Abstract
Background
Acute myeloid leukemia (AML) is a serious disease with complex etiology and marked variation in survival. Known prognostic factors include AML subtypes, age at diagnosis and sex. However, survival outcomes may vary across healthcare systems. In this study, we evaluated the survival patterns in individuals diagnosed with AML at ages 0–24 years in the US and England between prognostic features and across countries.
Methods
We obtained data on 4387 and 2194 subjects from the US Surveillance Epidemiology and End Result (SEER) registries and UK National Cancer Data Repository (NCDR). Subjects were diagnosed and followed in 1995–2014. Kaplan-Meier curve and stratified Cox proportional hazards regression were used.
Results
Overall risk of mortality was 23% lower in English patients compared to that in the US patients (adjusted hazard ratio (aHR), 95% confidence Interval (CI): 0.77, 0.71–0.84). Survival difference of similar extent was observed in subgroups of sex and age at diagnosis. However, mortality risks between two countries varied substantially across AML subtypes, especially in AML inv(16) (1.81, 0.61–5.34), AML with minimal differentiation (0.54, 0.25–1.17), AML without maturation (0.38, 0.20–0.74) and AML with maturation (0.52, 0.31–0.86).
Conclusions
Similar to the population trend, mortality risk across sex, age at diagnosis, and most AML subtypes was lower in England. Survival outcome for AML with and without maturation in England was better than the population trend, while that for AML inv (16) was worse. Our findings suggest that future etiologic and policy research may uncover the underlying mechanisms and contribute to closing these morality gaps.
Keywords: Acute myeloid leukemia, childhood, young adult, survival, US, England, prognostic factors
1. Introduction
Acute myeloid leukemia (AML) is a serious disease with complex etiology and marked variation in survival.1–5 The five-year survival rate is about 60–70%6, but it varies substantially by age at diagnosis and subtypes characterized based on morphology and cytogenetics.1,7–9 Sex and socioeconomic factors also have been reported as having contributed to survival variation.2,4,5 Favorable prognostic characteristics include younger age at diagnosis, acute promyelocytic leukemia (APL), inv(16), −t(8;21), t(15;17), trisomy 21, female sex, and higher socioeconomic status, while older age at diagnosis, genetic abnormalities such as del(5q) and t(9;22), male sex and lower socioeconomic status are indicators of poor prognosis.1–3,8–12 In addition, survival variation was observed across interactive subgroups of AML subtypes, age at diagnosis and sex.2,9,13
Survival outcomes associated with these prognostic features are modifiable and often vary with the standard of healthcare system. Timely diagnosis, tailored treatment and effective management can reduce these variations. Examining survival patterns across healthcare settings could lead to identifying factors for further improving overall survival. To this end, we conducted a study on survival outcome of childhood and young adult AML patients in the US and England who were diagnosed in 1995–2014. The US and England are two countries with similar degrees of human development14 yet different healthcare systems with respect to insurance and clinical practice. In England, all citizens are covered by the National Health Service, which is free at delivery.15 In the US, about 95% of children and 80% of adults 18–65 years are covered by public and private insurance, and citizens and permanent residents aged 65 years and older are eligible for Medicare, a government-run health insurance system.16 Also, in England, new therapeutic options are evaluated by a central agency (National Institute for Health Care and Excellence) prior to becoming part of standard of care. In the US, new drugs are approved by the Food and Drug Administration, and the National Comprehensive Cancer Network and the American Society of Clinical Oncology publish suggested guidelines for the care of patients with AML. Thus, the USA and England vary in terms of access to care and standardization of care.15 The aim of the present study is to evaluate the survival of patients diagnosed with AML at ages 0–24 years in the US and England between prognostic features and across countries. Increased knowledge of survival patterns across prognostic features in different health systems may help tailor care and management to be more precise, thereby reducing mortality at the population level.
2. Material and Methods
2.1 Study population
Eligible subjects were individuals diagnosed with AML in the US or England at ages 0–24 years and followed between 1995 and 2014. US data were obtained from the Surveillance Epidemiology and End Result (SEER) registry. English data were obtained from the UK National Cancer Data Repository (NCDR). Death certificate-only cases (n=10 from SEER, n=14 from NCDR) were excluded because of missing follow-up time since diagnosis.
Data on 4387 patients in the US were used for analysis. Currently, seventeen participating registries located in geographically diverse regions across the country collect and record demographic, clinical treatment and outcome data on patients using standardized coding manual.17,18 The SEER-covered population represents approximately 28% of the US population and is comparable to the general US population with regards to sex, race–ethnicity, and measures of poverty and education.19 Data were accessed under the SEER research data use agreement. Data on 2194 eligible patients in England were used for analysis. The National Cancer Registration and Analysis Service of Public Health England (PHE) collects patient-level demographic and vital information on all cases of cancer that occur in people living in the country. Inpatient and outpatient admissions and treatment details were sourced into the NCDR from the Hospital Episode Statistics. Data from primary care practices throughout the UK were linked through the Clinical Practice Research Datalink. More details on the NCDR have been published previously.20–22
2.2 Study variables
We investigated AML survival trends by clinical and demographic features. Variables common to both US and England data and parametrized in the same way were: vital status at the latest follow-up, follow-up duration, sex, age at diagnosis, number of primaries, year of diagnosis, and AML subtype classified by the International Classification of Diseases for Oncology, third Edition (ICD-O-3)/WHO 2008 definitions.
Vital status at the latest follow-up was recorded as alive/censored or deceased. Follow-up duration between time from diagnosis to event was recorded in months. We had access to quantitative and interval data on age at diagnosis from US and England data, respectively. Based on the homogeneity of hazard risk of mortality, we grouped age at diagnosis as 0–9, 10–14, and 15–24 years in the analysis. Number of primaries was dichotomized into “single/multiple”. Multiple primaries are defined as more than one synchronous cancer (diagnosed at the same time) or metachronous (diagnosed within 2 months apart) in the same individual.23 Year of diagnosis was grouped into 5-year intervals: 1995–1999, 2000–2004, 2005–2009, and 2010–2014.The AML subtypes’ ICD-O-3 / WHO 2008 definitions and italicized abbreviations used in this study are as follows—9840/3: Acute erythroid leukemia (M6 type), AEL; 9861/3: Acute myeloid leukemia, AML-NOS; 9866/3: Acute promyelocytic leukemia (AML with t(15;17)(q22;q12)) PML/RARA, APL; 9867/3: Acute myelomonocytic leukemia, AMML; 9910/3: Acute megakaryoblastic leukemia, AMKL; 9871/3: AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB-MYH11, AML inv(16); 9872/3: Acute myeloid leukemia with minimal differentiation, AML with minimal differentiation; 9873/3: Acute myeloid leukemia without maturation, AML without maturation; 9874/3: Acute myeloid leukemia with maturation, AML with maturation; and other subtypes (ICD-O-3 codes 9865/3, 9869/3, 9895/3, 9896/3, 9897/3, 9898/3, 9911/3, 9920/3). An index of multiple deprivation (IMD) encompassing a range of domains such as income, employment, education, access/barriers to services, and living environment/housing was used to adjust for community-level socio-economic effects. The English IMD 2010 was a rank variable of 5 levels derived from 36 indicators.24 We generated a comparable IMD as a rank variable using percentiles of the latent based linear combination of 22 socioeconomic status (SES) indicators from the 2010–2014 Census American Community Survey. Supplementary Table S1 displayed SES variables and their corresponding weights used to derive the latent variable.
2.3 Statistical analysis
Distribution of vital status was summarized by study variables. Kaplan–Meier curve was used to visualize the change in survival probability over time since diagnosis in each country among patients with a specific prognostic feature. Adherence to proportional hazard assumptions was determined based on the basis of smoothed plots of Schoenfeld residuals and log-negative log plots performed before and after stratifying on year interval of diagnosis.25. Therefore, we stratified the estimation of hazard ratios (HRs) associated with covariates in the uni- and multivariable proportional hazards models by year of diagnosis intervals and reported the pooled HR across strata. To assess the relationship between mortality and the prognostic factors simultaneously, we estimated adjusted HRs and associated 95% confidence intervals (CI) in a multivariable model for each country that included all prognostic factors. To examine between-country mortality risk contrast in different patient groups—males, females, patients diagnosed at different ages, and those with specific AML subtypes, we conducted uni- and multi-variable stratified analysis. All analyses were two-tailed with the level of significance at 5%. All statistical analysis was performed using R Statistical Software, version 3.3.1.26
3. Results
A total of 4387 and 2194 individuals with AML in the US and England were included in the analysis, respectively. Table 1 presented the relationship between mortality and study variable by country. Crude mortality proportion was higher in the US (41.6%) than in England (41.1%). In both countries, male, being diagnosed at ages 15 to 24, multiple primaries, and higher degree of regional deprivation were associated with higher mortality risk. There were some differences in mortality of patients with different AML subtypes between countries. Among patients in England, AML subtypes with the highest mortality proportions were AMML (44.7%), AML-NOS (45.9%), and other subtypes (50%). In the US, the subtypes were AMML (48.5%), AEL (48.1%), and AML with minimal differentiation (60.7%). Mortality reduced in both countries over the years by about 20% during the study period. These differences in proportions were also reflected in the Kaplan-Meier curves in Figure 1.
Table 1.
Demographic and clinical characteristics of AML patients diagnosed at ages 0–24 years in 1995–2014 in the US and England.
| Variables | UK (N = 2194) | US (N = 4387) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Death n (%) |
Alive n (%) |
uHR (95% CI) |
p | Death (%) |
Alive (%) |
uHR (95% CI) |
p | |
| Within-country | 901 (41.1) |
1293 (58.9) |
1827 (41.6) |
367 (58.4) |
||||
| Sex | ||||||||
| Female | 418 (40.5) |
613 (59.5) |
ref | – | 831 (40.0) |
1247 (60.0) |
ref | – |
| Male | 483 (41.5) |
680 (58.5) |
1.03 (0.90, 1.17) |
0.671 | 996 (43.2) |
1310 (56.8) |
1.12 (1.02, 1.23) |
0.018 |
| Age at diagnosis, years | ||||||||
| 0 to 9 | 284 (34.6) |
537 (65.4) |
ref | – | 586 (37.5) |
977 (62.5) |
ref | – |
| 10 to 14 | 133 (39.2) |
206 (60.8) |
1.15 (0.94, 1.42) |
0.177 | 306 (40.3) |
453 (59.7) |
1.07 (0.93, 1.23) |
0.355 |
| 15 to 24 | 484 (46.8) |
550 (53.2) |
1.47 (1.27, 1.7) |
<0.0001 | 935 (45.3) |
1129 (54.7) |
1.34 (1.21, 1.48) |
<0.0001 |
| Tumor Count | ||||||||
| 1 | 816 (39.8) |
1234 (60.2) |
ref | – | 1663 (40.2) |
2474 (59.8) |
ref | – |
| ≥2 | 85 (58.6) |
59 (41.4) |
1.7 (1.36, 2.13) |
<0.0001 | 164 (66.4) |
83 (33.6) |
2.55 (2.17, 3.00) |
<0.0001 |
| AML subtype | ||||||||
| APL | 36 (17.4) |
171 (82.6) |
ref | – | 128 (20.1) |
509 (79.9) |
ref | – |
| AEL | 11 (31.4) |
24 (68.6) |
1.80 (0.92, 3.54) |
0.088 | 25 (48.1) |
27 (51.9) |
2.80 (1.82, 3.03) |
<0.0001 |
| AML, NOS | 674 (45.9) |
796 (54.1) |
3.03 (2.17, 4.24) |
<0.0001 | 937 (47.1) |
998 (51.6) |
2.72 (2.26, 3.27) |
<0.0001 |
| AMML | 76 (44.7) |
94 (55.3) |
2.81 (1.89, 4.18) |
<0.0001 | 186 (48.5) |
223 (54.5) |
2.51 (2.01, 3.15) |
<0.0001 |
| AML inv(16) | 6 (33.3) |
12 (66.7) |
2.47 (1.04, 5.87) |
0.041 | 16 (15.5) |
87 (84.5) |
0.80 (0.48, 1.35) |
0.407 |
| AML, minimal differentiation | 9 (37.5) |
15 (62.5) |
2.18 (1.05, 4.54) |
0.036 | 85 (60.7) |
55 (39.3) |
3.48 (2.64, 4.58) |
<0.0001 |
| AML without maturation | 12 (26.7) |
33 (73.3) |
1.37 (0.71, 2.63) |
0.349 | 88 (47.3) |
98 (52.7) |
2.48 (1.89, 3.25) |
<0.0001 |
| AML with maturation | 21 (23.1) |
70 (76.9) |
1.09 (0.64, 1.87) |
0.749 | 104 (40.6) |
152 (59.4) |
2.08 (1.61, 2.70) |
<0.0001 |
| AMKL | 42 (39.6) |
64 (60.4) |
2.43 (1.55, 3.79) |
<0.0001 | 103 (41.0) |
148 (59.0) |
2.31 (1.78, 2.99) |
<0.0001 |
| Other | 14 (50.0) |
14 (50.0) |
4.29 (2.31, 7.97) |
<0.0001 | 155 (37.1) |
263 (62.9) |
2.40 (1.89, 3.03) |
<0.0001 |
| Year of diagnosis | ||||||||
| 1995 – 1999 | 255 (50.8) |
247 (49.2) |
ref | – | 290 (54.9) |
238 (45.1) |
ref | – |
| 2000 – 2004 | 239 (44.6) |
297 (55.4) |
0.86 (0.72, 1.03) |
0.105 | 539 (48.9) |
563 (51.1) |
0.86 (0.75, 0.99) |
0.039 |
| 2005 – 2009 | 215 (39.1) |
335 (60.9) |
0.77 (0.64, 0.92) |
0.005 | 463 (39.9) |
697 (60.1) |
0.69 (0.59, 0.79) |
<0.0001 |
| 2010 – 2014 | 192 (31.7) |
414 (68.3) |
0.69 (0.57, 0.84) |
0.0002 | 535 (33.5) |
1062 (66.5) |
0.75 (0.65, 0.86) |
<0.0001 |
| IMD2010 | ||||||||
| 1 - least deprived | 156 (40.4) |
230 (59.6) |
ref | – | 345 (39.4) |
530 (60.6) |
ref | – |
| 2 | 133 (33.8) |
260 (66.2) |
0.79 (0.63, 1.00) |
0.05 | 348 (41.0) |
502 (59.1) |
1.06 (0.91, 1.23) |
0.465 |
| 3 | 168 (42.2) |
230 (57.8) |
1.08 (0.86, 1.34) |
0.512 | 374 (41.0) |
542 (59.2) |
1.10 (0.95, 1.28) |
0. 186 |
| 4 | 196 (43.0) |
260 (57.0) |
1.11 (0.90, 1.36) |
0.351 | 417 (43.0) |
553 (57.1) |
1.16 (1.01, 1.34) |
0. 038 |
| 5 - most deprived | 248 (44.3) |
312 (55.7) |
1.13 (0.93, 1.39) |
0.22 | 343 (44.3) |
432 (55.7) |
1.30 (1.12, 1.51) |
0.0007 |
Figure 1.
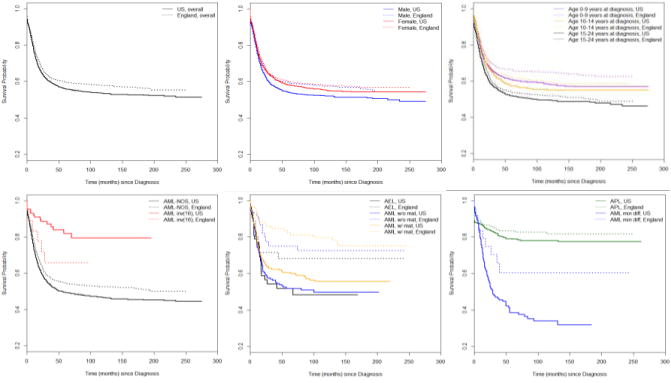
Kaplan-Meier curve for between-country comparison of survival probability in patients grouped by clinical features. Abbreviation used for AML subtypes: AML w/o mat: AML without maturation; AML w/mat: AML with maturation; AML min diff: AML with minimal differentiation.
Shown in Table 2 are the mutually adjusted associations between prognostic factors and AML survival in each country, stratified by year of diagnosis. After adjustment, the association between older age at diagnosis, multiple primaries, specific AML subtypes, and higher degree of socioeconomic deprivation and higher risk of mortality remained in both countries, though the extent varied. For example, the risk of mortality among patients diagnosed at 15–24 years was 1.51 (95% CI: 1.35–1.69) times as high in the US and 1.38 (1.18–1.62) times as high in England, compared to the hazard isk of mortality among children diagnosed at ages 0–9 years in the same country. Among the factors examined, AML subtype showed the most variable pattern of association with mortality between countries. Compared to patients with APL in their respective countries, US patients with subtypes other than AML inv(16) and UK patients with subtypes other than AML with and without maturation showed significantly higher risk of mortality.
Table 2.
Association between prognostic factors and overall survival. Adjusted hazard risks of mortality (aHR) and associated 95% confidence intervals (CI) were estimated using multivariable Cox proportional hazards model stratified by year of diagnosis.
| Variables | UK | US | ||
|---|---|---|---|---|
|
| ||||
| aHR (95% CI) | p | aHR (95% CI) | p | |
| Sex | ||||
| Female | ref | – | ref | – |
| Male | 1.02 (0.90, 1.17) | 0.726 | 1.06 (0.97, 1.16) | 0.221 |
| Age at diagnosis, years | ||||
| 0 to 9 | ref | – | ref | – |
| 10 to 14 | 1.30 (1.05, 1.60) | 0.017 | 1.14 (0.99, 1.32) | 0.067 |
| 15 to 24 | 1.38 (1.18, 1.62) | <0.0001 | 1.51 (1.35, 1.69) | <0.0001 |
| Tumor Count | ||||
| 1 | ref | – | ref | – |
| ≥2 | 1.56 (1.25, 1.96) | 0.0001 | 2.42 (2.04, 2.88) | <0.0001 |
| AML subtype | ||||
| APL | ref | – | ref | – |
| AEL | 2.08 (1.05, 4.10) | 0.035 | 3.20 (2.08, 4.92) | <0.0001 |
| AML-NOS | 2.99 (2.13, 4.19) | <0.0001 | 2.87 (2.38, 3.46) | <0.0001 |
| AMML | 3.23 (2.16, 4.82) | <0.0001 | 2.56 (2.04, 3.21) | <0.0001 |
| AML inv(16) | 2.47 (1.04, 5.90) | 0.041 | 0.85 (0.50, 1.42) | 0.527 |
| AML, minimal differentiation | 2.65 (1.27, 5.53) | 0.009 | 3.75 (2.85, 4.95) | <0.0001 |
| AML without maturation | 1.58 (0.82, 3.05) | 0.174 | 2.53 (1.92, 3.32) | <0.0001 |
| AML with maturation | 1.22 (0.71, 2.10) | 0.478 | 2.24 (1.72, 2.91) | <0.0001 |
| AMKL | 2.97 (1.88, 4.70) | <0.0001 | 3.04 (2.31, 4.00) | <0.0001 |
| Other | 4.54 (2.44, 8.47) | <0.0001 | 2.05 (1.60, 2.62) | <0.0001 |
| IMD2010 | ||||
| 1 - least deprived | ref | – | ref | – |
| 2 | 0.80 (0.63, 1.01) | 0.06 | 1.04 (0.89, 1.20) | 0.634 |
| 3 | 1.10 (0.89, 1.37) | 0.376 | 1.09 (0.94, 1.27) | 0.230 |
| 4 | 1.16 (0.94, 1.43) | 0.177 | 1.18 (1.03, 1.37) | 0.001 |
| 5 - most deprived | 1.16 (0.95, 1.42) | 0.142 | 1.29 (1.11, 1.50) | 0.001 |
Hazard ratios of mortality between England and the US were presented in Table 3. Overall, mortality risk was lower in England (unadjusted HR, 95% CI: 0.86, 0.79–0.94). After adjusting for sex, age at diagnosis, tumor count, subtype and socioeconomic differences, and stratifying by year of diagnosis intervals, this between-country difference persisted (adjusted HR, 95% CI: 0.77, 0.71–0.84).
Table 3.
Between-country (referent: US) comparison of hazard risk of mortality with associated 95% confidence intervals (CI) in patients grouped by clinical features. Unadjusted (uHR) and adjusted (aHR) hazard ratios were estimated using Cox proportional hazards model stratified by year of diagnosis.
| Variables | uHR (95% CI) (US=referent) | p | aHR* (95% CI) (US=referent) | p |
|---|---|---|---|---|
| Between country | 0.86 (0.79, 0.94) | 0.0002 | 0.77 (0.71, 0.84) | <0.0001 |
| Sex | ||||
| Female | 0.89 (0.79, 1.00) | 0.051 | 0.76 (0.68, 0.86) | <0.0001 |
| Male | 0.83 (0.74, 0.92) | 0.0006 | 0.77 (0.68, 0.87) | <0.0001 |
| Age at diagnosis, years | ||||
| 0 to 9 | 0.81 (0.70, 0.93) | 0.003 | 0.75 (0.65, 0.87) | 0.0001 |
| 10 to 14 | 0.86 (0.70, 1.06) | 0.153 | 0.86 (0.70, 1.06) | 0.159 |
| 15 to 24 | 0.88 (0.79, 0.99) | 0.031 | 0.75 (0.66, 0.84) | <0.0001 |
| Tumor Count | ||||
| 1 | 0.86 (0.79, 0.94) | 0.0007 | 0.79 (0.72, 0.86) | <0.0001 |
| ≥2 | 0.62 (0.47, 0.81) | 0.0007 | 0.58 (0.42, 0.80) | 0.001 |
| AML subtype | ||||
| APL | 0.76 (0.52, 1.10) | 0.151 | 0.77 (0.53, 1.12) | 0.170 |
| AEL | 0.70 (0.32, 1.55) | 0.382 | 1.00 (0.44, 2.26) | 0.991 |
| AML-NOS | 0.84 (0.76, 0.93) | 0.0007 | 0.77 (0.70, 0.86) | <0.0001 |
| AMML | 0.84 (0.64, 1.11) | 0.227 | 0.97 (0.73, 1.29) | 0.813 |
| AML inv(16) | 2.21 (0.81, 5.98) | 0.119 | 1.81 (0.61, 5.34) | 0.089 |
| AML, minimal differentiation | 0.37 (0.18, 0.77) | 0.008 | 0.54 (0.25, 1.17) | 0.119 |
| AML without maturation | 0.39 (0.21, 0.73) | 0.003 | 0.38 (0.20, 0.74) | 0.005 |
| AML with maturation | 0.40 (0.25, 0.65) | 0.0003 | 0.52 (0.31, 0.86) | 0.012 |
| AMKL | 0.77 (0.53, 1.11) | 0.163 | 0.71 (0.49, 1.04) | 0.081 |
| Other | 1.24 (0.70, 2.18) | 0.464 | 1.49 (0.84, 2.66) | 0.173 |
As an example, multivariable stratified proportional hazards model for males included age at diagnosis, tumor count, AML subtype, and regional deprivation; multivariable stratified proportional hazards model for APL patients included sex, age at diagnosis, tumor count, and regional deprivation.
After controlling for known prognostic factors in the multivariable analysis (Table 3), patients diagnosed in England were at about 23% lower risk of mortality than those who were diagnosed in the US. The similar extent of difference was observed between patients of two countries across subgroups of sex and age at diagnosis. Among subtypes, AML without maturation (adjusted HR, 95% CI: 0.38, 0.20–0.74), AML with maturation (0.52, 0.31–0.86), and AML-NOS (0.77, 0.70–0.86) were associated with substantially lower risk of mortality in England. While, although not significant, English patients with AML inv(16) showed a higher risk of mortality than their US counterparts (1.81, 0.61–5.34).
4. Discussion
In this study, we investigated heterogeneity in risk of mortality associated with prognostic features in young AML patients from two countries that had different health systems. We observed mortality difference between countries for a given prognostic feature, as well as survival differences across prognostic features within each country, using population-based data on patients diagnosed at ages 0–24 years in the US and England between 1995 and 2014.
Taken as a whole, survival outcome was better in England. We observed a similar trend in subgroups identified by sex and age at diagnosis. Mortality risk associated with AML subtypes showed large variation between two countries. Most subtypes are associated with lower risk of mortality in England compared to that in the US. AML with and without maturation in England were associated with risks of mortality that were even lower than the population-level trend. AML inv(16) has been widely reported as a favorable prognostic subtype.27–30 However, US data, but not English data, in our study sample supported this. Factors contributing to these between-country mortality differences warrant further investigation. We speculate that the observed survival differences in pediatric AML between two countries may be due in part to differences between health systems. Though we did not have insurance information on the study subjects, according to published reports, about 35% of children and young adults in the US were uninsured or underinsured during the study period.16,31 Lack of sufficient healthcare coverage has been associated with increased barriers to healthcare access including lack of a usual source of care, difficulty of obtaining referrals, and delayed care.31
Strength of this study include the novelty of this research question, as well as access to two large, high-quality datasets that capture diverse characteristics of young AML patients. This is a first study on survival of AML patients diagnosed during childhood and young adulthood in two health systems of two countries with comparable extent of economic development. Using well-validated and maintained population-based data from two countries, we were able to confirm previous findings, such as increased mortality risk in males compared to females7 and in patients diagnosed at older ages than younger ages,32 and assess survival by established prognostic features and compute risk estimates with optimal precision.
Like most epidemiological studies, this study was not without limitations. Treatment, insurance, and race/ethnicity are known/have been reported to be associated with survival disparity,1,33,34 but information on treatment was not available in SEER data, information on insurance was only available in SEER data for individuals diagnosed since 2007, and we did not have access to NCDR race-ethnicity data. Therefore, our findings could have been confounded by these factors. Race/ethnicity may be a proxy for socioeconomic and biological/genetic diversity. We control for the effect of socioeconomic disparity on the association between survival and prognostic factors by adjusting for area deprivation in the analysis. Also, register data such as those from SEER and NCDR have been routinely collected in the course of long follow-up time and are observational in nature. We applied precaution during the data analysis and used covariate adjustment and stratification to reduce the bias in estimates due to unmeasured confounders.
5. Conclusions
In conclusion, AML patients diagnosed between ages 0–24 years were at higher risk of mortality in the US compared to that in England. AML with and without maturation were associated with the largest survival benefit in England. While, AML inv(16), known to have favorable prognosis, was associated with higher risk mortality in England. Our findings suggest that there is potential for further improvement in both health systems, and factors contributing to these observed differences warrant future research.
Supplementary Material
Highlights.
Mortality in AML patients diagnosed at ages 0–24 years was lower in England than US.
Between-country mortality difference was similar across gender and age at diagnosis.
Mortality Risk tend to be lower in England for all AML subtypes except AML inv(16).
The difference was the largest for AML w/and w/o maturation.
Contrary to usual trend, mortality risk for AML inv(16) was much higher in England.
Acknowledgments
Authors received partial salary support from the National Institutes of Health (NIH) COBRE Grant 8P20GM103464-9 (PI: Shaffer) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under Grant no. U54-GM104941 (PI: Binder-Macleod). This project involves data derived from patient-level information collected by the NHS, as part of the care and support of cancer patients. The data is collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England (PHE). Access to the data was facilitated by the PHE Office for Data Release. The authors thank Shona Lucitt and Sean McPhail of Public Health England for help with NCDR data access.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hossain MJ, Xie L, Caywood EH. Prognostic factors of childhood and adolescent acute myeloid leukemia (AML) survival: evidence from four decades of US population data. Cancer Epidemiol. 2015;39(5):720–726. doi: 10.1016/j.canep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain MJ, Xie L. Sex disparity in childhood and young adult acute myeloid leukemia (AML) survival: Evidence from US population data. Cancer Epidemiol. 2015;39(6):892–900. doi: 10.1016/j.canep.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 4.Radhi M, Meshinchi S, Gamis A. Prognostic factors in pediatric acute myeloid leukemia. Curr Hematol Malig Rep. 2010;5(4):200–206. doi: 10.1007/s11899-010-0060-z. [DOI] [PubMed] [Google Scholar]
- 5.Knoble NB, Alderfer MA, Hossain MJ. Socioeconomic status (SES) and childhood acute myeloid leukemia (AML) mortality risk: Analysis of SEER data. Cancer Epidemiol. 2016;44:101–108. doi: 10.1016/j.canep.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childhood Leukemia Survival Rates. https://www.cancer.org/cancer/leukemia-in-children/detection-diagnosis-staging/survival-rates.html. Accessed May 30, 2017.
- 7.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. The Oncologist. 2007;12(3):341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 8.Tarlock K, Meshinchi S. Pediatric acute myeloid leukemia: biology and therapeutic implications of genomic variants. Pediatr Clin North Am. 2015;62(1):75–93. doi: 10.1016/j.pcl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Manola KN. Cytogenetics of pediatric acute myeloid leukemia. Eur J Haematol. 2009;83(5):391–405. doi: 10.1111/j.1600-0609.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 10.Petridou ET, Sergentanis T, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. doi: 10.1093/annonc/mdu572. 2014:mdu572. [DOI] [PubMed] [Google Scholar]
- 11.British Committee for Standards in Haematology. Milligan DW, Grimwade D, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135(4):450–474. doi: 10.1111/j.1365-2141.2006.06314.x. [DOI] [PubMed] [Google Scholar]
- 12.Kroll ME, Stiller CA, Murphy MFG, Carpenter LM. Childhood leukaemia and socioeconomic status in England and Wales 1976–2005: evidence of higher incidence in relatively affluent communities persists over time. Br J Cancer. 2011;105(11):1783–1787. doi: 10.1038/bjc.2011.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease. Blood Rev. 2003;17(2):71–97. doi: 10.1016/s0268-960x(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 14.United Nations Development Programme. Human Development Reports. http://hdr.undp.org/en/composite/HDI. Accessed May 30, 2017.
- 15.Redaniel MT, Pulte D, Jeffreys M. Survival disparities by age and country of diagnosis for patients with acute leukemia. Leuk Lymphoma. 2015;56(10):2787–2792. doi: 10.3109/10428194.2015.1014358. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics (US) Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US); 2016. http://www.ncbi.nlm.nih.gov/books/NBK367640/ [PubMed] [Google Scholar]
- 17.Ruhl J, Adamo M, Dickie L, Sun L, Johnson C. Hematopoietic and lymphoid neoplasm coding manual. Bethesda MD Natl Cancer Inst. 2015:25–29. [Google Scholar]
- 18.Adamo M, Ruhl J, Dickie L. SEER program coding and staging manual. Bethesda MD Natl Cancer Inst. 2016 https://seer.cancer.gov/manuals/2016/SPCSM_2016_maindoc.pdf. Accessed May 1, 2016.
- 19.Howlader N, Noone A-M, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- 20.National Cancer Data Repository. http://www.ncin.org.uk/collecting_and_using_data/national_cancer_data_repository/#. Accessed December 18, 2017.
- 21.PHE. Routes to diagnosis 2015 update: leukaemia: acute myeloid. 2016 Feb; http://www.ncin.org.uk/view?rid=3117. Accessed December 18, 2017.
- 22.Miller S. Cancer data flower in Public Health England (PHE) 2017 May; http://www.ncin.org.uk/view?rid=3220. Accessed December 18, 2017.
- 23.Amer MH. Multiple neoplasms, single primaries, and patient survival. Cancer Manag Res. 2014;6:119–134. doi: 10.2147/CMAR.S57378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne RA, Abel GA. UK indices of multiple deprivation-a way to make comparisons across constituent countries easier. Health Stat Q. 2012;53:22. [Google Scholar]
- 25.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14(15):1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 26.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. http://www.R-project.org. [Google Scholar]
- 27.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 28.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 29.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 30.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 31.Kogan MD, Newacheck PW, Blumberg SJ, et al. Underinsurance among children in the United States. N Engl J Med. 2010;363(9):841–851. doi: 10.1056/NEJMsa0909994. [DOI] [PubMed] [Google Scholar]
- 32.Razzouk BI, Estey E, Pounds S, et al. Impact of age on outcome of pediatric acute myeloid leukemia. Cancer. 2006;106(11):2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- 33.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108(1):74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gramatges MM, Rabin KR. The adolescent and young adult with cancer: state of the art– acute leukemias. Curr Oncol Rep. 2013;15(4):317–324. doi: 10.1007/s11912-013-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


