 results in loss of hematopoietic stem cell quiescence. J Exp Med. 2015;212:203–216. doi: 10.1084/jem.20132544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orelio C, Peeters M, Haak E, van der Horn K, Dzierzak E. Interleukin-1 regulates hematopoietic progenitor and stem cells in the midgestation mouse fetal liver. Haematologica. 2009;94:462–469. doi: 10.3324/haematol.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeian AH, Li CF, Wu CY, Zhang X, Delacerda J, You MJ, Han F, Cai Z, Jeong YS, Jin G, et al. A hypoxia-responsive TRAF6-ATM-H2AX signalling axis promotes HIF1α activation, tumorigenesis and metastasis. Nat Cell Biol. 2017;19:38–51. doi: 10.1038/ncb3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupec RA, Jundt F, Rebholz B, Eckelt B, Weindl G, Herzinger T, Flaig MJ, Moosmann S, Plewig G, Dörken B, et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity. 2005;22:479–491. doi: 10.1016/j.immuni.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Schuettpelz LG, Borgerding JN, Christopher MJ, Gopalan PK, Romine MP, Herman AC, Woloszynek JR, Greenbaum AM, Link DC. G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia. 2014;28:1851–1860. doi: 10.1038/leu.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, Hirst M, Hogge D, Marra M, Wells RA, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat Med. 2010;16:49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- Stein SJ, Baldwin AS. Deletion of the NF-κB subunit p65/RelA in the hematopoietic compartment leads to defects in hematopoietic stem cell function. Blood. 2013;121:5015–5024. doi: 10.1182/blood-2013-02-486142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G, Inagaki M, Takubo K, Mishina Y, Hess PR, Dean GA, Yoshimura A, Matsumoto K, Suda T, Ninomiya-Tsuji J. TAK1 (MAP3K7) signaling regulates hematopoietic stem cells through TNF-dependent and -independent mechanisms. PLoS ONE. 2012;7:e51073. doi: 10.1371/journal.pone.0051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HW, Liao HM, Peng WH, Lin HR, Chen CH, Chen GC. Atg9 interacts with dTRAF2/TRAF6 to regulate oxidative stress-induced JNK activation and autophagy induction. Dev Cell. 2013;27:489–503. doi: 10.1016/j.devcel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Tesio M, Tang Y, Müdder K, Saini M, von Paleske L, Macintyre E, Pasparakis M, Waisman A, Trumpp A. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J Exp Med. 2015;212:525–538. doi: 10.1084/jem.20141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney ME, Niederkorn M, Konno H, Matsumura T, Gohda J, Yoshida N, Akiyama T, Christie S, Fang J, Miller D, et al. Loss of Tifab, a del(5q) MDS gene, alters hematopoiesis through derepression of Toll-like receptor-TRAF6 signaling. J Exp Med. 2015;212:1967–1985. doi: 10.1084/jem.20141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varney ME, Choi K, Bolanos L, Christie S, Fang J, Grimes HL, Maciejewski JP, Inoue JI, Starczynowski DT. Epistasis between TIFAB and miR-146a: neighboring genes in del(5q) myelodysplastic syndrome. Leukemia. 2017;31:491–495. doi: 10.1038/leu.2016.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L, Baldwin AS, Jr, Friedman AD, Paz-Priel I. Loss of IKKβ but not NF-κB p65 skews differentiation towards myeloid over erythroid commitment and increases myeloid progenitor self-renewal and functional long-term hematopoietic stem cells. PLoS ONE. 2015;10:e0130441. doi: 10.1371/journal.pone.0130441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rodriguez S, Wang L, Wang S, Serezani H, Kapur R, Cardoso AA, Carlesso N. Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. Stem Cell Reports. 2016a;6:940–956. doi: 10.1016/j.stemcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li CF, Zhang L, Wu CY, Han L, Jin G, Rezaeian AH, Han F, Liu C, Xu C, et al. TRAF6 restricts p53 mitochondrial translocation, apoptosis, and tumor suppression. Mol Cell. 2016b;64:803–814. doi: 10.1016/j.molcel.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Rao DS, Boldin MP, Taganov KD, O’Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Xiu Y, Ashton J, Xing L, Morita Y, Jordan CT, Boyce BF. Noncanonical NF-κB signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions. Stem Cells. 2012;30:709–718. doi: 10.1002/stem.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Rao DS, O’Connell RM, Garcia-Flores Y, Baltimore D. MicroRNA-146a acts as a guardian of the quality and longevity of hematopoietic stem cells in mice. eLife. 2013;2:e00537. doi: 10.7554/eLife.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.