Abstract
Background
Inflammatory proteins could help identify individuals most likely to have gallbladder cancer (GBC) among those waiting for cholecystectomy.
Methods
We analyzed 49 circulating inflammation-related proteins in 144 patients with GBC and 150 patients with gallstones. We calculated age- and sex-adjusted odds ratios (ORs) and 95% CIs for protein quantiles and GBC versus gallstones. Using proteins associated with early GBC (stage 1–2) that were selected in stepwise logistic regression, we created an inflammation score and explored the potential utility for risk stratification.
Results
26 proteins (53%) had P values for the trend across categories ≤0.001, with associations for a one category increase ranging from 1·52 (95% CI: 1.20–1.94) for C-C motif ligand 4 to 4·00 (95% CI: 2·76–5·79) for interleukin (IL)-8. Soluble tumor necrosis factor receptor 2 (sTNFR2), IL-6, sTNFR1, C-C motif ligand 20 (CCL20), vascular cell adhesion molecule 1, IL-16, and granulocyte colony-stimulating factor had P values ≤0.001 for early GBC. Of those, IL-6, IL-16, CCL20, and STNFR1 were included in the inflammation score. In a high-risk setting with a pre-test disease risk of 10% (e.g., elderly patients) and using an inflammation score cutoff that provides 90% sensitivity, 39% of patients on the waiting list would be predicted to be positive, and 23% of those would be predicted to have GBC.
Conclusion
These results highlight the strong associations of inflammatory proteins with GBC risk and their potential clinical utility. Larger studies are needed to identify the most effective combinations of inflammatory proteins for detecting early GBC and precursor lesions.
Keywords: gallbladder cancer, risk stratification, inflammation, cytokines, chemokines
Introduction
Gallstones are the main risk factor for gallbladder cancer (GBC) [1, 2], a highly lethal disease with a 5-year relative survival rate of 17% across all stages [3]. Early stage (localized) tumors can be cured with cholecystectomy [4]. Thus, early detection and treatment is critical to improve survival among patients with GBC. Unfortunately, timely treatment of all individuals with gallstones through cholecystectomy is not practical given the high prevalence of gallstones, particularly in high-risk areas. In Chile, which has among the highest rates of GBC in the world, national policy dictates that individuals aged 35–49 who are diagnosed with gallstones are prioritized for cholecystectomy [5], leading to delayed treatment of older patients who are at higher GBC risk. This delay may be very important clinically since survival is much improved for early-stage cancers; 5-year survival is 41% for localized tumors, and nearly 90% for tumors confined to the muscularis, versus 11% for regional and 3% for distant tumors [4, 6].
Levels of circulating proteins related to inflammation might help stratify individuals awaiting cholecystectomy since gallbladder carcinogenesis is strongly tied to inflammation [7]. Inflammation involves multiple signaling pathways and molecules produced by various types of immune cells. Gallstones can cause inflammation [7]. Histopathologic changes indicative of inflammation in the gallbladder proceed the formation of gallstones in both animal models and humans [8–10], and gallstones have been associated with elevated levels of circulating cytokines [11]. However, circulating levels of inflammatory proteins are notably higher in patients with GBC than in patients with gallstones alone [11, 12]. We previously used multiplex cytokine panels to measure these proteins in circulation and in bile and showed strong elevations in patients with GBC compared to those with gallstones [12, 13].
The ability to prioritize the cholecystectomy waiting list according to the probability that GBC is present could help identify patients with early stages of GBC and thus increase the chances of curative treatment. These effects are particularly important in high-risk regions, such as Chile, Northern India, and Shanghai, China. In the present study, we evaluated the utility of using circulating inflammatory proteins to stratify patients waiting for cholecystectomy by their risk of prevalent GBC.
Methods
From June 1997 through May 2001, the Shanghai Biliary Tract Cancer Study enrolled 368 GBC cases [2, 14]. Newly diagnosed cancer cases were identified through a rapid-reporting system established between the Shanghai Cancer Institute and 42 collaborating hospitals in 10 urban districts of Shanghai, and 774 gallstone patients were frequency matched to patients with GBC on age, sex, and hospital. Over 74% of patients with GBC were confirmed by histology. Those without pathological tissue, generally due to unresectable tumours, were evaluated by a clinical review panel of four gastrointestinal surgeons and a pathologist, who reviewed imaging data and clinical and operative reports [2]. All patients were permanent residents of urban Shanghai between the ages of 35 and 74 without a previous non-skin cancer, and provided written informed consent. The U.S. National Cancer Institute and Shanghai Cancer Institute institutional review boards approved the study.
We evaluated circulating inflammatory proteins in 144 GBC cases and 150 randomly selected gallstone patients who either had serum collected prior to surgery or did not have surgery. We used the Milliplex (EMD Millipore, Billerica, MA) and the Meso Scale Discovery (MSD) Human Vascular Injury II (Meso Scale Diagnostics LLC, Rockville, MD, USA) kits to test for 68 inflammatory proteins. For the Milliplex assay serum samples were incubated with beads in 96-well plates, after which fluorescently labeled detection antibodies were added. A Bio-Plex instrument and Bio-Plex Manager 6.1 software (Bio-Rad, Hercules, CA, USA) were used to analyze the 96-well plates. The MSD plate-based ELISA assay was performed according to the manufacturer’s instructions. Briefly, serum samples were incubated with assay diluent, followed by incubation with a detection antibody. The MSD plates were then analyzed using the MSD Sector Imager 6000 plate reader and Discovery Workbench 3.0 software (Meso Scale Diagnostics LLC, Rockville, MD, USA).
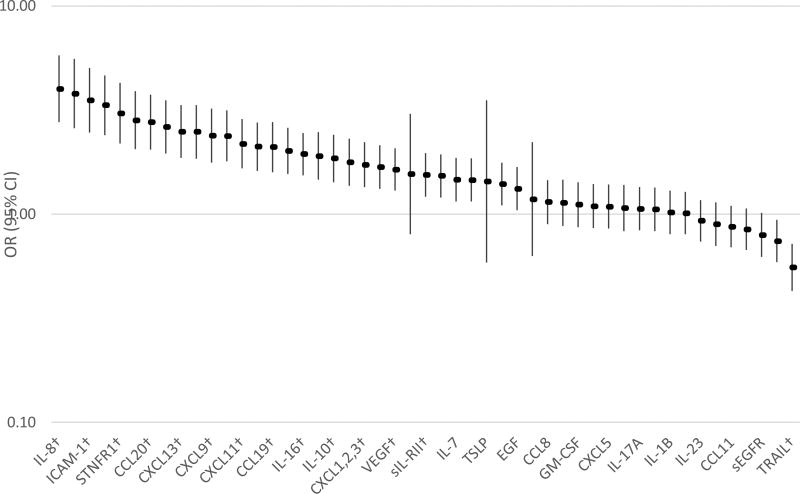
Some of these proteins have been previously reported in a subset of patients in the current study, which expanded the number of patients and tested new markers [12]. Quality control evaluations led us to drop 19 of 68 proteins (see supplemental methods), leaving 49 proteins for analysis, which are listed in Figure 1.
Figure 1.
Odds ratios (OR) and 95% confidence intervals (CIs)* between ordinal categories of inflammatory proteins in patients with gallbladder cancer (N=144) compared to those with gallstones (N=150).
*Adjusted for age and sex and matched on lot
†Passed Bonferroni correction (P<0·001)
Note: all markers had 4 categories except IL-29, IL-33, and TSLP, which had 2 categories
As performed in previous studies [15, 16], cutpoints were used to create categories that were determined by the proportion of subjects with detectable values as follows: (a) for proteins detectable in ≥75% of subjects, four categories were created based on quartiles of values above the lower limit of quantitation (LLOQ) using the distribution among gallstone controls (subjects with undetectable values were included in the lowest quartile); (b) for proteins detectable in 50–75% of subjects, four categories were created where the first category included all subjects with undetectable values and the next three categories were based on tertiles of values above the LLOQ; (c) for proteins detectable in 25–50% of subjects, three categories were created where the first category included all subjects with undetectable values and the next two categories were based on a median split of the values above the LLOQ; (d) for proteins detectable in <25% of subjects, two categories were created: one for undetectable values and the other for values above the LLOQ. These proteins were modeled both as categorically and ordinally (coded as 1, 2, 3, 4) to evaluate linear trend. For simplicity, we provide the ordinal ORs, which reflect the linear change per category.
Univariate associations between sociodemographic and behavioral characteristics and case-control status were investigated using the Kruskal-Wallis test for difference in medians and chi-square tests for categorical comparisons. To best control for potential differences by lot, we fit logistic models conditional on lot to calculate ORs and 95% CIs for associations between inflammatory proteins and GBC versus gallstones.
All models were adjusted for age (≤54,55–65, ≥66) and sex (male/female). In addition, we conducted stepwise linear regression among population-based controls to determine whether inflammation marker levels above the LLOQ were associated with: education (none/primary, junior middle, senior middle, some college), ever drinking, ever smoking, categorical body mass index (underweight, normal weight, overweight, obese), fasting status (fasting/not fasting), and history of diabetes. We required p < 0·05 for a variable to be entered into a model and p < 0·01 for that variable to be retained in the model. After identifying potential confounders through stepwise linear regression, we fit logistic regression models to determine whether the covariate changed the OR for the association between the categorical inflammation protein and GBC by more than 10% while adjusting for age and gender. No covariate changed the OR by more than 10%, so, the final models included only age and sex. At the time of analysis, we identified 11 samples (4 from patients with GBC and 7 from patients with gallstones) that were collected after surgery, but exclusion of these samples did not substantively affect the results [12]. We also considered multiple comparisons by applying a Bonferroni correction of α=0·001 (0·05/49 proteins analyzed).
After examining associations for all patients with GBC, we fit models restricted to early/localized GBC (stage 1 and 2) compared to all controls. For proteins that were associated with early GBC, we conducted stepwise logistic regression using α=0·05 to determine which proteins were associated with GBC (all cases combined) compared to gallstones taking the other proteins into account. For those proteins that remained in the model, we created an inflammation score. We created this score by summed the categorical values (i.e., 1, 2, 3, or 4) for the level of each protein weighted by the log-OR from the model for that protein and gallbladder cancer risk (using all gallbladder cancer cases, regardless of stage). We then divided the score into four categories based on the distribution in the gallstone controls.
Finally, we explored the potential clinical utility of proteins and the summary inflammation score to identify early GBC. We set the sensitivity for each at 90%, 80%, or 70% and varied the prevalence of GBC (i.e., assumed risk before testing for the marker) from 1% to 10%. We then used the biomarker webtool available at https://analysistools.nci.nih.gov/biomarkerTools/, originally described by Wentzensen and Wacholder [17], to estimate the specificity, number positive per 1,000 cholecystectomy patients screened, and positive predictive value (PPV, the risk of disease after a positive test).
Results
GBC patients were slightly older than gallstone patients (median age 67 versus 65, P=0.005) and were also less educated (P=0.0002), but they were similar to gallstone patients with respect to sex, smoking, drinking, diabetes, and obesity (Table 1). One-hundred-fifteen (80%) of the GBC patients had evidence of gallstones.
Table 1.
Characteristics of gallbladder cancer cases (GBC) and gallstone patients (GS) tested for inflammatory proteins in the Shanghai Biliary Tract Cancer Case-Control Study.
| GBC (N=144) | GS (N=150) | |
|---|---|---|
| Median age (range) | 67 (39–74) | 65 (34–74) |
| Male, N (%) | 45 (31.3) | 41 (27.3) |
| Ever smoker, N (%) | 42 (29.4) | 32 (21.3) |
| Ever drinker, N (%) | 24 (16.7) | 23 (15.3) |
| Self-reported diabetes, N (%) | 16 (11.2) | 22 (14.7) |
| Education, N (%) | ||
| None/Primary | 78 (54.2) | 52 (34.7) |
| Jr. Middle | 35 (24.3) | 33 (22.0) |
| Sr. Middle | 15 (10.4) | 43 (28.7) |
| Some college | 16 (11.1) | 22 (14.7) |
| Self-reported BMI 5 years prior to interview | ||
| Underweight: <18.5 | 14 (9.7) | 10 (6.7) |
| Normal: 18.5 24.9 | 73 (50.7) | 95 (63.3) |
| Overweight: 25 29.9 | 50 (34.7) | 40 (26.7) |
| Obese: ≥30.0 | 7 (4.9) | 5 (3.3) |
| Gallstones, N (%) | 115 (79.9) | 150 (100.0) |
Of the 49 proteins analyzed, 26 (53%) were associated with GBC risk at P≤0.001, with ordinal associations that ranged from OR=1·52 per category (95% CI: 1·20–1·94) for C-C motif ligand 4 (CCL4) to OR=4·00 (95% CI: 2·76–5·79) for interleukin 8 (IL-8) (Figure 1). Of these 26 proteins, most were associated with early GBC [Table 2, four categories for all proteins except interleukin 29 (IL-29), interleukin 33 (IL-33), and thymic stromal lymphopoietin (TSLP), which had two categories].
Table 2.
Odds ratios (ORs) and 95% CI* for ordinal associations of immune markers with early gallbladder cancer (GBC) (N=32) versus gallstones (N=150).
| Marker | OR | 95% CI | P† |
|---|---|---|---|
| IL-8 | 2.01 | 1.32–3.04 | 0.001 |
| sTNFR2 | 2.37 | 1.44–3.92 | 0.0007 |
| ICAM-1 | 2.16 | 1.35–3.44 | 0.001 |
| IL-6 | 2.26 | 1.45–3.51 | 0.0003 |
| STNFR1 | 2.63 | 1.56–4.45 | 0.0003 |
| CRP | 1.86 | 1.20–2.89 | 0.006 |
| CCL20 | 2.18 | 1.43–3.34 | 0.0003 |
| SAA | 1.73 | 1.16–2.57 | 0.008 |
| CXCL13 | 1.37 | 0.94–1.98 | 0.1 |
| VCAM1 | 2.23 | 1.40–3.56 | 0.0008 |
| CXCL9 | 1.51 | 1.02–2.25 | 0.04 |
| TNF-a | 2.07 | 1.33–3.23 | 0.001 |
| CXCL11 | 1.62 | 1.08–2.41 | 0.02 |
| CCL15 | 1.74 | 1.17–2.61 | 0.007 |
| CCL19 | 1.38 | 0.94–2.01 | 0.1 |
| CXCL6 | 1.61 | 1.09–2.38 | 0.02 |
| IL-16 | 2.75 | 1.79–4.21 | <.0001 |
| CXCL10 | 1.48 | 1.00–2.20 | 0.05 |
| IL-10 | 1.37 | 0.94–2.01 | 0.1 |
| CCL2 | 1.40 | 0.95–2.06 | 0.1 |
| CXCL1,2,3 | 1.36 | 0.94–1.98 | 0.1 |
| G-CSF | 1.82 | 1.28–2.61 | 0.001 |
| VEGF | 1.62 | 1.11–2.35 | 0.01 |
| sIL-RII | 1.20 | 0.83–1.74 | 0.3 |
| CCL4 | 1.11 | 0.77–1.59 | 0.6 |
| TRAIL | 0.67 | 0.45–0.99 | 0.04 |
Adjusted for age and sex and matched on lot
P value for the trend across categories of marker level
Seven proteins had P values of 0.001 or less for early GBC: soluble tumor necrosis factor receptor 2 (sTNFR2), interleukin 6 (IL-6), soluble tumor necrosis factor receptor 1 (sTNFR1), C-C motif ligand 20 (CCL20), vascular cell adhesion molecule 1 (VCAM-1), interleukin 16 (IL-16), and granulocyte colony-stimulating factor (G-CSF). When included together in a stepwise logistic regression model, IL-6, IL-16, CCL20, and sTNFR1 remained in the model. We then used these four proteins to create an inflammation score. Restricted to early GBC cases, the OR for quartile 4 versus 1 was 42·01 (95% CI: 4·65–379·25).
We also explored potential clinical utility by calculating the performance characteristics of the proteins associated with early GBC (Table 3). In general, higher disease prevalence produced higher PPVs. Also, using cutoffs that were more specific but less sensitive increased the PPV. Taking the inflammation score as an example, with a pre-test disease prevalence of 5%, which has been reported in an unselected group of cholecystectomy patients from a high-risk region [18], a cut-off that would provide 90% sensitivity would produce 362 positives out of every 1,000 cholecystectomy patients tested. Of those 362 positive patients, 45 (362*PPV of 12·4%) would have GBC. Lowering the sensitivity to 70% would lead to 147 positives out of every 1,000 patients tested, of whom 35 would have GBC.
Table 3.
Performance characteristics of seven inflammatory proteins associated with early/localized gallbladder cancer
| Disease Prevalence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 5% | 1% | |||||||||
| Marker | Sensitivity | Specificity | N positive per 1000 tested |
PPV * |
cNP V† |
N positive per 1000 tested |
PPV * |
cNP V† |
N positive per 1000 tested |
PPV * |
cNP V† |
| CCL20 | 90% | 40.5% | 626 | 14.4% | 2.7% | 610 | 7.4% | 1.3% | 574 | 1.5% | 0.2% |
| 80% | 58.0% | 458 | 17.5% | 3.7% | 439 | 9.1% | 1.8% | 407 | 1.9% | 0.3% | |
| 70% | 69.8% | 342 | 20.5% | 4.6% | 322 | 10.9% | 2.2% | 294 | 2.3% | 0.4% | |
| G-CSF | 90% | 31.0% | 711 | 12.7% | 3.5% | 701 | 6.4% | 1.7% | 665 | 1.3% | 0.3% |
| 80% | 47.9% | 549 | 14.6% | 4.4% | 535 | 7.5% | 2.2% | 503 | 1.5% | 0.4% | |
| 70% | 60.4% | 426 | 16.4% | 5.2% | 411 | 8.5% | 2.6% | 383 | 1.8% | 0.5% | |
| IL-6 (high-sensitivity) | 90% | 41.5% | 617 | 14.6% | 2.6% | 601 | 7.5% | 1.3% | 565 | 1.5% | 0.2% |
| 80% | 58.8% | 451 | 17.8% | 3.6% | 431 | 9.3% | 1.8% | 399 | 1.9% | 0.3% | |
| 70% | 70.6% | 335 | 20.9% | 4.5% | 314 | 11.1% | 2.2% | 286 | 2.4% | 0.4% | |
| IL-16 | 90% | 29.1% | 728 | 12.4% | 3.7% | 719 | 6.3% | 1.8% | 683 | 1.3% | 0.3% |
| 80% | 45.6% | 570 | 14.0% | 4.7% | 557 | 7.2% | 2.3% | 525 | 1.5% | 0.4% | |
| 70% | 58.2% | 446 | 15.7% | 5.4% | 432 | 8.1% | 2.6% | 404 | 1.7% | 0.5% | |
| sTNFR1 | 90% | 52.9% | 514 | 17.5% | 2.1% | 492 | 9.1% | 1.0% | 456 | 1.9% | 0.2% |
| 80% | 69.5% | 355 | 22.6% | 3.1% | 330 | 12.1% | 1.5% | 298 | 2.6% | 0.3% | |
| 70% | 79.6% | 254 | 27.6% | 4.0% | 229 | 15.3% | 1.9% | 201 | 3.4% | 0.4% | |
| sTNFR2 | 90% | 55.4% | 491 | 18.3% | 2.0% | 469 | 9.6% | 0.9% | 433 | 2.0% | 0.2% |
| 80% | 71.7% | 335 | 23.9% | 3.0% | 309 | 12.9% | 1.5% | 277 | 2.8% | 0.3% | |
| 70% | 81.3% | 238 | 29.4% | 3.9% | 213 | 16.5% | 1.9% | 185 | 3.7% | 0.4% | |
| VCAM-1 | 90% | 68.8% | 371 | 24.3% | 1.6% | 341 | 13.2% | 0.8% | 305 | 2.8% | 0.1% |
| 80% | 82.3% | 239 | 33.4% | 2.6% | 208 | 19.2% | 1.3% | 176 | 4.4% | 0.2% | |
| 70% | 89.3% | 166 | 42.1% | 3.6% | 137 | 25.6% | 1.7% | 109 | 6.2% | 0.3% | |
| Inflammation score‡ | 90% | 66.6% | 391 | 23.0% | 1.7% | 362 | 12.4% | 0.8% | 326 | 2.7% | 0.2% |
| 80% | 80.7% | 254 | 31.5% | 2.7% | 223 | 17.9% | 1.3% | 191 | 4.0% | 0.3% | |
| 70% | 88.2% | 176 % | 39.7 | 3.6% | 147 | 23.8% | 1.8% | 119 | 5.7% | 0.3% | |
PPV=positive predictive value, the risk of disease after a positive test
cNPV=complement of the negative predictive value (NPV), the risk of disease after a negative test result
Inflammation score=sum of the categorical values of 4 markers that remained in the stepwise logistics regression model (CCL20, IL-6, IL-16, sTNFR1), weighted by the log-OR from the overall GBC model for each marker.
In a population with a very high risk of GBC, such as cholecystectomy patients aged 70+, the risk of GBC prior to testing could be 10% or higher [18]. In this situation 100 of every 1000 patients would have GBC. Using an inflammation marker cut-off with 90% sensitivity would produce 391 test positives out of every 1,000 patients tested, and those positives would include 90 of the 100 patients with GBC. Lowering the sensitivity to 70% would lead to 176 positives out of every 1,000 patients tested, including 70 with GBC, but 30 GBC cases would test negative and be missed.
Discussion
Overall survival is quite poor (median ~6 months [19–21]) for GBC, which is a major public health burden in some parts of the world, including certain regions in Chile, India, and China. However, if detected at early stages, then survival is much higher [3, 4]. At these early stages, cholecystectomy can be curative; thus, it is extremely important to identify and treat early stage GBC [22]. Patients with gallstones are at increased risk for developing GBC. In areas with high GBC incidence rates, early stage GBC is relatively common [4]. However, such areas are also challenged limited resources and the burden of who to prioritize for surgery among all the patients with gallstones. This challenge is especially acute in a country like Chile, which has one of the highest risk of GBC in the world and prioritizes patients with gallstones aged 35–49, leading to delayed treatment of older patients who are at higher risk for GBC [5]. The results from our previous study of GBC and gallstone patients [12] suggested that measuring levels of circulating inflammatory proteins may help identify those patients who are more likely to have cancer. In the current study, we expanded the number of GBC and gallstone patients to more accurately assess the association between inflammatory proteins and GBC compared to patients with gallstones. We also explored the potential clinical utility of these proteins for use in prioritizing the cholecystectomy waiting list.
In this study, we confirmed that inflammatory proteins are strongly and robustly associated with GBC risk compared to patients with gallstones. Most proteins were associated with risk of early stage GBC, as well as GBC risk overall, and seven proteins had P values ≤0·001 for early GBC (sTNFR2, IL-6, sTNFR1, CCL20, VCAM-1, IL-16, and G-CSF). Of those, IL-6, IL-16, CCL20, and STNFR1 remained associated with GBC in multivariable logistic models. An inflammation score based on these 4 proteins was strongly associated with GBC risk compared to patients with gallstones (quartile 4 versus 1 OR for early GBC cases: 42·01, 95% CI: 4·65–379·25).
Should our findings be confirmed in future studies, these proteins may prove useful for risk stratification among patients waiting for cholecystectomy. For example, if the inflammation score were applied with 90% sensitivity in a high-risk setting such as Chile with a 5% disease prevalence [18], 90% of the GBC cases could be treated more quickly by prioritizing the 36% of the patients who test positive. In a higher risk setting with a pre-test disease risk of 10%, such as among elderly patients, 39% of the patients on the waiting list would be selected using a cutoff for the inflammation score that provides 90% sensitivity, and nearly one quarter of the positive patients would be found to have GBC. Reducing the sensitivity to 70% would select only 18% of patients on the waiting list, and 40% of the positives would have GBC.
Few studies have been conducted on inflammatory proteins and GBC. IL-6, which has been studied intensively in other cancers, is expressed by cholangiocarcinoma and stromal inflammatory cells and may contribute to the development of cholangiocarcinoma by promoting cell proliferation and survival [23–25]. One recent study evaluated cytokines in serum from 52 GBC cases and 30 age and sex matched healthy controls and found higher levels of IL-6 in GBC cases compared to normal controls [26]. G-CSF affects granulocytic precursor proliferation and differentiation, and G-CSF-producing gallbladder cancers have been reported in a number of case reports [27–29]. These tumors often co-express G-CSF and IL-6 [27, 29], which is supported in our study by the fact that IL-6 but not G-CSF remained in the stepwise logistic regression model. CCL20 is released by TH17 cells, which are increasingly understood to be important players in the tumor microenvironment, although their exact role remains unclear [30]. CCL20 appears to contribute to colorectal cancer by promoting cell proliferation and migration [31]. IL-16 regulates T cell growth, but the way in which it contributes to cancer differs by cancer site [32]. sTNFRII is associated with insulin resistance, and elevated circulating levels have been associated with the development of colorectal cancer [33], while elevated levels of sTNFR1 have been associated with increased cancer mortality [34]. Finally, in recent years the role of VCAM-1 in tumor growth, metastasis, and angiogenesis have been elucidated, highlighting its role in tumor progression and its potential as a target for cancer treatment.
GBC usually develops after progressing from chronic cholecystitis to dysplasia and then to cancer [35]. Progression from dysplasia to cancer is estimated to take 10 years [35]. Given this timeline, there is ample opportunity for screening and early detection. Our results suggest that inflammatory proteins may help identify early stage cancers among patients with gallstones waiting for cholecystectomy.
Our study has many strengths. It is one of the largest studies of inflammatory proteins and GBC to date. We used an assay that was extensively evaluated for reproducibility and reliability [36] and has been used in multiple epidemiologic studies [15, 16, 37–41]. We were able to demonstrate robust associations between inflammatory proteins and GBC. Misclassification of disease status was minimized through comprehensive pathology and clinical review, which provided nearly complete confirmation of case status.
Although our results are promising, we had a limited number of early cancers, making it difficult to evaluate combinations of proteins. In addition, current multiplex assays for inflammatory proteins tend to be expensive and are designed for research purposes. To apply these proteins to a clinical setting in the future, fast, economical assays that require a small volume of blood have to be developed and demonstrated to be reproducible. A larger study, ideally including gallbladder dysplasia as well as cancer, is needed to more fully investigate the performance of combinations of proteins. Additional replication is needed in other study populations including more patients. However, we previously observed similar associations between inflammation markers and gallbladder cancer compared to gallstones in both Shanghai and Chile [12], suggesting that these associations are robust across populations. As with any epidemiologic study, the results could be influenced by unmeasured confounders (e.g., exercise, stress, circadian rhythm). Processing time and time in storage might affect marker levels, but any such bias would be expected to be non-differential by case-control status, thus attenuating the results. Finally, although this study is to our knowledge the most comprehensive analyses of inflammatory proteins and GBC to date, there are many more immune-related proteins that could be useful in risk stratification and in gallbladder carcinogenesis and should be evaluated in future studies.
In conclusion, GBC is a major public health problem in some regions, including parts of Chile, India, and China, which has led to an urgent need for risk stratification. We evaluated the association between inflammatory proteins and GBC risk compared to gallstones to determine whether such proteins may be used to stratify people waiting for cholecystectomy based on risk. Given the strong and consistent associations we observed and the reasonable predicted performance in the setting of the cholecystectomy waiting list, these proteins may prove to have clinical utility. Additional studies are needed before clinical use, however, ideally with more proteins and including patients with precancerous lesions, to identify the best protein or combination of proteins to facilitate prioritization of the cholecystectomy waiting list in areas where demand is higher than the capacity of the health care system.
Supplementary Material
Highlights.
Inflammatory proteins can be used to stratify based on gallbladder cancer risk
These proteins may have clinical utility in high gallbladder cancer incidence areas
Additional studies are needed to identify the most effective combinations
Acknowledgments
The authors wish to thank the collaborating surgeons and pathologists in Shanghai for assistance in patient recruitment and pathology review; Chia–Rong Cheng, Lu Sun, and Kai Wu of the Shanghai Cancer Institute for coordinating data and specimen collection; Shelley Niwa and Karen Pettit of Westat for support with study and data management; and Michael Curry of Information Management Services, Inc., for analytical support.
Financial Support
This work was supported by a grant (Z01 CP010218) from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics and general funds from the Office of Research on Women’s Health, National Institutes of Health. Funding was awarded to Jill Koshiol. No other co-authors received grant funding for the work associated with this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study concept and design (Koshiol, Gao, Kemp, Shen, Hildesheim, Hsing, Wang, Pinto); acquisition of data (Koshiol, Gao, Corbel, Kemp, Shen, Rashid, Wang, Hsing, Pinto); statistical analysis (Koshiol, Pfeiffer); interpretation of data (Koshiol, Gao, Kemp, Hildesheim, Hsing, Rashid, Pfeiffer, Pinto); drafting of manuscript (Koshiol); critical review and revision of manuscript (Koshiol, Gao, Corbel, Kemp, Shen, Hildesheim, Hsing, Rashid, Wang, Wang, Pfeiffer, Pinto).
Conflicts of Interest: None.
References
- 1.Roa I, Ibacache G, Munoz S, de Aretxabala X. Gallbladder cancer in Chile: Pathologic characteristics of survival and prognostic factors: analysis of 1,366 cases. American journal of clinical pathology. 2014;141(5):675–82. doi: 10.1309/AJCPQT3ELN2BBCKA. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF., Jr Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97(11):1577–82. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshiol J, Ferreccio C, Devesa SS, Roa JC, Fraumeni JF., Jr Biliary Tract Cancer. Cancer Epidemiology and Prevention. 2017 [Google Scholar]
- 4.de Aretxabala X, Roa I, Hepp J, Maluenda F, Mordojovich G, Leon J, Roa JC. Early gallbladder cancer: is further treatment necessary? Journal of surgical oncology. 2009;100(7):589–93. doi: 10.1002/jso.21389. [DOI] [PubMed] [Google Scholar]
- 5.Health Ministries, Chilean Government, Clinical Guide: Preventive Cholecystectomy in Adults between the Age of 35 and 49. Clinical Guide Series 2010. 2010 [Google Scholar]
- 6.Koshiol J, Ferreccio C, Devesa SS, Roa JC, Fraumeni JF., Jr . Biliary Tract Cancers. In: Thun MJ, Linet MS, Cerhan JR, Haiman C, editors. Cancer Epidemiology and Prevention. Oxford University Press; 2017. [Google Scholar]
- 7.Espinoza JA, Bizama C, Garcia P, Ferreccio C, Javle M, Miquel JF, Koshiol J, Roa JC. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865(2):245–54. doi: 10.1016/j.bbcan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rege RV. Inflammatory cytokines alter human gallbladder epithelial cell absorption/secretion. J Gastrointest Surg. 2000;4(2):185–92. doi: 10.1016/s1091-255x(00)80055-4. [DOI] [PubMed] [Google Scholar]
- 9.van Erpecum KJ, Wang DQ, Moschetta A, Ferri D, Svelto M, Portincasa P, Hendrickx JJ, Schipper M, Calamita G. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res. 2006;47(1):32–41. doi: 10.1194/jlr.M500180-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. 2009;136(2):425–40. doi: 10.1053/j.gastro.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Kemp TJ, Gao YT, Corbel A, McGee EE, Wang B, Shen MC, Rashid A, Hsing AW, Hildesheim A, Pfeiffer RM, Pinto LA, Koshiol J. The Association of Circulating Inflammation Proteins and Gallstone Disease. doi: 10.1111/jgh.14265. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshiol J, Castro F, Kemp TJ, Gao YT, Roa JC, Wang B, Nogueira L, Araya JC, Shen MC, Rashid A, Hsing AW, Hildesheim A, Ferreccio C, Pfeiffer RM, Pinto LA. Association of inflammatory and other immune markers with gallbladder cancer: Results from two independent case-control studies. Cytokine. 2016;83:217–25. doi: 10.1016/j.cyto.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp TJ, Castro FA, Gao YT, Hildesheim A, Nogueira L, Wang BS, Sun L, Shelton G, Pfeiffer RM, Hsing AW, Pinto LA, Koshiol J. Application of multiplex arrays for cytokine and chemokine profiling of bile. Cytokine. 2015;73(1):84–90. doi: 10.1016/j.cyto.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu E, Sakoda LC, Gao YT, Rashid A, Shen MC, Wang BS, Deng J, Han TQ, Zhang BH, Fraumeni JF, Jr, Hsing AW. Aspirin use and risk of biliary tract cancer: a population-based study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1315–8. doi: 10.1158/1055-9965.EPI-05-0032. [DOI] [PubMed] [Google Scholar]
- 15.Shiels MS, Katki HA, Hildesheim A, Pfeiffer RM, Engels EA, Williams M, Kemp TJ, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating Inflammation Markers, Risk of Lung Cancer, and Utility for Risk Stratification. J Natl Cancer Inst. 2015;107(10) doi: 10.1093/jnci/djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105(24):1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentzensen N, Wacholder S. From differences in means between cases and controls to risk stratification: a business plan for biomarker development. Cancer Discov. 2013;3(2):148–57. doi: 10.1158/2159-8290.CD-12-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roa I, de Aretxabala X, Roa J, Araya JC, Villaseca M, Guzman P, Burgos L. Is gallbladder cancer a disease with bad prognosis? Revista medica de Chile. 2002;130(11):1295–302. [PubMed] [Google Scholar]
- 19.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, La Vecchia C. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20(1):146–59. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 20.Bertran E, Heise K, Andia ME, Ferreccio C. Gallbladder cancer: incidence and survival in a high-risk area of Chile. Int J Cancer. 2010;127(10):2446–54. doi: 10.1002/ijc.25421. [DOI] [PubMed] [Google Scholar]
- 21.Koshiol J, Ferreccio C, Devesa SS, Roa JC, J Fraumeni JF. Biliary Tract Cancer. Cancer Epidemiology and Prevention. 2017 [Google Scholar]
- 22.Roa I, de Aretxabala X. Gallbladder cancer in Chile: what have we learned? Current opinion in gastroenterology. 2015;31(3):269–75. doi: 10.1097/MOG.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 23.Brito AF, Abrantes AM, Encarnacao JC, Tralhao JG, Botelho MF. Cholangiocarcinoma: from molecular biology to treatment. Med Oncol. 2015;32(11):245. doi: 10.1007/s12032-015-0692-x. [DOI] [PubMed] [Google Scholar]
- 24.Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer. 2012;1(1):58–70. doi: 10.3978/j.issn.2224-4778.2011.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing gammadeltaT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139(4):869–81. doi: 10.1002/ijc.30134. [DOI] [PubMed] [Google Scholar]
- 27.Izumo W, Furukawa K, Katsuragawa H, Tezuka T, Furukawa T, Hataji K, Komatsu A, Shigematsu K, Yamamoto M. Granulocyte-colony stimulating factor-producing gallbladder carcinoma-include analysis all case reports: A case report. Int J Surg Case Rep. 2016;21:87–90. doi: 10.1016/j.ijscr.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanaya N, Aoki H, Yamasaki R, Morihiro T, Takeuchi H. Granulocyte Colony-Stimulating Factor-Producing Gallbladder Cancer. Acta Med Okayama. 2016;70(5):393–396. doi: 10.18926/AMO/54599. [DOI] [PubMed] [Google Scholar]
- 29.Suzumura K, Iimuro Y, Asano Y, Kuroda N, Hirano T, Yamanaka J, Okada T, Okamoto T, Torii I, Fujimoto J. Granulocyte-colony stimulating factor-producing gallbladder carcinoma. Int Surg. 2014;99(5):577–83. doi: 10.9738/INTSURG-D-13-00129.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi W, Huang X, Wang J. Correlation between Th17 cells and tumor microenvironment. Cell Immunol. 2013;285(1–2):18–22. doi: 10.1016/j.cellimm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Frick VO, Rubie C, Keilholz U, Ghadjar P. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: An overview. World J Gastroenterol. 2016;22(2):833–41. doi: 10.3748/wjg.v22.i2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richmond J, Tuzova M, Cruikshank W, Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J Cell Physiol. 2014;229(2):139–47. doi: 10.1002/jcp.24441. [DOI] [PubMed] [Google Scholar]
- 33.Aleman JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146(2):357–73. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlsson AC, Juhlin CC, Larsson TE, Larsson A, Ingelsson E, Sundstrom J, Lind L, Arnlov J. Soluble tumor necrosis factor receptor 1 (sTNFR1) is associated with increased total mortality due to cancer and cardiovascular causes - findings from two community based cohorts of elderly. Atherosclerosis. 2014;237(1):236–42. doi: 10.1016/j.atherosclerosis.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Roa I, de Aretxabala X, Araya JC, Roa J. Preneoplastic lesions in gallbladder cancer. Journal of surgical oncology. 2006;93(8):615–23. doi: 10.1002/jso.20527. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S, Purdue MP, Hsing AW, Pinto L, McCoy JP, Hildesheim A. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1902–11. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, Moore SC, Purdue MP, Wentzensen N, Hildesheim A, Shiels MS. Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2840–9. doi: 10.1158/1055-9965.EPI-14-0699-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang Kuhs KA, Hildesheim A, Trabert B, Kemp TJ, Purdue MP, Wentzensen N, Katki HA, Pinto LA, Loftfield E, Safaeian M, Chaturvedi AK, Shiels MS. Association between Regular Aspirin Use and Circulating Markers of Inflammation: A Study within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2015;24(5):825–32. doi: 10.1158/1055-9965.EPI-14-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loftfield E, Shiels MS, Graubard BI, Katki HA, Chaturvedi AK, Trabert B, Pinto LA, Kemp TJ, Shebl FM, Mayne ST, Wentzensen N, Purdue MP, Hildesheim A, Sinha R, Freedman ND. Associations of Coffee Drinking with Systemic Immune and Inflammatory Markers. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1052–60. doi: 10.1158/1055-9965.EPI-15-0038-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trabert B, Eldridge RC, Pfeiffer RM, Shiels MS, Kemp TJ, Guillemette C, Hartge P, Sherman ME, Brinton LA, Black A, Chaturvedi AK, Hildesheim A, Berndt SI, Safaeian M, Pinto L, Wentzensen N. Prediagnostic circulating inflammation markers and endometrial cancer risk in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Int J Cancer. 2017;140(3):600–610. doi: 10.1002/ijc.30478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trabert B, Pinto L, Hartge P, Kemp T, Black A, Sherman ME, Brinton LA, Pfeiffer RM, Shiels MS, Chaturvedi AK, Hildesheim A, Wentzensen N. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Gynecol Oncol. 2014;135(2):297–304. doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.