Abstract
Laminins are large heterotrimers composed of the α, β and γ subunits with distinct tissue-specific and developmentally regulated expression patterns. The laminin-α2 subunit, encoded by the LAMA2 gene, is mainly expressed in skeletal muscle, Schwann cells of the peripheral nerve and astrocytes and pericytes of the capillaries in the brain. Mutations in LAMA2 cause the most common type of congenital muscular dystrophies, called LAMA2 MD or MDC1A. The disorder manifests mostly as a muscular dystrophy but slowing of nerve conduction contributes to the disease. There are severe, non-ambulatory or milder, ambulatory variants, the latter resulting from reduced laminin-α2 expression and/or deficient laminin-α2 function. Lm-211 (α2β1γ1) is responsible for initiating basement membrane assembly. This is primarily accomplished by anchorage of Lm-211 to dystroglycan and α7β1 integrin receptors, polymerization, and binding to nidogen and other structural components. In LAMA2 MD, Lm-411 replaces Lm-211; however, Lm-411 lacks the ability to polymerize and bind to receptors. This results in a weakened basement membrane leading to the disease. The possibility of introducing structural repair proteins that correct the underlying abnormality is an attractive therapeutic goal. Recent studies in mouse models for LAMA2 MD reveal that introduction of laminin-binding linker proteins that restore lost functional activities can substantially ameliorate the disease. This review discusses the underlying mechanism of this repair and compares this approach to other developing therapies employing pharmacological treatments.
Introduction
Basement membranes (BMs) are cell-adherent extracellular matrices (ECMs) that are essential for the structural support, maintenance and differentiation of animal tissues [1–3]. Their assembly, structure and functions depend upon laminin heterotrimeric glycoproteins consisting of α-, β- and γ subunits. In mammals there are a total of five different α subunits (α1 – α5, encoded by LAMA1 to LAMA5), four different β chains (β1 – β4, encoded by LAMB1 – LAMB4) and three γ chains (γ1 – γ3, encoded by LAMC1 – LAMC3). During protein synthesis and secretion α, β and γ chains assemble via specific interactions of their coiled-regions. So far, 15 different laminins derived from the many possible combinations have been described, which differ in function and expression pattern [4, 5]. The essential role of the laminins for the tissue integrity and function is best supported by mouse studies showing that deletion of the gene encoding a particular chain results either in embryonic lethality or severe disorders after birth. For example, mouse embryos deficient for Lamc1 (gene coding for the γ1 chain), which is the γ chain of ten different laminins, die at pre-implantation stage [6].
The fundamental role of laminins is based on their creating of a primary scaffold that (1) attaches the extracellular matrix to the cell surface and (2) serves as a platform to which other extracellular matrix components, such as the nidogens and collagens, become stably attached. The main laminin receptors expressed on cell surfaces are α-dystroglycan, specific integrins and sulfated glycolipids (reviewed in [1, 2]). Binding of laminins to their receptors affects assembly of the BM, mechanical linkage of the cell to BM and signaling. We would like to emphasize that the receptor binding of the laminins in the context of laminin-deficient muscular dystrophy is particularly important for the assembly of the sarcolemmal and endoneurial BM and that the different receptors can, at least partially, compensate for each other.
LAMA2 mutations cause congenital muscular dystrophy
ECMs stabilize cells and tissues by providing solid-phase cell adhesion substrates, linking ECM to cytoskeleton [7]. The consequences of ECM linkage loss is particularly evident in the heritable muscular dystrophies and skin blistering diseases. In both cases, mutations in distinct laminins and collagens cause severe diseases [8, 9]. The reason for the severe phenotype is the disruption of the ECM-to-receptor-to-cytoskeletal linkage. In skin, the main receptors of the ECM components are particular integrins, which then connect to several cytoskeletal components [9]. In skeletal muscle, the main ECM receptors are α-dystroglycan (αDG) and α7β1 integrin and dystrophin is an important linker to the cytoskeleton. Mutation in the genes coding for those proteins cause muscular dystrophies supporting the concept that a disruption of transverse linkages from stroma to cytoskeleton results in a failure of sarcolemmal stability and muscle function [8, 10].
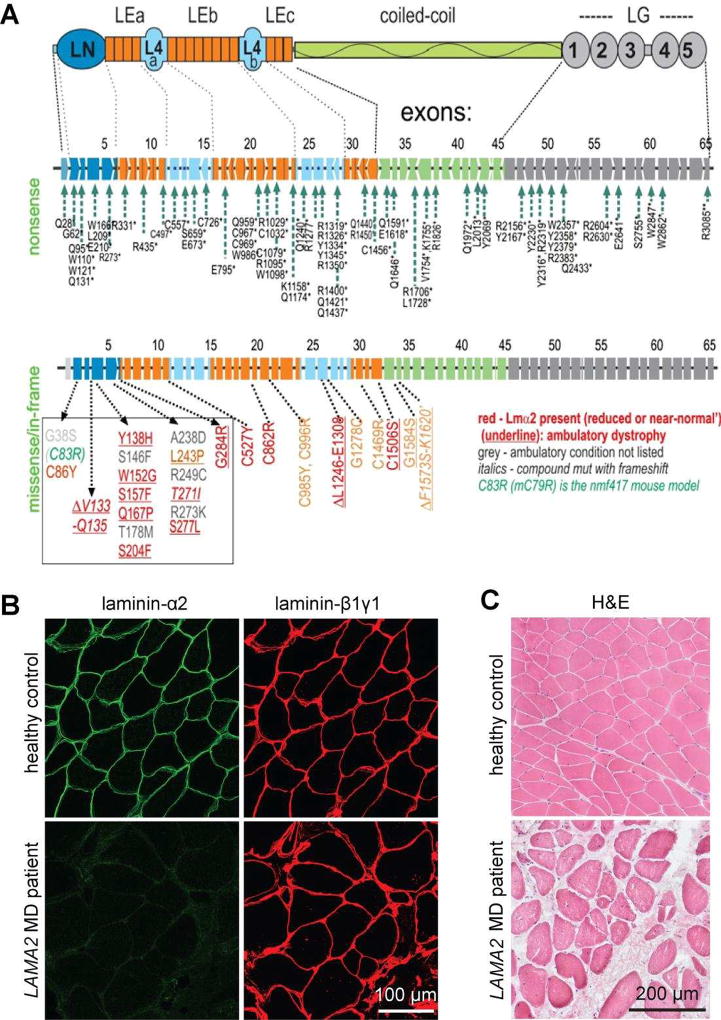
Laminin-211 (α2β1γ1 chain composition; abbreviated Lm-211) is expressed mainly in adult muscle and peripheral nerve. Mutations within the LAMA2 gene cause a particular subtype of congenital muscular dystrophies (CMDs), which are characterized as early onset, often very severe muscular dystrophies [8] (Bönnemann, this issue). LAMA2 MD (also called MDC1A) is the largest subgroup of the CMDs. A recent study of 249 CMD patients in United Kingdom [13] revealed that LAMA2 mutations were the most common (37.4%) followed by dystroglycanopathies (26.5%) and Ullrich-CMD (15.7%). Mutations within the LAMA2 gene are spread throughout the gene (Fig. 1A). The majority of LAMA2 mutations listed in the Leiden Data Bank are nonsense mutations that result in a premature chain truncation to cause a severe, non-ambulatory dystrophy (Fig. 1A). The patients with such mutations lack any laminin-α2 staining in muscle biopsies but express similar amounts of the β1 and the γ1 laminin chains as detected in healthy controls (Fig. 1B). As a consequence of laminin-α2 deficiency, myofibers become damaged under the load of myofiber contraction. Muscle fibers undergo apoptotic degeneration followed by regeneration, chronic inflammation, and fibrosis (Fig. 1C). Analysis of laminin-α2 deficient mice has shown that regeneration fails and causes regenerating cells to undergo apoptosis [14, 15]. Patients with null-expression mutations are floppy at birth, often fail to thrive, never ambulate, and often die of muscle wasting and respiratory failure by the second decade [8, 13].
Figure 1. Mutations and muscle phenotype in LAMA2 MD.
A. Domain structure of laminin-α2 (top), location of mutations that cause the severe, non-ambulatory form (middle) or the ambulatory form of LAMA2 MD (bottom). The mutations of the severe form span the entire length of the gene and its 65 exons and intervening introns. They completely lack expression of the laminin-α2 resulting from premature chain-termination mutations. The ambulatory dystrophy is typically seen with missense mutations causing reduced and sometimes even normal expression of laminin-α2, resulting from amino acid substitutions or small deletions. Note that 17 of these mutations are clustered in the N-terminal LN domain that enables the laminin to polymerize. B. Cross-sections of muscle biopsies from normal and LAMA2 MD patients stained with antibodies directed to laminin-α2 or laminin-β1 and –γ1. Note that LAMA2 patient biopsies are negative for laminin-α2 but express similar amount of the laminin-β1 and -γ1. C. Haematoxylineosin stained muscle cross-sections from healthy controls and LAMA2 MD patients. Note the loss of muscle fibers and replacement with non-muscle tissue and the heterogeneous size of muscle fibers in LAMA2 MD patients compared to healthy controls.
While the absence of laminin-α2 results primarily in skeletal muscle damage, it also affects the peripheral nerve and the brain. The peripheral nerve defect alters nerve conduction [16, 17] and there is a report that a LAMA2 mutation can also result in limb-girdle muscular dystrophy with involvement of the peripheral nerve [18]. In the brain, LAMA2 MD patients often show changes in white matter density detected by T2-weighted magnetic resonance deduced to reflect increased water density rather than changes in myelination [19–22]. Additional brain abnormalities are those of cerebellar hypoplasia and, rarely, occipital lobe neuronal migration defects [8]. A subset of patients exhibits seizures or moderate mental retardation.
Other mutations in LAMA2 cause milder forms of the disease [23]. They are often missense mutations localized to the 5’ region of LAMA2 (red in Fig. 1A) that result in only reduced or near-normal expression of laminin-α2. Based on the recent solution of the laminin LN domain by X-ray crystallography [24, 25], several of the dystrophic mutations (Q167P, Y138H, G284R) map to a polymerization patch on one face of the LN domain. Others (C86Y, W152G, S157F, S277L, S204F, L243P) reside in the interior of the LN domain and are likely to disrupt the protein fold. An additional eight mutations are located downstream of the LN domain on the short arm, one is an in-frame deletion within the L4b domain [26]. The molecular basis for the alteration of laminin function by these mutations is unknown given no activities other than LN domain spacing has been postulated to date. In summary, most of the nonsense mutations cause absence of laminin-α2 and thus result in severe, non-ambulatory forms of LAMA2 MD while some of the missense mutations are predicted to affect laminin polymerization because they reside in the LN domain that has been demonstrated to affect laminin self-assembly in vitro and on cultured cells (see below).
Animal models of LAMA2 MD
There are several autosomal recessive mouse models of Lama2 mutations that cover the range of clinical severity seen in humans (Table 1). The first of those mutants was identified more than 60 years ago as a spontaneous mutant, called dystrophia muscularis (dy/dy) [27]. While dy/dy mice represent an intermediate to severe form of LAMA2 MD, the other spontaneous mutants, called dy2J/dy2J [28] and dynmf417/dynmf417 [29] display milder forms. Besides the spontaneous mutants, dyW/dyW [14, 30] and dy3K /dy3K mice [31] were generated by homologous recombination to represent the severe forms of LAMA2 MD. Because the mutation in the dy/dy mice has not been mapped, dyW/dyW and dy3K /dy3K mice are the two models that are used as models for the severe form. The dy3K/dy3K mice are complete Lama2 knockout mice [31]. They show a very severe phenotype that becomes evident soon after birth; they do not thrive and develop severe muscular dystrophy evidenced by histology and functional weakness. They develop hindlimb paralysis but the mice die at an age of a few weeks before lameness becomes the predominant phenotype. A very similar phenotype is seen in dyW/dyW mice, which are the best characterized and most often used mouse model for LAMA2 MD. The median survival of dyW/dyW mice is between 5 to 16 weeks. The big variation seems to largely depend on housing conditions and the genetic background [32]. The overall phenotype of dyW/dyW mice is slightly less severe compared to dy3K /dy3K mice, which might be based on the presence of low levels of a truncated laminin-α2 fragment [33]. Because of the longer survival of dyW/dyW mice compared to dy3K /dy3K mice, the hindlimb paralysis becomes more dominant at later time points.
Table 1.
Mouse models of laminin-deficient muscular dystrophy
| mouse | mutation | laminin- α2 pro- tein |
median survival (weeks) |
body weight |
muscular dystrophy |
Ref. |
|---|---|---|---|---|---|---|
| dynmf417/dynmf417(dy7J/dy7J) | ENU-induced missense mutation | C79R mutation; normal level | no reduction reported | normal | mild | [29] |
| dy2J/dy2J | Spontaneous splice-site mutation | truncated and reduced level | no reduction reported | slight reduction | mild | [28] [34] |
| dy/dy | Unknown, spontaneous mutation | very low level to absent | 20–35 | reduction | moderate | [27, 107]; [108] [109] |
| dyW/dyW | targeted knockout | truncated; very low level to absent | 5–16 | strong reduction | severe | [76] [33] [32] |
| dy3K/dy3K | targeted knockout | absent | 3–5 | strong reduction | severe | [31] |
Mice representing the mild, ambulatory form of LAMA2 MD are dy2J/dy2J and dynmf417/dynmf417 mice. The Lama2 mutation in dy2J/dy2J mice is a splice donor defect that causes an in-frame 57 amino acid deletion (residues 34–90) of the α2LN domain [34, 35]. Muscle histology shows a reduction in cross-sectional area with rounded contours, fibrosis, chronic inflammation, and regeneration (measured by the presence of central nuclei). This is accompanied by prominent amyelination in the sciatic nerve and roots [36] and there is evidence of leakage of water from the blood-brain barrier capillaries [37, 38]. Starting from about 3 weeks of age, the mice exhibit a stereotyped behavior of retracting their hindlimbs when suspended by the tail. The mice display weakness of all limbs and several weeks after weaning develop permanent hindlimb extensor contractions. Weights are near-normal and survival is typically greater than one year. A very similar phenotype to the dy2J/dy2 mice has been described for dynmf417/dynmf417 mice [29]. A missense mutation in Lama2 converts the cysteine residue at position 79 to an arginine (C79R). This residue is located in the α2LN domain suggesting that impaired laminin polymerization (see below) contributes to the disease phenotype. Of note, levels of laminin-α2 in muscle and nerve are not reduced by immunostaining and the BM ultrastructure appears unchanged in dynmf417/dynmf417 mice [29] Thus, dy2J/dy2J and dynmf417/dynmf417 mice are good models of the LAMA2 MD patients who suffer from the less severe, ambulatory form of the disease. There are also zebrafish, dog, cat models for LAMA2 MD [39–41].
Pathogenic mechanisms of LAMA2 MD
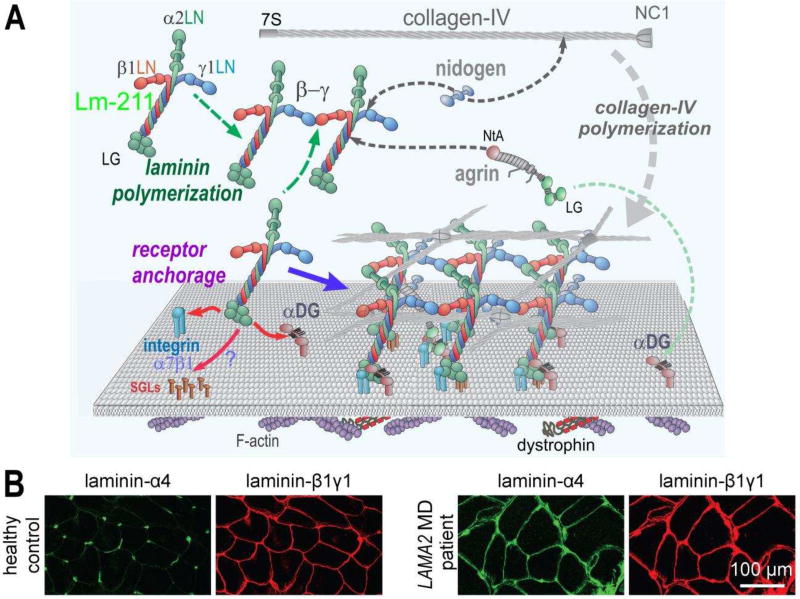
Laminins are essential for BM assembly, initiating the process by binding to surface receptors and receptor-like molecules (Fig. 2A). In muscle this initiating adhesion event is mediated by the principal laminin, Lm-211. The main laminin-binding loci responsible for initial anchorage to the cells are located in the LG domains of laminin-α2 chain [42, 43]. These domains bind to αDG, integrin α7β1, and very possibly to sulfated glycolipids (SGLs), similar to what was found in Schwann cells [44]. Although SGLs may substantially contribute to BM assembly by providing additional cell surface anchorage, it is αDG (via β-dystroglycan) and α7β1 integrin that establish critical BM anchorage to the underlying cytoskeleton [45, 46]. Of note, α7β1 integrin-binding is restricted in its binding repertoire, interacting only with α2, α1, and α5 laminins [47]. Integrin α7β1 binds to laminin domains LG1–3 and requires the presence of the adjacent coiled-coil domain [48, 49]. Binding of Lm-211 to αDG maps to the C-terminal LG1–3 and LG4–5 domains of laminin-α2 [2] and requires the presence of mannosyl O-linked carbohydrates [50]. A unique repeating disaccharide consisting of xylose and glucoronate, coded by the LARGE glycosyltransferase and located on the neck-like region of αDG, is required for binding to the LG domains of laminins, agrins, and perlecan [50–56]. A comparison of Dag1 (encoding α- and β-dystroglycan) and Itga7- (encoding α7 integrin) deficient muscles in mice indicates that the interaction of the BM with αDG is more critical for muscle maintenance than the α7β1 integrin, as Dag1-deficient but not Itga7-deficient muscle fibers become detached from the BM and renders fibers prone to contraction-induced injury [57]. As Lm-411 has barely detectable binding to αDG and Lm-511 binds poorly [58], the increase of these laminins is inadequate to compensate for the loss of Lm-211.
Figure 2. Normal and dystrophic basement membrane assembly and composition.
A. In skeletal muscle, Lm-211 forms the initial polymer nascent scaffolding by binding to integrin α7β1, αDG, and possibly also to sulfated glycolipids (SGL) via the LG domains. The α7β1 integrin and αDG form linkages through cellular adaptor proteins to actin and other cytoskeletal cables. Lm-21 1 also polymerizes through the three different LN domains that interact to form a ternary node. Nidogen-1 (and −2) binds to the laminin-γ1subunit (domain LEb3) and to collagen-IV, acting as a bridge, with the collagen polymerizing to form a second network. Agrin binds to the coiled-coil of the Lm-211 via its NtA domain and to αDG with the C-terminal LG domains, possibly acting as a collateral linker. All structural components become directly or indirectly tethered to cell receptors through the polymerizing laminin. Lm-411 is a component of the peripheral nerve Schwann cell basement membrane but absent in normal sarcolemmal basement membrane. B. Immuno-stained cross-section of skeletal muscle from a healthy control and a LAMA2 MD patient. In healthy controls, laminin-α4 is largely restricted to blood vessel and the neuromuscular junction. In LAMA2 MD patients, laminin-α4 is found in muscle basement membrane hereby substituting for laminin-α2.
Concomitant with receptor-mediated anchorage of Lm-211, laminin self-polymerization contributes to the formation of a provisional extracellular matrix. One effect of this self-polymerization is to confer an approximately three-fold increase of the amount of laminin bound to the surface of cultured myotubes and an even higher increase in Schwann cells [12, 43]. Polymerization occurs through ternary binding of the LN domains of the α-, β- and γ chains, each laminin forming three ternary nodes with adjacent laminins [24, 59–63]. Absence of any of the three different LN domains prevents polymerization [43, 62]. As in muscle all the expressed β- and the γ-laminins possess LN domains, the N-terminal end of the long α-laminins decides on the polymerization properties of the native heterotrimer. Thus, laminins that contain a short α-laminin, i.e. laminin-α3A or laminin-α4, are predicted not to polymerize. Laminin-α4 is normally found in the adult Schwann cell BM of peripheral nerve, in blood vessels of nerve and muscle, and at the neuromuscular junction, but it is not found in non-synaptic regions of the muscle BM [36, 64, 65]. Anchorage in nerve and vessels may depend upon interactions with sulfated glyco-lipids and CD-146 given that αDG and integrin interactions appear to be quite weak [47, 66–69].
The laminin accumulation by cell surface binding and self-polymerization provides the scaffold for nidogens (mainly nidogen-1) to form a critical high-affinity bridge between laminins and to type IV collagen [43]. This is achieved by the binding of nidogen-1 to the short arm of laminin-γ1 [70, 71] and its binding to collagen type IV [60, 70]. The resulting co-polymer of the laminin and the collagen type IV networks provides structural support that resists damage through muscle contractions and also acts as a signaling platform.
Loss of laminin-α2 in LAMA2 MD patients does not result in the loss of staining with antibodies directed against the β1- and the γ1 chain (Fig. 1B), suggesting compensatory expression of genes coding for other α chains. Indeed, muscle biopsies of LAMA2 MD patients are strongly positive for laminin-α4 [58] (illustrated in Fig. 2B). The same compensatory expression of laminin-α4 has been documented in dy/dy and dyW/dyW mice [72–74]. Laminin-α4 is expressed in skeletal muscle during embryonic development. It disappears after birth from the muscle BM except at the neuromuscular junction and it also outlines blood vessels in muscle [58, 64]. Besides laminin-α4, there is also some increase in the levels of laminin-α5 in dyW/dyW mice and in LAMA2 MD patients [58, 73].
In muscle, the loss of laminin-α2 and the compensatory presence of laminin-α4 increases the fraction of laminin that can be extracted with isotonic buffer, reflecting reduction of the tight attachment of Lm-411 to the muscle BM [12, 58]. The loose attachment of Lm-411 to muscle BM is paralleled by a complete lack of accumulation of recombinant Lm-411 to the surface of cultured C2C12 myotubes [58]. This loss of surface binding by Lm-411, which contrasts the still significant attachment of Lm-411 to BM in vivo, is thought to be largely due to low concentration of components assembling in an open culture system compared to the high concentration for assembly in the diffusion-limited potential space that becomes the BM in a tissue. Effectively, the readout of laminin accumulation in tissue culture system is very sensitive to differences that are obscured in vivo, a valuable characteristic for discerning among different repair treatments.
The reasons for the lack of Lm-411 accumulation in tissue culture and its poor incorporation into muscle BM are the weak binding of the laminin to αDG and to integrins [47, 69], absence of alternative receptors, and inability to self-polymerize. Similarly, the other compensatory laminin, Lm-511, although it binds well to several integrins (including α7β1) and polymerizes, binds only weakly to αDG compared to Lm-211 [47, 58, 75]. Thus, LAMA2 MD may well be a disease that is largely caused by the insufficient assembly of the laminin network and the poor connection to the plasma membrane of the underlying muscle fiber. Indeed, recent experiments that aimed at restoring those two functions have provided strong support for this hypothesis (see below).
Treatment strategies
Replacing lost laminin
The most obvious strategy to treat LAMA2 MD would be to express laminin-α2 using gene therapeutic approaches (Table 2). In a proof-of-principle experiment, Engvall and colleagues have shown that muscle-specific, transgenic expression of a cDNA encoding the human version of laminin-α2 is capable of restoring muscle histology and allowing dyW/dyW mice to survive for more than one year (there were no attempts made to measure lifespan). Because the transgene was not expressed in the Schwann cells of the peripheral nerve, hindlimb paralysis was still present [76]. In an analogous approach, the Durbeej laboratory expressed cDNA encoding laminin-α1 transgenically using the chicken β-actin (CAG) promoter [77, 78]. As this transgene is expressed ubiquitously (including Schwann cells in the peripheral nerve), laminin-α1-expressing dy3K /dy3K mice did not suffer from hindlimb paralysis and the mice survived for up to two years [78]. This work therefore shows that laminin-α1 can fully substitute for the function of laminin-α2. As cDNAs encoding laminin-α2 or laminin-α1 by far exceed the size limit of adeno-associated viral (AAV) vectors, this approach cannot be translated into a treatment of LAMA2 MD patients. An alternative to gene therapy could be the injection of recombinant Lm-111. Indeed, injection of EHS-derived Lm-111 into dyW/dyW mice has been shown to be efficacious [79]. However, translation of such a protein-based therapy remains a challenge because it may be difficult to efficiently incorporate a protein of the size of 1,000 kDa into the muscle BM after injection. Moreover, production of sufficient amounts of fully active, recombinant human Lm-111 has not yet been achieved. Another recent approach to increase LAMA1 expression has used electroporation of skeletal muscle to deliver a guide RNA (gRNA) that targets the Lama1 promoter and a “dead” version of Cas9 coupled with the VP160 transcription activation domain. Applied to mdx mice, a mouse model for Duchenne Muscular Dystrophy (DMD), this approach led to increased expression of Lama1 and detectable levels of Lm-111 [80]. While there is some controversy as to whether Lm-111 in DMD patients would result in a beneficial effect [81], such an approach, when applied to LAMA2 MD patients is likely to improve the dystrophy.
Table 2.
Basement membrane structural repair strategies evaluated in mouse models.
| Type of Repair | Mouse | Approach | Observed benefits | Comments | Ref. |
|---|---|---|---|---|---|
| LAMA1 gene replacement | dy3K/dy3K | Transgenic expression of laminin-α1 using general CAG promoter | Substantial repair of muscle and peripheral nerve with restoration of weight, strength and behavior. | This approach is not amenable to gene therapy as cDNA too large (9.3 kb) for somatic viral-mediated delivery. | [78] |
| Parenteral delivery of Lm-111 | dyW/dyW | EHS Lm-111 injected i.v., i.p. or i.m. | Lm-111 detected in sarcolemmal pattern. Median survival increased from ~83 to 292 days; slight weight and muscle force increases; increased activity; decreased inflammation. | EHS Lm-111 protein therapy also reported to improved mdx mice (DMD model); however, findings not replicated by laminin-α1 transgene in mdx. | [79, 110]; [81] |
| Exon skipping | dy3K/dy3K | PMO-mediated skipping of exon 4 of mouse premature mRNA. | Trend to improve survival. | Exon-skipping, under intense investigation for DMD, seems unlikely to be of general value for the LAMA2 gene because the exon borders do not match protein domain borders. | [83] |
| Laminin receptor anchorage | dyW/dyW | Transgenic muscle-expression of miniaturized agrin (mag) | Substantial increase in survival, fiber size, BM ultrastructure, weights, and walking time with reduced muscle creatine kinase activity. | Mag, by binding to the compensatory Lm-411, increases anchorage to αDG. AAV-mediated expression improved dyW/dyW dystrophy. | [73, 98] |
| Laminin polymerization | dy2J/dy2J | Transgenic muscle-expression of a laminin-nidogen fusion protein (αLNNd) | Restoration of fore-limb grip strength to normal. Forelimb muscle histology fiber sizes/appearance restored to normal with elimination of fibrosis. Regeneration greatly reduced. | Lm-211 is unable to polymerize. αLNNd binds to laminin-γ1 near the intersection of the laminin cross. It acts primarily by enabling polymerization of truncated Lm-211 expressed in dy2J/dy2J mice | [12] |
| Combined laminin anchorage + polymerization | dyW/dyW | Transgenic muscle expression of mag and αLNNd | Survival increased greater than five-fold to near-normal levels. Histology (fiber size/fibrosis) improved. Increased weights and muscle strength. | Improvement is mediated by enabling Lm-411 to polymerize and bind to αDG. Benefit was significantly greater than that seen with either transgene alone. Sets stage for double AAV somatic gene delivery. | [58] |
| Non-homologous end-joining correction of splicing defect | dy2J/dy2J | AAV delivery of CRISPR-Cas9 gene-editing components | Restored expression of full-length laminin-α2. Substantial improvement of muscle histology with reduced fibrosis. Increased mouse activity and muscle force of contraction. | General applicability for splice donor mutations. Would not correct nonsense and missense mutations. | [84] |
Exon-skipping to correct out-of-frame mutations has been used successfully to treat dystrophin-deficiency in mice and human. A first exon-skipping oligonucleotide has recently been conditionally approved by Food and Drug Administration (FDA) for the treatment of DMD [82]. Exon-skipping has also been discussed for LAMA2 MD but appears most likely to be problematic in that skipping of nearly all LAMA2 exons will result in either cysteine mispairing with improper domain folding and disulfide-bond formation and/or major loss of functional domains. In a recent trial to restore laminin-α2 in dy3K /dy3K mice, exon-skipping had some benefit but did not significantly increase lifespan [83].
AAV-mediated CRISPR-Cas9 genome editing, targeted to both the muscle and the nerve, was recently used to substantially ameliorate the muscle and peripheral nerve phenotype of the dy2J/dy2J mice by correcting the splicing defect [84]. The basic therapeutic design should have wide application for splice donor defects. In a recent study of congenital muscular dystrophies in U.K. it was reported that 19/89 LAMA2 MD arose from splice-site mutations.
Restoration of muscle BM by linker proteins
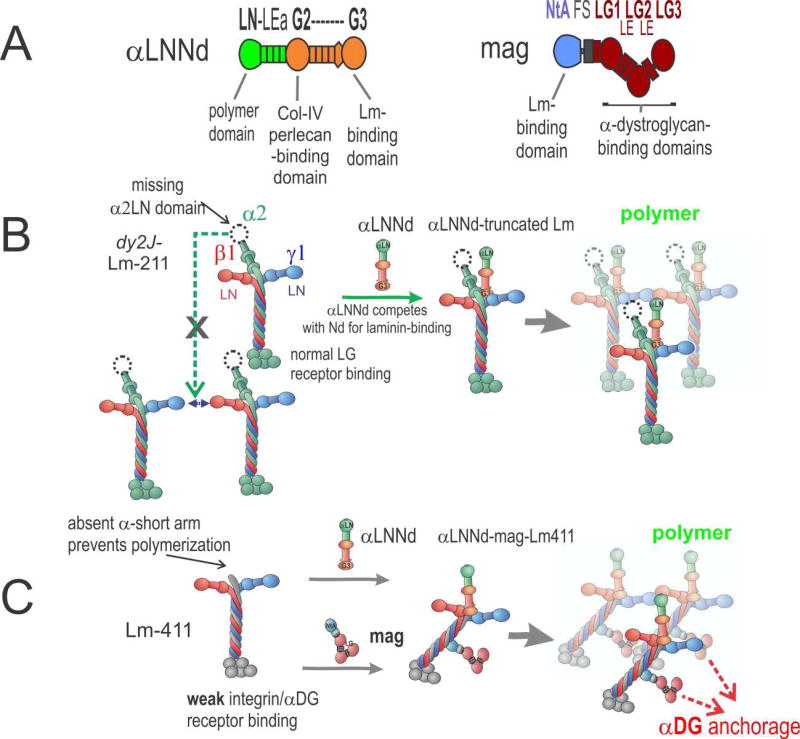
Another approach to restore the linkage of the muscle BM to the plasma membrane in LAMA2 MD patients uses the upregulated Lm-411 (and Lm-511) as scaffold for the attachment of small, bi-functional linker proteins. The first modification to make Lm-411 behave more like Lm-211 was made many years ago by using a specifically designed, miniaturized version of the protein agrin, called mini-agrin or briefly mag (Fig. 3) [73]. Agrin is concentrated at the neuromuscular junction where its motor neuron-derived splice isoforms are responsible for the formation of postsynaptic specializations [85]. In non-synaptic regions of the muscle fibers, expression of agrin is low [54] and thus is unlikely to effectively compensate for a loss of laminin-α2 in dyW/dyW mice [86]. Design of mag was based on work revealing the binding activities of the muscle (non-neural) agrin isoform. Mag consists of the high-affinity, laminin coiled-coil domain-binding NtA domain [87, 88] and one follistatin repeat fused to the LG/LE C-terminal domains responsible for high-affinity binding to αDG [89]. Thus, mag links Lm-411 with its extremely low affinity for αDG and α7β1, to αDG. Transgenic, muscle-specific expression of mag in dyW/dyW strongly improves muscle histology and survival [73]. While survival was not measured, mag greatly improved muscle histology and regeneration also in dy3K /dy3K mice [90]. In dyW/dyW mice, similar efficacy like mag was also achieved with a fusion protein between the agrin NtA domain and the αDG-binding domain V of perlecan [91], corroborating the idea that this disease-ameliorating effect is based on the linkage of Lm-411 to αDG. Finally, substantial repair was even achieved when expression of mag in dyW /dyW mice was initiated after birth [91].
Figure 3. αLNNd and mag repair of laminin function.
A. Domain structure and functional activities of αLNNd and mag. Regions derived from laminin-α1 are in green; regions derived from nidogen-1 are in orange. Mag is a miniaturized version of agrin with N-terminal regions (blue) and C-terminal parts (red). B. In the ambulatory form of LAMA2 MD and its dy2J/dy2J mouse model, a truncated version of Lm-211 (“dy2J–Lm-211”) is expressed. αLNNd binds to the nidogen-binding site and creates an artificial short arm with a functional LN domain. This enables polymerization and promotes assembly of a stable basement membrane. C. In the absence of laminin-α2, which causes the severe non-ambulatory form of LAMA2 MD, the amount of Lm-41 1 is increased. Lm-411 is unable to polymerize and binds poorly to integrin α7β1 and to αDG. Co-expression of αLNNd and mag provide the necessary domains for polymerization and αDG anchorage. Together the two linker proteins restore laminin assembly and muscle binding, which results in strong amelioration of the severe muscular dystrophy in dyW/dyW mice.
While mag improves Lm-411 binding to αDG, this approach is still unable to cause self-polymerization. To enable Lm-411 self-polymerization, another linker protein was generated that consists of the laminin-α1 LN-LEa domains fused to a nidogen-1 fragment extending from the G2 to the G3 domain [92]. This fusion protein, called αLNNd, was shown to bind to Lm-111 at the nidogen-binding locus (Lmγ1 domain LEb3) and to collagen-IV. Lm-111 binding is mediated by the C-terminal G3 domain of αLNNd while collagen-binding is mediated by the G2 domain (Fig. 3A,C). The presence of the α1LN domain enables polymerization of laminins that lack either the αLN domain or the entire α-short arm [92]. On cultured C2C12 myotubes, co-incubation of αLNNd with α-short arm-truncated Lm-111 restores binding to levels indistinguishable to full-length Lm-111 [12]. The idea that restoration of laminin self-polymerization may provide benefit to the mild forms of LAMA2 MD, was tested by the muscle-specific transgenic expression of αLNNd in dy2J/dy2J mice, which bears an internal in-frame α2LN deletion and is defective of self-polymerization [11]. Indeed, expression of αLNNd resulted in a strong amelioration of the dystrophic phenotype. The effect was manifested as a prevention of fibrosis and restoration of fore-limb grip strength, both to normal levels. αLNNd also improved myofiber shape, size, and numbers to control levels. It led to the restoration of near-normal levels of Lm-211 in the muscle BM, suggesting that this reflects improved assembly of the truncated Lm-211 expressed in dy2J/dy2J into the BM [12].
While the success with the αLNNd transgene was an exciting advance that also helped confirm the model of BM assembly, only a small fraction of patients would be candidates for repair with αLNNd alone. For most patients there is a complete or near-complete loss of Lm-211. As highlighted above and in Fig. 2C, there is substantial compensation by Lm-411 in these severe forms of the LAMA2 MD. If indeed self-polymerization and cell anchoring are the two key functions of Lm-211, restoration of self-polymerization should provide additional benefit to mag-expressing dyW /dyW mice, representing the severe form of LAMA2 MD. This hypothesis was well supported by the observation that combination of αLNNd and mag restores binding of Lm-411 to myotubes that reaches levels that are only observed with Lm-211, while either of the linker proteins led to a moderate increase in Lm-411 binding [58]. Most importantly, transgenic co-expression of αLNNd and mag in dyW /dyW mice completely restored BM stability with substantial recovery of muscle force and size, increased body weight, and the extension of survival from a few weeks to more than 1.5 years [58]. About one third of the double transgenic dyW/dyW mice lived more than two years, comparable to wild-type mice. These results are thus clear proof for the model that laminin self-polymerization and its anchorage to the muscle fiber surface are the two key activities that are needed to stabilize muscle BM.
The cDNAs for αLNNd and mag are in the size range that can be packaged into adeno-associated virus (AAV) with an appropriate promoter. AAV is one of the most promising of the somatic gene delivery systems in which high expression can be achieved in muscle and other tissues. The experience of human clinical trials with AAV to correct muscular dystrophies has been encouraging. While protein can be lost due to host cellular immune responses to transgene products and AAV capsid [93], this problem has been reduced by avoiding the creation of transgene neoantigens (note that the domains of αLNNd and mag are expressed in LAMA2 MD patients), by optimizing serotype, and by adding immunosuppressive therapy [94]. Promising success has been recently achieved in the treatment of limb-girdle dystrophy type 2D (α-sarcoglycan deficiency) with recombinant AAV with persistence of protein expression, restoration of the sarcoglycan-complex, and increased muscle fiber size [95]. Furthermore, simultaneous infection with two AAV vectors has been successfully accomplished for the delivery of dysferlin, dystrophin or CRISPR-Cas9 genome-editing components to muscle [84, 96, 97]. By the same token, it should be possible to separately deliver the two linker proteins αLNNd and mag to LAMA2 MD patients with paired AAV somatic gene delivery in muscle and peripheral nerve. It should further be noted that expression of mag using systemic infection with AAV has been shown to provide similar benefit to dyW/dyW mice as the transgenic expression [98]. Thus, while the combined repair strategy is more challenging than one using a single AAV, such strategy is compatible with existing technologies and limitations.
Interference with secondary events of disease
Treatment strategies using small molecules attempt to halt disease progression by interfering with downstream consequences of Lm-211 deficiency (Table 3). Such strategies are aimed to inhibit the apoptosis, inflammation and fibrosis that all result from the primary injury. Drug therapies to treat the sequelae of the dystrophy show improvements, but are limited in that they do not correct the underlying structural defect. The most advanced of those drugs is omigapil. It is an orally available deprenyl-analog that binds to GAPDH. The drug inhibits apoptosis and was originally developed by Novartis and is now further developed by Santhera Pharmaceuticals Ltd. Treatment of dyW/dyW and dy2J/dy2J mice was shown to ameliorate disease progression by inhibition of apoptosis, positively affecting fibrosis and improving respiration [99, 100]. Omigapil is currently being evaluated in a clinical phase 1 trial for congenital muscular dystrophies (CALLISTO, NCT01805024, Carsten Bönnemann and Reghan Foley, NINDS, NIH). Losartan is an antagonist of angiotensin-II type I receptor that is approved for the treatment of high blood pressure. The drug was found to reduce fibrosis by acting on TGF-β1 activity. In dyW/dyW and dy2J/dy2J mice, it inhibits TGF-β signaling, lowers TGF-β1 levels in serum, reduces fibrosis, and increases the number of myofibers [101–103]. Inhibition of inflammation in dy/dy mice with prednisolone, a potent steroid, improves survival and forelimb strength [104]. Bortezomib, a proteasome inhibitor, was evaluated in dy3K /dy3K mice [105] and was found to increase survival from a mean of 23.5 days to 32 days, to slightly increase weights and the number of quadriceps myofibers, and to decrease the proportion of caspase-3 positive fibers. However, the same treatment regimen in dy2J/dy2J mice did not show much benefit [105, 106]. All those treatments are potentially of value for the LAMA2 MD patients, it remains to be seen, however, whether they are efficacious enough to provide benefit in a clinical trial.
Table 3.
Drug treatments directed at sequelae of muscle damage evaluated in mouse models
| Inhibition target |
Mouse | Treatment | Observed Bene- fits |
Comments | Ref. |
|---|---|---|---|---|---|
| Inflammation | dyW/dyW | Prednisolone (10 µg/gm/wk p.o.) | Survival increase from 66 to 90% at 24 weeks; no change in weight; 16% increase in grip strength. | Long-term steroid use many side-effects that may make this drug problematic in LAMA2-MD. | [104, 111] |
| Protein degradation (proteosome genes) | dy3K/dy3K & dy2J/dy2J | Bortezomib (0.4 mg/kg i.v.) | Median survival, weight and fiber size in dy3K/dy3K mildly increased with decreased regeneration. | No beneficial effect in dy2J/dy2J mouse. | [105, 106] |
| Apoptosis | dyW/dyW & dy2J/dy2J | Omigapil (0.1–1 mg/kg oral) | Improves respiration, locomotion and weight and protects from early mortality Modest reduction of fibrosis. | Under phase 1 clinical trial for LAMA2 and collagen-VI deficiencies. | [99, 100] |
| Fibrosis | dyW/dyW & dy2J/dy2J | Losartan (0.6 mg/ml drinking water) | Decreases TGF-β signaling. Reduced fibrosis and inflammation. Improved locomotion, strength and weight. | Losartan is an angiotensin II receptor type 1 antagonist shown to block pro-fibrotic action of TGF-β. | [101–103] |
| Other | dyW/dyW | Doxycycline (6 mg/ml drinking water) | Median survival increased from 32 to 70 days; small weight increase; improved behavior and histology. | Tetracycline derivatives may benefit the dystrophy by apoptosis inhibition. | [112] |
Concluding Remarks
Considerable progress has been made in developing treatments for LAMA2 deficiency. The greatest degree of phenotypic improvement has been observed in mice in which the underlying structural defect was corrected. Nonetheless, significant improvements were also obtained with agents that reduce the untoward sequelae. We cannot be confident that a single therapy will suffice to effectively treat dystrophic patients. The different therapies are not mutually exclusive and it stands to reason that the most successful protocols may prove to be combinatorial ones that repair the structural defect but also separately reduce the apoptotic, inflammatory and fibrotic sequelae.
Highlights.
Basement membranes have reached center stage due to their involvement in many physiological and pathological conditions.
Genetic diseases of basement membranes are debilitating and affect many organs resulting in diverse phenotypes.
We provide an overview of the field and discuss developmental, structural and biochemical aspects of basement membranes.
We introduce the special issue and outline key components of basement membrane biology.
Acknowledgments
This review was supported by NIH grant R01-DK36425 (to PDY) and grants from the Cantons of Basel-Stadt, Basel-Landschaft, Swiss Foundation for Research on Muscle Diseases, Association Francaise contre les Myopathies, and Neuromuscular Research Association Basel (to MAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Current Pharmaceutical Design. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3(2):a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald PR, Lustig A, Steinmetz MO, Kammerer RA. Laminin chain assembly is regulated by specific coiled-coil interactions. J Struct Biol. 2010;170(2):398–405. doi: 10.1016/j.jsb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Smyth N, Vatansever HS, Meyer M, Frie C, Paulsson M, Edgar D. The targeted deletion of the LAMC1 gene. Ann N Y Acad Sci. 1998;857:283–6. doi: 10.1111/j.1749-6632.1998.tb10133.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Guo SS, Fassler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215(4):445–456. doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez-Mallebrera C, Brown SC, Sewry CA, Muntoni F. Congenital muscular dystrophy: molecular and cellular aspects. Cell Mol Life Sci. 2005;62(7–8):809–23. doi: 10.1007/s00018-004-4510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uitto J, Has C, Vahidnezhad H, Youssefian L, Bruckner-Tuderman L. Molecular pathology of the basement membrane zone in heritable blistering diseases:: The paradigm of epidermolysis bullosa. Matrix Biol. 2017;57–58:76–85. doi: 10.1016/j.matbio.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218(2):213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Colognato H, Yurchenco PD. The laminin alpha2 expressed by dystrophic dy(2J) mice is defective in its ability to form polymers. Curr Biol. 1999;9(22):1327–1330. doi: 10.1016/s0960-9822(00)80056-1. [DOI] [PubMed] [Google Scholar]
- 12.McKee KK, Crosson SC, Meinen S, Reinhard JR, Ruegg MA, Yurchenco PD. Chimeric protein repair of laminin polymerization ameliorates muscular dystrophy phenotype. J Clin Invest. 2017;127(3):1075–1089. doi: 10.1172/JCI90854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sframeli M, Sarkozy A, Bertoli M, Astrea G, Hudson J, Scoto M, Mein R, Yau M, Phadke R, Feng L, Sewry C, Fen ANS, Longman C, McCullagh G, Straub V, Robb S, Manzur A, Bushby K, Muntoni F. Congenital muscular dystrophies in the UK population: Clinical and molecular spectrum of a large cohort diagnosed over a 12-year period. Neuromuscul Disord. 2017;27(9):793–803. doi: 10.1016/j.nmd.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest. 1999;79(12):1601–13. [PubMed] [Google Scholar]
- 15.Meinen S, Lin S, Thurnherr R, Erb M, Meier T, Ruegg MA. Apoptosis inhibitors and mini-agrin have additive benefits in congenital muscular dystrophy mice. EMBO Mol Med. 2011;3(8):465–79. doi: 10.1002/emmm.201100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shorer Z, Philpot J, Muntoni F, Sewry C, Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. J Child Neurol. 1995;10(6):472–5. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- 17.Mercuri E, Pennock J, Goodwin F, Sewry C, Cowan F, Dubowitz L, Dubowitz V, Muntoni F. Sequential study of central and peripheral nervous system involvement in an infant with merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 1996;6(6):425–9. doi: 10.1016/s0960-8966(96)00383-5. [DOI] [PubMed] [Google Scholar]
- 18.Chan SH, Foley AR, Phadke R, Mathew AA, Pitt M, Sewry C, Muntoni F. Limb girdle muscular dystrophy due to LAMA2 mutations: diagnostic difficulties due to associated peripheral neuropathy. Neuromuscul Disord. 2014;24(8):677–83. doi: 10.1016/j.nmd.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Alkan A, Sigirci A, Kutlu R, Aslan M, Doganay S, Yakinci C. Merosin-negative congenital muscular dystrophy: diffusion-weighted imaging findings of brain. J Child Neurol. 2007;22(5):655–9. doi: 10.1177/0883073807303219. [DOI] [PubMed] [Google Scholar]
- 20.Sunada Y, Edgar TS, Lotz BP, Rust RS, Campbell KP. Merosin-negative congenital muscular dystrophy associated with extensive brain abnormalities. Neurology. 1995;45(11):2084–9. doi: 10.1212/wnl.45.11.2084. [DOI] [PubMed] [Google Scholar]
- 21.Tan E, Topaloglu H, Sewry C, Zorlu Y, Naom I, Erdem S, M DA, Muntoni F, Dubowitz V. Late onset muscular dystrophy with cerebral white matter changes due to partial merosin deficiency. Neuromuscul Disord. 1997;7(2):85–9. doi: 10.1016/s0960-8966(96)00421-x. [DOI] [PubMed] [Google Scholar]
- 22.Caro PA, Scavina M, Hoffman E, Pegoraro E, Marks HG. MR imaging findings in children with merosin-deficient congenital muscular dystrophy. AJNR Am J Neuroradiol. 1999;20(2):324–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnemann CG, Wang CH, Quijano-Roy S, Deconinck N, Bertini E, Ferreiro A, Muntoni F, Sewry C, Beroud C, Mathews KD, Moore SA, Bellini J, Rutkowski A, North KN. Diagnostic approach to the congenital muscular dystrophies. Neuromuscul Disord. 2014;24(4):289–311. doi: 10.1016/j.nmd.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain SA, Carafoli F, Hohenester E. Determinants of laminin polymerization revealed by the structure of the alpha5 chain amino-terminal region. EMBO Rep. 2011;12(3):276–82. doi: 10.1038/embor.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carafoli F, Hussain SA, Hohenester E. Crystal structures of the network-forming short-arm tips of the laminin beta1 and gamma1 chains. PLoS One. 2012;7(7):e42473. doi: 10.1371/journal.pone.0042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allamand V, Sunada Y, Salih MA, Straub V, Ozo CO, Al-Turaiki MH, Akbar M, Kolo T, Colognato H, Zhang X, Sorokin LM, Yurchenco PD, Tryggvason K, Campbell KP. Mild congenital muscular dystrophy in two patients with an internally deleted laminin alpha2-chain. Hum Mol Genet. 1997;6(5):747–52. doi: 10.1093/hmg/6.5.747. [DOI] [PubMed] [Google Scholar]
- 27.Michelson AM, Russell ES, Harman PJ. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955;41(12):1079–84. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier H, Southard JL. Muscular dystrophy in the mouse caused by an allele at the dy-locus. Life Sci. 1970;9(3):137–44. doi: 10.1016/0024-3205(70)90306-1. [DOI] [PubMed] [Google Scholar]
- 29.Patton BL, Wang B, Tarumi YS, Seburn KL, Burgess RW. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. J Cell Sci. 2008;121(Pt. 10):1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- 30.Kuang W, Xu H, Vachon PH, Engvall E. Disruption of the lama2 gene in embryonic stem cells: laminin alpha 2 is necessary for sustenance of mature muscle cells. Exp Cell Res. 1998;241(1):117–25. doi: 10.1006/excr.1998.4025. [DOI] [PubMed] [Google Scholar]
- 31.Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415(1):33–9. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 32.Willmann R, Gordish-Dressman H, Meinen S, Ruegg MA, Yu Q, Nagaraju K, Kumar A, Girgenrath M, Coffey CBM, Cruz V, Van Ry PM, Bogdanik L, Lutz C, Rutkowski A, Burkin DJ. Improving Reproducibility of Phenotypic Assessments in the DyW Mouse Model of Laminin-alpha2 Related Congenital Muscular Dystrophy. J Neuromuscul Dis. 2017;4(2):115–126. doi: 10.3233/JND-170217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo LT, Zhang XU, Kuang W, Xu H, Liu LA, Vilquin JT, Miyagoe-Suzuki Y, Takeda S, Ruegg MA, Wewer UM, Engvall E. Laminin alpha2 deficiency and muscular dystrophy; genotype-phenotype correlation in mutant mice. Neuromuscul Disord. 2003;13(3):207–15. doi: 10.1016/s0960-8966(02)00266-3. [DOI] [PubMed] [Google Scholar]
- 34.Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994;8(3):297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- 35.Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995;4(6):1055–61. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- 36.Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and −8. J Cell Biol. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menezes MJ, McClenahan FK, Leiton CV, Aranmolate A, Shan X, Colognato H. The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J Neurosci. 2014;34(46):15260–80. doi: 10.1523/JNEUROSCI.3678-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Brien DP, Johnson GC, Liu LA, Guo LT, Engvall E, Powell HC, Shelton GD. Laminin alpha2 (merosin)-deficient muscular dystrophy and demyelinating neuropathy in two cats. J Neurol Sci. 2001;189(1–2):37–43. doi: 10.1016/s0022-510x(01)00559-7. [DOI] [PubMed] [Google Scholar]
- 40.Hall TE, Bryson-Richardson RJ, Berger S, Jacoby AS, Cole NJ, Hollway GE, Berger J, Currie PD. The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc Natl Acad Sci U S A. 2007;104(17):7092–7. doi: 10.1073/pnas.0700942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelton GD, Liu LA, Guo LT, Smith GK, Christiansen JS, Thomas WB, Smith MO, Kline KL, March PA, Flegel T, Engvall E. Muscular dystrophy in female dogs. J Vet Intern Med. 2001;15(3):240–4. doi: 10.1892/0891-6640(2001)015<0240:mdifd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157(7):1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee KK, Harrison D, Capizzi S, Yurchenco PD. Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem. 2007;282(29):21437–47. doi: 10.1074/jbc.M702963200. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169(1):179–89. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belanto JJ, Mader TL, Eckhoff MD, Strandjord DM, Banks GB, Gardner MK, Lowe DA, Ervasti JM. Microtubule binding distinguishes dystrophin from utrophin. Proc Natl Acad Sci U S A. 2014;111(15):5723–8. doi: 10.1073/pnas.1323842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezniczek GA, Konieczny P, Nikolic B, Reipert S, Schneller D, Abrahamsberg C, Davies KE, Winder SJ, Wiche G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J Cell Biol. 2007;176(7):965–77. doi: 10.1083/jcb.200604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K. Ligand-binding specificities of laminin-binding integrins: A comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25(3):189–197. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Sung U, O'Rear JJ, Yurchenco PD. Localization of heparin binding activity in recombinant laminin G domain. Eur. J. Biochem. 1997;250(1):138–43. doi: 10.1111/j.1432-1033.1997.00138.x. [DOI] [PubMed] [Google Scholar]
- 49.Ido H, Nakamura A, Kobayashi R, Ito S, Li S, Futaki S, Sekiguchi K. The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin gamma chains in integrin binding by laminins. J Biol Chem. 2007;282(15):11144–54. doi: 10.1074/jbc.M609402200. [DOI] [PubMed] [Google Scholar]
- 50.Briggs DC, Yoshida-Moriguchi T, Zheng T, Venzke D, Anderson ME, Strazzulli A, Moracci M, Yu L, Hohenester E, Campbell KP. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat Chem Biol. 2016;12(10):810–4. doi: 10.1038/nchembio.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, Campbell KP. Molecular Recognition by LARGE Is Essential for Expression of Functional Dystroglycan. Cell. 2004;117(7):953–64. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335(6064):93–6. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LV, Fujita S, Fukuta-Ohi H, Shimizu T, Ruegg MA, Matsumura K. Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J Biol Chem. 1996;271(38):23418–23. doi: 10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]
- 54.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273(1):600–5. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 55.Hohenester E, Tisi D, Talts JF, Timpl R. The crystal structure of a laminin G-like module reveals the molecular basis of alpha-dystroglycan binding to laminins, perlecan, and agrin. Mol Cell. 1999;4(5):783–92. doi: 10.1016/s1097-2765(00)80388-3. [DOI] [PubMed] [Google Scholar]
- 56.Talts JF, Andac Z, Gorhing W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. Embo J. 1999;18(4):863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han R, Kanagawa M, Yoshida-Moriguchi T, Rader EP, Ng RA, Michele DE, Muirhead DE, Kunz S, Moore SA, Iannaccone ST, Miyake K, McNeil PL, Mayer U, Oldstone MB, Faulkner JA, Campbell KP. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of alpha-dystroglycan. Proc Natl Acad Sci U S A. 2009;106(31):12573–9. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinhard JR, Lin S, McKee KK, Meinen S, Crosson SC, Sury M, Hobbs S, Maier G, Yurchenco PD, Ruegg MA. Linker proteins restore basement membrane and correct LAMA2-related muscular dystrophy in mice. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yurchenco PD, Tsilibary EC, Charonis AS, Furthmayr H. Laminin polymerization in vitro. Evidence for a two-step assembly with domain specificity. J.Biol.Chem. 1985;260:7636–7644. [PubMed] [Google Scholar]
- 60.Yurchenco PD, Cheng YS, Colognato H. Laminin forms an independent network in basement membranes. J Cell Biol. 1992;117(5):1119–33. doi: 10.1083/jcb.117.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yurchenco PD, Cheng YS. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 1993;268:17286–17299. [PubMed] [Google Scholar]
- 62.Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272(50):31525–32. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 63.Garbe JH, Gohring W, Mann K, Timpl R, Sasaki T. Complete sequence, recombinant analysis and binding to laminins and sulphated ligands of the N-terminal domains of laminin [alpha]3B and [alpha]5 chains. Biochem J. 2002;362(Pt 1):213–221. doi: 10.1042/0264-6021:3620213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–21. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rogers RS, Nishimune H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017;57–58:86–105. doi: 10.1016/j.matbio.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189(1):4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa T, Wondimu Z, Oikawa Y, Gentilcore G, Kiessling R, Egyhazi Brage S, Hansson J, Patarroyo M. Laminins 411 and 421 differentially promote tumor cell migration via alpha6beta1 integrin and MCAM (CD146) Matrix Biol. 2014;38:69–83. doi: 10.1016/j.matbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Ishikawa T, Wondimu Z, Oikawa Y, Ingerpuu S, Virtanen I, Patarroyo M. Monoclonal antibodies to human laminin alpha4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of alpha6beta1 integrin and MCAM to alpha4-laminins. Matrix Biol. 2014;36:5–14. doi: 10.1016/j.matbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin {alpha}4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275(45):35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- 70.Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, Chu ML. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO. J. 1991;10:3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poschl E, Mayer U, Stetefeld J, Baumgartner R, Holak TA, Huber R, Timpl R. Site-directed mutagenesis and structural interpretation of the nidogen binding site of the laminin gamma1 chain. Embo J. 1996;15(19):5154–9. [PMC free article] [PubMed] [Google Scholar]
- 72.Patton BL, Connoll AM, Martin PT, Cunningham JM, Mehta S, Pestronk A, Miner JH, Sanes JR. Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul Disord. 1999;9(6–7):423–33. doi: 10.1016/s0960-8966(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 73.Moll J, Barzaghi P, Lin S, Bezakova G, Lochmuller H, Engvall E, Muller U, Ruegg MA. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413(6853):302–7. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 74.Ringelmann B, Roder C, Hallmann R, Maley M, Davies M, Grounds M, Sorokin L. Expression of Laminin alpha1, alpha2, alpha4, and alpha5 Chains, Fibronectin, and Tenascin-C in Skeletal Muscle of Dystrophic 129ReJ dy/dy Mice. Exp Cell Res. 1999;246(1):165–182. doi: 10.1006/excr.1998.4244. [DOI] [PubMed] [Google Scholar]
- 75.Yu H, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem. J. 2003;371(Pt 2):289–99. doi: 10.1042/BJ20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient Congenital Muscular Dystrophy. Partial genetic correction in two mouse models. J Clin Invest. 1998;102(4):844–52. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gawlik K, Miyagoe-Suzuki Y, Ekblom P, Takeda S, Durbeej M. Laminin {alpha}1 chain reduces muscular dystrophy in laminin {alpha}2 chain deficient mice. Hum Mol Genet. 2004;13(16):1775–1784. doi: 10.1093/hmg/ddh190. [DOI] [PubMed] [Google Scholar]
- 78.Gawlik KI, Li JY, Petersen A, Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum Mol Genet. 2006;15(18):2690–700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 79.Rooney JE, Knapp JR, Hodges BL, Wuebbles RD, Burkin DJ. Laminin-111 protein therapy reduces muscle pathology and improves viability of a mouse model of merosin-deficient congenital muscular dystrophy. Am J Pathol. 2012;180(4):1593–602. doi: 10.1016/j.ajpath.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perrin A, Rousseau J, Tremblay JP. Increased Expression of Laminin Subunit Alpha 1 Chain by dCas9-VP160. Mol Ther Nucleic Acids. 2017;6:68–79. doi: 10.1016/j.omtn.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gawlik KI, Oliveira BM, Durbeej M. Transgenic expression of Laminin alpha1 chain does not prevent muscle disease in the mdx mouse model for duchenne muscular dystrophy. Am J Pathol. 2011;178(4):1728–37. doi: 10.1016/j.ajpath.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aartsma-Rus A, Straub V, Hemmings R, Haas M, Schlosser-Weber G, Stoyanova-Beninska V, Mercuri E, Muntoni F, Sepodes B, Vroom E, Balabanov P. Development of Exon Skipping Therapies for Duchenne Muscular Dystrophy: A Critical Review and a Perspective on the Outstanding Issues. Nucleic Acid Ther. 2017;27(5):251–259. doi: 10.1089/nat.2017.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aoki Y, Nagata T, Yokota T, Nakamura A, Wood MJ, Partridge T, Takeda S. Highly efficient in vivo delivery of PMO into regenerating myotubes and rescue in laminin-alpha2 chain-null congenital muscular dystrophy mice. Hum Mol Genet. 2013;22(24):4914–28. doi: 10.1093/hmg/ddt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kemaladewi DU, Maino E, Hyatt E, Hou H, Ding M, Place KM, Zhu X, Bassi P, Baghestani Z, Deshwar AG, Merico D, Xiong HY, Frey BJ, Wilson MD, Ivakine EA, Cohn RD. Correction of a splicing defect in a mouse model of congenital muscular dystrophy type 1A using a homology-directed-repair-independent mechanism. Nat Med. 2017;23(8):984–989. doi: 10.1038/nm.4367. [DOI] [PubMed] [Google Scholar]
- 85.Tintignac LA, Brenner HR, Ruegg MA. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol Rev. 2015;95(3):809–52. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- 86.Eusebio A, Oliveri F, Barzaghi P, Ruegg MA. Expression of mouse agrin in normal, denervated and dystrophic muscle. Neuromuscul Disord. 2003;13(5):408–15. doi: 10.1016/s0960-8966(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 87.Denzer AJ, Brandenberger R, Gesemann M, Chiquet M, Ruegg MA. Agrin binds to the nerve-muscle basal lamina via laminin. J Cell Biol. 1997;137(3):671–83. doi: 10.1083/jcb.137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. Embo J. 1998;17(2):335–43. doi: 10.1093/emboj/17.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gesemann M, Cavalli V, Denzer AJ, Brancaccio A, Schumacher B, Ruegg MA. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 1996;16(4):755–67. doi: 10.1016/s0896-6273(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 90.Bentzinger CF, Barzaghi P, Lin S, Ruegg MA. Overexpression of mini-agrin in skeletal muscle increases muscle integrity and regenerative capacity in laminin-{alpha}2-deficient mice. Faseb J. 2005;19(8):934–42. doi: 10.1096/fj.04-3376com. [DOI] [PubMed] [Google Scholar]
- 91.Meinen S, Barzaghi P, Lin S, Lochmuller H, Ruegg MA. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages. J Cell Biol. 2007;176(7):979–93. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKee KK, Capizzi S, Yurchenco PD. Scaffold-forming and adhesive contributions of synthetic laminin-binding proteins to basement membrane assembly. J Biol Chem. 2009;284(13):8984–8994. doi: 10.1074/jbc.M809719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z, Tapscott SJ, Chamberlain JS, Storb R. Immunity and AAV-Mediated Gene Therapy for Muscular Dystrophies in Large Animal Models and Human Trials. Front Microbiol. 2011;2:201. doi: 10.3389/fmicb.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D, Zhong L, Nahid MA, Gao G. The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert Opin Drug Deliv. 2014;11(3):345–64. doi: 10.1517/17425247.2014.871258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mendell JR, Rodino-Klapac L, Sahenk Z, Malik V, Kaspar BK, Walker CM, Clark KR. Gene therapy for muscular dystrophy: Lessons learned and path forward. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grose WE, Clark KR, Griffin D, Malik V, Shontz KM, Montgomery CL, Lewis S, Brown RH, Jr, Janssen PM, Mendell JR, Rodino-Klapac LR. Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS ONE. 2012;7(6):e39233. doi: 10.1371/journal.pone.0039233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Odom GL, Gregorevic P, Allen JM, Chamberlain JS. Gene therapy of mdx mice with large truncated dystrophins generated by recombination using rAAV6. Mol Ther. 2011;19(1):36–45. doi: 10.1038/mt.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qiao C, Li J, Zhu T, Draviam R, Watkins S, Ye X, Chen C, Li J, Xiao X. Amelioration of laminin-{alpha}2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proc Natl Acad Sci U S A. 2005;102(34):11999–2004. doi: 10.1073/pnas.0502137102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Erb M, Meinen S, Barzaghi P, Sumanovski LT, Courdier-Fruh I, Ruegg MA, Meier T. Omigapil ameliorates the pathology of muscle dystrophy caused by laminin-alpha2 deficiency. J Pharmacol Exp Ther. 2009;331(3):787–95. doi: 10.1124/jpet.109.160754. [DOI] [PubMed] [Google Scholar]
- 100.Yu Q, Sali A, Van der Meulen J, Creeden BK, Gordish-Dressman H, Rutkowski A, Rayavarapu S, Uaesoontrachoon K, Huynh T, Nagaraju K, Spurney CF. Omigapil treatment decreases fibrosis and improves respiratory rate in dy(2J) mouse model of congenital muscular dystrophy. PLoS ONE. 2013;8(6):e65468. doi: 10.1371/journal.pone.0065468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Accorsi A, Kumar A, Rhee Y, Miller A, Girgenrath M. IGF-1/GH axis enhances losartan treatment in Lama2-related muscular dystrophy. Hum Mol Genet. 2016;25(21):4624–4634. doi: 10.1093/hmg/ddw291. [DOI] [PubMed] [Google Scholar]
- 102.Elbaz M, Yanay N, Aga-Mizrachi S, Brunschwig Z, Kassis I, Ettinger K, Barak V, Nevo Y. Losartan, a therapeutic candidate in congenital muscular dystrophy: studies in the dy(2J)/dy(2J) mouse. Ann Neurol. 2012;71(5):699–708. doi: 10.1002/ana.22694. [DOI] [PubMed] [Google Scholar]
- 103.Meinen S, Lin S, Ruegg MA. Angiotensin II type 1 receptor antagonists alleviate muscle pathology in the mouse model for laminin-alpha2-deficient congenital muscular dystrophy (MDC1A) Skelet Muscle. 2012;2(1):18. doi: 10.1186/2044-5040-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Connolly AM, Keeling RM, Streif EM, Pestronk A, Mehta S. Complement 3 deficiency and oral prednisolone improve strength and prolong survival of laminin alpha2-deficient mice. J Neuroimmunol. 2002;127(1–2):80–7. doi: 10.1016/s0165-5728(02)00104-2. [DOI] [PubMed] [Google Scholar]
- 105.Korner Z, Fontes-Oliveira CC, Holmberg J, Carmignac V, Durbeej M. Bortezomib partially improves laminin alpha2 chain-deficient muscular dystrophy. Am J Pathol. 2014;184(5):1518–28. doi: 10.1016/j.ajpath.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 106.Korner Z, Durbeej M. Bortezomib Does Not Reduce Muscular Dystrophy in the dy2J/dy2J Mouse Model of Laminin alpha2 Chain-Deficient Muscular Dystrophy. PLoS ONE. 2016;11(1):e0146471. doi: 10.1371/journal.pone.0146471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Russell ES, Silvers WK, Loosli R, Wolfe HG, Southard JL. New genetically homogeneous background for dystrophic mice and their normal counterparts. Science. 1962;135(3508):1061–2. doi: 10.1126/science.135.3508.1061. [DOI] [PubMed] [Google Scholar]
- 108.Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc.Natl.Acad.Sci.U.S.A. 1994;91:5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J.Biol.Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- 110.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2009;106(19):7991–6. doi: 10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Connolly AM, Schierbecker J, Renna R, Florence J. High dose weekly oral prednisone improves strength in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12(10):917–25. doi: 10.1016/s0960-8966(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 112.Girgenrath M, Beermann ML, Vishnudas VK, Homma S, Miller JB. Pathology is alleviated by doxycycline in a laminin-alpha2-null model of congenital muscular dystrophy. Ann Neurol. 2009;65(1):47–56. doi: 10.1002/ana.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]