Abstract
Fatty acid drug discovery (FADD) is defined as the identification of novel, specialized bioactive mediators that are derived from fatty acids and have precise pharmacological/therapeutic potential. A number of reports indicate that dietary intake of omega-3 fatty acids and limited intake of omega-6 promotes overall health benefits. In 1929, Burr and Burr indicated the significant role of essential fatty acids for survival and functional health of many organs. In reference to specific dietary benefits of differential omega-3 fatty acids, docosahexaenoic and eicosapentaenoic acids (DHA and EPA) are transformed to monohydroxy, dihydroxy, trihydroxy, and other complex mediators during infection, injury, and exercise to resolve inflammation. The presented FADD approach describes the metabolic transformation of DHA and EPA in response to injury, infection, and exercise to govern uncontrolled inflammation. Metabolic transformation of DHA and EPA into a number of pro-resolving molecules exemplifies a novel, inexpensive approach compared to traditional, expensive drug discovery. DHA and EPA have been recommended for prevention of cardiovascular disease since 1970. Therefore, the FADD approach is relevant to cardiovascular disease and resolution of inflammation in many injury models. Future research demands identification of novel action targets, receptors for biomolecules, mechanism(s), and drug-interactions with resolvins in order to maintain homeostasis.
Keywords: inflammation, lipid mediators, lipoxygenase, macrophages, neutrophils, Omega-3 fatty acids, resolvins, resolution
2. Introduction
Fatty acids are functionally diverse, caloric energy molecules involved in several cellular signalling events that are associated with various physiological processes after metabolism (Zárate et al., 2017). Dietary intake of specific polyunsaturated fatty acids (PUFAs; linolenic and linoleic acid) protects from a wide range of diseases including heart disease and cancer, to neurological and metabolic disorders. There are several reports and findings demonstrating that balanced intake of omega-3 and omega-6 PUFAs provide overall health benefits. The therapeutic potential of fatty acids primarily relies on their metabolic intermediates and lipid mediator bioactives. Fatty acid metabolism is largely mediated through an oxygenation process catalyzed by three classes of enzymes: lipoxygenase (LOX), cyclooxygenase (COX) and cytochrome P450 (CYP) epoxygenase that results in an array of fatty acid-derived metabolic lipid mediators (Massey and Nicolaou, 2011). Two major omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), gain the attention of researchers worldwide to transform into pro-resolving biomolecules, enabling a novel and inexpensive approach to drug discovery. Recent emerging reports indicate that DHA- and EPA-derived lipid mediators facilitate resolution of inflammation in the tissue to protect against cardiac disorders (Mittal et al., 2010). The Fatty Acid Drug Discovery (FADD) outlook is a novel application of fatty acid-derived lipid mediators for specific therapeutic implication. Fatty acid metabolic transformation is under the regulation of LOXs, COXs, and CYP and results in a group of bioactive lipid mediators with pro-inflammatory and anti-inflammatory activities (Wanga et al., 2014). The catalyzing enzyme interaction of fatty acid-based precursors and oxygenation results in formation of specialized pro-resolving mediators (SPM; AA-Lipoxin, EPA- E series Resolvins and DHA- D series Resolvins and Protectins and Maresins) and pro-inflammatory lipid mediators (LTs and PGs) (Serhan, 2014). The resolvins, both D- and E-series, are reported as key lipid mediators in the diverse resolution of the inflammatory process. Mass spectrometry-based analytical technology allows the identification of selective metabolic transformations of fatty acids, which is essential for SPM biosynthesis utilizing dietary PUFAs (DHA and EPA) and their intermediates to facilitate resolution of inflammation (Zhang, Guodong et al., 2014). In the presented review, we address the differential applications of lipid mediators in the course and resolution of inflammation with a model of drug discovery, particularly for fatty acid-derived molecules.
3. Primer on drug discovery
The cost of traditional drug discovery escalates each year, now reaching more than 2.6 billion dollars, including the price of failure and the opportunity cost (Mullard, 2014). Moreover, medicine without adverse effects is rare due to complex human metabolism, diet-drug interaction, and disease pathology. Hiring chemists to perform an array of drug discovery steps, conducting post-marketing surveillance, and monitoring adverse effects on a large number of patients contributes significantly to the cost of the new drug. An alternative route that reduces the enormous cost of the drug discovery is recycling or repurposing known molecules for different uses (Cressey, 2011). However, this approach to control obesity, for example, is ineffective without consideration of the extensive evolution of human metabolism and progressive sedentary lifestyle. Thus, to overcome the failure in drug discovery and to develop novel drugs for metabolic dysregulation, alternative approaches are urgently needed. The human metabolism evolves with time and works non-stop from birth to death to adapt to injury, infection, stress (e.g., exercise, change in circadian rhythm, etc.), and climate change (different seasons/year). The body responds to injury and stress with the adaptive response and forms differential molecules using the available substrate. Therefore, precise chemical knowledge of substrate-enzyme interaction (protein, fat, carbohydrate and amino acid) and information on degradation products will provide unique bioactive options.
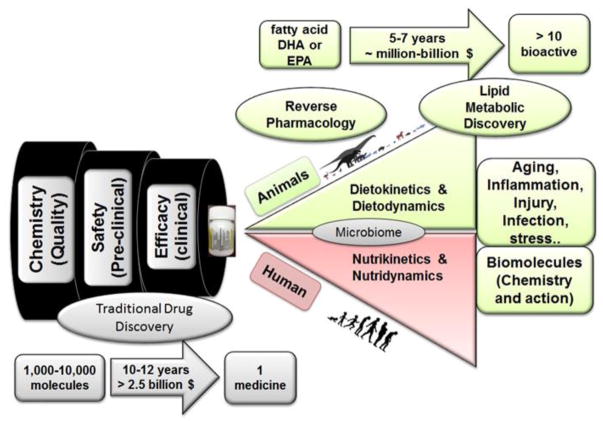
In the presented summary, we review two omega-3 fatty acids, DHA and EPA, which form endogenous specialized biomolecules that resolve inflammation in response to injury, infection, and exercise. The pharmaceutical industry is using the template of these molecules to develop novel medicine in the form of lipids, short and long acting peptides, proteins, or gases (hydrogen sulphide, carbon monoxide, and nitric oxide) (Corminboeuf and Leroy, 2015; Perretti et al., 2015; Serhan, 2014). We demonstrate the process of novel drug development using traditional drug discovery, emphasizing specifically the quality, safety, and efficacy before reaching the market in Figure 1. Through the examples of DHA and EPA, the second half of the figure suggests that studying the absorption, distribution, metabolism, and excretion of any diet/nutrient will allow us to harness the benefit of reverse pharmacology (Patwardhan and Vaidya, 2010) (Figure 1). This process, during which previously identified molecular targets are used as drug candidates for intervention in pathological conditions, is an efficient approach to hone in on specific drug candidates faster than the classical pharmacology approach. For example, in reverse pharmacology, a novel molecule is identified, and a genomic study takes place. Compounds are then screened, identified, and functional studies are performed to target a specific drug candidate. This is opposite from the traditional pharmacologic process, which first attempts to understand the pharmacokinetics and functional activity of a compound, then identifies a drug candidate, and finally studies the genomics and molecular mechanisms associated with the drug (Takenaka, 2001). Of course, reverse pharmacology will not be the direct solution for neglected, orphan, and genetic diseases, but extensive knowledge of metabolism from birth to aging will provide a strategy for adapting necessary steps to treat a patient for many diseases. For the alternative drug discovery route, some governing bodies, such as the UK Medical Research Council, US National Institutes of Health, and the National Center for Advancing Translational Sciences, are making progress in collaborating with academic institutions to produce more basic discoveries for the advancement of medical therapeutic programs.
Figure 1. Process for Fatty Acid Drug Discovery (FADD) approach.
Illustration depicts the processes of traditional versus reverse pharmacology. Using fatty acids as drug biosynthesizing candidates, reverse pharmacology is a feasible, more cost-effective method to develop novel molecules to combat diseases associated with conditions such as aging, inflammation, injury, and infection. eicosapentaenoic acid (EPA); docosahexaenoic acid (DHA)
4. Primer on fatty acids, nutrition, and obesity
The global prevalence of obesity in men and women is directly linked to lack of healthy diet, practices of imbalanced lifestyle (disturbed sleep and wake cycle), and a number of obesity-related chronic diseases that include cardiovascular events (Djousse et al., 2012; Flegal et al., 2012; Gregg and Shaw, 2017; Held et al., 2012; Sowers, 2003). There are more than 100 factors that contribute to the development of obesity. Recent genomic data indicates that individual responses to fat molecules are much more complex than expected (Locke et al., 2015). Therefore, it is advisable to follow healthy eating practices by limiting emphasis on high and low fat/carbohydrate diet. The overall diet effect varies with age, lifestyle, intestinal microbiome, circadian rhythms (what time you eat), and lipid processing enzymes (lipases, lipoxygenases), which modify progression or repression of various diseases such as cardiovascular disease. Thus, dietary and nutrition research linking to obesity-related outcome surprisingly leads to more questions and convolution than a precise answer (Casazza et al., 2013). Until now, diet and obesity research has directed its focus on energetics and micronutrients such as protein, carbohydrates, and high/low fat, but very few groups have recognized that not all fats are created equal (Halade et al., 2012; Ramsden et al., 2011; Spector and Kim, 2015). Moreover, the requirement of fat or metabolic energy intake changes as the individual transitions throughout life and subsequently regulates immune cell phenotype and milieu. In this review, we propose two emerging and essential concepts of diet-enzyme interaction and diet-body interaction to form bioactive mediators. This is exemplified with therapeutic application of potent specialized pro-resolving biomolecules using the Fatty Acid Drug Discovery (FADD) approach. As the title indicates, we address two major concepts in the present study; the first concept is the role of EPA and DHA in health and second is metabolic transformation into a wide range of bioactive lipids, including specialized pro-resolving mediators (SPMs). FADD-derived novel biomolecules are proposed as a solution to resolve inflammation in differential acute and chronic disease pathology, including myocardial infarction, rather than a micronutrient approach. From the perspective of diet and nutrition, it is important to focus on dietokinetics or nutritkinetics, (Motilva et al., 2015; van Velzen et al., 2009) which can be defined as the body’s ability with the help of enzymes to absorb, distribute, store, metabolize, and excrete fat similar to any other dietary substance. The key functional effect of diet/nutrients on the body is termed “dietodynamics” or “nutridynamics,” which can be defined as the effect(s) of metabolized biomolecules on the body in homeostatic conditions or in disease pathology. Some reports on fat will measure outcome irrespective of nutritkinetics or dietokinetics. Dietodynamics or nutridynamics can be studied in many interventions with specific emphasis on one macronutrient. Now, with advances in mass spectrometry-based lipidomic and metabolomic analytical technology, it is feasible to determine that specific fatty acids or lipid molecules can be metabolized into a number of bioactive molecules in response to stress, exercise, infection, or injury with the help of different metabolizing body enzymes (lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome p450 (CYP) epoxygenase). The availability of lipid substrates and the activity of specific enzymes are prime factors to determining the nutrikinetics or nutridynamics for individual homeostasis, disease progression, resolution, and repression of disease pathology. Of note, these pathways are of significant importance for pain, infection, immunity, inflammation, stress, and exercise to develop novel pharmaceutical agents and have been since the beginning of drug discovery. Importantly, many drugs like aspirin, ibuprofen, diclofenac, misoprostol, paracetamol, montelukast, zafirlukast, pranlukast, zileuton, atorvastatin, simvastatin, lovastatin, cerivastatin, and sulindac interfere with these complex metabolic pathways.
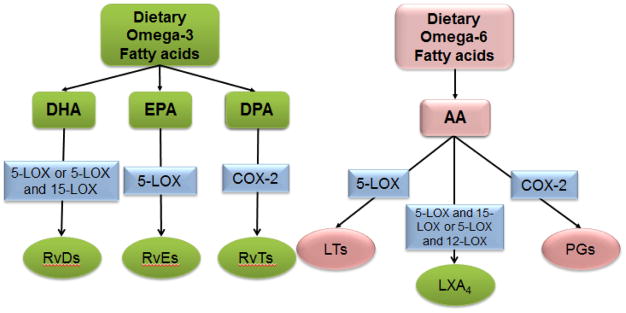
The metabolic transformation of omega-3 fatty acids (DHA and EPA) and omega-6 fatty acids (arachidonic acid; AA) into various mediators is provided in Figure 2. To exemplify the FADD approach, metabolic transformation of DHA and EPA into biomolecules is displayed in Figures 3 and 4 respectively. DHA and EPA are transformed into various species of monohydroxy DHA/EPA, dihydroxy DHA/EPA, and trihydroxy DHA/EPA with varying degrees of pro-resolving capacity. DHA- and EPA-derived lipid resolvin biomolecules are metabolites of marine oil component with enormous potential to resolve inflammation (Serhan, 2014). Therefore, DHA and EPA arguably contribute to preventing cardiovascular disease and, more specifically, chronic heart failure (De Caterina, 2011). In focusing on the effects of DHA, it could be postulated that the bioactive fatty acid may be a pro-resolving agent in heart failure pathology. To accomplish this, reverse pharmacology can be employed to other fatty acids as method that focuses on a translational, or bench-to-bedside approach. This is based on traditional observation, advancement of precise structural elucidation from the Serhan laboratory, and nutritional immunology knowledge that apply to reversing the discovery trend from classical laboratory-to-clinic pathway to preventive, precise, personalized, and prognostic therapy (Dalli and Serhan, 2017; Serhan et al., 2017).
Figure 2.
Metabolism of omega-3 and omega-6 dietary fatty acids and respective lipid mediators. Arachidonic acid (AA); eicosapentaenoic acid (EPA); docosahexaenoic acid (DHA); docosapentaenoic acid (DPA); 5-lipoxygenase (5-LOX); 12-lipoxygenase (12-LOX); 15-lipoxygenase (15-LOX); cyclooxygenase (COX); leukotriene (LT); lipoxin A4 (LXA4); prostaglandins (PGs); E-series resolvins (RvEs); D-series resolvins (RvDs); 13-series resolvins (RvTs).
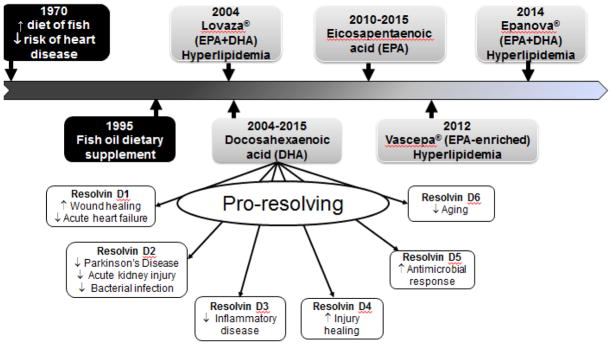
Figure 3.
Estimated chronological depiction of major points in the development of D-series resolvin mediators controlling inflammation and promoting resolution of inflammation. Timeline displays the estimated progression of the fish oil-enriched diet to the development of drugs for the treatment of hyperlipidemia to the advancement of D-series mediators from DHA.
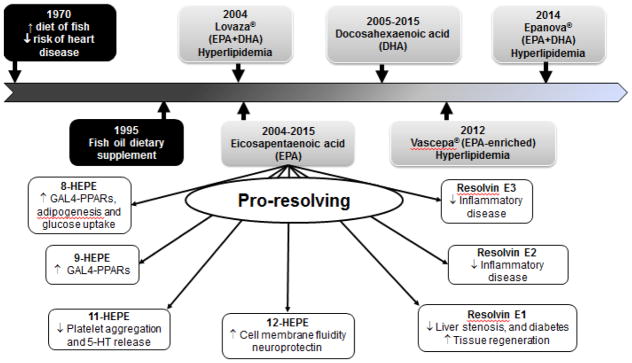
Figure 4.
Estimated chronological depiction of major points in the development of E-series resolvin mediators controlling inflammation and promoting resolution of inflammation. Timeline displays the progression of the fish oil-enriched diet to the development of drugs for the treatment of hyperlipidemia to the advancement of E-series mediators from EPA.
5. Essential fatty acids in health and disease
In 1929, Burr and Burr performed the first landmark survival study in rats without fatty acid droplets in their diet. Data from this study suggest that dietary fatty acids are essential for health and to prevent a series of diseases that were otherwise observed in rats fed a fat-free diet (Burr and Burr, 1973; Smith and Mukhopadhyay, 2012; Spector and Kim, 2015). Burr et al. coined the term “essential fatty acids” by identifying the fatty acids that cannot be synthesized by the body and, therefore, are critical for optimal health. Burr and Burr described the fat-free diet in great detail with the complete description of fat deficiency disease. The results proved that dietary fat is required to stimulate growth and prevent disease in humans and rodents (Burr and Burr, 1973; Hibbeln et al., 2006; Spector and Kim, 2015). The human body primarily depends on essential fatty acids in the diet, as well as the intake of fatty acid precursors, which are endogenously elongated and desaturated into long chain fatty acids. Accordingly, physiological and metabolic functions are mediated by the availability of long-chain polyunsaturated fatty acids (PUFA: >20 carbon chain length) with high levels of unsaturation (3–6 double bonds in structure). Later in 1970, Danish physicians Jorn Dyerberg and colleagues observed that Greenland Eskimos consuming fish-enriched nutrition had low incidences of heart disease (Bang et al., 1980). Fast forward to 1957, the use of fish oil as a dietary supplement or a DHA- (22:6n-3) and EPA- (20:5n-3) enriched diet was, and still is, recommended in order to prevent cardiovascular disease by many societies, including the American Heart Association and the European Cardiovascular Society (Dalen and Devries, 2014). As a result, the therapeutic drug Omacor (Reliant Inc. USA), currently known as Lovaza® by GlaxoSmithKline in North America (St Petersberg, FL, USA), was originally developed (Pronova Biocare, Lysaker, Norway) and approved for post-infarct patients in Europe since 2001 (Calder, 2013). These concentrated omega-3-acid ethyl esters (1000 mg to 4000 g/day) are approved for the treatment of hyperlipidemia, or high levels of marine lipids in the blood (2014; Lavie et al., 2009). As of today, the dietokinetics or nutrikinetics and dietodynamics or nutridynamics of these fatty acids in the physiological and pathological setting that spans from birth to aging are unclear. In contrast, fatty acids with one or more double bonds in trans-configuration, or trans-fatty acids, induce cardiovascular events and systemic inflammation (Mozaffarian et al., 2004a; Mozaffarian et al., 2004b). During this slow development in fatty acid knowledge and health benefits, Ancel Keys’ seven countries study results mislead with the conclusion that fat is the primary factor for cardiovascular disease, without accounting nutrikinetics and nutridynamics in rodents or humans (Keys, 1957; Keys et al., 1984). Traditionally, there are three major classes of circulating lipoproteins based on lipid and protein composition: 1) very low-density lipoproteins (VLDL), 2) low-density lipoproteins (LDL), and 3) high-density lipoproteins (HDL). With the advancement of lipid-protein research, further sub-classes within these classes of complexes and the various molecular components have emerged (2002). The lipid-lowering therapy is focused on LDL-lowering agents to reduce atherosclerotic cardiovascular disease risk (Lloyd-Jones et al., 2016). Of note, the overall presumed hypothesis that trans fats are unsafe was originally developed by Kummerow (Kummerow, 1979). With the advancement of the LIPID MAPS initiative, the lipids have been further reclassified into six major classes (Fahy et al., 2011; Fahy et al., 2005; Fahy et al., 2009; Fahy et al., 2007). Lack of quantitative analytical technology would be the primary cause of overall misunderstanding in fat convolution. Recent evidence suggests that single nucleotide polymorphisms in the fatty acid desaturase 1 and 2 (FADS 1/2) gene cluster influence an individual’s response to fat via either omega-3 or -6 fatty acids, affecting the subsequent production of pro-inflammatory and pro-resolving molecules (Roke and Mutch, 2014).
In general, fatty acids represent 30–35% of an individual’s total energy intake and are obtained from various sources, including plants, animals, and microbes, presented in Table 1 (Simopoulos, 2000). Fatty acids act as gene regulators and are essential components of the cell and mitochondrial lipid bilayer membranes (Royce et al., 2013). An increase in cis-unsaturated fatty acid percentage enhances membrane fluidity and also alters associated physiological functions (Yang et al., 2011). The role of fatty acids is not only restricted to governing membrane fluidity, but they also activate several native metabolic pathways useful in fighting life-threatening disorders, such as α-secretase-dependent amyloid precursor protein processing, which helps control Alzheimer’s disease (Sioen et al., 2006). Fatty acids are delivered to the heart using fatty-acid-binding proteins (FABP). In fact, several membrane-associated FABP have been reported, collectively also referred to as fatty acid transporters for cellular entry of fatty acids (Glatz et al., 2010). The cytoplasmic FABPs allow binding of fatty acids and uptake into cytoplasm via different organelles for various functions, such as by lipid droplets for storage; by endoplasmic reticulum for signalling, trafficking and membrane synthesis; by mitochondria or peroxisomes for oxidation; and by the nucleus for the control of lipid-mediated transcriptional programs (Schaap et al., 1999).
Table 1.
List of common dietary fatty acids, source and physicochemical significance in the context of human pathophysiology.
| Fatty Acid | Chemical Formula | Carbon backbone and Saturation | Physiological Significance | Source |
|---|---|---|---|---|
| Palmitic acid | C16H32O2 | 16 & Saturated | Energy source | Palm oil, meat and dairy fats |
| Stearic acid | C18H36O2 | 18 & Saturated | Energy source | Meat fat, cocoa butter |
| Oleic acid | C18H34O2 | 18 & Monounsaturated | Blood pressure regulation | Canola oil, sunflower oil and almond |
| Linoleic acid | C18H32O2 | 18 & Polyunsaturated | AA biosynthesis | Corn, safflower, soyabeen and sunflower |
| Linolenic acid | C18H30O2 | 18 & Polyunsaturated | Biosynthesis of EPA and DHA | Walnuts, Canola oil, cotton seed and sunflower oil |
| Arachidonic acid | C20H32O2 | 20 & Polyunsaturated | Source for inflammatory mediators and pro- resolving lipoxins | Meat and poultry eggs |
| Docosahexaenoic acid | C22H32O2 | 22 & Polyunsaturated | Source of D-series resolvins, protectins and maresins | Fatty fish, fish oil and algal oil |
| Eicosapentaenoic acid | C20H30O2 | 20 & Polyunsaturated | Source of E series resolvins | Fatty fish and fish oil |
Common fatty acids were classified on the basis of the number of carbon atoms and extent of carbon-carbon saturation. Furthermore, the extent and number of double bonds in the carbon backbone define a fatty acid as either saturated or unsaturated (Table 1). Based on dietary requirements, fatty acids are classified into two major classes: essential and non-essential (Russo, 2009). Naturally occurring fatty acids are often derived from olive oil, soybean oil, legumes/pulses, flaxseed, almonds, and a variety of nuts and seeds. In general, the human body depends on the dietary source of the fatty acids that are required for normal physiology and cannot synthesize several vital fatty acids, which must be obtained from the diet. Two fatty acids are known to be an essential for humans: alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid) (Chang et al., 2009). Among all the natural sources, vegetable oils are good sources of essential fats; however, the desired ratio of omega-6 to omega-3 is important and should be ~ 5:1 (Kohli and Levy, 2009). Excess intake of omega-6 in aging populations leads to obesity, bone loss, and many other chronic inflammatory disease (Alvheim et al., 2014; Fernandes, 2008; Halade et al., 2011a; Halade et al., 2010c; Lopategi et al., 2016; Patterson et al., 2012; Simopoulos, 2002, 2016). Essential fatty acids are beneficial in attenuating a number of diverse pathological conditions such as cardiovascular disease, chronic pulmonary airway disorders, neurodegeneration, and cancer (Das and Fams, 2003; De Caterina, 2011). The optimum intake of fatty acids in the diet improves cardiovascular functions, including anti-inflammatory properties, reduced major coronary events and improved anti-platelet effect (Akbar et al., 2005; Mori et al., 2003; Wanga et al., 2014). With urbanization, the levels of omega-6 fatty acids, such as AA and LA, have increased in the American diet, but the levels of conditional fatty acids such as EPA and DHA remain unchanged or even reduced. Of note, DHA and EPA act as precursors for several bioactive metabolites (eicosanoids/lipid mediators) that are beneficial in the prevention or treatment of several diseases (Figure 2) (Wolk et al., 2006). With recent advancement in lipidomics and metabolomics mass spectrometry technology, it is feasible to determine the nanogram and picogram levels of DHA and EPA-derived lipid metabolites. Furthermore, a series of studies in-vitro and in-vivo has explored the potential of these molecules to resolve inflammation, which is completely different than inhibition of inflammation (Bannenberg, 2009). It has been reported that DHA and EPA are centrally associated with the pathophysiology of atherosclerosis and chronic inflammation (Simopoulos, 1991).
Clinical interventions and trials established that EPA and DHA lower cardiovascular risk in patients with type 2 diabetes without adversely affecting glycemic control (Bradberry and Hilleman, 2013). Recent studies have demonstrated that Lovaza®, an omega-3 fatty acid ethyl ester, is effective in coronary heart disease by regulation of severe hypertriglyceridemia. Lovaza® is commercially available in capsule dosage form by Glaxo-Smith-Kline to treat hyperlipidemia. Clinical studies carried out since 2000 have revealed the potential of omega-3 fatty acids to protect against cardiac disorders and their derivatives, including EPA, DHA, ALA, and IPE (icosapent ethyl; contains high-purity EPA ethyl ester). During clinical trials, omega-3 fatty acids with brand names, such as GISSI–Prevenzione, JELIS, ORIGIN, and Su.Fol.Om3, etc., were studied in different patients groups. Each clinical trial was attributed to a specific group of patients with its pros and cons, but the omega-3 fatty acids were overall effective in controlling cardiac disorders (Herrera et al., 2015).
At the same time, the negative report of the omega-3 products cannot be ignored. In spite of their benefits, omega-3 fatty acids were reported ineffective in reducing cardiovascular risk when consumed at a lower dose or in combination with different fatty acids (Eckel, 2010). Surprisingly, a clinical study demonstrated omega-3 fatty acid supplementation failed to provide a protective role in statistically lowering the risk of mortality in the cardiac death, sudden death, myocardial infarction, or stroke (Rizos et al., 2012). Meta-analysis studies suggest the use of omega-3 PUFAs and reconsideration of supplement dose, adherence, baseline intake, and CVD risk. Despite these negative aspects, EPA and DHA both are associated with the prevention and management of cardiovascular disease, hyperlipidemia, hyperinsulinemia, and possibly type 2 diabetes (T2D) by lowering triacylglycerol concentrations (Merched et al., 2008). Interestingly, EPA and DHA were reported beneficial in coronary heart disease, not only by altering serum lipids, but also by reducing blood and ventricular arrhythmias (Abedi and Sahari, 2014; Kain and Halade, 2017; Tomio et al., 2013; Uderhardt et al., 2012; von Schacky, 2007).
6. Not all fats are created equal - elongation, diversified, and contrasting effects
FADS are the best categorized genes shown to influence circulating and membrane PUFAs. FADS encode fatty acid desaturase enzymes that regulate the endogenous metabolism of cis omega-3 and omega-6 fatty acids, termed FADS1 and FADS2, respectively. These biologically-relevant candidate genes encode the delta-5 and delta-6 desaturases, which participate in the metabolic conversion of the essential fatty acid linoleic acid (LA) to longer chain omega-6 fatty acids. Stoffel and his colleagues confirmed that fatty acids are not only essential constituents for membrane phospholipids, but are also precursors for eicosanoids, anandamides, and docosanoids. The deficiency of essential fatty acids and eicosanoids allows a normal lifespan for male and female FADS2 (synonym- delta-6 fatty acid desaturase; D6D) null mice, but impairs reproductive abilities (Stoffel et al., 2008). Furthermore, Stoffel extended his findings and developed auxotrophic FADS2 mutant mice (omega-6-fatty acid desaturase) that are resistant to obesity. The essential fatty-acid-deprived FADS2 null mice were obesity-resistant and enriched with eicosa-5,11,14-trienoic acid. Altered lipid metabolism in FADS2 null mice suggested that membrane-bound phospholipid species are critical for the structural and operative functions in lipid homeostasis in normal physiology, as well as pathophysiology (Stoffel et al., 2014). With the observed specific and divergent effects of different fatty acids, it is the consensus in the scientific and medical communities that individuals should be prescribed more omega-3 and fewer omega-6 PUFAs in order to achieve optimum cardiovascular benefits (Harris and Shearer, 2014; Patterson et al., 2012). However, an excess of omega-6 fatty acid remains elevated in the Western diet. The availability of linoleic acid in the diet increased in the 20th century from 2.79% to 7.21%, with limited change in α-linolenic acid intake, which increased from 0.39% to 0.72% from dietary energy source. Fatty acids alter the physiology and pathology of target organs, providing that the metabolism of fatty acids depends on the activity of enzymes, such as stearoyl-CoA desaturase (SCD), delta-5 (D5D) and delta-6-desaturase (D6D). Three-weeks-long, controlled diet studies of saturated fats and PUFA in humans suggested that saturated fat intake stimulated SCD and D6D activity, while D5D activity was lowered. This all occurred without impacting levels of DHA or EPA. In fact, docosapentaenoic acid (DPA) levels were significantly higher after a saturated fat diet intake, suggesting an increase in D6D activity. Moreover, humans who were provided with saturated fats and PUFA had the same concentration of AA, mainly derived from LA, which contributes to the formation of eicosanoids (Warensjo et al., 2008). Evidence confirms that palmitic acid, but not stearic acid, leads to increased mortality, particularly as a result of cardiovascular disease. Intake of PUFA (LA) reduced the severity of cardiovascular disease, which was independent of desaturase activity (Warensjo et al., 2008).
Metabolic changes in fatty acid products in the heart are also controlled by D6D activity. Hyperactivity of D6D leads to the conversion of LA into AA, and the presence of the AA metabolites is reflected in dilated cardiomyopathy. In heart failure, there are higher levels of AA in membrane phospholipids due to higher activity of D6D, but utilization of these fatty acids remains unclear. Fatty acids are either used for energy or for formation of eicosanoids or docosanoids. Inhibition of D6D (SC-26196) reversed phospholipid remodeling and reduced the levels of AA and DHA in heart failure rat models and also reduced levels of 5- hydroxy eicosatetraenoic acid (HETE), 12-HETE, 15-HETE, and TXA2. As a result of the inhibition of D6D, there is altered phospholipid membrane remodeling in the heart and improved ventricular function with reduced fibrotic hypertrophy (Le et al., 2014). Thus, the overall intake of omega-6 increased, but the intake of omega-3 fatty acids, such as DHA, EPA, and DPA, had been continuously lowered, suggesting the predisposition of human health to many pro-inflammatory conditions (Blasbalg et al., 2011). American dietary guidelines recommend 1 gram of EPA+DHA per day to be given to coronary heart disease patients and 3–4 grams of EPA+DHA per day to patients with high triglyceride levels. Very high doses of fish oil products (about 3–12 grams) can lower triglycerides by 20–50%, though they have no benefit in preventing pancreatitis, which is a major risk in patients with high triglycerides (2014). The use of fish oil to lower the risk of mortality, cardiac death, sudden death, myocardial infarction, and stroke are based on relative and absolute measures of cardiovascular health and include associations that cannot be ignored (Rizos et al., 2012). These diversified, paradoxical, and multidimensional findings provide rationale to develop a theory about DHA and EPA lipid metabolites that can be extrapolated to DPA and other fatty acids in order to provide strong rational and pharmacological evidence in disease pathology. In fact, recent evidence indicates that novel omega-3 DPA oxygenated products (RvTs) are catalyzed by COX-2 and exert potent anti-inflammatory and tissue-protective actions in an in vivo murine hind limb ischemia reperfusion model of second organ injury (Dalli et al., 2013).
7. Omega-3 and omega-6 fatty acids imbalance in disease pathology
Ramsden et al. performed a randomized, single-blinded trial and established that high omega-3 fatty acids combined with low omega-6 fatty acids promotes more anti-nociceptive, or pain-relieving mediators than low omega-6 fatty acids alone (Ramsden et al., 2013). This trial provides evidence that a combination of dietary omega-3 fatty acids with a concurrent reduction of omega-6 fatty acids reduces chronic daily headache pain. This specific trial was conceptualized with the aim to observe anti-nociceptive outcomes, either due to low omega-6 fatty acids or high omega-3 fatty acids plus low omega-6 fatty acids. However, the outcome was negative only in low omega-6 fatty acids group, which suggests that an optimum balance is required in order to reduce chromic pain. Reduced pain in a clinical setting in a low omega-6 fatty acid plus high omega-3 fatty acids group was supported by a marked increase of anti-nociceptive omega-3 derivatives 18-HEPE and 17-HDHA (precursors to E- and D-series resolvins) (Ramon et al., 2012; Ramsden et al., 2013; Tjonahen et al., 2006). Omega-6 AA, which was significantly reduced in erythrocytes by the high omega-3 and low omega-6 fatty acids, but not in the omega-6 fatty acids intervention, is the biosynthetic precursor to a variety of pro-nociceptive and vasoactive lipid mediators implicated in headache pathogenesis (Ramsden et al., 2013). Furthermore, high omega-3 fatty acid plus low omega-6 fatty acid intervention significantly reduces numerous hydroxy octadecadienoic acid (HODE) and HETE derivatives of omega-3 LA and omega-6 AA, compared to baseline. The majority of HODEs and HETEs have putative pro-nociceptive properties by acting as endogenous ligands for the vanilloid (TRPV1) receptor channel (i.e., endovanilloids) (Hwang et al., 2000; Patwardhan et al., 2010; Ramsden et al., 2013). No beneficial outcome of pain reduction was observed in a controlled trial to test the efficacy of a diet of only omega-3 fatty acids (DHA+EPA) (Pradalier et al., 2001). Conversely, an intervention using a low dose of EPA+DHA yielded comparative results to high omega-3 fatty acids plus low omega-6 fatty acids intervention. The outcome from this study exemplifies that a dietary omega-6-lowering component is essential in order to reduce chronic pain, as well as to observe the maximum benefit of omega-3 fatty acids in disease pathology (Ramsden et al., 2013). Recent investigations have suggested that lower plasma levels of omega-3 fatty acids leads to an increased risk of cardiac death. Moreover, the beneficial versus harmful effect of omega-6 fatty acids debate is still ongoing (Calder and Deckelbaum, 2011). It has been proposed that omega-3 index is a new risk factor for death from coronary artery disease, especially following sudden cardiac arrest from a study using human samples (Schacky, 2010). It is important to note that catalyzing enzymes define the fate of omega-3 LA, EPA, and DHA.
8. Fatty acid processing enzymes: lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450 (CYP) epoxygenase
In physiological and pathological stress, fatty acids undergo a series of enzymatic or non-enzymatic breakdowns to produce potent bioactive lipid mediators with diverse pharmacological activity (Funk, 2006; Levy, 2010). Three major pathways associated in fatty acid metabolism are LOX, COX, and CYP. The COX, LOX and CYP pathways result in production of mediators that initiate, resolve, or progress inflammation depending on the type of activity and fatty acid substrate (Halade et al., 2016; Halade et al., 2017; Khanapure et al., 2007; Kim et al., 2008). It has been established that eicosanoids play an important role in the initiation, progression, and resolution of inflammation (Uderhardt et al., 2012). Leukocyte-type 12/15-lipoxygenase (12/15-LOX) has been identified as a major enzyme in the production of anti-inflammatory lipid mediators (lipoxins) (Uderhardt and Kronke, 2012). However, 5-LOX is involved in the generation of leukotrienes (LTs), which initiate inflammation via production of 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HpETE) (Radmark and Samuelsson, 2009). Further, HpETE acts as precursor for the lipoxins including LXA4 and LXB4. In fact, in 2009 Gerhard Kronke et al. demonstrated that deletion of the leukocyte-type 12/15-LOX gene leads to uncontrolled inflammation and tissue damage (Kronke et al., 2009).
Studies have shown enhanced inflammatory gene expression and decreased levels of LXA4 in 12/15-LOX-deficient mice. These findings were further supported by the anti-inflammatory and tissue-protective role of 12/15-LOX reported by Olof Radmark et al. (Radmark and Samuelsson, 2009). Future experiments with major emphasis on a substrate- and organ-specific role of 12/15 LOX is essential for tissue and organ regeneration and restoration of function. In this context, cardiac tissue could be an ideal target to explore 12/15-LOXs’ activity and robustness towards the biosynthesis of pro-resolving lipid mediators. Fatty acid metabolism varies among different tissues, mostly adipose tissue, skeletal muscle, and liver, which possess complex mechanisms. For an enzyme, the activity remains a function of its structural configuration and kinetic constants, hence profiling this enzyme at proteomic and biophysical levels will lead to the understanding of molecular insights specific to particular tissues/organs. Similarily, the distribution of fatty acids in different tissues and organs is not homogeneous. Therefore, the enzyme activity (12/15 LOXs) and biosynthesis of pro-resolving lipid mediators in the course of inflammation resolution also varies depending on type and availability of fatty acid substrate. To understand and explore pro-resolving lipid mediator(s) biosynthesis in varying substrates (quantitative and qualitative), immediate attention is required to explore the efficacy of 12/15-LOXs in diverse disease pathologies.
LOXs are complex enzymes, involved in oxidative and redox metabolism of PUFAs. They also possess an affinity for different substrates, which results in various oxylipid mediators (Radmark et al., 2007). The enzyme 15-LOX catalyzes the production of 15 (S)-HpETE as a key intermediate from AA and acts as a precursor for lipoxin, which possesses excellent anti-inflammatory activity. Additionally, 15-LOX also catalyzes the production of 17-(S)-HDHA from docosahexaenoic acid (DHA), which is further metabolized to produce resolvins (D-series) (Xu et al., 2006). The outcome of such unique catalysis results in the production of specialized pro-resolving mediators (SPM), including protectins and maresins. An enzyme’s ability to catalyze multiple biochemical reactions is known as enzyme promiscuity or, more precisely, catalytic promiscuity (Khersonsky and Tawfik, 2010). Furthermore, resolvins and 5-LOXs are involved in the catalysis of 5(S)-Hp-ETE, 15(S)-HpETE, 17-(S)-HpDHA, 15(S)-HpEPA that results in protectins, which actively participate in the resolution of inflammation (Bannenberg and Serhan, 2010; Hao and Breyer, 2007). 12-LOX exists as two isoforms, platelet-type and leukocyte-type, which catalyzes the formation of 12-HpETE from AA. Both isoforms of 12-LOX share 60% identity at the amino acid level and strongly differ in substrate specificity and enzyme kinetics (McDonnell et al., 2001; McDonnell et al., 2002). One possible reason for enzyme promiscuity in the LOX enzyme is due to the unique catalytic site, which rapidly transitions to develop an affinity for various substrates. The concept of enzyme promiscuity in the LOX enzyme is operational in time-dependent manner in inflammation and disease pathology. A detailed understanding of LOX catalytic promiscuity will result in a novel approach in drug development process (Verma and Pulicherla, 2016). LOXs are the key enzymes in oxidative breakdown of these lipids, resulting in bioactive lipid mediators and offering a broad spectrum of physiological function (Massey and Nicolaou, 2013). A detailed scheme of metabolites produced by a series of enzymatic activity is shown in Figure 2. Furthermore, CYP epoxygenase is reported as a key enzyme in the biosynthesis of bioactive lipid mediators with significant biological importance (Fischer R, 2014; Lands, 2012). CYP epoxyegnase has shown potent cardiovascular protective action and potential for coronary artery disease drug development. A preclinical study in patients referred for coronary angiography demonstrated lower EET metabolite levels, secondary to suppressed EET biosynthesis (Oni-Orisan et al., 2016). However, lipid biochemistry has not been explored completely, as several mediators, such as 20-HEPE and 20-HDPE, are derived from EPA and DHA respectively under activity of CYP hydroxylase (Johnson et al., 2015). Along with LOXs, COXs (COX-1 and COX-2) are membrane-associated homodimer hemoproteins actively involved in oxygenation of lipids, which results in an array of bioactive lipid mediators with diverse pharmacological activities (Picot et al., 1994). Naturally occurring COXs exist in two isoforms and possess peroxidase and oxygenase active sites on the same molecule (Smith et al., 2000). The function of COX is largely dependent on cofactor attachment (heme), which is responsible for substrate activation of the active site by oxidizing tyrosine residues (Tyr385). In a sequential manner using the same active site, COXs are involved in two oxygenation reactions (first in 11R configuration and second in 15S configuration) using AA as a substrate, resulting in prostaglandin endoperoxides (Rouzer and Marnett, 2003). Thus, similar to LOXs, COXs are dioxygenases and two isoforms, COX-1 and COX-2, catalyze similar reactions for prostanoid biosynthesis. AA remains the prime substrate for COXs, while other fatty acids have an affinity for COXs in different capacities. It has also been reported that selective COX-2 inhibitors are key drug molecules resulting in a higher percentage of myocardial infarction and stroke events. The selective COX-2 inhibitors are associated with inhibition of prostacyclin (PGI2), lipoxins, resolvins, and endothelial nitric oxide (e-NO) biosynthesis (Das, 2005). Metabolism and dietary intake of essential fatty acids via COXs and LOXs drive pathways to reduce the percentage of global disease burden including coronary heart disease, stroke, diabetes mellitus, hypertension, cancer, depression, schizophrenia, Alzheimer’s disease, and vascular diseases (Das, 2008; Kain and Halade, 2017).
9. DHA metabolites in pro-resolution therapy
Omega-3 fatty acids play an important role in preventing the negative effects that are associated with chronic inflammation. Omega-3 substrates such as EPA, DHA, and DPA, are metabolized into intermediate lipid mediators including monohydroxy, dihydroxy, and trihydroxy molecules. Many of the dihydroxy and trihydroxy molecules, which carry potential bioactivity, are referred to as resolution phase interaction products (resolvins; D series and E-series depending on DHA or EPA metabolite). Direct cell-cell interaction of leukocytes and platelets organizes synthesis of DHA-, EPA-, and DPA-derived biomolecules, which limit the activity of pro-inflammatory leukocytes, while also increasing the activity of pro-resolving inflammatory mediators (Serhan, 2014). To exemplify the promise for FADD, this section of the review focuses exclusively on the DHA and EPA bioactives, commonly known as SPMs. DHA metabolites are commonly referred to as D-series resolvins 1-6 (RvD 1-6), protectins, and maresins. Our study identified that DHA is more effective in extending the lifespan of lupus-prone inflamed mice (Halade et al., 2010a) and attenuates breast cancer bone metastasis (Haworth et al., 2008). Similarly, EPA transforms to E-series Resolvins (RvE1-3) and AA to lipoxins, prostaglandins, and leukotrienes (Serhan, 2014).
9.1 Monohydroxy DHA
Wound healing is the first step in the response mechanism to cutaneous or sterile injury, such as myocardial infarction or stroke, which is characterized by immune cell trafficking. At the local tissue level, healing is a programmed event that is coordinated by the generation of SPMs, which are produced by the interaction of platelets, neutrophils, and macrophages at the site of injury or in the spleen reservoir. The generation of specialized resolving mediators in the acute inflammatory phase, overlapping with resolving response programs, may lead to the termination of injury-mediated inflammation. In genetically diabetic mice (db/db), apoptotic neutrophil clearance is delayed and DHA metabolites levels are lowered, despite having bioequivalent levels of DHA molecules in the cutaneous wound healing model. Reduced levels of monohydroxy DHA metabolites (4-HDHA, 14-HDHA and 17-HDHA) significantly impair cutaneous wound healing and continue non-resolving inflammation, suggesting the presence of chronic inflammation (Tourki and Halade, 2017). Monohydroxy DHA serves as the precursor for these resolving molecules, such as maresins, protectins, and resolvins. It has been shown that Resolvin D1 (RvD1) administration improved healing of diabetic wounds, as RvD1 stimulates macrophage phagocytosis and emigration from healing site. Topical application of RvD1 suppressed diabetes-mediated defective wound healing in mice (Tang et al., 2013). Thus, it can be determined that the diabetes-mediated defective systemic wound healing inflammatory response is ameliorated by RvD1 to promote a compensatory resolving response.
Omega-3 fatty acids produce molecules called hydroxy-docosahexaenoic acids (HDHA) using LOX. A study performed by Sapieha and colleagues showed that the presence of 4-HDHA in the human blood increases 20-fold compared to that of 7-HDHA when associated with blood clotting (Sapieha et al., 2011). The high concentration of 4-HDHA compared to that of 7-HDHA was also observed in isolated mouse neutrophils in a study that aimed to determine the activity of 5-LOX in the presence of calcium ionophore with exogenous DHA. This report showed that the presence of 5-LOX-derived 4-HDHA and the PPARγ receptor have a role in the anti-angiogenesis that is associated with omega-3 PUFAs (Sapieha et al., 2011). A study performed by Ramon et al. found that another molecule, DHA-derived 17-HDHA, can aid the adaptive immune response initiated by vaccines. Administration of 17-HDHA in conjunction with an influenza vaccine increases antibody production in a mouse model. This response elicited better protection against a pandemic H1N1 infection compared to that of the wild-type (Ramon et al., 2014). DHA-derived 17-HDHA has a direct role in the differentiation of B cells, initiating antibody secretion. Ramon and colleagues reported 100% survival rate of the mice that were injected with the vaccine and 17-HDHA, as compared to a previous study that showed 80% survival in mice only treated with the vaccine, indicating the potency of this lipid-derived pro-resolving mediator (Ramon et al., 2012).
9.2 Resolvin D1
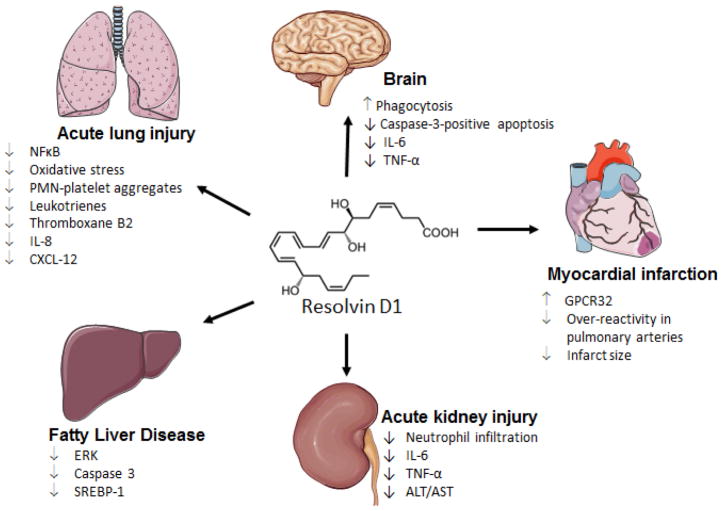
RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid) is one of a series of six common pro-resolving molecules (RvD1-6) that are derived from DHA. RvD1 pharmacological potency has been tested in-vitro and in a number of in-vivo disease pathology models (Table 2) (Cianci et al., 2016; Croasdell et al., 2015; Gilbert et al., 2015a; Hodges et al., 2016; Kain et al., 2015; Norling et al., 2012; Titos et al., 2011). In the surgery-induced myocardial infarction model, during which the left anterior descending coronary artery is permanently ligated, it was observed that RvD1 accelerated neutrophil clearance and increased macrophage clearance from the injured left ventricle, preventing immune cell accumulation at the sit of injury (Kain et al., 2015). In response to the occlusion of the left coronary artery caused by this sterile ligation procedure in mice, there is rapid trafficking of neutrophils, monocytes, and macrophages, which leads to fibrotic scarring on left ventricle (Kain et al., 2014; Lopez et al., 2015; Ma et al., 2013). Post-MI myocardium injury recruits immune cells from the spleen reservoir and stimulates repair of damaged myocardium. Metabolomic profiling of the spleen indicates that the spleen triggers the production of lipid mediators, including RvD1, Maresin 1 and Lipoxin A4, which promote self-resolution of inflammation at the left ventricular site. Subcutaneous administration of RvD1 increases the clearance of neutrophils from the spleen and promotes macrophage clearance from the healing site. RvD1 acts primarily on the ALX/FPR2 receptor in spleen and left ventricle to promote effective resolution of inflammation. It is of importance to note that the mRNA expression of ALX/FPR2 receptor is >50% higher in the spleen than the LV. This indicates the presence of a cardiosplenic axis, which suggests that the spleen plays a pivotal role in post-MI cardiac healing and remodeling. Thus, the overall effective coordination of neutrophil and macrophage kinetics by SPM in the spleen and left ventricle promotes healing and can, therefore, delay heart failure. A reperfusion study confirmed that intraventricular administration of RvD1 reduces myocardium infarct size; however, presence of linoleic acid attenuates the cardioprotection offered by RvD1 (Gilbert et al., 2015b) (Gilbert et al., 2016).
Table 2.
Novel finding on RvD1 in different model of resolution pathobiology
| Pathobiology mode | Mechanistic finding | Reference |
|---|---|---|
| Mice ischemia reperfusion – acute kidney injury –(Duffield et al., 2006) | ↓ leukocyte accumulation, ↑ activation of reparative macrophages and ↓ interstitial fibrosis protection from ischemic acute kidney injury | Duffield et al., 2006 |
| Mice - diet-induced obesity- murine (Titos et al., 2011) | ↓ Th1 cytokines, ↑ macrophage arginase-1 and other M2 marker expression | Titos et al., 2011 |
| Mice - type 2 diabetes- murine (Tang et al., 2013) | ↑ resolution of peritonitis, ↓ apoptotic thymocytes and ↑ macrophage phagocytosis | Tang et al., 2013 |
| Mice - ischemia reperfusion (Shinohara et al., 2014) | ↓ polymorphonuclear leukocyte lung infiltration and platelet aggregates ↓ leukotrienes and thromboxane B2 |
Shinohara et al., 2014 |
| Mice - corneal allotransplantation – (Hua et al., 2014) | ↓ dendritic cell maturation, ↑corneal allograft survival and ↓ host T-cell infiltration and corneal angiogenesis | Hua et al., 2014 |
| Mice - myocardial infarction (Kain et al., 2015) | ↑ fractional shortening, ↓ left ventricular hypertrophy, ↓ pulmonary edema, ↑ clearance of macrophages and neutrophils post-injury, and ↑ inflammation resolution | Kain et al., 2015 |
| Mice - ischemia/reperfusion liver injury (Kang and Lee, 2016) | ↓ myeloperoxidase activity and Cxcl1 and Cxcl2 mRNA expression and ↑ M2 polarization | Kang et al., 2016 |
| Mice - rheumatoid arthritis (Norling et al., 2016) | ↓ arthritis severity, cachexia, hind-paw edema, and paw leukocyte infiltration | Norling et al., 2017 |
A study performed by Norling et al. showed that RvD1 reduced polymorphonuclear (PMN) leukocytes in an acute peritonitis human cell model. The key mechanism is largely controlled by RvD1 interacting with PMN cell receptors, FPR2/ALX and GPR32. In vivo, Norling used a peritonitis murine model to show that there is a decrease in pro-inflammatory leukocyte recruitment in wild-type mice when treated with RvD1. This was not seen in the fpr2-null mice (Norling et al., 2012). This indicates the importance of direct interaction of RvD1 with the FPR2 receptor for anti-inflammatory actions. In a study of RvD1 action in an obesity mouse model, the metabolite was shown to cause the responding macrophages to express an M2-like phenotype, which confers anti-inflammatory characteristics. This reduces the activity of inflammatory cytokines, such as TNF-α and IL-6, which both contribute to inflammation and, consequently, heart failure post-MI (Titos et al., 2011). Receptors for RvD1 have been identified and described; the human GPR32 receptor has no other identified ligands other than RvD1, whereas the ALX receptor is shared by both RvD1 and lipoxin A4 (Cheng et al., 2016). In the permanent coronary ligation mice model that induces-myocardial infarction, Kain et al. demonstrated an improvement in fractional shortening and reduction of the occurrence of fibrosis post-cardiac injury when treated with DHA-derived RvD1 metabolite, which exemplifies its therapeutic utility in heart failure (Kain et al., 2015) (Figure 5 showing multiple activities of RvD1 in resolution of inflammation) (Hua et al., 2014; Kain et al., 2014; Kang and Lee, 2016; Norling et al., 2016; Serhan, 2014; Shinohara et al., 2014). As expected, in a myocardial ischemia-reperfusion model of injury, RvD1 reduces infarct size by modulating phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway (Gilbert et al., 2015a). PI3K is the important signal transduction enzyme responsible for the RISK signaling pathway. The PI3K/Akt pathway itself limits pro-inflammatory response (Rice et al., 2016) and promotes myocyte survival (Fujio et al., 2000), which leads to cardio-protection. Gilbert et al. showed that RvD1 has cardio-protective features, as treatment with higher doses (0.3 μg) of RvD1 was shown to increase the expression the PI3K/Akt pathway (Gilbert et al., 2015a). RvD1 has also been shown to be associated with decreased inflammation and oxidative stress in a cigarette-induced emphysema mouse model. This study concluded that the administration of the pro-resolving mediator, RvD1, aids in the attenuation of chronic lung disease (Li et al., 2016).
Figure 5.
Sketch indicating the beneficial potential and mechanism of Resolvin D1 in resolution pathophysiology.
9.3 Resolvin D2
The next DHA-derived SPM is RvD2 (7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid), as it has been shown to play an anti-inflammatory role in different infection and injury models (Chiang et al., 2015; Duffield et al., 2006; Spite et al., 2009; Tian et al., 2015). According to Tian et al., RvD2 impedes lipopolysaccharide-induced inflammation by the down-regulation of IL-6, NO, TNF-α, and IL-1-β in a rat model of Parkinson’s disease (Tian et al., 2015). Similarly, it was shown that RvD2 prevents the activity of reactive oxygen species, which are known to cause tissue injury during the inflammatory response. In a murine model of ischemic acute kidney injury, it was shown that levels of resolvins RvD2, RvD3 and RvD4 are increased in plasma during the inflammatory phase. In this study, the mice also received exogenous DHA. RvD2, which was not observed endogenously in renal tissue, increased in both the plasma and renal tissue (Duffield et al., 2006). In the presence of RvD2 and bacterial infection in mice, the activity of macrophages was increased, while there was a decrease in the response of cytokines (IL-1β, IL-6, IL-23, and TNF-α) and neutrophils (Spite et al., 2009). This is especially important, as the role of macrophages in the resolution of inflammation is essential for clearing cell debris. It is unclear whether resolvins digest the necrotic cell organelle or activate signals to remove the organelle from the injured or infected area. Chiang et al. identified the RvD2 ligand primarily present in peripheral blood PMNs, monocytes, and macrophages and its ability to induce its pro-resolving role using the GPR18 receptor (Chiang et al., 2015). In this study, wild-type mice and GPR18 knockout mice were both given exogenous RvD2 in the presence of a bacterial infection. The gpr18-null mice showed non-resolving infection due to higher levels of PMN infiltration, a deficiency in the phagocytosis of dying/dead cells (efferocytosis), and lower activity of macrophage-controlled phagocytosis. Treatment of the wild-type mice with RvD2 had the opposite effect, suggesting that there is a positive, pro-resolving reaction that occurs with the binding of RvD2 to GPR18 (Chiang et al., 2015).
9.4 Resolvin D3
RvD3 (4S,10,17S-trihydroxy-5E,7E,9E,13Z,15E,19Z-docosahexaenoic acid) and has been argued to be one of the most potent of the D-series resolvins. RvD3 reduces the infiltration and trans-endothelial migration of neutrophils in acute inflammation. Similar to RvD1, this resolvin stimulates the proliferation of IL-10, which is a potent inhibitory factor of cytokine synthesis (Lefkowitz, 2016). RvD3 mediated actions reduce the occurrence of collateral tissue damage that is associated with prolonged inflammatory response. RvD3 also has been observed to stimulate the phagocytosis of apoptotic neutrophils via macrophages, referred to as efferocytosis (Nair et al., 2016; Oh and Lagakos, 2011). Though these characteristics are similar to those of RvD1 and RvD2, RvD3 has been shown to persist throughout the resolution timeframe post-injury, while RvD1 and RvD2 are present only in early resolution (Oh and Lagakos, 2011). The persistence of this pro-resolving molecule in the resolution phase of injury recovery could be key to overall success and development of a solution to inflammatory disease. RvD3 is of special interest because it controls the dysregulated inflammation in aging populations. In a study performed by Arnardottir and colleagues reported the resolution of inflammation in aging mice that were studied administered RvD1 and RvD3. Increased levels of RvD1 and RvD3 treatment stimulated increased monocyte-accelerated resolution of acute inflammation via efferocytosis (Arnardottir et al., 2014).
9.5 Resolvin D4
RvD4 (4S,5,17S-trihydroxy-6E,8E,10E,13E,15Z,19Z-docosahexaenoic acid) is less characterized than some of the other resolvins. However, RvD4 has been shown to act in a similar way to the other DHA resolvin metabolites in that it reduces the activity of neutrophils at the site of injury and increases the activity of macrophages in the clearance of apoptotic bodies (Raetz et al., 2009). It is important to explore the abundance of pro-resolving lipid mediators, lack of DHA substrate or deficiency of DHA processing LOX enzymes and their impact on resolving post-injury inflammatory response, compared to a younger model. During S. aureus infection, RvD4 reduces neutrophilic infiltration and, with time, enhances the uptake of apoptotic neutrophils by human dermal fibroblasts at concentrations as low as 0.1 nM. RvD4 shows pro-resolution properties, remaining at bioactive levels (~ pM) in the late stages of resolution (Velazquez and Grodecki-Pena, 2016).
9.6 Resolvin D5
Resolvin D5 (7S,17S-dihydroxy-5Z,8E,10Z,13Z,15E,19Z-docosahexaenoic acid) regulates the uptake of Escherichia coli (E. coli) by phagocytes in mice while also downregulating the expression of NF-κβ and TNF-α. In conjunction with appropriate antibiotics, RvD5, along with RvD1 and protectin D1, host antimicrobial response is increased, allowing for a higher survival rate of an otherwise lethal bacterial infection (Chiang et al., 2012). The downregulation of NF-κβ and TNF-α by RvD5 could be beneficial in the heart failure model, as both of these factors are increased in the heart post-cardiac injury. A recent aging metabolome study described that RvD6 levels (4S,17S-dihydroxy-5E,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid), were decreased in aging process indicating the role of lipid metabolism in progressive aging (Jove et al., 2016).
9.7 Resolvin D6
Much like RvD4, RvD6 (4S, 17S-dihydroxy-5E,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid) is not as extensively characterized as other resolvins. A human aging metabolome study suggests that RvD6 levels are decreased with increasing age (Jove et al., 2016). This could be an indicator that the reduction in abundance of pro-resolving lipid mediators has a possible significant impact on the aging and healing process. RvD6 is thought to also have qualities that support the resolution of inflammation post-injury (Pietilainen et al., 2007).
9.8 Protectin D1
Similar to RvD1-6, protectin D1 (PD1) (10R,17S,dihydroxy-4Z,7Z,11E,13E,15Z,19X-docosahexaenoic acid) is a metabolite of DHA that also exhibits pro-resolving properties. PD1 was originally described as neuroprotectin D1 due to its anti-inflammatory effects in the brain ischemia mouse model (Bazan, 2007). PD1 shares many of these characteristics with the other D-series resolvins, as it lowers the activity of PMNs, down-regulates the activation of pro-inflammatory molecules (NFκβ and COX-2), and increases macrophage phagocytosis of apoptotic cells. PD1 also is engaged in damage control in the inflammatory model, as it reduces the occurrence of apoptosis induced by oxidative stress (Schwab et al., 2007). PD1 has been described to confer cell survival in a human ARPE-19 (retinal pigment epithelia) cell model. It inhibited the activation of COX-2 and NF-κβ, which are genes that have pro-inflammatory characteristics. By these means, the infarct area in brain ischemia is reduced, conferring better survival (Bazan, 2006).
9.9 Maresins
In the carotid artery ligation mouse model, Akagi et al. hypothesized that treatment with RvD2 or another pro-resolving D-series lipid mediator, Maresin 1 (MaR1) (7R,14S-dihydroxy-4Z,8E,10E,12Z,16Z,19Z-docosahexaenoic acid), prevented neointimal hyperplasia, or the thickening of arterial walls post-injury (Akagi et al., 2015). Both RvD2 and MaR1 treatments decreased the amount of superoxide that the cytokine TNF-α produced. The density and trafficking of neutrophils to the site of injury was reduced with both treatments, though infiltration was reduced by 56% by MaR1 and only 48% by RvD2. Phagocytic macrophage activity was reduced by 48% and 43% in this model by treatment with RvD2 and MaR1, respectively. Thus, RvD1 and MaR1 showed potency to reduce neointimal hyperplasia and contribute to translational use in disease pathology (Akagi et al., 2015). These pro-resolving lipid mediators have been shown to confer healing in tissue post-injury. MaR1 has been shown to play a potent anti-inflammatory role in the resolution of chronic inflammation in asthma. In a mouse model of allergic inflammation, Krisnamoorthy and colleagues showed that MaR1 limits the activity of ILC2, which are innate lymphoid cells that are significant in the propagation of allergic inflammation. Exogenous treatment of inflammation with MaR1 increased the activity of regulatory T cells, which inhibit the activity of ILC2 in mice (Krishnamoorthy et al., 2015). Furthermore, MaR1 treatment has been shown to initiate the resolution phase in allergic inflammation of the lungs to occur faster and more readily, decreasing the number of eosinophils in the bronchoalveolar lung fluid. Cytokines such as IL-5 and IL-13 were decreased in the resolution phase as well, due to the decreased activity of ILC2. Accordingly, the amount of active TGF-β, which alters the profile of CD4+ T cells, and regulatory T cells were also increased (Krishnamoorthy et al., 2015). Like other monhydroxy and dihydroxy DHA metabolites, MaR1 also exhibits characteristics that cause pro-resolving effects due to its ability to block PMN-sustained infiltration to the site of inflammation, while causing an increase in macrophage phagocytosis (Serhan et al., 2009).
10. EPA-derived bioactives with potential pro-resolving activity
10.1 Monohydroxy EPA
Despite the explored potential of EPA and EPA bioactives in human physiology, inflammation, infection, injury models, and cardiac disorders, the biological conversion of EPA from ALA remains unrealible and severely restricted. Overall, only 6% of dietary ALA is converted to active EPA, and a high intake of omega-6 fatty acids decreases this to 2.4%–3% (Gerster, 1998). Furthermore, EPA undergoes a series of metabolic degradation under the regulation of various LOXs to produce bioactive lipid mediators in response to stress, i.e., exercise, injury or infection. COX-2 and/or aspirin or cytochrome p450 induced EPA oxidative catalysis and resulted in 18-HEPE via an intermediate 18-HpEPE (Serhan and Petasis, 2011). 5-LOX and 15-LOX possess an affinity for 18-HEPE and catalase production of resolvin (RvE1 and RvE2 by 5-LOX; RvE3 by 15-LOX) (Oh et al., 2011). It has been established that RvEs regulate immune cell trafficking and promote the resolution of inflammation in a variety of murine translational models.
Recently, a few more EPA metabolites, including 5 hydroxyeicosapentaenoic acids (HEPE), 8-HEPE, 9-HEPE, 12-HEPE and 18-HEPE (hydroxylation products of EPA) were tested in many experimental models and reported distinct pharmacological properties (Calviello et al., 2013). E-series resolvins modulate peroxisome-proliferator-activated receptors (PPARs) and represent a class of the nuclear receptor superfamily associated with fatty acid storage and their catabolism. Yamada et al. have demonstrated the role of EPA in up-regulation of PPARs and the subsequent catabolism of fatty acids in 3T3-F442A, NIH-3T3, and C2C12 cell cultures (Yamada et al., 2014). Furthermore, HEPEs also control endometriotic lesion development, but the complete physiological functions of HEPEs are yet to be explored (Tomio et al., 2013). With the advancement of mass spectrometry technology and the structural elucidation effort from the Serhan laboratory, EPA and EPA-derived lipid mediators remain ideal candidates to be studied for the resolution of inflammation (Dalli et al., 2018; Serhan, 2014). However, these omega-3 fatty acid derivatives have shown a significant variation in their pharmacology. Nording et al., found individual responsiveness to omega-3 fatty acids in healthy individuals is greatly variable and measurable, and this can be used as a marker for disease risk and determining metabolic phenotype (Nording et al., 2013). Additionally, 18-HEPE, an EPA (omega-3 fatty acid) metabolite, has shown to be effective in limiting cardiac remodeling. Use of fat-1 mice allowed the investigation of 18-HEPE and its reduction of macrophage-mediated pro-inflamamtory activation of cardiac fibroblasts in a murine model of hypertrophy. The study has revealed the EPA, and 18-HEPE are beneficial, not only for inhibiting fibrosis in cardiac tissue but also for improving cardiac function (Endo et al., 2014). Administration of 18-HEPE to mice prevented pressure-overload-induced cardiac fibrosis and inflammation. These findings support previous studies carried out by Tavazzi et al. and Yokoyama et al. (Tavazzi et al., 2008; Yokoyama et al., 2007). These studies aimed to investigate the effect of a fish diet on prevention of major coronary events in hypercholesterolaemic individuals. These two studies were carried out using randomized clinical trials with dietary supplementation of omega-3 PUFAs and protective role in atherothrombotic cardiovascular disease, including arrhythmias (Tavazzi et al., 2008; Yokoyama et al., 2007). Patients supplemented with omega-3 fatty acids had improved cardiac function and reduced mortality due to chronic heart failure. Furthermore, Nodari et al. demonstrated that omega-3 fatty acids improve left ventricular functional capacity and decrease the circulating concentrations of inflammatory cytokines such as TNF-α and IL-6 in non-ischemic dilated cardiomyopathy (Nodari et al., 2011). The bioactive lipid mediators derived from EPA metabolism were reported as having several other physiological functions such as pro-inflammatory signaling and neuroprotection in addition to the resolution of inflammation (Bazan, 2007). The PGE3 derived from the COX-mediated metabolism of eicosapentaenoic acid (EPA) reduces angiogenesis, cell proliferation and cell invasion (Connolly, 1999). These activities suggest that PGE3 plays a role in the protection against cancer development and its metastasis (Yang et al., 2014). It has also been reported that the EPA-derived lipid mediator PGJ3 helps in fighting against leukemia (Weylandt et al., 2015). There has been increasing belief that omega-3 PUFAs and their metabolites can result in the development of new generation anti-tumor drugs (Azrad et al., 2013). The lipid signaling plays an important role in tumor development and downregulation, resulting in expression of pro-inflammatory and pro-tumorigenic eicosanoids from ARA in the tumor microenvironment (Wanga et al., 2014). These findings suggested that eicosanoid metabolism and subsequent inhibition of prostaglandin E2 (PGE2) production contribute to EPA-elicited anti-proliferative activity in various cancers. Recent research shows that the lipid signaling is dysregulated in tumor tissues, leading to increased production of pro-inflammatory and pro-tumorigenic eicosanoids from ARA in the tumor microenvironment (Kang, 2013). The over activity of ARA-derived mediators leads to up-regulation of pro-tumorigenic lipid- metabolizing enzymes, including COX-2, 5-LOX and CYP epoxygenases, as well as down-regulation of anti-tumorigenic enzyme 15-LOX1 (Zhang, G. et al., 2014). These modifications at the gene level provide a supportive microenvironment for tumor development and progression. Both EPA and DHA were reported having an inhibitory effect in the ARA-derived 6-series lipid mediators via multiple mechanisms, including the reduced release of ARA from membrane phospholipids, inhibition of the enzymatic activities of the metabolizing enzymes, and direct competition with ARA for the enzymatic conversions (Shao et al., 2014). The bioactive lipid mediators produced under the activities of COX-2, LOX, and CYP were reported to have strong anti-tumorigenic activities (Qian and Xu, 2014). Recent findings suggest that omega-3 fatty acids possess the potential for controlling type 2 diabetes (T2D) in addition to its anti-tumor property. A random clinical trial study carried out in 2010 by Suzanne Hendrich suggested a dietary intake of omega-3 fatty acids, both DHA and EPA, improves plasma triglycerides in human clinical studies of people with T2D (Hendrich, 2010).
10.2 Resolvin E1
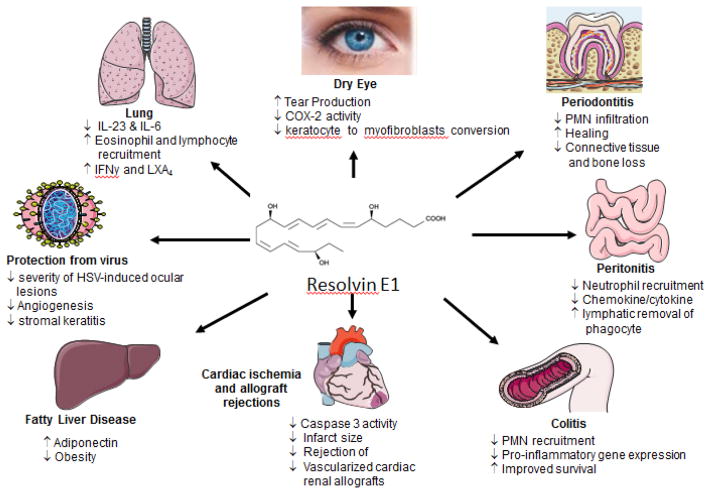
The RvE1 (5S,12R,18R-trihydroxyeicosapentaenoic acid) is derived from the oxygenation of EPA (Endo et al., 2014). There is significantly growing interest in EPA-derived molecules, also E-series resolvin, to explore their therapeutic potential in the eye, lung, and periodontal disease pathologies (Table 3) (Cianci et al., 2016; Croasdell et al., 2015; Hasturk, H. et al., 2007; Hodges et al., 2017; Lee et al., 2015). Many E-series resolvins act within a local inflammatory milieu to stop leukocyte recruitment and promote resolution (Arita, 2012). RvE1 possesses broad substrate affinity and executes actions by regulating and/or inhibiting many pathways. In the course of resolution of inflammation, RvE1 mediates its action by blocking PMN transendothelial migration and facilitating apical PMN clearance from mucosal epithelial cells (Serhan et al., 2000). Furthermore, RvE1 also inhibits NF-κβ activation, a central pathway that links various inflammation signaling by LTB4-BLT1 attenuation result in inhibition of superoxide species (Arita et al., 2007; Campbell EL, 2007). Stereoselective biosynthesis suggested that RvE1 (RvE1; (5S, 12R, 18R)-trihydroxy-6Z, 8E, 10E, 14Z, 16E-eicosapentaenoic acid) is an oxygenase product with diverse biological activity (Oh et al., 2011). RvE1 is tested in reperfusion injury using open-chest rat model of ischemia-reperfusion. The cardioprotective action of RvE1 was reported to be quite different from other resolvin and protectins. In the study of H9c2 cell line, it is shown that RvE1 acts directly on cardiomyocyte (Figure 6 showing multiple activity of RvE1) (Arita et al., 2005; Dona et al., 2008; Fredman et al., 2010; Freire et al., 2017; Hasturk, H. et al., 2007; Haworth et al., 2008; Ishida et al., 2010; Keyes et al., 2010; Lee et al., 2016; Li et al., 2010; Schwab et al., 2007). RvE1 offers protection in reperfusion injury by limiting leukocyte diapedesis (Serhan et al., 2008). Additionally, RvE1 attenuates caspase-3 activity along with Bax and Bcl-2 in the rat model of chronic inflammation (carrageenan-sponge implant model) (El Kebir and Filep, 2013). In addition to offering cardiac protection, RvE1 was reported beneficial in inhibition of corneal inflammation. In addition, RvE1 is effective in lipopolysaccharides (LPS), antibiotic-killed Pseudomonas aeruginosa and Staphylococcus aureus-induced inflammation in C57BL/6 mouse corneas. Results have shown a complete inhibition of cytokine production in C57BL/6 mouse corneas, which provides evidence for an anti-inflammatory effect on human corneal epithelial cells (Ma et al., 2015). In addition to above-mentioned effects, RvE1 also indicated to improve wound healing without apoptosis of corneal epithelial cells (Hasturk, Hatice et al., 2007; Serhan, 2009). EPA plays an important role in the regulation of serum glucose levels and E-series resolvins to offer protection against diabetes (Boden and Shulman, 2002; Jump, 2011). In recent findings, RvE1 is effective in rescuing impaired neutrophil phagocytosis in T2D (Herrera et al., 2015). RvE1 was reported to enhance neutrophil phagocytosis in obese T2D mice overexpressing human RvE1 receptor. Some reports suggest that EPA as omega-3 fatty acid reduces hypersensitivity and asthmatic complications. Hiram et al. investigated the beneficial role of RvE1, which is resolving human arterial hyperactivity via the resolution of inflammatory markers (Hiram et al., 2015). Furthermore, RvE1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses, which provides clear evidence of combating allergic complications (Flesher et al., 2014; Hisada et al., 2009). RvE1 was also reported to attenuate T cell priming in the draining lymph nodes and effector T cell activation in the skin, which led to reduced skin inflammation (Sawada et al., 2015).
Table 3.
Novel finding of RvE1 in different model of resolution pathophysiology
| Pathobiology model | Mechanistic finding | Reference |
|---|---|---|
| Female BALBc and male FVB mice – peritonitis colitis (Arita et al., 2005) | protection of intestinal inflammation and ↓ colitis | Arita et al., 2005 |
| New Zealand White rabbits - periodontal disease (Hasturk H, 2007) | ↓ systemic inflammation and ↓ C-reactive protein and IL-1beta | Hasturk et al., 2007 |
| Platelet-rich plasm and human blood – platelet activation (Dona et al., 2008) | ↓ leukocyte rolling ↓ ADP-induced platelet aggregation | Dona et al., 2008 |
| FVB mice – lung injury (Haworth et al., 2008) | ↓ IL-23 and IL-6 ↑ resolution of allergic airway inflammation | Haworth et al., 2008 |
| Human platelet – platelet activation (Fredman et al., 2010) | ↓ ADP-stimulated P-selectin mobilization and actin polymerization | Fredman et al., 2010 |
| Male C57BL/6J mice – lung injury (Seki et al., 2010) | ↓ bacterial infection and acute lung injury | Seki et al., 2010 |
| Female C57BL/6 mice – dextran-induced colitis (Ishida et al., 2010) | ↓ pro-inflammatory responses of macrophages | Ishida et al., 2010 |
| Dry eye mouse model (Li et al., 2010) | ↑ tear production, ↓ inflammation, and ↓ inducible COX-2 | Li et al., 2010 |
| Female BALB/c mice – pulmonary inflammation (El Kebir and Filep, 2013) | ↑ neutrophil apoptosis and ↑ activation of caspase 3 and 8 | Kebir et al., 2012 |
| Mice - LPS, P. aeruginosa and S. aureus infection (Lee et al., 2015) | ↓ corneal Inflammation | Lee et. al., 2015 |
| Rats – periodontitis and dysbiosis (Lee et al., 2016) | ↓ bone loss, ↓ inflammatory gene expression, and ↓ osteoclast density | Lee et al., 2016 |
| Human - neutrophils and monocyte/macrophages- (Freire et al., 2017) | RvE1 receptor leukotriene B4 (BLT-1) on neutrophils ERV-1/ChemR23 on monocyte/macrophages |
Freire et al., 2017 |
Figure 6.
Sketch indicating the beneficial potential and mechanism of Resolvin E1 in resolution pathophysiology.
10.3 Resolvin E2
RvE2; a 5S,18-dihydroxy-eicosapentaenoic is a new addition to the E-series resolvin family (de Souza et al., 2006). Bioactive lipid mediator RvE2 (RvE2) was identified with anti-inflammatory and pro-resolving activity via inhibiting PMN leukocyte infiltration. RvE2 mediates increased production of anti-inflammatory cytokines and inhibits the synthesis of IL-1, TNF- α, and other major pro-inflammatory cytokines in order to help in tissue homeostasis during resolution of inflammation (Locke et al., 2015). Now it is confirmed that human recombinant 5-LOX generates RvE2 from EPA and a common precursor of E-series resolvins, namely, 18-HEPE possesses similar biological activity (Tjonahen et al., 2006). Recently, the complete structure of RvE2 was confirmed by using total organic synthesis (Ogawa S, 2009). Sungwhan et. al., reported leukocyte-directed actions of RvE2 as one of the local mediators of tissue homeostasis during inflammation resolution (Oh et al., 2012). A study using human PMNs provided clear evidence that RvE2 mediates resolution of inflammation via leukocyte G-protein-coupled receptors (R-23), activating chemotaxis of human neutrophils, phagocytosis and anti-inflammatory cytokine production (Oh et al., 2012)
10.4 Resolvin E3
The physiological functions of RvE1 and RvE2, both of which are biosynthesized by neutrophils via the 5LOX pathway, have been studied in the last few years (N., 2007). A recent addition is a novel E-series resolving 17,18 dihydroxyeicosapentaenoic acid (17, 18 diHEPE) with enhanced pro-resolving activity (Isobe et al., 2012). In addition to 17,18-diHEPE, a few more bioactive lipid mediators intermediates were reported, including 8, 18 dihydroxyeicosapentaenoic acid (8,18 diHEPE); 11,18-diHEPE; and 12,18-diHEPE from 18-HEPE (Schneider et al., 2007). 8,18 diHEPE is involved in the overexpression of GAL4-PPARα, GAL4-PPARγ, and GAL4-PPARδ. The PPARs are a member of nuclear receptor family and are involved in regulation and storage of lipids. Further, 11/18- and 12/18-diHEPE are reported to reduce pplatelet aggregation and 5-HT release, while increasing cell membrane fluidity and neuroprotection respectively (Buckley et al., 2014). The 5-LOXs mediate biosynthesis of RvE1 and RvE2 in neutrophils, while 12/15-LOXs catalyze the biosynthesis of RvE3 in eosinophils (Gronert et al., 2005). The unique nature of LOXs strongly suggests catalytic promiscuity in enzymes associated with lipid mediators biosynthesis. 5-LOX and 15-LOX show broad affinity to 18-HpEPE to biosynthesize different HpEPEs. Scientific reports also provide clear evidence that RvE3 plays an essential role in maintaining renal functions by regulating oxidative damage and lipid peroxide activity. A recent report suggests that there is a decrease in eicosanoids and docosanoids metabolite levels after oral administration of a higher dose of arachidonic acid (Katakura et al., 2015). The decrease in plasma level of eicosanoids and docosanoids metabolites is due to change in ROS and lipid peroxide after long-term use of arachidonic acid. The study provides substantial evidence for the beneficial role of EPA-derived lipid mediators for the resolution of inflammation (Powell et al., 1995).
11. Classical examples for other fatty acids for FADD
Similar to DHA and EPA, α-linoleic acid and AA are transformed into numerous bioactive lipid species. It is important to study when and where these metabolites are formed and how they relate to physiology, disease pathology, and/or resolution of inflammation (Halade and Kain, 2017; Kain and Halade, 2017). DHA is transformed to monohydroxy DHA, D-series resolvins, protectins and maresins. EPA is transformed to many HEPE molecules (5-, 8-, 9-, 11-, 12-, 18-HEPE), RvE1, RvE2, and RvE3 (Endo et al., 2014). Similarly, AA, EPA, and DHA act as precursors to cyp450 epoxygenase that lead to a generation of different EETs (Oni-Orisan et al., 2015). Our current review is exclusively focused on the metabolic transformation of DHA and EPA to diverse molecules with significant biological action in disease pathology, specifically to resolve inflammation. With the advancement of mass spectrometry technology, other fatty acid biomolecules, such as hydroxy fatty acids (HFA) have recently been added as new entries to lipid signaling (Dalli et al., 2018; Yore et al., 2014). In white adipose tissue, FAHFAs are synthesized in response to signals from carbohydrate-responsive element-binding protein (ChREBP) and streamed into circulation to promote insulin release and glucose uptake (Yore et al., 2014). Furthermore, nitro fatty acids (e.g., nitro -oleaic acid, -linoleic and -conjugated linoleic acid) (Delmastro-Greenwood et al., 2015; Halade et al., 2009, 2010b; Halade et al., 2011b; Woodcock et al., 2018) and omega-6 derived endocannabinoids (Alvheim et al., 2012) are other biomolecules that qualify for FADD and biological action to maintain homeostatic physiology and disease pathology. This presented approach can be used for other fatty acids, including short and medium chain fatty acids, saturated fatty acids and different highly unsaturated fatty acids. Application of mass spectrometry, the detection and profiling challenge has been crossed, as now many lipid biomolecules can be measured in biospecimens, including breath (Lundstrom et al., 2011). Future challenges include integrating the action of these local producing autocoids, lipid mediators, eicosanoids or local hormones in response to a diverse array of enzymes and receptors in disease pathology, inflammation, and injury. Simultaneously, the enormous contribution of 100 trillion catalytic microbiome flora and the influence of short and medium chain fatty acids (e.g., caprylic acid, capric acid, butyric acid, valeric acid, etc.) need to be considered for the modification and formation of these mediators at the physiological level in healthy subjects.
12. Pitfalls of old and current novel technologies
Lipidome represents all molecules including lipids, fatty acids, and their metabolites, all which possess great structural diversity and complexity (Han, 2016). The association of fatty acids and their metabolic products (lipid mediators) in human diseases have remained unknown for a long time, but lipid profiling remains limited due to lack of cutting-edge quantitative technology in lipidomics. It is important to note here, the lipidome of an organism, tissue, and cells varies in healthy and pathological conditions (Hadj Ahmed et al., 2017). Further, there is organ/tissue- specific variation in fatty acids and variation in the associated metabolite profile in many diseases including cardiac disorders, inflammation, neurological and other metabolic disorders (Siguel and Lerman). Some reports in literature measure lipids with direct infusion of the sample to mass spectrometry without chromatographic separation of lipid biomolecules. Lipid measurements largely rely on chromatographic separation of lipids, fatty acids, and associated metabolites using mass spectrometry. Now the lipids field is developing (LIPID MAPS) to drive the lipidome databases similar to proteome, though it has been quite challenging to profile a novel bioactive(s) and associated metabolites. Advancement in the analytical techniques such as MS-based technologies (MALDI-TOF, MALDI-MS/MS, and LCMS/MS, MS-based bioactive lipid imaging) have merged to advance lipidome studies, but at the same time, there is need to understand the mechanism of lipids in systems biology (Halade and Kain, 2017; Junot et al., 2014; Ma et al., 2015). There have been difficulties to determine the trafficking of lipids, fatty acids, and associated metabolites as all of these molecules are complex to tag with reporter molecules unless it is conjugated with a protein and/or chemical (fluorescent tagging) (Schultz et al., 2010). Radiolabeled lipids, fatty acids, and associated metabolites remain a primary option in early days of lipid profiling, quantification, and localization (Wakabayashi et al., 1994). Similar to other biomolecules, including nucleic acids and proteins (via mutational analysis), lipids and fatty acids are difficult to modify to study their physiological significance. Hence, a novel approach is essential to study the metabolic transformation of fatty acid to bioactive lipid mediators involved in diseases (pro-inflammatory) or reparative mechanisms to resolve inflammation (anti-inflammatory).
13 Future prospective on DHA and EPA metabolites and their mechanism of action
Traditionally, lipids were considered energy sources with powerful brick and mortar capacity that separates the interior cell cytoplasm from the external stress or injury. Lipids are building blocks of cell membrane components and, in response to external stimuli, fatty acids breakdown in to endogenous molecules to be involved various signalling processes and regulate different physiological events (Glatz, 2011). At this time, there has been an increasing emphasis on studying fatty acids and their associated metabolites for their therapeutic applications. Despite dynamic efforts, the molecular characterization of biomolecules remains difficult in context with physiological events as these molecules require meticulous sample preparation and prudent extraction methodology (Dalli et al., 2018; DH, 2003). The major point of FADD is translational and has direct relation to enzymes (LOXs and COXs), thus providing the opportunity for prognostic, personalized, and precise medical discovery. It remains a major challenge to study affinity of fatty acids with metabolizing enzymes and the rate-limiting step in metabolic events. With the emerging, cutting-edge quantitative lipidomics and proteomics offer the ability to determine the lipidome of a cells and even whole organisms (Tumanov and Kamphorst, 2017). Based on chemical structural resemblance to FADD biomolecules, the development of new mimetics is emerging as an approach to study binding affinity and feed-forward activation or suppression of metabolizing enzymes (Proschak et al., 2017). Indeed, we offer a list of efforts that are imperative for advancing the field of fatty acid research and subsequent FADD:
Stability of DHA and EPA metabolites (monohydroxy DHA/EPA, resolvins, maresins and protectins) in healing and non-healing inflamed organs, milieu, or biological compartments
Optimal requirement of fatty acids substrates DHA and EPA and check point to ensure presence of LOX enzyme to metabolize the substrate
Supply of dietary DHA and EPA to feed LOX enzymes for metabolic transformation into biomolecules in response to injury or infection
Lipids trafficking in response to injury, infection, exercise or aging
Organ-specific verses immune-cell-specific receptor(s) to initiate pharmacological action
Impact of pro-inflammatory molecules like leukotrienes and prostaglandins in receptor sensitization or desensitization with lower levels of DHA and EPA
Identification and validation of novel and functional sensor for monohydroxy DHA and EPA, D- and E-series resolvins, maresin and protectins
14. Conclusion
With the advancement of lipidomics technology and an enormous contribution from the Serhan laboratory and others in structural elucidation, much progress has been made to capitalize the molecular and cellular signaling of DHA and EPA biomolecules in disease pathology. Thus, DHA and EPA transformation to a number of lipid biomolecules exemplifies the successful launching of Fatty Acid Drug Discovery (FADD). The emphasis on lipid absorption, distribution, storage, metabolism and excretion (dietokinetics or nutrikinetics) from birth to aging, as well as lipid utilization (nutridynamics or dietodynamics), will be an active an area of research for novel biomolecules. In the area of personalized and precise medicine, it is important to personalize diet to precisely treat infection and injury based on age (infant, teen, adult, aging or senescent) as well as average energy expenditure. What is unclear at this point is how drug treatment, aging, and other factors (diet, microbiome, or epigenetics) decrease omega 3 (DHA and EPA) storage and metabolism to form different lipid mediators. DHA- and EPA-derived pro-resolving lipid mediators could have undetected levels or could be lowered as a result of stress due to senescent pathophysiology. In addition, the pro-inflammatory burden in obese subjects could also contribute to decreased metabolism and use of DHA and EPA-derived lipid mediators. This defect may be at the enzymatic level (low LOX and COX activity) or substrate level (low DHA and EPA levels), but future studies of novel DHA- and EPA-derived immunoresolvents such as resolvins, lipoxins maresins, and protectins will provide a foundation to make progress in resolution pharmacology and physiology. Thus, novel approaches can be used to potentiate the action of DHA and EPA biomolecules using the FADD approach in the lipid world.
Acknowledgments
The author would like to acknowledge Servier Medical Art use for figures. The lead and corresponding author acknowledges the peer-interactive discussion and collaborative support from Dr. Charles N. Serhan, Brigham and Women’s Hospital and funding from AT006704 and HL132989 to GVH.
List of abbreviations
- AA
Arachidonic acid
- ALA
α-linolenic acid
- ChREBP
carbohydrate responsive element-binding protein
- COX
Cyclooxygenase
- CVD
Cardiovascular disease
- CYP
Cytochrome p450
- D5D
Delta-5 desaturase
- D6D
Delta-6-desaturase
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic Acids
- FABP
Fatty acid binding protein
- FADD
Fatty acid drug discovery
- FADS
Fatty acid desaturase
- HETE
Hydroxy eicosatetraenoic acid
- HODE
Hydroxy octadecadienoic acid
- HPETE
Hydroperoxy eicosatetraenoic acid
- IL
Interleukin
- IPE
Icosapent ethyl
- LA
Linoleic acid
- LOX
Lipoxygenase
- LPS
Lipopolysaccharides
- LTs
Leukotrienes
- LXs
Lipoxins
- MI
Myocardial infarction
- NF-κβ
Nuclear factor kappa β
- NIH
National Institutes of Health
- NO
Nitric oxide
- PGs
Prostaglandins
- PI3K/Akt
Phosphoinositide 3-kinase/protein kinase
- PMNs
Polymorphonuclear leukocytes
- PUFA
Polyunsaturated fatty acid
- SCD
Stearoyl-CoA desaturase
- SPM
Specialized pro-resolving mediators
- T2D
Type 2 diabetes
- TNF
Tumor necrosis factor
Footnotes
Conflict of Interest- The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106(25):3143–3421. [PubMed] [Google Scholar]
- 2.Fish oil supplements. Jama. 312(8):839–840. doi: 10.1001/jama.2014.9758. [DOI] [PubMed] [Google Scholar]
- 3.Abedi E, Sahari MA. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food science & nutrition. 2014;2(5):443–463. doi: 10.1002/fsn3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015;29(6):2504–2513. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvheim AR, Malde MK, Osei-Hyiaman D, Hong Lin Y, Pawlosky RJ, Madsen L, Kristiansen K, Froyland L, Hibbeln JR. Dietary Linoleic Acid Elevates Endogenous 2-AG and Anandamide and Induces Obesity. Obesity. 2012 doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvheim AR, Torstensen BE, Lin YH, Lillefosse HH, Lock EJ, Madsen L, Froyland L, Hibbeln JR, Malde MK. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids. 2014;49(1):59–69. doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arita M. Mediator lipidomics in acute inflammation and resolution. Journal of biochemistry. 2012;152(4):313–319. doi: 10.1093/jb/mvs092. [DOI] [PubMed] [Google Scholar]
- 9.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of immunology (Baltimore, Md : 1950) 2007;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 10.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging Delays Resolution of Acute Inflammation in Mice: Reprogramming the Host Response with Novel Nanoproresolving Medicines. Journal of immunology (Baltimore, Md: 1950) 2014 doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated Fatty acids with cancer risk and progression. Frontiers in oncology. 2013;3(3):224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. The American journal of clinical nutrition. 1980;33(12):2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 14.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochimica et biophysica acta. 2010;1801(12):1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannenberg GL. Resolvins: Current understanding and future potential in the control of inflammation. Current Opinion in Drug Discovery & Development. 2009;12(5):644–658. [PubMed] [Google Scholar]
- 16.Bazan NG. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Advances in experimental medicine and biology. 2006;572:531–540. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]
- 17.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Current Opinion in Clinical Nutrition & Metabolic Care. 2007;10(2):136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 18.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. European Journal of Clinical Investigation. 2002;32(s3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 20.Bradberry JC, Hilleman DE. Overview of Omega-3 Fatty Acid Therapies. Pharmacy and Therapeutics. 2013;38(11):681–691. [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley Christopher D, Gilroy Derek W, Serhan Charles N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burr GO, Burr MM. Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutrition reviews. 1973;31(8):248–249. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 23.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? British journal of clinical pharmacology. 2013;75(3):645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calder PC, Deckelbaum RJ. Harmful, harmless or helpful? The n-6 fatty acid debate goes on. Curr Opin Clin Nutr Metab Care. 2011;14(2):113–114. doi: 10.1097/MCO.0b013e328343d895. [DOI] [PubMed] [Google Scholar]
- 25.Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of omega-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. BioMed research international. 2013;2013:743171. doi: 10.1155/2013/743171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell ELLN, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21(12):3162–3270. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 27.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, McIver K, Mehta T, Menachemi N, Newby PK, Pate R, Rolls BJ, Sen B, Smith DL, Jr, Thomas DM, Allison DB. Myths, presumptions, and facts about obesity. The New England journal of medicine. 2013;368(5):446–454. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta neurologica Taiwanica. 2009;18(4):231–241. [PubMed] [Google Scholar]
- 29.Cheng X, He S, Yuan J, Miao S, Gao H, Zhang J, Li Y, Peng W, Wu P. Lipoxin A attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free radical biology & medicine. 2016;93:52–66. doi: 10.1016/j.freeradbiomed.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. The Journal of experimental medicine. 2015 doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484(7395):524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, Colas RA, Dalli J, Serhan CN, Romano M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem cells translational medicine. 2016;5(1):20–32. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connolly DPRaJM. Omega-3 fatty acids as cancer chemo preventive agents. Pharmacol Ther. 1999;83(2):217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 34.Corminboeuf O, Leroy X. FPR2/ALXR agonists and the resolution of inflammation. Journal of medicinal chemistry. 2015;58(2):537–559. doi: 10.1021/jm501051x. [DOI] [PubMed] [Google Scholar]
- 35.Cressey D. Traditional drug-discovery model ripe for reform. Nature. 2011;471(7336):17–18. doi: 10.1038/471017a. [DOI] [PubMed] [Google Scholar]
- 36.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. American journal of physiology Lung cellular and molecular physiology. 2015;309(8):L888–901. doi: 10.1152/ajplung.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalen JE, Devries S. Diets to prevent coronary heart disease 1957–2013: what have we learned? The American journal of medicine. 2014;127(5):364–369. doi: 10.1016/j.amjmed.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Dalli J, Colas RA, Serhan CN. Novel n-3 Immunoresolvents: Structures and Actions. Scientific Reports. 2013;3:1940. doi: 10.1038/srep01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalli J, Colas RA, Walker ME, Serhan CN. Lipid Mediator Metabolomics Via LC-MS/MS Profiling and Analysis. Methods in molecular biology (Clifton, NJ) 2018;1730:59–72. doi: 10.1007/978-1-4939-7592-1_4. [DOI] [PubMed] [Google Scholar]
- 40.Dalli J, Serhan CN. Pro-Resolving Mediators in Regulating and Conferring Macrophage Function. Frontiers in immunology. 2017;8:1400. doi: 10.3389/fimmu.2017.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das UN. COX-2 inhibitors and metabolism of essential fatty acids. Med Sci Monit. 2005;11(7):RA233–237. [PubMed] [Google Scholar]
- 42.Das UN. Can essential fatty acids reduce the burden of disease(s)? Lipids in Health and Disease. 2008;7(1):9. doi: 10.1186/1476-511X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das UN, Fams Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition. 2003;19(1):62–65. doi: 10.1016/s0899-9007(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 44.De Caterina R. n-3 fatty acids in cardiovascular disease. The New England journal of medicine. 2011;364(25):2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 45.de Souza PM, Newson J, Gilroy DW. Targeting lipoxygenases with care. Chemistry & biology. 2006;13(11):1121–1122. doi: 10.1016/j.chembiol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Delmastro-Greenwood M, Hughan KS, Vitturi DA, Salvatore SR, Grimes G, Potti G, Shiva S, Schopfer FJ, Gladwin MT, Freeman BA, Gelhaus Wendell S. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free radical biology & medicine. 2015;89:333–341. doi: 10.1016/j.freeradbiomed.2015.07.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DHM Methodologic challenges in designing clinical studies to measure differences in the bioequivalence of n-3 fatty acids. Mol Cell Biochem. 2003;246(1–2):83–90. [PubMed] [Google Scholar]
- 48.Djousse L, Bartz TM, Ix JH, Zieman SJ, Delaney JA, Mukamal KJ, Gottdiener JS, Siscovick DS, Kizer JR. Adiposity and incident heart failure in older adults: the cardiovascular health study. Obesity. 2012;20(9):1936–1941. doi: 10.1038/oby.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112(3):848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. Journal of immunology (Baltimore, Md : 1950) 2006;177(9):5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 51.Eckel RH. The fish oil story remains fishy. Circulation. 2010;122(21):2110–2112. doi: 10.1161/CIRCULATIONAHA.110.986976. [DOI] [PubMed] [Google Scholar]
- 52.El Kebir D, Filep JG. Targeting neutrophil apoptosis for enhancing the resolution of inflammation. Cells. 2013;2(2):330–348. doi: 10.3390/cells2020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endo J, Sano M, Isobe Y, Fukuda K, Kang JX, Arai H, Arita M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. The Journal of experimental medicine. 2014;211(8):1673–1687. doi: 10.1084/jem.20132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fahy E, Cotter D, Sud M, Subramaniam S. Lipid classification, structures and tools. Biochimica et biophysica acta. 2011;1811(11):637–647. doi: 10.1016/j.bbalip.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CR, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA. A comprehensive classification system for lipids. Journal of lipid research. 2005;46(5):839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. Journal of lipid research. 2009;50(Suppl):S9–14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic acids research. 2007;35(Web Server issue):W606–612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandes G. Progress in nutritional immunology. Immunologic research. 2008;40(3):244–261. doi: 10.1007/s12026-007-0021-3. [DOI] [PubMed] [Google Scholar]
- 59.Fischer RKA, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M, Luft FC, Weylandt K, Schunck WH. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. Journal of lipid research. 2014;55(6):1150–1164. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 61.Flesher RP, Herbert C, Kumar RK. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clinical science. 2014;126(11):805–814. doi: 10.1042/CS20130623. [DOI] [PubMed] [Google Scholar]
- 62.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(10):2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freire MO, Dalli J, Serhan CN, Van Dyke TE. Neutrophil Resolvin E1 Receptor Expression and Function in Type 2 Diabetes. Journal of immunology (Baltimore, Md : 1950) 2017;198(2):718–728. doi: 10.4049/jimmunol.1601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt Promotes Survival of Cardiomyocytes In Vitro and Protects Against Ischemia-Reperfusion Injury in Mouse Heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Funk CD. Lipoxygenase pathways as mediators of early inflammatory events in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(6):1204–1206. doi: 10.1161/01.ATV.0000222960.43792.ff. [DOI] [PubMed] [Google Scholar]
- 66.Gerster H. Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res. 1998;68(3):159–173. [PubMed] [Google Scholar]
- 67.Gilbert K, Bernier J, Bourque-Riel V, Malick M, Rousseau G. Resolvin D1 reduces infarct size through a phosphoinositide 3-kinase/protein kinase B mechanism. Journal of cardiovascular pharmacology. 2015a doi: 10.1097/FJC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert K, Bernier J, Bourque-Riel V, Malick M, Rousseau G. Resolvin D1 Reduces Infarct Size Through a Phosphoinositide 3-Kinase/Protein Kinase B Mechanism. Journal of cardiovascular pharmacology. 2015b;66(1):72–79. doi: 10.1097/FJC.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 69.Gilbert K, Malick M, Madingou N, Bourque-Riel V, Touchette C, Rousseau G. Linoleic acid attenuates cardioprotection induced by resolvin D1. The Journal of nutritional biochemistry. 2016;31:122–126. doi: 10.1016/j.jnutbio.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Glatz J. Challenges in Fatty Acid and Lipid Physiology. Frontiers in Physiology. 2011;2(45) doi: 10.3389/fphys.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glatz JFC, Luiken JJFP, Bonen A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiological Reviews. 2010;90(1):367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 72.Gregg EW, Shaw JE. Global Health Effects of Overweight and Obesity. The New England journal of medicine. 2017 doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- 73.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, MLS A role for the mouse 12/15lipoxygenase pathway in promoting epithelial wound healing and host defense. The Journal of biological chemistry. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 74.Hadj Ahmed S, Kaoubaa N, Kharroubi W, Zarrouk A, Najjar MF, Batbout F, Gamra H, Lizard G, Hammami M. Association of plasma fatty acid alteration with the severity of coronary artery disease lesions in Tunisian patients. Lipids in Health and Disease. 2017;16(1):154. doi: 10.1186/s12944-017-0538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental gerontology. 2011a;46(1):43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halade GV, Jin YF, Lindsey ML. Roles of saturated vs. polyunsaturated fat in heart failure survival: not all fats are created equal. Cardiovascular research. 2012;93(1):4–5. doi: 10.1093/cvr/cvr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halade GV, Kain V. Obesity and Cardiometabolic Defects in Heart Failure Pathology. Comprehensive Physiology. 2017;7(4):1463–1477. doi: 10.1002/cphy.c170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging. 2016;8(11):2611–2634. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halade GV, Kain V, Ingle KA, Prabhu SD. Interaction of 12/15 Lipoxygenase with Fatty Acids Alters the Leukocyte Kinetics Leading to Improved Post-myocardial Infarction Healing. American journal of physiology. Heart and circulatory physiology. 2017 doi: 10.1152/ajpheart.00040.2017. ajpheart.00040.02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. Journal of immunology (Baltimore, Md : 1950) 2010a;184(9):5280–5286. doi: 10.4049/jimmunol.0903282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halade GV, Rahman MM, Fernandes G. Effect of CLA isomers and their mixture on aging C57Bl/6J mice. European journal of nutrition. 2009;48(7):409–418. doi: 10.1007/s00394-009-0029-7. [DOI] [PubMed] [Google Scholar]
- 82.Halade GV, Rahman MM, Fernandes G. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice. The Journal of nutritional biochemistry. 2010b;21(4):332–337. doi: 10.1016/j.jnutbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. The Journal of nutritional biochemistry. 2010c;21(12):1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. The Journal of nutritional biochemistry. 2011b;22(5):459–469. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han X. Lipidomics for studying metabolism. Nature Reviews Endocrinology. 2016;12:668. doi: 10.1038/nrendo.2016.98. [DOI] [PubMed] [Google Scholar]
- 86.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney international. 2007;71(11):1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 87.Harris WS, Shearer GC. Omega-6 fatty acids and cardiovascular disease: friend, not foe? Circulation. 2014;130(18):1562–1564. doi: 10.1161/CIRCULATIONAHA.114.012534. [DOI] [PubMed] [Google Scholar]
- 88.Hasturk HKA, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. J Immuno. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 89.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. The Journal of Immunology. 2007;179(10):7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 90.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. Journal of immunology (Baltimore, Md : 1950) 2007;179(10):7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 91.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nature immunology. 2008;9(8):873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Held C, Iqbal R, Lear SA, Rosengren A, Islam S, Mathew J, Yusuf S. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. European heart journal. 2012;33(4):452–466. doi: 10.1093/eurheartj/ehr432. [DOI] [PubMed] [Google Scholar]
- 93.Hendrich S. (n-3) Fatty Acids: Clinical Trials in People with Type 2 Diabetes. Advances in nutrition. 2010;1(1):3–7. doi: 10.3945/an.110.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herrera BS, Hasturk H, Kantarci A, Freire MO, Nguyen O, Kansal S, Van Dyke TE. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infection and immunity. 2015;83(2):792–801. doi: 10.1128/IAI.02444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. The American journal of clinical nutrition. 2006;83(6 Suppl):1483s–1493s. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 96.Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C, Fortin S, Rousseau E. Resolvin E1 normalizes contractility, Ca2+sensitivity and smooth muscle cell migration rate in TNF-α- and IL-6-pretreated Human Pulmonary Arteries. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2015;309(8) doi: 10.1152/ajplung.00177.2015. ajplung.00177.02015. [DOI] [PubMed] [Google Scholar]
- 97.Hisada T, Ishizuka T, Aoki H, Mori M. Resolvin E1 as a novel agent for the treatment of asthma. Expert Opinion on Therapeutic Targets. 2009;13(5):513–522. doi: 10.1517/14728220902865622. [DOI] [PubMed] [Google Scholar]
- 98.Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, Dartt DA. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal immunology. 2016 doi: 10.1038/mi.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, Dartt DA. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal immunology. 2017;10(1):46–57. doi: 10.1038/mi.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hua J, Jin Y, Chen Y, Inomata T, Lee H, Chauhan SK, Petasis NA, Serhan CN, Dana R. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investigative ophthalmology & visual science. 2014;55(9):5944–5951. doi: 10.1167/iovs.14-14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(11):6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflammatory bowel diseases. 2010;16(1):87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. The Journal of biological chemistry. 2012;287(13):10525–10534. doi: 10.1074/jbc.M112.340612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson AL, Edson KZ, Totah RA, Rettie AE. Cytochrome P450 omega-Hydroxylases in Inflammation and Cancer. Advances in pharmacology. 2015;74:223–262. doi: 10.1016/bs.apha.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jove M, Mate I, Naudi A, Mota-Martorell N, Portero-Otin M, De la Fuente M, Pamplona R. Human Aging Is a Metabolome-related Matter of Gender. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71(5):578–585. doi: 10.1093/gerona/glv074. [DOI] [PubMed] [Google Scholar]
- 106.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14(2):115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Junot C, Fenaille F, Colsch B, Bécher F. High resolution mass spectrometry based techniques at the crossroads of metabolic pathways. Mass Spectrometry Reviews. 2014;33(6):471–500. doi: 10.1002/mas.21401. [DOI] [PubMed] [Google Scholar]
- 108.Kain V, Halade GV. Metabolic and Biochemical Stressors in Diabetic Cardiomyopathy. Frontiers in cardiovascular medicine. 2017;4:31. doi: 10.3389/fcvm.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. Journal of molecular and cellular cardiology. 2015;84:24–35. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic research in cardiology. 2014;109(6):444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 111.Kang JW, Lee SM. Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing M2 macrophage polarization and efferocytosis. Biochimica et biophysica acta. 2016;1861(9 Pt A):1025–1035. doi: 10.1016/j.bbalip.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Kang JX, Liu A. The role of the tissue omega-6/omega-3 fatty acid ratio in regulating tumor angiogenesis. Cancer Metastasis Rev. 2013;32(1):201–210. doi: 10.1007/s10555-012-9401-9. [DOI] [PubMed] [Google Scholar]
- 113.Katakura M, Hashimoto M, Inoue T, Mamun AA, Tanabe Y, Arita M, Shido O. Chronic Arachidonic Acid Administration Decreases Docosahexaenoic Acid- and Eicosapentaenoic Acid-Derived Metabolites in Kidneys of Aged Rats. PloS one. 2015;10(10):e0140884. doi: 10.1371/journal.pone.0140884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. American journal of physiology Heart and circulatory physiology. 2010;299(1):H153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 115.Keys A. Diet and the epidemiology of coronary heart disease. Journal of the American Medical Association. 1957;164(17):1912–1919. doi: 10.1001/jama.1957.62980170024007e. [DOI] [PubMed] [Google Scholar]
- 116.Keys A, Menotti A, Aravanis C, Blackburn H, Djordevic BS, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, et al. The seven countries study: 2,289 deaths in 15 years. Preventive medicine. 1984;13(2):141–154. doi: 10.1016/0091-7435(84)90047-1. [DOI] [PubMed] [Google Scholar]
- 117.Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Current topics in medicinal chemistry. 2007;7(3):311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 118.Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annual review of biochemistry. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 119.Kim SY, Kim TB, Moon KA, Kim TJ, Shin D, Cho YS, Moon HB, Lee KY. Regulation of pro-inflammatory responses by lipoxygenases via intracellular reactive oxygen species in vitro and in vivo. Experimental & molecular medicine. 2008;40(4):461–476. doi: 10.3858/emm.2008.40.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. British journal of pharmacology. 2009;158(4):960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. Journal of immunology (Baltimore, Md : 1950) 2015;194(3):863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, Zarbock A, Koenders MI, Axmann R, Zwerina J, Baenckler HW, van den Berg W, Voll RE, Kuhn H, Joosten LA, Schett G. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. Journal of immunology (Baltimore, Md : 1950) 2009;183(5):3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 123.Kummerow FA. Nutrition imbalance and angiotoxins as dietary risk factors in coronary heart disease. The American journal of clinical nutrition. 1979;32(1):58–83. doi: 10.1093/ajcn/32.1.58. [DOI] [PubMed] [Google Scholar]
- 124.Lands B. Consequences of essential fatty acids. Nutrients. 2012;4(9):1338–1357. doi: 10.3390/nu4091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54(7):585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 126.Le CH, Mulligan CM, Routh MA, Bouma GJ, Frye MA, Jeckel KM, Sparagna GC, Lynch JM, Moore RL, McCune SA, Bristow M, Zarini S, Murphy RC, Chicco AJ. Delta-6-desaturase links polyunsaturated fatty acid metabolism with phospholipid remodeling and disease progression in heart failure. Circulation Heart failure. 2014;7(1):172–183. doi: 10.1161/CIRCHEARTFAILURE.113.000744. [DOI] [PubMed] [Google Scholar]
- 127.Lee CT, Teles R, Kantarci A, Chen T, McCafferty J, Starr JR, Brito LC, Paster BJ, Van Dyke TE. Resolvin E1 Reverses Experimental Periodontitis and Dysbiosis. Journal of immunology (Baltimore, Md : 1950) 2016;197(7):2796–2806. doi: 10.4049/jimmunol.1600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee JE, Sun Y, Gjorstrup P, Pearlman E. Inhibition of Corneal Inflammation by the Resolvin E1. Investigative ophthalmology & visual science. 2015;56(4):2728–2736. doi: 10.1167/iovs.14-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lefkowitz RJ. Alfred Goodman Gilman (1941–2015) Nature. 2016;529(7586):284. doi: 10.1038/529284a. [DOI] [PubMed] [Google Scholar]
- 130.Levy BD. Resolvins and protectins: Natural pharmacophores for resolution biology. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2010;82(4–6):327–332. doi: 10.1016/j.plefa.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26(5):431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li X, Hu H, Wang Y, Xue M, Li X, Cheng W, Xuan Y, Yin J, Yang N, Yan S. Valsartan Attenuates KIR2.1 by Downregulating the Th1 Immune Response in Rats Following Myocardial Infarction. Journal of cardiovascular pharmacology. 2016;67(3):252–259. doi: 10.1097/FJC.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 133.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC., Jr 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68(1):92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 134.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Grassler J, Gronberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson A, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindstrom J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Muller G, Muller-Nurasyid M, Musk AW, Nagaraja R, Nothen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundstrom J, Swertz MA, Swift AJ, Syvanen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gadin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van‘t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, Mu TC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Illig T, Jacobs KB, Jarvelin MR, Jockel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimaki T, Lyssenko V, Mannisto S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tonjes A, Tregouet DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Volker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Perusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O'Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK LifeLines Cohort S, International Endogene C, Heath AC, Consortium AD, Group ABW, Consortium CAD, Consortium CK, Glgc Icbp, Investigators M, Consortium MI, Consortium P, ReproGen C, Consortium G. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopategi A, Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Titos E, Claria J. Role of bioactive lipid mediators in obese adipose tissue inflammation and endocrine dysfunction. Molecular and cellular endocrinology. 2016;419:44–59. doi: 10.1016/j.mce.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 136.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. American journal of physiology Heart and circulatory physiology. 2015;308(4):H269–280. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lundstrom SL, Balgoma D, Wheelock AM, Haeggstrom JZ, Dahlen SE, Wheelock CE. Lipid mediator profiling in pulmonary disease. Current pharmaceutical biotechnology. 2011;12(7):1026–1052. doi: 10.2174/138920111795909087. [DOI] [PubMed] [Google Scholar]
- 138.Ma S, Yim SH, Lee SG, Kim EB, Lee SR, Chang KT, Buffenstein R, Lewis KN, Park TJ, Miller RA, Clish CB, Gladyshev VN. Organization of the Mammalian Metabolome according to Organ Function, Lineage Specialization, and Longevity. Cell metabolism. 2015;22(2):332–343. doi: 10.1016/j.cmet.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circulation research. 2013;112(4):675–688. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Massey Karen A, Nicolaou A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochemical Society Transactions. 2011;39(5):1240–1246. doi: 10.1042/BST0391240. [DOI] [PubMed] [Google Scholar]
- 141.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radical Biology and Medicine. 2013;59:45–55. doi: 10.1016/j.freeradbiomed.2012.08.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McDonnell M, Davis W, Jr, Li H, Funk CD. Characterization of the murine epidermal 12/15-lipoxygenase. Prostaglandins Other Lipid Mediat. 2001;63(3):93–107. doi: 10.1016/s0090-6980(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 143.McDonnell M, Li H, Funk CD. Characterization of epidermal 12(S) and 12(R) lipoxygenase. Eicosanoids & other bioactive lipids in cancer. Inflammation & radiation injury. 2002;5:147–153. [Google Scholar]
- 144.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. The FASEB Journal. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mittal A, Ranganath V, Nichani A. Omega fatty acids and resolution of inflammation: A new twist in an old tale. Journal of Indian Society of Periodontology. 2010;14(1):3–7. doi: 10.4103/0972-124X.65426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free radical biology & medicine. 2003;35(7):772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 147.Motilva MJ, Serra A, Rubio L. Nutrikinetic studies of food bioactive compounds: from in vitro to in vivo approaches. International journal of food sciences and nutrition. 2015;66(Suppl 1):S41–52. doi: 10.3109/09637486.2015.1025721. [DOI] [PubMed] [Google Scholar]
- 148.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. The American journal of clinical nutrition. 2004a;79(4):606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. trans fatty acids and systemic inflammation in heart failure. The American journal of clinical nutrition. 2004b;80(6):1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mullard A. New drugs cost US$2.6 billion to develop. Nature Reviews Drug Discovery. 2014;13(12):877–877. [Google Scholar]
- 151.NSC Resolution phase of inflammation. Novel endogenous anti-inflammatory And proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 152.Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. The New England journal of medicine. 2016;374(6):555–561. doi: 10.1056/NEJMoa1508808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei Cas L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011;57(7):870–879. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 154.Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, Hogg RJ, Trygg J, Hammock BD, Zivkovic AM. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PloS one. 2013;8(10):e76575. doi: 10.1371/journal.pone.0076575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(8):1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, Irimia D, Serhan CN, Perretti M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI insight. 2016;1(5):e85922. doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ogawa SUD, Yokokura Y, Arai H, Arita M, Inoue M. Total synthesis and bioactivity of resolvin E2. Org Lett. 2009;11:3602–3605. doi: 10.1021/ol901350g. [DOI] [PubMed] [Google Scholar]
- 158.Oh DY, Lagakos WS. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2011;14(4):322–327. doi: 10.1097/MCO.0b013e3283479230. [DOI] [PubMed] [Google Scholar]
- 159.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin E2 Formation and Impact in Inflammation Resolution. The Journal of Immunology. 2012;188(9):4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. The Journal of clinical investigation. 2011;121(2):569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oni-Orisan A, Edin ML, Lee JA, Wells MA, Christensen ES, Vendrov KC, Lih FB, Tomer KB, Bai X, Taylor JM, Stouffer GA, Zeldin DC, Lee CR. Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: a targeted metabolomics study. 2015 doi: 10.1194/jlr.M061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Oni-Orisan A, Edin ML, Lee JA, Wells MA, Christensen ES, Vendrov KC, Lih FB, Tomer KB, Bai X, Taylor JM, Stouffer GA, Zeldin DC, Lee CR. Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: a targeted metabolomics study. Journal of lipid research. 2016;57(1):109–119. doi: 10.1194/jlr.M061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. Journal of nutrition and metabolism. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. The Journal of clinical investigation. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Patwardhan B, Vaidya AD. Natural products drug discovery: accelerating the clinical candidate development using reverse pharmacology approaches. Indian journal of experimental biology. 2010;48(3):220–227. [PubMed] [Google Scholar]
- 166.Perretti M, Leroy X, Bland EJ, Montero-Melendez T. Resolution Pharmacology: Opportunities for Therapeutic Innovation in Inflammation. Trends in pharmacological sciences. 2015;36(11):737–755. doi: 10.1016/j.tips.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 167.Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 168.Pietilainen KH, Sysi-Aho M, Rissanen A, Seppanen-Laakso T, Yki-Jarvinen H, Kaprio J, Oresic M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects--a monozygotic twin study. PloS one. 2007;2(2):e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Powell WS, Chung D, Gravel S. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. The Journal of Immunology. 1995;154(8):4123–4132. [PubMed] [Google Scholar]
- 170.Pradalier A, Bakouche P, Baudesson G, Delage A, Cornaille-Lafage G, Launay JM, Biason P. Failure of omega-3 polyunsaturated fatty acids in prevention of migraine: a double-blind study versus placebo. Cephalalgia: an international journal of headache. 2001;21(8):818–822. doi: 10.1046/j.1468-2982.2001.218240.x. [DOI] [PubMed] [Google Scholar]
- 171.Proschak E, Heitel P, Kalinowsky L, Merk D. Opportunities and Challenges for Fatty Acid Mimetics in Drug Discovery. Journal of medicinal chemistry. 2017;60(13):5235–5266. doi: 10.1021/acs.jmedchem.6b01287. [DOI] [PubMed] [Google Scholar]
- 172.Qian S, Xu Y. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomedical Journal. 2014;0(0):0. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Radmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. Journal of lipid research. 2009;50(Suppl):S40–45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends in biochemical sciences. 2007;32(7):332–341. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 175.Raetz CR, Guan Z, Ingram BO, Six DA, Song F, Wang X, Zhao J. Discovery of new biosynthetic pathways: the lipid A story. Journal of lipid research. 2009;50(Suppl):S103–108. doi: 10.1194/jlr.R800060-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, Phipps RP. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? Journal of immunology. 2014;193(12):6031–6040. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. Journal of immunology (Baltimore, Md : 1950) 2012;189(2):1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, Barden A, Lynch C, Coble R, Mas E, Palsson O, Barrow DA, Mann JD. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154(11):2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ramsden CE, Hibbeln JR, Majchrzak-Hong SF. All PUFAs are not created equal: absence of CHD benefit specific to linoleic acid in randomized controlled trials and prospective observational cohorts. World review of nutrition and dietetics. 2011;102:30–43. doi: 10.1159/000327789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rice HB, Bernasconi A, Maki KC, Harris WS, von Schacky C, Calder PC. Conducting omega-3 clinical trials with cardiovascular outcomes: Proceedings of a workshop held at ISSFAL 2014. Prostaglandins, leukotrienes, and essential fatty acids. 2016 doi: 10.1016/j.plefa.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 181.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. Jama. 2012:308. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 182.Roke K, Mutch DM. The role of FADS1/2 polymorphisms on cardiometabolic markers and fatty acid profiles in young adults consuming fish oil supplements. Nutrients. 2014;6(6):2290–2304. doi: 10.3390/nu6062290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rouzer CA, Marnett LJ. Mechanism of Free Radical Oxygenation of Polyunsaturated Fatty Acids by Cyclooxygenases. Chemical Reviews. 2003;103(6):2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 184.Royce LA, Liu P, Stebbins MJ, Hanson BC, Jarboe LR. The damaging effects of short chain fatty acids on Escherichia coli membranes. Applied Microbiology and Biotechnology. 2013;97(18):8317–8327. doi: 10.1007/s00253-013-5113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Russo GL. Dietary n–6 and n–3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochemical Pharmacology. 2009;77(6):937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 186.Sapieha P, Stahl A, Chen J, Seaward MR, Willett KL, Krah NM, Dennison RJ, Connor KM, Aderman CM, Liclican E, Carughi A, Perelman D, Kanaoka Y, Sangiovanni JP, Gronert K, Smith LE. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Science translational medicine. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sawada Y, Honda T, Hanakawa S, Nakamizo S, Murata T, Ueharaguchi-Tanada Y, Ono S, Amano W, Nakajima S, Egawa G, Tanizaki H, Otsuka A, Kitoh A, Dainichi T, Ogawa N, Kobayashi Y, Yokomizo T, Arita M, Nakamura M, Miyachi Y, Kabashima K. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. The Journal of experimental medicine. 2015;212(11):1921–1930. doi: 10.1084/jem.20150381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schaap FG, Binas B, Danneberg H, van der Vusse GJ, Glatz JF. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circulation research. 1999;85(4):329–337. doi: 10.1161/01.res.85.4.329. [DOI] [PubMed] [Google Scholar]
- 189.Schacky V. Omega 3 essential fatty acids Vs Cardiac diseases the contribution of omega 3 index. Cell Bio Mol. 2010;56:93–101. [PubMed] [Google Scholar]
- 190.Schneider C, Pratt DA, Porter NA, Brash AR. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chemistry & biology. 2007;14(5):473–488. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schultz C, Neef AB, Gadella TW, Goedhart J. Labeling Lipids for Imaging in Fixed Cells. Cold Spring Harbor Protocols. 2010;2010(7) doi: 10.1101/pdb.prot5458. pdb.prot5458. [DOI] [PubMed] [Google Scholar]
- 192.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. Journal of immunology (Baltimore, Md : 1950) 2010;184(2):836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. Journal of Thrombosis and Haemostasis. 2009;7:44–48. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- 195.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Serhan CN, Chiang N, Dalli J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Molecular aspects of medicine. 2017 doi: 10.1016/j.mam.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2–Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. The Journal of experimental medicine. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111(10):5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annual review of pathology. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shao Z, Fu Z, Stahl A, Joyal JS, Hatton C, Juan A, Hurst C, Evans L, Cui Z, Pei D, Gong Y, Xu D, Tian K, Bogardus H, Edin ML, Lih F, Sapieha P, Chen J, Panigrahy D, Hellstrom A, Zeldin DC, Smith LE. Cytochrome P450 2C8 omega3-long-chain polyunsaturated fatty acid metabolites increase mouse retinal pathologic neovascularization--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(3):581–586. doi: 10.1161/ATVBAHA.113.302927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shinohara M, Kibi M, Riley IR, Chiang N, Dalli J, Kraft BD, Piantadosi CA, Choi AM, Serhan CN. Cell-cell interactions and bronchoconstrictor eicosanoid reduction with inhaled carbon monoxide and resolvin D1. American journal of physiology Lung cellular and molecular physiology. 2014;307(10):L746–757. doi: 10.1152/ajplung.00166.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Siguel EN, Lerman RH. Altered fatty acid metabolism in patients with angiographically documented coronary artery disease. Metabolism - Clinical and Experimental. 43(8):982–993. doi: 10.1016/0026-0495(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 204.Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poult Sci. 2000;79(7):961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 205.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. The American journal of clinical nutrition. 1991;58:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 206.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 207.Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sioen IA, Pynaert I, Matthys C, De Backer G, Van Camp J, De Henauw S. Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids. 2006;41(5):415–422. doi: 10.1007/s11745-006-5115-5. [DOI] [PubMed] [Google Scholar]
- 209.Smith W, Mukhopadhyay R. Essential fatty acids: the work of George and Mildred Burr. The Journal of biological chemistry. 2012;287(42):35439–35441. doi: 10.1074/jbc.O112.000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry. 2000:69. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 211.Sowers JR. Obesity as a cardiovascular risk factor. The American journal of medicine. 2003;115(Suppl 8A):37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 212.Spector AA, Kim HY. Discovery of essential fatty acids. Journal of lipid research. 2015;56(1):11–21. doi: 10.1194/jlr.R055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stoffel W, Hammels I, Jenke B, Binczek E, Schmidt-Soltau I, Brodesser S, Odenthal M, Thevis M. Obesity resistance and deregulation of lipogenesis in Delta6-fatty acid desaturase (FADS2) deficiency. EMBO reports. 2014;15(1):110–120. doi: 10.1002/embr.201338041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stoffel W, Holz B, Jenke B, Binczek E, Gunter RH, Kiss C, Karakesisoglou I, Thevis M, Weber AA, Arnhold S, Addicks K. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. The EMBO journal. 2008;27(17):2281–2292. doi: 10.1038/emboj.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Takenaka T. Classical vs reverse pharmacology in drug discovery. BJU international. 2001;88(Suppl 2):7–10. doi: 10.1111/j.1464-410x.2001.00112.x. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 217.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62(2):618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, Gissi HFI. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 219.Tian Y, Zhang Y, Zhang R, Qiao S, Fan J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson’s disease rat model. Biochemical and biophysical research communications. 2015;460(3):799–805. doi: 10.1016/j.bbrc.2015.03.109. [DOI] [PubMed] [Google Scholar]
- 220.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. Journal of immunology. 2011;187(10):5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 221.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chemistry & biology. 2006;13(11):1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 222.Tomio K, Kawana K, Taguchi A, Isobe Y, Iwamoto R, Yamashita A, Kojima S, Mori M, Nagamatsu T, Arimoto T, Oda K, Osuga Y, Taketani Y, Kang JX, Arai H, Arita M, Kozuma S, Fujii T. Omega-3 polyunsaturated Fatty acids suppress the cystic lesion formation of peritoneal endometriosis in transgenic mouse models. PloS one. 2013;8(9):e73085. doi: 10.1371/journal.pone.0073085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tourki B, Halade G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. Faseb j. 2017;31(10):4226–4239. doi: 10.1096/fj.201700109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tumanov S, Kamphorst JJ. Recent advances in expanding the coverage of the lipidome. Current Opinion in Biotechnology. 2017;43(Supplement C):127–133. doi: 10.1016/j.copbio.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, Sarter K, Frey B, Rothe T, Voll R, Nimmerjahn F, Bochkov VN, Schett G, Kronke G. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 226.Uderhardt S, Kronke G. 12/15-lipoxygenase during the regulation of inflammation, immunity, and self-tolerance. Journal of molecular medicine. 2012;90(11):1247–1256. doi: 10.1007/s00109-012-0954-4. [DOI] [PubMed] [Google Scholar]
- 227.van Velzen EJ, Westerhuis JA, van Duynhoven JP, van Dorsten FA, Grun CH, Jacobs DM, Duchateau GS, Vis DJ, Smilde AK. Phenotyping tea consumers by nutrikinetic analysis of polyphenolic end-metabolites. Journal of proteome research. 2009;8(7):3317–3330. doi: 10.1021/pr801071p. [DOI] [PubMed] [Google Scholar]
- 228.Velazquez F, Grodecki-Pena A. CD43 Functions as an E-Selectin Ligand for Th17 Cells In Vitro and Is Required for Rolling on the Vascular Endothelium and Th17 Cell Recruitment during. Inflammation In Vivo. 2016;196(3):1305–1316. doi: 10.4049/jimmunol.1501171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Verma MK, Pulicherla KK. Enzyme promiscuity in earthworm serine protease: substrate versatility and therapeutic potential. Amino Acids. 2016;48(4):941–948. doi: 10.1007/s00726-015-2162-3. [DOI] [PubMed] [Google Scholar]
- 230.von Schacky C. Omega-3 fatty acids and cardiovascular disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2007;10(2):129–135. doi: 10.1097/MCO.0b013e3280127af0. [DOI] [PubMed] [Google Scholar]
- 231.Wakabayashi S, Freed LM, Bell JM, Rapoport SI. In vivo Cerebral Incorporation of Radiolabeled Fatty Acids after Acute Unilateral Orbital Enucleation in Adult Hooded Long-Evans Rats. Journal of Cerebral Blood Flow & Metabolism. 1994;14(2):312–323. doi: 10.1038/jcbfm.1994.38. [DOI] [PubMed] [Google Scholar]
- 232.Wanga W, Zhua Julia, Lyua Fei, Panigrahy Dipak, Ferrarac Katherine W, BH, Zhanga G. ω-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins & other Lipid Mediators. 2014;113:13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Warensjo E, Riserus U, Gustafsson IB, Mohsen R, Cederholm T, Vessby B. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2008;18(10):683–690. doi: 10.1016/j.numecd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 234.Weylandt KH, Chen Yong Q, Lim Kyu, Su Hui-Min, Cittadinia Achille, Calviello G. W3 PUFAs in the Prevention and Cure of Inflammatory, Degenerative, and Neoplastic Diseases 2014. BioMed research international. 2015;2015(695875):2. doi: 10.1155/2015/695875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumptionand renal cell carcinoma incidence in women. Jama. 2006;296(11):1371–1377. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 236.Woodcock CC, Huang Y, Woodcock SR, Salvatore SR, Singh B, Golin-Bisello F, Davidson NE, Neumann CA, Freeman BA, Wendell SG. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion, and tumor growth. The Journal of biological chemistry. 2018;293(4):1120–1137. doi: 10.1074/jbc.M117.814368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xu ZG, Li SL, Lanting L, Kim YS, Shanmugam N, Reddy MA, Natarajan R. Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: potential role in diabetic nephropathy. Kidney international. 2006;69(3):512–519. doi: 10.1038/sj.ki.5000137. [DOI] [PubMed] [Google Scholar]
- 238.Yamada H, Oshiro E, Kikuchi S, Hakozaki M, Takahashi H, Kimura K. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. Journal of lipid research. 2014;55(5):895–904. doi: 10.1194/jlr.M047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang P, Jiang Y, Fischer SM. Prostaglandin E3 metabolism and cancer. Cancer Letters. 2014;348(1):1–11. doi: 10.1016/j.canlet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yang X, Sheng W, Sun GY, Lee JC. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochemistry international. 2011;58(3):321–329. doi: 10.1016/j.neuint.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPAlisI. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 242.Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159(2):318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zárate R, el Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clinical and Translational Medicine. 2017;6(1):25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Progress in Lipid Research. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Progress in lipid research. 2014;53:108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]