Abstract
The centromeric DNA of fission yeast is arranged with a central core flanked by repeated sequences. The centromere-associated proteins, Mis6p and Cnp1p (SpCENP-A), associate exclusively with central core DNA, whereas the Swi6 protein binds the surrounding repeats. Here, electron microscopy and immunofluorescence light microscopy reveal that the central core and flanking regions occupy distinct positions within a heterochromatic domain. An “anchor” structure containing the Ndc80 protein resides between this heterochromatic domain and the spindle pole body. The organization of centromere-associated proteins in fission yeast is reminiscent of the multilayered structures of human kinetochores, indicating that such domain structure is conserved in eukaryotes.
INTRODUCTION
Centromere function requires the proper orchestration of several subfunctions, such as kinetochore assembly, sister chromatid cohesion, binding of kinetochore microtubules, orientation of sister kinetochores to opposite poles, and their movement toward the spindle poles. Centromere structure may be organized so as to accomplish these functions in different, separable domains. Although centromere functions have been scrutinized in several genetically tractable model organisms, such as Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Drosophila melanogaster, detailed structural studies have been limited or lacking in all these organisms. The most comprehensive molecular view of a centromere is available in Saccharomyces cerevisiae. The “point centromeres” of this organism are built on a single nucleosome with what contains the histone H3 variant CSE4 (CENP-A), the CBF3 DNA-binding complex, and 12 additional proteins already identified (Pluta et al., 1995; Pidoux and Allshire, 2000). However, the compact size of these centromeres (125 bp) renders them too small for fine structural analysis. The “regional” centromeres of Drosophila (420 kb) are more typical of the centromeres found in the vast majority of eukaryotes (Murphy and Karpen, 1995). Here, the DNA is quite well characterized (Sun et al., 1997) and the centromeric DNA has been shown to display an epigenetic structure (reviewed by Karpen and Allshire, 1997). Drosophila kinetochores also appear bilaminar by electron microscopy (EM; Goldstein, 1981), but the positions of centromere-binding proteins within these structures have not been determined. In contrast, the centromeric DNA of humans is not well understood, but the fine structure of their kinetochores has been studied extensively, particularly through the binding of autoantibodies from human patients with scleroderma. These immunoglobulins react with several distinct centromere proteins (CENPs; Brenner et al., 1981; Earnshaw and Migeon, 1985; Earnshaw and Rothfield, 1985). As seen by EM, the human metaphase centromere is multilayered and contains several substructures: a fibrous corona, an outer and inner plate, and the space between them. Underlying these plates is the heterochromatic region that underlies the inner plate (reviewed by Pluta et al., 1995). Each of these substructures appears to comprise a distinct protein composition. The fibrous corona contains CENP-E, dynein, and dynactin, the outer CENP-F, the inner plate contains CENP-C and CENP-A, and the underlying heterochromatin contains CENP-B, INCENP, HP1, and Suvar3–9 (Saitoh et al., 1992; Cooke et al., 1997, 1990; Vafa and Sullivan, 1997; Warburton et al., 1997; Yao et al., 1997; Aagaard et al., 1999).
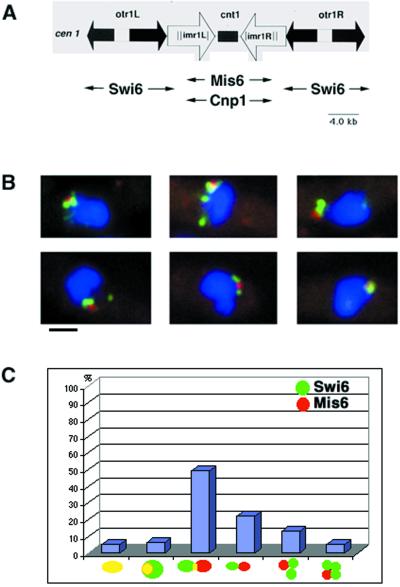
In fission yeast, the centromere DNA has been functionally defined (reviewed by Pidoux and Allshire, 2000). Fission yeast centromeres occupy 40–100 kb on the chromosome and all three have a symmetric organization. A central core sequence (CC/cnt) is flanked by arrays of repeated (inner imr/B and outer otr/K+L) sequences (Clarke et al., 1986; Chikashige et al., 1989; Clarke and Baum, 1990). These chromosomal elements also show clear epigenetic structure (Steiner and Clarke, 1994; Ekwall et al., 1997). There is no similarity between sequences of centromeric DNA in S. pombe, S. cerevisiae, Drosophila and humans, but on the basis of their size and organization, S. pombe centromeres can be classified as “regional” (Pluta et al., 1995). In S. pombe several CENPs have been identified: Swi6, Chp1, Cnp1, Mis6, Mis12, Ndc80, Nuf2, and Spc24 (Ekwall et al., 1995; Saitoh et al., 1997; Doe et al., 1998; Goshima et al., 1999; Takahashi et al., 2000) (Wigge and Kilmartin, 2001). Chromatin immunoprecipitation cross-linking experiments have demonstrated that Cnp1 (S. pombe CENP-A) and Mis6 proteins both bind to the central core region but not the flanking regions. Conversely, the chromodomain proteins Swi6 and Chp1 bind the flanking repeats but not the central core region. This indicates that there are two distinct structural and functional domains in S. pombe centromeres (Partridge et al., 2000) (Goshima et al., 1999; Saitoh et al., 1997; Takahashi et al., 2000; see Figure 1A).
Figure 1.
The central core and flanking regions are cytologically distinct. (A) Schematic representation of fission yeast centromere structure and binding proteins. The schematic structure of cen1 is depicted together with the chromatin cross-linking chromatin immunoprecipitation data indicating where Swi6, Mis6 (Partridge et al., 2000), and Cnp1 proteins (Takashi et al., 2000) bind to the centromere DNA. (B) IF pictures showing the localization of Swi6 (green) and Mis6-HA (red) proteins in interphase nuclei. Swi6 binds to all heterochromatic regions in S. pombe nuclei, i.e., centromeres, telomeres, and mat2/3 regions, but the major signal in interphase cells represents the clustered centromeres (Ekwall et al., 1995). DAPI (blue) was used to stain the DNA. Bar, 2.0 μm. (C) Statistical analysis of the relative positions of Swi6 and Mis6 at centromeres. Yellow color indicates colocalization in the merged picture (n = 100).
In the work reported here we are exploring the organization of centromere-binding proteins in fission yeast during interphase. Studies by immunofluorescence in vertebrates have shown that several such proteins are localized during interphase as if they were still associated with centromeric DNA, e.g., CENP-A, -B, and -C (Pudenko et al., 1997), whereas others are not, e.g., CENP-E (Cooke et al., 1997). The difficulty of studying the organization of centromere-binding proteins during interphase in vertebrates is that neither the position nor the orientation of these structures appears to be controlled at this cell cycle time. In fission yeast, on the other hand, the centromeres are localized to a specific part of the nucleus that lies immediately beneath the nuclear envelope, opposite the spindle pole body (SPB; which is situated in the cytoplasm; Funabiki et al., 1993; Ekwall et al., 1995; Ding et al., 1997). This situation makes it possible to know approximately where all the centromere-binding proteins will be positioned and to measure their relative positions with reference to the SPB. In this study we used light and electron microscopic immunolocalization to find these relative positions and to determine whether the centromere-binding proteins are organized during interphase. Our evidence implies that these proteins are ordered and that their order can be related to the part of the centromere to which they bind. We infer that the portion of the fission yeast chromosome that is essential for normal segregation at mitosis is rigorously positioned during interphase, both before and after SPB replication. Despite the differences in their DNA sequences, the centromeres of fission yeast appear similar in their design to those of humans, suggesting that a multilayered organization may be conserved in many eukaryotes. The functional implications of this observation are discussed.
MATERIALS AND METHODS
S. pombe strains carrying the markers mis6-3xHA-LEU2+ (Saitoh et al., 1997), cut12-Pk-ura4+ (Bridge et al., 1998), and ndc80-GFP-kanMX6 (Wigge and Kilmartin, 2001) were prepared for immunofluorescence microscopy (IF) by the formaldehyde fixation procedure (Hagan and Hyams, 1988) with some modifications. Log-phase cultures were incubated for 5–30 min in YES + 1.2 M sorbitol before harvest. PEMAL (PEM + 5 or 0.03% milk, 0.1 M l-lysine HCl, cleared by centrifugation during 30 min at 20,000 × g) was used instead of PEMBAL. Primary antibodies were mouse anti-hemagglutinin (HA; Boehringer and Mannheim, Indianapolis, IN), mouse anti-Pk (Serotec, Oxford, UK), rabbit anti-green fluorescent protein (GFP; Molecular Probes, Eugene, OR), rabbit anti-Swi6 (Ekwall et al., 1995), and sheep anti-Cnp1 (Mellone and Allshire, unpublished data). Fluorescein isothiocyanate or Texas Red-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA ) or Sigma (St. Louis, MO). Cells were visualized with the use of an Axioskop II microscope (Zeiss, Oberkochen, Germany) equipped with a C4742–95 charge-coupled device camera (Hamamatsu, Middlesex, NJ). A 100× objective lens with NA 1.3 produced images with a calculated resolution of 211 nm.
For EM localization of GFP fusion proteins, samples of S. pombe cells harboring GFP-Swi6 (Pidoux et al., 2000), GFP-Cnp1 (Mellone and Allshire, unpublished data), and Ndc80-GFP (Wigge and Kilmartin, 2001) before or after were prepared by a modification of the methods of (Ding et al., 1997). In brief, S. pombe cells grown in liquid cultures were harvested by centrifugation and frozen in a high-pressure freezer (Balzers, Lichtenstein) with 2300 bar within 0.6–0.7 s. Frozen samples were freeze-substituted into 1% formaldehyde in methanol at −93°C for 10 h, warmed to −61°C for 6 h, warmed to −38°C for 1 h, and embedded in Lowicryl K11M. Serial sectioning was to a section thickness of 30–50 nm.
Immunostaining was carried out after blocking overnight in 0.1 M phosphate buffer, pH 7.4, with 10% bovine serum albumin or 10% donkey serum for 1.5 h and addition of rabbit antibodies to GFP (A11122, Molecular Probes) diluted 1:100 in the same buffer at 4°C. GFP fusion proteins were followed by protein A conjugated to 10-nm colloidal gold (Au10) or donkey anti-rabbit antibodies conjugated to 12-nm colloidal gold (Au12) for 2 h. Cells were postfixed in 2% glutaraldehyde for 15 min and poststained with uranyl acetate for 7 min and lead citrate for 4 min. The average labeling densities on the heterochromatin domains in G2 cells were 162 ± 43 Au10/μm2 for Swi6 and 13 ± 14 Au10/μm2 for Cnp1. The background staining of gold in the nucleus was 13 ± 4/μm2 for Swi6 and <2/μm2 for Cnp1. The nonspecific background staining in the cytoplasm was 3 ± 4 and 1 ± 2 Au10/μm2, respectively. Serial sections were imaged in a Leo906 80-kV electron microscope, the resulting EM pictures were scanned with a snapscan (Agfa, Ridgefield Park, NJ), and three-dimensional (3-D) computer models were generated with the IMOD software package (Kremer et al., 1996).
RESULTS
Central Core and Flanking Domains Are Cytologically Distinct
Immunofluorescence microscopy (IF), with antibodies that recognized Swi6 and Mis6 (represented by an HA-tagged allele; Saitoh et al., 1997), was carried out with unsynchronized, log-phase cultures of S. pombe. Swi6 localizes to all heterochromatic regions in S. pombe nuclei, i.e., centromeres, telomeres, and mat2/3 regions, but the major signal in interphase cells corresponds to the centromeres, which are clustered near the SPB (Ekwall et al., 1995). Double immunolabeling of Mis6-HA (red) and Swi6 (green) indicated that Swi6p and Mis6p colocalized in only a minority (11%) of cells (Figure 1, B and C). In 100 cells analyzed, 49% of the signals were partially overlapping but clearly distinct, 22% of the signals were adjacent but not overlapping, and 18% of cells showed two to three Swi6 spots surrounding a Mis6 spot (Figure 1C). In control experiments double immunolabeling of Mis6-HA (red) and Cnp1 (green) resulted in 100% colocalization (n = 50). Thus, Mis6p and Swi6p, proteins with previously described distinctions in their DNA-binding domains (Partridge et al., 2000), show cytologically distinct localizations. The variability of the localizations we have seen also suggest that the relative positions of these two centromere subdomains are highly dynamic in interphase cells.
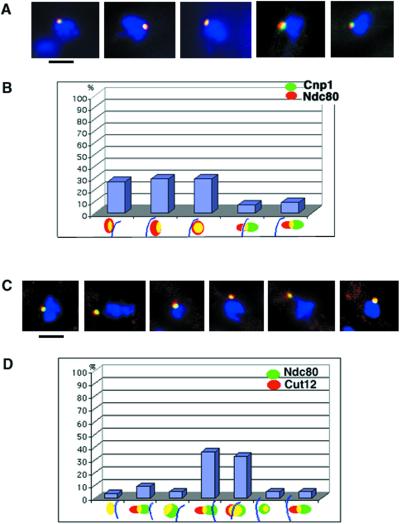
This finding prompted us to investigate the location of these CENPs relative to the newly described CENP, Ndc80 (Wigge and Kilmartin, 2001). With the use of antibodies directed against the Cnp1 protein (green) and the GFP (red), which was present as a chimera with Ndc80, we learned that these two proteins were partially colocalized in all interphase cells (Figure 2, A and B). Based on 50 cells analyzed, the Ndc80 signal was generally larger than the Cnp1 signal; in 14% of cells the Ndc80 signal protruded toward the nuclear periphery (Figure 2B; columns 4 and 5). An Ndc80 homologue has been purified with the SPB preparations from S. cerevisiae,; therefore, we looked to see whether the S. pombe protein colocalized with Cut12p, a protein that resides near the inner face of the SPB, adjacent to the nucleus (Osborne et al., 1994; Bridge et al., 1998; Wigge et al., 1998; Wigge and Kilmartin, 2001). Ndc80p and Cut12p signals colocalized to some extent in all interphase cells (Figure 3, C and D), whereas the Cut12 signal was not clearly separable from that of Ndc80; it localized peripherally to Ndc80 relative to the 4′,6-diamidino-2-phenylindole (DAPI)-stained chromosomes (blue arc) in 83% of cells (N = 50; Figure 2D, columns 2, 4, 5, and 7). Because the Ndc80 signal also overlapped with Cnp1 we concluded that Ndc80 occupies a position between the central core (Cnp1p) and the nuclear face of the SPB (Cut12p). Taken together these results indicated that there may be at least three distinct layers in the centromeres of S. pombe. Moreover, the position of the centromere appears to be dynamic in interphase cells. It was, however, difficult to resolve the structural components of the kinetochore by light microscopy.
Figure 2.
Ndc80 localizes with Cnp1 domain and extends toward the SPB. (A) IF pictures showing the localization of Cnp1 (green) and Ndc80 (red) proteins in interphase nuclei. Cells expressing Ndc80-GFP were stained with anti-GFP and anti-Cnp1 antibodies. (C) IF pictures showing the relative localization of Ndc80-GFP (green) and Cut12-Pk (red) proteins in interphase nuclei. Cells harboring the Cut12-Pk protein fusion were stained with anti-Pk and anti-Cnp1 antibodies (Mellone and Allshire, unpublished data). (A and C) DAPI (blue) was used to stain the DNA. Bar, 2.0 μm. (B and D) Statistical analysis of the relative positions of the markers as indicated in Figures B and D (n = 50 for each graph). Yellow color indicates colocalization in the merged picture and the blue arc indicates the position of the DAPI-stained chromatin.
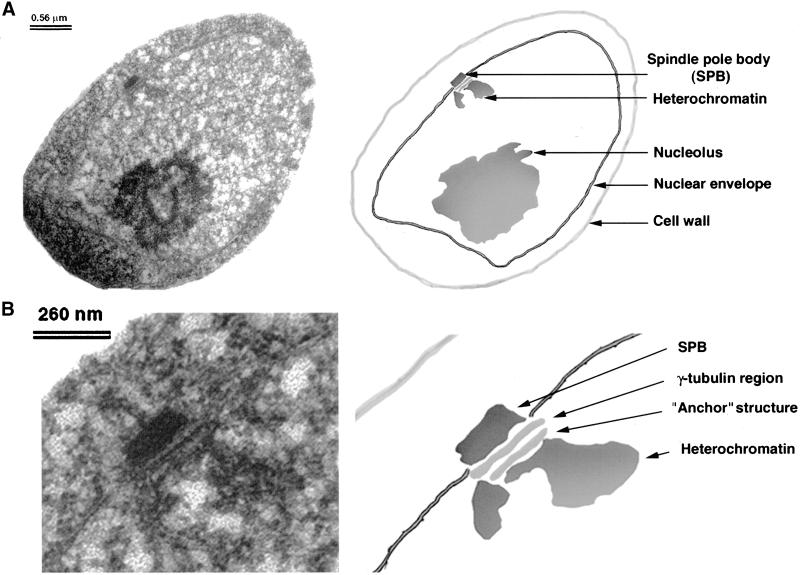
Figure 3.
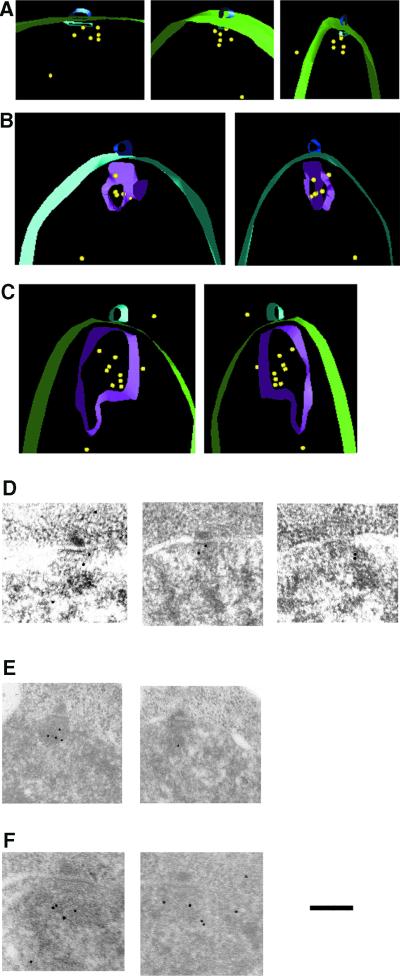
EM analysis of S. pombe centromeres. (A) A low-magnification EM micrograph of a section through a HPF-fixed and K11M-embedded interphase S. pombe cell. The cellular structures are indicated: cell wall, nuclear envelope, nucleolus, heterochromatin region, and SPB. Bar, 0.56 μm. (B) A higher magnification of the same cell. The nuclear structures indicated are SPB γ-tubulin region, anchor structure, and heterochromatin. Bar, 260 nm
EM Analysis of Centromeric Heterochromatin
To obtain more detailed information about fission yeast centromeres, log-phase cultures of S. pombe were immobilized by high pressure freezing (HPF), fixed by freeze substitution, embedded, and analyzed by EM. With this procedure the structures of nuclei and microtubules were generally well preserved. In addition to the nucleolus, the nucleus, containing another structure that stained more darkly than the surrounding nucleoplasm, was observed near the SPB (Figure 3). This structure was 200–300 nm wide and amorphous but generally of round shape. Based on its staining and its intranuclear position near the SPB, we inferred that this structure was centromeric heterochromatin (see below). At higher magnification we noticed another plate-like structure, ∼250 nm wide and 20 nm thick, lying between the presumed centromeric heterochromatin and the previously described osmiophilic region where γ-tubulin is bound to the inner surface of the nuclear envelope, just opposite the SPB (Figure 3B; Ding et al., 1997). We will refer to this new structure as the centromere “anchor,” because it appears to base the centromeric heterochromatin near the γ-tubulin region that lies proximal to the nuclear envelope.
Fission yeast centromeres have previously been shown to cluster in close to the SPB (Funabiki et al., 1993). To confirm the identity of the electron-dense material as centromeric heterochromatin, we carried out immuno-EM with antibodies that recognized the Swi6 component of this material. Antibodies against GFP were used to detect the GFP-Swi6 fusion protein (Pidoux et al., 2000), because these were known to perform well in immuno-EM (Zeng et al., 1999). The total number of gold particles (Au10) corresponding to GFP-Swi6 on the heterochromatin structure was determined to compare whether the amount of Swi6 varied between the interphase (single SPB) and prophase (duplicated SPB) stages of the cell cycle (Figure 4, A and B; see MATERIALS AND METHODS). On average 5 ± 3 and 6 ± 2 Au10, respectively, were bound to the each cross-section of the flanking domain at these two stages. Swi6 was present throughout the heterochromatin domain but not in the anchor structure. The heterochromatin domain and anchor structures persisted during the entire G2 phase of the cell cycle, because they were also apparent at prophase stages when the SPB was duplicated (Figure 4B).
Figure 4.
Swi6 is part of the heterochromatic domain, as detected by staining with antibodies and Au10. (A) Representative micrographs showing Swi6 localization in G2 cells with single SPBs. (B) Swi6 immuno-EM picture showing parts of nuclei from late interphase or prophase cells with duplicated SPB. (C) Different angular views of a 3-D model showing the GFP-Swi6 immuno-EM localization (yellow). The increased intranuclear background labeling in the 3-D model compared with the Swi6 IF localization (Figure 1) is most likely caused by loss of Swi6 antigen during the IF processing because the diffuse haze of Swi6 is also observed by live analysis of GFP-Swi6 (Pidoux et al. 2000). The positions of the nuclear envelope (green), the SPB (light blue), cytoplasmic microtubules (dark blue), and the boundary of the heterochromatin domain (pink) are indicated.
3-D EM Analysis of the Central Core and Flanking Regions
The IF localizations of Swi6, Mis6 and Cnp1 described above indicated that the central core region of the centromere was often positioned differently from the flanking centromeric region. To determine the position of Swi6 within the 3-D structure of the interphase heterochromatin, cells carrying a GFP-Swi6 fusion were subjected to HPF, and serial sections of 30–50 nm were cut, stained with antibodies of interest, and imaged in the EM. Serial images of the labeled sections were aligned and 3-D models were constructed with the use of the IMOD software (see MATERIALS AND METHODS). In the resulting models GFP-Swi6Au10 was localized in the peripheral portion of the centromeric heterochromatin region, in some instances just outside the electron-dense region of that structure (Figure 4C).
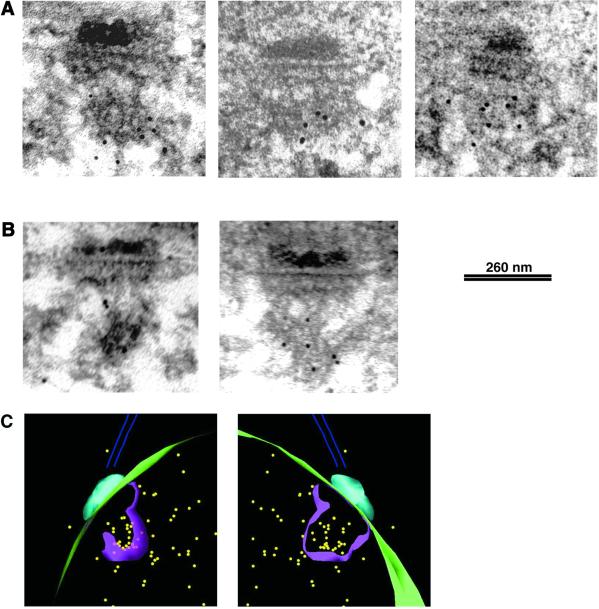
To compare the position of Cnp1 with that of Swi6, cells carrying a GFP-Cnp1 fusion were prepared for immuno-EM and 3-D models were constructed as described above. In the resulting models GFP-Cnp1Au10 occupied a more central position within centromeric heterochromatin than that seen with Swi6p (compare with Figures 4 and 5); in some cases the Cnp1-GFP was adjacent to the anchor structure visible by EM (Figure 5, A, B, D, and E). Thus, Swi6 bound to centromere flanking region chromatin, imr and otr, is situated at the periphery of the clustered centromeric heterochromatin, whereas Cnp1, which binds to central core (cnt) region, is less abundant and localized centrally within the heterochromatin domain.
Figure 5.
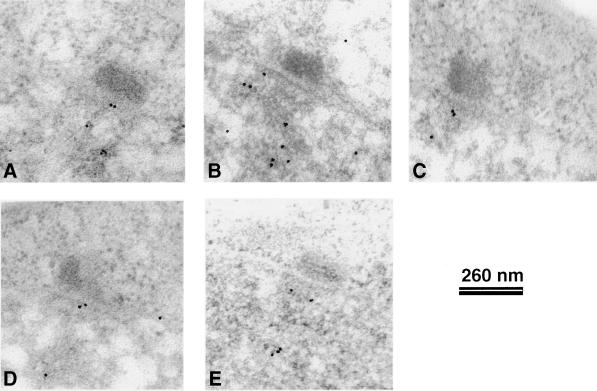
Cnp1 has a central localization within the heterochromatin domain. (A–C). Different angular views of 3-D models showing the GFP-Cnp1 immuno-EM localization (yellow). (A) The contours of the nuclear envelope (green) the SPB (light blue), and the anchor structure (dark blue) are indicated. (B) The contours of the nuclear envelope (green), the SPB (light blue), the anchor structure (dark blue), and the heterochromatin domain (pink) are indicated. (C) The contours of the nuclear envelope (green), the SPB (light blue), and the heterochromatin domain (pink) are indicated. (D–F) The primary micrographs representing the GFP-Cnp1 immuno-EM localization. (D) These three sections were used to create the 3-D model in A; (E) the two sections were used to create the 3-D model in B; and (D) the two sections were used to create the 3-D model in C. Bar, 260 nm.
Ndc80 Is Part of the Centromere Anchor Structure
Because the IF signals from Ndc80 localizes between Cnp1 and the SPB, we looked to see whether Ndc80 was part of the anchor structure. Immuno-EM was carried out as described above but with a strain expressing the Ndc80-GFP fusion (Wigge and Kilmartin, 2001). Some of the Ndc80 gold signal was located centrally within the heterochromatin domain, and some was indeed present on the anchor structure situated between the heterochromatin domain and the γ-tubulin region (Figure 6). The labeling density for Ndc80-GFP detected by anti-rabbit Au12 was 467 ± 103 on the 250-nm-wide and 20-nm-thick anchor structure and 44 ± 9 Au12/μm2 on the centromeric heterochromatin. The background in the nucleus and cytoplasm was 9 ± 6 and ≤1 Au12/μm2, respectively.
Figure 6.
Ndc80 is part of the anchor domain. (A–E) Representative micrographs showing Ndc80-GFP immunolocalization in nuclei of G2 cells with single SPBs. Anti-rabbit-Au12 was used to detect Ndc80-GFP.
A Multilayered Organization of S. pombe Centromeres
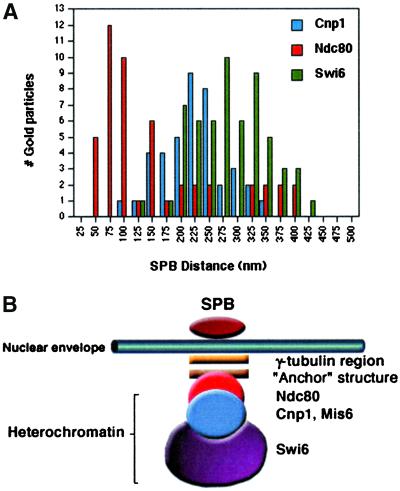
To investigate how the distinct protein-binding domains within S. pombe centromeres corresponded to the observed structures, the distances from the nuclear face of the SPB to the gold particles representing the immuno-EM positions of Ndc80, Cnp1, and Swi6 signals were measured. The average distance to all the Ndc80 gold was 142 ± 106 nm (n = 49); to the Cnp1 gold it was 213 ± 59 nm (n = 37); and to the Swi6 gold it was 278 ± 65 nm (n = 58). The distributions of all these distances are shown graphically in Figure 7 A. A statistical analysis of these distributions of markers suggested that the corresponding proteins occupied significantly different positions relative to the SPB (p < 0.00001). It follows that they occupy different positions within the centromere structure. Only the Ndc80 protein was detected (and not Cnp1 or Swi6) within the region most proximal to the SPB (40–80 nm) corresponding to the anchor structure. Furthermore, within the heterochromatic domain most distal to the rest of the chromatin, 350–450 nm from the SPB, only Swi6 protein was present (and not Cnp1 or Ndc80). The distribution of Cnp1 peaks between that of Swi6 and Ndc80, consistent with the central position occupied by Cnp1 with the 3-D models in Figure 4C and Figure 5. Therefore, as indicated in the schematic model (Figure 7B) we concluded that the central core (Cnp1), the flanking centromere region (Swi6), and the anchor structure (Ndc80) occupy distinct layers. These probably correspond to different domains within the S. pombe centromere.
Figure 7.
S. pombe centromeres are multilayered. (A) Histogram showing the distribution of SPB-gold distances for Ndc80, Cnp1, and Swi6 proteins in interphase cells. (B) Schematic model of S. pombe centromere cluster in interphase cells. The distribution of Ndc80, Cnp1, and Swi6 proteins are indicated in relation to the observed centromeric anchor and heterochromatin structures.
DISCUSSION
A Centromere Domain Structure Is Conserved from S. pombe to Human
In this work we have obtained evidence for a structure in the centromeric heterochromatin and the associated chromatin-SPB anchor in S. pombe cells at different stages of interphase. Although there is little precedence for the direct visualization of distinct chromosomal domains in yeast cells, these structures can now be compared with the equivalent structures in human cells.
Specimen preparation by HPF and subsequent analysis by EM have resulted in a slightly modified view of the human metaphase kinetochore structure, compared with that seen by conventional EM (McEwen et al., 1998). Nonetheless, the basic organization of these centromere-associated structures was the same as that seen by conventional fixation, with an outer region (the plate-like structures) being distinct from the underlying heterochromatin. With the use of HPF and EM techniques on S. pombe cells we have found a striking similarity between structure of the interphase centromere structure and the metaphase centromere of humans. It is not clear why S. pombe centromeres are organized in this manner throughout G2, because the mammalian centromeres seem to unfold in S phase and then refold during G2 to reappear as typical kinetochore structures in late G2 (He and Brinkley, 1996). It could perhaps be a reflection of the shorter S. pombe cell cycles allowing approximately one order of magnitude less time for unfolding to occur. Another possibility is that maintenance of the connection between centromeres and the SPB serves a specific function in maintaining the S. pombe chromatin organization during G2.
It is interesting to note that the positions of some CENPs are conserved within the multilayered kinetochore structures from S. pombe to human. First we show that Cnp1 (S. pombe CENP-A) occupies a central position within interphase kinetochores distinct from Swi6. Recent studies, including live analysis, indicate that, although the human kinetochore unfolds and refolds during interphase, the human prekinetochore interphase structure remains ordered in interphase so that CENP-A localization is limited to the edge of a larger CENP-B heterochromatin domain even before the typical double dot structure appears in G2 (Pudenko et al., 1997; Sugimoto et al., 2000). At metaphase CENP-A is a component of the inner plate in human centromere (Vafa and Sullivan, 1997). Second, the more peripheral position of chromodomain protein Swi6 is reminiscent of the localization of HP1 to the underlying heterochromatin in humans. A third parallel is the localization of Ndc80 to the anchor structure in S. pombe, whereas the human homologue of Ndc80, HEC, is localized to the outer part of HeLa cell centromeres (Wigge and Kilmartin, 2001). It is possible that this conservation of position reflects centromere functions that are conserved across a broad range of eukaryotes. Because the anchor structure is adjacent to the γ-tubulin region from which microtubules are nucleated in mitosis, the anchor structure may carry out a function in mitosis analogous to the outer plate structure in human centromeres, perhaps harboring microtubule interactions as discussed by Wigge and Kilmartin (2001). The S. pombe central core and the human inner plate could have a similar function. Interestingly, cells with a central core marker mis6–302 temperature-sensitive allele show both defective interphase centromere clustering and a mitotic missegregation defect of chromosomes, which is consistent with a biorientation defect of sister kinetochores (Saitoh et al., 1997). Mis6 is required to recruit Cnp1 to the central core (Takahashi et al., 2000). Hence, it is possible that Mis6- and Cnp1-mediated clustering is a prerequisite for correct biorientation and that biorientation is the conserved function for the inner plate. Based on their position within the structure it is also conceivable that the flanking region and the underlying heterochromatin in human centromere have similar functions. S. pombe cells with mutations that disturb the integrity of the flanking regions such as swi6, rik1, clr4, and csp7-12 display a typical lagging chromosome phenotype in anaphase, but interphase clustering of centromeres is normal (Ekwall et al., 1996, 1999).We are hopeful that this work will open the way for detailed EM studies of the putative defective centromere structures corresponding to the distinct functional defects in these mutants.
ACKNOWLEDGMENTS
We thank members of the Karl Ekwall laboratory, Dr. I. Hagan, and Dr. A. Önfelt for critically reading the manuscript. We thank Dr. I. Hagan for the Cut12-Pk strains, Dr. J. Kilmartin for generously providing the Ndc80-GFP strain before publication, and Prof. M. Yanagida for the gift of Mis6-HA strain. Work in the Karl Ekwall laboratory was supported by the following grants: MFR K2000-31X-12562, NFR 09920-302, and CF 4284-B99 and a Junior Individual Grant from the Swedish Foundation for Strategic Research. Work carried out in Boulder was supported by RR00592 from the National Center for Research Resources.
REFERENCES
- Aagaard L, Laible G, Selenko P, Schmid M, Dorn R, Schotta G, Kuhfittig S, Wolf A, Lebersorger A, Singh PB, Reuter G, Jenuwein T. Functional mammalian homologues of the Drosophila PEV-modifier Su(var)3–9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J. 1999;18:1923–1938. doi: 10.1093/emboj/18.7.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, Pepper D, Berns MW, Tan E, Brinkley BR. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981;91:95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge AJ, Morphew M, Bartlett R, Hagan IM. The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev. 1998;12:927–942. doi: 10.1101/gad.12.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, Niwa O, Yanagida M. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell. 1989;57:739–751. doi: 10.1016/0092-8674(89)90789-7. [DOI] [PubMed] [Google Scholar]
- Clarke L, Amstutz H, Fishel B, Carbon J. Analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1986;83:8253–8257. doi: 10.1073/pnas.83.21.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L, Baum MP. Functional analysis of a centromere from fission yeast: a role for centromere specific repeated DNA sequences. Mol Cell Biol. 1990;10:1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Bernat RL, Earnshaw WC. CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol. 1990;110:1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke CA, Schaar B, Yen TJ, Earnshaw WC. Localization of CENP E in the fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew DM, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CL, Wang G, Chow C, Fricker MD, Singh PB, Mellor EJ. The fission yeast chromo domain encoding gene chp1(+) is required for chromosome segregation and shows a genetic interaction with alpha-tubulin. Nucleic Acids Res. 1998;26:4222–4229. doi: 10.1093/nar/26.18.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92:290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91:313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Ekwall K, Cranston G, Allshire RC. Fission yeast mutants that alleviate transcriptional silencing in centromeric flanking repeats and disrupt chromosome segregation. Genetics. 1999;153:1153–1169. doi: 10.1093/genetics/153.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwall K, Javerzat JP, Lorentz A, Schmidt H, Cranston G, Allshire R. The chromodomain protein Swi6: a key component at fission yeast centromeres. Science. 1995;269:1429–1431. doi: 10.1126/science.7660126. [DOI] [PubMed] [Google Scholar]
- Ekwall K, Nimmo ER, Javerzat JP, Borgstrom B, Egel R, Cranston G, Allshire R. Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localization of the chromo domain protein Swi6p and impair centromere function, Pt 11. J Cell Sci. 1996;109:2637–2648. doi: 10.1242/jcs.109.11.2637. [DOI] [PubMed] [Google Scholar]
- Ekwall K, Olsson T, Turner BM, Cranston G, Allshire RC. Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell. 1997;91:1021–1032. doi: 10.1016/s0092-8674(00)80492-4. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS. Kinetochore structure and its role in chromosome orientation during the first meiotic division in male D. melanogaster. Cell. 1981;25:591–602. doi: 10.1016/0092-8674(81)90167-7. [DOI] [PubMed] [Google Scholar]
- Goshima G, Saitoh S, Yanagida M. Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 1999;13:1664–1677. doi: 10.1101/gad.13.13.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- He D, Brinkley BR. Structure and dynamic organization of centromeres/prekinetochores in the nucleus of mammalian cells. J Cell Sci. 1996;109:2693–2704. doi: 10.1242/jcs.109.11.2693. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Allshire RC. The case for epigenetic effects on centromere identity and function. Trends Genet. 1997;13:489–496. doi: 10.1016/s0168-9525(97)01298-5. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Hsieh CE, Mattheyses AL, Rieder CL. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107:366–375. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TD, Karpen GH. Localization of centromere function in a Drosophila minichromosome. Cell. 1995;82:599–609. doi: 10.1016/0092-8674(95)90032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MA, Schlenstedt G, Jinks T, Silver PA. Nuf2, a spindle pole body-associated protein required for nuclear division in yeast. J Cell Biol. 1994;125:853–866. doi: 10.1083/jcb.125.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JF, Borgstrom B, Allshire RC. Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev. 2000;14:783–791. [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Allshire RC. Centromeres: getting a grip of chromosomes. Curr Opin Cell Biol. 2000;12:308–319. doi: 10.1016/s0955-0674(00)00094-6. [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Uzawa S, Perry PE, Cande WZ, Allshire RC. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast [in process citation] J Cell Sci. 2000;113:4177–4191. doi: 10.1242/jcs.113.23.4177. [DOI] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC. The centromere: hub of chromosomal activities. Science. 1995;270:1591–1594. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- Pudenko AS, Kudryavtsev IS, Zatsepina OV, Chentsov Yu S. Spatial association of prekinetochores and chromocentres in the interphase nuclei of mouse cultured fibroblasts. Membr Cell Biol. 1997;11:449–461. [PubMed] [Google Scholar]
- Saitoh H, Tomkiel J, Cooke CA, Ratrie Hd, Maurer M, Rothfield NF, Earnshaw WC. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992;70:115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Steiner NC, Clarke L. A novel epigenetic effect can alter centromere function in fission yeast. Cell. 1994;79:865–874. doi: 10.1016/0092-8674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Fukuda R, Himeno M. Centromere/kinetochore localization of human centromere protein A (CENP- A) exogenously expressed as a fusion to green fluorescent protein. Cell Struct Funct. 2000;25:253–261. doi: 10.1247/csf.25.253. [DOI] [PubMed] [Google Scholar]
- Sun X, Wahlstrom J, Karpen G. Molecular structure of a functional Drosophila centromere. Cell. 1997;91:1007–1019. doi: 10.1016/s0092-8674(00)80491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Chen ES, Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288:2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Vafa O, Sullivan KF. Chromatin containing CENP A and alpha satellite DNA is a major component of the inner kinetochore plate. Curr Biol. 1997;7:897–900. doi: 10.1016/s0960-9822(06)00381-2. [DOI] [PubMed] [Google Scholar]
- Warburton PE, Cooke CA, Bourassa S, Vafa O, Sullivan BA, Stetten G, Gimelli G, Warburton D, Tyler-Smith C, Sullivan KF, Poirier GG, Earnshaw WC. Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr Biol. 1997;7:901–904. doi: 10.1016/s0960-9822(06)00382-4. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Jensen ON, Holmes S, Soues S, Mann M, Kilmartin JV. Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J Cell Biol. 1998;141:967–977. doi: 10.1083/jcb.141.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge PA, Kilmartin JV. The Ndc80 complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol. 2001;152:349–360. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol. 1997;139:435–447. doi: 10.1083/jcb.139.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Kahana JA, Silver PA, Morphew MK, McIntosh JR, Fitch IT, Carbon J, Saunders WS. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J Cell Biol. 1999;146:415–425. doi: 10.1083/jcb.146.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]