Abstract
BACKGROUND
Hormone receptor positive breast cancer is the most common subtype; better tools to identify which patients in this group would derive clear benefit from chemotherapy are needed.
PURPOSE
To evaluate the prognostic potential of diffusion-weighted MRI (DWI) by investigating associations with pathologic biomarkers and a genomic assay for 10-year recurrence risk.
STUDY TYPE
Retrospective.
SUBJECTS
107 consecutive patients (from 2/2010 to 1/2013) with ER positive/HER2neu negative invasive breast cancer who had the 21-gene recurrence score (RS) test (Oncotype DX, GenomicHealth, Inc).
FIELD STRENGTH/SEQUENCE
Each subject underwent pre-surgical 3T breast MRI, which included DWI (b=0, 800 s/mm2).
ASSESSMENT
Apparent diffusion coefficient (ADC) and contrast-to-noise ratio (CNR) were measured for each lesion by a fifth year radiology resident. Pathological markers (Nottingham histologic grade, Ki-67, RS) were determined from pathology reports. Medical records were reviewed to assess recurrence-free survival.
STATISTICAL TESTS
RS was stratified into low (<18), moderate (18–30), and high (>30) risk groups. Associations of DWI characteristics with pathologic biomarkers were evaluated by binary or ordinal logistic regression as appropriate, with adjustment for multiple comparisons. Post-hoc comparisons between specific groups were also performed.
RESULTS
ADCmean (OR=0.61 per 1-SD increase, adj. p=0.044) and CNR (OR=1.76 per 1-SD increase, adj. p=0.026) were significantly associated with increasing tumor grade. DWI CNR was also significantly associated with a high (Ki-67≥14%) proliferation rate (OR=2.55 per 1-SD increase, adj. p=0.026). While there were no statistically significant linear associations in ADC (adj. p = 0.80–0.85) and CNR (adj. p=0.56) across all three RS groups by ordinal logistic regression, post-hoc analyses suggested that high RS lesions exhibited lower ADCmean (p=0.037) and ADCmax (p=0.004) values and higher CNR (p=0.008) compared to lesions with a low or moderate RS.
DATA CONCLUSION
DWI characteristics correlated with tumor grade, proliferation index and RS, and may potentially help to identify those with highest recurrence risk and most potential benefit from chemotherapy.
Keywords: diffusion-weighted imaging (DWI), breast cancer, Oncotype DX 21-gene recurrence score, adjuvant chemotherapy, prognostic biomarker
INTRODUCTION
Management of breast cancer is highly individualized and routinely based upon disease burden (stage), standard pathologic features, and tumor molecular expression signatures. In particular, the decision to administer chemotherapy prior to starting prolonged endocrine therapy for small tumors that express the estrogen and/or progesterone receptor (ER/PR) but do not overexpress the HER2 receptor is challenging. Although there is evidence for chemotherapy benefit for women diagnosed with this heterogeneous group of tumors with a low mean recurrence rate (1), the decision to recommend chemotherapy in addition to hormone therapy must be balanced with serious side effects (2). Identification of specific patients in this group who are more likely to recur and/or who would derive clear benefit from chemotherapy is therefore of interest.
Several multigene expression assays for early stage breast cancer have been developed to provide a more detailed molecular portrait for accurate prognosis. One widely used assay is the 21-gene Recurrence Score (RS) (Oncotype DX, Genomic Health, Inc). RS prognosticates 10-year recurrence risk (3) in hormone receptor positive, HER2 negative breast cancer. Moreover, RS also predicts likelihood of benefit from adjuvant chemotherapy as measured by absolute decrease in 10-year distant recurrence rate in both node negative and positive patients (4,5) and predicts the likelihood of pathologic complete response to neoadjuvant treatment (6). Because of its usefulness in guiding treatment decisions, RS is now incorporated into both American Society of Clinical Oncology (ASCO) (7) and the National Comprehensive Cancer Network (NCCN) (8) treatment guidelines, which specifically recommends the test for hormone receptor positive, HER2 negative, node negative tumors, with ongoing larger trials such as SWOG-S1007/RxPonder (9) potentially extending current recommendations to node-positive patients in the near future. In clinical settings, RS significantly influences recommendation for adjuvant chemotherapy (10), potentially reducing unnecessary chemotherapy use (11).
Despite the described benefits, use of RS is currently limited due to its expense (approximately $4,000). Consequently, there is room for development of alternative markers that may provide prognostic value for less cost or supplemental markers to identify individuals whose care would warrant the RS test. Imaging markers obtained from dynamic contrast enhanced (DCE) MRI, which is already often obtained for preoperative evaluation, may address this need. The literature suggests that tumor morphology and kinetic features on DCE-MRI correlate with RS and may be predictive of disease recurrence (12–16). In addition to routine DCE-MRI, diffusion-weighted imaging (DWI), a fast and non-contrast enhanced technique increasingly incorporated into routine breast MRI protocols, may be of supplemental benefit (17). While DCE-MRI provides information about tumor vascularity, DWI reflects water mobility in tissue, characterizing tumor cellularity. Recent studies have shown DWI measurements to correlate with prognostic markers such as Nottingham grade (18,19), Ki-67 score (20–23), and expression of HER2 (22,24) and hormonal receptors (21,24) in invasive breast cancer. However, few studies specifically evaluate ER positive cancers (25), and apparently, only one published study to date has specifically investigated DWI as a potential predictor of RS (26). Thus the purpose of this study was to confirm associations of DWI characteristics with Nottingham grade and Ki-67 score for the specific subgroup of ER positive, HER2-negative invasive breast cancers, and to further explore correlations between DWI characteristics and RS.
MATERIALS and METHODS
Our study was approved by our Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. Requirements for informed consent were waived due to the retrospective nature of the study.
Subjects and Lesions
Our study cohort was obtained by querying our cancer consortium clinical data repository, which compiles data from a variety of sources that include our institutional pathology database, clinical MRI database, and the regional tumor registry. We identified women at least 18 years old who were diagnosed with invasive breast cancer and subsequently underwent both pre-surgical breast MR imaging and central RS testing of their tumors from February 1, 2010 to January 31, 2013. For this study, all subjects were required to have undergone the standard breast MRI examination at our institution, which was performed on a 3 tesla (T) scanner and included DWI. From this database inquiry, we identified 111 consecutive women with biopsy-proven hormone receptor positive, HER2 negative invasive breast cancer meeting study inclusion criteria.
Four subjects were excluded from the study: three with obscuration of the breast lesion on MRI due to post-biopsy changes (large hematoma n=1, seroma n=1, and susceptibility artifact from biopsy clip n=1); one for poor lesion visibility on DWI (a small 6mm non-mass enhancement), making the ADC measurements unreliable. Thus, the final cohort included 107 women (median age 56, range 32–75 years) with hormone receptor positive (ER and/or PR), HER2 negative invasive cancer.
Pathologic Assessment
All biopsy specimens pathologically assessed at our institution included verification of invasive cancer, tumor grade (by Nottingham histologic score reflecting tumor cell differentiation based on tubule formation, nuclear grade, and mitotic rate), ER and PR status (by Allred score for expression, where score ≥3 is positive), HER2 status (positive or negative by immunohistochemistry and/or fluorescence in situ hybridization), and Ki-67 proliferation index. Twenty-five tissue samples that were pathologically assessed at outside institutions did not include assessment of Ki-67. A previously proposed prognostic Ki-67 cut point of 14% (27,28) was used to differentiate tumors with low (<14%) and high proliferation rates. RS were stratified into pre-specified risk groups of low (<18), moderate (18–30), and high (>30), which have been validated in retrospective studies (3,4).
Long Term Follow-Up
Medical records were reviewed on July 19, 2017 to identify patients who had breast cancer recurrence and determine their length of recurrence free survival (RFS). Recurrence was defined as any new breast cancer-related diagnosis (local or distant metastasis) after completion of treatment. Recurrence-free survival was defined as the time from definitive (final) surgical treatment to the date of pathologic diagnosis of cancer recurrence or time of last clinical visit in non-recurring patients. A total of 4 recurrences were identified. For each patient who recurred, medical records were reviewed to extract additional associated patient characteristics including genetic mutation, surgical, radiation, and medical treatment received, surgical margins, and site(s) of metastasis. The mean ADC and CNR and RS of tumors that recurred were also noted for comparison with those of the study population.
MRI Acquisition
All patients underwent breast MRI after core biopsy, with a median time between biopsy and MRI of 15 days (range 4 to 63 days). Breast MR examinations were performed on a Philips Achieva Tx 3T scanner using a dedicated bilateral 16-channel breast coil (MammoTrak, Philips Healthcare). The MRI sequences were acquired in the axial orientation and included T2-weighted fast spin echo, T1-weighted non-fat-suppressed, T1-weighted fat-suppressed DCE-MRI, and DWI.
DCE-MRI included one pre- and three postcontrast T1-weighted fat suppressed three-dimensional fast gradient echo (eTHRIVE) sequences, acquired with parallel imaging technique (SENSitivity Encoding; SENSE). The following imaging parameters were utilized: repetition time/echo time: 5.96 ms/3.09 ms, flip angle: 10°, matrix size: 440×660, field of view: 22×33 cm, number of slices: 280, slice thickness: 1.3 mm, in plane voxel size: 0.5 mm. The contrast agent administered was 0.1 mmol/kg-body weight gadoteridol (ProHance, Bracco Diagnostics) delivered at 2 cc/s followed by a 20 mL saline flush. DCE-MRI acquisition time was 2 minutes 57 seconds per sequence, with center k-space acquired at 2 minutes; total post-contrast scan time was 8 minutes 51 seconds.
DWI was performed immediately following the DCE-MRI acquisition (typically 9 minutes post-contrast injection), and was acquired using a diffusion-weighted echo-planar imaging sequence with parallel imaging and fat suppression (Spectral Attenuated Inversion Recovery; SPAIR). The following imaging parameters were utilized: repetition time/echo time: 5336 ms/61 ms; reduction factor: 3; averages: 2; matrix size: 240×240; field of view: 36×36 cm; number of slices: 30; slice thickness: 5 mm; gap: 0. Diffusion gradients were applied in six directions with b values of 0 and 800 s/mm2. Acquisition time was 3 minutes 28 seconds.
Image Analysis
DCE-MRI scans were clinically interpreted by one of four fellowship-trained radiologists specializing in breast imaging. Lesion characteristics including lesion type (mass, non-mass enhancement, focus), size, location, and BI-RADS assessment (29) were recorded at the time of interpretation. This information was entered into our clinical database along with detailed histopathology for each lesion and later extracted for the purposes of this study.
DWI scans were retrospectively evaluated by a radiology resident (V.N.) under the direct guidance and supervision of a fellowship-trained radiologist (H.R.) specializing in breast imaging with over 5 years of breast MRI experience. DWI was analyzed with custom software developed in Java language and incorporating open source image analysis tools (ImageJ, National Institutes of Health), as previously described (30). ADC maps were calculated based on a standard monoexponential model
| (1) |
in units mm2/s, where b is the maximum b-value (800 s/mm2), S0 is the signal intensity at b = 0 s/mm2, and SD is the diffusion weighted signal intensity at b = 800 s/mm2.
The approaches for measuring ADC for tumor and normal tissue regions have been described in detail previously (31,32), and are briefly summarized here. Lesion locations were identified from clinical radiology reports and DCE-MR images. Regions of interest (ROIs) were defined on the b = 800 s/mm2 diffusion-weighted images for the lesion and normal appearing breast tissue in the contralateral breast, which were propagated to ADC maps. T2-weighted images were referenced in order to avoid areas of cyst and necrosis. For lesions, the analysis software further enabled thresholding (if necessary) to exclude voxels within the ROI with very low signal on b=800 s/mm2 image, representing intervening fibroglandular, cystic and/or adipose tissue, important for restricting measures to only viable solid tumor voxels and especially useful for measuring small and non-mass lesions (31). Mean, standard deviation, maximum, and minimum ADC values (ADCmean, ADCstdev, ADCmax, and ADCmin, respectively) and mean DWI signal intensity (on b=800 s/mm2 images) were calculated for non-excluded lesion ROI voxels. To measure normal tissue, an ROI was defined in the contralateral breast at the same slice level as the lesion (if possible) including the entire breast fibroglandular tissue area. Thresholding was used as needed to exclude voxels within the ROI with very low signal on the b=0 s/mm2 image, representing intervening fat, as previously described (31,32). Mean ADC and mean DWI signal intensity (on b=800 s/mm2 images) were calculated for non-excluded voxels in the normal tissue ROI.
Finally, the contrast-to-noise ratio (CNR) of lesion-to-normal tissue was calculated as previously described (33):
| (2) |
where μlesion and μtissue are the mean DWI signal intensities (b = 800 s/mm2) for lesion and normal tissue ROIs, respectively, and σlesion and σtissue are the corresponding standard deviations. CNR > 0 indicates higher signal intensity in the lesion versus normal tissue.
Statistical Analysis
For the primary analysis, associations between lesion DWI measurements (ADCmean; ADCstdev; ADCmax; ADCmin; CNR) and pathologic biomarkers (Nottingham grade; Ki-67; RS risk group) were analyzed using binary and ordinal logistic regression as appropriate, with p-value adjustment for multiple comparisons using Benjamini and Hochberg’s procedure to control the false discovery rate (FDR) at ≤ 5% (34). Additional logistic regression analyses were performed without adjustment for multiple comparisons. These included evaluating associations between lesion morphology on imaging (type: mass vs. NME; maximum diameter) and pathologic biomarkers, post-hoc comparisons of DWI measurements between specific groups, post-hoc multivariate analyses, and pairwise correlation analysis of DWI measurements using Spearman’s rank correlation. All DWI measurements were standardized by their mean and standard deviation (SD) so that each logistic regression odds ratio (OR) represents the change per 1-SD increase in the measurement. All analyses were conducted with the statistical computing language R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-sided tests were used with statistical significance defined as p<0.05.
RESULTS
Pathologic characteristics of 107 hormone receptor positive (ER and/or PR), HER2 negative invasive cancer included in the final assessment are summarized in Table 1. Lesions included 93 (87%) invasive ductal carcinomas, 13 (12%) invasive lobular carcinomas, and 1 (1%) mixed lobular and ductal histologies. Lesions were predominantly Stage I and II (35), with 83/107 (77.6%) patients node-negative. The median RS was 19 (range: 0–36), including 50 (47%) low RS, 51 (48%) moderate RS, and 6 (5%) high RS. By most recent follow-up, 103 of the 107 (96%) study patients remained breast cancer free (median follow-up time 59 months, range 0 to 84 months) and four experienced breast cancer recurrence (median RFS 25 months, range 14 to 83 months).
Table 1.
Lesion Characteristics (n=107).
| N (%) | ||
|---|---|---|
| Histology | Invasive ductal carcinoma | 93 (87.0) |
| Invasive lobular carcinoma | 13 (12.0) | |
| Mixed ductal and lobular | 1 (1.0) | |
| AJCC Stage | IA | 60 (56.1%) |
| IB | 3 (2.8%) | |
| IIA | 25 (24.3%) | |
| IIB | 13 (12.1%) | |
| IIIA | 5 (4.7%) | |
| Nottingham tumor grade | 1 | 31 (29.0) |
| 2 | 53 (49.5) | |
| 3 | 23 (21.5) | |
| Ki-67† | <14% | 44 (53.7) |
| ≥14% | 38 (46.3) | |
| 21-Gene Recurrence Score | Low (<18) | 50 (46.7) |
| Moderate (18 – 30) | 51 (47.7) | |
| High (>30) | 6 (5.6) | |
| Progesterone receptor | Positive | 98 (91.6) |
| Negative | 9 (8.4) | |
| Follow-up Status | Recurrence | 4 (3.7%) |
| No Recurrence (< 5 years F/U) | 52 (48.6%) | |
| No Recurrence (≥ 5 years F/U) | 51 (47.7%) |
Abbreviations: AJCC = American Joint Committee on Cancer 7th edition, NME = non-mass enhancement; ADC = apparent diffusion coefficient; DWI = diffusion weighted imaging; CNR = contrast-to-noise ratio; F/U= follow-up
25 Lesions missing Ki-67 results were excluded; Ki-67 was not consistently collected on patients referred from outside institutions
On DCE-MRI, 96/107 (90%) lesions were masses, and maximum diameters ranged from 7 to 130 mm (median = 19 mm). On DWI, ADCmean ranged from 0.46 to 1.90 (median = 1.09) ×10−3 mm2/s, ADCstdev ranged from 0.09 to 0.58 (median = 0.23) ×10−3 mm2/s, ADCmax ranged from 0.70 to 3.12 (median = 1.77) ×10−3 mm2/s, ADCmin ranged from 0.12 to 1.51 (median = 0.68) ×10−3 mm2/s, and CNR ranged from −1.2 to 6.3 (median = 2.4). Summary of imaging characteristics stratified by pathologic biomarker subgroups are given in Table 2. No significant associations of morphologic type and size on MRI with pathologic biomarkers of Nottingham grade, Ki-67 score, and RS were detected (p = 0.32 – 0.91 for lesion type and p = 0.18 – 0.77 for size).
Table 2.
Summary of imaging features stratified by pathologic subgroups (n=107 lesions).
| Nottingham Grade* | Ki-67 Status* | 21-Gene Recurrence Score* | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Variable | Grade 1 (N=31) |
Grade 2 (N=53) |
Grade 3 (N=23) |
<14% (N=44) |
≥14% (N=38) |
Low (N=50) |
Moderate (N=51) |
High (N=6) |
|
| Lesion type mass (vs. NME) | 27 (87.1) | 49 (92.5) | 20 (87.0) | 40 (90.9) | 32 (84.2) | 47 (94.0) | 43 (84.3) | 6 (100.0) | |
| Max diameter (cm) | 17 (12 – 22) | 20 (15 – 28) | 20 (16 – 26) | 18 (12 – 26) | 18 (15 – 24) | 21 (16 – 28) | 17 (14 – 24) | 16 (12 – 19) | |
| ADC (×10−3 mm2/s) | |||||||||
| Mean | 1.24 ± 0.31 | 1.16 ± 0.28 | 1.03 ± 0.26 | 1.20 ± 0.30 | 1.15 ± 0.27 | 1.15 ± 0.27 | 1.18 ± 0.30 | 0.91 ± 0.32 | |
| SD | 0.30 ± 0.12 | 0.25 ± 0.11 | 0.25 ± 0.13 | 0.27 ± 0.12 | 0.28 ± 0.13 | 0.26 ± 0.12 | 0.28 ± 0.12 | 0.18 ± 0.05 | |
| Min | 0.70 ± 0.24 | 0.71 ± 0.27 | 0.56 ± 0.23 | 0.69 ± 0.24 | 0.66 ± 0.22 | 0.66 ± 0.23 | 0.70 ± 0.28 | 0.59 ± 0.34 | |
| Max | 1.98 ± 0.51 | 1.80 ± 0.43 | 1.70 ± 0.52 | 1.87 ± 0.49 | 1.86 ± 0.48 | 1.82 ± 0.47 | 1.90 ± 0.47 | 1.28 ± 0.34 | |
| CNR | 2.0 ± 1.3 | 2.4 ± 1.2 | 3.1 ± 1.3 | 2.0 ± 1.2 | 2.9 ± 1.1 | 2.4 ± 1.2 | 2.3 ± 1.3 | 3.9 ± 1.1 | |
Abbreviations: NME = non-mass enhancement; ADC = apparent diffusion coefficient; DWI = diffusion weighted imaging; CNR = contrast-to-noise ratio; SD = standard deviation.
Values are no. (%), median (inter-quartile range), or mean ± SD.
Correlation of DWI Characteristics with Prognostic Pathologic Factors
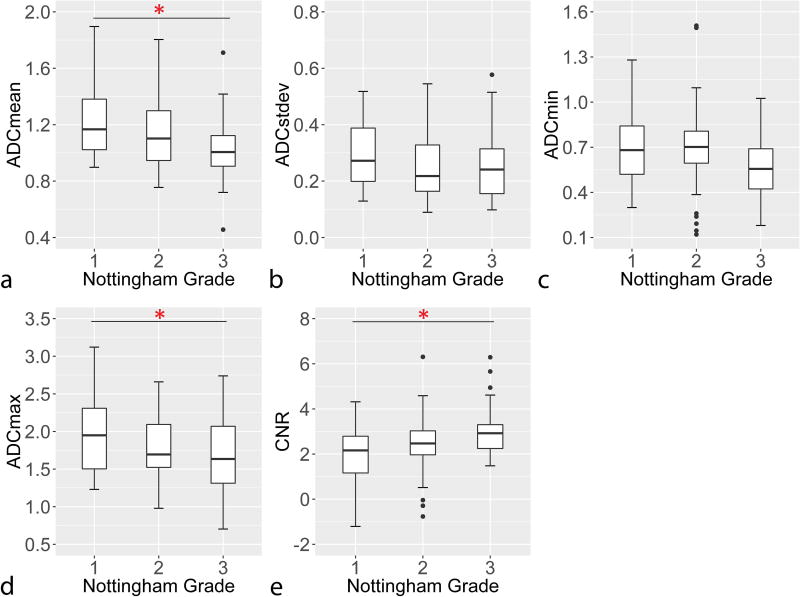
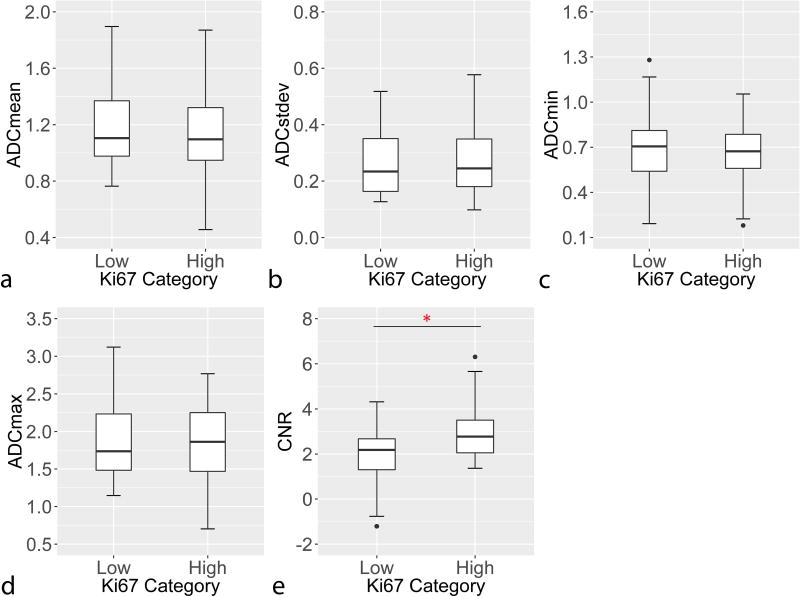
In comparison with Nottingham Grade, ADC measures were observed to generally decrease with increasing grade, while CNR measures increased, Figure 1. Both tumor ADCmean (OR=0.61 per 1-SD increase, padj=0.044) and DWI CNR (OR=1.76 per 1-SD increase, padj =0.026) were significantly associated with Nottingham Grade (Table 3). Similarly, lesions with high Ki-67 proliferation rates (≥ 14%) demonstrated higher DWI CNR than lesions with lower proliferation rates, Figure 2, which was statistically significant (OR=2.55 per 1-SD increase, padj=0.026, Table 3).
Figure 1.
Association Nottingham tumor grade with a) ADCmean, b) ADCstdev, c) ADCmin, d) ADCmax, and e) DWI CNR. Ordinal logistic regression analysis showed ADCmean decreased (a; adj. p = 0.044) and DWI CNR increased (e; adj. p = 0.026) with increasing tumor grade. Asterisk (*) denotes statistical significance (p<0.05) after correction for multiple comparisons.
Table 3.
Associations between DWI measurements and pathologic biomarkers.
| Nottingham Grade* | Ki-67 Status† | 21-Gene Recurrence Score* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Variable | OR‡ | (95% CI) | Adj. P- value§ |
OR‡ | (95% CI) | Adj. P- value§ |
OR‡ | (95% CI) | Adj. P- value§ |
|
| ADC | ||||||||||
| Mean | 0.61 | (0.42–0.88) | 0.044 | 0.83 | (0.53–1.31) | 0.78 | 0.92 | (0.63–1.34) | 0.84 | |
| SD | 0.74 | (0.51–1.07) | 0.27 | 1.15 | (0.75–1.76) | 0.80 | 0.95 | (0.66–1.37) | 0.85 | |
| Min | 0.72 | (0.50–1.03) | 0.22 | 0.87 | (0.53–1.44) | 0.80 | 1.05 | (0.72–1.53) | 0.85 | |
| Max | 0.64 | (0.44–0.94) | 0.082 | 0.99 | (0.64–1.53) | 0.95 | 0.90 | (0.62–1.31) | 0.80 | |
| DWI CNR | 1.76 | (1.21–2.57) | 0.026 | 2.55 | (1.37–4.76) | 0.026 | 1.25 | (0.85–1.83) | 0.56 | |
Abbreviations: ADC = apparent diffusion coefficient; DWI = diffusion weighted imaging; CNR = contrast-to-noise ratio, OR = odds ratio, CI = confidence interval, SD = standard deviation.
Ordinal logistic regression with Nottingham grade or Oncotype DX Recurrence Score as the dependent variable;
Binary logistic regression with Ki-67 ≥14% as the dependent variable;
Odds ratio per 1-SD increase in ADC or CNR;
Wald-test p-value from logistic regression, adjusted for multiple comparisons (15 in total) using the Benjamini and Hochberg procedure to control the false discovery rate at ≤5%.
Figure 2.
Association of with Ki-67 proliferation rate with a) ADCmean, b) ADCstdev, c) ADCmin, d) ADCmax, and e) DWI CNR. Lesions with high Ki-67 (≥14%) exhibited significantly higher DWI CNR (e) versus low Ki-67 lesions (<14%; adj. p = 0.026). Asterisk (*) denotes statistical significance (p<0.05) after correction for multiple comparisons.
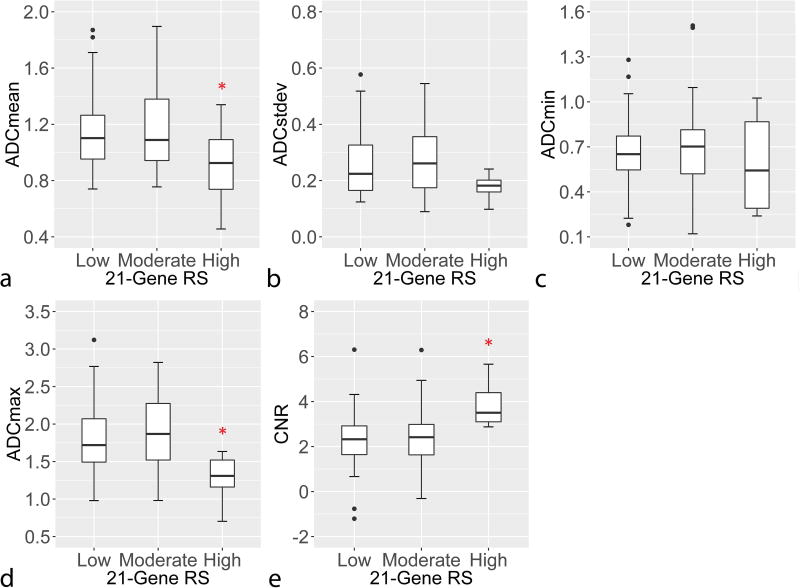
With respect to RS, the high risk group was observed to be distinct in terms of ADC and CNR from the low and moderate risk groups, which themselves appeared to be similar, rather than DWI metrics steadily increasing or decreasing across RS categories, Figure 3. This was confirmed in that the overall linear associations between the DWI parameters and RS (evaluated across the three risk categories) were not significant by ordinal logistic regression (padj = 0.56 – 0.85, Table 3), but the high risk group had significantly lower ADCmean (p=0.037) and ADCmax (p=0.009) and higher CNR (p=0.008) than the combined low and moderate risk group by post hoc group-wise comparisons (Table 4). Examples of DCE-MRI, DWI, and ADC images of tumors with low versus high RS are provided in Figures 4 and 5. Alternatively, there were no significant differences detected in DWI measures when comparing the combined high and moderate risk group versus the low risk group (p=0.62 – 0.92).
Figure 3.
Association of 21-Gene Recurrence Scores (RS) rate with a) ADCmean, b) ADCstdev, c) ADCmin, d) ADCmax, and e) DWI CNR. While associations were not statistical significant for any of the DWI parameters vs. RS by ordinal logistic regression analysis, ADCmean (a), ADCmax (d) and CNR (e) significantly differentiated high RS from low and moderate RS (p<0.05) in post-hoc comparisons. Asterisk (*) denotes significant difference (p<0.05) between high and low/moderate RS groups by post-hoc comparison without adjustment for multiple comparisons.
Table 4.
Post-hoc analysis of high vs. low/moderate 21-Gene Recurrence Score.
| 21-Gene Recurrence Score | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | Low/Moderate (N=101) |
High (N=6) |
OR* | (95% CI) | P-value† | |
| ADC (×10−3 mm2/s) | Mean | 1.17 ± 0.28 | 0.91 ± 0.32 | 0.25 | (0.07–0.92) | 0.037 |
| SD | 0.27 ± 0.12 | 0.18 ± 0.05 | 0.28 | (0.06–1.21) | 0.089 | |
| Min | 0.68 ± 0.25 | 0.59 ± 0.34 | 0.69 | (0.29–1.67) | 0.41 | |
| Max | 1.86 ± 0.47 | 1.28 ± 0.34 | 0.15 | (0.04–0.63) | 0.009 | |
| DWI CNR | 2.4 ± 1.3 | 3.9 ± 1.1 | 3.05 | (1.34–6.94) | 0.008 | |
Abbreviations: ADC = apparent diffusion coefficient; CI = confidence interval; DWI = diffusion weighted imaging; CNR = contrast-to-noise ratio; OR = odds ratio; CI = confidence interval; SD = standard deviation.
Odds ratio for high Oncotype DX Recurrence Score per 1-SD increase in ADC or CNR;
Wald-test p-value from logistic regression without adjustment for multiple comparisons.
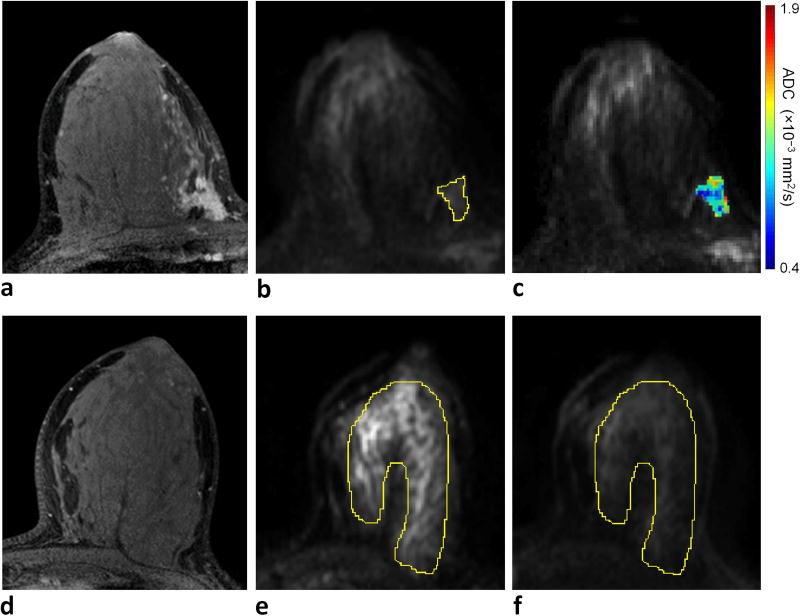
Figure 4.
Example of invasive lobular carcinoma (grade 2/3) with low RS (RS = 17) detected in a 33-year-old female. a) DCE-MRI demonstrates a 47 mm right breast mass at 3 o’clock, 60 mm from the nipple. On DWI, the lesion exhibits b) relatively low signal intensity on b = 800 s/mm2 diffusion-weighted image with CNR = 1.1 (lesion ROI contour is shown), and c) moderate to low ADC (ADCmean = 1.15 × 10−3 mm2/s). For CNR calculation, d) normal fibroglandular tissue was identified on DCE-MRI at a corresponding slice level in the contralateral breast, e) an ROI was defined on the b = 0 s/mm2 image, where breast tissue is most visible, to cover the largest tissue area possible, f) which was then propagated to the b = 800 s/mm2 image to measure the mean DWI signal intensity within the ROI.
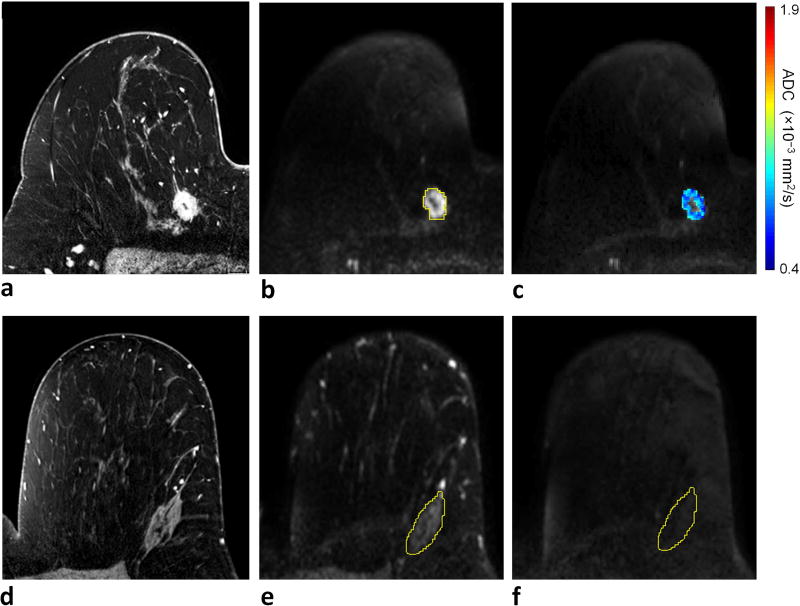
Figure 5.
Example of invasive ductal carcinoma (grade 3/3) with high RS (RS = 34) detected in a 58-year-old female. a) DCE-MRI demonstrates a 17 mm right breast mass at 1 o'clock, 114 mm from the nipple. On DWI, the lesion exhibits b) high signal intensity on b = 800 s/mm2 diffusion-weighted image with CNR = 4.6 (lesion ROI contour is shown), and c) very low ADC (ADCmean = 0.79 × 10−3 mm2/s). For CNR calculation, d) normal fibroglandular tissue was identified on DCE-MRI at a corresponding slice level in the contralateral breast, e) an ROI was defined on the b = 0 s/mm2 image, where breast tissue is most visible, to cover the largest tissue area possible, f) which then propagated to the b = 800 s/mm2 image to measure the mean DWI signal intensity within the ROI.
Among the DWI metrics that were associated with one or more of the pathological biomarkers, ADCmax and ADCmean were positively correlated (r=0.71, p<0.001) while DWI CNR was not significantly correlated with either ADCmax (r= −0.14, p=0.15) or ADCmean (r= −0.15, p=0.13). In a multivariate ordinal logistic regression model, ADCmean (OR=0.66 per 1-SD increase, p=0.031) and DWI CNR (OR=1.65 per 1-SD increase, p=0.011) were independently associated with Nottingham Grade. An exploratory multivariate binary logistic regression model of high RS (vs. low and moderate RS) suggested that ADCmax (OR=0.14 per 1-SD increase, p=0.016) and DWI CNR (OR=3.11 per 1-SD increase, p=0.020) were each independently associated with high RS, though this assessment is based on only six high risk lesions.
Recurrence-Free Survival Outcomes
Disease recurrence was detected in 4 patients over the follow-up period. Of the 103 patients without recurrence, 52 had < 5years of follow-up and 51 had ≥5 years of follow-up. Of those with disease recurrence, two had low RS and two had moderate RS scores, Table 5. Two out of four recurrences occurred in patients whose treatment were nonstandard. (Recurrent Patient #2 with low RS [RS = 17] had several unique extenuating circumstances of young age [33 years], BRCA2 mutation, and positive chest wall surgical margins despite several re-excisions after mastectomy. This patient received full treatment with chemotherapy along with aromatase inhibitor, but was diagnosed with metastases to the bilateral ovaries 20 months after surgery. Recurrent Patient #3 with moderate RS scores declined chemotherapy and developed bone metastases 14 months after surgery.) Of the remaining two patients who received standard treatment, Recurrent Patient #1 with a low RS (RS = 10) who recurred did not receive chemotherapy, was treated with lumpectomy followed by radiation and aromatase inhibitor, and recurred 83 months later with multiple chest and abdominal metastases. On DWI, this patient’s tumor exhibited relatively low ADC (ADCmean = 0.94 ×10−3 mm2/s) and high CNR (3.3). Recurrent Patient #4 with moderate RS (RS = 27) received chemotherapy in addition to aromatase inhibitor and presented with axillary lymph node metastasis 30 months after surgery. On DWI, although this patient’s tumor exhibited a mean ADC score typical of a low/moderate RS, it exhibited a notably high CNR (4.1), which was almost a standard deviation higher than the average CNR of the high RS group. The low number of recurrence events in the study cohort did not allow for statistical analysis.
Table 5.
Patients who exhibited breast cancer recurrence in long term follow-up (n=4).
| Recurrent Patient ID |
Age (years) |
Histology, AJCC Stage, Grade |
Oncotype DX Recurrence Score |
ADCmean (×10−3 mm2/s) |
CNR | Chemotherapy? | RFS (months) |
Disease Recurrence |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 56 | IDC, IB, Grade 2 | 10/Low | 0.94 | 3.3 | No | 83 | Multiple Distant Metastases |
| Patient 2 | 33 | ILC, IIB, Grade 2 | 17/Low | 1.15 | 1.1 | Yes | 20 | Ovarian Metastases |
| Patient 3 | 59 | IDC, IIA, Grade 3 | 22/Moderate | 1.12 | 2.2 | No | 14 | Bone Metastases |
| Patient 4 | 49 | IDC, IA, Grade 3 | 27/Moderate | 1.21 | 4.9 | Yes | 30 | Axillary LN Metastasis |
Abbreviations: AJCC = American Joint Committee on Cancer 7th edition; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; RFS = recurrence free survival; DCIS = ductal carcinoma in situ; LN = lymph node
DISCUSSION
In this retrospective study, we evaluated associations of DWI characteristics of hormone receptor positive (ER and/or PR), HER2 negative invasive breast cancers with 21-gene RS recurrence risk and other prognostic factors. We found that ADCmean and DWI CNR correlated with proliferation rate (Ki-67 ≥ 14%) and Nottingham histologic grade, which agrees with prior studies in invasive breast cancers. Post-hoc analyses suggested that tumor ADC (max and mean) and CNR were also associated with high RS. Our findings suggest that incorporating DWI into breast cancer evaluations may provide additional biological characterization to help in assessing prognosis and guiding clinical management.
The prognostic value of breast tumor MRI characteristics is an emerging area of research. Several studies have recently reported ADC measures to be associated with tumor grade and proliferation (18–23). However, these prior DWI studies have not focused specifically on hormone receptor positive, HER2 negative invasive breast cancers, the most common but heterogeneous group of breast cancers, for which chemotherapy recommendation is variable in practice. Our study validates associations between DWI characteristics and tumor aggressiveness, as measured by Ki-67 and Nottingham histologic grade in this specific cancer subtype.
Furthermore, in post-hoc analysis we found that lesions classified as high risk by RS had lower ADC (mean and max) and higher DWI CNR than low and moderate risk lesions. Only one prior study to date directly explored correlation between DWI and RS in ER-positive, PR-positive, HER2 negative invasive cancers, which demonstrated lower ADC in lesions with higher RS (26). However, those promising findings were based on a small study population (n = 31) containing only a single tumor with high RS score. Our study supports an association between ADC and RS in a larger patient cohort and adds to growing literature showing associations between RS and computer-extracted DCE-MRI lesion characteristics related to size, morphology, kinetics, and texture (12–16). In addition to lesion ADCmean values investigated in the prior study (26), our study evaluated ADCmax, ADCmin, and CNR metrics as well. Biologically, ADC values—particularly ADCmax—would be lowest in homogeneous lesions with high cell density and higher in more heterogeneous or diffuse lesions and/or those with cystic components. On the other hand, minimum ADC values would likely vary less across lesions of varying heterogeneity provided each contains at least some small regions of high cell density. This may explain why ADCmax and ADCmean appeared to be associated with high RS while ADCmin did not. The standard deviation of ADC values (ADCstdev) also tended to be lower in high RS versus low and moderate RS cancers, which supports this hypothesis of lower microstructural heterogeneity in the high RS cancers. Our observation that DWI CNR was higher in the high risk group compared to the low and moderate risk groups suggests that the higher risk lesions are more distinct from normal tissue on DWI, likely related to higher relative cellularity and more restricted diffusion.
Exploratory multivariate binary logistic regression modeling, although based on a small number of high RS lesions, suggested that DWI CNR and ADCmax were each independently associated with high RS. Following the same trend, multivariate modeling also suggested that ADCmean and DWI CNR were independently associated with Nottingham Grade. Accordingly, our findings suggest ADC and CNR measures may be complementary but independent prognostic factors, warranting further study in larger cohorts.
Long-term follow-up provided some interesting insights, although the low numbers of recurrences in the study cohort precluded any statistical analysis. In the case of one patient with low RS who did not receive chemotherapy and recurred with distant metastases 83 months later, low ADCmean and high CNR might have suggested a more aggressive tumor phenotype warranting chemotherapy despite low RS score. Similarly, in another patient with moderate RS who recurred, very high CNR despite moderate ADCmean not only suggests a more aggressive phenotype but also highlights our finding that CNR and ADC may be independent predictive factors. Further investigation to explore the added value of quantitative imaging markers for recurrence prediction modeling may improve outcomes for personalized treatment strategies.
Our study had several limitations. Regarding the MRI technique, we evaluated a limited set of common DWI tumor characteristics. Further study using additional histogram metrics and/or radiomics features to assess lesion morphology and heterogeneity could provide greater insights. As demonstrated by prior DCE-MRI studies, advanced computerized image feature extraction techniques to measure a wider range of textural characteristics may demonstrate additional prognostic value. The slice thickness of our DWI acquisition was 5 mm in order to achieve adequate signal-to-noise ratio, which could result in partial volume averaging. Higher-spatial resolution techniques hold potential to improve the ability to characterize DWI features of breast lesions, in particular for small and non-mass type lesions (36). Lesions were evaluated on MRI after core needle biopsy, and the presence of post biopsy changes may have affected the MRI measurements, although care was taken to exclude obvious hematomas and seromas for quantitative measurements. All DWI examinations were performed after DCE-MRI and it is possible that signal intensity on DWI could be affected by residual gadolinium, although we have previously shown no significant effect on breast tumor ADC measures using our imaging protocol (30).
Regarding our study population, although we incorporated all three risk groups of RS, a larger proportion of patients were in the low and moderate risk groups compared to the high risk group. The previous study by Thakur et al (26) also had a similar limitation, which is presumably due to selection bias of patients who undergo genomic testing, based on clinical decisions between physicians and patients (i.e., clinicians may more likely order testing in patients they feel to be of unclear risk and are undecided whether benefits from chemotherapy treatment outweigh the risk). Our study also included 20 patients with node-positive disease. Although more recent studies have suggested both prognostic and predictive benefit of Oncotype Dx RS in node-positive cancers (5,37), current NCCN guidelines only recommend the use of RS in in node-negative, ER-positive cancers (8). In our analysis, “low”, “moderate”, and “high” RS scores were defined in accordance to original cutoffs published by Paik et al (3,4). More recent studies have used a lower RS (<11) to define low recurrence risk (38,39); this new definition has been adopted in the upcoming 2018 American Joint Committee on Cancer (AJCC) 8th edition staging guidelines (40). Lastly, while the associations of DWI parameters with Nottingham Grade and Ki-67 were statistically significant after accounting for the number of comparisons in the primary analysis, the associations of the DWI parameters with high RS were detected during a subsequent post-hoc analysis and require further validation in a larger study population..
In conclusion, we found associations between DWI characteristics and multiple prognostic factors including RS, suggesting that these imaging biomarkers may reflect recurrence risk and likelihood of benefit from chemotherapy. More specifically, our findings suggest that DWI measures of ADCmean, ADCmax and CNR may help discriminate higher risk lesions with more aggressive biology within the most common and diverse subgroup of hormone receptor positive/HER2 negative breast cancers. This is clinically relevant given that patients with a high RS have been shown retrospectively to benefit the most from adjuvant chemotherapy treatment while the lower risk groups have minimal benefit (4). These results suggest that DWI may provide helpful prognostic information for newly diagnosed breast cancers, and support the inclusion of DWI in preoperative MRI examinations used to evaluate extent of disease. In limited resource settings where costly genetic testing cannot be performed on all patients with hormone receptor positive breast cancer (the most common subtype), DWI, along with DCE-MRI, may serve as a potential alternative or a triaging tool in identifying the subset of high risk patients who would benefit from the genetic test. Further investigation of MRI and DWI biomarkers is warranted to determine the associations with long-term clinical outcomes and to incorporate MRI characteristics into current predictive risk models for personalized breast cancer treatment.
Acknowledgments
Grant Support: This work was supported by NIH grants R01 CA151326 and P50 CA138293 and funding by the Safeway Foundation.
References
- 1.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. Journal of the National Cancer Institute. 1997;89(22):1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 2.Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015;24(Suppl 2):S149–153. doi: 10.1016/j.breast.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England journal of medicine. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. The Lancet Oncology. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(29):7265–7277. doi: 10.1200/JCO.2005.02.0818. [DOI] [PubMed] [Google Scholar]
- 7.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 8.Febbo PG, Ladanyi M, Aldape KD, et al. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9(Suppl 5):S1–32. doi: 10.6004/jnccn.2011.0137. quiz S33. [DOI] [PubMed] [Google Scholar]
- 9.SWOG-S1007: A Phase III, Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy +/− Chemotherapy in Patients With 1–3 Positive Nodes, Hormone Receptor-Positive and HER2-Negative Breast Cancer With Recurrence Score (RS) of 25 or Less. RxPONDER: A Clinical Trial Rx for Positive Node, Endocrine Responsive Breast Cancer. [Accessed September 8, 2017]; Available at: https://clinicaltrials.gov/ct2/show/NCT01272037.
- 10.Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA. Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. Journal of oncology practice. 2013;9(4):182–187. doi: 10.1200/JOP.2012.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider JG, Khalil DN. Why does Oncotype DX recurrence score reduce adjuvant chemotherapy use? Breast cancer research and treatment. 2012;134(3):1125–1132. doi: 10.1007/s10549-012-2134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashraf AB, Daye D, Gavenonis S, et al. Identification of intrinsic imaging phenotypes for breast cancer tumors: preliminary associations with gene expression profiles. Radiology. 2014;272(2):374–384. doi: 10.1148/radiol.14131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Zhu Y, Burnside ES, et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology. 2016;281(2):382–391. doi: 10.1148/radiol.2016152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton EJ, Oh JH, Dashevsky BZ, et al. Breast cancer subtype intertumor heterogeneity: MRI-based features predict results of a genomic assay. Journal of magnetic resonance imaging : JMRI. 2015;42(5):1398–1406. doi: 10.1002/jmri.24890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan T, Bloch BN, Plecha D, et al. A Radio-genomics Approach for Identifying High Risk Estrogen Receptor-positive Breast Cancers on DCE-MRI: Preliminary Results in Predicting OncotypeDX Risk Scores. Scientific reports. 2016;6:21394. doi: 10.1038/srep21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dialani V, Gaur S, Mehta TS, et al. Prediction of Low versus High Recurrence Scores in Estrogen Receptor-Positive, Lymph Node-Negative Invasive Breast Cancer on the Basis of Radiologic-Pathologic Features: Comparison with Oncotype DX Test Recurrence Scores. Radiology. 2016;280(2):370–378. doi: 10.1148/radiol.2016151149. [DOI] [PubMed] [Google Scholar]
- 17.Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. Journal of magnetic resonance imaging : JMRI. 2017;45(2):337–355. doi: 10.1002/jmri.25479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costantini M, Belli P, Rinaldi P, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clinical radiology. 2010;65(12):1005–1012. doi: 10.1016/j.crad.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Cipolla V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, de Felice C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. European journal of radiology. 2014;83(12):2144–2150. doi: 10.1016/j.ejrad.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Shin JK, Kim JY. Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: Associations between quantitative MR parameters and Ki-67 proliferation status. Journal of magnetic resonance imaging : JMRI. 2017;45(1):94–102. doi: 10.1002/jmri.25348. [DOI] [PubMed] [Google Scholar]
- 21.Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. The British journal of radiology. 2012;85(1016):e474–479. doi: 10.1259/bjr/79381464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EJ, Kim SH, Park GE, et al. Histogram analysis of apparent diffusion coefficient at 3.0t: Correlation with prognostic factors and subtypes of invasive ductal carcinoma. Journal of magnetic resonance imaging : JMRI. 2015;42(6):1666–1678. doi: 10.1002/jmri.24934. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima K, Yamano T, Fukushima K, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. European journal of radiology. 2016;85(5):943–949. doi: 10.1016/j.ejrad.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jeh SK, Kim SH, Kim HS, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. Journal of magnetic resonance imaging : JMRI. 2011;33(1):102–109. doi: 10.1002/jmri.22400. [DOI] [PubMed] [Google Scholar]
- 25.Mori N, Ota H, Mugikura S, et al. Luminal-type breast cancer: correlation of apparent diffusion coefficients with the Ki-67 labeling index. Radiology. 2015;274(1):66–73. doi: 10.1148/radiol.14140283. [DOI] [PubMed] [Google Scholar]
- 26.Thakur SB, Durando M, Milans S, et al. Apparent diffusion coefficient in estrogen receptor-positive and lymph node-negative invasive breast cancers at 3.0T DW-MRI: A potential predictor for an oncotype Dx test recurrence score. Journal of magnetic resonance imaging : JMRI. 2017 doi: 10.1002/jmri.25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. Journal of the National Cancer Institute. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feeley LP, Mulligan AM, Pinnaduwage D, Bull SB, Andrulis IL. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27(4):554–561. doi: 10.1038/modpathol.2013.153. [DOI] [PubMed] [Google Scholar]
- 29.(ACR) ACoR. Breast imaging reporting and data system atlas (BI-RADS Atlas) Reston, VA: 2003. [Google Scholar]
- 30.Nguyen VT, Rahbar H, Olson ML, Liu CL, Lehman CD, Partridge SC. Diffusion-weighted imaging: Effects of intravascular contrast agents on apparent diffusion coefficient measures of breast malignancies at 3 tesla. Journal of magnetic resonance imaging : JMRI. 2015 doi: 10.1002/jmri.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahbar H, Kurland BF, Olson ML, et al. Diffusion-Weighted Breast Magnetic Resonance Imaging: A Semiautomated Voxel Selection Technique Improves Interreader Reproducibility of Apparent Diffusion Coefficient Measurements. Journal of computer assisted tomography. 2016;40(3):428–435. doi: 10.1097/RCT.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald ES, Schopp JG, Peacock S, et al. Diffusion-weighted MRI: association between patient characteristics and apparent diffusion coefficients of normal breast fibroglandular tissue at 3 T. AJR American journal of roentgenology. 2014;202(5):W496–502. doi: 10.2214/AJR.13.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahbar H, Partridge SC, Demartini WB, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology. 2012;263(2):374–382. doi: 10.1148/radiol.12111368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- 35.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 36.Wisner DJ, Rogers N, Deshpande VS, et al. High-resolution diffusion-weighted imaging for the separation of benign from malignant BI-RADS 4/5 lesions found on breast MRI at 3T. Journal of magnetic resonance imaging : JMRI. 2014;40(3):674–681. doi: 10.1002/jmri.24416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 38.Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(20):2341–2349. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 39.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8. Springer International Publishing; 2017. [Google Scholar]