Abstract
High salt intake is one of the major dietary determinants of hypertension and cardiovascular disease in Japan and throughout the world. Although dietary salt restriction may be of clinical benefit in salt-sensitive individuals, many individuals may not wish, or be able to, reduce their intake of salt. Thus, identification of functional foods that can help protect against mechanistic abnormalities mediating salt-induced hypertension is an issue of considerable medical and scientific interest. According to the “vasodysfunction” theory of salt-induced hypertension, the hemodynamic abnormality initiating salt-induced increases in blood pressure usually involves subnormal vasodilation and abnormally increased vascular resistance in response to increased salt intake. Because disturbances in nitric oxide activity can contribute to subnormal vasodilator responses to increased salt intake that often mediate blood pressure salt sensitivity, increased intake of functional foods that support nitric oxide activity may help to reduce the risk for salt-induced hypertension. Mounting evidence indicates that increased consumption of traditional Japanese vegetables and other vegetables with high nitrate content such as table beets and kale can promote the formation of nitric oxide through an endothelial independent pathway that involves reduction of dietary nitrate to nitrite and nitric oxide. In addition, recent studies in animal models have demonstrated that modest increases in nitrate intake can substantially attenuate the initiation of salt-induced hypertension. These observations are: 1) consistent with the view that increased intake of many traditional Japanese vegetables and other nitrate rich vegetables, and of functional foods derived from such vegetables, may help maintain healthy blood pressure despite a high salt diet; 2) support government recommendations to increase vegetable intake in the Japanese population.
Keywords: Salt sensitivity, Salt, Hypertension, Sodium, Nitrate, Blood pressure
Introduction
Japanese-style diets [1–3] and Mediterranean-style diets [4] are associated with lower cardiovascular risk and greater longevity than many other diets worldwide. Nevertheless, according to the Global Burden of Disease Study, dietary hazards that promote cardiovascular disease remain among the top risk factors for death and disability in Japan [5]. In Japanese, as in other population groups, high salt intake is widely considered to be one of the major dietary determinants of high blood pressure and cardiovascular disease [6–9]. Thus, there is ongoing interest in developing improved dietary approaches to reducing the risk for salt-induced hypertension and cardiovascular disease in Japan as in other parts of the world.
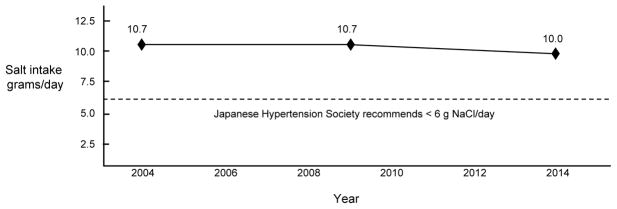
Although dietary salt restriction may be of clinical benefit in salt-sensitive individuals, many individuals may not wish, or be able, to reduce their intake of salt to the level recommended by medical authorities in Japan (<6 grams of salt/day) [8]. As discussed by Okuda and colleagues, salt intake decreased substantially in various regions of Japan between 1950 and 1990 [10]. However over the past two decades, it appears that educational efforts in Japan and throughout the world have met with only modest success in achieving further reductions in salt intake at the population level [11]. For example, Figure 1 shows that in Japan, average daily salt intake (~10 grams/day) changed very little between 2004 and 2014 and has remained well above the maximum level of 6 grams/day recommended by the Japanese Society of Hypertension [8, 10, 12]. In most other countries, average salt intake also exceeds the levels recommended by scientific agencies and governmental authorities [13]. Takahashi and colleagues found that even in Japanese hypertensive patients who are well aware of the dietary guidelines on salt intake, average salt intake is nearly 10 grams/day and is similar to that of patients with low awareness of the dietary guidelines [14].
Figure 1. Average daily salt intake in Japanese adults (males and females combined).
Data taken from Kawano and colleagues for 2004 [82] and from the Japan Ministry of Health, Labor, and Welfare for 2009 and 2014 [83]. Average daily salt intake (~10 grams/day) changed very little between 2004 and 2014 and has remained well above the maximum level of 6 grams/day recommended by the Japanese Society of Hypertension [8, 10, 12].
Functional Foods and Reducing Risk For Salt-Induced Hypertension
Given limited recent progress in achieving reductions in average daily salt intake in Japan and elsewhere, and considering ongoing debate about the desirability of efforts to reduce salt intake at the population level [15–18], it is important to consider alternative dietary approaches to reducing risk for salt-induced hypertension. In this regard, increased intake of functional foods with ingredients that help protect against salt-induced increases in blood pressure may represent a promising dietary approach to reducing the risk for salt-induced hypertension in the population. Increased consumption of such functional foods might reduce the risk for salt-induced hypertension even if further reductions in average salt intake are not achieved.
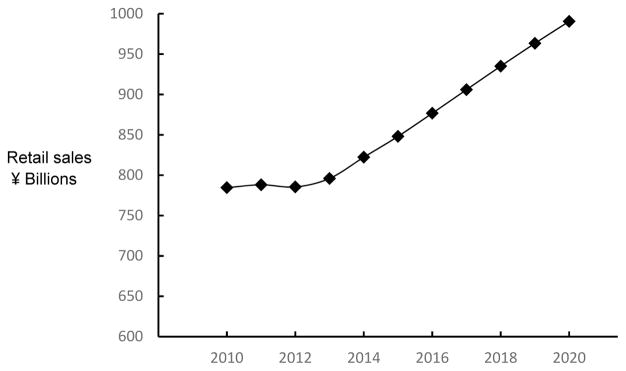
The term “functional food” appears to have originated in Japan [19], and broadly refers to foods with physiologically active components that provide health benefits beyond basic nutrition [20]. In contrast to other countries, Japan has formulated an explicit and detailed regulatory approval process for functional foods. Through this process, the Japanese Ministry of Health has approved the labeling of food products with various health benefits, including claims related to the control of blood pressure [21]. A variety of functional foods and nutrients with antihypertensive properties have been described that may help to maintain healthy blood pressure [22, 23]. Recently, Japanese regulations for the labeling of certain types of functional foods were modified [21] and retail sales of functional foods appear to be substantially increasing (Fig. 2) [24].
Figure 2. Annual retail sales of functional foods in Japan, current and projected [24].
Retail sales of functional foods are substantially increasing in Japan [24].
Improved understanding of the mechanistic abnormalities that initiate salt-induced hypertension could provide insight into specific types of functional foods that might be useful for maintaining normal blood pressure in subjects consuming a high-salt diet. Accordingly, we next discuss the hemodynamic abnormalities most commonly involved in the initiation of salt-induced hypertension. We then consider how some groups of functional foods may ameliorate those abnormalities and help people maintain healthy blood pressure despite consumption of a high-salt diet.
A Contemporary Mechanistic Framework for Initiation of Most Instances of Salt-induced Hypertension
For decades, cardiologists have widely embraced the historical view of Guyton and colleagues on the pathogenesis of salt sensitivity and salt-induced hypertension. This view holds that salt-induced hypertension is usually initiated by a subnormal renal ability to excrete a salt load which causes abnormal increases in sodium balance, blood volume, cardiac output, and therefore blood pressure [25–33]. Thus, the historical view has encouraged the use of preventive interventions against hypertension that are based on reducing salt intake or increasing salt excretion. However, as we have discussed in detail elsewhere, the results of salt-loading studies in humans and animals that include appropriate normal controls (salt-resistant subjects with normal blood pressure), have called into question Guyton’s historical conceptual framework for initiation of salt-induced increases in blood pressure [34–36].
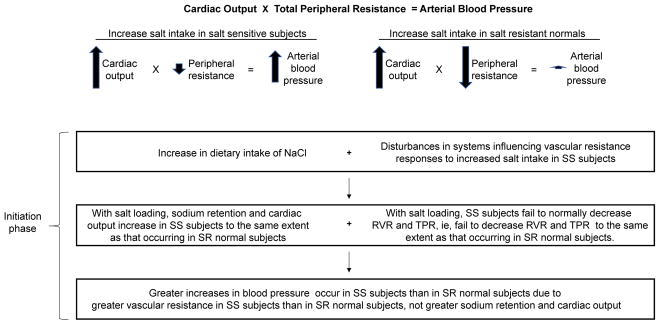
In contrast to the historical conceptual framework, an alternative conceptual framework for salt sensitivity (termed the “vasodysfunction” framework) holds that initiation of salt-induced hypertension is not usually caused by abnormal (greater) increases in sodium retention and cardiac output in salt-sensitive subjects compared to those in salt-loaded normal controls (Fig. 3) [34, 36]. Rather, initiation of salt-induced hypertension is often caused by subnormal vasodilation and abnormal vascular resistance responses to increased salt intake in salt-sensitive subjects compared to those in salt-resistant normal controls (Fig. 3) [34, 36, 37].
Figure 3. The vasodysfunction framework for the usual pathogenesis of salt-induced increases in blood pressure.
According to this conceptual framework, initiation of salt-induced hypertension (the acute phase) is hemodynamically mediated by an abnormal vascular resistance response to increases in salt intake, together with normal salt-induced increases in cardiac output.
RVR, renal vascular resistance; SS, salt sensitive; SR, salt resistant; TPR, total peripheral resistance.
Adapted from Kurtz et al. [36], with permission.
In salt-resistant normal individuals, increases in salt intake are usually associated with increases in cardiac output together with vasodilation and decreases in vascular resistance that prevent salt-induced increases in sodium retention and cardiac output from initiating hypertension [35–37]. Thus, according to the vasodysfunction theory (Fig. 3), interventions that promote vasodilation and normal vascular resistance responses to increases in salt intake may protect against the initiation of salt-induced increases in blood pressure. This raises the questions: 1) which of the mechanisms that mediate vasodilation and normal vascular resistance responses to increases in salt intake are impaired in salt-sensitive subjects? 2) what types of functional foods might help to correct those mechanisms and thereby reduce the risk for salt-induced hypertension ?
Mechanisms Mediating Subnormal Vasodilation and Abnormal Vascular Resistance Responses to Increases in Salt Intake
In salt-sensitive subjects, disturbances in a variety of mechanisms may cause subnormal vasodilation and abnormal vascular resistance responses to increases in salt intake [34, 36]. For example, in salt-sensitive subjects, the failure to normally vasodilate and reduce vascular resistance in response to increases in salt intake may be related to: 1) structural alterations in blood vessels that occur with aging and/or atherosclerosis and physically prevent normal salt-induced vasodilation and decreases in vascular resistance, and or 2) functional disturbances (reduced vasodilator and/or enhanced vasoconstrictor mechanisms) that interfere with factors that promote salt-induced vasodilation and decreases in vascular resistance. Functional disturbances that impair normal vasodilatory responses to salt, and hence risk for salt sensitivity, may be amenable to correction by modulation of pathways that promote vasodilation and decreases in vascular resistance. Accordingly, identification of functional foods that ameliorate mechanistic disturbances causing abnormal vascular resistance responses to a high-salt diet may provide new opportunities for reducing the risk for salt-induced hypertension.
The Role of Alterations in Nitric Oxide Activity in Determining Blood Pressure Responses to Increases in Salt Intake
Nitric oxide (NO) is an important determinant of arterial blood pressure, and impaired NO bioactivity is associated with hypertension [38]. With respect to the influence of NO on vascular resistance, NO has direct effects on the vasculature that promote arterial dilation, and indirect effects involving inhibition of sympathetic nerve activity that buffer vasoconstriction [38]. Over 25 years ago, Chen and Sanders discovered that in Dahl salt-sensitive (Dahl S) rats, the most widely used animal model of salt sensitivity, subnormal NO responses to increases in salt intake may be critically involved in the initiation of salt-induced disturbances in vascular resistance and hypertension [39]. Subsequently, a considerable body of evidence has emerged from human and animal studies consistent with the view that NO-related pathways often play important roles in determining vascular resistance and blood pressure responses to increases in salt intake [34, 40–49].
In normal individuals (salt-resistant subjects with normal blood pressure), increases in salt intake increase cardiac output and induce vasodilation and decreases in vascular resistance that are mediated at least in part by flow-mediated, endothelial-dependent increases in NO activity [34]. In normal salt-resistant subjects, this vasodilatory response to increases in salt intake serves to protect against the pressor effects of salt-induced increases in cardiac output [34]. In contrast, many salt-sensitive subjects exhibit a subnormal ability to vasodilate and reduce vascular resistance with salt loading [34]. In salt-sensitive subjects, this abnormal vascular resistance response to increases in salt intake, together with normal salt-induced increases in cardiac output, initiates salt-induced increases in blood pressure. The salt-induced abnormality in vascular resistance appears to often involve a subnormal ability to increase NO activity in response to increases in salt intake [34, 39, 41–49].
Having originally discovered that subnormal NO activity might contribute to the initiation of salt-induced increases in blood pressure in Dahl S rats, Chen and Sanders explored whether interventions aimed at augmenting NO activity might be useful for the prevention of salt-induced hypertension [39]. They found that supplemental administration of L-arginine, the substrate for enzymatic generation of NO by NO synthase in the classical NO production pathway, could entirely prevent salt-induced hypertension in Dahl S rats [39]. Later, studies by Siani et al. suggested that in normotensive humans consuming low normal amounts of arginine (~3.75 grams/day), increasing L-arginine intake to ~10 grams per day, through a diet rich in lentils and nuts, could reduce blood pressure [50].
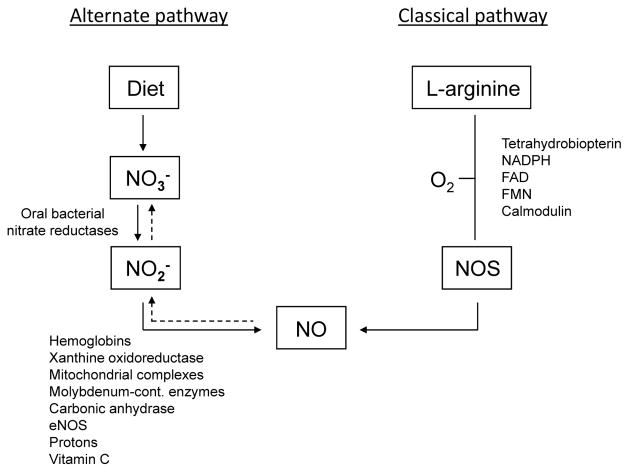
Although supplemental arginine may augment NO activity and reduce blood pressure in some circumstances [51, 52], Zand and colleagues have pointed out various issues with L-arginine supplementation including the concern that patients with endothelial dysfunction have an impaired ability to convert L-arginine to NO [53]. In addition, Higashi and colleagues reported that in salt sensitive subjects, salt loading may impair the ability of L-arginine to increase endothelial NO production in the renal vasculature and decrease renal vascular resistance [54]. In this regard, it is interesting that many investigators have begun to strongly emphasize the potential biomedical importance of an alternate pathway for generating NO that does not require L-arginine and NO synthase [53, 55–61]. This alternative pathway involves the stepwise reduction of nitrate to nitrite to NO (Figs 4 and 5) and as discussed by Zand et al. provides an endothelial independent source of NO [53]. Moreover, it has been proposed that increasing intake of nitrate-rich vegetables can safely increase NO activity through this alternative pathway and reduce blood pressure [58, 60–63].
Figure 4. The classic and alternative pathways for generation of nitric oxide (NO).
The classic pathway involves generation of NO through L-arginine. In the presence of molecular oxygen and several cofactors, nitric oxide synthases (NOS) catalyze the oxidation of the amino acid L-arginine to NO. NO is oxidized to nitrite (NO2-) and nitrate (NO3-) (dashed arrows). The alternative pathway involves generation of NO through reduction of nitrate and nitrite. Endogenously generated nitrate and nitrate from the diet can be reduced to nitrite by oral bacteria. Nitrite is absorbed systemically, and via several enzymatic and non-enzymatic pathways reduced to NO. Adapted from Hezel and Weitzberg with permission [84].
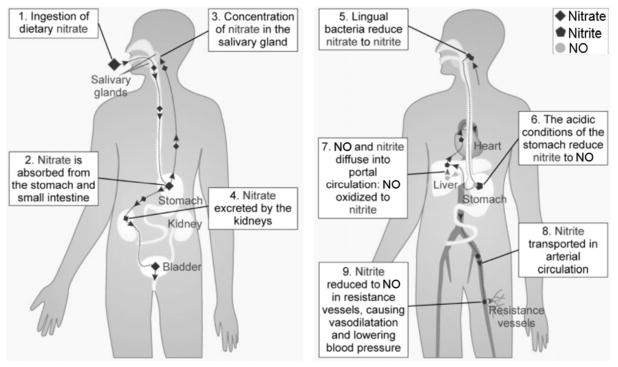
Figure 5. Processing of dietary nitrate through the alternative pathway for generation of NO (nitrate-nitrite-NO pathway).
Dietary nitrate enters the entero-salivary circulation and is exposed to oral anaerobic bacteria which express nitrate reductases that reduce nitrate to nitrite. The salivary nitrite is swallowed into the stomach where some of the nitrite is converted to NO and other NO species by gastric acid while most of the nitrite enters into the lower gastrointestinal tract unchanged and is absorbed into the systemic circulation [84]. In the vasculature, various enzymatic and non-enzymatic processes facilitate reduction of nitrite to NO which promotes decreases in vascular tone. Adapted from Webb et al., with permission [85]. Note that antibiotics or antiseptic mouthwashes and proton pump inhibitors can interfere with the functioning of this alternative pathway by decreasing oral bacteria or decreasing gastric acid secretion [84].
Effects of Augmenting Nitrate Intake on Blood Pressure
Recent meta-analyses support the view that augmenting nitrate intake by administration of nitrate salts or foods with a high nitrate content can reduce blood pressure [64, 65]. However, it has also been pointed out that the blood pressure trial results have varied considerably [58, 65, 66]. For example, it appears that some patient groups may tend to be resistant to the blood pressure-lowering effects of nitrate supplementation (e.g. diabetic subjects, older subjects, etc) [58, 67, 68]. Considering substantial variation in susceptibility to the blood pressure-lowering effects of dietary nitrate, identification of individuals most likely to benefit from augmenting nitrate intake is an issue of considerable medical interest. Because disturbances in NO activity may play an important role in the pathogenesis of salt sensitivity [34], increased dietary intake of foods with high nitrate content may be of particular value in normotensive people for reducing the risk of developing salt-induced hypertension.
Functional Foods With High Nitrate Content to Reduce the Risk for Salt-Induced Hypertension
Carlstrom and colleagues reported experimental results indicating that increased intake of nitrate may be useful for attenuating salt-induced increases in blood pressure [69]. They found that in unilaterally nephrectomized rats administered a very high-salt diet, adding sodium nitrate to the diet greatly attenuates the hypertension otherwise induced by administration of the high-salt diet alone (Fig. 6) [69]. Treatment with sodium nitrate did not affect food intake or urinary excretion of sodium [69]. Thus, the lower blood pressure in rats treated with sodium nitrate could not be attributed to a lower intake of sodium. If anything, the rats treated with sodium nitrate and the high-salt diet may have consumed more sodium than the rats treated with the high-salt diet alone. Remarkably, the amount of nitrate required to protect against the adverse effects of the high-salt diet was relatively small. Specifically, Carlstrom and colleagues found that addition to the diet of 0.025 parts nitrate for every 1 part added salt (by weight) was sufficient to substantially protect against salt-induced increases in blood pressure, cardiac hypertrophy, cardiac fibrosis, renal fibrosis, and proteinuria [69].
Figure 6. Increased intake of sodium nitrate significantly attenuates salt-induced hypertension.
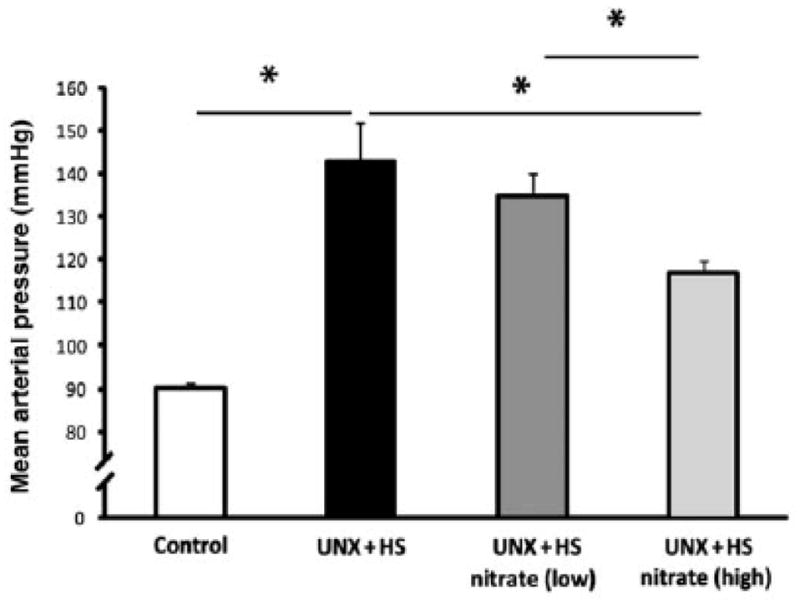
In unilaterally nephrectomized rats fed a high salt diet (4% NaCl), Carlstrom and colleagues found that oral administration of sodium nitrate provides significant protection against salt-induced increases in mean arterial pressure (as measured by radioltelemetry) [69].
UNX, unilateral nephrectomy; HS, high salt diet; Nitrate-low, dietary supplementation with a low amount of nitrate (approximately 0.0025 parts nitrate by weight for every 1 part added salt by weight); Nitrate-high, dietary supplementation with a higher amount of nitrate (approximately 0.025 parts nitrate by weight for every 1 part added salt by weight). Values are means ± SEM. *p < 0.05.
Adapted from Carlstrom et al., with permission [69].
The findings of Carlstrom and colleagues [69] imply that dietary addition of small amounts of nitrate relative to added salt may be sufficient to reduce the risk for salt-induced increases in blood pressure. While caution is advised when extrapolating results from animal studies to humans, the studies of Carlstrom and colleagues suggest that increasing dietary intake of nitrate by an additional 250 mg/day in normotensive individuals may be sufficient to protect against the pressor effect of ingesting 5 to 10 grams of salt/day. According to the analysis of Hord and colleagues, consumption of a vegetable-rich diet consistent with the dietary guidelines followed in the Dietary Approaches to Stop Hypertension (DASH) trial could readily increase average nitrate intake by 250 mg/day or more (with the use of high nitrate vegetables in the diet) [63]). In fact, simply supplementing a diet with several average weight (80 gram) beets per day, or less than a cup of fresh spinach per day, could provide an additional 250 mg/day of dietary nitrate (assuming average vegetable nitrate levels similar to those reported by Hord and colleagues [63]).
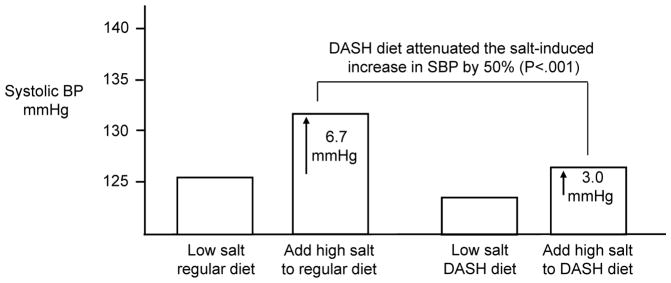
The potential value of vegetable-rich diets in reducing the risk for salt-induced increases in blood pressure is illustrated by studies examining the effects of the DASH diet on blood pressure responses to changes in salt intake. As shown in Figure 7, Sacks and colleagues found that consumption of the DASH diet caused clinically significant attenuation of salt-induced increases in blood pressure [70]. While a variety of factors in DASH-style diets such as potassium could have beneficial effects on blood pressure, many investigators have proposed that nitrate may be a particularly important antihypertensive component in DASH-style diets (through its capacity to promote increases in NO activity, Figs 4 and 5) [58, 60–63].
Figure 7. The Dietary Approaches to Stop Hypertension (DASH) diet significantly attenuates salt-induced increases in systolic blood pressure (SBP).
In response to increasing salt intake from 50 mmol/day to 150 mmol/day, the mean increase in SBP in subjects consuming a DASH-style diet (3 mmHg) was 50% less than that in subjects consuming a control diet typical of diets in the USA, 6.7 mmHg (p<0.001) [70].
As with DASH-style diets, the antihypertensive effects of Japanese-style diets and Mediterranean-style diets have also been proposed to be mediated, at least in part, through effects on nitrate metabolism and NO activity. For example, Sobko and colleagues found that blood pressure was lower in normal subjects fed a diet containing traditional Japanese vegetables with a high nitrate content than when fed a control diet lacking those vegetables [71]. Plasma levels of nitrate and nitrite precursors of NO were significantly greater during administration of the vegetable-rich diet than during administration of the control diet [71].
Potassium is widely considered to be another important factor that may contribute to cardioprotective effects of vegetable-rich diets [72–76] and is a nutrient recognized by the Japanese government in the evaluation of foods with functional claims for maintaining healthy blood pressure. As discussed by Houston [76], potassium also has vasodilatory effects, and studies in humans suggest that the capacity of supplemental potassium to attenuate salt-induced increases in blood pressure may be mediated partly through effects on NO activity [46]. It should be noted that beetroot (table beet), a vegetable that has attracted considerable attention for its antihypertensive properties [58, 64, 77], is particularly rich in both nitrate and potassium.
Implications for Public Health Efforts To Reduce the Risk for Salt-Induced Hypertension
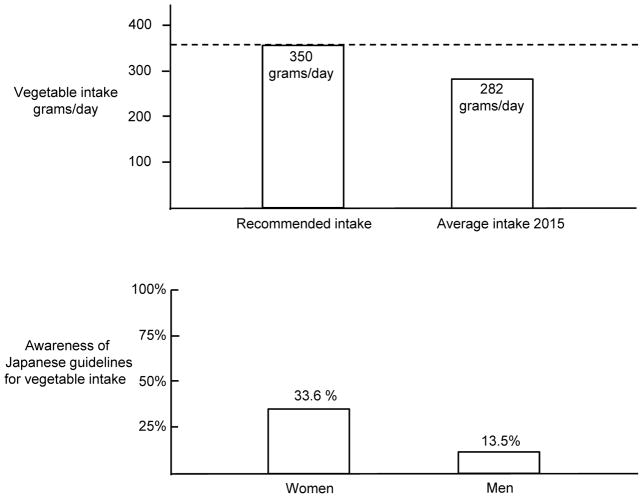
As part of the national health promotion campaign “Healthy Japan 21”, the Ministry of Health, Labor, and Welfare recommends that Japanese people increase their vegetable intake to a target goal of 350 grams per day [78]. Currently, vegetable intake in Japan is below the recommended target and averages approximately 280 grams per day (Fig. 8, top panel) [78]. Given that a variety of traditional Japanese vegetables have a high nitrate content [71], increasing vegetable intake to the target goal of 350 grams per day could result in substantial increases in nitrate intake and potassium intake which might help to reduce the risk for salt-induced increases in blood pressure. While concerns have been raised about health risks of taking supplemental nitrate/nitrite salts (mainly nitrite, nitrite toxicity is a result of its rapid reaction with hemoglobin, which may cause methemoglobinemia) [79], there is little or no evidence of health risks associated with the slow and controlled release of nitrate that occurs with increased intake of vegetables. As discussed by Hord and colleagues and Bryan and Ivy, the benefits of vegetable and fruit consumption outweigh any theoretical risks from nitrate present in those foods [63, 80].
Figure 8. Vegetable intake in Japan and awareness of Japanese guidelines on vegetable intake. Top panel.
Vegetable intake in Japan is below the level of intake recommended by the Japanese government [78]. Bottom panel: Low awareness among Japanese men and women of government recommendations on vegetable intake [78]. Less than 34% of Japanese women and 14% of Japanese men appear to be aware of current government recommendations for vegetable intake [78].
Unfortunately, it appears that only a small percentage of Japanese women and men are familiar with the target goals for vegetable intake recommended by the Japanese government [78]. According to the studies of Wang and colleagues, less than 34% of Japanese women and 14% of Japanese men appear to be aware of current government recommendations for vegetable intake (Fig. 8, bottom panel) [78]. Thus, further activities to educate the public about the benefits of increasing vegetable intake should be encouraged. In light of the findings of Sobko et al. and others, traditional Japanese vegetables with a high nitrate content such as Osaka shirona, burdock, komatsuna, hakusai, spinach, etc., and non-traditional vegetables with a high nitrate content such as table beets and kale, are of particular interest for their potential to support the production of NO and maintenance of healthy blood pressure [60–62, 71, 81]. We suggest that increased intake of vegetables with high nitrate content, and increased intake of functional food products prepared from high nitrate vegetables, be considered as an additional approach for reducing the risk of salt-induced hypertension in the general population.
Highlights.
Salt-induced hypertension is a major cause of morbidity and mortality in Japan
Subnormal nitric oxide activity mediates initiation of salt-induced hypertension
Increased dietary nitrate increases nitric oxide activity and reduces blood pressure
Many Japanese vegetables are rich in nitrate and promote healthy blood pressure
High nitrate vegetables may be considered functional foods for blood pressure control
Acknowledgments
Disclosures and acknowledgements
National Center for Research Resources, M0RR-00079, US Public Health Service; National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230; Praemium Academiae award of the Czech Academy of Sciences to MP; and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamori Y, Sagara M, Arai Y, Kobayashi H, Kishimoto K, Matsuno I, et al. Soy and fish as features of the Japanese diet and cardiovascular disease risks. PLoS One. 2017;12:e0176039. doi: 10.1371/journal.pone.0176039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurotani K, Akter S, Kashino I, Goto A, Mizoue T, Noda M, et al. Quality of diet and mortality among Japanese men and women: Japan Public Health Center based prospective study. BMJ. 2016;352:i1209. doi: 10.1136/bmj.i1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamori Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp Clin Cardiol. 2006;11:94–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Ros E, Martínez-González MA, Estruch R, Salas-Salvadó J, Fitó M, Martínez JA, et al. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv Nutr. 2014;5:330S–6S. doi: 10.3945/an.113.005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Risk Factor Collaborators. [Access date, January 28, 2018];Global Burden of Disease Profile: Japan. 2010 http://www.healthdata.org/sites/default/files/files/country_profiles/GBD/ihme_gbd_country_report_japan.pdf.
- 6.Takase H, Sugiura T, Kimura G, Ohte N, Dohi Y. Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population. J Am Heart Assoc. 2015;4:e001959. doi: 10.1161/JAHA.115.001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iso H. Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb. 2011;18:83–8. doi: 10.5551/jat.6866. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, Ando K, Tsuchihashi T, Yoshita K, Watanabe Y, Kawarazaki H, et al. Report of the Salt Reduction Committee of the Japanese Society of Hypertension (2) Goal and strategies of dietary salt reduction in the management of hypertension. Hypertens Res. 2013;36:1020–5. doi: 10.1038/hr.2013.105. [DOI] [PubMed] [Google Scholar]
- 9.Ando K, Kawarazaki H, Miura K, Matsuura H, Watanabe Y, Yoshita K, et al. Report of the Salt Reduction Committee of the Japanese Society of Hypertension (1) Role of salt in hypertension and cardiovascular diseases. Hypertens Res. 2013;36:1009–19. doi: 10.1038/hr.2013.102. [DOI] [PubMed] [Google Scholar]
- 10.Okuda N, Okayama A, Miura K, Yoshita K, Saito S, Nakagawa H, et al. Food sources of dietary sodium in the Japanese adult population: the international study of macro-/micronutrients and blood pressure (INTERMAP) Eur J Nutr. 2017;56:1269–80. doi: 10.1007/s00394-016-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberio AM, Sumar N, Trieu K, Lorenzetti DL, Tarasuk V, Webster J, et al. Population-level interventions in government jurisdictions for dietary sodium reduction: a Cochrane Review. Int J Epidemiol. 2017;46:1551–405. doi: 10.1093/ije/dyw361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uechi K, Asakura K, Masayasu S, Sasaki S. Within-country variation of salt intake assessed via urinary excretion in Japan: a multilevel analysis in all 47 prefectures. Hypertens Res. 2017;40:598–605. doi: 10.1038/hr.2016.185. [DOI] [PubMed] [Google Scholar]
- 13.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi N, Tanabe K, Adachi T, Nakashima R, Sugamori T, Endo A, et al. Awareness of salt restriction is not reflected in the actual salt intake in Japanese hypertensive patients. Clin Exp Hypertens. 2015;37:388–92. doi: 10.3109/10641963.2014.987392. [DOI] [PubMed] [Google Scholar]
- 15.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–75. doi: 10.1016/S0140-6736(16)30467-6. [DOI] [PubMed] [Google Scholar]
- 16.Graudal N, Hubeck-Graudal T, Jurgens G, McCarron DA. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta-analysis. Adv Nutr. 2015;6:169–77. doi: 10.3945/an.114.007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolarz-Skrzypek K, Staessen JA. Reducing salt intake for prevention of cardiovascular disease--times are changing. Adv Chronic Kidney Dis. 2015;22:108–15. doi: 10.1053/j.ackd.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Alderman MH. Dietary sodium: where science and policy diverge. Am J Hypertens. 2016;29:424–7. doi: 10.1093/ajh/hpu256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T. Health claims on functional foods: the Japanese regulations and an international comparison. Nutr Res Rev. 2003;16:241–52. doi: 10.1079/NRR200363. [DOI] [PubMed] [Google Scholar]
- 20.Henry CJ. Functional foods. Eur J Clin Nutr. 2010;64:657–9. doi: 10.1038/ejcn.2010.101. [DOI] [PubMed] [Google Scholar]
- 21.Kamioka H, Tsutani K, Origasa H, Yoshizaki T, Kitayuguchi J, Shimada M, et al. Quality of systematic reviews of the Foods with Function Claims registered at the Consumer Affairs Agency Web site in Japan: a prospective systematic review. Nutr Res. 2017;40:21–31. doi: 10.1016/j.nutres.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Hieda K, Sunagawa Y, Katanasaka Y, Hasegawa K, Morimoto T. Pharmacological effect of functional foods with a hypotensive action. Nihon Yakurigaku Zasshi. 2015;146:33–9. doi: 10.1254/fpj.146.33. [DOI] [PubMed] [Google Scholar]
- 23.Borghi C, Cicero AF. Nutraceuticals with a clinically detectable blood pressure lowering effect: a review of available randomized clinical trials and their meta-analyses. Br J Clin Pharmacol. 2017;83:163–71. doi: 10.1111/bcp.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Market Access Secretariat, Agriculture and Agri-Food Canada. [Access date, January 28, 2018];Functional foods and beverages in Japan, Global Access Report. 2016 http://www.agr.gc.ca/eng/industry-markets-and-trade/international-agri-food-marketintelligence/asia/market-intelligence/functional-foods-and-beverages-injapan/?id=1461767215372.
- 25.Guyton AC. Circulatory physiology III. Philadelphia: W.B. Saunders; 1980. Arterial pressure and hypertension. [Google Scholar]
- 26.Guyton AC, Manning RD, Jr, Hall JE, Norman RA, Jr, Young DB, Pan YJ. The pathogenic role of the kidney. J Cardiovasc Pharmacol. 1984;6(Suppl 1):S151–61. doi: 10.1097/00005344-198400061-00025. [DOI] [PubMed] [Google Scholar]
- 27.Guyton AC, Hall JE, Coleman TG, Manning RD, Norman RA., Jr . The dominant role of the kidneys in long-term arterial pressure regulation in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, diagnosis, and management. 2. New York: Raven Press, Ltd; 1995. pp. 1311–26. [Google Scholar]
- 28.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 29.Cruz DN, Simon DB, Nelson-Williams C, Farhi A, Finberg K, Burleson L, et al. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension. 2001;37:1458–64. doi: 10.1161/01.hyp.37.6.1458. [DOI] [PubMed] [Google Scholar]
- 30.Brands MW. Chronic blood pressure control. Compr Physiol. 2012;2:2481–94. doi: 10.1002/cphy.c100056. [DOI] [PubMed] [Google Scholar]
- 31.Crowley SD, Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest. 2014;124:2341–7. doi: 10.1172/JCI72274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JE. Guyton and Hall textbook of medical physiology. 13. Philadelphia: Elsevier; 2015. [Google Scholar]
- 33.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, et al. Salt sensitivity of blood pressure: A scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 34.Morris RC, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133:881–93. doi: 10.1161/CIRCULATIONAHA.115.017923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int. 2016;90:965–73. doi: 10.1016/j.kint.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC., Jr The American Heart Association Scientific Statement on Salt Sensitivity of Blood Pressure: Prompting consideration of alternative conceptual frameworks for the pathogenesis of salt sensitivity ? J Hypertens. 2017;25:2214–25. doi: 10.1097/HJH.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 37.Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28:1362–9. doi: 10.1681/ASN.2016080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens. 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–67. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito S. Nitric oxide in the kidney. Curr Opin Nephrol Hypertens. 1995;4:23–30. doi: 10.1097/00041552-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Matsuoka H, Itoh S, Kimoto M, Kohno K, Tamai O, Wada Y, et al. Asymmetrical dimethylarginine, an endogenous nitric oxide synthase inhibitor, in experimental hypertension. Hypertension. 1997;29:242–7. doi: 10.1161/01.hyp.29.1.242. [DOI] [PubMed] [Google Scholar]
- 42.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension. 1999;33:1008–12. doi: 10.1161/01.hyp.33.4.1008. [DOI] [PubMed] [Google Scholar]
- 43.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–61. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 44.Bragulat E, de la Sierra A. Salt intake, endothelial dysfunction, and salt-sensitive hypertension. J Clin Hypertens. 2002;4:41–6. doi: 10.1111/j.1524-6175.2002.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manning RD, Jr, Meng S, Tian N. Renal and vascular oxidative stress and salt sensitivity of arterial pressure. Acta Physiol Scand. 2003;179:243–50. doi: 10.1046/j.0001-6772.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 46.Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt sensitive Asians. Hypertension. 2006;48:724–9. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 47.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC., Jr Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–5. doi: 10.1161/HYPERTENSIONAHA.111.170175. [DOI] [PubMed] [Google Scholar]
- 48.Toda N, Arakawa K. Salt-induced hemodynamic regulation mediated by nitric oxide. J Hypertens. 2011;29:415–24. doi: 10.1097/HJH.0b013e328341d19e. [DOI] [PubMed] [Google Scholar]
- 49.Cao Y, Mu JJ, Fang Y, Yuan ZY, Liu FQ. Impact of high salt independent of blood pressure on PRMT/ADMA/DDAH pathway in the aorta of Dahl salt-sensitive rats. Int J Mol Sci. 2013;14:8062–72. doi: 10.3390/ijms14048062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13:547–51. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 51.Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959–65. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Boger RH. The pharmacodynamics of L-arginine. Altern Ther Health Med. 2014;20:48–54. [PubMed] [Google Scholar]
- 53.Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res. 2011;31:262–9. doi: 10.1016/j.nutres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Higashi Y, Oshima T, Watanabe M, Matsuura H, Kajiyama G. Renal response to L-arginine in salt-sensitive patients with essential hypertension. Hypertension. 1996;27:643–8. doi: 10.1161/01.hyp.27.3.643. [DOI] [PubMed] [Google Scholar]
- 55.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–32. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 57.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med. 2016;279:315–36. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 58.Gee LC, Ahluwalia A. Dietary nitrate lowers blood pressure: epidemiological, preclinical experimental and clinical trial evidence. Curr Hypertens Rep. 2016;18:17. doi: 10.1007/s11906-015-0623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bryan NS, Tribble G, Angelov N. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr Hypertens Rep. 2017;19:33. doi: 10.1007/s11906-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 60.Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is rocket science - Why dietary nitrate is hard to beet! Part I: Twists and turns in the realisation of the nitrate - nitrite - NO pathway. Br J Clin Pharmacol. 2017;83:129–39. doi: 10.1111/bcp.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mills CE, Khatri J, Maskell P, Odongerel C, Webb AJ. It is rocket science - why dietary nitrate is hard to Beet! part II: further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br J Clin Pharmacol. 2017;83:140–51. doi: 10.1111/bcp.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–62. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 64.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and metaanalysis. J Nutr. 2013;143:818–26. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 65.Ashor AW, Lara J, Siervo M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: a systematic review and meta-analysis. J Hypertens. 2017;35:1353–9. doi: 10.1097/HJH.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 66.d’El-Rei J, Cunha AR, Trindade M, Neves MF. Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int J Hypertens. 2016;2016:6791519. doi: 10.1155/2016/6791519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med. 2013;60:89–97. doi: 10.1016/j.freeradbiomed.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 68.Siervo M, Lara J, Jajja A, Sutyarjoko A, Ashor AW, Brandt K, et al. Ageing modifies the effects of beetroot juice supplementation on 24-hour blood pressure variability: An individual participant meta-analysis. Nitric Oxide. 2015;47:97–105. doi: 10.1016/j.niox.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, et al. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–85. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 70.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 71.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–40. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adrogue HJ, Madias NE. Sodium surfeit and potassium deficit: Keys to the pathogenesis of hypertension. J Am Soc Hypertens. 2014;8:203–13. doi: 10.1016/j.jash.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Okayama A, Okuda N, Miura K, Okamura T, Hayakawa T, Akasaka H, et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open. 2016;6:e011632. doi: 10.1136/bmjopen-2016-011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okuda N, Miura K, Okayama A, Okamura T, Abbott RD, Nishi N, et al. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: a 24-year follow-up of the NIPPON DATA80 Study. Eur J Clin Nutr. 2015;69:482. doi: 10.1038/ejcn.2014.276. [DOI] [PubMed] [Google Scholar]
- 76.Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13:309–17. doi: 10.1007/s11906-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 77.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–7. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang DH, Kogashiwa M, Mori N, Yamashita S, Fujii W, Ueda N, et al. Psychosocial determinants of fruit and vegetable consumption in a Japanese population. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13080786. pii: E786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundberg JO, Larsen FJ, Weitzberg E. Supplementation with nitrate and nitrite salts in exercise: a word of caution. J Appl Physiol (1985) 2011;111:616–7. doi: 10.1152/japplphysiol.00521.2011. [DOI] [PubMed] [Google Scholar]
- 80.Bryan NS, Ivy JL. Inorganic nitrite and nitrate: evidence to support consideration as dietary nutrients. Nutr Res. 2015;35:643–54. doi: 10.1016/j.nutres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Matsuda R, Watanabe T, Ikarashi A, Shiramasa Y, Maitani T. Estimation of the daily intake of nitrate based on analysis of total diet samples. Shokuhin Eiseigaku Zasshi. 2009;50:29–33. doi: 10.3358/shokueishi.50.29. [DOI] [PubMed] [Google Scholar]
- 82.Kawano Y, Ando K, Matsuura H, Tsuchihashi T, Fujita T, Ueshima H, et al. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (1) Rationale for salt restriction and salt-restriction target level for the management of hypertension. Hypertens Res. 2007;30:879–86. doi: 10.1291/hypres.30.879. [DOI] [PubMed] [Google Scholar]
- 83.Ministry of Health, Labor, and Welfare. Healthy Japan. 21(second term) [Google Scholar]
- 84.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 85.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]