Abstract
Background
Female breast cancer incidence rates have been increasing in Portugal for years. We, therefore, conducted the first nationwide breast cancer study to assess regional differences.
Methods
Cases were obtained from population-based cancer registries covering the country’s Mainland (South, North, Centre), as well as the two Autonomous Regions (Azores and Madeira), for the time-period 1998 through 2011. Analyses were restricted to ages 30 to 84 years and stratified by region. We used the age-period-cohort (APC) framework to complement standard descriptive techniques and to forecast future trends. Estimable APC parameters included net drift, longitudinal age-specific incidence rate curves, and fitted age-specific incidence rate ratios.
Results
There were 71 545 breast cancer cases diagnosed in Portugal at ages 30 to 84 years from 1998 to 2011. The South presented the highest age-standardized rate (155.8/100 000), while the North presented the fastest rate of increase (3.6%/year). Age-specific statistical interactions were observed between regions. Younger women in the North revealed a decreased risk of developing breast cancer compared to women from the same age group in the South and Centre, while that risk was reversed in older women (p<0.05). We estimate that from 2014 onwards, the North might rank first among all regions.
Conclusion
The variant patterns observed could be due to a combination of different screening practices and/or exposure to risk factors across regions. Disease heterogeneity among younger and older women may also explain part of the differences in age-specific rates. These results justify continued monitoring of breast cancer incidence by region.
Keywords: Breast cancer, Incidence, Trends, Portugal
1. Introduction
Breast cancer is the most frequent cancer and the leading cause of cancer mortality among Portuguese women, with an estimated 6 088 new cases and 1 570 deaths in 2012, accounting for 30% of all cancer cases and 16% of all cancer deaths (1). Notwithstanding the recent declines in incidence rates observed in some western countries, attributed to reductions in the use of postmenopausal hormone replacement therapy and to plateaus in participation of mammography screening (2), cancer registries operating in Portugal have reported increases in breast cancer age-adjusted incidence rates (3–6). These registries cover the Southern, Northern, and Central parts of the country’s mainland, representing around 95% of the whole population, as well as the archipelagos of the Azores and Madeira, both located in the north Atlantic Ocean, and representing the remaining 5%.
Temporal increases in breast cancer incidence can be driven by factors related to 1) age at diagnosis, 2) events that impact all ages at a given point in time such as changes in screening or diagnostic practice patterns (period or secular effects), and/or 3) factors that vary from one generation to the next such as changes in reproductive patterns (birth-cohort) (7). To account for the effects of these three interrelated variables, cancer surveillance researchers have used age-period-cohort (APC) models (8). APC modeling is a mathematical approach that provides a unique set of estimable functions and parameters in order to better understand the impact of age, period, and cohort effects on disease trends (9).
In this study, we aim to describe breast cancer incidence temporal trends in Portugal by complementing standard descriptive methods with the APC framework. We will specifically analyze past and expected trends by South, North, Centre, Azores, and Madeira geographic regions to further hypothesize for differences across the country. To our knowledge, no studies describing these trends have ever been published, and certainly no head to head comparison between geographic regions have ever been performed.
2. Methods
Invasive breast cancer cases (ICD-10 code C50) by single age and district/region of residence were obtained for the 14-year time-period 1998 through 2011 from four population-based cancer registries that cover the entire country of Portugal. Only first primary breast tumors, as defined by international coding rules (10), have been included in the analyses. Female population estimates by single age and district/region of residence for the same period were obtained from Statistics Portugal. We restricted analyses to ages 30 to 84 years to avoid low counts at extreme ages. Results were stratified by geographic regions (South, North, Centre, Azores, and Madeira) and three age groups according to the likelihood of mammographic screening: age 30–44 years (unlikely to be screened and a surrogate to early onset breast cancers), age 45–69 years (likely to be screened), and age 70–84 years (unlikely to be screened and a surrogate to late onset breast cancers).
Age-standardized (ASR) incidence rates expressed per 100 000 woman-years were computed by the direct method by using the European standard population as the reference (11). The estimated annual percentage change (EAPC) of the ASR was computed using weighted log-linear regression (12). Cumulative risks measuring the risk which a woman would have of developing breast cancer during the age span 0–74 years, assuming no other causes of death were in operation, were assessed for each region (11).
We used the age-period-cohort (APC) framework to complement standard descriptive techniques as well as to forecast future incidence trends. APC models were fitted to one-year periods so there were 55 single-year age groups (30, 31, …, 84 years), 14 single-year calendar periods (1998, 1999, …, 2011), and 68 partially overlapping 2-year birth cohorts (1914, 1916, …, 1981) (13). Estimable APC parameters included net drift, longitudinal and cross-sectional age-specific incidence rate curves, and fitted age-specific incidence rate ratios (IRR) (14).
In brief, net drift is the APC model analog of the EAPC and it measures the overall long-term secular trend that is attributable to both calendar time (period or secular effects) and the successive cohorts enrolled in the study (birth-cohort effects). The longitudinal and cross-sectional curves are two different ways of summarizing the age-associated natural history. However, contrary to the cross-sectional curve, the longitudinal curve, also named age-at-onset curve, is adjusted for period and cohort effects. It is constructed by extrapolating from observed age-specific rates of each birth cohort to estimate past, current, and future rates for a referent cohort, thus taking into account the experience of all cohorts in the study (see Supplementary Fig. 1). The choice of the referent cohort is arbitrary, but has been conventionally set to the mid-cohort because it corresponds to the birth cohort tracking the longest amount of time within the registry (15).
We used the Wald test to obtain P values to assess statistical interactions between a pairwise of age-specific longitudinal curves (e.g., North vs. South) and the Bonferroni test to correct for multiple comparisons (16). Briefly, an “interaction” is said to occur when an unknown factor in some way alters the effect of an exposure–disease relationship (17). The interaction could be quantitative or qualitative. A quantitative interaction occurs when the unknown factor modifies only the magnitude of the effect (e.g., higher or lower rates in one region compared to another). Graphically, no reversal or crossing of the IRR with aging is observed. By contrast, a qualitative interaction occurs when there is a change not only in magnitude but in the direction of the effect (e.g., compared to a different region, younger women would present a lower risk of developing breast cancer while that risk would be reversed in older women). Regions with no interaction would be parallel on the log scale or proportional on the absolute scale (18).
Finally, we also projected ASRs up to 2025, as described elsewhere (19). Estimates are obtained by multiplying the estimated longitudinal age incidence rate curve in a referent birth cohort by the rate ratio between birth-specific cohorts and the referent cohort. Statistical analyses and graphical plotting were performed in Matlab, version R2016a (MathWorks Inc, Natick, MA, USA). Goodness of fit for each APC model was assessed by the square root of the usual over-dispersion parameter σ2, and by similarity between observed and fitted rates (19). All hypothesis tests were two-sided, and P values <0.05 were considered statistically significant.
3. Results
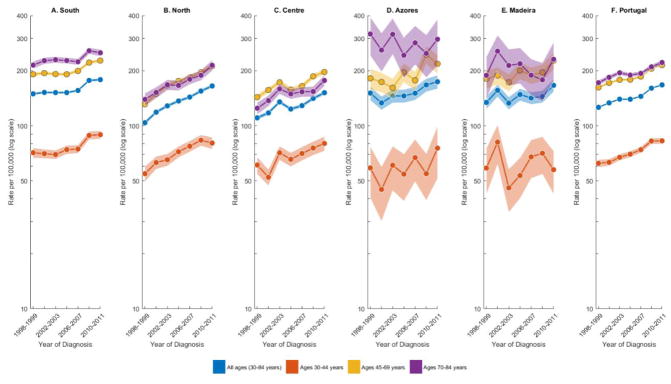
During the time period 1998 through 2011, there were 71 545 breast cancer cases diagnosed in Portugal between the ages 30 through 84 years (Table 1). Almost half of these cases (47.5%) were among Southern women, 27.7% among Northern women, 20.6% among Centre women, and the remaining 4.2% among women living in the Portuguese Autonomous Regions (Azores and Madeira). APC models were successfully fitted to the observed incidence data, with the models presenting negligible over-dispersion values (data not shown) and fitted confidence bands (shaded areas) consistently following close to observed rates (dots) (Fig. 1). APC age effects are shown in Supplementary Fig. 1, and period and cohort effects in Supplementary Fig. 2.
Table 1.
Breast cancer incidence in Portugal in women ages 30 to 84 years old, by region and age group, 1998–2011
| South* | North† | Centre§ | Azores¶ | Madeira* | Portugal | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Districts/Regions covered | Beja, Faro, Évora, Lisboa, Portalegre, Santarém, Setúbal | Braga, Bragança, Porto, Viana do Castelo, Vila Real | Aveiro, Castelo Branco, Coimbra, Guarda, Leiria, Viseu | Archipelago of the Azores | Archipelago of Madeira | South, North, Centre Azores, Madeira | |
|
| |||||||
| Population in Census 2011 (30–84 years)** | 1 563 312 | 1 124 437 | 836 755 | 75 065 | 91 756 | 3 691 325 | |
|
| |||||||
| Number of cases | 34 016 (≈2 430/year) | 19 804 (≈1 415/year) | 14 742 (≈1 055/year) | 1 394 (≈100/year) | 1 589 (≈115/year) | 71 545 (≈5 110/year) | |
|
| |||||||
| ASR per 100 000 woman-years (95% CI) | 30–44 | 73.03 (65.33 to 80.74) | 66.91 (58.77 to 75.06) | 64.28 (54.56 to 73.99) | 50.62 (23.57 to 77.68) | 55.86 (29.67 to 82.04) | 68.11 (63.39 to 72.82) |
| 45–69 | 200.41 (189.93 to 210.90) | 171.58 (159.99 to 183.18) | 166.78 (153.68 to 179.88) | 182.10 (134.39 to 229.79) | 184.62 (140.81 to 228.42) | 183.45 (176.88 to 190.01) | |
| 70–84 | 230.16 (211.99 to 248.32) | 172.42 (152.54 to 192.30) | 149.54 (130.43 to 168.65) | 258.63 (163.67 to 353.60) | 195.09 (119.73 to 270.44) | 193.71 (182.72 to 204.70) | |
| 30–84 | 155.83 (149.38 to 162.29) | 132.42 (125.36 to 139.49) | 126.50 (118.53 to 134.47) | 140.99 (112.37 to 169.62) | 137.45 (111.39 to 163.52) | 141.29 (137.28 to 145.30) | |
|
| |||||||
| Cum. risk (0 to 74 years) | 7.1% | 6.1% | 5.8% | 6.5% | 6.4% | 6.5% | |
|
| |||||||
| EAPC (%/year) (95% CI) | 30–44 | 2.31 (1.45 to 3.18) | 3.41 (2.12 to 4.72) | 2.73 (1.24 to 4.24) | 1.49 (−0.98 to 4.02) | 0.99 (−3.50 to 5.69) | 2.74 (2.13 to 3.35) |
| 45–69 | 1.53 (0.78 to 2.28) | 3.65 (3.01 to 4.28) | 2.26 (1.07 to 3.47) | 2.40 (0.27 to 4.57) | 1.90 (−0.40 to 4.26) | 2.26 (1.74 to 2.78) | |
| 70–84 | 1.14 (0.40 to 1.89) | 3.50 (2.81 to 4.20) | 1.89 (0.80 to 3.01) | −0.32 (−3.50 to 2.95) | 0.05 (−2.90 to 3.09) | 1.80 (1.14 to 2.47) | |
| 30–84 | 1.61 (0.92 to 2.30) | 3.58 (3.08 to 4.08) | 2.32 (1.31 to 3.33) | 1.69 (0.07 to 3.34) | 1.46 (−0.50 to 3.46) | 2.28 (1.83 to 2.73) | |
|
| |||||||
| Net drift (%/year) (95% CI) | 30–44 | 1.91 (1.07 to 2.75) | 3.07 (2.01 to 4.14) | 2.26 (0.52 to 4.03) | 1.23 (−3.14 to 5.80) | 0.74 (−3.24 to 4.87) | 2.59 (1.97 to 3.21) |
| 45–69 | 1.39 (1.03 to 1.75) | 3.78 (3.27 to 4.30) | 2.16 (1.62 to 2.71) | 2.04 (0.20 to 3.92) | 1.14 (−0.55 to 2.85) | 2.19 (1.95 to 2.44) | |
| 70–84 | 1.62 (1.06 to 2.18) | 3.85 (2.97 to 4.75) | 2.45 (1.52 to 3.40) | −2.14 (−4.58 to 0.36) | −0.35 (−3.17 to 2.54) | 2.25 (1.84 to 2.65) | |
| 30–84 | 1.49 (1.22 to 1.77) | 3.66 (3.28 to 4.04) | 2.20 (1.76 to 2.63) | 1.29 (−0.11 to 2.71) | 0.46 (−0.83 to 1.76) | 2.23 (2.04 to 2.43) | |
ASR, Age-standardized rate for the European population; CI, Confidence interval; EAPC, Estimated annual percentage change of the ASR.
Covered by Southern Region Cancer Registry
Covered by Northern Region Cancer Registry
Covered by Centre Region Cancer Registry
Covered by Azores Region Cancer Registry
Source: Statistics Portugal
Fig. 1.
Trends in age-standardized rates for breast cancer incidence in Portugal (overall) and by region among women aged 30–84 years and among women in specific age-groups (30–44, 45–69, and 70–84 years), 1998–2011. Two-year age intervals and two-year calendar periods were used to smooth the APC curves. The rates are plotted on the log scale. Shaded bands represent 95% confidence limits.
The South presented the highest ASR (155.8 per 100 000 woman-years), followed by the North (132.4/100 000) and Centre (126.5/100 000) (Table 1). A similar ranking was observed by age group as well as for the cumulative risk of developing breast cancer before the age of 75. In the 14-year study, the ASR increased 1.6% (95% confidence interval, 0.9 to 2.3) per year among Southern women, 3.6%/year (3.1 to 4.1) among Northern women, and 2.3%/year (1.3 to 3.3) among Centre women. Statistically significantly different EAPCs were also observed in all age groups, with South and Centre presenting the fastest rate of increase in the age group 30 to 44 years. Net drifts, measuring the overall log-linear trend in the period and cohort effects, were almost equal to the EAPCs, but presented narrower confidence intervals.
Fig. 1 presents overall age-standardized and age-specific temporal trends in breast cancer incidence for each geographic region as well as for the whole country. Excluding the Azores and Madeira, which depicted unstable rates due to a low number of cases, breast cancer incidence rates increased throughout the 14-year study period in all age groups, with the steepest increases being observed in the North. Age groups 45–69 and 70–84 presented similar rates in the North, and in the Centre, rates in the middle age groups (45–69) even surpassed rates in older ages (70–84).
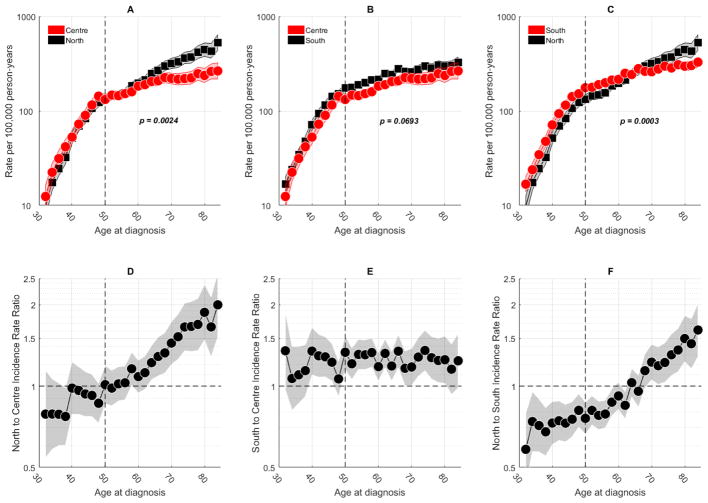
Fig. 2 shows the APC fitted age-at-onset curves on a pairwise comparison between two of the three main regions. After correcting for multiple comparisons through the Bonferroni test, the P-values for the null hypothesis of no interactions between regions were statistically significantly different for North vs. Centre (Fig. 2A) and North vs. South (Fig. 2C). In both pair of regions, qualitative age interactions were observed (Fig. 2D and Fig. 2F, respectively), with the IRR crossing the reference line for the older ages. These interactions were more pronounced in the North vs. South, where IRR values were always statistically significantly different before and after the crossover.
Fig. 2.
Age-specific rates and corresponding inter-regions rate ratios for breast cancer incidence in women aged 30–84 years, in Portugal, 1998–2011. A vertical reference line identifies median age at menopause. Two-year age intervals and two-year calendar periods were used to smooth curves. Shaded bands represent 95% confidence limits. Rates and rate ratios are plotted on the log scale. (A–C) Pairwise longitudinal fitted age-at-onset curves between regions. Adjusted P-values (Bonferroni test) are shown. (D–F) Inter-regions age-specific rate ratios.
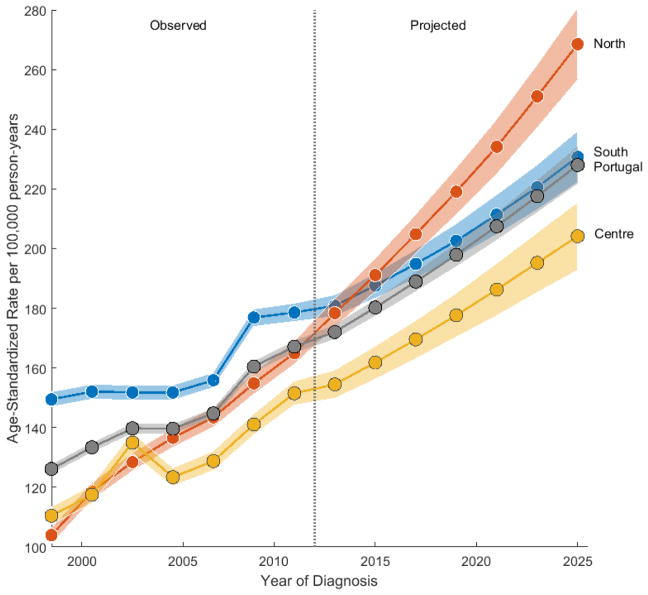
Fig. 3 shows the observed and projected incidence rates for each of the three main regions. The South always presented the highest ASR across the observed period, after which forecasts show a crossover between the South and the North, with the latter ranking first up to 2025, when a rate approximately 1.2 and 1.3 times higher is attained among Northern women (268.5 per 100 000 woman-years), compared to the South (230.8) and the Centre (204.1), respectively.
Fig. 3.
Overall observed (1998–2011) and projected (2012–2025) age-standardized rate trends for breast cancer incidence in women aged 30–84 years, by region and in Portugal (overall). Vertical reference line separates observed from forecast period. Two-year age intervals and two-year calendar periods were used to smooth curves. The rates are plotted on a linear scale. Shaded bands represent 95% confidence limits.
4. Discussion
Complementing standard descriptive epidemiology with novel statistical methods such as the APC framework can lead to a clearer understanding of a disease’s pattern in a defined population. In our study, we have used this approach for the first time to analyze breast cancer incidence trends in Portugal, by region. Estimated APC parameters included, among others, the net drift, which measures the overall log-linear trend by calendar period and birth cohort. Given the similarity of the net drift to the EAPC, we used the more familiar EAPC as the summary measure for the interpretation of the overall secular trends. Also, given that the Azores and Madeira represent only 5% of the whole country, we decided to focus our main discussion in women living on the mainland.
The risk of developing breast cancer is associated with higher socio-economic status (20), and this could explain the highest rates observed in the South, as this region by far has greater income and education (21, 22). Conversely, factors such as screening effects could have played a major role on the EAPCs observed in the North, and to a lesser extent in the Centre. Portugal established a pilot screening study in the Centre region in 1990, initially funded by the Commission of European Communities and coordinated by the Centre branch of the Portuguese League Against Cancer (LPCC) (23). This study was later transformed into a national screening program financed by the country’s health system, but always under the coordination of LPCC’s regional branches, except in Algarve, located in the South, where a different institution has been in charge of the program (24). Geographic coverage still varies widely, with the Centre being the only region where rollout was considered complete during the study period. Also, not all regions follow the same target age for eligible women (25). Except for the North (26), there are no published studies from each regional program, namely for the period 1998–2011, so it is not possible to compare their performance to better help understand our results.
In a recent report though, the Centre is referred to as the region attaining the highest participation rate (82%) (27). This in part could explain the higher rates observed in the Centre for the age group 45–69, when compared to age group 70–84 (Fig. 1). Conversely, the higher EAPC in the North could have been caused by a more pronounced effect of the so-called “prevalent wave” (28), especially in more recent years, given that the rollout in this region is close to complete now. As for the South, we anticipate that full coverage will be challenging as 73% of the Lisbon Metropolitan Area, the largest population concentration in Portugal, located in the South, is still lacking an organized screening program (27). Regardless of differences in ASRs and EAPCs, an increasing trend in breast cancer incidence rates was common to all regions and at all ages. Part of these trends may be attributed to birth-cohort changes, which in turn could be related to the adoption of a westernized lifestyle among Portuguese women, who are now more likely to be obese (29), delay childbearing (30), have lower parity (31), and have increased usage of contraceptive methods (32).
Worth noting is that women unlikely to have been screened (i.e., those under age 45 years) (Table 1) have expressed an EAPC as high as the one observed for women in age groups older than or equal to 45 years (the case for the North), or even higher (the case for the South and the Centre). This is particularly worrisome as an increase in risk among younger women due to an increase in exposure to risk factors (i.e., cohort or generational effect) would be more plausible than something affecting all age groups at a given point in time, such as screening and/or patient ascertainment (i.e., calendar-period effect). Additional analytical studies are thus necessary to identify possible causes for this striking trend. Besides, early-onset (usually ER-negative) breast cancers are proportionally more aggressive than late-onset (usually ER-positive) tumors (33). Future research stratifying by hormone-receptor status are also warranted to further elucidate these results.
Age-specific incidence curves were substantially different depending on the type of curve used, i.e., cross-sectional or longitudinal. In contrast to the standard cross-sectional curve (Supplemental Fig. 1B), the APC-fitted longitudinal curve (Supplemental Fig. 1A) has clearly shown an increased risk of breast cancer with advancing age. Our analysis thus confirms that the standard cross-sectional curve is not the best way to represent the age-associated natural history of the disease, at least for breast cancer. This happens because the cross-sectional curve reflects the experience of older cohorts at older ages and younger cohorts at younger ages (34), meaning that when there is a progressive increase in risk from one generation to the next (e.g., a decline in total fertility rate), the cross-sectional curve gives the false impression of a decreased risk among older women (20). Since larger positive EAPCs only intensify this bias (34), we have opted to use longitudinal (i.e., forward-looking or prospective) curves for regional pairwise comparisons (Fig. 2, A–F). A notable finding from our analysis is that younger women in the North reveal a decreased risk of developing breast cancer compared to women from the same age group in the Centre (Fig. 2D) and the South (Fig. 2F), although more pronounced in the later, while that risk is reversed in older women. A potential explanation for this phenomenon may be different screening practices, which could have led to a higher detection rate of more indolent cancers (mainly hormone-receptor positive) among older women (35), namely in the North. These interactions could also represent surrogates for age-dependent etiological heterogeneity (33). Additional studies stratifying by age, year, tumor characteristics, and smaller geographic areas such as districts could help further assess differences and identify patterns of geographic heterogeneity.
Our forecasts have shown that breast cancer age-adjusted incidence rates are expected to rise in all three main regions (Fig. 3). Given the expected increase in population and in life expectancy (36), the burden of the disease is also expected to increase. At least for the North, this is in accordance with a previous study (37). In fact, if the drastic trend observed in this region upholds, it will not take long before women from the North present the highest risk for being diagnosed with breast cancer. It should be noted, however, that these projections should be viewed with caution as they do not foresee changes in factors affecting trends, not least in the long term.
There are limitations associated with the approach we have taken in our study. First, it is limited by its descriptive nature, although it provides direction and helps identify the needs for targeted analytic studies (14). Second, due to the identifiability problem of the APC model, we were not able to separate the linear component of cohort effects from the linear component of period effects, meaning that we could not definitely quantify how much of the linear trend is attributed to either cohort or period effects (9). Third, we could not categorize results by tumor characteristics, which could have helped us identify the effects of the introduction and dissemination of screening (38), namely in the North and the Centre. Finally, although the registries follow the same rules for abstracting and coding of tumors, different levels of completeness of cancer registration and case ascertainment could have influenced the data reported to this study, and ultimately its results (39).
In conclusion, we have demonstrated that during the period 1998 through 2011 breast cancer incidence rates in Portugal increased at all ages and across all regions, with the South presenting the highest age-adjusted rate and the North the fastest rate of increase. The use of APC parameters, namely the age-specific longitudinal (age-at-onset) curve, has enabled us to observe a more realistic age-associated natural history of the disease as well as different age-interactions between regions, which in turn could be related to different cohort effects and/or screening practices. Our forecasts have also shown that Northern rates might soon surpass Southern rates. From a cancer surveillance perspective, these are all key reasons to put things in motion to tackle risk factors for breast cancer, such as fighting the obesity trend, disseminating access to early detection programs, and preparing the health system to respond accordingly.
Supplementary Material
Highlights.
Age-adjusted breast cancer incidence rates increased in all regions and at all ages.
Southern Portugal presented the highest age-adjusted rate.
Northern Portugal presented the fastest rate of increase.
Women under age 45 years have expressed higher EAPCs than women age 45+ years.
Forecasts have shown that Northern rates might soon surpass Southern rates.
Acknowledgments
This research was supported in part by an appointment (GFL) to the National Cancer Institute (NCI) Research Participation Program administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the National Institutes of Health. This study was also supported in part by the Fulbright Commission; the Luso-American Foundation for the Development; the Regional Directorate for Science and Technology; and by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD.
We thank the reviewers for helpful comments, which have greatly improved the content of this manuscript. Additionally, the authors thank Dr. Brenda Edwards from the NCI for her comments to a preliminary version of this paper, and the NIH Library Writing Center for manuscript editing assistance.
Abbreviations
- APC
age-period-cohort
- ICD-10
International Classification of Diseases, Tenth Revision
- ASR
age-standardized rate
- EAPC
estimated annual percentage change
- IRR
incidence rate ratio
- ER
estrogen-receptor
- WHO
World Health Organization
Footnotes
- Study concepts – GFL, ABM, WFA
- Study design – GFL, SPK, WFA
- Data acquisition – GFL, JB, CC, AM
- Quality control of data and algorithms – GFL, SPK, JB, CC, AM
- Data analysis and interpretation – All authors
- Statistical analysis – GFL, SPK, WFA
- Manuscript preparation – GFL, WFA
- Manuscript editing – All authors
- Manuscript review – All authors
Conflict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [updated 4/13/2017. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495–506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 3.RORENO. Registo Oncológico Nacional 2007. Porto: Instituto Português de Oncologia do Porto Francisco Gentil, E.P.E; 2013. [Google Scholar]
- 4.ROR-Centro. Registo Oncológico Nacional 2008. Coimbra: Instituto Português de Oncologia de Coimbra Francisco Gentil, E.P.E; 2014. [Google Scholar]
- 5.ROR-Sul. Registo Oncológico Nacional 2009. Lisboa: Instituto Português de Oncologia de Lisboa Francisco Gentil, E.P.E; 2015. [Google Scholar]
- 6.RORENO. Registo Oncológico Nacional 2010. Porto: Instituto Português de Oncologia do Porto Francisco Gentil, E.P.E; 2016. [Google Scholar]
- 7.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006;36:19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 8.Anderson WF. Cancer surveillance research. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1669–71. doi: 10.1158/1055-9965.EPI-09-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holford TR, Armitage P, Colton T. Age-Period-Cohort Analysis. In: Everitt B, Howell D, editors. Encyclopedia of Biostatistics. John Wiley & Sons, Ltd; 2005. pp. 82–99. [Google Scholar]
- 10.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology: ICD-O. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 11.Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, Muir CS, Skeet RG, editors. Cancer registration: principles and methods. IARC Sci Publ No. 95. Lyon, France: International Agency for Research on Cancer; 1991. pp. 126–58. [PubMed] [Google Scholar]
- 12.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Holford TR. The estimation of age, period and cohort effects for vital rates. Biometrics. 1983;39(2):311–24. [PubMed] [Google Scholar]
- 14.Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev. 2011;20(7):1263–8. doi: 10.1158/1055-9965.EPI-11-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernyavskiy P, Little MP, Rosenberg PS. A unified approach for assessing heterogeneity in age-period-cohort model parameters using random effects. Stat Methods Med Res. 2017 doi: 10.1177/0962280217713033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Darlington RB. Encyclopedia of Statistics in Behavioral Science. John Wiley & Sons, Ltd; 2005. Multiple Testing. [Google Scholar]
- 17.Jatoi I, Anderson WF. Qualitative age interactions in breast cancer studies: a mini-review. Future Oncol. 2010;6(11):1781–8. doi: 10.2217/fon.10.139. [DOI] [PubMed] [Google Scholar]
- 18.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100(24):1804–14. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg PS, Barker KA, Anderson WF. Estrogen Receptor Status and the Future Burden of Invasive and In Situ Breast Cancers in the United States. J Natl Cancer Inst. 2015;107(9):djv159. doi: 10.1093/jnci/djv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkin DM, Bray F, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 21.Statistics Portugal. Per capita purchasing power by Geographic localization (NUTS - 2013) [Available from: https://www.ine.pt/
- 22.Statistics Portugal. Higher education rate (Series 1998 - %) of resident population aged between 25 and 64 years old by Place of residence (NUTS - 2002) and Age group. [Available from: https://www.ine.pt/
- 23.IARC. Breast Cancer Screening. Lyon, France: International Agency for Research on Cancer; 2002. p. 229. [Google Scholar]
- 24.Dourado F, Carreira H, Lunet N. Mammography use for breast cancer screening in Portugal: results from the 2005/2006 National Health Survey. Eur J Public Health. 2013;23(3):386–92. doi: 10.1093/eurpub/cks103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer. 2018;142(1):44–56. doi: 10.1002/ijc.31043. [DOI] [PubMed] [Google Scholar]
- 26.Bento MJ, Goncalves G, Aguiar A, Castro C, Veloso V, Rodrigues V. Performance indicators evaluation of the population-based breast cancer screening programme in Northern Portugal using the European Guidelines. Cancer Epidemiol. 2015;39(5):783–9. doi: 10.1016/j.canep.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 27.da Saúde Direção-Geral. Lisboa: Direção-Geral da Saúde; 2016. Avaliação e Monitorização dos Rastreios Oncológicos Organizados de Base Populacional de Portugal – 2015. [Available from: https://www.dgs.pt/ [Google Scholar]
- 28.Ellis L, Woods LM, Esteve J, Eloranta S, Coleman MP, Rachet B. Cancer incidence, survival and mortality: explaining the concepts. Int J Cancer. 2014;135(8):1774–82. doi: 10.1002/ijc.28990. [DOI] [PubMed] [Google Scholar]
- 29.Marques-Vidal P, Ravasco P, Paccaud F. Differing trends in the association between obesity and self-reported health in Portugal and Switzerland. Data from national health surveys 1992–2007. BMC Public Health. 2012;12:588. doi: 10.1186/1471-2458-12-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statistics Portugal. Mean age of women at birth of first child. [Available from: https://www.ine.pt/
- 31.World Bank. Fertility rate, total (births per woman) [Available from: http://data.worldbank.org/indicator/SP.DYN.TFRT.IN.
- 32.World Bank. Contraceptive prevalence, any methods (% of women ages 15–49) [Available from: http://data.worldbank.org/indicator/SP.DYN.CONU.ZS.
- 33.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8):dju165. doi: 10.1093/jnci/dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296–302. doi: 10.1158/1055-9965.EPI-14-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144(1):1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Almeida Simoes J, Figueiredo Augusto G, Fronteira I, Hernandez-Quevedo C. Portugal: Health System Review. Health systems in transition. 2017;19(2):1–184. [PubMed] [Google Scholar]
- 37.Castro C, Antunes L, Lunet N, Bento MJ. Cancer incidence predictions in the North of Portugal: keeping population-based cancer registration up to date. Eur J Cancer Prev. 2016;25(5):472–80. doi: 10.1097/CEJ.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 38.Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. 2014;2014(49):187–97. doi: 10.1093/jncimonographs/lgu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer. 2009;45(5):756–64. doi: 10.1016/j.ejca.2008.11.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.