Abstract
We previously showed that C57BL/6J mice fed high fat diet (HFD) supplemented with 1% grape polyphenols (GP) for 12 weeks developed a bloom of Akkermansia muciniphila with attenuated metabolic syndrome symptoms. Here we investigated early timing of GP-induced effects and the responsible class of grape polyphenols. Mice were fed HFD, low-fat diet (LFD), or formulations supplemented with GP (HFD-GP, LFD-GP) for 14 days. Mice fed HFD-GP, but not LFD-GP, showed improved oral glucose tolerance compared to controls. A. muciniphila bloom occurred earlier in mice fed LFD-GP than HFD-GP; however, timing was dependent on baseline A. muciniphila levels rather than dietary fat. Mice gavaged for 10 days with GP extract (GPE) or grape proanthocyanidins (PAC), each delivering 360 mg PAC/ kg body weight, induced a bloom of fecal and cecal A. muciniphila, the rate of which depended on initial A. muciniphila abundance. Grape PAC were sufficient to induce a bloom of A. muciniphila independent of specific intestinal gene expression changes. Gut microbial community analysis and in vitro inhibition of A. muciniphila by GPE or PAC suggest that the A. muciniphila bloom in vivo occurs via indirect mechanisms.
Keywords: Akkermansia, grape, gut, microbes, polyphenols, proanthocyanidins
Graphical abstract
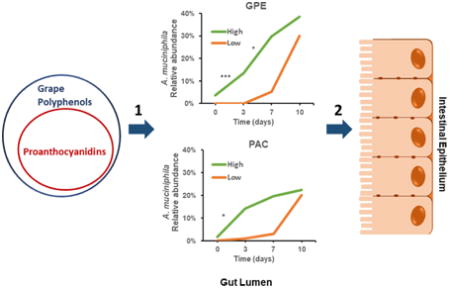
1. Introduction
Metabolic syndrome (MetS) defines co-occurrence of at least three of five symptoms (hyperglycemia, insulin resistance, hypertension, dyslipidemia, and central obesity [1]), which develop due to a combination of poor diet, sedentary lifestyle, and genetic predisposition. Underlying MetS is a state of chronic low grade inflammation, possibly associated with an increase in pro-inflammatory gut microbe-derived lipopolysaccharide (LPS), which leaks into systemic circulation due to impaired gut barrier integrity [2]. MetS is a precursor to type-2 diabetes (T2D), which an estimated 10% of adults worldwide will develop by 2040 [3]. Polypharmacological interventions that treat individual symptoms of MetS have not curtailed T2D prevalence, therefore lifestyle and dietary adjustments may be a more effective approach to preserving metabolic health.
Dietary polyphenols present in fruit, vegetables, nuts, teas, and spices, are associated with reduced risk of metabolic and cardiovascular disease [4], despite generally poor absorption into circulation [5-7]. Recent evidence suggests that poorly absorbed fruit polyphenols mediate host systemic effects in association with alterations in gut microbial composition, although cause-effect relationships remain to be established. Compared to high fat diet (HFD) controls, our prior studies showed that mice fed HFD formulated with Concord grape polyphenols (GP) for 12 weeks had increased abundance of the gut Verrucomicrobium Akkermansia muciniphila in association with leaner phenotype, less intestinal and systemic inflammation, improved oral glucose tolerance, and intestinal gene expression related to improved gut barrier and metabolic resilience [8]. Similar results were demonstrated in mice fed a high-fat, high sucrose (HFHS) diet supplemented with cranberry extract for 8 weeks [9] and in mice fed a HFHS diet supplemented with polymeric proanthocyanidins (pentamers and larger) from apple juice for 20 weeks [10].
Major compounds contained in grape berries, cranberries, and apples include flavan-3-ols (catechins and epicatechin), proanthocyanidins (PAC; polymers of flavan-3-ols), anthocyanin pigments, flavonols, and phenolic acids [11, 12]. Mice fed HFD supplemented with a polyphenol-rich red lettuce variety rich in chlorogenic acid, anthocyanins, and flavonols, but undetectable levels of PAC, demonstrated improved oral glucose tolerance after 9 weeks of supplementation, but did not show a bloom in A. muciniphila after 12 weeks [13] further suggesting that PAC may be the compounds responsible for the GP-induced bloom. Here we investigated the timing of the GP-induced A. muciniphila bloom and identify the polyphenol class sufficient to promote this effect.
2. Materials and Methods
2.1 Diets
Polyphenol-protein complexes allow stable and concentrated delivery of polyphenols in a food-based formulation while permitting release of bioactive polyphenols from the protein matrix [11, 14, 15]. Grape polyphenol-soy protein isolate (GP-SPI) complex containing 10% total polyphenols extracted from frozen grape pomace (Welch Foods Inc, Concord, MA) was prepared as previously described [8, 11]. Mice were fed the following ingredient-matched diet formulations (Research Diets, New Brunswick, NJ): 1) high fat diet containing 10% SPI (HFD); 2) HFD formulated with 10% GP-SPI delivering 1% GP (HFD-GP); 3) low fat diet containing 10% SPI (LFD); and 4) LFD formulated with 10% GP-SPI delivering 1% GP (LFD-GP) (Supplementary Tables 1 & 2).
2.2 Mice
All mice (male C57BL/6J) were purchased from Jackson Laboratory (Bar Harbor, ME) at age 5 weeks and fed ad libitum with free access to water in a room with a temperature of 24 ± 1 °C and a 12:12 h light-dark cycle (7 am – 7 pm). Protocols were approved by Rutgers University Institutional Care and Use Committee and followed federal and state laws.
2.2.1. 14-day time course experiments
Two independent studies were performed. 1) Pair-housed mice were fed LFD for one week and at age 6 weeks switched to receive HFD or HFD-GP (12 mice/ group) for 14 days. 2) Pair-housed mice (n = 24), purchased several weeks later, were fed LFD for one week then at age 6 weeks evenly split into two groups that received LFD or LFD-GP.
2.2.2. GPE vs. PAC 10-day time course experiment
Purified oligomeric proanthocyanidins from grape seeds (PAC standard; Sigma, catalog no. 1298219) were solubilized in 0.5% aq. ethanol (100 mg/mL) on day of gavage. Grape polyphenol extract (GPE) was prepared from Concord grape pomace (Welch Foods Inc, Concord MA). Individually housed mice (n = 30) were fed LFD ad libitum. At age 6 weeks, mice were randomized to three groups (10 mice/ group) and gavaged daily for 10 days with: 1) GPE delivering 360 mg PAC/kg body wt.; 2) PAC dissolved in 0.5% aq. ethanol delivering 360 mg /kg body wt. or; 3) 0.5% aq. ethanol (100 μL dose).
2.3. A. muciniphila in vitro experiments
A. muciniphila (ATCC, BAA-835) was cultured overnight in liquid medium prepared as previously described [16-18]. Diluted cultures were plated on solid agar medium containing increasing concentrations SPE column-purified GPE (GPECP) or PAC standard delivering equivalent amounts of proanthocyanidins.
2.4. Data deposition
DNA sequences encoding bacterial and archaeal 16S rRNA V4 region reported in this paper have been deposited in the Sequence Read Archive (SRA) under the accession number SRP119480.
Detailed experimental procedures are provided in online supporting materials.
3. Results
3.1. GP supplementation promotes metabolic resilience
Two cohorts of mice were fed either HFD or LFD with and without GP supplementation to investigate short-term effects of GP on host phenotypes and microbial community structure. In each case, mice were fed LFD during a one-week acclimation period and data from baseline oral glucose tolerance tests (OGTT, Fig. 1A-B day 0) were used to assign 6-week old male mice (n = 12 per group) to the control or GP-supplemented group to ensure groups had similar baseline OGT (p > 0.05). Compared to the HFD group, mice in HFD-GP group showed significantly better OGT after 14 days (Fig. 1A day 14 blood glucose area under the curve, unpaired, 2-tailed t-test, p = 0.023), which is consistent with our previous study where mice fed HFD-GP had improved OGT at 3 weeks [8]. Mice in LFD and LFD-GP groups showed no difference in OGT (Fig. 1B, day 14 blood glucose area under the curve, unpaired t-test, p = 0.25). Consistent with improved metabolic status, after 14 days serum insulin was significantly decreased in HFD-GP group compared to HFD group (p = 00066), but similar to serum insulin levels in the LFD and LFD-GP groups (T-test, p > 0.05; Table 1). With respect to serum LPS, there were no significant differences between HFD and HFD-GP groups (p = 0.9) or LFD and LFD-GP groups (p = 0.2; Table 1).
Figure 1. Effect of 14 days of GP supplementation on oral glucose tolerance.
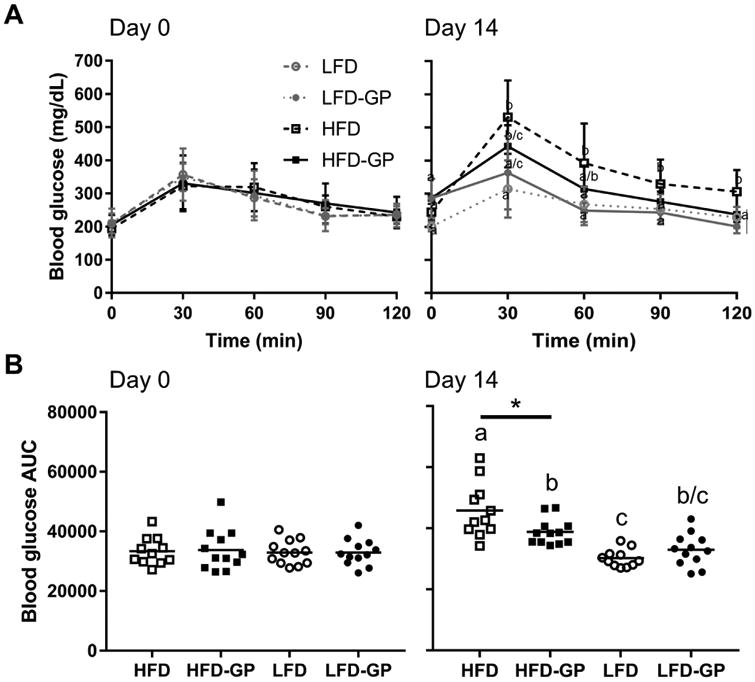
A Oral glucose tolerance tests were performed at baseline (day 0) and after 14 days on diets. Blood glucose concentrations (mg/dL) expressed as mean ± SD (n= 11 – 12 mice per group) were measured at the indicated time points (0-120 min) following oral administration of 2 g/kg glucose. B. Area under the curve (AUC) representation of data in A. was determined for individual mice, horizontal bar represents mean for each group. Between-group difference by diet base was determined by unpaired t-test (* = p < 0.05), while difference across all four groups was determined by one-way ANOVA followed by Tukey's multiple comparisons test. Different letters indicate significant difference between groups (p < 0.05) and the same letter indicates no difference.
Table 1. Serum Biochemistry.
| HFD | HFD-GP | LFD | LFD-GP | |
|---|---|---|---|---|
| Insulin (pg/mL) | 1884 ± 434 | 1207 ± 378 *** | 1423 ± 862 | 1198 ± 873 |
| LPS (ng/mL) | 0.51 ± 0.26 | 0.74 ± 0.49 | 0.56 ± 0.27 | 0.58 ± 0.24 |
T-test, unpaired, two tailed:
p = 0.00066
Food consumed per day was not significantly different between HFD and HFD-GP groups of mice (p= 0.10) or LFD and LFD-GP groups of mice (p= 0.30), indicating diets with and without GP had comparable palatability (Table 2). The average daily dose of total polyphenols (TP, as gallic acid equivalents) consumed by mice in the HFD-GP group was not significantly different from that consumed by mice in the LFD-GP group (p= 0.17; Table 2). Compared to HFD or LFD controls, GP supplementation did not affect body weight gain over the 14-day period (Supplementary Fig. 1A). Except for fat mass in the LFD-GP group, fat and lean mass significantly increased within all groups from day 0 to day 14 (Supplementary Fig. 1B), as determined by EchoMRI. Mice receiving HFD, but not HFD-GP, had significantly greater adiposity than mice on LF-based diets (Supplementary Fig. 1B). Lean mass was similar among the four diet groups, both before and after 14 days of GP supplementation (Supplementary Fig. 1B).
Table 2. Food and Total Polyphenols Consumed.
| HFD (n= 11) | HFD-GP (n= 12) | LFD (n= 12) | LFD-GP (n= 12) | |
|---|---|---|---|---|
| Food consumed (g/day/mouse) | 2.6 ± 0.5 | 3.2 ± 0.3 | 2.8 ± 0.2 | 2.9 ± 0.1 |
| Total Polyphenols consumed (mg/day/mouse) | - | 32.5 ± 3.1 | - | 29.3 ± 1.2 |
Unpaired, 2-tailed t-test performed on HFD or LFD control vs. supplemented groups Mice housed two per cage, six cages per group
Diet energy content (kCal/g) was measured by bomb calorimetry to confirm diets were isocaloric; measured values were somewhat higher than the values derived by calculation (Supplementary Tables 2 & 3). Bomb calorimetry of fecal samples collected after 14 days on diets showed that compared to LFD controls, the fecal samples from the LFD-GP group had greater energy content (t-test, p = 0.012). As studies were not performed with single housed mice in metabolic cages to allow collection of total feces produced over a fixed time period, it is unclear whether GP can reduce energy absorbed from diet, or affect other factors such as intestinal transit time. HFD and HFD-GP groups showed no differences in fecal energy content (Supplementary Table 3).
3.2. Short-term GP exposure promotes intestinal gene expression changes independent of dietary fat
In our prior study, mice fed HFD-GP for 12 weeks demonstrated several gene expression changes consistent with improved energy metabolism, improved gut barrier integrity, and decreased tissue inflammation compared to HFD-fed animals [8]. In the present study, a subset of previously observed gene expression changes was observed in the jejunum and ileum, but not in colon tissues (p > 0.05), after mice were fed HFD-GP for 14 days (Fig. 2A-C). Compared to HFD-fed controls, mice in the HFD-GP group had significantly decreased gene expression of Glut-2 in ileum tissue (p = 0.0038; Fig. 2B), but elevated Glut-2 in jejunum tissue (p = 0.033; Fig. 2A). Polyphenols are known to inhibit SGLT1 and Glut-2 transporters responsible for glucose absorption [19-21]; elevated jejunal Glut-2 gene expression could be a compensatory mechanism in response to such inhibition. Mice fed HFD-GP diet had lower expression of cytokine IL-6 in jejunum tissues (p = 0.014; Fig. 2A), and lower expression of iNOS in ileum tissues (p = 0.0094; Fig. 2B), suggesting attenuation of HFD-induced intestinal inflammation. Tight junction proteins ZO-1 (encoded by Tjp1) and occludin control the permeability of the intestinal epithelium [22]. Mice fed HFD-GP showed significant increase in ileal Tjp1 (p = 0.0009; Fig. 2B), suggesting improved gut barrier integrity. GP exposure for 14 days did not induce gene expression changes in occludin, GCG, TNFα, or Angptl4/Fiaf (p > 0.05; Fig. 2A-C) that were previously observed after 12 weeks of HFD or HFD-GP feeding [8].
Figure 2. Effect of 14 days of GP supplementation on intestinal gene expression and cecal weights.
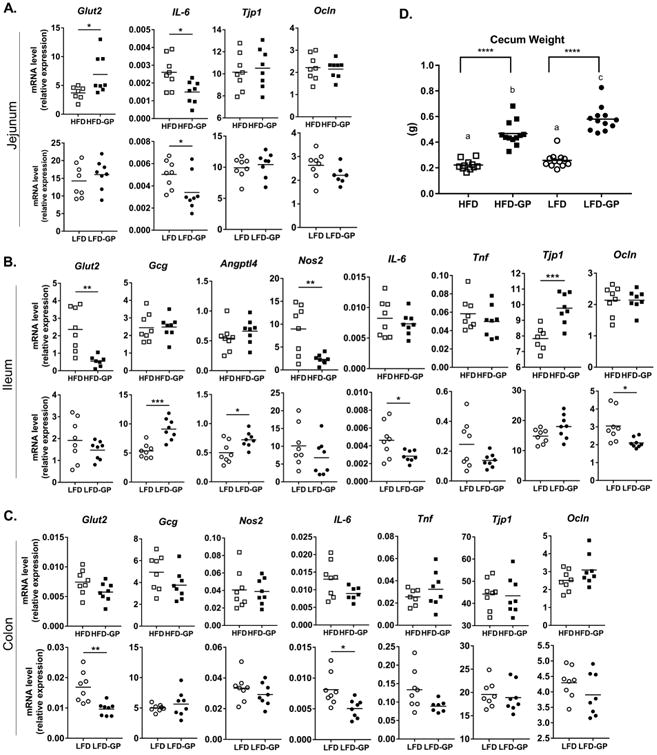
Relative mRNA levels of selected genes expressed in A. jejunum, B. ileum, and C. colon tissues was determined by qPCR. Target mRNA was normalized to HMBS as endogenous control and data were analyzed according to the 2-ΔCT method. Data are mean ± SD (n=8 samples per group) and the average of technical duplicates are used for each sample. Between-group difference was determined by unpaired, 2-tailed t-test: *p<0.05; **p<0.01; ***p<0.001. D. Cecal weights (g) of individual mice. Significant difference between diet groups in panels A - C is signified by letters a, b, or c; different letters indicate significant difference (p<0.05) between groups and the same letter indicates no difference. **** = p < 0.001.
Compared to LFD-fed animals, mice fed LFD-GP showed decreased IL-6 expression in jejunum, ileum, and colon tissues (Fig. 2A-C). Mice fed LFD-GP displayed increased ileal gene expression of Angptl4 (p = 0.016) and GCG (p = 0.0003; Fig. 2B), markers consistent with metabolic health. Angptl4 stimulates fatty acid oxidation and limits fat deposition in peripheral tissue [23] while GCG encodes pre-proglucagon, which is cleaved to glucagon-like peptide-1 (GLP-1), an incretin that promotes insulin production, and GLP-2, which promotes the integrity of the mucosal and intestinal epithelium barrier [24]. The LFD-GP group had lower occludin expression in ileum (p = 0.02; Fig. 2B), inconsistent with improved gut barrier, but this result may be due to two individuals with unusually high expression in the LFD group. The LFD-GP group had lower Glut-2 expression in colon (p = 0.0038; Fig. 2C).
3.3. GP supplementation rapidly reshapes gut microbial community structure
16S rRNA gene sequencing was performed on fecal samples collected on days 0, 2, 5, 7, 9, and 14 from 6 mice (one cage-mate per cage), along with matched cecal samples. Ceca from mice that consumed HFD-GP or LFD-GP were enlarged and twice the mass of ceca from mice fed LFD or HFD (Figure 2D), while liver weights were similar between all four diet groups (Supplementary Fig. 1C). GP supplementation of HFD or LFD significantly decreased OTU richness (Figure 3A) in fecal samples within 2 days and in cecal samples at endpoint, suggesting the A. muciniphila bloom occurs at expense of other taxa. Shannon index is a microbial diversity measure that scales OTUs based on community evenness. Regardless of the GP-mediated decrease in OTU richness, GP supplementation did not change evenness of fecal microbial communities, except for day 14 where Shannon index was higher for HFD-GP group (p < 0.05, Figure 3B). Shannon index diversity results suggest that in fecal samples, while GP reduced overall OTU richness, there are also changes in the remaining OTUs that contribute to overall evenness of the community. In contrast, GP supplementation decreased evenness of cecal microbial communities regardless of dietary fat, as indicated by a lower Shannon index (p < 0.01, Figure 3B).
Figure 3. GP altered microbial composition in both HFD and LFD conditions. A-B.
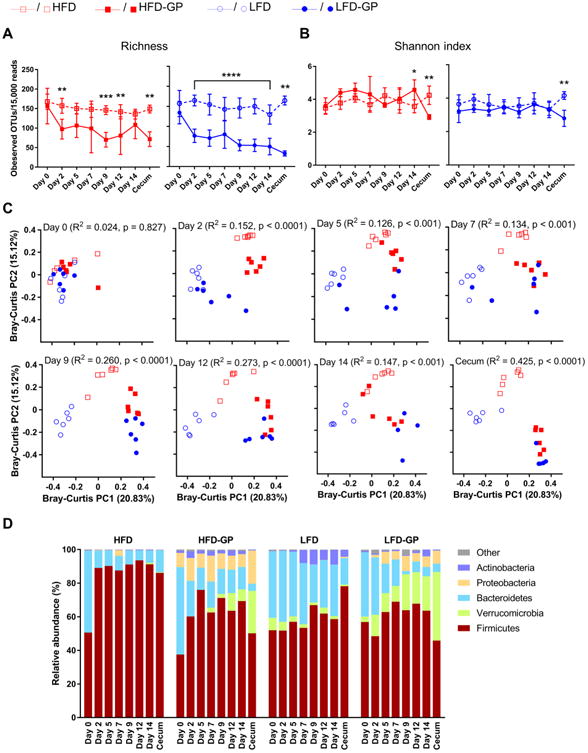
Microbiota α diversity as calculated by (A) OTU richness and (B) Shannon index of fecal and cecal samples by diet and day of study. Asterisks represent significant differences between GP-supplemented groups and control groups determined by repeated measurement two-way ANOVA followed by Sidak's multiple comparisons tests (for fecal samples from Day 0-Day 14) or Mann-Whitney tests (for cecal samples from Day 14), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. C. Bray-Curtis principal coordinate plots of gut microbial communities by day of study. ADONIS tests were performed to assess differential clustering caused by GP supplementation and diet base. R2 and p values representing the percentages of overall variation explained by GP supplementation and corresponding significance are listed, while the effect of diet base and other analyses by diet base/supplementation can be found in Supplementary Table 4. D. Relative abundance of the five dominant bacterial phyla. Low-abundance phyla (< 0.3%) were combined into the Other category. In panels A-C, analyses were performed on 15,000 sequences per sample, while non-rarefied data was used in panel D. n = 6 mice per group.
Principal coordinate analysis (PCoA) showed that baseline fecal microbial communities of mice, all on LFD, clustered together (Figure 3C, Baseline); however, dissimilarities in microbiotas were detectable between the cohort of mice used for the HFD-based study and the cohort used for the LFD-based study (Supplementary Table 4, ADONIS; overall effects with respect to assigned diet base: R2= 0.15, p< 0.01 and effects of diet base by supplementation status: HFD vs. LFD, R2= 0.285, p< 0.01; HFD-GP vs. LFD-GP, R2= 0.184, p< 0.05). Two days into the intervention period, microbial communities were distinguishable by diet group (significance considered at p< 0.05) and they continued to differentiate over the 14-day supplementation period (Fig. 3C and Supplementary Table 4). Over the time course the microbial communities from LFD-GP and HFD-GP groups clustered together on the PCoA plot (Fig. 3C); after 14 days dissimilarities were detectable between fecal microbial communities, but not cecal microbial communities (Supplementary Table 4, ADONIS, day 14 fecal samples LFD-GP vs. HFD-GP: R2= 0.229, p< 0.05; cecal samples LFD-GP vs. HFD-GP: R= 0.230, p = 0.054).
Relative abundance of bacterial taxa by diet and day of fecal sample collection are illustrated in Fig. 3D and details of significant differences within diet group (i.e. change from baseline) and between group differences (i.e. comparisons at matched time points) are presented in Supplementary Fig 2. Regardless of diet base, GP supplementation increased abundance of the Verrucomicrobia phylum, of which Akkermansia muciniphila is the sole member in mice (Fig. 3D and Supplementary Fig. 2). Baseline fecal samples of mice used for the HFD-based experiment exhibited very low relative abundances of A. muciniphila (HFD at baseline: 0.003% ± 0.0006%; HFD-GP at baseline: 0.003% ± 0.0005%); however, these increased measurably by day 7-14, particularly in the GP supplemented group (mean ± SEM across days 7-14; HFD: 0.2% ± 0.17%; HFD-GP: 5.7% ± 2.1%). The A. muciniphila bloom was most notable in cecal samples (HFD-GP: 22.1% ± 10.32%; HFD: 0.45% ± 0.23%; p< 0.0001; Fig. 3B and Supplementary Fig. 2A). Relative abundance of Firmicutes increased in fecal samples collected from HFD (baseline: 52.3% ± 3.5% vs. day 14: 91.1% ± 2.6%, p< 0.05) and HFD-GP (baseline: 39.7% ± 5.7% vs. day 14: 69.0% ± 9.9%; p< 0.05) groups; however, GP supplementation significantly suppressed the HFD-induction of Firmicutes in both fecal and cecal samples (Fig. 3B and Supplementary Fig. 2B). Indeed, levels of Firmicutes in fecal samples were indistinguishable (p > 0.05) at all time points between HFD-GP and LFD-based treatments, which was not the case for the HFD control group. Levels of Bacteroidetes were suppressed in HFD-fed mice compared to baseline, regardless of GP supplementation (Fig. 3B and Supplementary Fig. 2C). Compared to time-matched HFD samples, the HFD-GP group had increased levels of Proteobacteria in day-2 fecal samples as well as cecal samples and increased levels of Actinobacteria (Fig. 3B and Supplementary Fig. 2C, F).
Starting from day 9 through 14, the average relative abundance of A. muciniphila in fecal and cecal samples from the LFD-GP group exceeded 17% (mean ± SEM across days 9-14: 20.2% ± 2.5%), a significant increase compared to baseline levels (3.2% ± 1.9%) and compared to time-matched samples from the LFD group (across days 9-14: 2.3% ± 0.8%; at baseline: 7.4% ± 2.7%) (Fig. 3B and Supplementary Fig. 2A). Increased A. muciniphila in the LFD-GP group was accompanied by significant reduction in Bacteroidetes compared to LFD group and significant increase in Proteobacteria compared to baseline (Fig. 3B and Supplementary Fig. 2A-C). Compared to LFD group, relative abundance of Firmicutes was decreased in LFD-GP cecal samples, but similar in fecal samples of LFD and LFD-GP groups (Fig. 3B and Supplementary Fig. 2D).
Independent of dietary fat and as early as 2 days post-treatment, GP supplementation induced dynamic genus level changes, with functional relevance to fiber digestion and gut barrier integrity (Supplementary Fig. 3). Consistent with improved gut barrier function, GP supplementation lowered Oscillibacter, which has been correlated with HFD-induced weight gain, decreased transepithelial resistance, and lower ZO-1 gene expression in proximal colon [25]. GP decreased Clostridium IV, Intestinimonas, and Acetatifactor genera, associated with metabolism of fiber and short chain fatty acid (SCFA) production [26-28]; however, this was accompanied by increases in Blautia and Akkermansia, which also produce SCFAs [29]. GP was associated with decreased Gemella, which inhabit mucus and were reported to be enriched in colorectal cancer patients [30] and contribute to salivary dysbiosis in subjects with inflammatory bowel disease [31]. GP correlated with decreased Romboutsia, which has been isolated from human colon [32], but functional characteristics remain undetermined. Genera Enterococcus and Streptococcus include commensal probiotic, lactic acid producing strains in addition to strains associated with antibiotic resistance and disease [33]. Genus Weissella, originally classified as members of genus Lactobacillus, include strains of probiotic, lactic acid producing bacteria isolated from fermented foods while some strains are associated with infection [34].
C57BL/6J mice (n= 3) were fed LFD-GP for 7 days and then returned to LFD to determine if the GP-induced A. muciniphila bloom could be reversed. Relative abundance of A. muciniphila significantly increased within 7 days of GP supplementation and was returned to pre-supplementation levels by 21 days after GP removal (Supplementary Fig. 4A). Total bacteria and archaeal 16S rRNA gene counts per gram of feces were similar (p > 0.05) at all time points (Supplementary Fig. 4B) suggesting that GP supplementation does not affect total fecal bacterial and archaeal load.
3.4. Proanthocyanidins promote intestinal bloom of A. muciniphila before specific changes in host gene expression
The grape pomace extract (GPE) used as the source of GP is a mixture of several classes of compounds including proanthocyanidins (PACs), catechins, flavonols, anthocyanins, and hydroxycinnamic acids [11], in addition to extractable, soluble fiber [35]. As the most abundant class of polyphenols, we hypothesized that PACs, which are concentrated in seeds and skin, could be sufficient to induce a bloom in A. muciniphila. This was bolstered by our previous finding of no Akkermansia bloom with a high-polyphenol lettuce variety that did not contain PACs [13], as well as evidence of an Akkermansia bloom with cranberry extract rich in PAC [12] and apple PAC [10]. We therefore compared the ability of GPE or a standard of purified oligomeric PACs from grape seeds (PAC standard) to promote the intestinal bloom of A. muciniphila in a 10-day dosing study. We also hypothesized that this A. muciniphila bloom would occur independently and prior specific host gene expression changes associated with metabolic health.
GPE was prepared as described in methods. Total polyphenols in GPE accounted for 11.2 % of the dry extract weight as determined by Folin-Ciocalteu assay [36]. PACs accounted for 90 % of the total polyphenols in GPE (or 10% of dry weight), as determined by the DMAC method [37]. Anthocyanins contributed 0.23 % of total polyphenols in the GPE, as determined by the pH differential method [38]. Using same colorimetric method, total polyphenols quantified in the PAC standard accounted for 80% of the dry weight, 84% of total polyphenols could be attributed to PAC compounds, and monomeric anthocyanins were not detected. To identify and compare the levels of the most abundant proanthocyanidin compounds in GPE and PAC standard, samples were separated by UPLC followed by high resolution mass spectrometry. Supplementary Figs. 5A-B show the total ion current (TIC) chromatogram and individual chromatograms depicting the relative abundance of ions with mass/charge ratios (m/z) corresponding to the most abundant PAC monomers, oligomers (2 – 5 degrees of polymerization, DP), and their gallate derivatives detected during full scan mode of GPE and PAC samples. For each chromatogram, areas of individual peaks were integrated and summed to give total peak area as an estimate of ion abundance. Based on total peak area, the PAC standard had 2.5 times more monomeric flavan-3-ols (catechin/epicatechin) and 13 – 38 times more proanthocyanidins compared to GPE. Supplementary Fig. 5C illustrates the comparative abundance of catechin/epicatechin monomers and the most abundant type B proanthocyanidins (i.e. dimers, trimers, dimer gallates, trimer gallates, tetramers and pentamers) detected in GPE and PAC samples.
After a one-week acclimation period on LFD, six-week old mice were randomly assigned to be gavaged daily for 10 days with GPE (delivering 360 mg total PACs/ kg), PAC standard (360 mg/kg), or vehicle (0.5% ethanol in water). The relative abundance of fecal A. muciniphila started increasing as early as day 3 for individual animals treated with GPE or PAC standard; however, A. muciniphila was significantly increased for GPE (p = 0.0005) and PAC standard-treated (p = 0.035) groups at day 10 in comparison to the control group (Fig. 4A).
Figure 4. Comparison of PAC vs. GPE treatment on A. muciniphila bloom and cecal weight.
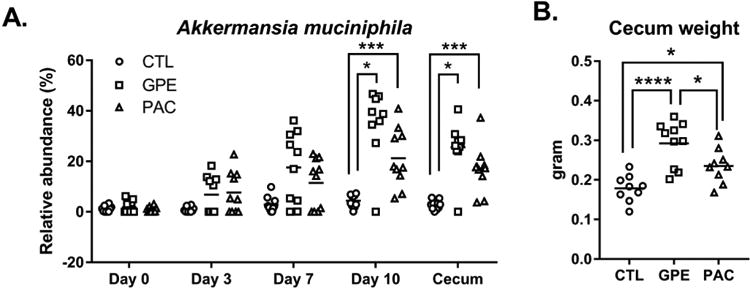
A qPCR quantification of A. muciniphila relative to total bacteria in fecal samples collected on days 0, 3, 7, and 10 during oral gavage with PAC, GPE, or vehicle as well as cecal content after euthanasia on day 10. Kruskal-Wallis test was used to detect differences between groups followed by pair wise comparisons using Dunn's multiple comparison test to detect differences between vehicle vs. GPE and vehicle vs. PAC groups. B. Comparison of cecal weights (g) of individual mice in each group. One-way ANOVA was performed to evaluate differences between the 3 groups followed by Tukey post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
There appeared to be marked baseline (day 0) variations in relative abundance of A. muciniphila, more easily observed when data are plotted on a log scale as opposed to linear scale, which emphasized differences at later time points (Supplementary Figs. 6A-B). We hypothesized that mice with higher levels of A. muciniphila at baseline would develop a GPE- or PAC-induced bloom more rapidly. qPCR analysis of day 0 fecal samples revealed that mice with high (> 0.01%) and low baseline levels of A. muciniphila were evenly distributed among control, GPE, and PAC standard groups (Supplementary Table 7). A. muciniphila bloom rates for each mouse was calculated for day 0 – 3, day 4 – 7 and day 8 – 10 time periods and within each treatment group the bloom rates were compared between subsets of mice having high and low levels of A. muciniphila. Compared to mice with a low relative abundance of A. muciniphila at baseline, mice starting with a high relative abundance showed a more rapid GPE- induced A. muciniphila bloom (0% per day vs. 3.3 % per day, p < 0.0079) and PAC-induced A. muciniphila bloom (0.3% per day vs. 4.2% per day, p < 0.016) within the first three days (Supplemental Table 7). There was one super-responder that started with a low (0.00005%) relative abundance A. muciniphila and increased to 4.9 % by day 3 (Supplemental Table 7). Subsets of mice with low and high baseline levels of A. muciniphila within the control group showed no differences in bloom rate (Supplemental Table 7).
Total bacterial load, estimated as total bacteria and archaea 16S rRNA gene counts per gram of fecal sample extracted (Supplementary Fig. 6C), was not significantly different between GPE, PAC and control groups on day 0 or day 7, consistent with our previous data [8]. Non-A. muciniphila 16S rRNA gene counts per gram of fecal sample, representing non-A. muciniphila bacterial load, was similar between treatment groups on day 0, but significantly decreased (p = 0.02) in the GPE group on day 7 (Supplementary Fig. 6C). Cecal weights of PAC-treated mice were significantly higher than that of control-treated mice, while the GPE-treated group showed higher cecal weight than both control and PAC groups (Fig. 4B). Body weights, cumulative food consumption, and liver weights were similar among control and treated groups after 10 days of treatment (Supplementary Fig. 6D-F).
Based on the rapid GP-induced gut microbial changes we hypothesized that the A. muciniphila bloom would occur before host gene expression changes. PACs caused greater increase in IL-6 (p = 0.033) and TNF (p = 0.048), relative to GPE treatment, in jejunum and colon tissues respectively (Supplementary Fig. 7 A & C). This increase in inflammatory mediators may be an effect of the purified PAC standard compared to PAC within a mixture of other GPE compounds including soluble fibers that may have opposing effects. Increased Glut-2 (p = 0.011) was detected in ileum tissues of GPE-treated mice relative to the control group (Supplementary Fig. 7 B). Compared to control, ten days of GPE or PAC standard treatments did not induce significant changes in gene expression of iNOS, GCG, Angptl4, Tjp-1, occludin, TNFα, Muc-2, or Muc-3 (p > 0.05; Supplementary Fig. 7 A-C). These data indicate that the GPE and PAC standard-induced bloom in A. muciniphila precedes, and possibly directly or indirectly promotes, these host gene expression changes associated with metabolic resilience.
3.5. Effect of GPE or PACs on cultured A. muciniphila
The observed bloom in A. muciniphila could be due to a direct growth-promoting effect of GPE or the PAC standard. Alternatively, A. muciniphila growth may be indirectly promoted by GPE- or PAC-mediated suppression of microbes that limit growth of A. muciniphila, either through competition for growth substrate or other means, or via cross-feeding activities. To directly evaluate the effect of GPE and PAC on A. muciniphila growth, two dilutions of pure culture were plated on agar medium containing increasing concentrations of C18 column-purified GPEcp or PAC, adjusted to pH 7.1. Compared to control, A. muciniphila growth was inhibited with concentrations of 0.03 mg – 1.0 mg proanthocyanidins/mL (Fig 5).
Figure 5. Grape polyphenols and proanthocyanidins inhibit the in vitro growth of A. muciniphila.
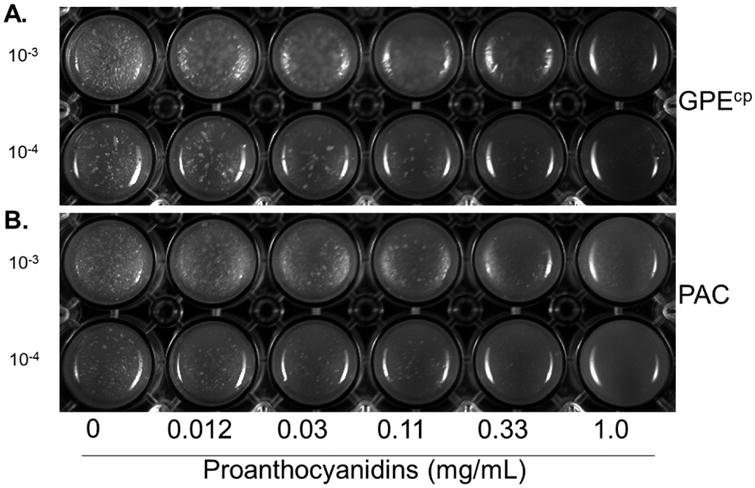
GPEcp and PAC standard were adjusted to pH 7.1, dilutions were prepared and added to molten agar to achieve 1.0, 0.33, 0.11, 0.03, or 0.012 mg of proanthocyanidin (B2 equivalents) per mL medium. A. muciniphila culture (OD = 0.875) was diluted 10-3 and 10-4 and 30 μL aliquots were spread on the surface of solid medium impregnated with A. GPEcp or B. PAC standard. Photograph (representative of two independent experiments) shows 24-well plates incubated under anaerobic conditions at 37°C after 3 days.
4. Discussion
We previously showed that feeding mice HFD supplemented with 1% GP for 12 weeks resulted in a bloom of gut bacterium, A. muciniphila, in association with intestinal gene expression changes consistent with metabolic health [8]. Here we characterized the timing of GP effects, and showed that GP can promote an A. muciniphila bloom within two weeks (Fig. 3, Supplementary Figs. 2 and 4). Genus level analysis showed that GP supplementation altered the abundance of microbes associated with fermentation of resistant fibers, gut barrier function, and probiotic activity(Supplementary Fig. 3). Furthermore, the GPE- or PAC-induced A. muciniphila bloom occurred prior to significant changes in the expression of several host genes (Fig. 4A and Supplementary Fig. 7). The speed of A. muciniphila bloom induction was dependent on its baseline levels (Supplementary Fig. 6A-B and Supplementary Table 7). Mice were sourced from the same vendor and came from the same breeding facility (Jax East); however, different cohorts of animals had different starting levels of A. muciniphila (Fig. 3B) and, furthermore, animals received within a single shipment also showed vastly different baseline levels of A. muciniphila (Supplementary Figs. 6A-B and Supplementary Table 7) ranging from 0.00005% - 6.19% relative abundance. While such variations may be better controlled by using litter mates, achieving adequate numbers of mice of same gender and age for all groups is not feasible as mice generally have a litter size of 8-10 pups (4-5 males/litter) therefore pups are typically pooled at age 21 days to fulfill orders (personal communication). Such differences in baseline A. muciniphila abundance, in addition to plant source and extraction methods, may explain why the bloom is not consistently observed in other recent studies of grape pomace extract [39].
GP supplementation of HFD for 14 days induced major changes in gut microbial community structure; however, changes in host gene expression consistent with improved metabolic status observed over this period were much less pronounced than changes observed in our previous 12-week study [7]. Limited changes in the intestinal gene expression were seen after 14 days of GP supplementation of HFD or LFD (Figs. 2A-C). Another study reported increased gene expression of Muc2 in mice administered grape seed extract in drinking water for 12 weeks [40], which we have not observed in our studies. Relative timing of GP effect on gut microbiota and molecular markers of gut health, suggests that the bloom in A. muciniphila precedes and possibly promotes beneficial changes in the gut.
Dietary intake of polyphenols in the human diet is estimated at greater than 2 g per day from food and supplements [41] and total PAC intake from all food sources was recently estimated to be 68 – 192 mg/day [42] or 1.0 mg/kg/day – 2.9 mg/kg/day for human males assuming average weight of 75 kg. Mice in our studies ingested an estimated 500 mg PAC/kg body weight/day and using 12.3 as species conversion factor [43] this murine dose converts to a human dose of 40 .6 mg/kg/day, or 14 - 40 times the estimated range for human intake. The present dose was chosen to remain consistent with our previous study [8], but future work will determine whether lower doses of PAC show similar gut microbiota and host effects. In a recent clinical study where subjects consumed sweetened dried cranberries daily for 2 weeks there was a trend towards increased fecal Akkermansia indicating that effects in mice relate to human subjects [44].
Biochemical characterization by LC-MS confirmed that the GPE used in this study contained the broad range of polyphenols previously reported [11]. In contrast, the PAC standard (derived from grape seeds) was purified to contain mainly catechin/epicatechin monomers and PAC oligomers (dimers to pentamers) whose relative quantities decreased with increasing degrees of polymerization (Supplementary Fig. 5C). The GPE used for oral administration to the mice (10 % proanthocyanidins relative to dry weight) was prepared the same way as for production of GP-SPI ingredient, to approximate the range and levels of polyphenols incorporated in HFD-GP and LFD-GP. In contrast, for testing growth of A. muciniphila in vitro, the GPE was further subject to SPE column-purification to obtain GPEcp, enriched for PAC compounds (50 % PAC relative to dry weight). Standard phytochemical methods were used to standardize the levels of PAC delivered in GPE or GPEcp vs. the PAC standard; however, due to the complex chemistries of these mixtures the levels of individual PAC are never equimolar. While PAC were identified as sufficient to induce the A. muciniphila bloom, there may be other grape-derived compounds that contribute to the effect as levels of A. muciniphila achieved with PAC were somewhat lower than those achieved with GPE, but still significant compared to control. In addition to polyphenols, soluble fibers such as pectins, inulin, gums, and water-soluble hemicellulose (e.g. arabinoxylan) can be extracted from grape pomace [35]. Soluble fibers in GPE could therefore explain the trend of its greater activity with respect to the A. muciniphila bloom in comparison to purified PAC (Fig. 4A).
A recent study of grape seed polyphenol extract (GSPE) gavaged to mice over 7 weeks showed no change in A. muciniphila; however, compositional analysis to confirm presence and levels of PACs in this GSPE test material was not reported [45]. In a study where mice were fed HFHS diet in conjunction with oligomeric PAC (OP, monomer - tetramer) or polymeric PAC (PP, pentamer to undecamer and larger) for 20 weeks, only mice supplemented with PP showed significant increase in A. muciniphila [10]. It is unclear why oligomeric PAC from grape and apple showed different effects on A. muciniphila in mice. Nevertheless, the present demonstration of the powerful prebiotic effects of dietary PAC provides an important mechanistic clue as to how these poorly bioavailable antioxidant compounds abundant in fruits and berries may benefit human health, as demonstrated in several epidemiological studies [4, 46-49].
A. muciniphila levels inversely correlated with onset of inflammation and insulin resistance in HFD-fed mice [50], while administration of live A. muciniphila to HFD-fed mice attenuated symptoms of metabolic syndrome [51]. It was recently reported that administration of pasteurized, non-replicative A. muciniphila enhanced the metabolic benefits of the bacterium in HFD-fed mice [17, 52]. Amuc_1100*, a thermostable outer-membrane protein of A. muciniphila, was demonstrated to partially recapitulate the beneficial effects A. muciniphila in HFD-fed mice through interaction with host toll-like receptor 2 (TLR2) [17, 52]. These results help to explain how the A. muciniphila bloom may produce beneficial metabolic outcomes, while our data link dietary polyphenols, such as grape PACs, to the A. muciniphila bloom. Although compositionally defined LFD has a fat content similar to murine chow, LFD promotes metabolic stress as it is formulated with cellulose rather than soluble fibers, which undergo fermentation by gut bacteria resulting in production of bacterial metabolites (e.g. short chain fatty acids) that decrease intestinal inflammation and support intestinal health [53]. Compared to mice fed LFD, mice fed LFD-GP had lower expression of inflammatory IL-6 in all intestinal segments, indicating that GP can attenuate LFD-induced metabolic stress (Figure 2).
A. muciniphila metabolizes mucin and has been localized to the loose outer layer of mucus, suggesting a separate bacterial niche. Mucus, however, is continually and rapidly shed into the intestinal lumen therefore bacteria associated with the outer mucus layer, such as A. muciniphila, will ultimately end up among the luminal bacterial population and must compete for resources in the lumen. A recent study that compared microbial communities in mucus layer and luminal content from caecum and colon of stable defined medium density microbiota (sDMDM) gnotobiotic mice found that the microbial community of the outer mucus layer differed in composition from the lumen; however, all constituents of isobiotic microbiota were present at some level in both compartments [54]. Furthermore, Akkermansia abundances were similar in cecal content vs. cecal mucus as well as in colon content vs. colon mucus [54]. In vitro growth of A. muciniphila was inhibited by GPEcp or PAC standard delivering at least 0.03 mg/mL of PAC (Fig. 5A-B) suggesting that PAC do not directly induce A. muciniphila bloom.
There are several plausible explanations for the A. muciniphila bloom that warrant further investigation. PAC as well as other GPE compounds may confer a selective growth advantage to A. muciniphila by suppressing competitor microbes within the gut microbial community. GP-supplementation rapidly decreased OTU richness (Fig. 3A) and GPE administration decreased non-A. muciniphila 16S rRNA gene counts/g feces (Supplementary Fig. 6C), but further work is needed to understand how specific alterations may promote the A. muciniphila bloom. Alternatively, cross-feeding activities among the community may favor growth of A. muciniphila. Powerful antioxidant activity of PAC may give A. muciniphila an ecological advantage over more oxygen-tolerant bacteria in the gut. PAC-induced change in expression of host genes other than those investigated in the study may also play a role. Understanding how PAC can influence the gut microbiota and intestinal milieu represents a novel approach to treating and preventing metabolic disease.
Supplementary Material
Acknowledgments
We thank Taina Spicer, Madeline Bandomer, and Shikha Ranka for technical assistance.
Funding: This work was funded by the National Institutes of Health (IR, DER 1R01AT008618-01; DER, K01-AT008829; PJT, R01HL122593; RNC, F32DK101154). PJT is a Nadia's Gift Foundation Innovator supported, in part, by the Damon Runyon Cancer Research Foundation (DRR-42-16), the UCSF Program for Breakthrough Biomedical Research (partially funded by the Sandler Foundation), and the Searle Scholars Program.
We also thank Linfei Zhou for assistance with data analysis. All authors read and approved the final manuscript.
Footnotes
Author Contributions: DER and LZ designed the experiments. DER, LZ, RNC, IR, and PJT wrote the manuscript. LZ, PK, KM, HK, RMD, KT, and DER performed experiments and data analyses. AP performed analytical chemistry. RNC and PJT performed 16S rRNA gene sequencing; LZ and RNC performed bioinformatics and statistical analyses.
Competing interests: DER and IR have equity interest in Nutrasorb LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–88. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IDF Diabetes Atlas. 7. International Diabetes Foundation; Brussels, Belgium: 2015. [Google Scholar]
- 4.Visioli F, De La Lastra CA, Andres-Lacueva C, Aviram M, Calhau C, Cassano A, et al. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51:524–46. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 5.Felgines C, Krisa S, Mauray A, Besson C, Lamaison JL, Scalbert A, et al. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br J Nutr. 2010;103:1738–45. doi: 10.1017/S0007114510000061. [DOI] [PubMed] [Google Scholar]
- 6.Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C -tracer study. Am J Clin Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 7.Choy YY, Jaggers GK, Oteiza PI, Waterhouse AL. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J Agric Food Chem. 2013;61:121–7. doi: 10.1021/jf301939e. [DOI] [PubMed] [Google Scholar]
- 8.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes. 2015;64:2847–58. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–83. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 10.Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016;6:31208. doi: 10.1038/srep31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roopchand DE, Kuhn P, Krueger CG, Moskal K, Lila MA, Raskin I. Concord grape pomace polyphenols complexed to soy protein isolate are stable and hypoglycemic in diabetic mice. J Agric Food Chem. 2013;61:11428–33. doi: 10.1021/jf403238e. [DOI] [PubMed] [Google Scholar]
- 12.Anhe FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2014 doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 13.Cheng DM, Roopchand DE, Poulev A, Kuhn P, Armas I, Johnson WD, et al. High phenolics Rutgers Scarlet Lettuce improves glucose metabolism in high fat diet-induced obese mice. Mol Nutr Food Res. 2016;60:2367–78. doi: 10.1002/mnfr.201600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roopchand DE, Grace MH, Kuhn P, Cheng DM, Plundrich N, Pouleva A, et al. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012;131:1193–200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roopchand DE, Kuhn P, Rojo LE, Lila MA, Raskin I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol Res. 2013;68:59–67. doi: 10.1016/j.phrs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 17.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 18.Stams AJ, Van Dijk JB, Dijkema C, Plugge CM. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol. 1993;59:1114–9. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005;579:1653–7. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Kwon O, Chen S, Daruwala R, Eck P, Park JB, et al. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J Biol Chem. 2002;277:15252–60. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- 21.Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2010;54:1773–80. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- 22.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. Journal of Lipid Research. 2002;43:1770–2. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 24.Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–48. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 25.Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bui TP, Shetty SA, Lagkouvardos I, Ritari J, Chamlagain B, Douillard FP, et al. Comparative genomics and physiology of the butyrate-producing bacterium Intestinimonas butyriciproducens. Environ Microbiol Rep. 2016;8:1024–37. doi: 10.1111/1758-2229.12483. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer N, Desmarchelier C, Blaut M, Daniel H, Haller D, Clavel T. Acetatifactor muris gen. nov., sp. nov., a novel bacterium isolated from the intestine of an obese mouse. Arch Microbiol. 2012;194:901–7. doi: 10.1007/s00203-012-0822-1. [DOI] [PubMed] [Google Scholar]
- 29.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said HS, Suda W, Nakagome S, Chinen H, Oshima K, Kim S, et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014;21:15–25. doi: 10.1093/dnares/dst037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricaboni D, Mailhe M, Khelaifia S, Raoult D, Million M. Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 2016;12:6–7. doi: 10.1016/j.nmni.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745–67. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KW, Park JY, Jeong HR, Heo HJ, Han NS, Kim JH. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe. 2012;18:96–102. doi: 10.1016/j.anaerobe.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Zhu M, Shi T, Guo C, Huang Y, Chen Y, et al. Recovery of dietary fiber and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions. Food Funct. 2017;8:341–51. doi: 10.1039/c6fo01423b. [DOI] [PubMed] [Google Scholar]
- 36.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Oxidants and Antioxidants, Pt A. 1999;299:152–78. [Google Scholar]
- 37.Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, et al. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90:1473–8. doi: 10.1002/jsfa.3966. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–78. [PubMed] [Google Scholar]
- 39.Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Ståhlman M, et al. Reduced obesity, diabetes and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab. 2017 doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 40.Yang G, Xue Y, Zhang H, Du M, Zhu MJ. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice. Br J Nutr. 2015;114:15–23. doi: 10.1017/S0007114515001415. [DOI] [PubMed] [Google Scholar]
- 41.Espin JC, Gonzalez-Sarrias A, Tomas-Barberan FA. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 42.EFSA Panel on Dietetic Products NaA. Safety of cranberry extract powder as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal. 2017;15:4777. doi: 10.2903/j.efsa.2017.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bekiares N, Krueger CG, Meudt JJ, Shanmuganayagam D, Reed JD. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects. OMICS. 2017 doi: 10.1089/omi.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Zhao S, Wang J, Shi J, Sun Y, Wang W, et al. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol Nutr Food Res. 2017 doi: 10.1002/mnfr.201601082. [DOI] [PubMed] [Google Scholar]
- 46.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr. 2014;144:335–43. doi: 10.3945/jn.113.184945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi M, Rosato V, Bosetti C, Lagiou P, Parpinel M, Bertuccio P, et al. Flavonoids, proanthocyanidins, and the risk of stomach cancer. Cancer Causes Control. 2010;21:1597–604. doi: 10.1007/s10552-010-9588-4. [DOI] [PubMed] [Google Scholar]
- 48.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev. 2012;70:491–508. doi: 10.1111/j.1753-4887.2012.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu YJ, Zhan J, Liu XL, Wang Y, Ji J, He QQ. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr. 2014;33:59–63. doi: 10.1016/j.clnu.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anhe FF, Marette A. A microbial protein that alleviates metabolic syndrome. Nat Med. 2017;23:11–2. doi: 10.1038/nm.4261. [DOI] [PubMed] [Google Scholar]
- 53.Chassaing B, Garenaux E, Carriere J, Rolhion N, Guerardel Y, Barnich N, et al. Analysis of the sigmaE regulon in Crohn's disease-associated Escherichia coli revealed involvement of the waaWVL operon in biofilm formation. J Bacteriol. 2015;197:1451–65. doi: 10.1128/JB.02499-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Limenitakis JP, Fuhrer T, Geuking MB, Lawson MA, Wyss M, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


