Abstract
Is covert visuospatial attention–selective processing of information in the absence of eye movements–preserved in adults with Attention-Deficit/Hyperactivity Disorder (ADHD)? Previous findings are inconclusive due to inconsistent terminology and suboptimal methodology. To settle this question, we used well-established spatial cueing protocols to investigate the perceptual effects of voluntary and involuntary attention on an orientation discrimination task for a group of adults with ADHD and their neurotypical age- and gender-matched controls. In both groups, voluntary attention significantly improved accuracy and decreased reaction times at the relevant location, but impaired accuracy and slowed reaction times at irrelevant locations, relative to a distributed attention condition. Likewise, involuntary attention improved accuracy and speeded responses. Critically, the magnitudes of all these orienting and reorienting attention effects were indistinguishable between groups. Thus, these counterintuitive findings indicate that spatial covert attention remains functionally intact in adults with ADHD.
Keywords: ADHD, visual perception, selective attention, orienting, reorienting, microsaccades, performance fields
INTRODUCTION
Despite symptoms of “inattention” as a qualitatively defining feature (American Psychiatric Association [APA], 2013), surprisingly, much is still unknown about attention in Attention-Deficit/Hyperactivity Disorder (ADHD). ADHD is a neurodevelopmental disorder characterized by a heterogeneous set of persistent maladaptive behaviors and neurocognitive impairments. Initially conceptualized as a behavioral disorder of hyperactivity and heightened impulsivity in children, the notion of deficient attention was introduced in the 3rd edition of the Diagnostic and Statistical Manual of Mental Disorders (APA, 1980; Barkley, 2007). Many years later it was recognized that ADHD may persist through adolescence and even onset in adulthood (Barkley, 2007). Estimated to affect 5–6% of the worldwide adult population (Polanczyk et al., 2007), a burgeoning literature shows adults with ADHD exhibit abnormalities in several domains, including, but not limited to, response precision, cognitive flexibility, working memory, temporal information processing, response inhibition, and our cognitive process of interest, attention (Hervey et al., 2004; Mueller et al., 2017; Pievsky & McGrath, 2017).
Attention is not a unitary concept (Carrasco, 2011; Posner, 2014). The cognitive requirements of many of the “classic” tasks adopted to probe attentional functioning in ADHD (e.g. Continuous Performance Task, Stroop tasks, Attention Network Test) involve several distinct attentional and executive functions (Hervey et al., 2004; Mueller et al., 2017). For example, high performance on Stroop and flanker tasks, in which observers are asked to ignore distracting features or other stimuli, requires strong executive interference control and response inhibition in addition to intact selective attention. This combination of task demands is unfortunate, as patients’ most reliable deficits lie under the umbrella of executive functions, which encompass response inhibition, reward response, decision-making, and motivational processes, among others (Barkley, 1997; Willcutt et al., 2005; Pievsky & McGrath, 2017). Thus, any of these factors could be responsible for observed behavioral differences between groups. Moreover, terminology spanning clinical and experimental adult ADHD research has been inconsistent and imprecise.
There are significant gaps regarding which types of attention are deficient in ADHD. Research in adults with ADHD on sustained attention reports significant limitations in their ability to continuously perform a task over a prolonged period (e.g. minutes) (Marchetta et al., 2007; Dankner et al., 2017; Mueller et al., 2017). There has been surprisingly little research on selective attention, i.e. the preferential processing of one stimulus in the presence of other distracting stimuli (Tsal et al., 2005; Mueller et al., 2017). A few studies have argued that ADHD adults show heightened distractibility to irrelevant distractors (Marchetta et al., 2007; Tucha et al., 2008; Marzinzik et al., 2012; Godefroid & Wiersema, 2017). In contrast to the rich literature on visuospatial orienting in children (e.g., Tsal et al., 2005; Ortega et al., 2013; review by Huang-Pollock & Nigg, 2003), only five studies have explicitly investigated the perceptual effects of spatial “orienting” and “reorienting” of selective attention (Posner, 1980) in adults with ADHD (Tomporowski et al., 1994; Epstein et al., 1997; Epstein et al., 2001; Oberlin et al., 2005; Dhar et al., 2008). These studies suggest that orienting and reorienting may be functionally spared in the disorder, but suboptimal methodology complicates their interpretation (see Discussion).
For the first time, we isolate, manipulate and measure the visual perceptual effects of both covert voluntary (endogenous) and involuntary (exogenous) spatial attention in adults with mild-to-moderate ADHD and their age- and gender-matched neurotypical (NT) controls. The perceptual consequences of these types of attention are often the same in NT observers; both increase contrast sensitivity, enhance spatial resolution, accelerate the rate of information accrual, and even alter stimulus appearance. However, these two types of selective attention can differ according to task demands and stimuli, exhibit different temporal dynamics, and are supported by partially overlapping and interactive yet distinct neural networks (Carrasco, 2011). Given that these types of attention can result in different perceptual consequences (Carrasco, 2011), and that their functional roles differ, it is critical to assess both types of attention in this population as only one, both or none could be spared. Understanding whether these types of attention are fully functional or impaired in the ADHD brain is informative for developing more sophisticated neuropsychological and neurobiological models of the disorder. We measured both accuracy and RT for an orientation discrimination task. In addition, we compared microsaccades (MS) of these two groups as they differ in continuous sustained tasks (Fried et al., 2014; Dankner et al., 2017), and have been linked to some perceptual and attentional tasks (Rucci & Poletti, 2015). In two separate psychophysical experiments, we used central and peripheral cues to directly manipulate either endogenous (Experiment 1) or exogenous (Experiment 2) attention, respectively.
EXPERIMENT 1: ENDOGENOUS ATTENTION
METHOD
Observers
To be included in the study, all adult observers had to possess normal or corrected-to-normal vision. Observers in the ADHD group were clinically diagnosed with DSM-IV-TR ADHD (APA, 2000) according to the Adult ADHD Clinical Diagnostic Scale v.1.2 (Adler & Spencer, 2004), and Structured Clinical Interview for DSM-IV, Research Version, Non-patient Edition (SCID-I/NP; First et al., 2002). We did not exclude potential observers on the basis of age, race, gender, ADHD severity or comorbidities. Fourteen adults with ADHD (Table S1) and 14 age- and gender-matched NT controls (M age=31.0, SD=8.5; 7 F) participated in Experiment 1; all observers had attended college and some graduate school. Our sample size was similar to previous studies reporting significant performance differences between ADHD and NT groups in purported tasks of focused and sustained attention (reviews, Frazier et al., 2004; Schoechlin & Engel, 2005), and similar or larger than studies that found intact attention effects in other special populations (autism: Grubb, Behrmann, Egan, Minshew, Carrasco & Heeger, 2013; Grubb, Behrmann, Egan, Minshew, Heeger, & Carrasco, 2013; amblyopia: Roberts et al., 2016) and neurotypical observers (e.g., Carrasco & Yeshurun, 1998; Carrasco, Penpeci-Talgar & Cameron, 2001; Carrasco, Ling & Read, 2004; White et al., 2015; Dugué et al., 2016). All experimental procedures were in agreement with the Helsinki declaration and approved by the New York University and NYU School of Medicine Institutional Review Boards. All observers (except for author M.R., control observer) were naïve to the experimental hypotheses and signed written consent.
Apparatus & set-up
Observers were tested in the same dimly lit, sound-attenuated room for both experiments. Stimuli were programmed on an Apple iMac MC413LL/A 21.5” Desktop (3.06 GHz Intel Core 2 Duo) using MATLAB (MathWorks, Massachusetts, USA) in conjunction with the MGL toolbox (http://justingardner.net/mgl). They were presented at a viewing distance of 57 cm on a 21" IBM P260 CRT monitor (1280×960 pix resolution, 90 Hz refresh rate), calibrated and linearized using a Photo Research (Chatworth, CA) PR-650 SpectraScan Colorimeter. Observers performed the experiments using a forehead and chin rest to ensure head stabilization. Eye movements were monitored using an EyeLink 1000 Desktop Mount eye tracker (SR Research, Ontario, Canada).
Stimuli
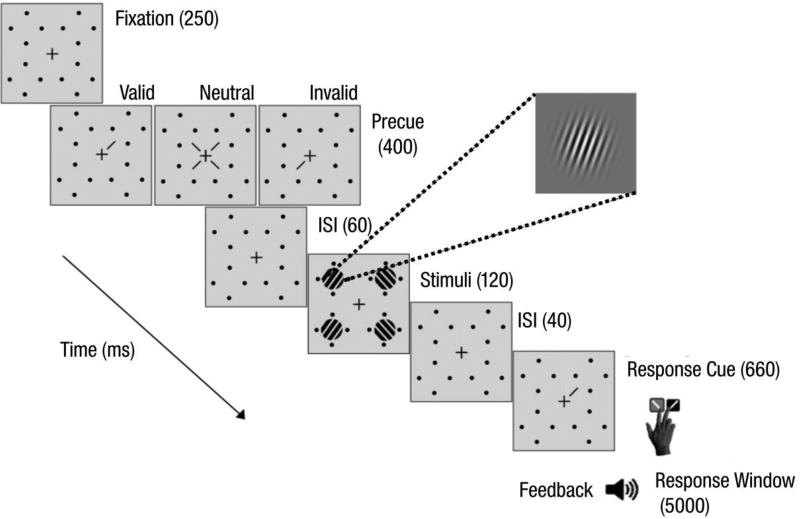
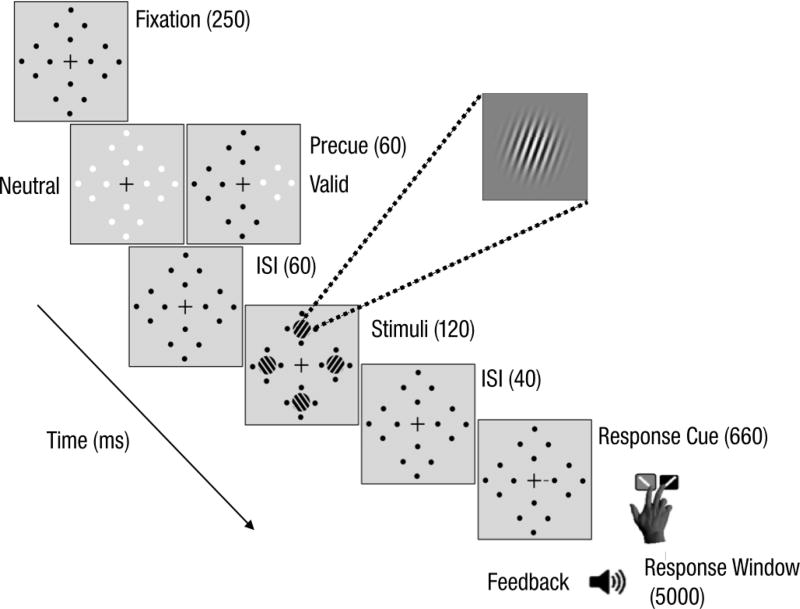
Observers were asked to fixate on a black, centrally-placed cross (0.5° across) throughout the trial (Fig 1). Four placeholders–each comprised of four black dots (0.05° radius) arranged in a circle 0.5° from the location of an upcoming Gabor patch stimulus (to prevent masking)–were always presented on the screen to reduce location uncertainty. The target and three distractor stimuli were all 3.2° wide, 4-cpd Gabor patches (contrast-defined sinusoidal gratings embedded in a Gaussian envelope, σ=0.46°), randomly and independently tilted ±20° from vertical, centered at 6.4° eccentricity along the diagonals, and with the same mean luminance as the uniform grey background. To manipulate endogenous spatial attention, we presented a central precue—either a single 0.88° line or four 0.28° lines (all 0.14° thick)—0.38° from the center of the fixation cross, which pointed to one or all (neutral, distributed condition) of the possible target locations. The response cue indicated the target location by pointing to one placeholder (that matched the single central precue for valid trials and mismatched for invalid trials) and eliminated location uncertainty at the response time for all conditions.
Fig 1.
Trial sequence for Experiment 1: Endogenous attention.
Procedure
Observers performed the same experimental procedure across two hour-long behavioral sessions. They completed about 18 blocks of 60 trials each for a total of 1,080 trials; 648 trials in the valid cue condition (60% of all trials), and 216 trials each in the invalid (20% of all trials) and neutral cue (20% of all trials) conditions. At the beginning of the first session, observers completed practice blocks (24 trials each, 100% stimulus contrast) until they could perform the task reliably above chance. Then, they underwent a staircase procedure (neutral cues only) where we obtained their individual stimulus contrast thresholds yielding 80% accuracy. The contrast of the Gabor patch stimuli was initially set at each individual’s threshold performance around 80%. The required stimulus contrast did not differ between the ADHD (M=32.1±9.1%) and NT controls (M=19.6±7.5%; t(26)=1.07; p>.1, Cohen’s d=0.4; Scaled JZS Bayes Factor=1.9, according to Rouder et al., 2009). If observers made an eye movement ≥1° from the fixation cross between initiation and stimulus offset, the trial would immediately abort and the text, "Please fixate," would appear at the center of the screen. These trials were rerun at the end of the block. Both groups broke fixation (ADHD: M=2.86±0.5; NT: M=2.40±0.9) with similar frequency per block (independent samples t-test: t(26)=0.45, p>.1, Cohen’s d=0.2; Scaled JZS Bayes Factor=2.6). The ADHD group broke fixation in 4.8% of all trials; the NT group in 4.0%.
Task & trial sequence
Observers performed a two-alternative forced-choice (2AFC) orientation discrimination task binocularly while endogenous spatial attention was manipulated via presentation of either a single (80% of all trials, of which 75% of trials were valid and 25% trials were invalid) or distributed central precue (20% of all trials; Fig 1). On every trial, observers were encouraged to respond as accurately as possible, without time stress. After 250 ms, the precue was presented for 400 ms, after which there was a brief interstimulus interval (ISI) of 60 ms. The 460 ms stimulus-onset-asynchrony (SOA) between precue onset and stimulus was designed to ensure that all observers had ample time to voluntarily deploy their endogenous attention (Müller & Rabbitt, 1989; Nakayama & Mackeben, 1989; Liu et al., 2007; review by Carrasco, 2011). After the interval, the target and distractor Gabor patches appeared simultaneously inside the placeholders for 120 msi. There was a brief 40-ms ISI between display offset and the response cue, which remained on the screen for 660 ms. An auditory tone indicated the beginning of the 5000-ms response window, in which observers had to report the target orientation (clockwise or counterclockwise relative to vertical) using one of two keyboard presses ('1' for clockwise, '2' for counterclockwise) with their right hand. Observer response terminated the response window, after which there was a mandatory 1000-ms intertrial interval. Auditory feedback was provided at the end of each trial and visual feedback indicating observers’ accuracy and number of fixation breaks was presented at the end of each block.
RESULTS
Overall performance
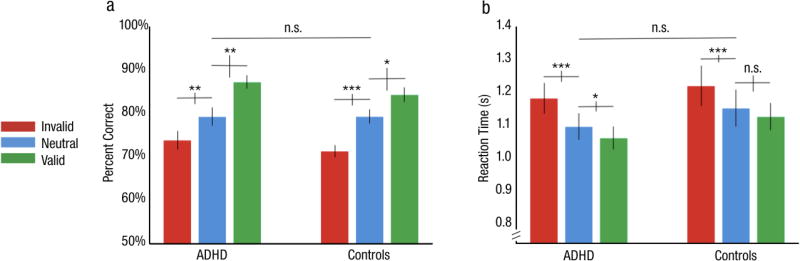
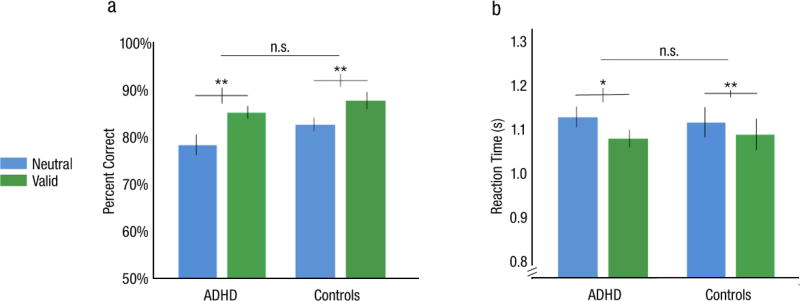
Overall accuracy in the neutral cueing condition was similar in the ADHD (M=79.3±2.1%) and NT (M=79.3±1.6%) observers, confirming that task difficulty was matched across groups (Fig 2a). A 2-way mixed design ANOVA revealed a main effect of cue (F(1.41, 36.8)=41.3, p<.001, ηp2=.61), but neither the main effect of group (F(1, 26)=1.1, p>.1, ηp2=.04) nor its interaction with cue condition (F<1, ηp2=.02) were significant, indicating no differences in overall accuracy or the magnitude of the attention effect between groups.
Fig 2.
Performance in Experiment 1: Endogenous attention. a) Percent accuracy. b) Reaction times. Error bars are ±1 SEM. * = p<.05, ** = p<.01, *** = p<.001.
To confirm that any non-significant results were not simply due to a lack of statistical power to find differences between groups, we calculated Bayesian information criterion probabilities (pBIC) that represent the strength of evidence in favor of the null (H0)—a non-significant main effect or interaction—or alternative (H1)—a significant main effect or interaction—hypotheses given our data set D (Masson, 2011). A value of pBIC between .75–.95 and a Bayes factor >3 are considered positive evidence. The Bayes factor analysis of the main effect of group provided evidence in favor of the null with an odds of 1.55 to 1: pBIC(H0|D)=.61 and pBIC(H1|D)=.39. The analysis of the interaction between cue and group, the test of greatest interest to this study, provided positive evidence in favor of the null hypothesis with an odds of 4.78 to 1: pBIC(H0|D)=.83 and pBIC(H1|D)=.17.
A corresponding 2-way mixed ANOVA of RT found a similar pattern of results (Fig 2b; see Supplementary Information). In both groups, accuracy was significantly higher for the valid (ADHD: M=87.3±1.5%; NT: M=84.4±1.6%) and lower for the invalid (ADHD: M=73.9±2.1%; NT: M=71.3±1.3%) compared to the neutral cueing condition (Fig 2a). The Bayes factor analysis found positive evidence in support of the null hypothesis that there was no significant difference in overall RT between groups with an odds of 3.02 to 1: pBIC(H0|D)=.75 and pBIC(H1|D)=.25. We also found evidence in favor of the alternative hypothesis of a significant cue × group interaction, with the ADHD group exhibiting a greater benefit of the endogenous cue, faster responses during the valid- than the neutral- cueing condition (BF < .01; pBIC(H0|D)<.01, pBIC(H1|D)=.99).
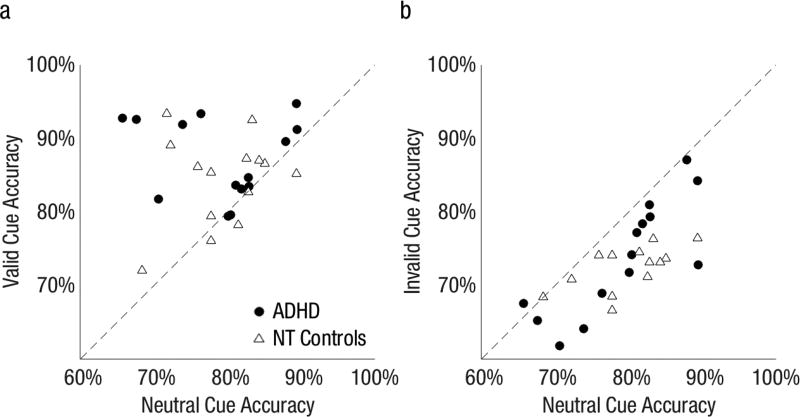
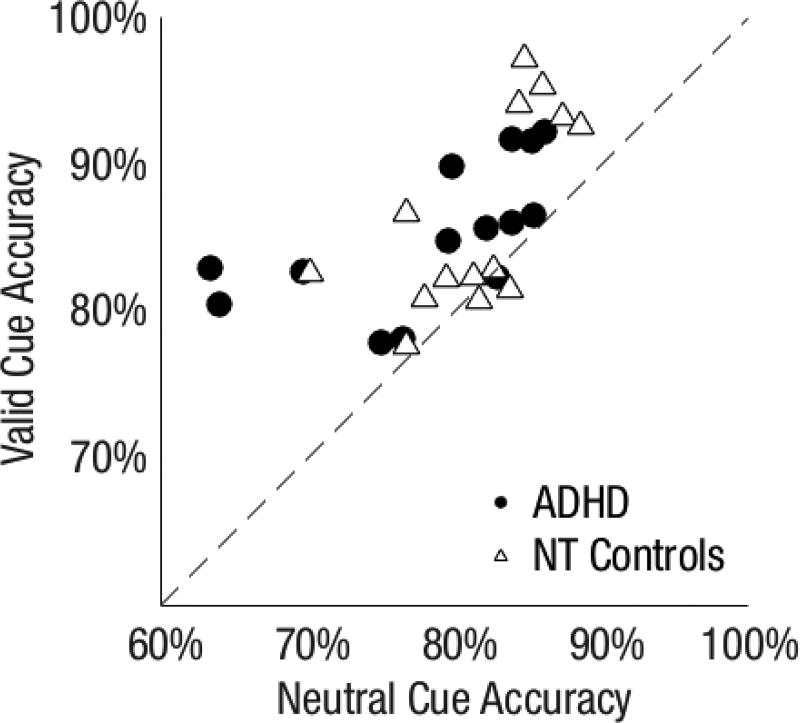
Fig 3 shows that the variance of individual endogenous attention benefits and costs were similar in both groups and present for all but a few observers, who fall on the diagonal line.
Fig 3.
Performance accuracy for individual observers in Experiment 1. a) Valid versus neutral cue condition. b) Invalid versus neutral cue condition. The farther from the diagonal, the greater the attention (a) benefit and (b) cost.
Microsaccades
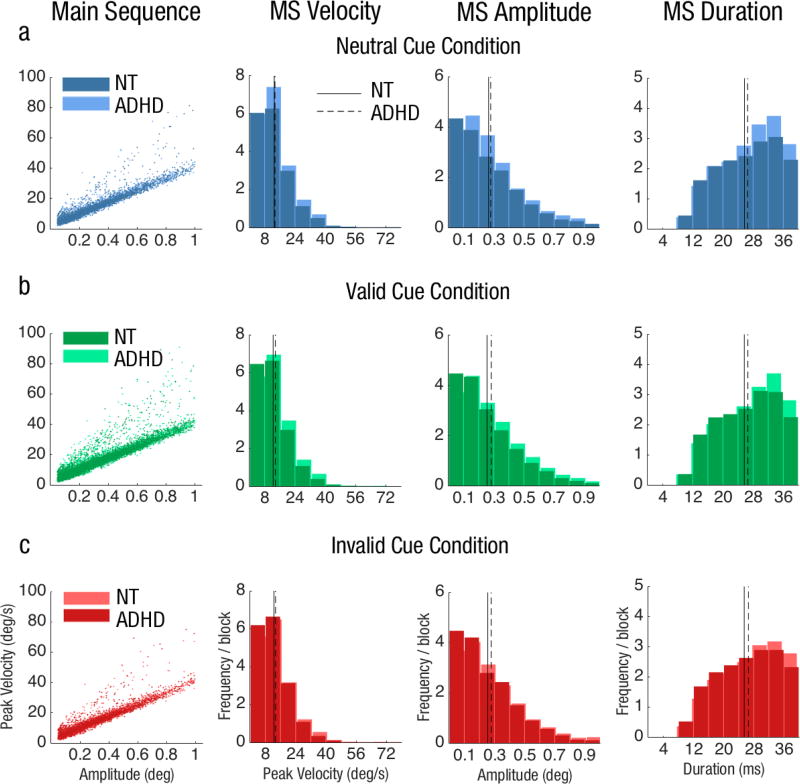
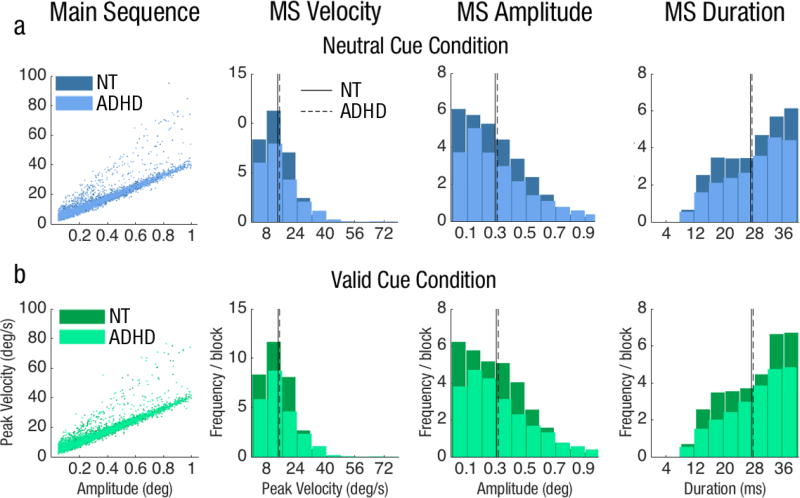
The average frequency of MS throughout the trial per block did not significantly differ among attention conditions (valid: M=30.9±2.07, neutral: M=30.8±2.29 and invalid: M=30.1±1.94); or between groups (ADHD: M=153.6±15.1, NT: M=153.9±14.7; both ps>.1). MS in each condition followed the main sequence: the higher the amplitude, the faster the velocity (column 1, Fig 4). Furthermore, their kinematics (i.e., peak velocity, amplitude and duration) did not differ as a function of attention condition or group (all ps>.1; columns 2–4, Fig 4).
Fig 4.
MS Kinematics for both groups in Experiment 1: Endogenous attention for the a) neutral cue condition, b) valid cue condition and c) invalid cue condition. MS frequency for the valid condition was divided by three to normalize the trial probability within each block. Distributions of MS velocity (column 2), amplitude (column 3), and duration (column 4) in terms of average frequency per block for each group.
In sum, adults with ADHD demonstrate the classic benefit in both accuracy and RT of voluntarily orienting to a spatial location which they will be subsequently asked about, as well as cost of initially orienting to the incorrect location. The magnitude and pattern of these typical attentional effects are indistinguishable from those found in NT adults. Moreover, the oculomotor correlates of these behavioral effects were similar between both groups.
EXPERIMENT 2: EXOGENOUS ATTENTION
In Experiment 1, we demonstrated that voluntary covert orienting and reorienting of selective visual attention remains functionally intact in adults with ADHD. Psychophysical, neuroimaging, and neurophysiological studies indicate that the two types of attention are supported by interactive and partially overlapping yet distinct neural systems (Carrasco, 2011; Posner, 2014). In this experiment, we investigated whether exogenous selective attention is also preserved in adults with ADHD, employing essentially the same task as in Experiment 1. Moreover, our task design enabled us to directly assess, for the first time, the spatial distribution of attention across the visual field in adults with ADHD. Some studies on neuropsychological disorders often comorbid with ADHD, e.g. autism spectrum disorder (e.g., Keehn et al., 2013) have reported differences in the spatial distribution of attention across the visual field, but others have found a similar distribution (Grubb, Behrmann, Egan, Minshew, Carrasco & Heeger, 2013; Grubb, Behrmann, Egan, Minshew, Heeger, & Carrasco, 2013). We investigated potential perceptual and attentional asymmetries at isoeccentric locations (see Supplementary Information).
METHOD
Observers
Inclusion and exclusion criteria were the same as in Experiment 1. Fourteen adults with ADHD (6 also participated in Experiment 1; see Table S1) and 14 age- and gender-matched NT controls (M age=30.9, SD=8.0; 7 F) participated in Experiment 2; all observers had attended college and some graduate school. The ADHD group was mainly comprised of individuals exhibiting mild-to-moderate ADHD symptomology, according to the Conner's Adult ADHD Rating Scale (CAARS; Conners et al., 1999).
Apparatus & set-up
They were identical to Experiment 1.
Stimuli
They were identical to Experiment 1 except for the stimuli locations and form of the precue (Fig 5). We presented the placeholders and Gabor patches at the cardinal axes. To manipulate exogenous attention, the dots of either one–valid peripheral precue–or all four–neutral precue– placeholders grew in size (to 0.16° radius) and the color changed from black to white. The response cue (a 0.8° line placed 0.3° from the central fixation cross) indicated the target location by pointing to one placeholder (matching the peripheral precue location in the valid condition).
Fig 5.
Trial sequence for Experiment 2: Exogenous attention.
Procedure
The procedure was identical to Experiment 1 except that: (a) there were no invalid cues; with exogenous attention, the benefit of the valid cue is the same regardless of whether there are invalid trials or not (Giordano et al., 2009; Carrasco, 2011); (b) both groups of observers completed about 20 experimental blocks of 48 trials each. There was not a significant difference in the mean contrast required by the ADHD (M=34.2±7.5%) and NT controls (M=25.3±7.4%; t(26)=0.8, p>.1; Scaled JZS Bayes Factor=2.2, according to Rouder et al., 2009). Trials in which observers broke fixation were cancelled and excluded from analyses. Both groups broke fixation (ADHD: M=2.09±0.6; NT: M=1.02±0.4) with similar frequency per block (independent samples t-test: t(26)=1.45, p>.1; Scaled JZS Bayes Factor=1.3).
Task & trial sequence
Observers performed the same 2AFC orientation discrimination task binocularly while exogenous spatial attention was manipulated via presentation of either a valid peripheral (50% of trials) or a neutral, distributed (50% of trials) precue (Fig 5). The sequence was the same as in Experiment 1, except that the precue duration was only 60 ms. The 120 ms SOA between precue onset and stimulus was designed to optimize the attentional effects of the exogenous cue and prevent any voluntary deployment of attention (Müller & Rabbitt, 1989; Nakayama & Mackeben, 1989; Liu et al., 2007; review by Carrasco, 2011).
RESULTS
Overall performance
Once again, task difficulty in the neutral condition was well equated between groups (ADHD: M=78.4±2.1%; NT: M=81.5±1.3%; Fig 6a). A 2-way mixed design ANOVA of accuracy revealed a significant main effect of cue (F(1, 26)=31.2, p<.001, ηp2=.55), but neither the main effect of group (F(1, 26)=1.09, p>.1, ηp2=.04) nor their interaction (F<1, ηp2=.03) was significant (Fig 6a). The Bayes factor analysis of the main effect of group provided evidence in favor of the null with an odds of 2.22 to 1: pBIC(H0|D)=.69 and pBIC(H1|D)=.31. The analysis of the interaction between cue and group, the test of greatest interest to this study, provided positive evidence in favor of the null hypothesis with an odds of 4.56 to 1: pBIC(H0|D)=.82 and pBIC(H1|D)=.18.
Fig 6.
Performance in Experiment 2: Exogenous attention. a) Accuracy. b) Reaction times. Error bars are ±1 SEM. * = p<.05, ** = p<.01, *** = p<.001.
A corresponding analysis of RT found a similar pattern of results (Fig 6b; see Supplementary Information). In both groups, accuracy was higher in the valid (ADHD: M=85.2±1.3%; NT: M=86.4±1.8%) than in the neutral cueing condition (Figure 6a; ADHD: t(13)=4.25, p=.001, 95% CI=[3.4,10.4], Cohen’s d=1.1; NT: t(13)=3.63, p=.003, 95% CI=[2.0,7.8], Cohen’s d=1.0). The Bayes factor analysis found positive evidence in support of the null hypothesis that there was no significant difference in overall RT between groups with an odds of 4.72 to 1: pBIC(H0|D)=.83 and pBIC(H1|D)=.17. The Bayes factor analysis favored the null hypothesis regarding the interaction between cueing condition and group with odds of 2.60 to 1: pBIC(H0|D)=.72 and pBIC(H1|D)=.28. Thus, both groups exhibited the classic exogenous attention benefit to the same extent.
The variance of individual exogenous attention accuracy benefits was similar for both groups and present for all but a few observers, whose data are along the diagonal line (Fig 7).
Fig 7.
Valid versus neutral cue condition accuracy for individual observers in Experiment 2. The farther above the diagonal, the greater the attention benefit.
Microsaccades
The average frequency of MS throughout the trial per block did not significantly differ between attention conditions (valid: M=45.6±4.45, neutral: M=43.6±4.35; p>.1) or between groups (ADHD: M=73.5±9.8, NT: M=105±13.6; p>.07). MS in all conditions followed the main sequence; the higher the amplitude, the faster the speed (column 1, Fig 8). Further, their kinematics (i.e. peak velocity, amplitude and duration) did not differ as a function of attention condition or group (all ps>.1; columns 2–4, Fig 8).
Fig 8.
MS Kinematics for both groups in Experiment 2: Exogenous attention for the a) neutral cue condition and b) valid cue condition. MS follow the main sequence in all conditions (column 1). Distributions of MS velocity (column 2), amplitude (column 3), and duration (column 4) in terms of average frequency per block for each group. Due to a hardware failure, the data for 13 of 14 observers per group is shown.
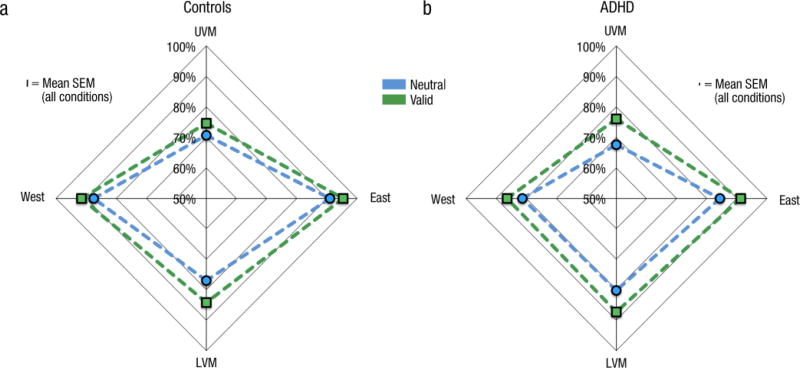
This experiment revealed that the benefit of inflexible and involuntary exogenous attentional orienting remains functionally intact in adults with ADHD. Moreover, this experiment showed for the first time that adults with ADHD possess canonical performance fields (Fig 9; see Supplementary Information): task performance in both groups was better (to an equal extent) at both locations along the horizontal meridian than along the vertical meridian. Both groups were also significantly better at the LVM than UVM. Furthermore, the benefit of exogenous attention was similar across locations thus preserving the shape of the performance fields. These findings are consistent with those of NT adults (Carrasco, Talgar & Cameron, 2001; Cameron et al., 2002; Carrasco, Giordano & McElree, 2004; Abrams et al., 2012).
Fig 9.
Performance fields for Experiment 2. Accuracy in the valid and neutral cue conditions plotted as a function of target location for the (a) NT controls and (b) adults with ADHD.
GENERAL DISCUSSION
This study shows that covert orienting and reorienting of selective attention–as assessed by our basic task and stimuli–is spared in adults with mild-to-moderate ADHD. This is the first study to investigate the perceptual effects of both endogenous (Experiment 1) and exogenous (Experiment 2) covert attention in a group of adults with mild-to-moderate ADHD and their age- and gender-matched NT controls. We employed a spatial cueing task that is well established in NT observers (Carrasco, 2011), and has been used to assess selective visual attention in other special populations, i.e., autism spectrum disorder (Grubb, Behrmann, Egan, Minshew, Carrasco & Heeger, 2013; Grubb, Behrmann, Egan, Minshew, Heeger, & Carrasco, 2013) and individuals with amblyopia (Roberts et al., 2016). The adults with ADHD demonstrated significant and similarly sized benefits of endogenous and exogenous attention to those shown by NT observers. Moreover, in Experiment 1, the cost of deploying endogenous attention to the incorrect target was the same in both groups. A complementary Bayes factor analysis verified that the nonsignificant group × cue interaction was not due to a lack of statistical power. A recent review of 34 meta-analyses reported that observers with ADHD performed worse than healthy controls in 96% of the sampled neurocognitive tasks (Pievsky & McGrath, 2017). When weighted by the number of aggregated studies in each meta-analysis, the standardized mean difference (SMD) was .56, a medium-sized effect according to typical benchmarks (Cohen, 1988). Importantly, the mean SMD of the 84 studies with 50 or few observers was not meaningfully affected by a smaller sample size.
Given reports of substantial differences between ADHD and NT groups on a diverse array of neuropsychological tasks, including some purported to tax “sustained” and “focused” attention, in studies with similar sample sizes to ours (reviews, Frazier et al., 2004; Schoechlin & Engel, 2005), and the results of our Bayesian analyses, we feel confident that our study was powerful enough to detect significant differences between groups, had there been any. In both experiments, we ruled out speed-accuracy tradeoffs, and the RT benefits and costs were similar for both groups. Moreover, the overall endogenous RT effect increased with age for both groups. In contrast to reports of greater intraindividual variability in ADHD than controls (Kofler et al., 2013), RT variability did not differ for the two groups. This likely reflects our emphasis on accuracy and the timing of the response window. In summary, voluntary orienting and reorienting, as well as involuntary orienting of covert, selective visual attention remain functionally intact in adults with ADHD.
Some studies had suggested that orienting and reorienting may be functionally spared in ADHD, but suboptimal methodology limits their interpretation. Three studies on covert attention employed adaptations of the classic Posner spatial cueing task (Posner, 1980), in which an observer must detect a peripheral stimulus as fast as possible while their attention is voluntarily or involuntarily drawn to one hemifield via presentation of a spatial cue (Tomporowski et al., 1994; Epstein et al., 1997; Epstein et al., 2001). These studies employed reaction time (RT) as their primary dependent measure; however, RT differences may reflect criterion shifts (Wickelgren, 1977; Carrasco & McElree, 2001), which are more likely in detection than discrimination tasks, and differences in processing speed or sensitivity. Therefore, the reported cueing effects could be attributed to criterion differences between attention conditions rather than to perceptual enhancements. Moreover, the long stimulus-onset-asynchronies used (>200 ms) allowed for eye movements, thus potentially confounding the effects attributed to covert attention. Lastly, peripheral cues have been used to study involuntary attention with a cue-to-stimulus asynchrony (Oberlin et al., 2005) past its maximal effect. Voluntary and involuntary orienting peak by around 300 and 120 ms after cue onset, respectively (Müller & Rabbitt, 1989; Nakayama & Mackeben, 1989; Liu et al., 2007; review by Carrasco, 2011). In this study, we overcame all of these methodological concerns.
Only a few studies have directly investigated eye movements in adults with ADHD (e.g., Gooding & Basso, 2008; Fried et al., 2014; Dankner et al., 2017). Some studies have shown that when instructed to move their eyes, ADHD do as well as NT. However, they show some deficits of control with delayed saccade and antisaccade tasks (Gooding & Basso, 2008). In this study, the frequency of saccades did not differ between the groups; this may not be surprising given the relatively fast temporal demands of our task. Recent studies employing a long sustained attention task found differences in MS rate between ADHD and NT (Fried et al., 2014; Danker et al., 2017). We also analyzed observers’ MS to investigate whether these oculomotor correlates of perception would parallel our behavioral results. We found no differences in MS frequency or kinematics between the group of adults with ADHD and NT.
Experiment 2 revealed that in both the neutral and attention conditions, adults with ADHD exhibit the same canonical performance fields as the control group and other NT observers. These novel results indicate that the perceptual sensitivity of ADHD adults as well as the extent and the distribution of exogenous attention are consistent with those of the general population (Carrasco, Penpeci-Talgar & Cameron, 2001; Cameron et al., 2002; Carrasco, Giordano & McElree., 2004; Abrams et al., 2012). In line with findings of preserved perceptual abilities (Kim et al., 2014a) and exogenous attention on color appearance (Kim et al., 2014b), the present study rules out an early perceptual or attentional deficit as a contributing factor to explain the diverse symptomology of the disorder.
Given documented differences in sustained attention and temporal expectation (Hervey et al., 2004; Marchetta et al., 2007; Fried et al., 2014; Dankner et al., 2017; Mueller et al., 2017), as well as literature documenting significant impairments in a diverse set of neurocognitive tasks (reviews: Mueller et al., 2017; Pievsky & McGrath, 2017), it is conceivable that differences between groups could emerge with selective attention using harder tasks and/or with more distractors or with an ADHD group with more severe symptomology. Our goal was to isolate the effects of selective attention in ADHD without taxing executive function, and thus these possibilities were outside the scope this study.
A main feature of ADHD, and partly why its diagnosis remains controversial despite decades of research, is that it is heterogeneous (Castellanos & Tannock, 2002; Mueller et al., 2017). Patients diagnosed with the same ADHD label under the current DSM-5 (APA, 2013) likely suffer from distinct disorders (Milich et al., 2001) with unique severity of symptomology, etiologies and biological bases; however, there is not a consensus as to the ecological validity of proposed subdivisions (Castellanos & Tannock, 2002). Nevertheless, potential individual differences in selective attention according to subtype and/or severity are open and interesting research questions. The observers for which we had severity scores would mainly be classified as exhibiting mild-to-moderate symptomology (Experiment 2). We cannot report the severity makeup of our ADHD group in Experiment 1 because we could only obtain severity scores for some observers.
We are agnostic regarding whether the underlying neural mechanisms or substrates of attention are the same in adults with ADHD. In fact, substantial anatomical, neuroimaging, and neurophysiological evidence suggest that they are not (Cortese et al., 2012; Mueller et al., 2017). Structurally, studies have found global reductions in gray matter, local gray matter reductions of the prefrontal cortex, anterior cingulate cortex (Seidman et al., 2006) and bilateral early visual areas (Ahrendts et al., 2011), and differences in white matter microarchitecture (Yoncheva et al., 2016). Further, they exhibit abnormal brain activity and disrupted functional connectivity (Konrad & Eickhoff, 2010) between several areas implicated in attentional processing (Cortese et al., 2012). For example, studies have found hypoactivation in dorsolateral and ventral prefrontal cortex, anterior cingulate cortex, and the basal ganglia, as well as hyperactivation in posterior regions of parietal and occipital cortex (Cortese et al., 2012). Further research is needed to link the evidence of difference in brain structure with behavioral differences and similarities between adults with ADHD and NTs.
CONCLUSIONS
The current diagnostic criteria of adult ADHD rely on a combination of both cognitive and neurobehavioral symptoms and are often assessed using self-report questionnaires and clinical interview, which can be incomplete, unreliable, and vulnerable to biases. Thus, discovery and clinical implementation of more objective psychometric measures of attentional processes would be valuable. The present study indicates that the perceptual effects of endogenous and exogenous attention are intact in adults with mild-to-moderate ADHD–they improve perception across the visual field. The basic psychophysical attention task we used, if corroborated with larger samples including observers with more pronounced ADHD symptoms, and tested with other clinical groups, could have translational potential; if incorporated into the clinical diagnostic battery of tests, together with others in which executive function is taxed (e.g., working memory), it could help in the differential diagnosis of ADHD and other conditions–e.g. depression (Paelecke-Habermann et al., 2005; Hammar & Ardal, 2009), schizophrenia (Wang et al., 2005) and anxiety (Pacheco-Unguetti et al., 2010)–in which selective attention, although not always optimally manipulated, has been reported to be compromised.
Supplementary Material
Acknowledgments
Funding was provided by the National Institutes of Health National Eye Institute grant RO1 EY016200 to MC and the National Science Foundation Graduate Research Fellowship Program grant DGE1342536 to MR. Thanks to Dr. Yuliya Yoncheva for assisting with verifying ADHD diagnoses and members of the Carrasco lab for helpful comments on the manuscript.
Footnotes
Except for one 53 yo observer, for whom the stimuli were displayed for 180 ms so that she could perform the task above chance.
Contributions. MR, BA, and MC designed the study. MR and BA recruited observers, conducted data collection and analyses. FXC verified ADHD diagnoses. MR and MC wrote the manuscript; BA and FXC provided feedback.
References
- Abrams J, Nizam A, Carrasco M. Isoeccentric locations are not equivalent: the extent of the vertical meridian asymmetry. Vision Research. 2012;52(1):70–78. doi: 10.1016/j.visres.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L, Spencer T. The adult ADHD clinical diagnostic scale (ACDS) V 1.2. New York University School of Medicine; New York: 2004. [Google Scholar]
- Ahrendts J, Rüsch N, Wilke M, Philipsen A, Eickhoff SB, Glauche V, Tebartz van Elst L. Visual cortex abnormalities in adults with ADHD: a structural MRI study. The World Journal of Biological Psychiatry. 2011;12(4):260–270. doi: 10.3109/15622975.2010.518624. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: Author; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text rev. 4. Washington, DC: Author; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, D.C.: Author; 2013. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. ADHD in adults: History, diagnosis, and impairments. [Retrieved June, 2017];ContinuingEdCourses. 2007 from http://www.continuingedcourses.net/active/courses/course034.php.
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42(8):949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: the past 25 years. Vision Research. 2011;51(13):1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM, McElree B. Temporal performance fields: visual and attentional factors. Vision Research. 2004;44(12):1351–1365. doi: 10.1016/j.visres.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences. 2001;98(9):5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Cameron EL. Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision. 2001;15:61–75. doi: 10.1163/15685680152692015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Talgar CP, Cameron EL. Characterizing visual performance fields: effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision. 2001;15(1):61–75. doi: 10.1163/15685680152692015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Yeshurun Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. Journal of Experimental Psychology: Human Perception & Performance. 1998;24(2):673–692. doi: 10.1037//0096-1523.24.2.673. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Conners CK, Erhardt D, Sparrow EP. Conners' adult ADHD rating scales (CAARS): technical manual. Toronto: Multi-Health Systems; 1999. [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. The American Journal of Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner Y, Shalev L, Carrasco M, Yuval-Greenberg S. Prestimulus inhibition of saccades in adults with and without Attention-Deficit/Hyperactivity Disorder as an index of temporal expectations. Psychological Science. 2017 doi: 10.1177/0956797617694863. 956797617694863. [DOI] [PubMed] [Google Scholar]
- Dhar M, Been PH, Minderaa RB, Althaus M. Distinct information processing characteristics in dyslexia and ADHD during a covert orienting task: An event-related potential study. Clinical Neurophysiology. 2008;119(9):2011–2025. doi: 10.1016/j.clinph.2008.05.027. [DOI] [PubMed] [Google Scholar]
- Dugué L, Roberts M, Carrasco M. Attention reorients periodically. Current Biology. 2016;26(12):1595–1601. doi: 10.1016/j.cub.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Erhardt D, March JS, Swanson JM. Asymmetrical hemispheric control of visual-spatial attention in adults with attention deficit hyperactivity disorder. Neuropsychology. 1997;11(4):467. doi: 10.1037/0894-4105.11.4.467. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Johnson DE, Varia IM, Conners CK. Neuropsychological assessment of response inhibition in adults with ADHD. Journal of Clinical and Experimental Neuropsychology. 2001;23(3):362–371. doi: 10.1076/jcen.23.3.362.1186. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/NP) New York State Psychiatric Institute, New York: Biometrics Research; 2002. [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Fried M, Tsitsiashvili E, Bonneh YS, Sterkin A, Wygananski-Jaffe T, Epstein T, Polat U. ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vision Research. 2014;101:62–72. doi: 10.1016/j.visres.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Giordano AM, McElree B, Carrasco M. On the automaticity and flexibility of covert attention: a speed-accuracy trade-off analysis. Journal of Vision. 2009;9(3:30):1–10. doi: 10.1167/9.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroid E, Wiersema JR. Impaired processing of task-irrelevant salient information in adults with attention-deficit/hyperactivity disorder: Evidence from event-related potentials. Journal of Abnormal Psychology. 2017;126(1):52. doi: 10.1037/abn0000221. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain & Cognition. 2008;68(3):371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Carrasco M, Heeger DJ. Endogenous spatial attention: evidence for intact functioning in adults with autism. Autism Research. 2013;6(2):108–118. doi: 10.1002/aur.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Heeger DJ, Carrasco M. Exogenous spatial attention: evidence for intact functioning in adults with autism spectrum disorder. Journal of Vision. 2013;13(14) doi: 10.1167/13.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar Å, Årdal G. Cognitive Functioning in Major Depression – A Summary. Frontiers in Human Neuroscience. 2009;3:26. doi: 10.3389/neuro.09.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: the case of visuospatial orienting. Clinical Psychology Review. 2003;23(6):801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller RA, Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience & Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Al-Haj M, Chen S, Fuller S, Jain U, Carrasco M, Tannock R. Colour vision in ADHD: Part 1-Testing the retinal dopaminergic hypothesis. Behavioral and Brain Functions. 2014;10(1):1. doi: 10.1186/1744-9081-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Al-Haj M, Fuller S, Chen S, Jain U, Carrasco M, Tannock R. Color vision in ADHD: Part 2-Does attention influence color perception? Behavioral and Brain Functions. 2014;10(1):1. doi: 10.1186/1744-9081-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Heeger DJ, Carrasco M. Neural correlates of the visual vertical meridian asymmetry. Journal of Vision. 2006;6(11):1294–1306. doi: 10.1167/6.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Stevens ST, Carrasco M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Research. 2007;47(1):108–113. doi: 10.1016/j.visres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Marchetta ND, Hurks PP, De Sonneville LM, Krabbendam L, Jolles J. Sustained and focused attention deficits in adult ADHD. Journal of Attention Disorders. 2007;11(6):664–676. doi: 10.1177/1087054707305108. [DOI] [PubMed] [Google Scholar]
- Marzinzik F, Wahl M, Kruger D, Gentschow L, Colla M, Klostermann F. Abnormal distracter processing in adults with attention-deficit-hyperactivity disorder. PLoS One. 2012;7(3):e33691. doi: 10.1371/journal.pone.0033691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson ME. A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behavior Research Methods. 2011;43(3):679–690. doi: 10.3758/s13428-010-0049-5. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8(4):463–488. [Google Scholar]
- Mueller A, Hong DS, Shepard S, Moore T. Linking ADHD to the neural circuitry of attention. Trends in Cognitive Science. 2017;21(6):474–488. doi: 10.1016/j.tics.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15(2):315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29(11):1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Alford JL, Marrocco RT. Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behavioural Brain Research. 2005;165(1):1–11. doi: 10.1016/j.bbr.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Ortega R, López V, Carrasco X, Anllo-Vento L, Aboitiz F. Exogenous orienting of visual-spatial attention in ADHD children. Brain Research. 2013;1493:68–79. doi: 10.1016/j.brainres.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science. 2010;21(2):298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89(1):125–135. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Pievsky MA, McGrath RE. The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses. Archives of Clinical Neuropsychology. 2017:1–15. doi: 10.1093/arclin/acx055. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. The Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner M. Orienting of attention: Then and now. The Quarterly Journal of Experimental Psychology (Hove) 2014;30:1–12. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Roberts M, Cymerman R, Smith RT, Kiorpes L, Carrasco M. Covert spatial attention is functionally intact in amblyopic human adults. Journal of Vision. 2016;16(15):1–19. doi: 10.1167/16.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychonomic Bulletin & Review. 2009;16(2):225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- Rucci M, Poletti M. Control and functions of fixational eye movements. Annual Review of Vision Science. 2015;1:499–518. doi: 10.1146/annurev-vision-082114-035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoechlin C, Engel RR. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Archives Clinical Neuropsychology. 2005;20(6):727–744. doi: 10.1016/j.acn.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Aleardi M. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60(10):1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Tinsley V, Hager LD. Visuospatial attentional shifts and choice responses of adults and ADHD and non-ADHD children. Perceptual and Motor Skills. 1994;79(3 suppl):1479–1490. doi: 10.2466/pms.1994.79.3f.1479. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Shalev L, Mevorach C. The diversity of attention deficits in ADHD: the prevalence of four cognitive factors in ADHD versus controls. Journal of Learning Disabilities. 2005;38(2):142–157. doi: 10.1177/00222194050380020401. [DOI] [PubMed] [Google Scholar]
- Tucha L, Tucha O, Laufkötter R, Walitza S, Klein H, Lange K. Neuropsychological assessment of attention in adults with different subtypes of attention-deficit/hyperactivity disorder. Journal of Neural Transmission. 2008;115(2):269–278. doi: 10.1007/s00702-007-0836-z. [DOI] [PubMed] [Google Scholar]
- Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophrenia Research. 2005;78(2):235–241. doi: 10.1016/j.schres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- White A, Rolfs M, Carrasco M. Stimulus competition mediates the joint effects of spatial and feature-based attention. Journal of Vision. 2015;15(14):7, 1–21. doi: 10.1167/15.14.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickelgren WA. Speed-accuracy tradeoff and information processing dynamics. Acta Psychologica. 1977;41(1):67–85. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yoncheva YN, Somandepalli K, Reiss PT, Kelly C, Di Martino A, Lazar M, Castellanos FX. Mode of anisotropy reveals global diffusion alterations in Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(2):137–145. doi: 10.1016/j.jaac.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.