Abstract
Circulating tumor cells (CTCs) are a major contributor of cancer metastases and hold a promising prognostic significance in cancer detection. Performing functional and molecular characterization of CTCs provides in-depth knowledge about this lethal disease. Researchers are making efforts to design devices and develop assays for enumeration of CTCs with a high capture and detection efficiency from whole blood of cancer patients. The existing and on-going research on CTC isolation methods has revealed cell characteristics which are helpful in cancer monitoring and designing of targeted cancer treatments. In this review paper, a brief summary of existing CTC isolation methods is presented. We also discuss methods of detaching CTC from functionalized surfaces (functional assays/devices) and their further use for ex-vivo culturing that aid in studies regarding molecular properties that encourage metastatic seeding. In the clinical applications section, we discuss a number of cases that CTCs can play a key role for monitoring metastases, drug treatment response, and heterogeneity profiling regarding biomarkers and gene expression studies that bring treatment design further towards personalized medicine.
Keywords: Circulating Tumor cells (CTCs), Cell enrichment, Point of care, Liquid biopsy, Personalized therapies
1. Introduction
According to the Centers for Disease and Control Prevention (CDC), cancer records the second highest reason of death worldwide, with more than 90% of deaths being caused by cancer cell metastasis (Hong and Zu, 2013; Wicha and Hayes, 2011). This is largely due to the fact that malignant tumors have the ability to shed tumor cells that invade surrounding tissue and enter the lymphatic and circulatory systems (Asghar et al., 2012b). These cells, known as circulating tumor cells (CTCs), ultimately establish new metastasis at other tissue and organ sites throughout the body (Chaffer and Weinberg, 2011; Harouaka et al., 2014; Maheswaran and Haber, 2010; Wan et al., 2011).
CTCs were first discovered more than hundred years ago by Thomas Asworth (Ashworth, 1869). Since then, many studies have focused on discovering efficient CTC detection and isolation techniques, with the prospects of using CTCs as a ‘liquid biopsy’ for peripheral blood analyses and an early biomarker for response to systemic therapies (Alix-Panabières and Pantel, 2013; Diaz and Bardelli, 2014; Lianidou, Evi S., 2014; Wan et al., 2012; Wang et al., 2013). However, a number of challenges are associated with CTC isolation, detection, and downstream analysis. CTCs are sparse, approximately 1–100 cells can be found per milliliter of blood, along with 106–107 red blood cells (Asghar et al., 2013; Hafeez et al., 2012; Ilyas et al., 2014a; Ilyas et al., 2014b; Miller et al., 2009). Increasing blood sample volumes is a possible resolution that provides more accurate measurements, but comes with its own time constraints and patient care challenges. CTC heterogeneity is another major obstacle, as various groups of CTCs have significant variations in surface expression of biomarkers (Attard and de Bono, 2011; Ignatiadis and Dawson, 2014). Currently, CellSearch, the exclusively US-FDA (Food and Drug Administration) cleared device for CTC detection is a prognostic indicator for breast, prostate and colorectal cancer (Krebs et al., 2011; Sieuwerts et al., 2009). A great clinical need still exists for low-cost, non-invasive, and efficient CTC detection and isolation devices. Herein, we review the most promising CTC isolation methods and apply more focus on future directions of CTC technology (Figure 1). The different isolation methods are categorized on the basis of specific CTC characteristics such as physical properties (size, elasticity, surface charge) (Asghar et al., 2012b; Gascoyne et al., 2009; Moon et al., 2011; Müller et al., 2005; Vona et al., 2000; Zheng et al., 2011), biological characteristics such as cellular function (Alix-Panabières, 2012; Lu et al., 2010) and the expression of tumor-specific surface proteins (Allard et al., 2004; Helzer et al., 2009; Lu et al., 2013b; McKeown and Sarosi, 2013; Riethdorf et al., 2007; Stott et al., 2010; Talasaz et al., 2009). After isolating CTCs from patient samples, releasing CTCs from the capturing substrate presents with challenges. The successful detachment of CTCs is an important step for establishing ex-vivo CTC cultures and obtaining morphological information. In this paper, we review downstream processing steps, describing CTC release from substrate with the use of various enzymatic actions, aptamers and polymers. Protocols and success rates for culturing CTCs from cancer patients demonstrating heterogeneous CTC morphological properties are also discussed, and a description of ex-vivo CTC culturing under various cell culture conditions for disease model development is provided. Moreover, the clinical aspects of CTCs are described, and examples of how CTCs can participate in monitoring metastasis and drug therapy responses are discussed.
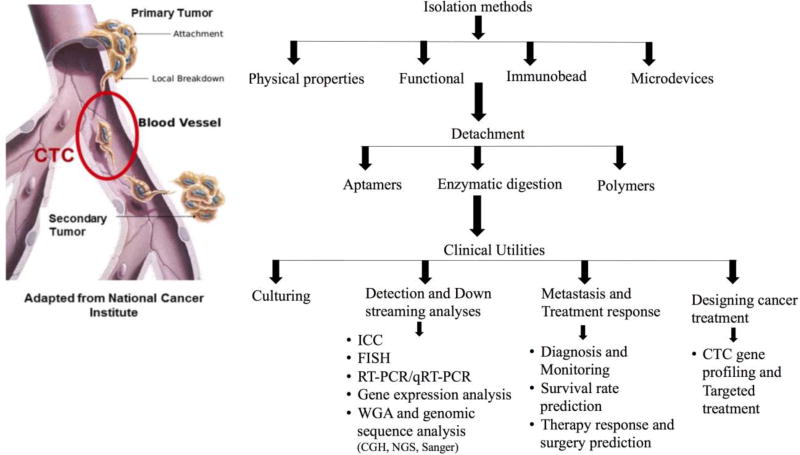
Figure 1.
Outline of existing isolation, detection and characterization techniques and promising future clinical utilities.
2. CTC Isolation Methods
Since the discovery of CTCs, several isolation techniques have been developed. However, these techniques are often limited by the presence of extremely low number of CTCs in patient blood (1–100 cells per mL), as well as their fragile and heterogeneous nature (Alix-Panabières and Pantel, 2013; Zheng et al., 2013). CTC fragility becomes a concern when the cells need to be detached from the various chips and membranes that are used to isolate them. We discuss detachment after introducing the major CTC isolation methods developed thus far. Most of the existing technologies consist of a two-step process of cell enrichment and subsequent detection. Cell enrichment involves capturing CTCs based on their physical properties, including size, elasticity, density, and charge (Gascoyne et al., 2009; Moon et al., 2011; Müller et al., 2005; Vona et al., 2000; Zheng et al., 2011), and various biological characteristics, such as cellular functions (Alix-Panabières, 2012) and tumor-specific surface proteins (Allard et al., 2004; Helzer et al., 2009; Lu et al., 2013b; McKeown and Sarosi, 2013; Riethdorf et al., 2007; Stott et al., 2010; Talasaz et al., 2009). Detection methods then allow for single-cell level specificity when counting CTCs and further separating them from normal blood cells. These detection methods include visual microscopy, immunostaining, biomechanical discrimination and polymerase chain reaction (PCR) (Alix-Panabières and Pantel, 2013).
2.1 Physical Property-Based Assays
Enrichment via physical properties, such as size and membrane capacitance, allows one to isolate CTCs quickly without labeling (Kim et al., 2016). Unfortunately, these techniques present certain limitations, as current technologies lack specificity and yield less pure results than functional assays due to cell heterogeneity (Hong and Zu, 2013; Wang et al., 2013). Dielectrophoretic field-flow fractionation (DEP-FFF) employs separation by size and polarizability using membrane capacitance and can process 30 million cells within 30 min with high recovery rates. However, it requires very specific parameters such as cell type and electric field frequency (Gascoyne et al., 2009; Zieglschmid et al., 2005). Metacell filtraction device, isolation by size of epithelial tumor cells (ISET), ScreenCellCyto, and dead flow fractionation techniques all use size to select for CTCs (De Giorgi et al., 2010; Dolfus et al., 2015; Hou et al., 2013; Vona et al., 2004; Wang et al., 2013). With the exception of Metacell, these size-based techniques quickly isolate CTCs, which are usually larger in size than other blood cells, but fail to enrich smaller CTCs and those with similar deformability to leukocytes (Dolfus et al., 2015; Joosse et al., 2015; Zheng et al., 2011). It is also difficult to release the captured CTCs from porous membranes for downstream analyses. To overcome this challenge, a Parsotrix method is developed which is a size-based selection method that involves a cassette device for collecting CTCs that are readily available for subsequent studies, overcoming the detachment limitation (Joosse et al., 2015). In summary, size-based CTC isolation methods provide high throughput, however these methods find limited applicability in clinical settings due to heterogeneity of CTCs in term of their size.
2.2 Functional Assays
Functional assays to detect only viable CTCs may overcome some of the limitations of physical heterogeneity. However, current CTC methods based on cell functional properties face issues regarding product purity. These include analyzing CD45 protein levels and collagen adhesion matrix (CAM) removal and uptake, using the EPISPOT assay (Epithelial Immunospot) (Alix-Panabières, 2012) and CAM assay (Vita Assay) (Lu et al., 2010), respectively. The CAM assay measures CTC invasiveness via CAM protein uptake. It produces results with high sensitivity and specificity, but requires over 12 hours for isolation and may fail to isolate more heterogeneous cells due to its biomarker dependence (Monteiro-Riviere et al., 2009). CD45 is a common leukocyte antigen used during the EPISPOT assay to remove leukocytes via CD45 depletion. The assay then detects proteins released by or shed from CTCs during short-term cultures (Alix-Panabières, 2012). Though a very promising technique, problems in EPISPOT detection arise when antigen levels are lower or binding efficiency is reduced (Kalyuzhny, 2005).
Elevated telomerase activity has been associated with malignancy in most cancer types (Blackburn, 2000) and can be used to detect CTCs via a telomerase-specific replication selective adenovirus in a method known as TelomeScan (Kojima et al., 2009). The virus replicates in cancer cells only and marks them with green fluorescence protein (GFP). However, in a breast cancer clinical trials, there are significant discrepancies between CellSearch detection and TelomeScan detection. A probable cause of these differences may be that TelomeScan may more effectively detect epithelial to mesenchymal transition (EMT) or EpCAM negative tumor cells, but also detect hematopoietic stem cells for false-positive results (Kim et al., 2011).
Another method that reportedly overcomes limitations of cell size heterogeneity involves generating nanoroughess on glass surfaces via reactive ion etching (RIE). RIE is then combined with photolithography to make specific patterns on the glass surface that favor CTC attachment to normal cell attachment on the basis of adhesion preferences (Chen et al., 2012b). This is primarily due to focal adhesion or Fanconi Anemia (FA) density. FA protein is responsible for DNA damage repair (Nalepa et al., 2013). Although nanorough glass surfaces show adhesion towards the cancer cells, these surfaces provide low CTC capture purity due to significant nonspecific binding of other blood cells.
2.3 Immunobead Assays
The most common methods of CTC enrichment involve immunobead assays and microdevices. Immunobead assays use either positive selection to target tumor-associated biomarkers or negative selection to remove blood cells with common leukocyte biomarkers. Epithelial cell adhesion molecules (EpCAM) are often used in positive selection, and CD45 for negative selection. Magnetic beads functionalized with antibodies specifically attach to these antigens, and cells are subsequently removed by applying a magnetic field. CellSearch, an immunobead assay that uses anti-EpCAM antibodies, is currently the only US FDA approved device for CTC detection in breast, prostate and colorectal cancer (Harouaka et al., 2014; Kim et al., 2012). For this reason, it is commonly used as a standard for other detection and enrichment methods (Beije et al., 2015). Other popular EpCAM targeting assays include MagSweeper (Talasaz et al., 2009), AdnaTest (Zieglschmid et al., 2005), and IsoFlux (Harb et al., 2013). These immunobead assays provide more pure yields, but require longer sample processing times than size-based enrichment methods (Hong and Zu, 2013). Furthermore, CTCs that undergo EMT are EpCAM negative and are not isolated by anti-EpCAM assays. Negative selection is a one possible solution. For negative selection, CD45 depletion is most commonly used and often in-conjunction with other label-independent techniques such as red blood cell lysis. However, these processes may also result in the unintended removal of less conventional CTCs (Lustberg et al., 2012) that express CD45, resulting in underestimates of CTC numbers (Joosse et al., 2015; Lustberg et al., 2012).
2.4 Microdevices and Microfluidic Platforms
Microdevices and microfluidic platforms have revolutionized the healthcare system and found tremendous applications including biological and chemical analysis, fertility analysis, cell sorting, point-of-care diagnosis, infectious disease diagnostics, DNA sequencing, and tissue engineering (Asghar et al., 2015; Asghar et al., 2011a; Asghar et al., 2011b; Asghar et al., 2012a; Asghar et al., 2010; Asghar et al., 2016a; Asghar et al., 2014; Asghar et al., 2016b; Billo et al., 2011; Coarsey et al., 2017; Fennell and Asghar, 2017; Ilyas et al., 2013; Islam et al., 2014; Kanakasabapathy et al., 2017; Rappa et al., 2016; Safavieh et al., 2017; Shafiee et al., 2015; Sher et al., 2017; Vidyala et al., 2011; Yu et al., 2017). These microdevices can be functionalized with various antibody “cocktails” in addition to standard anti-EpCAM proteins, enabling more precise isolation of CTCs. Common biomarkers targeted in CTC isolation include epithelial cell membrane antigens that are relevant to cancer therapy, epithelial growth factor receptor (EGFR) and human epidermal folate binding protein receptor, mucin-1 (MUCI), TROP-2, growth factor receptor 2 (HER2 or EGFR/ERBB2), and mesenchymal stem cell antigens (CD318, N-cadherin and c-MET) (Pecot et al., 2011).
One of the microdevices developed, the CTC-Chip, consists of microposts coated with anti-EpCAM antibodies (McKeown and Sarosi, 2013; Stott et al., 2010). The original chip has now been developed into one with herringbone groove patterns known as the Herringbone (HB) CTC-Chip. With the new design, blood-flow within the channels becomes less streamlined and capture efficiency increases as more CTCs contact antibodies lining the chip’s inner walls. Though cell viability after CTC-Chip and HB-Chip is often compromised, the HB-Chip provides more comprehensive data, primarily through its capture of CTC clusters, and may become important tool for studying tumor metastasis (Nagrath et al., 2007; Sarioglu et al., 2015). A new Ephesia CTC-Chip combines immunobead technology with microfluidics. The beads self-assemble onto a series of magnetic traps for rapid isolation and enumeration of CTCs at 90–94% capture efficiencies and non-specific capture rates below 0.4%. Current challenges involve increasing the processing capacity of larger sample volumes (Alix-Panabières et al., 2012; Karabacak et al., 2014; Saliba et al., 2010).
The CTC iChip is another example of successful isolation technology combinations. It integrates size-based enrichment with either EpCAM-based positive enrichment or CD45 negative depletion with 97% capture yield and 8 mL/h processing rates (Karabacak et al., 2014; Ozkumur et al., 2013). The size-based enrichment process removes unnucleated cells using a micropost array, and antibody-mediated white blood cell (WBC) removal ultimately yields label free, viable CTCs (Karabacak et al., 2014; Ozkumur et al., 2013). This technology is limited to isolation of single cells and 2–4 cell clusters, not tumor microemboli. A device specific to CTC cluster capture, known as Cluster Chip, has recently been developed to account for this issue (Sarioglu et al., 2015). CTC clusters enter microfluidic pores and are trapped by stacked rows of triangular pillars, while single cells flow freely. The clusters are then released via flow reversal. The device’s flow rates are extremely slow compared to size-based filters and help to protect cell viability. However, capture yield is low compared to the CTC-Chip (Sarioglu et al., 2015).
Another approach uses standing acoustic-wave fields inside microchannels to capture CTCs based on various physical properties, including size, density, and compressibility. In this particular method, an array of pressure nodes and antinodes is maintained inside a microfluidic channel at a tilted angel to the direction in which fluid flows. In this way, all cells experience different acoustic radiation forces that result in varying movement trajectories, ultimately separating the cells. This method efficiently isolates CTCs (with the recovery rate of 83–90% which is obtained with high input and attachment of polydimethylsiloxane (PDMS) microfluidic channel to the substrate for diminishing the removal of WBCs) without damaging the integrity, functionality and viability of the cells (Li, P. et al., 2015). Magnetic levitation is also investigated to isolate cancer cells based on their specific densities compared to other blood cells (Durmus et al., 2015). MagDense platform is developed which levitates lung, breast, colorectal, and esophageal carcinoma cells at different heights compared to other blood cells as cancers cells shows lower densities (Durmus et al., 2015). Further studies are needed to investigate the CTC capture efficiency and specificity of this approach from clinical samples.
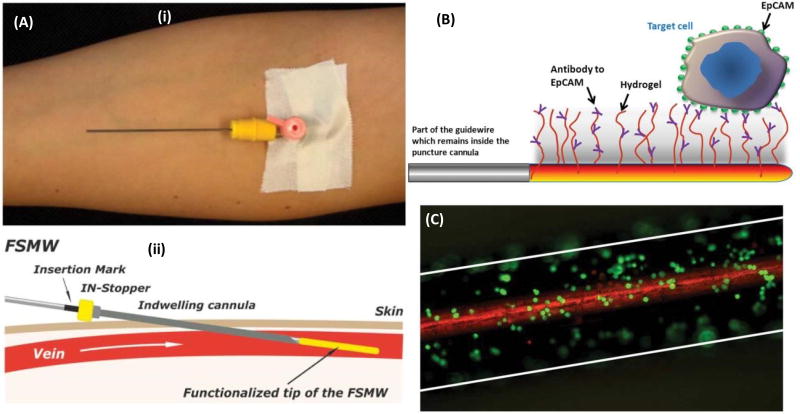
All the aforementioned technologies have been designed for cell capture ex vivo. However, a new technology, known as the GILUPI GmbH CellCollector, applies an anti-EpCAM wire directly into the peripheral arm vein and captures CTCs with remarkable efficiency, processing approximately 1.5 L of blood in 30 minutes (Figure 2) (Saucedo-Zeni et al., 2012). For testing 24 cancer patients (non-small cell lung cancer and breast cancer) were examined, in which 22 out of 24 were detected with CTC with the detection rate of 91.6% (Saucedo-Zeni et al., 2012). Though more studies need to be done to verify its clinical applicability, the nanodetector has shown no evident side effects in past applications (Alix-Panabières and Pantel, 2013). The ability to analyze such large blood volumes increases the device’s sensitivity and makes it a promising candidate for future CTC studies. Additional uses include for detection of fetal trophoblast cells in pregnant women (Krebs et al., 2014; Luecke et al., 2015). Advantages and limitations of various CTC isolation and detection technologies are summarized in Table 1.
Figure 2.
(A) (i) Insertion of the functionalized structured medical Seldinger guidewire (FSMW) into the vein through a conventional cylindrical (instrument is also known as GILUPI GmbH CellCollector). (ii) Wire (anti-EpCAM-antibody-functionalized) is slowly inserted inside the cannula till surface is in contact with the blood flow in vein lumen. (B) Seldinger guidewire-Gold-plated with a size of 200 nm- coated with polycarboxylate hydrogel (functionalized with anti-EpCAM-antibodies) which capture circulating cells expressing EpCAM antigen on surface. (C) Image illustrating breast cancer cells (SK-BR-3) captured by functionalized guidewire. Reprinted with permissions from (Saucedo-Zeni et al., 2012).
Table 1.
List of CTC isolation methods and techniques with their advantages and limitations.
| Name | Basic Properties | Advantages | Limitations |
|---|---|---|---|
|
| |||
| Acoustophoresis (Deshmukh et al., 2014; Gossett et al., 2010; Li, P. et al., 2015). | Cells are suspended in fluid and are exposed to ultrasound waves and pressure amplitude | The strength of the acoustic radiation force is dependent upon the volume of the particle, the density of both the particle and the fluid | With direct pressure source, any instability in the pressure source can cause deviation in the intended flow lines |
| This results in an acoustic radiation force | Isolation factors include the size of the particle and its compressibility | ||
| The radiation force will then push particles towards the pressure anode or pressure anti-anode, ultimately separating the particles | |||
|
| |||
| AdnaTest (Andreopoulou et al., 2012; Müller et al., 2012; Zieglschmid et al., 2005) | Separation by way of anti-EpCAM and anti-MUC1 antibody-targeting immunomagnetic beads | The variety of selection markers (antibodies) allows for the possibility of characterizing cells for multiple markers, all simultaneously | Possible false-positive finding due to expression of a selection marker being present in other cells other than CTCs, such as with nucleic acid contamination. |
| CTCs are then detected via RT-PCR assay for tumor-associated transcripts | Antibody cocktails are specific to a certain cancer type | CTCs that undergo EMT are EpCAM negative | |
| Cells are not viable after detection | |||
|
| |||
| CAM assay (Lu et al., 2010; Monteiro-Riviere et al., 2009) | Used as a functional cell separation method based on CTC invasiveness compared to other cells. | High sensitivity and specificity, leading to effective enrichment and identification based on CTC invasiveness. | Isolation step requires more than 12 hours |
| CTCs can be identified using the CAM uptake criteria. | Downstream analysis is possible. | Biomarker dependent | |
|
| |||
| CellSearch (Attard et al., 2009; Beije et al., 2015; Harouaka et al., 2014; Hong and Zu, 2013; Kim et al., 2012; Swennenhuis et al., 2009) | Separation by anti-EpCAM targeting immunomagnetic beads | The only FDA-approved blood test for the use in patient care diagnostics, used for metastatic breast, prostate, are colorectal cancer | CTCs expressing low levels of EpCAM are unlikely to be captured |
| Cells captured appear to be more apoptotic | |||
|
| |||
| CTC-Chip (McKeown and Sarosi, 2013; Nagrath et al., 2007) | Unique microfluidic approach | High sensitivity and specificity | Long blood processing time (1.67mL/hr) |
| Use of antibody (anti-EpCAM) microposts | 99% success rate in identifying CTCs in the peripheral blood of metastatic pancreatic, prostate, colon cancer, lung, and breast patients | Cells no longer viable | |
| Controlled laminar flow conditions | |||
|
| |||
| CTC Cluster-Chip (Sarioglu et al., 2015) | Utilizes bifurcating traps to capture CTCs, release via flow reversal | CTC clusters can be isolated | CTC clusters were identified in only 30–40% of patients suffering from metastatic breast, prostate, and melanoma cancers |
| Sensitive enough to capture CTC clusters as small as 2 cells | Identification of CTCs is lower versus the CTC-Chip | ||
| Does not require the use of tumor specific markers for isolation | |||
| Low flow rates preserve cell viability | |||
|
| |||
| CTC-iChip (Karabacak et al., 2014; Ozkumur et al., 2013) (Bioengineering, 2014) | Size-based enrichment with either EpCAM-based positive enrichment or CD45 negative depletion | High throughput | Limited to single or small 2–4 cell clusters, not tumor microemboli |
| Microfluidic design for rapid sorting | Fast processing time | ||
| Magnetically labeled target cells are sorted out | More sensitive in detecting low levels of CTCs, versus commercial technology | ||
| Inertial based capture platform | Capacity to isolate CTCs by methods either dependent or independent of tumor membrane epitopes, making this method applicable to all cancers | ||
|
| |||
| Dielectrophoresis (DEP) (Gascoyne et al., 2009; Gossett et al., 2010; Zieglschmid et al., 2005) | Separation can be positive or negative, which will affect the position of the cell in the field | Can be used for selective isolation of cells | Requires specific parameters such as cell type and electric field frequency. |
| Cells are placed in a non-uniform electric field, the field then imparts a net force on the cell due to an induced or permanent dipole | Either AC or DC currents may be used | DC current can result in a electrochemical reaction on the electrode surface, causing a reduction in convective flow. This reduction can cause cell isolation to be inhibited, as well as free radical generation that results in cellular damage. | |
| Isolation based on polarizability and size | |||
|
| |||
| DynaBeads (Hardingham et al., 1993) | Magnetic separation and isolation based on binding to desired target and beads responding to magnetic field | Can be pre-coupled with biomolecules with an affinity for a desired target, such as: antibodies, proteins, antigens, or DNA/RNA probes | Only 3 types of DynaBeads are available for human tumor cell isolation |
|
| |||
| Ephesia CTC Chip (Alix-Panabières et al., 2012; Karabacak et al., 2014; Saliba et al., 2010) | Isolation by way of immunomagnetic sorting coupled with microfluidics | Capture efficiency range of 90–94% | Currently has not been formatted to accommodate large volumes of blood |
| Sample flows across immobilized magnetic beads | Ephesia technology can allow for a more flexible platform to perform advanced cell biology testing on cancer cells | ||
| Required sample volumes are one-tenth those of flow cytometry | |||
|
| |||
| EPISPOT (Alix-Panabières, 2012; Kalyuzhny, 2005; Millner et al., 2013) | Removes leukocytes via CD45 depletion and during short-term cell cultures, detects specific marker proteins shed/ secreted/ released from single epithelial cancer cells | This assay creates a new way to detect viable CTCs and DTCs (disseminated tumor cells) | Requires efficient antigen binding and specific epitope presentation |
| An adaptation of the enzyme-linked immunospot (ELISPOT) technology | With an extension of a multi-parameter analysis, this could reveal the CTC/DTC protein fingerprint | Demands high antigen levels | |
| Transition into in vitro cultures may decrease cell viability and reduce detection rates | |||
|
| |||
| GILUPI CellCollector Nanodetector (Alix-Panabières and Pantel, 2013; Krebs et al., 2014; Luecke et al., 2015; Maheswaran et al., 2008; Nagrath et al., 2007; Saucedo-Zeni et al., 2012) | Ex vivo (FSMW) Functionalized Structured Medical Wire is antibody (anti-EpCAM) coated and applied into peripheral arm vein | Overcomes sample blood volume limitations | Only used for extraction of CTCs directly from patient’s bloodstream, not for use of extraction in blood sample |
| Isolation occurs in vivo | Increases diagnostic sensitivity of CTC isolation | More studies need to be done to verify clinical applicability | |
| No evident side effects | |||
|
| |||
| Herringbone CTC-Chip (Sarioglu et al., 2015; Stott et al., 2010; Wang et al., 2016) | An advanced CTC-chip utilizing a strategy of including herringbones or surface ridges in the walls of the device to disrupt streamlines and encourage collisions between antibody-coated walls and CTCs | Future goal is to be used for large scale clinical applications Successfully isolated 93% CTCs from patients with prostate cancer | It does not consider the inherent particulate properties of cells. |
| Captures CTC clusters | Cells are no longer viable | ||
|
| |||
| ISET (De Giorgi et al., 2010; Joosse et al., 2015; Vona et al., 2004; Zheng et al., 2011) | Utilizes a filter-based, size exclusion approach to isolate epithelial cells | High throughput | CTCs can be damaged or fragmented due to multi-step cell processes |
| Downstream morphological studies can be performed | CTC heterogeneity regarding morphology and size | ||
|
| |||
| IsoFlux Rare Cell Access System (Harb et al., 2013) | Uses next generation sequencing (NGS), with quantitative polymerase chain reactions (qPCR), fluorescence in situ hybridization (FISH), and immunofluorescence | High sensitivity of CTCs from many tumor types | Maximum daily analysis is 12 samples |
| Uses flow control and immunomagnetic capture to increase CTC isolation | The variety of selection markers (antibodies) allows for the possibility of characterizing cells for multiple markers, all simultaneously | CTC may have biomarker heterogeneity Those that undergo EMT are EpCAM negative | |
| Multiple kits for lab usage are available, both for cell enrichment, and downstream analysis | |||
|
| |||
| MagSweeper (Ao et al., 2016; Powell et al., 2012; Talasaz et al., 2009) | Separation by way of anti-EpCAM antibody-targeting immunomagnetic beads | Isolates CTCs without contaminating leukocytes | The CTCs possessing low or no EpCAM expression will be over-looked (CTCs that undergo EMT are EpCAM negative) |
| The variety of selection markers (antibodies) allows for the possibility of characterizing cells for multiple markers, all simultaneously | |||
|
| |||
| MagDense (Durmus et al., 2015) | Magnetic levitation based on specific densities of CTCs | Platform enables single-cell density measurements and imaging | Further studies are required for capture efficiency and specificity for CTCs |
|
| |||
| Metacell Filtration (Dolfus et al., 2015) | Size based separation technique driven by capillary-action | Filtration techniques are sensitive enough to allow cytomorphological and immunocytochemical analysis of CTCs | Filters have a larger pore size (8µm) versus ScreenCellCyto (6.5µm) |
| Commercial device used for isolation of CTCs, allowing for cytological identification | |||
|
| |||
| Microfluidic Silicon Chip (Sequist et al., 2009) | Antibody based | Antibody coated microposts maximize CTC-antibody interactions | Further CTC separation from microfluidic device is challenging |
|
| |||
| NanoVelcro Microfluidic Device (Zhe et al., 2011) | Microfluidic device uses tiny rods coated with antibodies | This is a more advanced prototype that adds a new transparent substrate for isolating CTCs | Only EpCAM-positive CTCs are detected |
| After blood passes through, a laser is used to extract CTCs | |||
| Uses 3 color immunofluorescence for detection of CTCs | |||
|
| |||
| Negative Enrichment QMS (Ignatiadis and Reinholz, 2011; Jing et al., 2007) | A negative selection technique for cell enrichment followed by separation by Quadrupole Magnetic Flow Sorter (QMS) | Improved sample yield and purity | CTC expressing CD-45 maybe inadvertently remove from the sample |
| High throughput and cost effective | Contamination with WBC’s might result in unintentional loss of CTCs | ||
|
| |||
| OncoQuick® (Rosenberg, R et al., 2002; Rosenberg, R. et al., 2002) | Polypropylene tube is inserted above the separation medium | High throughput, inexpensive | Loss of sample while depleting mononuclear cells |
| The medium allows for elimination of erythrocytes, granulocytes, lymphocytes, and mononuclear cells | Increases sensitivity and specificity for tumor cells | Detection depends upon only cytokeratine-20 biomarker | |
| Significant reduction in co-enriched number of mononuclear cells, with a high CTC recovery rate | |||
|
| |||
| Parsortrix (Joosse et al., 2015; Xu et al., 2015) | Isolation of CTCs based on size and compressibility | Purity of CTCs harvested is 3.1% | CTC heterogeneity regarding size |
| Ability to capture CTC clusters | |||
| Harvests CTCs with both epithelial and mesenchymal features | |||
| Uses cassette device to for easy detachment | |||
|
| |||
| pluriSelect (Nadezhda Frolova, 2012) | Antibody based CTC separation pluriSelect uses pluriBead® carrying a tumor-associated anti-EpCAM antibody | Non-magnetic cell separation, can be added directly to a whole blood sample | Currently limited for use in colon carcinoma diagnostics |
|
| |||
| Reactive Ion Etching (RIE) (Chen et al., 2012a; Nalepa et al., 2013) | Adhesion of the CTCs on the nano-roughsurface regardless of size and without using any capture antibody. | The capture efficiency is up to 80% within one hour of cell incubation | Suboptima l capture purity |
| Does not require EpCAM expression for the cell capture | |||
|
| |||
| RoboSep/EasySep (Wang et al., 2013; Wilcox et al., 2009) | Magnetically label and separate cells by positive or negative selection | High throughput | Rely on biomarkers for capturing the cells |
| Fully automated instrument, reducing possibilities of cross contamination | |||
| Design for a customized cell separation protocol can be created by stem cell technologies | |||
|
| |||
| ScreenCellCyto (Dolfus et al., 2015; Wang et al., 2013) | Size-exclusion based isolation method | In addition to ScreenCell devices being used for isolation of CTCs, they are also used for isolation of Circulating Fetal Cells (CFCs) from maternal peripheral blood for prenatal diagnostics | Poor specificity |
| Peripheral blood sample is mixed with filtration buffer | Filters in only 3 minutes | Unable to capture CTCs smaller than WBCs | |
| Diluted sample is then filtered by aspiration created by a vacuum tube collector | Not dependent upon EpCAM | ||
| Disposable | |||
|
| |||
| TelomeScan (Kim et al., 2011; Kojima et al., 2009) | Detects elevated telomerase activity via a telomerase-specific replication selective adenovirus | May more effectively detect epithelial to mesenchymal transition (EMT) or EpCAM negative tumor cells | May also detect hematopoietic stem cells for false-positive results |
3. CTC Detachment from Surfaces
Another important challenge that still has not been overcome for many isolation techniques is to effectively release CTCs from the substrate without impacting the cells. Detachment from filters, immunoaffinity chips, and other substrates require the removal of receptor-ligand interactions and/or focal adhesions (Zheng et al., 2013). Release methods using sheer stress may reduce viability and influence function of these fragile cells (Albuquerque et al., 2000; Born et al., 1992; Chowdhury et al., 2010). Therefore, less invasive methods of detachment have been developed for cancer cells, using temperature, light, electrodes, and aptamers. In this paper, we will discuss the three common methods of detachment technology specific to CTCs, using enzymatic digestion, aptamers, and pH-responsive polymers.
3.1 Enzymatic digestion
Employing enzymes to digest the extracellular matrix and detach cells is the standard method of cell release (Zheng et al., 2013). However, this method is known to degrade other cells’ membrane proteins and cell-to-cell junction proteins (Keizer et al., 1988). Still, there are several accounts of successful enzymatic release of CTCs (Adams et al., 2008; Dharmasiri et al., 2009). CTC release efficiency of approximately 100% is found in antibody-functionalized microfluidic devices using 0.25% w/w trypsin, though effects on cellular function have yet to be thoroughly analyzed in these studies (Adams et al., 2008; Dharmasiri et al., 2009). Another nanofilm developed for use in microdevices provides 95% release efficiency with 90% cell viability. Briefly, layer-by-layer (LbL) assembly was used to form layers of anionic and cationic polymers, and alginate lyase for enzymatic degradation of the nanofilms. The released cells were then successfully cultured for 5 days afterwards, providing functional evidence of viability (Li, W. et al., 2015). Similarly, nucleases can be used to detach cells from aptamer-functionalized substrates. Endonuclease treatment using BamHI at 37°C for 30 min detached CTCs from polyacrylamide hydrogels at approximately 99% efficiency and at a faster rate than trypsin, with little damage to cell receptors (Li et al., 2013). Nevertheless, further studies must be done analyzing cell viability and function to investigate if enzymatic degradation is a suitable method for downstream CTC cultures.
3.2 Aptamers for CTC Detachment
Aptamers are newly emerged powerful tool to study CTCs (Dickey and Giangrande, 2016). These are single stranded oligonucleotides (RNA or DNA) of small MW (8–15 KDa) with some characteristics typically not found in antibodies (Chen et al., 2016). The advantages to using aptamers for CTC isolation is that they provide a high stability resistance to a spectrum of harsh conditions (urea, organic solvents, denaturation), have negligible toxicity and immunogenicity and oriented surface immobilization, thus offering a very efficient and non-invasive detachment method. These features allow for tumor cell penetration and blood clearance, not typical for antibodies. Furthermore, aptamers can be developed against the binding targets in the range between small compounds to large cell membrane or transmembrane proteins on the CTCs. However, there is an evidence that aptamers are unqiue in that changes in environmental conditions such as temperature or pH can alter their three dimensional confirmations and cause them to lose their affinity to the target molecule (Zheng et al., 2013). Precautions must be taken, however, when using temperature to alter affinity binding, as temperatures around 45–53°C can harm tumor cell functions in vitro (Walsh et al., 2007).
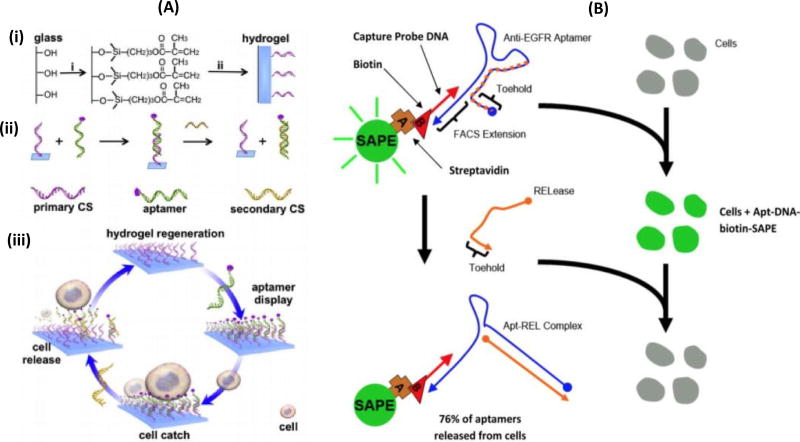
As an alternative to temperature and pH-mediated aptamer release, complementary oligonucleotide sequences can be designed to preferentially bind to aptamers and produce changes in aptamer conformation (Figure 3). In one study, researchers effectively released CTCs from anti-EGFR aptamers attached to glass beads using an oligonucleotide known as RELease (Figure 3B). Cells, when bind with aptamers in the presence of RELease molecules, produces fluorescence because of the selective binding. This opens the hairpin structure and release 76% of the aptamers from cellular surface. About 92% of cells were released using this technique, compared to only 69% of cells via soft resuspension (Wan et al., 2012). In another report, researchers were able to recover about 95% of cells in 10 min using this method, while maintaining 99% cell viability (Zhang et al., 2012) (Figure 3A).
Figure 3.
(A) Aptamer-mediated CTC capture and release via complementary oligonucleotide sequences (CS= complementary sequences) (Zhang et al., 2012). (i) explain about the basic structure of the of the hydrogel immobilized on the glass slide (ii) illustration of the aptamer sequence hybridized with complementary sequences (CSs) (iii) represents the process of cell release. The stable aptamer hybridized with triggering CSs, forming a new hybridized state that leads to rapid dissociation of the aptamer from the hydrogel hence releasing the cells from the hydrogel. (B) Illustrating the cell binding and aptamer formed by SAPE of anti-EGFR aptamer – DNA and biotin, and addition of RELease particles. After washing, the fluorescence cells (caused by selective binding with aptamer) completely hybridizes with anti-EGFR aptamer because of the presences of RELease RNA. This leads to opening up its herpin sturucture which releases 76% of anti-EGFR aptamer from the cellular surface. (Wan et al., 2012)
3.3 Polymers for CTC Detachment
Similar to aptamers, polymers can be designed to respond to changes in external conditions by reversibly changing their conformation via dissolution or deformation (de las Heras Alarcón et al., 2005). The pH-responsive polymers are synthesized by linking structures with weakly acidic and basic functional groups to a hydrophobic base. Once the groups become ionized, groups of similar charges cause repulsions and the polymer expands. Conversely, groups can lose their charges and cause the polymer to contract. Ionization of polymers can also directly affect affinity to extracellular matrix (ECM) proteins, as these are negatively charged under physiological conditions (Bajpai et al., 2008). For example, chitosan is a weak base and its functional groups are deprotonated under strongly basic environments (pH > 7.4), resulting in a negatively charged surface from which ECM proteins dissociate (Yeh and Lin, 2008). Using this method and a pH of 7.65, 90% of CTCs were detached in approximately 1 hour, while viability was preserved at 95% (Chen et al., 2012b). When designing pH-responsive polymers, pH values must be regulated carefully, since proton or hydroxyl group localization may pose a damage to cell viability or influence cell functions. These polymers should also allow high binding specificity and efficiency during preliminary detection and capture techniques (Phillips et al., 2008).
A nanostructured coating was also introduced that can be released from Herringbone CTC Chips via temperature, and can be used for bulk detachment, mechanical stimulation, and for single cell detachment. Gelatin and streptavidin are deposited in alternate layers using LbL assembly, with polystyrene nanoparticles coated with streptavidin covering 22% of the gelatin outer layer. The final product can respond to stimuli at even 135 nm thicknesses, allowing the nanocoating to be used in microdevices. Disturbing cell function is not a concern when using temperature stimulation because the nanocoating dissolves at only 37°C. Cell viability and recovery were 88.3% and 93.2%, respectively, using this method. Alternatively, a frequency-controlled microtip can dissolve 145–215 µm regions of the nanocoating using 15–30 Hz vibrations, releasing cells with 91.5% viability. All cells were then cultured and grew in confluent colonies after 7 days (Reátegui et al., 2015). However, the effects of mechanical vibrations on cancer cells are not yet clear.
In summary, current technologies that hold the great potential for CTC detachment include oligonucleotide-mediated aptamer release, and stimuli-responsive polymers. Further considerations for developing new release methods should involve biocompatibility, point-of-care integration, detachment efficiency, cell viability, and compatibility with microdevices.
4. Culturing CTCs
For therapies that target tumor metastasis and can be tailored to patient-specific tumor characteristics, the culturing of CTCs is an essential step in drug discovery. MetaCell filtration device is a capillary action driven, size-based separation method that isolates and enriches viable CTCs from peripheral blood samples using a porous polycarbonate membrane. CTCs isolated using this filtration method are available for downstream analysis because the cells remain healthy and unharmed, as this method does not require any lysing solution or targeting antibodies for CTC isolation (Cegan et al., 2014; Kolostova et al., 2014a; Kolostova et al., 2015a; Kolostova et al., 2015b; Kolostova et al., 2014b). In a study performed on prostate cancer patients, CTCs were isolated and proliferated in vitro at a 64.3% success rate (18 out of 28 CTC-positive peripheral blood samples) (Kolostova et al., 2014a). CTCs were kept viable for 14–28 days by transferring the membrane filter to a cultivation plate and culturing cells directly on the membrane using FBS-enriched RPMI medium (10%), under normal cancer cell incubation environments (37°C, 5% CO2). CTCs were also cultured directly on the well surface after 14 days of membrane culture. It was observed that CTCs under culture conditions become larger, paler, and more elongated. The morphologies of those CTCs growing on the membrane resembled epithelial cells and conglomerating stem cells, while the more prominent nucleoli of the CTCs growing on the well bottom suggested greater plasticity and invasiveness than those growing on the membrane (Kolostova et al., 2014a). Similar methods were used to successfully culture CTCs isolated from lung cancer orthotopic mouse models (Kolostova et al., 2014b), ovarian, cervical, and endometrial cancer (Kolostova et al., 2015b), gastric cancer (Kolostova et al., 2015a), urinary bladder cancer (Cegan et al., 2014), and esophageal cancer (Kolostova et al., 2014b).
It has been reported that other CTC isolation methods such as conventional CTC-Chip, CTC-iChip, MagSweeper and EPISPOT can also serve as effective isolation methods for subsequent CTC culture (Alix-Panabières, 2012; Alix-Panabières et al., 2007; Ameri et al., 2010; Helzer et al., 2009; Ozkumur et al., 2013; Yu et al., 2014). Using orthotopic murine models derived from prostate cancer cell lines, CTCs were collected and cultured on a plastic CTC-chip. After 5 days, cells began forming individual colonies, and by the 12th day of culture, colonies were expanding with less than 1% cell death (Helzer et al., 2009). Current efforts are being made to investigate extracellular matrix microarrays that enable testing of cell lines against a multitude of conditions. Studies have already used these microarrays for isolation and culturing of CTCs captured from mice engrafted with primary human pancreatic tumors (Gach et al., 2014).
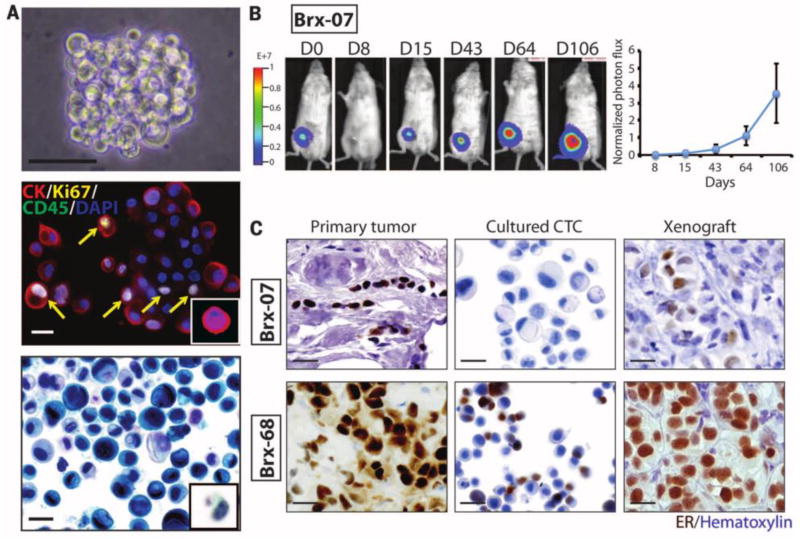
Though successfully done by very few studies, ex vivo cultures of CTCs may produce more accurate and sustainable disease models than the previously introduced adherent membrane cultures and can be used to form CTC-derived tumors. In one study, researchers conducted xenograft mouse models studies with injected human cancer cell lines. Isolated CTCs were then successfully cultured ex vivo under hypoxic conditions and injected into other mice to form CTC-derived tumors. Hypoxic atmosphere is known to cause increase in tumor metastasis and even larger and more aggressive xenografts (Ameri et al., 2010; Gilkes et al., 2014; Spill et al., 2016). The resulting CTC-derived tumors were larger and metastasized more aggressively than the original cell line tumors, ultimately demonstrating the stem-cell like properties of CTCs (Ameri et al., 2010). However, careful considerations must be taken when using xenograft mouse models established with human cancer cell lines. Metastasis may occur late or even not at all. Isolation of human patient CTCs for direct ex-vivo culture may instead provide more accurate timelines for metastasis (Yu et al., 2011). In a study, investigators have demonstrated to form xenograft mouse models by implanting patient-derived CTC cell lines (Figure 4) (Yu et al., 2014). Scientists used various techniques to optimize CTC culture proliferation ex vivo, including hypoxic conditions and nonadherant cultures. CTCs captured from estrogen receptor-positive (ER+) breast cancer samples, using the CTC-iChip, were cultured and yielded a proliferative index of approximately 30% (range 24 to 32%). After testing four culture protocols, cells were found to grow at best as tumor spheres under 4% O2, with serum-fee media, basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF). CTC cultures grouped together while separating themselves from the cancer cell lines and individual, uncultured CTCs (Yu et al., 2014). Scientists also observed that in adherent monolayer culture, the CTCs were unable to survive after several cell divisions and therefore stressed the importance of nonadherent cultures (Yu et al., 2014). This is likely due to their similarity to undifferentiated human mammary epithelial cells (HMECs), which proliferate as nonadherent mammospheres (Dontu et al., 2003).
Figure 4.
Ex-vivo CTC (breast cancer) culture (A) Nonadherent CTC cultures (B) mouse xenografts derived from cultured CTCs implanted in mammary fat pad. (C) Orignal breast tumors and corresponding CTCs culture and two different CTC lines derived from mouse xenografts. (Yu et al., 2014)
When preparing CTC cell lines using peripheral blood samples, it is also important to consider the patients’ treatment status [9]. It is reported that there is a higher likelihood of culturing success with increased tumor burden. CTC cell lines were created successfully from 6 out of the 36 patients, and 9 of the failed attempts were responding to breast cancer treatment. Cell lines from several different treatment time points were also generated for three patients, and in those cases, CTC cell line generation was unsuccessful during the early stages of treatment [9]. Downstream cytological analysis introduced many possibilities for CTC cell line studies. Cultured CTCs were very similar to captured CTCs and were used for various drug screenings, incorporating functional testing and genotyping into the drug susceptibility predictions. The CTC-derived tumors were immunohistochemically and histologically similar to the primary tumor. The study did not, however, detect increased expression of pathways related to stem cell activity in CTC cultures when compared with cancer cell lines.
Future studies should be done characterizing the effects of various isolation techniques on CTC morphology and function before these techniques can be confidently used for clinical analyses and CTC culture. There is a great need to optimize conditions for CTC culture and better understand differences between nonadherent CTC cell lines and adherent cell lines, CTC cultures and patient samples or murine models. With a better understanding, all of these technologies have potential for patient-specific drug susceptibility testing and mutational profiles for cancer patients in the future.
5. Detection and Downstream Analysis
CTCs can be detected after enrichment via fluorescence in situ hybridization (FISH) for genome amplification, immunocytochemistry (ICC) for protein markers, and RT-PCR/qRT-PCR for quantifying specific RNA and DNA sequences. ICC is used in majority of the enrichment techniques, and there are a variety of ways that cells are characterized as CTCs. CellSearch requires the cell to be greater than 4µm in diameter, negative for CD45 markers, and positive for cytokeratin (CK) protein marker (form intermediate filaments in epithelial cells) and 4,6-diamino-2-phenylindole (DAPI) nuclear dye (Joosse et al., 2015). Additional labels can be used for therapy-relevant markers, such as prostate-specific antigen (PSA), androgen receptor (AR) (Gasch et al., 2013; Riethdorf et al., 2007), asCK, E-cadherin, vimentin (VIM), N-cadherin (CDH2), and CD133 (a stem cell marker) EMT-associated biomarkers (Armstrong et al., 2011). ICC can also be coupled with size-based and microfluidic isolation techniques, as well as the GILUPI GmbH CellCollector (Maheswaran et al., 2008; Nagrath et al., 2007; Saucedo-Zeni et al., 2012).
Targeting mRNA transcription factors with qRT-PCR offers a highly specific detection method that can be coupled with various isolation techniques, given properly designed primers and target gene selection (Joosse et al., 2015). However, challenges are faced when quantifying gene expression in unpurified samples, leading to false-negatives and false-positives (Pantel et al., 2008; Paterlini-Brechot and Benali, 2007). This is also a hurdle for FISH analyses (Attard et al., 2009). Cells captured using CellSearch appear to be more apoptotic and less likely to generate FISH signals (Attard et al., 2009; Swennenhuis et al., 2009). However, combinations of target genes, biomarkers, and protocols show promise in overcoming these numerous obstacles (Attard et al., 2009; Swennenhuis et al., 2009).
Other molecular profiling methods focus on the genomic identities of single CTCs. Singe cell molecular profiling requires highly pure samples and whole genome amplification (WGA) (Krebs et al., 2014; Punnoose et al., 2012). After amplification, cells can be genotyped using comparative genome hybridization (CGH), Sanger sequencing or next-generation sequencing (NGS) for each single cell (Voet et al., 2013). These techniques have already been performed to analyze genetic changes over an entire cell cycle and for comparing point mutations in CTCs to primary tumors (De Roock et al., 2010; Heitzer et al., 2013; Voet et al., 2013). However, these techniques should be further refined. The number of cells needed for accurate representations of the cancer should be determined (Parkinson et al., 2012). WGA can cause genetic alterations that may be accounted for by using other CTC samples and leukocytes as controls for detecting mutation patterns in singular CTCs (Dean et al., 2002; Krebs et al., 2014).
In addition to sequencing studies, gene-expression analysis must be performed to quantify the phenotypic effects of various genetic and epigenetic modifications. However, the delicate nature of RNA samples compared to DNA create significant challenges, and determining the relevant changes in expression is difficult among the signals produced by other cell processes and experimental procedures (Asare et al., 2008; Peeters et al., 2013). Techniques like smart-seq, next generation sequencing have been developed to better detect highly expressed RNA transcripts in CTCs derived from melanoma and prostate cancer patients (Cann et al., 2012; Ramsköld et al., 2012; Tang et al., 2009). Most CTC analysis techniques are still at a rudimental stage and are very costly, the area, which needs further development.
6. Metastasis and Treatment Response
6.1 Diagnosis and Monitoring
As motioned earlier, CTC as noninvasive “Liquid biopsy” is a real-time possibility to monitoring malignancies. It has significant advantage over the conventional solid biopsy (Lianidou, Evi S, 2014). In liquid biology, certain amount of blood (∼7.5 ml, in the case of CellSearch method) is required to detect CTCs whereas solid biopsy involves invasion inside the tissues. Moreover, the sample collection in cancers like osteoblastic metastasis in prostate cancer is challenging for solid biopsy and does not provide information about the tumor genome evolution over the time and its spatially heterogeneous nature (Chen et al., 2016; Lianidou, Evi S., 2014). CTCs as liquid biopsy can revolutionize the cancer diagnosis and monitoring in the patients (Alix-Panabières and Pantel, 2013). A study carried out on pancreatic ductal adenocarcinoma (PDAC) patient revealed that CTCs helped in diagnosis and staging of the disease progression. CTCs were detected in 54 out of 72 patients with the use of 1st gen NanoVelcro CTC chip, which confirmed the presence of PDAC. In addition, investigators reported that the presence of >3 CTCs in 4ml of blood helped in discriminating between local/regional and metastatic stages in the patients (Ankeny et al., 2016).
The cellular morphology and nuclear size of CTCs can reveal disease progression. A team collected the blood samples from patients at the different stages of prostate cancer from localized to advance metastatic castration-resistant disease. They identified 3 different subsets of the CTCs ranging from size < 8.54 µm- vsnCTCs (very small nuclear CTCs), 8.54 µm- snCTCs (small nuclear CTCs) and >14.99 µm - lnCTCs (large nuclear CTCs). Both vsnCTCs and snCTCs were present in the patients at the metastatic stage of cancer and vsnCTCs occurred higher in number in visceral organs like lungs or liver. Further in this investigation, 28 prostate cancer patients who had progressed through next generation hormonal maneuvers were examined. It was shown that 15 out of 28 were found with visceral lesions and 13 had bone disease, 6 (non-visceral metastatic patients) out of these 13 developed visceral lesions during their follow-up. Within 86–196 days prior to radiographic detection, 4 of the patients were detected with vsnCTC with the absence of visceral lesions at the time of analysis. Investigators observed the reduction of number of vsnCTC after the initiation of the anti-cancer therapy. This is supporting evidence that the CTC morphology reveals disease progression and can also assist in therapeutic inventions (Chen et al., 2015).
6.2 Survival Rate Prediction
CTCs could be an alternative for predicting the feedback of the treatment in cancer patients. Studies have been done to reveal that the quantification of CTCs is useful in predicting the response of the patients towards the drug and overall survival (OS) rate (Miyamoto et al., 2012). To monitor chemotherapy treatment, imaging techniques are often coupled with blood tumor marker levels, but these indicators may take up to several months to show various drug responses (Boss et al., 2008; Mohler et al., 2012). CTC levels in the blood, however, has been shown to signify changes within a few weeks (Hayes and Smerage, 2008; Saad and Abraham, 2008), before the onset of symptoms (Rao et al., 2012), and seem to yield greater accuracy (Budd et al., 2006). Most of these studies focused on CTC enumeration that has proven a strong correlations with OS rate in patients with melanoma (Mocellin et al., 2004), metastatic lung (Hou et al., 2012; Krebs et al., 2011), colorectal (Cohen et al., 2008), prostate (de Bono, Johann S. et al., 2008), and breast cancer (Budd et al., 2006; Cristofanilli et al., 2004; Riethdorf et al., 2007). The CTC count was monitored in patients suffering from mCRPC (metastatic castrate-resistant prostate cancer), revealed that the patient having more than 5 CTCs/7.5 mL in their blood has poor OS rate in comparison with patient having less than 5 CTCs per mL (de Bono, Johann S et al., 2008). Similar pattern was also observed in breast cancer patients which showed that the patient with persistently elevated CTC counts (>5 CTCs/7.5 mL at baseline and during investigation) exhibited significantly worse OS rate (Krebs et al., 2010). In another study, it has been demonstrated that CTC count is directly related to disease severity. A total count of 177 patients were examined with an average age of 58.0±13.4 years (median 58). From the count studies- before the investigation starts, 10 patients died as they had extremely large level of CTCs in their blood (9, 11, 15, 24, 111, 126, 301, 1143, 4648, and 23,618 CTCs/7.5 ml of blood). 49 patients out of 169 patients, had ≥5 CTCs /7.5 ml of blood, posses free survival rate with lower median progress of about 2.1 months and lower median OS of about 8.2 months. Remaining 114 patients had <5 CTCs/ 7.5 ml of blood and had progress free survival rate of about 7 months and overall survival rate of >18 months. In this study, elevated level of CTCs even after treatment investigation represented that the patients received feeble treatment. In summary, CTC count can be helpful in disease prognosis and management, hence patients might get benefit from alternative therapies during treatment (Cristofanilli et al., 2004).
6.3 Therapy Response and Surgery Prediction
CTCs could be useful in analyzing effects of anti-cancer drugs on their therapeutic targets and the analysis of these CTCs can identify the early detection of the resistance to therapy. A study conducted on metastatic breast cancer (MBC), showed CTCs prognostic importance in patients. The patient with the reduced CTCs number after 21 days of therapy was kept on the same therapy whereas the patient with the increase in the number of CTCs after 21 days was subjected to different chemotherapy (Smerage et al., 2014) Another report that used CellSearch to demonstrate the patient outcome, based on CTC count, has shown some interesting results. The trial (S0500 reported in 2014) failed after one cycle of first-line chemotherapy as it showed lack in overall survival in MBC patients. It showed persistant increase in the CTC count after 21 days of first-line chemotherapy (Smerage et al., 2014). This increase in the number of CTCs might be due to ineffective treatment for the patient and a need for change the treatment options or therapeutic drugs based on CTC count during the chemotherapy. These studies reveal that CTCs can provide more accurate prognosis to allow oncologists to get a clear picture of the treatment outcomes. Another study revealed that CTCs could be employed to follow therapy responses and monitoring disease progression (Lu et al., 2013a). A clinical study showed positive response of the therapy on the metastatic prostate cancer patients. 32 metastatic and 8 localized cancer patients diagnosed with CTCs with the help of 1st generation NanoVelcro Assay, were recruited for this study. As the therapy continued, the CTC quantification was performed with serial enumeration, and within 4–10 weeks of treatment statistical reduction in the CTC count was observed. However, in another case, this assay helped in revealing a negative response of the therapy (Lu et al., 2013a).
CTCs can provide new biomarkers that can help to view insight evolution of signaling responses. A study was designed in prostate cancer patients to depict “Androgen receptor (AR) signaling pathways” as a new biomarker to track castration-resistant disease. Androgen deprivation therapy (ADT) is used for the patients with metastatic prostate cancer as an initial response. However, progression of the disease was observed in the patients showing that the tumor cells resume proliferation despite of the continuous treatment (termed castration-resistant prostate cancer or CRPC) (Stott et al., 2010). In most of the cases, prostate metastatic cancer spread to bone that limits the sample collection to monitor the mechanism of drug resistance (Yuan and Balk, 2009). The “second generation” microfluidic chip (Stott et al., 2010) coated against PSA (prostate specific antigen) and PSMA (cell surface protein) (Lee et al., 2002) antibodies was adopted for broad capture of CTCs, followed by CTC characterization for AR signaling status. Results revealed that 4 out of 5 patients were detected with CTCs in metastatic prostate cancer patient before starting the androgen deprivation treatment. In the end of the treatment, it was observed that majority of CTCs have shown transformation “AR-on” (PSA+/PSMA-) to the “AR-off” phenotype within one month, followed by the complete disappearance of CTCs by 3 months after initiation of therapy. These results showed that monitoring of CTCs derived from the cancer patients could be a dynamic biomarker showing the effect of cancer drugs on their therapeutic targets by tracking the activity of the signaling pathway (AR pathway) (Miyamoto et al., 2012).
CTCs could also help in solving critical issue for the men with prostate cancer. It could assist oncologists to gain more information about making the decision for the treatments or surgeries in these patients. The patients with prostate cancer have to face a complicated decision of whether to go for surgery to eradicate prostate, reason being if the cancer has spread beyond the prostate gland, then the chances of potential surgical failure are more. However, scientists have observed that CTC test could be beneficial for surgery prediction. In a study, researchers examined 138 men with prostate cancer who went through surgery to remove prostate (Olsson et al., 1996). These patients were subjected to tests for CTCs presence. The results revealed that the patients with CTC positive were more likely to experience surgical failure compared with CTCs negative patients (Nemeroff, 2010). This shows that prediction and monitoring of CTC levels in cancer patients holds important information about how efficient and effective the treatment or surgery would be.
7. CTCs and Development of Targeted Therapeutics
7.1 CTCs Gene Profiling and Targeted Treatment
Currently, almost all the cancer therapies are based on analysis of primary tumor but these therapies fail to encounter metastatic cells, which is the main reason of metastatic relapse years after primary tumor diagnosis and surgical resection (Heitzer et al., 2013; Lohr et al., 2014; Uhr and Pantel, 2011). Molecular characterization of the CTCs from the metastatic cancer patients could be favorable in evaluating the potential therapeutic targeting and finding the fundamental cause for the abnormal functioning of these cells (Meng et al., 2004; Wan et al., 2013). An extensive study has been carried out in genotypic and phenotypic characterization profiling of CTCs (Fehm et al., 2010; Ignatiadis and Dawson, 2014; Meng et al., 2004). These studies can provide better understanding in context of treatment choice and the discovery of personalized medicine. Presently, the characterization of CTCs has been carried out on different tumor types, that does not limit to breast cancer but also includes prostate and lung cancer types (Jiang et al., 2010; Maheswaran et al., 2008; Miyamoto et al., 2012). A team working on gene profiling of breast cancer patients, aimed to observe heterogeneity and expression pattern of genes in CTCs (Powell et al., 2012). Cell lines (breast cancer) T47D, MCF7, MDA-MB-231, and SKBR3, and both primary as well as metastatic breast cancer samples were obtained for gene analysis. In the circulating tumor gene profiling, gene expression of 510 patients was studied and the cells were isolated by the MagSweeper method. This study was designed to analyze cells of 1700 cases (breast cancer) that indicate three standard genes i.e. GAPDH, ACTB and UBB to establish gene expression and statistical analysis of the results. The total of 31 of the 87 investigated genes were frequently noticeable in 15% of the cells (CTCs) scrutinized. Apart from 3 standard genes, the other 28 overexpressed genes along with few more genes from different studies, listed in Table 2 according to their functional categories. This study revealed that the molecular gene profiling of tumor cells provides a heterogeneous framework of the disease.
Table 2.
Gene categorization/grouping on the basis of functional categories after the evaluation of CTCs
It was analyzed that, not all the CTCs were responsible for seeding metastasis in the patients. Only few CTCs have the capacity to do so. CTCs with missing ER, HER2 or PR expression are more aggressive and have a higher potential of forming metastasis in less time and these tumors have limited targeted treatment options (Sørlie et al., 2001) (Bosch et al., 2010; Korsching et al., 2008). This is the reason for the failure of most of the therapies that target these biomarkers. Since these CTCs lose the expression of ER/PR/HER2 biomarkers in them; hence the therapies targeting these biomarkers fail to encounter these cells. These findings also acknowledged that few genes such as S100A9, S100A4, and NPTN were associated to metastasis and had a striking expression. The genes like ZEB2, TGFβ1, VIM, CXCR4 and FOXC1, are correlated to initiation and conservation of EMT, a process which leads towards the hikes of cell invasion by changing the epithelial cells to mesenchymal cells, biologically and morphologically (Barcellos-Hoff and Akhurst, 2009; Bertran et al., 2009; Boye and Mælandsmo, 2010; Chua et al., 2007; Kalluri and Weinberg, 2009; Padua and Massagué, 2009; Polyak and Weinberg, 2009; Rodriguez-Pinto et al., 2009; Thiery, 2002).
In a different study carried out on colorectal cancer (CRC) patients, the CTC DNA was amplified followed by sanger sequencing revealed that PIK3CA, KRAS and BRAF are the mutant genes which may effect the response of anti-EGFR therapy (Zhang et al., 2014). In another study, pancreatic CTCs from the mouse were subjected to RNA sequencing. It was found that CTCs were enriched with WNT-2 gene. In pancreatic cancer cells, WNT-2 genes suppress anoikis, increase anchorage independence sphere formation and upturn the metastatic propensity. Similarly, in humans’ pancreatic cancer cells, the WNT gene leads to the formation of non-adherent tumors. Thus, the molecular profiling of CTCs may act as a candidate therapeutic target as the pancreatic CTCs revealed the presence of WNT signaling in some human cases (Yu et al., 2012).
CTCs could play an important role in drug trials (Fehm et al., 2010; Ignatiadis and Dawson, 2014). The amplification of HER2- Human epidermal growth factor receptor 2 is a main cause of 20% of breast cancers in women. It is considered as one of the biomarkers for MBC patients. The patients with an over-expression of HER2 status are usually subjected to HER2–targeted treatment. In one study, 254 patients with MBC were tested to check HER2 status with the help of CellSearch (Fehm et al., 2010). It was observed that the women having original tumor with HER2 +ve genes also had HER2 +ve CTCs, but there were certain number of cases in which it was observed that the women with HER2 -ve original tumor possess HER2 +ve CTCs (Riethdorf et al., 2010). Formerly evaluated with lapatinib-1500 mg/day (is a EGFR coupled with HER2 tyrosine kinase inhibitor used in combination for HER2 +ve breast cancer patient treatment) in first line of therapy, population was subjected for CTC isolation. The result showed that out of 139 (HER2- negative) patients, 96 were CTC positive, out of which 7 were HER2 positive. When these 7 patients were treated with lapatinib, lack of objective response against lapatinib was observed in one of the patient and disease prevailed longer despite of lower HER2 shift. It was related to the failure of the phase II trial, that was unable to explain any significant interest of the lapatinib therapy in patients with HER2 -ve MBC and having overexpression CTCs with HER2-protein (Baselga et al., 2012; Chen, Y.-J. et al., 2013; Pestrin et al., 2012). However, further detailed studies of CTC characterization can overcome the obstacle of having less information about the biomarkers and it might be possible in future that the CTCs could be evaluated not only for personalized therapies but also for molecular screening of the tissues (Wan et al., 2013), as the attempts have been conducted to study CTCs with mutational outline from different cancer types.
8. Summary
The discovery of CTCs as a liquid biopsy has revolutionized the prognosis of cancer. CTCs hold great possibilities for early disease detection, metastasis monitoring and personalizing cancer therapies. Despite these advantages, the clinical use of CTCs as a “liquid biopsy” comes with significant challenges related to CTC heterogeneity, fragility, and incomplete gene expression knowledge. Methods for isolating, detaching, and detecting these cells continue to present with needs for increased specificity, sensitivity, and throughput. Culturing and analysis techniques need to be optimized to better understand the morphological and functional properties of CTC cell lines derived from patient samples. Further studies can then be performed regarding optimal treatment plans using CTCs derived from patient samples, regarding molecular and gene analysis for effective disease monitoring and discovery of new drug targets.
Acknowledgments
We would like to thank Mazhar Sher for reviewing the manuscript and providing invaluable comments and suggestions. We acknowledge research support from NIH R15AI127214, Institute for Sensing and Embedded Networking Systems Engineering (I-SENSE) Research Initiative Award, Humanity in Science Award, FAU Faculty Mentoring Award, and a start-up research support from College of Engineering and Computer Science, Florida Atlantic University, Boca Raton, FL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interests.
References
- Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Gottert J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. Journal of the American Chemical Society. 2008;130(27):8633–8641. doi: 10.1021/ja8015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Research. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque MLC, Waters CM, Savla U, Schnaper HW, Flozak AS. Shear stress enhances human endothelial cell wound closure in vitro. American Journal of Physiology-Heart and Circulatory Physiology. 2000;279(1):H293–H302. doi: 10.1152/ajpheart.2000.279.1.H293. [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients, Minimal Residual Disease and Circulating Tumor Cells in Breast Cancer. Springer; 2012. pp. 69–76. [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clinical chemistry. 2013;59(1):110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annual review of medicine. 2012;63:199–215. doi: 10.1146/annurev-med-062310-094219. [DOI] [PubMed] [Google Scholar]
- Alix-Panabières C, Vendrell J-P, Pellé O, Rebillard X, Riethdorf S, Müller V, Fabbro M, Pantel K. Detection and characterization of putative metastatic precursor cells in cancer patients. Clinical chemistry. 2007;53(3):537–539. doi: 10.1373/clinchem.2006.079509. [DOI] [PubMed] [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen LWMM. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical Cancer Research. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Ameri K, Luong R, Zhang H, Powell A, Montgomery K, Espinosa I, Bouley D, Harris A, Jeffrey S. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. British journal of cancer. 2010;102(3):561–569. doi: 10.1038/sj.bjc.6605491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreopoulou E, Yang LY, Rangel K, Reuben J, Hsu L, Krishnamurthy S, Valero V, Fritsche H, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect™ versus Veridex CellSearch™ system. International journal of cancer. 2012;130(7):1590–1597. doi: 10.1002/ijc.26111. [DOI] [PubMed] [Google Scholar]
- Ankeny J, Court C, Hou S, Li Q, Song M, Wu D, Chen J, Lee T, Lin M, Sho S. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. British journal of cancer. 2016;114(12):1367–1375. doi: 10.1038/bjc.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Z, Cote RJ, Datar RH. Affinity-Based Enrichment of Circulating Tumor Cells, Circulating Tumor Cells. Springer; 2016. pp. 17–28. [Google Scholar]
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular Cancer Research. 2011;9(8):997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare AL, Kolchinsky SA, Gao Z, Wang R, Raddassi K, Bourcier K, Seyfert-Margolis V. Differential gene expression profiles are dependent upon method of peripheral blood collection and RNA isolation. BMC genomics. 2008;9(1):474. doi: 10.1186/1471-2164-9-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Materials Today. 2015;18(10):539–553. doi: 10.1016/j.mattod.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W, Ilyas A, Billo JA, Iqbal SM. Shrinking of solid-state nanopores by direct thermal heating. Nanoscale research letters. 2011a;6(1):372. doi: 10.1186/1556-276X-6-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W, Ilyas A, Deshmukh RR, Sumitsawan S, Timmons RB, Iqbal SM. Pulsed plasma polymerization for controlling shrinkage and surface composition of nanopores. Nanotechnology. 2011b;22(28):285304. doi: 10.1088/0957-4484/22/28/285304. [DOI] [PubMed] [Google Scholar]
- Asghar W, Islam M, Wadajkar AS, Wan Y, Ilyas A, Nguyen KT, Iqbal SM. PLGA micro-and nanoparticles loaded into gelatin scaffold for controlled drug release. IEEE Transactions on Nanotechnology. 2012a;11(3):546–553. [Google Scholar]
- Asghar W, Ramachandran PP, Adewumi A, Noor MR, Iqbal SM. Rapid nanomanufacturing of metallic break junctions using focused ion beam scratching and electromigration. Journal of Manufacturing Science and Engineering. 2010;132(3):030911. [Google Scholar]
- Asghar W, Shafiee H, Chen P, Tasoglu S, Guven S, Gurkan UA, Demirci U. In vitro three-dimensional cancer culture models, Cancer Targeted Drug Delivery. Springer; 2013. pp. 635–665. [Google Scholar]
- Asghar W, Shafiee H, Velasco V, Sah VR, Guo S, El Assal R, Inci F, Rajagopalan A, Jahangir M, Anchan RM. Toxicology study of single-walled carbon nanotubes and reduced graphene oxide in human sperm. Scientific reports. 2016a;6:30270. doi: 10.1038/srep30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W, Velasco V, Kingsley JL, Shoukat MS, Shafiee H, Anchan RM, Mutter GL, Tüzel E, Demirci U. Selection of functional human sperm with higher DN A integrity and fewer reactive oxygen species. Advanced healthcare materials. 2014;3(10):1671–1679. doi: 10.1002/adhm.201400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar W, Wan Y, Ilyas A, Bachoo R, Kim Y-t, Iqbal SM. Electrical fingerprinting, 3D profiling and detection of tumor cells with solid-state micropores. Lab on a Chip. 2012b;12(13):2345–2352. doi: 10.1039/c2lc21012f. [DOI] [PubMed] [Google Scholar]
- Asghar W, Yuksekkaya M, Shafiee H, Zhang M, Ozen MO, Inci F, Kocakulak M, Demirci U. Engineering long shelf life multi-layer biologically active surfaces on microfluidic devices for point of care applications. Scientific reports. 2016b;6:21163. doi: 10.1038/srep21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14(3):146–149. [Google Scholar]
- Attard G, de Bono JS. Utilizing circulating tumor cells: challenges and pitfalls. Current opinion in genetics & development. 2011;21(1):50–58. doi: 10.1016/j.gde.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Attard G, Swennenhuis JF, Olmos D, Reid AHM, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Research. 2009;69(7):2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- Bajpai A, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Progress in Polymer Science. 2008;33(11):1088–1118. [Google Scholar]
- Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-β in breast cancer: too much, too late. Breast cancer research. 2009;11(1):1–6. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. The Lancet. 2012;379(9816):633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beije N, Jager A, Sleijfer S. Circulating tumor cell enumeration by the CellSearch system: The clinician’s guide to breast cancer treatment? Cancer treatment reviews. 2015;41(2):144–150. doi: 10.1016/j.ctrv.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Bertran E, Caja L, Navarro E, Sancho P, Mainez J, Murillo MM, Vinyals A, Fabra À, Fabregat I. Role of CXCR4/SDF-1α in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-β. Cellular signalling. 2009;21(11):1595–1606. doi: 10.1016/j.cellsig.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Billo JA, Asghar W, Iqbal SM. An implementation for the detection and analysis of negative peaks in an applied current signal across a silicon nanopore, Micro-and Nanotechnology Sensors, Systems, and Applications III. International Society for Optics and Photonics. 2011:80312T. [Google Scholar]
- Bioengineering, N.I.o.B.I.a. Tiny technology enables improved detection of circulating tumor cells, ScienceDaily 2014 [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Born C, Zhang Z, Al-Rubeai M, Thomas C. Estimation of disruption of animal cells by laminar shear stress. Biotechnology and bioengineering. 1992;40(9):1004–1010. doi: 10.1002/bit.260400903. [DOI] [PubMed] [Google Scholar]
- Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer treatment reviews. 2010;36(3):206–215. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Boss DS, Olmos RV, Sinaasappel M, Beijnen JH, Schellens JHM. Application of PET/CT in the development of novel anticancer drugs. The oncologist. 2008;13(1):25–38. doi: 10.1634/theoncologist.2007-0097. [DOI] [PubMed] [Google Scholar]
- Boye K, Mælandsmo GM. S100A4 and metastasis: a small actor playing many roles. The American journal of pathology. 2010;176(2):528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LWMM. Circulating tumor cells versus imaging— predicting overall survival in metastatic breast cancer. Clinical Cancer Research. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- Cann GM, Gulzar ZG, Cooper S, Li R, Luo S, Tat M, Stuart S, Schroth G, Srinivas S, Ronaghi M. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PloS one. 2012;7(11):e49144. doi: 10.1371/journal.pone.0049144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegan M, Kolostova K, Matkowski R, Broul M, Schraml J, Fiutowski M, Bobek V. In vitro culturing of viable circulating tumor cells of urinary bladder cancer. International journal of clinical and experimental pathology. 2014;7(10):7164. [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- Chen CL, Mahalingam D, Osmulski P, Jadhav RR, Wang CM, Leach RJ, Chang TC, Weitman SD, Kumar AP, Sun L. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. The Prostate. 2013;73(8):813–826. doi: 10.1002/pros.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-F, Zhu Y, Lu Y-T, Hodara E, Hou S, Agopian VG, Tomlinson JS, Posadas EM, Tseng H-R. Clinical Applications of NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Theranostics. 2016;6(9):1425. doi: 10.7150/thno.15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Ho H, Lichterman J, Lu YT, Zhang Y, Garcia MA, Chen SF, Liang AJ, Hodara E, Zhau HE. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer. 2015;121(18):3240–3251. doi: 10.1002/cncr.29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Weng S, Zhang F, Allen S, Li X, Bao L, Lam RH, Macoska JA, Merajver SD, Fu J. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS nano. 2012a;7(1):566–575. doi: 10.1021/nn304719q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Weng S, Zhang F, Allen S, Li X, Bao L, Lam RHW, Macoska JA, Merajver SD, Fu J. Nanoroughened surfaces for efficient capture of circulating tumor cells without using capture antibodies. ACS nano. 2012b;7(1):566–575. doi: 10.1021/nn304719q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-J, Yeh M-H, Yu M-C, Wei Y-L, Chen W-S, Chen J-Y, Shih C-Y, Tu C-Y, Chen C-H, Hsia T-C. Lapatinib-induced NF-kappaB activation sensitizes triple-negative breast cancer cells to proteasome inhibitors. Breast Cancer Research. 2013;15(6):1–14. doi: 10.1186/bcr3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury F, Na S, Li D, Poh Y-C, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature materials. 2010;9(1):82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26(5):711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- Coarsey CT, Esiobu N, Narayanan R, Pavlovic M, Shafiee H, Asghar W. Strategies in Ebola virus disease (EVD) diagnostics at the point of care. Critical reviews in microbiology. 2017;43(6):779–798. doi: 10.1080/1040841X.2017.1313814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. Journal of clinical oncology. 2008;26(19):3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical Cancer Research. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LWWM, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical Cancer Research. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- De Giorgi V, Pinzani P, Salvianti F, Grazzini M, Orlando C, Lotti T, Pazzagli M, Massi D. Circulating benign nevus cells detected by ISET technique: warning for melanoma molecular diagnosis. Archives of dermatology. 2010;146(10):1120–1124. doi: 10.1001/archdermatol.2010.264. [DOI] [PubMed] [Google Scholar]
- de las Heras Alarcón C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chemical Society Reviews. 2005;34(3):276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. The lancet oncology. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L, Wu X, Faruqi AF, Bray-Ward P, Sun Z, Zong Q, Du Y, Du J. Comprehensive human genome amplification using multiple displacement amplification. Proceedings of the National Academy of Sciences. 2002;99(8):5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh S, Brzozka Z, Laurell T, Augustsson P. Acoustic radiation forces at liquid interfaces impact the performance of acoustophoresis. Lab on a Chip. 2014;14(17):3394–3400. doi: 10.1039/c4lc00572d. [DOI] [PubMed] [Google Scholar]
- Dharmasiri U, Balamurugan S, Adams AA, Okagbare PI, Obubuafo A, Soper SA. Highly efficient capture and enumeration of low abundance prostate cancer cells using prostate-specific membrane antigen aptamers immobilized to a polymeric microfluidic device. Electrophoresis. 2009;30(18):3289–3300. doi: 10.1002/elps.200900141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. Journal of Clinical Oncology. 2014;32(6):579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey DD, Giangrande PH. Oligonucleotide aptamers: A next-generation technology for the capture and detection of circulating tumor cells. Methods. 2016;97:94–103. doi: 10.1016/j.ymeth.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfus C, Piton N, Toure E, Sabourin J-C. Circulating tumor cell isolation: the assets of filtration methods with polycarbonate track-etched filters. Chinese Journal of Cancer Research. 2015;27(5):479. doi: 10.3978/j.issn.1000-9604.2015.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmus NG, Tekin HC, Guven S, Sridhar K, Yildiz AA, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U. Magnetic levitation of single cells. Proceedings of the National Academy of Sciences. 2015;112(28):E3661–E3668. doi: 10.1073/pnas.1509250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Löhberg CR, Solomayer E, Rack B. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast cancer research and treatment. 2010;124(2):403–412. doi: 10.1007/s10549-010-1163-x. [DOI] [PubMed] [Google Scholar]
- Fennell R, Asghar W. Image Sensor Road Map and Solid-State Imaging Devices. Nano World. 2017;1(4) [Google Scholar]
- Gach PC, Attayek PJ, Whittlesey RL, Yeh JJ, Allbritton NL. Micropallet arrays for the capture, isolation and culture of circulating tumor cells from whole blood of mice engrafted with primary human pancreatic adenocarcinoma. Biosensors and Bioelectronics. 2014;54:476–483. doi: 10.1016/j.bios.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, Mauermann O, Izbicki JR, Pantel K, Riethdorf S. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clinical chemistry. 2013;59(1):252–260. doi: 10.1373/clinchem.2012.188557. [DOI] [PubMed] [Google Scholar]
- Gascoyne PRC, Noshari J, Anderson TJ, Becker FF. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30(8):1388–1398. doi: 10.1002/elps.200800373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nature Reviews Cancer. 2014;14(6):430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee B-N, Tin S, De Laurentiis M, Parker CA, Alvarez RH. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Molecular cancer therapeutics. 2012;11(11):2526–2534. doi: 10.1158/1535-7163.MCT-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett DR, Weaver WM, Mach AJ, Hur SC, Tse HTK, Lee W, Amini H, Di Carlo D. Label-free cell separation and sorting in microfluidic systems. Analytical and bioanalytical chemistry. 2010;397(8):3249–3267. doi: 10.1007/s00216-010-3721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez A, Asghar W, Rafique MM, Iqbal SM, Butt AR. GPU-based real-time detection and analysis of biological targets using solid-state nanopores. Medical & biological engineering & computing. 2012;50(6):605–615. doi: 10.1007/s11517-012-0893-9. [DOI] [PubMed] [Google Scholar]
- Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, Ionescu-Zanetti C. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Translational oncology. 2013;6(5):528–IN521. doi: 10.1593/tlo.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham JE, Kotasek D, Farmer B, Butler RN, Mi J-X, Sage RE, Dobrovic A. Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer research. 1993;53(15):3455–3458. [PubMed] [Google Scholar]
- Harouaka RA, Zhou M-D, Yeh Y-T, Khan WJ, Das A, Liu X, Christ CC, Dicker DT, Baney TS, Kaifi JT. Flexible micro spring array device for high-throughput enrichment of viable circulating tumor cells. Clinical chemistry. 2014;60(2):323–333. doi: 10.1373/clinchem.2013.206805. [DOI] [PubMed] [Google Scholar]
- Hayes DF, Smerage J. Is there a role for circulating tumor cells in the management of breast cancer? Clinical Cancer Research. 2008;14(12):3646–3650. doi: 10.1158/1078-0432.CCR-07-4481. [DOI] [PubMed] [Google Scholar]
- Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, Hoffmann EM, Lax S, Waldispuehl-Geigl J, Mauermann O, Lackner C. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer research. 2013;73(10):2965–2975. doi: 10.1158/0008-5472.CAN-12-4140. [DOI] [PubMed] [Google Scholar]
- Helzer KT, Barnes HE, Day L, Harvey J, Billings PR, Forsyth A. Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer research. 2009;69(19):7860–7866. doi: 10.1158/0008-5472.CAN-09-0801. [DOI] [PubMed] [Google Scholar]
- Hong B, Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3(6):377. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan DS-W, Lim W-T, Han J, Bhagat AAS, Lim CT. Isolation and retrieval of circulating tumor cells using centrifugal forces. Scientific reports. 2013:3. doi: 10.1038/srep01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J-M, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, Priest LJC, Greystoke A, Zhou C, Morris K. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. Journal of Clinical Oncology. 2012;30(5):525–532. doi: 10.1200/JCO.2010.33.3716. [DOI] [PubMed] [Google Scholar]
- Ignatiadis M, Dawson SJ. Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality? Annals of oncology. 2014;25(12):2304–2313. doi: 10.1093/annonc/mdu480. [DOI] [PubMed] [Google Scholar]
- Ignatiadis M, Reinholz M. Minimal residual disease and circulating tumor cells in breast cancer. Breast Cancer Research. 2011;13(5):1. doi: 10.1186/bcr2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas A, Asghar W, Ahmed S, Lotan Y, Hsieh J-T, Kim Y-t, Iqbal SM. Electrophysiological analysis of biopsy samples using elasticity as an inherent cell marker for cancer detection. Analytical Methods. 2014a;6(18):7166–7174. [Google Scholar]
- Ilyas A, Asghar W, Kim Y-t, Iqbal SM. Parallel recognition of cancer cells using an addressable array of solid-state micropores. Biosensors and Bioelectronics. 2014b;62:343–349. doi: 10.1016/j.bios.2014.06.048. [DOI] [PubMed] [Google Scholar]
- Ilyas A, Islam M, Asghar W, Menon JU, Wadajkar AS, Nguyen KT, Iqbal SM. Salt-leaching synthesis of porous PLGA nanoparticles. IEEE Transactions on Nanotechnology. 2013;12(6):1082–1088. [Google Scholar]
- Islam M, Asghar W, Young-tae K, Iqbal SM. Cell elasticity-based microfluidic label-free isolation of metastatic tumor cells. British Journal of Medicine and Medical Research. 2014;4(11):2129. [Google Scholar]
- Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clinical chemistry. 2010;56(9):1492–1495. doi: 10.1373/clinchem.2010.143297. [DOI] [PubMed] [Google Scholar]
- Jing Y, Moore LR, Schneider T, Williams PS, Chalmers JJ, Farag SS, Bolwell B, Zborowski M. Negative selection of hematopoietic progenitor cells by continuous magnetophoresis. Experimental hematology. 2007;35(4):662–672. doi: 10.1016/j.exphem.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Joosse SA, Gorges TM, Pantel K. Biology, detection, and clinical implications of circulating tumor cells. EMBO molecular medicine. 2015;7(1):1–11. doi: 10.15252/emmm.201303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast cancer research. 2008;10(5):R80. doi: 10.1186/bcr2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE. Handbook of ELISPOT: methods and protocols. Springer Science & Business Media; 2005. [Google Scholar]
- Kanakasabapathy MK, Pandya HJ, Draz MS, Chug MK, Sadasivam M, Kumar S, Etemad B, Yogesh V, Safavieh M, Asghar W. Rapid, label-free CD4 testing using a smartphone compatible device. Lab on a Chip. 2017;17(17):2910–2919. doi: 10.1039/c7lc00273d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen P-i. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nature protocols. 2014;9(3):694–710. doi: 10.1038/nprot.2014.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer GD, Visser W, Vliem M, Figdor CG. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. The Journal of Immunology. 1988;140(5):1393–1400. [PubMed] [Google Scholar]
- Kim MS, Sim TS, Kim YJ, Kim SS, Jeong H, Park J-M, Moon H-S, Kim SI, Gurel O, Lee SS. SSA-MOA: a novel CTC isolation platform using selective size amplification (SSA) and a multi-obstacle architecture (MOA) filter. Lab on a Chip. 2012;12(16):2874–2880. doi: 10.1039/c2lc40065k. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Masago A, Tamaki Y, Akazawa K, Tsukamoto F, Sato J, Ozawa T, Tsujino Y, Noguchi S. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast cancer research and treatment. 2011;128(3):765–773. doi: 10.1007/s10549-011-1603-2. [DOI] [PubMed] [Google Scholar]
- Kim T-H, Lim M, Park J, Oh JM, Kim H, Jeong H, Lee SJ, Park HC, Jung S, Kim BC. FAST: Size-Selective, Clog-Free Isolation of Rare Cancer Cells from Whole Blood at a Liquid–Liquid Interface. Analytical Chemistry. 2016 doi: 10.1021/acs.analchem.6b03534. [DOI] [PubMed] [Google Scholar]
- Kojima T, Hashimoto Y, Watanabe Y, Kagawa S, Uno F, Kuroda S, Tazawa H, Kyo S, Mizuguchi H, Urata Y. A simple biological imaging system for detecting viable human circulating tumor cells. The Journal of clinical investigation. 2009;119(10):3172–3181. doi: 10.1172/JCI38609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolostova K, Broul M, Schraml J, Cegan M, Matkowski R, Fiutowski M, Bobek V. Circulating tumor cells in localized prostate cancer: isolation, cultivation in vitro and relationship to T-stage and Gleason score. Anticancer research. 2014a;34(7):3641–3646. [PubMed] [Google Scholar]
- Kolostova K, Matkowski R, Gürlich R, Grabowski K, Soter K, Lischke R, Schützner J, Bobek V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2015a:1–8. doi: 10.1007/s10616-015-9866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. American journal of translational research. 2015b;7(7):1203. [PMC free article] [PubMed] [Google Scholar]
- Kolostova K, Zhang Y, Hoffman RM, Bobek V. In vitro culture and characterization of human lung cancer circulating tumor cells isolated by size exclusion from an orthotopic nude-mouse model expressing fluorescent protein. Journal of fluorescence. 2014b;24(5):1531–1536. doi: 10.1007/s10895-014-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching E, Jeffrey SS, Meinerz W, Decker T, Boecker W, Buerger H. Basal carcinoma of the breast revisited: an old entity with new interpretations. Journal of clinical pathology. 2008;61(5):553–560. doi: 10.1136/jcp.2008.055475. [DOI] [PubMed] [Google Scholar]
- Krebs MG, Hou J-M, Ward TH, Blackhall FH, Dive C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Therapeutic advances in medical oncology. 2010;2(6):351–365. doi: 10.1177/1758834010378414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells [mdash] biology and biomarkers. Nature reviews Clinical oncology. 2014;11(3):129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou J-M, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. Journal of clinical oncology. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- Lee S-J, Kim H-S, Yu R, Lee K, Gardner TA, Jung C, Jeng M-H, Yeung F, Cheng L, Kao C. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Molecular Therapy. 2002;6(3):415–421. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang P-H, Truica CI, Drabick JJ, El-Deiry WS, Dao M. Acoustic separation of circulating tumor cells. Proceedings of the National Academy of Sciences. 2015;112(16):4970–4975. doi: 10.1073/pnas.1504484112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen N, Zhang Z, Wang Y. Endonuclease-responsive aptamer-functionalized hydrogel coating for sequential catch and release of cancer cells. Biomaterials. 2013;34(2):460–469. doi: 10.1016/j.biomaterials.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Li W, Reátegui E, Park M-H, Castleberry S, Deng JZ, Hsu B, Mayner S, Jensen AE, Sequist LV, Maheswaran S. Biodegradable nano-films for capture and non-invasive release of circulating tumor cells. Biomaterials. 2015;65:93–102. doi: 10.1016/j.biomaterials.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianidou ES. Circulating tumor cell isolation: a marathon race worth running. Clinical chemistry. 2014;60(2):287–289. doi: 10.1373/clinchem.2013.216010. [DOI] [PubMed] [Google Scholar]
- Lianidou ES. Molecular characterization of circulating tumor cells: Holy Grail for personalized cancer treatment? Clinical chemistry. 2014;60(10):1249–1251. doi: 10.1373/clinchem.2014.230144. [DOI] [PubMed] [Google Scholar]
- Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz-Gordillo P, Francis J, Zhang C-Z, Shalek AK, Satija R. Whole exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nature biotechnology. 2014;32(5):479. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Fan T, Zhao Q, Zeng W, Zaslavsky E, Chen JJ, Frohman MA, Golightly MG, Madajewicz S, Chen WT. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. International journal of cancer. 2010;126(3):669–683. doi: 10.1002/ijc.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-T, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, Song M, Xu X, OuYang W-H, OuYang WW-L. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods. 2013a;64(2):144–152. doi: 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-T, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, Song M, Xu X, OuYang W-H, OuYang WWL. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods. 2013b;64(2):144–152. doi: 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke K, Gasiorowski L, Herold S, Brychta N, Gallerani G, Krahn T, Fabbri F, Dyszkiewicz W, Schumann C. The GILUPI CellCollector as an in vivo tool for circulating tumor cell enumeration and molecular characterization in lung cancer patients. ASCO Annual Meeting Proceedings; 2015. p. e22035. [Google Scholar]
- Lustberg M, Jatana KR, Zborowski M, Chalmers JJ. Emerging technologies for CTC detection based on depletion of normal cells, Minimal Residual Disease and Circulating Tumor Cells in Breast Cancer. Springer; 2012. pp. 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Current opinion in genetics & development. 2010;20(1):96–99. doi: 10.1016/j.gde.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW. Detection of mutations in EGFR in circulating lung-cancer cells. New England Journal of Medicine. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M, Sarosi E. THE CTC CHIP: A MICROFLUIDIC TECHNOLOGY UTILIZING ANTIBODIES FOR THE DETECTION OF CANCEROUS TUMOR CELLS 2013 [Google Scholar]
- Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C. HER-2 gene amplification can be acquired as breast cancer progresses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(25):9393–9398. doi: 10.1073/pnas.0402993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. Journal of oncology 2010. 2009 doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner LM, Linder MW, Valdes R. Circulating tumor cells: a review of present methods and the need to identify heterogeneous phenotypes. Annals of Clinical & Laboratory Science. 2013;43(3):295–304. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, Smas ME, Lord JB, Brannigan BW, Trautwein J. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer discovery. 2012;2(11):995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Del Fiore P, Guarnieri L, Scalerta R, Foletto M, Chiarion V, Pilati P, Nitti D, Lise M, Rossi CR. Molecular detection of circulating tumor cells is an independent prognostic factor in patients with high-risk cutaneous melanoma. International journal of cancer. 2004;111(5):741–745. doi: 10.1002/ijc.20347. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D’Amico AV, Eastham JA, Enke CA, Farrington T, Higano CS. Prostate cancer, version 3.2012 featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2012;10(9):1081–1087. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N, Inman A, Zhang L. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicology and applied pharmacology. 2009;234(2):222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- Moon H-S, Kwon K, Kim S-I, Han H, Sohn J, Lee S, Jung H-I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP) Lab on a Chip. 2011;11(6):1118–1125. doi: 10.1039/c0lc00345j. [DOI] [PubMed] [Google Scholar]
- Müller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, Aktas B, Kasimir-Bauer S, Pantel K, Fehm T. Prognostic impact of circulating tumor cells assessed with the CellSearch System and AdnaTest Breast in metastatic breast cancer patients: the DETECT study. 2012 doi: 10.1186/bcr3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, Jänicke F, Pantel K. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clinical Cancer Research. 2005;11(10):3678–3685. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- Nadezhda Frolova CM, Anne Rasser, Ulrich Sack, Jan-Michael Heinrich. In: A new approach for screening of colorectal cancer circulating tumor cells (CTC) using EpCAM-pluriBeads. Leipzig Uo., editor. 2012. pluriSelect. [Google Scholar]
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalepa G, Enzor R, Sun Z, Marchal C, Park S-J, Yang Y, Tedeschi L, Kelich S, Hanenberg H, Clapp DW. Fanconi anemia signaling network regulates the spindle assembly checkpoint. The Journal of clinical investigation. 2013;123(9):3839–3847. doi: 10.1172/JCI67364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff S. Circulating Tumor Cell Assays: A Major Advance in Cancer Treatment, LifeExtension. Life Extension. 2010:2. [Google Scholar]
- Olsson C, De Vries G, Benson M, Raffo A, Buttyan R, Cama C, O’Toole K, Katz A. The use of RT-PCR for prostate-specific antigen assay to predict potential surgical failures before radical prostatectomy: molecular staging of prostate cancer. British journal of urology. 1996;77(3):411–417. doi: 10.1046/j.1464-410x.1996.90616.x. [DOI] [PubMed] [Google Scholar]
- Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen P-i, Morgan B, Trautwein J. Inertial focusing for tumor antigen-dependent and-independent sorting of rare circulating tumor cells. Science translational medicine. 2013;5(179):179ra147–179ra147. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Massagué J. Roles of TGFβ in metastasis. Cell research. 2009;19(1):89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Reviews Cancer. 2008;8(5):329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- Parkinson DR, Dracopoli N, Petty BG, Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar P, Lee JSH. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10(1):138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer letters. 2007;253(2):180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW. A novel platform for detection of CK+ and CK-CTCs. Cancer discovery. 2011;1(7):580–586. doi: 10.1158/2159-8290.CD-11-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters DJE, De Laere B, Van den Eynden GG, Van Laere SJ, Rothe F, Ignatiadis M, Sieuwerts AM, Lambrechts D, Rutten A, van Dam PA. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. British journal of cancer. 2013;108(6):1358–1367. doi: 10.1038/bjc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestrin M, Bessi S, Puglisi F, Minisini AM, Masci G, Battelli N, Ravaioli A, Gianni L, Di Marsico R, Tondini C. Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast cancer research and treatment. 2012;134(1):283–289. doi: 10.1007/s10549-012-2045-1. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Xu Y, Xia Z, Fan ZH, Tan W. Enrichment of cancer cells using aptamers immobilized on a microfluidic channel. Analytical chemistry. 2008;81(3):1033–1039. doi: 10.1021/ac802092j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierga J-Y, Bidard F-C, Denis MG, De Cremoux P. Prognostic value of peripheral blood double detection of CK19 and MUC1 mRNA positive cells detected by RT - quantitative PCR in 94 breast cancer patients with a follow up of 9 years. Molecular oncology. 2007;1(3):267–268. doi: 10.1016/j.molonc.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, Telli ML, Advani RH, Carlson RW, Mollick JA. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PloS one. 2012;7(5):e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, Hughes BGM, Hicks RJ, Hampton GM, Amler LC, Pirzkall A. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: Association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clinical Cancer Research. 2012;18(8):2391–2401. doi: 10.1158/1078-0432.CCR-11-3148. [DOI] [PubMed] [Google Scholar]
- Ramsköld D, Luo S, Wang Y-C, Li R, Deng Q, Faridani OR, Daniels GA, Khrebtukova I, Loring JF, Laurent LC. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nature biotechnology. 2012;30(8):777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GC, Larson C, Repollet M, Rutner H, Terstappen LWMM, O’Hara SM, Gross S. Analysis of circulating tumor cells, fragments, and debris. Google Patents 2012 [Google Scholar]
- Rappa KL, Rodriguez HF, Hakkarainen GC, Anchan RM, Mutter GL, Asghar W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnology advances. 2016;34(5):578–587. doi: 10.1016/j.biotechadv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Reátegui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S. Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells. Advanced Materials. 2015;27(9):1593–1599. doi: 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clinical Cancer Research. 2007;13(3):920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clinical Cancer Research. 2010;16(9):2634–2645. doi: 10.1158/1078-0432.CCR-09-2042. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pinto D, Sparkowski J, Keough MP, Phoenix KN, Vumbaca F, Han DK, Gundelfinger ED, Beesley P, Claffey KP. Identification of novel tumor antigens with patient-derived immune-selected antibodies. Cancer immunology, immunotherapy. 2009;58(2):221–234. doi: 10.1007/s00262-008-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Gertler R, Friederichs J, Fuehrer K, Dahm M, Phelps R, Thorban S, Nekarda H, Siewert J. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–158. doi: 10.1002/cyto.10161. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Gertler R, Friederichs J, Fuehrer K, Dahm M, Phelps R, Thorban S, Nekarda H, Siewert JR. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–158. doi: 10.1002/cyto.10161. [DOI] [PubMed] [Google Scholar]
- Saad A, Abraham J. Role of tumor markers and circulating tumors cells in the management of breast cancer. Oncology. 2008;22(7):726–731. [PubMed] [Google Scholar]
- Safavieh M, Coarsey C, Esiobu N, Memic A, Vyas JM, Shafiee H, Asghar W. Advances in Candida detection platforms for clinical and point-of-care applications. Critical reviews in biotechnology. 2017;37(4):441–458. doi: 10.3109/07388551.2016.1167667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba A-E, Saias L, Psychari E, Minc N, Simon D, Bidard F-C, Mathiot C, Pierga J-Y, Fraisier V, Salamero J. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proceedings of the National Academy of Sciences. 2010;107(33):14524–14529. doi: 10.1073/pnas.1001515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, Engstrom A, Zhu H, Sundaresan TK, Miyamoto DT. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nature methods. 2015;12(7):685–691. doi: 10.1038/nmeth.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo-Zeni N, Mewes S, Niestroj R, Gasiorowski L, Murawa D, Nowaczyk P, Tomasi T, Weber E, Dworacki G, Morgenthaler NG. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. International journal of oncology. 2012;41(4):1241–1250. doi: 10.3892/ijo.2012.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist LV, Nagrath S, Toner M, Haber DA, Lynch TJ. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. Journal of Thoracic Oncology. 2009;4(3):281–283. doi: 10.1097/JTO.0b013e3181989565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee H, Asghar W, Inci F, Yuksekkaya M, Jahangir M, Zhang MH, Durmus NG, Gurkan UA, Kuritzkes DR, Demirci U. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Scientific reports. 2015;5:8719. doi: 10.1038/srep08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher M, Zhuang R, Demirci U, Asghar W. based analytical devices for clinical diagnosis: recent advances in the fabrication techniques and sensing mechanisms. Expert review of molecular diagnostics. 2017;17(4):351–366. doi: 10.1080/14737159.2017.1285228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JWM, Gratama J-W, Sleijfer S, Foekens JA. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. Journal of the National Cancer Institute. 2009;101(1):61–66. doi: 10.1093/jnci/djn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke MA, Lew DL. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. Journal of Clinical Oncology. 2014;32(31):3483–3489. doi: 10.1200/JCO.2014.56.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG. Global gene expression profiling of circulating tumor cells. Cancer research. 2005;65(12):4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Van De Rijn M, Jeffrey SS. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Current opinion in biotechnology. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott SL, Hsu C-H, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences. 2010;107(43):18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- Swennenhuis JF, Tibbe AGJ, Levink R, Sipkema RCJ, Terstappen LWMM. Characterization of circulating tumor cells by fluorescence in situ hybridization. Cytometry Part A. 2009;75(6):520–527. doi: 10.1002/cyto.a.20718. [DOI] [PubMed] [Google Scholar]
- Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh K-H, Yu W, Xiao W, Davis MM, Pease RF, Mindrinos MN. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proceedings of the National Academy of Sciences. 2009;106(10):3970–3975. doi: 10.1073/pnas.0813188106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A. mRNA-Seq whole-transcriptome analysis of a single cell. Nature methods. 2009;6(5):377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Ting DT, Wittner BS, Ligorio M, Jordan NV, Shah AM, Miyamoto DT, Aceto N, Bersani F, Brannigan BW, Xega K. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell reports. 2014;8(6):1905–1918. doi: 10.1016/j.celrep.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proceedings of the National Academy of Sciences. 2011;108(30):12396–12400. doi: 10.1073/pnas.1106613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyala SD, Asghar W, Iqbal SM. Porous organic nanolayers for coating of solid-state devices. Journal of nanobiotechnology. 2011;9(1):18. doi: 10.1186/1477-3155-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet T, Kumar P, Van Loo P, Cooke SL, Marshall J, Lin M-L, Esteki MZ, Van der Aa N, Mateiu L, McBride DJ. Single-cell paired-end genome sequencing reveals structural variation per cell cycle. Nucleic acids research. 2013;41(12):6119–6138. doi: 10.1093/nar/gkt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, Mineur A, Franco D, Lacour B, Pol S. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39(3):792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. The American journal of pathology. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LP, Anderson JK, Baker MR, Han B, Hsieh J-T, Lotan Y, Cadeddu JA. In vitro assessment of the efficacy of thermal therapy in human renal cell carcinoma. Urology. 2007;70(2):380–384. doi: 10.1016/j.urology.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nature medicine. 2013;19(11):1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Wan Y, Liu Y, Allen PB, Asghar W, Mahmood MAI, Tan J, Duhon H, Kim Y-t, Ellington AD, Iqbal SM. Capture, isolation and release of cancer cells with aptamer-functionalized glass bead array. Lab on a Chip. 2012;12(22):4693–4701. doi: 10.1039/c2lc21251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Tan J, Asghar W, Kim Y-t, Liu Y, Iqbal SM. Velocity effect on aptamer-based circulating tumor cell isolation in microfluidic devices. The Journal of Physical Chemistry B. 2011;115(47):13891–13896. doi: 10.1021/jp205511m. [DOI] [PubMed] [Google Scholar]
- Wang L, Asghar W, Demirci U, Wan Y. Nanostructured substrates for isolation of circulating tumor cells. Nano today. 2013;8(4):374–387. doi: 10.1016/j.nantod.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Thomas A, Lee E, Yang S, Cheng X, Liu Y. Highly efficient and selective isolation of rare tumor cells using a microfluidic chip with wavy-herringbone micro-patterned surfaces. Analyst. 2016;141(7):2228–2237. doi: 10.1039/c6an00236f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. Journal of Clinical Oncology. 2011;29(12):1508–1511. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, Novak AJ, Witzig TE, Feldman AL, Pittelkow MR. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114(14):2936–2944. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mao X, Imrali A, Syed F, Mutsvangwa K, Berney D, Cathcart P, Hines J, Shamash J, Lu Y-J. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PloS one. 2015;10(9):e0138032. doi: 10.1371/journal.pone.0138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H-Y, Lin J-C. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. Journal of Biomaterials Science, Polymer Edition. 2008;19(3):291–310. doi: 10.1163/156856208783720985. [DOI] [PubMed] [Google Scholar]
- Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345(6193):216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. The Journal of cell biology. 2011;192(3):373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487(7408):510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Rubin M, Geevarughese S, Pino J, Rodriguez H, Asghar W. Emerging technologies for home-based semen analysis. Andrology. 2017 doi: 10.1111/andr.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration, Urologic Oncology: Seminars and Original Investigations. Elsevier; 2009. pp. 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zheng J, Yang Y, Lu J, Gao J, Lu T, Sun J, Jiang H, Zhu Y, Zheng Y. Molecular spectrum of KRAS, NRAS, BRAF and PIK3CA mutations in Chinese colorectal cancer patients: analysis of 1,110 cases. Scientific reports. 2014;5:18678–18678. doi: 10.1038/srep18678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen N, Li S, Battig MR, Wang Y. Programmable hydrogels for controlled cell catch and release using hybridized aptamers and complementary sequences. Journal of the American Chemical Society. 2012;134(38):15716–15719. doi: 10.1021/ja307717w. [DOI] [PubMed] [Google Scholar]
- Zhe X, Cher ML, Bonfil RD. Circulating tumor cells: finding the needle in the haystack. Am J Cancer Res. 2011;1(6):740–751. [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Iqbal SM, Wan Y. Cell detachment: Post-isolation challenges. Biotechnology advances. 2013;31(8):1664–1675. doi: 10.1016/j.biotechadv.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Zheng S, Lin HK, Lu B, Williams A, Datar R, Cote RJ, Tai Y-C. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomedical microdevices. 2011;13(1):203–213. doi: 10.1007/s10544-010-9485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W-F, Li J, Yu L-C, Wu Y, Tang X-P, Hu Y-M, Chen Y-C. Prognostic value of EpCAM/MUC1 mRNA-positive cells in non-small cell lung cancer patients. Tumor Biology. 2014;35(2):1211–1219. doi: 10.1007/s13277-013-1162-8. [DOI] [PubMed] [Google Scholar]
- Zieglschmid V, Hollmann C, Gutierrez B, Alberti W, Strothoff D, Gross E, BÖCher O. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer research. 2005;25(3A):1803–1810. [PubMed] [Google Scholar]