Abstract
Background
To treat skin color disorders, such as vitiligo or burns, melanocytes are transplanted for tissue regeneration. However, melanocyte distribution in the human body varies with age and location, making it difficult to select the optimal donor skin to achieve a desired color match. Determining the correlations with the desired skin color measurement based on CIELAB color, epidermal melanocyte numbers, and melanin content of individual melanocytes is critical for clinical application.
Method
Fifteen foreskin samples from Asian young adults were analyzed for skin color, melanocyte ratio (melanocyte proportion in the epidermis), and melanin concentration. Furthermore, an equation was developed based on CIELAB color with melanocyte ratio, melanin concentration, and the product of melanocyte ratio and melanin concentration. The equation was validated by seeding different ratios of keratinocytes and melanocytes in tissue-engineered skin substitutes, and the degree of fitness in expected skin color was confirmed.
Results
Linear regression analysis revealed a significant strong negative correlation (r = − 0.847, R2 = 0.717) between CIELAB L* value and the product of the epidermal melanocyte ratio and cell-based melanin concentration. Furthermore, the results showed that an optimal skin color match was achieved by the formula.
Discussion
We found that L* value was correlated with the value obtained from multiplying the epidermal melanocyte ratio (R) and melanin content (M) and that this correlation was more significant than either L* vs M or L* vs R. This suggests that more accurate prediction of skin color can be achieved by considering both R and M. Therefore, precise skin color match in treating vitiligo or burn patients would be potentially achievable based on extensive collection of skin data from people of Asian descent.
Keywords: Hypopigmentation, Color reader, CIELAB color space, Skin tissue engineering, Pigment cell
Introduction
Evaluating skin color match for treating skin color disorder requires an objective measurement to quantify visual skin color into numerous levels. The principles of color measurement established by the Commission International d’Eclairage (CIE) have been widely applied to skin. Color values have been obtained by using reflectance spectroscopy, expressed in terms of color space L∗ value, hue angle, and chroma values (Weatherall & Coombs, 1992). Differences of trichromatic color vision between individuals are identified in terms of one, two, or all three CIE color-space parameters: L∗ value, a∗ value, and b∗ value (CIELAB). The L∗ value, which correlates to perceived lightness and ranges from absolute black (0) to absolute white (+100), is the most sensitive of the trichromatic values to skin color change. Because the method is quantitative and the principles are internationally recognized, these color-space parameters are proposed for the unambiguous communication of skin color information that relates directly to visual observations of clinical importance or scientific interest (Stevenson et al., 2012).
Medical therapy for hypopigmentation disorders has improved in recent years; however, complete repigmentation and perfect skin color match seem to be unsatisfactory in most patients treated (Iannella et al., 2016). Therefore, a variety of surgical grafting techniques have been performed to treat skin color disorders that do not respond to medical treatment, such as split thickness grafts, cultured autologous melanocytes, minigrafts, suction blister grafts, and non-cultured epidermal suspension, among others (Gauthier & Benzekri, 2012). These techniques contain different advantages and disadvantages with respect to cost, time consumption, treatment area and location, need for equipment, and possible outcome of abnormal appearance (Gauthier & Benzekri, 2012). A previous study reported that cultured skin substitutes (CSS) fabricated from autologous keratinocytes and fibroblasts seeded onto collagen-glycosaminoglycan substrates could be applied to excised, full-thickness burns on five patients. Spontaneous repigmentation of CSS treatment from passenger melanocytes in keratinocyte culture was found within 2 months after grafting (Harriger et al., 1995). Kahn & Cohen (1995) used very thin epithelial sheet grafts harvested by dermatome from pigmented donor areas and then covered it with petrolatum gauze on five patients with stable vitiligo, which all resulted in excellent repigmentation and no scarring developed. Gupta, Shroff & Gupta (1999) collected the donor epidermal sheets from the blisters developed through the cutaneous suction apparatus, and then grafted onto the denuded skin lesion site. Alternatively, Falabella (2001) implanted very small dermo-epidermal grafts on recipient sites prepared with minipunches of similar size. Mulekar (2003) and Van Geel et al. (2001) both grafted non-cultured melanocyte-keratinocyte suspensions onto previously dermabraded vitiligo lesions and achieved a high repigmentation. Transplantation of pure primary melanocyte cultures has also been proposed with a better response, relatively homogeneous skin color, and capability for use on larger lesion areas (Chen et al., 2004; Kaufmann et al., 1998; Lontz et al., 1994; Olsson & Juhlin, 2002).
Currently, the outcomes of repigmentation through pigment skin tissue engineering or pigment cell transfer are still unpredictable. Previous studies indicated that regulating cutaneous pigmentation in cultured skin substitutes was feasible by titration of human melanocytes and keratinocytes (Swope, Supp & Boyce, 2002; Swope et al., 1997). However, epithelial melanocytes used in Swope’s experiments came from a single donation; thus, the influence of different sources on skin pigmentation could not be determined. The aim of this work was to identify how melanocyte numbers and activity from different Asian people modulate skin color via a well-defined correlation between skin color and melanocytes. A total of 15 human adult foreskin samples donated from Asian individuals were analyzed and the correlations among skin color determined by CIELAB color-space parameter: L∗ value, epidermal melanocyte ratio, and melanin content per 106 melanocytes was investigated. Finally, we evaluated the feasibility of applying this skin color relationship in skin tissue engineering.
Materials and Methods
Materials
Gibco Company (New York, NY, USA) supplied Trypsin-EDTA solution (10 ×) and Penicillin-Streptomycin (10,000 units/ml penicillin G sodium, 10,000 µg/ml streptomycin sulfate in 0.85% saline). Melanocyte culture medium contains Medium 254 (Cascade Biologics Inc., Portland, OR, Portland), 1% Human Melanocyte Growth Supplement (HMGS, Cascade Biologics Inc., Portland, OR, Portland), and 1% Penicillin-Streptomycin. The EpiLife® keratinocyte medium (contain 0.06 mM calcium chloride) was obtained from Cascade Biologics Inc. (Portland, OR, Portland). Poly (ε-caprolactone) (PCL, CAPA 6500, MW 50,000 g/mol) was purchased from Solvay (Warrington, UK). Type I collagen from calfskin was purchased from Sigma (St. Louis, MO, USA). Dichloromethane (DCM) was purchased from J. T. Baker (Phillipsburg, NJ, USA).
Inclusion criteria for skin samples collection
A total of 15 Asian young adult foreskin samples were collected during circumcision surgery in Tri-Service General Hospital, R.O.C. Mean age of patients was 24.47 ± 1.03 years old, ranging from 21 to 38 years. The study protocol was reviewed and approved by the Institutional Review Board (IRB) in the Tri-Service General Hospital, R.O.C. (TSGHIRB No.: 095-05-0068). Written informed consent was obtained from each donor.
Primary culture of human epidermal keratinocytes and melanocytes
Primary human epidermal keratinocytes (PHEKs) and melanocytes (PHEMs) were isolated from equal size (1 cm × 1 cm in square) of human young adult foreskin samples obtained in the surgery of circumcision. For culture of PHEKs, the foreskin sample was initially immersed in 10 ml of 0.2% Dispase II solution (Sigma, St. Louis, MO, USA) at 4 °C for 48 h and diced in pieces, followed by incubation in 0.05% Trypsin-EDTA solution for 15 min. The pelleted cells were obtained by a centrifugation at 1,300 rpm for 5 min, seeded in a fibronectin/collagen (AthenaES, Baltimore, MD, USA) coated flask and cultured in EpiLife® keratinocyte medium at 37 °C in 5% CO2. For primary culture of PHEMs, the epidermal cell cultured in the EpiLife® medium were transferred to melanocyte culture medium after the epidermal primary culture and incubated at 37 °C in 5% CO2. Highly selected culture of PHEMs was then obtained after passage 2.
Skin color measurement
Foreskin samples were obtained immediately after surgery, blood and adipose tissues were removed, and color was measured in triplicate for each sample ex vivo using a color reader CR-10 (Konica Minolta, Osaka, Japan). The color differences were displayed in terms of trichromatic L∗, a∗, and b∗ values as determined by the CIE. Since the L∗ value correlates to perceived color brightness (black vs. white) and is the most sensitive trichromatic value for measuring skin pigmentation in the pilot study, it was chosen rather than a∗ and b∗ values to represent measured skin color in this study. The L∗ value was measured in triplicate by detecting the top surface of the collected foreskin sample and recorded as mean ± SE (standard error).
Analysis of melanocyte ratio in epidermal cells
Fluorescent-activated cell sorting (FACS) was used to distinguish melanocytes from epidermal cells. Epidermal cell suspensions of foreskin were immediately prepared after surgery, which were then centrifuged at 300 × g for 5 min and the cell pellets were treated with a Cytofix/CytoPerm Plus kit (BD, Franklin Lakes, NJ, USA) for subsequent flow cytometry. Briefly, melanocytes were isolated from epidermal cells by adherence. Next, the cells were permeabilized with 200 µl Cytofix/CytoPerm solution for 20 min at 4 °C and washed with 1 ml Perm/Wash Buffer (BD, Franklin Lakes, NJ, USA) twice. For labeling melanocytes, permeabilized cells were stained with mouse anti-human melan-A IgG (Santa Cruz Biotechnology, CA, USA) and incubated with fluorescein isothiocyanate-conjugated (FITC) goat anti-mouse IgG (Jackson, PA, USA). Negative controls for melan-A staining consisted of cells stained with FITC goat anti-mouse IgG only. The samples were then analyzed with five replicates being used for each sample, the samples were analyzed on a FACS Calibur flow cytometer using CellQuest software (BD, Franklin Lakes, NJ, USA); the mean value was then obtained.
Determination of melanin concentration
The melanin production of melanocytes from distinct foreskin samples in term of total melanin content per 106 melanocytes was measured by a spectrophotometric assay. Briefly, Purified primary human epidermal melanocytes (PHEMs) (106 cells per pellet) were lysed with 1 M NaOH at 80 °C for 2 h. After centrifugation at 12, 000 × g for 10 min at room temperature, the supernatants were transferred to fresh tubes and melanin content was determined in triplicate for each sample by measuring the absorbance at 490 nm in a spectrophotometer and expressed as microgram of melanin per 106 cells. Synthetic melanin (Sigma, St. Louis, MO, USA) was used to plot a standard curve.
Preparation of collagen/PCL membranous scaffolds
Collagen/polycaprolactone (PCL) scaffolds were prepared as described previously (Dai et al., 2004). Briefly, type I collagen was dissolved in 1% acetic acid, generating a 0.25% w/v collagen solution. The collagen solution was poured into a round glass vial (diameter 2.5 cm and height 4.5 cm) and frozen at −20 °C for 50 min, followed by lyophilizing in a freeze-dryer (DRC-1100, Eyela, Japan) for 24 h. The PCL/dichloromethane (DCM) solution (2.5% w/v) was then added to the freeze-dried collagen matrix to prepare a 1:20 w/w collagen/PCL scaffold. The glass vial was kept open overnight to allow DCM evaporation.
Color measurement of pigmented tissue-engineered skin substitutes based on collagen/PCL scaffold
Pigmented tissue engineered skin substitutes (diameter 2.5 cm) with primary human epidermal keratinocytes (PHEKs) and/or PHEMs (total cell density of 4.72 ×104 cells/cm2) in various ratios were prepared based on the L∗ value of calculated skin color relationship including L40 (melanocyte ratio was 1.79), and L50 (melanocyte ratio was 4.83) (L∗ values as 40 and 50 respectively) groups. Epilife serum-free medium was used to incubate the skin substitute at 37 °C in 5% CO2. The 6-well tissue culture plastics without cells served as the blank group. The color of pigmented tissue-engineered skin substitutes was measured with a color reader at various time points (10, 20, 30, 40, 50, 60, and 70 days).
The analysis of correlation for skin color and melanocytes
To estimate the relationships of epidermal melanocyte ratio or melanin concentration vs skin lightness based on foreskin CIELAB L∗ value, linear regression analysis was performed. The coefficient of determination (R2) and Pearson’s correlation coefficient (r) were calculated to measure goodness of fit of a statistical model and the strength and direction of the linear relationship.
Statistical analysis
The continuous data such as L∗ value of foreskin samples and tissue-engineered skin substitutes, melanin amount, and the melanocyte ratio in epidermal cells were shown as “mean ± SD (SD: standard deviation)”. The variables were grouped first, comparing mean values in categories, Pearson’s correlation coefficient (r) and coefficient of determination consecutively. Simple linear regression analysis was used afterwards to evaluate the combined effect of several variables and to estimate the coefficient of determination. Statistical Product and Service Solutions (SPSS) was used to perform a stepwise forward selection procedure. Thus, for each iterative loop of this procedure one more variable was integrated into the new formula. All statistical result is statistically significant when the P value is less than 0.05 (P < 0.05). Statistical analysis was performed using Statistical Package for the Social Sciences, Version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Measurement of skin color (L∗ value)
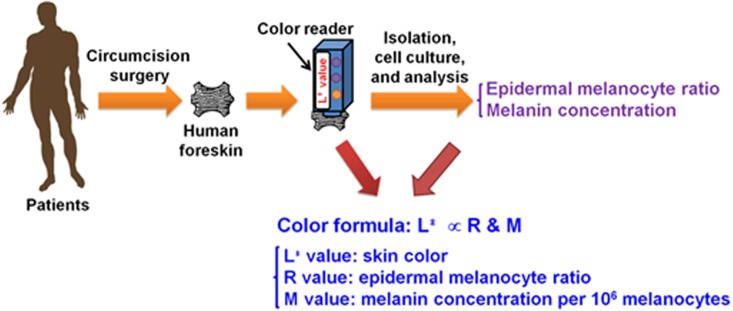
The study design of measuring skin color of foreskin samples, melanin concentration, and epidermal melanocyte ratios for establishing a relationship with skin color is shown in Fig. 1. The skin color of 15 human young adult foreskin samples was measured immediately after surgery by a color reader ex vivo yielding values from 39.43 ± 0.21 to 52.37 ± 1.90 as determined by CIELAB color-space parameter: L∗ value (Table 1).
Figure 1. The illustration of the process for measurement of skin color and formulation of the relation among L∗ value, epidermal melanocyte ratio and melanin concentration.
Table 1. Patient profile of foreskin and demographic data (n = 3 for L∗ value and melanin concentration; n = 5 for epidermal melanocyte ratio; values are mean ± SD)a.
| Samples (No.) | Age (years) | L∗ value | Epidermal melanocyte ratio (%) | Melanin concentration (µg/106 melanocytes) |
|---|---|---|---|---|
| 1 | 23 | 39.43 ± 0.21 | 1.96 ± 0.09 | 68.96 ± 0.20 |
| 2 | 25 | 47.27 ± 1.90 | 3.18 ± 0.24 | 34.61 ± 0.39 |
| 3 | 24 | 41.20 ± 2.17 | 3.13 ± 0.10 | 41.50 ± 0.34 |
| 4 | 24 | 46.97 ± 1.52 | 1.40 ± 0.28 | 81.91 ± 1.92 |
| 5 | 24 | 52.13 ± 1.62 | 1.97 ± 0.31 | 25.88 ± 0.11 |
| 6 | 22 | 52.37 ± 1.90 | 1.44 ± 0.09 | 21.97 ± 0.62 |
| 7 | 22 | 45.93 ± 0.59 | 2.70 ± 0.10 | 47.43 ± 0.72 |
| 8 | 26 | 49.30 ± 0.60 | 3.49 ± 0.14 | 24.31 ± 0.58 |
| 9 | 24 | 44.07 ± 0.15 | 2.44 ± 0.10 | 43.32 ± 2.66 |
| 10 | 22 | 47.53 ± 2.37 | 2.71 ± 0.52 | 26.39 ± 0.68 |
| 11 | 21 | 48.70 ± 2.81 | 3.40 ± 0.22 | 26.97 ± 0.27 |
| 12 | 38 | 49.73 ± 1.15 | 3.58 ± 0.07 | 23.39 ± 0.32 |
| 13 | 23 | 47.87 ± 3.26 | 3.23 ± 0.22 | 17.99 ± 0.50 |
| 14 | 25 | 45.87 ± 2.18 | 1.68 ± 0.12 | 67.66 ± 0.46 |
| 15 | 24 | 50.60 ± 1.40 | 1.41 ± 0.14 | 36.07 ± 0.20 |
Notes.
n, the number of tests performed on each individual sample.
Melanocyte ratio in epidermal cells (R)
The concentration of melanocytes in foreskin epidermal cell suspensions was measured using FACS. The results showed ratios of melanocytes to epidermal cells ranging from 1.40 ± 0.28 to 3.58 ± 0.07% (Table 1).
Epidermal melanin production (M)
To determine the melanin productivity of melanocyte isolated from each foreskin, PHEMs were cultured for two passages reaching a purity of 99.4% and analyzed using immunohistochemistry assays and FACS (Fig. 2). The results showed that the melanin production of 15 PHEMs ranged from 17.99 ± 0.50 to 81.91 ± 1.92μg/106 cells (Table 1).
Figure 2. Determination of epidermal melanocyte ratio (100×; scale bar: 100 µm).
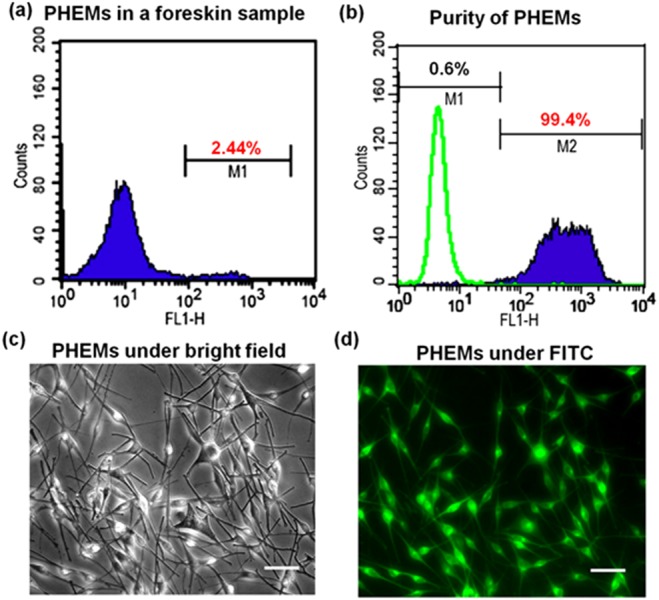
(A) The foreskin samples were treated immediately with the FACS method. The results showed up to 2.44% of melanocytes (M1) in a human adult foreskin sample. (B) The results of fluorescent-activated cell sorting (FACS) showed up to 99.4% purity of melanocytes (M2) in a selected PHEMs culture. (C) Selected primary culture of human epidermal melanocytes (PHEMs) was shown in bright field. (D) The PHEMs in selected primary culture were labeled by an anti-melan antibody incorporated with fluorescein isothiocyanate (FITC).
The analysis of correlation for skin color
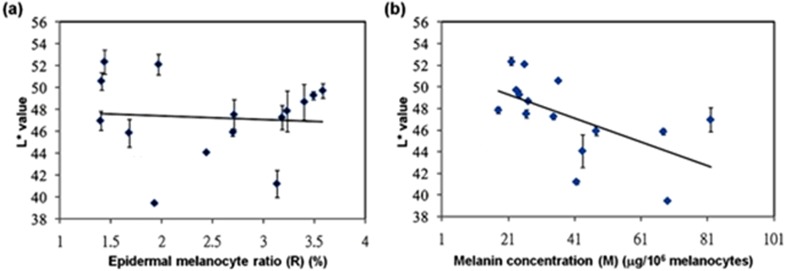
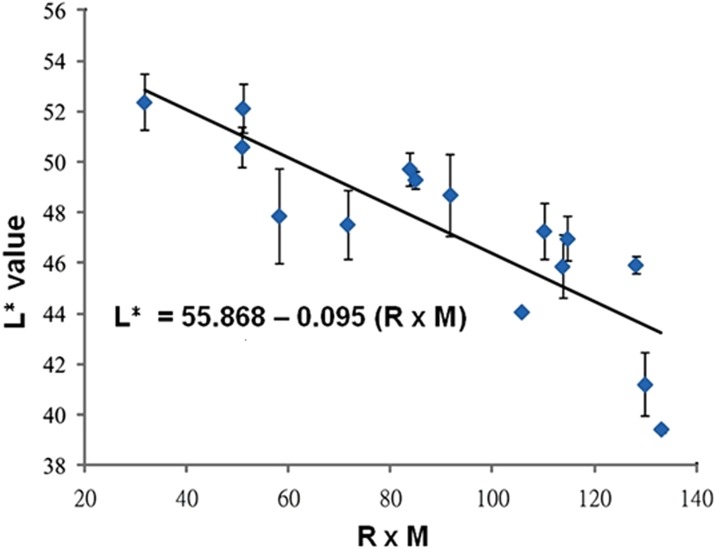
We next examined the correlation between skin color and melanin concentration. Plots of L∗ value against epidermal melanocyte ratio and L∗ value against melanin concentration per 106 melanocytes were generated based on the data shown in Table 1 (Fig. 3). The results revealed no correlation between L∗ value and epidermal melanocyte ratio (r = − 0.081, R2 = 0.0066). However, L∗ value was negatively correlated with melanin concentration (r = − 0.592, R2 = 0.3503) (P = 0.02) (Fig. 3). However, there were significant associations between the L∗ value with the production of epidermal melanocyte ratio and melanin content. A further linear regression analysis showed a significantly strong negative correlation (r = − 0.847, R2 = 0.717) between L∗ value and the epidermal melanocyte ratio multiplied by cell-based melanin concentration (Fig. 4). Given these results, an equation describing skin color relationship was generated: as L∗ = a × (M × R) + b (M: melanin concentration per 106 melanocytes; R: epidermal melanocyte ratio; a = − 0.095; and b = 55.872).
Figure 3. Statistical correlations between skin color-space parameter: L∗ value and epidermal melanocyte ratio or melanin concentration.
(A) L∗ value corresponding to epidermal melanocyte ratio expresses a random distribution where the relation between them seem to be fairly weak. Correlation coefficient, r = − 0.081 (P = 0.774) and coefficient of determination, R2 = 0.0066. (B) A slight trend of a higher L∗ value corresponding to a higher melanin concentration per 106 melanocytes was observed. Correlation coefficient, r = − 0.592 (P = 0.02) and coefficient of determination, R2 = 0.3503.
Figure 4. Statistical correlations between skin color-space parameter: L∗ value and the product of epidermal melanocyte ratio and melanin concentration.
A strong correlation was shown between L∗ value and the value of epidermal melanocyte ratio (R) (%) multiplied by melanin concentration (M) (µg/106 melanocytes). Correlation coefficient, r = − 0.847 (P < 0.001) and coefficient of determination, R2 = 0.717.
Application of the skin color relationship in skin tissue engineering
As shown in Fig. 5A, collagen/PCL constructs at 70 days were ranked in order of different shades of black as PHEMs > L40 > L50 > PHEKs > Blank. The result of the L∗ value profile of the collagen/PCL constructs is shown in Fig. 5B. Obvious changes of the L∗ values were noted in 20 days post-culture for all groups except the blank control group. After 70 days, the L∗ value of L40, and L50 groups were 43.50 ± 1.87, and 50.67 ± 0.55, respectively, whereas PHEMs and PHEKs exhibited values of 30.30 ± 0.56 and 79.73 ± 0.60, respectively.
Figure 5. (A) The gross appearance of the collagen/PCL constructs at 70 days; (B) the L∗ value profiles of the collagen/PCL constructs during a period of 70 days.
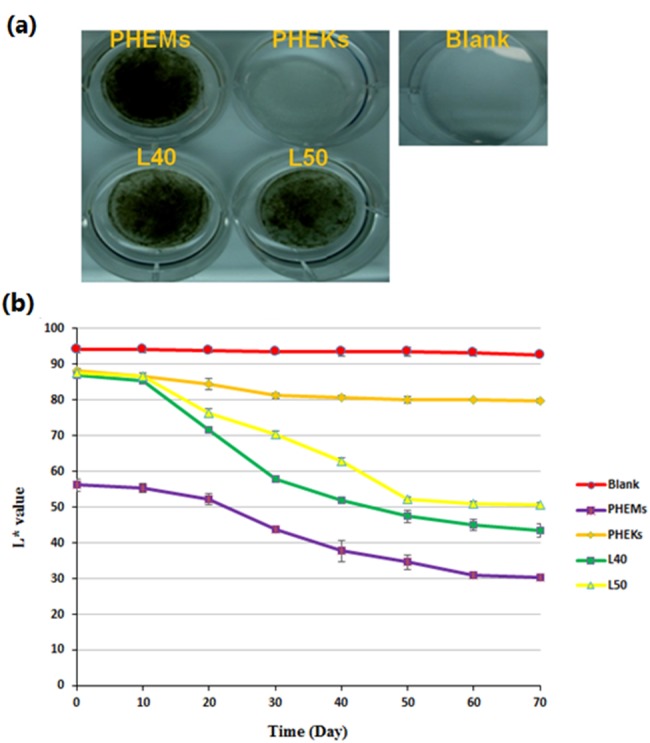
The 6-well tissue culture plastics without cells served as the blank group.
Discussion
To date, there is no method to accurately estimate transplanted skin pigments via a skin color formula used to fabricate tissue-engineered skin. Numerous previous studies have shown that skin constitutive pigmentation is determined by melanin production levels. For example, Alaluf et al. (2002b) performed correlation analysis and found the best correlation between the L∗ value and total melanin content in the epidermis. Del Bino et al. (2015) reported that total melanin content, including eumelanin and pheomelanin content, determines the constitutive skin pigmentation. Wakamatsu et al. (2006) indicated that cell-based melanin production and the predominant biological forms of melanin produced by melanocytes affect skin pigmentation. Additionally, implanting different numbers of melanocytes influences skin color. Swope, Supp & Boyce (2002) implanted 1.1 × 102, 1.1 × 103, and 1.1 × 104 human melanocytes/cm2 into athymic mice and found that mice with the highest density of melanocytes were significantly darker than mice in the other groups. Duval et al. (2014) confirmed that dermal fibroblasts influence the degree of skin pigmentation by measuring quantitative parameters related to skin color, melanin content, and melanocyte numbers in an in vitro skin system. Brankov, Prodanovic & Hurley (2016) found that pigmented basal cell carcinomas (BCCs) have a higher mean melanocyte count compared to non-pigmented BCCs, indicating that the pigment is increased not only because of increased melanin, but also because of increased melanocyte counts. The density of melanocytes varies with the body site, with approximately 900 melanocytes per square mm on the back and approximately 1,500 melanocytes per square mm in the genital region (Thingnes et al., 2012). Therefore, for the practical treatment of patients with skin color disorder, the implanted skin should be evaluated for both melanin production and melanocyte count from the donor site for precise color matching. We predict that regulating skin color can be determined by native cell-based melanin production (M) and melanocyte numbers in the epidermis (R). We found that the L∗ value was correlated with the value of the multiplicative product of the epidermal melanocyte ratio (R) and melanin content (M), and these values were much higher than those of the two aforementioned parameters individually, suggesting that considering the epidermal melanocyte ratio and melanin content strengthens the prediction of skin color. Moreover, we performed statistical analysis and found a negative correlation between M and R (r = − 0.5359, P = 0.04). On normalizing M to R (M∕R) vs L∗, a low correlation (r = 0.3340, P = 0.22) was observed. This result also agreed with the result that the product of M and R is close to a fixed value.
Although skin pigmentation is known to be regulated by melanocytes, the factors affecting and regulating ethnic skin color are further to be determined (Hoath & Leahy, 2003; Snell & Bischitz, 1963). Based on our previous research, skin color is essentially affected by the ethnicity of the individual, while melanocyte density and differentiation are also influenced by the environmental factors, such as ultraviolet radiation (UVR) and factors secreted by neighboring keratinocytes and fibroblasts (Dai et al., 2018). The melanocortin 1 receptor (MC1R), a G protein-coupled receptor that regulates the quantity and quality of melanin production, is the major determinant of the pigment phenotype of the skin. Three agonists, such as alpha-melanocyte stimulating hormone, adrenocorticotrophic hormone, and proopiomelanocortin, can activate MC1R via the cyclase/cAMP/protein kinase A signaling pathway. Next, the cAMP response element binding protein is phosphorylated, resulting in the transcriptional induction of microphthalmia-associated transcription factor (MITF). MITF is involved in regulating the expression of melanogenic proteins, such as tyrosinase (TYR), tyrosinase-related protein 1, and tyrosinase-related protein 2 to regulate skin color (Gillbro & Olsson, 2011). MITF activity can also be regulated by different transcription factors or mediators secreted by keratinocytes and fibroblasts, such as basic fibroblast growth factor, stem cell factor, endothelin-1, prostaglandins, and leukotrienes. Based on a genome-wide association study, polymorphisms in three genes, including SLC24A5, TYR, and SLC45A2, showed highly significant associations with melanin content in the skin (Stokowski et al., 2007; Wilson et al., 2013). The SLC24A5 gene encoding the NCKX5 protein, as a potassium-dependent sodium-calcium exchanger, exhibits lower exchange activity, resulting in reduced melanogenesis and lighter skin in individuals (Wilson et al., 2013). These regulatory genes in melanocytes are suggested to differ from race to race or even among individuals and lead to different melanin production capabilities (Han, Choi & Son, 2006). Alaluf et al. (2002a) studied the ethnic variation of melanin content and composition in the human skin from photoprotected and photoexposed areas on human bodies. They found that melanosome size plays a significant role in the variation of different ethnic skin types; African skin had the largest melanosomes followed in turn by Indian, Mexican, Chinese, and European. In addition, the levels of light-colored, alkali-soluble melanin including pheomelanin and DHICA-enriched eumelanin in photoprotected skin areas for European, Chinese, and African skin are estimated as 43.2, 34.4, and 15.1%, respectively (Alaluf et al., 2002a).
In our study, L∗ value was correlated with melanin content but not epidermal melanocyte ratio. A similar density of pigment-producing melanocytes in the skin (∼1,000/mm2) has been found in skin types from different races (Staricco & Pinkus, 1957). However, the skin color of people from the same group may vary because of different living habits, such as the time spent outdoors, diet, or the use of sunscreen, among other factors. Taiwanese people comprise a multi-racial population, including aborigines and immigrants from the mainland. This area was colonized by the Dutch and Japanese in the past. Therefore, the skin color may reflect different racial characteristics showing high variation in skin samples in this study. In fact, there are few more crucial factors affecting final skin color, including the amount of melanin, melanin composition, and melanosome size across skin from a range of ethnicities. In addition, the higher level of melanin production in darker skin was due to the continuously higher level of tyrosinase activity in melanocytes. Taken together, the amount of melanin in the epidermis plays an important role in skin color of individuals from different races, which agrees with our result that melanin content was significantly correlated with L∗ value of CIELAB color space.
Given that the native melanin-producing capability of a melanocyte depends on genetic factors (Fitzpatrick, Miyamoto & Ishikawa, 1967; Szabo et al., 1969; Wakamatsu et al., 2006), in clinical applications for autogenous pigment cell therapy, the cell-based melanin content is constant in the same individual, and the predicted skin color matches represented as L∗ value could be detected from the normal skin area adjacent to the hypopigmented lesion site. Thus, a meticulously estimated epidermal melanocyte ratio for the purpose of autogenous pigment cell therapy applications would be attained based on the color formula, which presents as L∗ = 0.095 × (M × R) + 55.872 (M: melanin concentration per 106 melanocytes; R: epidermal melanocyte ratio). Moreover, when culturing various melanocyte ratios in the collagen/PCL scaffolds, the results from in vitro experiments confirmed that the use of this skin color formula is feasible. The L∗ value of L40 and L50 groups was 43.50 ± 1.87 and 50.67 ± 0.55 respectively, very near the predicted value. Therefore, predicting skin color is possible via collecting skin data for establishing the skin color formula. In the clinic, the product of melanin production and melanocyte distribution (M × R) is indicative of the darkness of skin color. The density of melanocytes differs in different parts of the body. For skin autograft, a plastic surgeon often obtains skin grafts from different body sites for implantation at the recipient site. Based on the skin color formula derived in our study, we can more precisely predict skin color by measuring melanin production by the donor skin and adjusting the ratio of melanocytes and keratinocytes to produce a specific color in transplanted skin to treat patients with skin color disorders, such as vitiligo or burn. The cell sheet technique or plasma gel can be used, adjusting the ratio of melanocytes and keratinocytes to prepare transplanted skins suitable for different skin color lesions.
The skin color formula still has some limitations that should be addressed before its clinical application. Swope et al. found that cutaneous pigments would be present as a function of melanocyte density and time after grafting due to the depletion of human melanocytes in CSS in an animal model (Swope et al., 1997; Swope, Supp & Boyce, 2002). Furthermore, skin color is suggested to be determined by several other factors including the total quantity of melanin, the proportion between the brown–black eumelanin and the yellow–red pheomelanin, and its distribution involved in the epidermis (Naysmith et al., 2004). The loge values of eumelanin/pheomelanin ratio were inversely related to the color variables b∗ (yellow–blue) (R2 = 0.51, P < 0.001), a∗(red–green)(R2 = 0.47, P < 0.001), and to a lesser extent L∗ values (R2 = 0.22, P < 0.001) (Naysmith et al., 2004). On the other hand, three of cell types including melanocytes, keratinocytes, and fibroblasts actively participate in regulating skin pigmentation through secreted factors and their receptors, and their interactions may determine skin pigmentation (Yin et al., 2014). The other chromophores present in the skin include oxyhemoglobin, reduced hemoglobin, and carotene, which may influence the true skin color. Skin blood flow increases with increases in hemoglobin (Tsuchida, Fukuda & Kamata, 1991), and a∗ values correlate linearly well with hemoglobin levels. The a∗ value indicates the redness of the skin color and is mainly influenced by the degree of vascularization and the stretching of the skin over surrounding tissues. Therefore, all three CIE color-space parameters: L∗ value, a∗ value, and b∗ value should be discussed in the future for perfect match of the skin color used in clinical application.
Conclusions
A formula for estimating skin color based on CIELAB L∗ value, epidermal melanocyte ratio, and melanin concentration was generated from people of Asian descent. This skin color formula may serve as a useful methodology for determining distinct pigment cell concentrations for cell therapy or for developing a pigmented skin tissue engineering model for transplantation and pharmaceutical screening applications in vitro.
Supplemental Information
Funding Statement
This study was supported in part by Ministry of Science and Technology, R.O.C. (NSC 96-2314-B-016-037-MY2), Ministry of Economic Affairs, R.O.C. and Institute for Information Industry and Department of Industrial Technology, R.O.C. (98-EC-17-A-19-S2-0090), Ministry of National Defense, R.O.C. (MAB-105-043, MAB-106-034), National Defense Medical Center, Tri-Service General Hospital, R.O.C. (TSGH-C106-112), and Zuoying Branch of Kaohsiung Armed Forces General Hospital, R.O.C. (ZBH 105-07, ZBH 106-11). It was also supported by C.Y. Foundation for Advancement of Education, Sciences and Medicine, R.O.C. and Cheng Han Educational Foundation, R.O.C. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wen-Shyan Huang conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Yi-Wen Wang performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Kun-Che Hung, Pai-Shan Hsieh and Keng-Yen Fu analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Lien-Guo Dai analyzed the data, prepared figures and/or tables, approved the final draft.
Nien-Hsien Liou, Kuo-Hsing Ma and Jiang-Chuan Liu authored or reviewed drafts of the paper, approved the final draft.
Niann-Tzyy Dai conceived and designed the experiments, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The study protocol was reviewed and approved by the Institutional Review Board in the Tri-Service General Hospital, R.O.C. 095-05-0068.
Data Availability
The following information was supplied regarding data availability:
Dai, Niann-Tzyy; Huang, Wen-Shyan; Wang, Yi-Wen; Hung, Kun-Che; Fu, Keng-Yen; Dai, Lien-Guo; Liou, Nien-Hsien; Liu, Jiang-Chuan (2018): Analysis of skin pigmentation. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5797755.v1.
Dai, Niann-Tzyy; Huang, Wen-Shyan; Wang, Yi-Wen; Hung, Kun-Che; Hsieh, Pai-Shan; Fu, Keng-Yen; Dai, Lien-Guo; Liou, Nien-Hsien; Ma, Kuo-Hsing; Liu, Jiang-Chuan (2018): Measurement of L value in tissue engineering skin. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5797749.v2.
References
- Alaluf et al. (2002a).Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Research. 2002a;15:112–118. doi: 10.1034/j.1600-0749.2002.1o071.x. [DOI] [PubMed] [Google Scholar]
- Alaluf et al. (2002b).Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Research. 2002b;15:119–126. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- Brankov, Prodanovic & Hurley (2016).Brankov N, Prodanovic EM, Hurley MY. Pigmented basal cell carcinoma: increased melanin or increased melanocytes? Journal of Cutaneous Pathology. 2016;43:1139–1142. doi: 10.1111/cup.12819. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2004).Chen YF, Yang PY, Hu DN, Kuo FS, Hung CS, Hung CM. Treatment of vitiligo by transplantation of cultured pure melanocyte suspension: analysis of 120 cases. Journal of the American Academy of Dermatology. 2004;51:68–74. doi: 10.1016/j.jaad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Dai et al. (2018).Dai NT, Chang HI, Wang YW, Fu KY, Huang TC, Huang NC, Li JK, Hsieh PS, Dai LG, Hsu CK, Maitz PK. Restoration of skin pigmentation after deep partial or full-thickness burn injury. Advanced Drug Delivery Reviews. 2018;123:155–164. doi: 10.1016/j.addr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Dai et al. (2004).Dai NT, Williamson MR, Khammo N, Adams EF, Coombes AG. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials. 2004;25:4263–4271. doi: 10.1016/j.biomaterials.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Del Bino et al. (2015).Del Bino S, Ito S, Sok J, Nakanishi Y, Bastien P, Wakamatsu K, Bernerd F. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell & Melanoma Research. 2015;28:707–717. doi: 10.1111/pcmr.12410.. [DOI] [PubMed] [Google Scholar]
- Duval et al. (2014).Duval C, Cohen C, Chagnoleau C, Flouret V, Bourreau E, Bernerd F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: impact of photo-aging. PLOS ONE. 2014;9:e114182. doi: 10.1371/journal.pone.0114182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falabella (2001).Falabella R. Surgical therapies for vitiligo and other leukodermas, part 1: minigrafting and suction epidermal grafting. Dermatologic Therapy. 2001;14:7–14. doi: 10.1046/j.1529-8019.2001.014001007.x. [DOI] [Google Scholar]
- Fitzpatrick, Miyamoto & Ishikawa (1967).Fitzpatrick TB, Miyamoto M, Ishikawa K. The evolution of concepts of melanin biology. Archives of Dermatology. 1967;96:305–323. doi: 10.1001/archderm.1967.01610030083015. [DOI] [PubMed] [Google Scholar]
- Gauthier & Benzekri (2012).Gauthier Y, Benzekri L. Non-cultured epidermal suspension in vitiligo: from laboratory to clinic. Indian Journal of Dermatology, Venereology and Leprology. 2012;78:59–63. doi: 10.4103/0378-6323.90947. [DOI] [PubMed] [Google Scholar]
- Gillbro & Olsson (2011).Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. International Journal of Cosmetic Science. 2011;33:210–221. doi: 10.1111/j.1468-2494.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- Gupta, Shroff & Gupta (1999).Gupta S, Shroff S, Gupta S. Modified technique of suction blistering for epidermal grafting in vitiligo. International Journal of Dermatology. 1999;38:306–309. doi: 10.1046/j.1365-4362.1999.00702.x. [DOI] [PubMed] [Google Scholar]
- Han, Choi & Son (2006).Han K, Choi T, Son D. Skin color of Koreans: statistical evaluation of affecting factors. Skin Research and Technology. 2006;12:170–177. doi: 10.1111/j.0909-752X.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- Harriger et al. (1995).Harriger MD, Warden GD, Greenhalgh DG, Kagan RJ, Boyce ST. Pigmentation and microanatomy of skin regenerated from composite grafts of cultured cells and biopolymers applied to full-thickness burn wounds. Transplantation. 1995;59:702–707. doi: 10.1097/00007890-199503150-00011. [DOI] [PubMed] [Google Scholar]
- Hoath & Leahy (2003).Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. Journal of Investigative Dermatology. 2003;121:1440–1446. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- Iannella et al. (2016).Iannella G, Greco A, Didona D, Didona B, Granata G, Manno A, Pasquariello B, Magliulo G. Vitiligo: pathogenesis, clinical variants and treatment approaches. Autoimmunity Reviews. 2016;15:335–343. doi: 10.1016/j.autrev.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Kahn & Cohen (1995).Kahn AM, Cohen MJ. Vitiligo: treatment by dermabrasion and epithelial sheet grafting. Journal of the American Academy of Dermatology. 1995;33:646–648. doi: 10.1016/0190-9622(95)91287-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann et al. (1998).Kaufmann R, Greiner D, Kippenberger S, Bernd A. Grafting of in vitro cultured melanocytes onto laser-ablated lesions in vitiligo. Acta Dermato-Venereologica. 1998;78:136–138. doi: 10.1080/000155598433485. [DOI] [PubMed] [Google Scholar]
- Lontz et al. (1994).Lontz W, Olsson MJ, Moellmann G, Lerner AB. Pigment cell transplantation for treatment of vitiligo: a progress report. Journal of the American Academy of Dermatology. 1994;30:591–597. doi: 10.1016/S0190-9622(94)70067-2. [DOI] [PubMed] [Google Scholar]
- Mulekar (2003).Mulekar SV. Melanocyte-keratinocyte cell transplantation for stable vitiligo. International Journal of Dermatology. 2003;42:132–136. doi: 10.1046/j.1365-4362.2003.01628.x. [DOI] [PubMed] [Google Scholar]
- Naysmith et al. (2004).Naysmith L, Waterston K, Ha T, Flanagan N, Bisset Y, Ray A, Wakamatsu K, Ito S, Rees JL. Quantitative measures of the effect of the melanocortin 1 receptor on human pigmentary status. Journal of Investigative Dermatology. 2004;122:423–428. doi: 10.1046/j.0022-202X.2004.22221.x. [DOI] [PubMed] [Google Scholar]
- Olsson & Juhlin (2002).Olsson MJ, Juhlin L. Long-term follow-up of leucoderma patients treated with transplants of autologous cultured melanocytes, ultrathin epidermal sheets and basal cell layer suspension. British Journal of Dermatology. 2002;147:893–904. doi: 10.1046/j.1365-2133.2002.04837.x. [DOI] [PubMed] [Google Scholar]
- Snell & Bischitz (1963).Snell RS, Bischitz PG. The melanocytes and melanin in human abdominal wall skin: a survey made at different ages in both sexes and during pregnancy. Journal of Anatomy. 1963;97:361–376. [PMC free article] [PubMed] [Google Scholar]
- Staricco & Pinkus (1957).Staricco RJ, Pinkus H. Quantitative and qualitative data on the pigment cells of adult human epidermis. Journal of Investigative Dermatology. 1957;28:33–45. doi: 10.1038/jid.1957.4. [DOI] [PubMed] [Google Scholar]
- Stevenson et al. (2012).Stevenson JM, Weatherall IL, Litilejohn RP, Seman DL. A comparison of two different instruments for measuring venison CIELAB values and colour assessment by a trained panel. New Zealand Journal of Agricultural Research. 2012;34:207–211. doi: 10.1080/00288233.1991.10423361. [DOI] [Google Scholar]
- Stokowski et al. (2007).Stokowski RP, Pant PV, Dadd T, Fereday A, Hinds DA, Jarman C, Filsell W, Ginger RS, Green MR, Van der Ouderaa FJ, Cox DR. A genomewide association study of skin pigmentation in a South Asian population. American Journal of Human Genetics. 2007;81:1119–1132. doi: 10.1086/522235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope, Supp & Boyce (2002).Swope VB, Supp AP, Boyce ST. Regulation of cutaneous pigmentation by titration of human melanocytes in cultured skin substitutes grafted to athymic mice. Wound Repair and Regeneration. 2002;10:378–386. doi: 10.1046/j.1524-475X.2002.10607.x. [DOI] [PubMed] [Google Scholar]
- Swope et al. (1997).Swope VB, Supp AP, Cornelius JR, Babcock GF, Boyce ST. Regulation of pigmentation in cultured skin substitutes by cytometric sorting of melanocytes and keratinocytes. Journal of Investigative Dermatology. 1997;109:289–295. doi: 10.1111/1523-1747.ep12335766. [DOI] [PubMed] [Google Scholar]
- Szabo et al. (1969).Szabo G, Gerald AB, Pathak MA, Fitzpatrick TB. Racial differences in the fate of melanosomes in human epidermis. Nature. 1969;222:1081–1082. doi: 10.1038/2221081a0. [DOI] [PubMed] [Google Scholar]
- Thingnes et al. (2012).Thingnes J, Lavelle TJ, Hovig E, Omholt SW. Understanding the melanocyte distribution in human epidermis: an agent-based computational model approach. PLOS ONE. 2012;7:e40377. doi: 10.1371/journal.pone.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida, Fukuda & Kamata (1991).Tsuchida Y, Fukuda O, Kamata S. The correlation of skin blood flow with age, total cholesterol, hematocrit, blood pressure, and hemoglobin. Plastic and Reconstructive Surgery. 1991;88:844–850. doi: 10.1097/00006534-199111000-00017. [DOI] [PubMed] [Google Scholar]
- Van Geel et al. (2001).Van Geel N, Ongenae K, De Mil M, Naeyaert JM. Modified technique of autologous noncultured epidermal cell transplantation for repigmenting vitiligo: a pilot study. Dermatologic Surgery. 2001;27:873–876. doi: 10.1046/j.1524-4725.2001.01045.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu et al. (2006).Wakamatsu K, Kavanagh R, Kadekaro AL, Terzieva S, Sturm RA, Leachman S, Abdel-Malek Z, Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Research. 2006;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Weatherall & Coombs (1992).Weatherall IL, Coombs BD. Skin color measurements in terms of CIELAB color space values. Journal of Investigative Dermatology. 1992;99:468–473. doi: 10.1111/1523-1747.ep12616156. [DOI] [PubMed] [Google Scholar]
- Wilson et al. (2013).Wilson S, Ginger RS, Dadd T, Gunn D, Lim FL, Sawicka M, Sandel M, Schnetkamp PP, Green MR. NCKX5, a natural regulator of human skin colour variation, regulates the expression of key pigment genes MC1R and alpha-MSH and alters cholesterol homeostasis in normal human melanocytes. Advances in Experimental Medicine and Biology. 2013;961:95–107. doi: 10.1007/978-1-4614-4756-6_9. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2014).Yin L, Coelho SG, Ebsen D, Smuda C, Mahns A, Miller SA, Beer JZ, Kolbe L, Hearing VJ. Epidermal gene expression and ethnic pigmentation variations among individuals of Asian, European and African ancestry. Experimental Dermatology. 2014;23:731–735. doi: 10.1111/exd.12518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Dai, Niann-Tzyy; Huang, Wen-Shyan; Wang, Yi-Wen; Hung, Kun-Che; Fu, Keng-Yen; Dai, Lien-Guo; Liou, Nien-Hsien; Liu, Jiang-Chuan (2018): Analysis of skin pigmentation. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5797755.v1.
Dai, Niann-Tzyy; Huang, Wen-Shyan; Wang, Yi-Wen; Hung, Kun-Che; Hsieh, Pai-Shan; Fu, Keng-Yen; Dai, Lien-Guo; Liou, Nien-Hsien; Ma, Kuo-Hsing; Liu, Jiang-Chuan (2018): Measurement of L value in tissue engineering skin. figshare. Dataset. https://doi.org/10.6084/m9.figshare.5797749.v2.