Abstract
BACKGROUND
The number of deaths from prescription opioids in the United States continues to increase and remains a major public health concern. Opioid-related deaths parallel prescribing trends and post-operative opioids are a significant source of opioids in the community. Our objective was to identify opioid prescribing and use patterns after surgery to inform evidenced-based practices.
STUDY DESIGN
Data from a 340-bed academic medical institution and its affiliated outpatient surgical facility included: 1) retrospective medical record data, 2) prospective telephone questionnaire and medical record data. Retrospective data included patients discharged after 19 procedures types from July 2015–June 2016 (n=10,112). Prospective data included a consecutive sample of general and orthopaedic surgery, and urology patients undergoing one of 13 procedures from July 2016–February 2017 (n=539). Primary outcomes were the quantity of opioid prescribed and used in morphine milligram equivalents (MME), and the proportion of patients receiving instructions on disposal and non-opioid strategies.
RESULTS
In the retrospective dataset, 76% of patients received an opioid after surgery and 87% of prescriptions were prescribed by residents or advanced practice providers. Median prescription size ranged from 0–503 MME with wide interquartile ranges (IQR) for most procedures. In the prospective dataset, there were 359 participants (67% participation rate). Of these, 92% of patients received an opioid and the median proportion used was 27%, or 24 MME (IQR 0–96). Only 18% of patients received disposal instructions, while 84% of all patients received instructions on non-opioid strategies.
CONCLUSIONS
Median opioid use after surgery was 27% of the total prescribed and only 18% of patients reported receiving disposal instructions. Significant variability in opioid prescribing and use after surgery warrants investigation into contributing factors.
Keywords: postoperative pain, opioids, drug utilization, surgical procedures, prescribing patterns
Introduction
The number of deaths from prescription opioids in the United States continues to increase (1) and remains a major public health concern despite evidence that preventative measures and more conservative prescribing efforts can curtail this trend (2). Between 1999 and 2008 the opioid overdose death rate increased four-fold as opioid pain reliever sales increased (3). Several studies have outlined other risks of prescription opioid use. These include opioid dependence or prolonged opioid use after taking opioids for acute (4) or post-operative pain (5–8), as well as prescription opioid abuse leading to heroin use (9–11).
State resources and education efforts such as the 2016 Centers for Disease Control and Prevention (CDC) prescriber guidelines on safe and effective opioid prescribing have largely been focused on chronic pain and substance abuse treatment (12). Recent studies have shown that opioid prescribing for acute pain, particularly in the post-operative setting, remains a significant source of opioids in the community. More specifically, they have demonstrated that there is considerable variability in the amount prescribed after routine procedures (13–15), that the amount prescribed after these procedures has increased over time (14), and that patients generally use much less opioid medication than they are prescribed (15–20). However, many of these studies are limited by small sample size, findings expressed in number of pills or proportions rather than morphine milligram equivalents (MME), and reliance on patient recall of medication use weeks to months after surgery. Furthermore, the utility of findings on opioid use after surgery tend only to be generalizable in relation to the specific procedures studied and thus there remains a gap in the literature regarding many procedures.
The purpose of this study was to collect prospective data in the immediate post-operative period after common surgical procedures across multiple surgical specialties. Our specific aims were: 1) to describe current prescribing practices at discharge from surgery, and 2) to determine patient-reported opioid use after surgery. Our broader clinical goal is to inform evidence-based practices that provide effective post-operative pain management while minimizing the amount of unused opioid medication in the community.
Methods
Study design
This study involved two different datasets and methods of data collection. The first, a retrospective review of prescribing patterns was used to inform the second, a prospective telephone questionnaire and chart review focusing on opioid use after surgery.
Retrospective review of prescription data
We collected data on opioid prescriptions written at the time of discharge after inpatient or outpatient surgical procedures over a one-year period (July 1, 2015–June 30, 2016). These dates were chosen to coincide with the academic calendar to ensure that resident clinical level would remain consistent over the course of data collection.
Prospective telephone questionnaire and chart review data
We then conducted a prospective telephone questionnaire and chart review of patients approximately one week after discharge from a surgical procedure. We designed a telephone questionnaire with input from a committee of surgeons and researchers, as there is no validated instrument available in the literature to address our aims. Data collection took place between July 2016 and February 2017 and patients were recruited in a staggered fashion with additional procedures added over time. We recruited patients using our operating room scheduling system and contacted each patient by telephone five to seven days after discharge from their surgical procedure. A total of four attempts were made over two days at different times of day to maximize the likelihood of patient contact.
If patient contact was made, a 10-minute telephone questionnaire was conducted inquiring about discharge instructions received on pain management and medication disposal, whether an opioid was prescribed, and how much of the opioid medication was used. A verbal count of the number of remaining pills was obtained from the patient whenever possible, as well as consent to review their medical record for details about their opioid prescription history, surgery and post-operative recovery. If patients were still using the prescribed medication at the initial telephone call, a second call was made one week later to determine the total amount of pain medication used. If patient consent was obtained, a chart review was performed.
Data were collected and managed using REDCap electronic data capture tools hosted at the University of Vermont (21).
Setting and Patients
The study was carried out in a 340-bed academic medical center and its affiliated outpatient surgery facility, which includes 14 surgical specialties and an annual surgical volume of approximately 20,000 cases. We obtained a waiver of consent for the collection of data in the retrospective dataset and limited data in the prospective dataset. All patient information gathered prior to patient consent in the prospective dataset were limited to certain details necessary to identify, screen, and contact patients to participate in the study. At the time of telephone contact, consent was obtained to collect the remainder of the data from the prospective dataset, including opioid prescription and post-operative recovery details. Both study protocols were approved by the University of Vermont Institutional Review Board Committee on Human Research in Medical Settings.
Retrospective review of prescription data
We included patients 18 years and older who were discharged to home after one of 19 procedures in general surgery, orthopaedic surgery, urology, and gynecology, during the study period. Patients discharged to a skilled nursing or rehabilitation facility were excluded under the assumption that post-operative pain control would be managed by a provider other than the surgeon or surgery team.
Prospective telephone questionnaire and chart review data
We obtained a consecutive sample of patients who underwent one of 13 common procedures in general surgery, orthopaedic surgery, or urology during the recruitment period. The selection of procedures for each surgical specialty was based on the most commonly performed procedures at our institution (identified during the retrospective prescription database review) and specifically included a range of invasiveness and anticipated post-operative pain. We screened patients by their procedure listed in the scheduling system only for those surgeons who agreed to have their patients called as part of the study. Gynecology procedures were not included in this portion of the study due to resource limitations.
Exclusion criteria at enrollment were bilateral or multiple procedures performed at the same time, repeat procedure, recent procedure (within the last month), known malignancy, under the age of 18, urgent or emergent procedure (other than appendectomy), and previous enrollment in the study. Patients who underwent partial mastectomy for malignancy were exempt from the malignancy exclusion criterion due to the minimally invasive nature of the procedure. Patients excluded after enrollment were those who were unable to communicate independently by telephone (whether due to mental status, disability, or language barrier), pregnant, discharged to a skilled nursing or rehabilitation facility, experienced a procedure complication, readmitted to the hospital, remained in the hospital four or more days after surgery, refused to participate, or could not be reached. Our aim was to collect data from at least 10 patients per procedure in order for any findings to be considered clinically relevant.
Between July 2016 and February 2017, 844 patients were screened into the study. Of these, 305 met one or more exclusion criterion and 539 patients remained eligible for the telephone questionnaire.
Measurements
Morphine milligram equivalents (MME) for each opioid prescription (including tramadol) were calculated using the conversion formulae recommended by the Centers for Disease Control (22) and the Washington State Agency Medical Directors’ Group guidelines (23). During data collection we noted that tramadol was occasionally prescribed in addition to another opioid, and we attempted to clarify when this occurred in our findings.
Retrospective review of prescription data
For each patient discharged after a surgical procedure, we extracted the following data from the electronic medical record: procedure name and CPT code, attending name and specialty, prescription date, medication name, strength, and quantity for all Schedule II–IV opioids and tramadol, as well as the name and type of prescriber (attending, resident, or advanced practice provider – henceforth referred to as APP – which included advanced practice nurse practitioners and physician assistants). We analyzed data from 10,112 patients who underwent 11,566 procedures between July 1, 2015 and June 30, 2016.
Prospective telephone questionnaire and chart review data
We collected demographic data and details of the patient’s surgery, recovery period, and opioid prescription history, in addition to their questionnaire data. Our primary outcome measures were the quantity and proportion of opioid prescribed and used after surgery, and the proportion of patients who received instructions on medication disposal and non-opioid pain management strategies.
Data Analysis
Retrospective review of prescription data
We used descriptive statistics to summarize characteristics associated with common procedures in general and orthopaedic surgery, urology and gynecology, including the number of procedures performed annually, inpatient versus outpatient, length of stay, category of opioid prescribed at discharge (if any), total MME prescribed, and the role of the prescriber (attending, resident, or APP).
Prospective telephone questionnaire and chart review data
We tabulated the frequency of specific surgical procedures according to surgical specialty. We characterized distributions of MME prescribed, MME used, and proportion of MME used with univariate statistics (medians, quartiles, and ranges). We also calculated the relative frequencies of responses regarding opioid use, non-opioid pain management, and medication disposal. Finally, we constructed violin plots to visualize smoothed, empirical distributions of the MMEs prescribed and used for specific surgical procedures. Violin shapes are mirrored kernel densities of the distribution of values (24).
All analyses were performed using Stata (StataCorp), version 14 (25).
Results
Retrospective review of prescription data
Of the 10,112 patients identified, 57% were female and the median patient age was 56. Outpatient procedures constituted 65% of all procedures and 76% of patients were discharged with a prescription for either an opioid or tramadol. In this academic setting, most of the prescriptions for opioids were written by residents (63%) or APPs (24%) (Table 1). The most commonly prescribed opioid medications were oxycodone (44%) and hydromorphone (31%). Median prescription sizes for each category of opioid, described in number of pills and MME per prescription, are provided in Table 2.
Table 1.
Characteristics of Patients, Procedures, and Prescriptions: A 2015–2016 Retrospective Dataset of Electronic Medical Record Data
| Characteristic | Data |
|---|---|
| Patient, (N=10,112) | |
| Age, y, median (Q1–Q3) | 56 (42–67) |
| Sex, female, n (%) | 5,757 (57) |
| Insurance type, n (%) | |
| Commercial | 5,014 (50) |
| Medicare | 3,134 (31) |
| Medicaid | 1,150 (11) |
| Other | 814 (8) |
| Procedure, (N=11,566), n (%) | |
| Specialty | |
| Orthopaedic surgery | 5,100 (44) |
| General surgery | 3,153 (27) |
| Gynecology | 1,680 (15) |
| Urology | 1,633 (14) |
| Outpatient procedure | 7,471 (65) |
| Prescription combinations per procedure | |
| No opioid or tramadol | 2,785 (24) |
| Opioid only | 7,463 (65) |
| Tramadol only | 369 (3) |
| Opioid and tramadol | 949 (8) |
| Prescription, (N=10,402), n (%) | |
| Prescriber role | |
| Resident | 6,581 (63) |
| Advanced practice provider | 2,464 (24) |
| Attending | 1,357 (13) |
Q1, 1st quartile; Q3, 3rd quartile.
Table 2.
Characteristics of Medication Prescribed: A 2015–2016 Retrospective Dataset of Electronic Medical Record Data
| Medication prescribed, (N=10,402) | n | % | Number of pills/Rx, median | MME/Rx, median |
|---|---|---|---|---|
| Oxycodone | 4,630 | 44 | 30 | 225 |
| Hydromorphone | 3,204 | 31 | 25 | 200 |
| Tramadol | 1,318 | 13 | 50 | 250 |
| Hydrocodone | 805 | 8 | 20 | 100 |
| Other opioid | 445 | 4 | – | – |
MME, morphine milligram equivalents; Rx, prescription.
To understand the relative contribution of each surgical specialty to potentially divertible medication we also calculated the annual volume of opioids prescribed by specialty: orthopaedic surgery (1,518,097 MME per year), general surgery (580,983 MME per year), gynecology (218,282 MME per year), and urology (143,552 MME per year).
Table 3 summarizes the opioid prescribing patterns after common surgical procedures for each of the four surgical specialties. For certain procedures (e.g. partial mastectomy), many patients were not prescribed any opioid medication, which is reflected in the first (Q1) and third (Q3) quartiles that are listed as 0 MME. The highest rates of tramadol prescribing occurred after hip (78%) and knee arthroplasty (65%), and lumbar arthrodesis (61%), with a typical prescription being 50 tablets of 50 mg tramadol (50 MME).
Table 3.
Prescriptions at Discharge after Selected Surgical Procedures: A 2015–2016 Retrospective Dataset of Electronic Medical Record Data
| Surgical procedure | Procedure frequency*, n | Opioid prescription rate†, % | Tramadol prescription rate†, % | MME prescribed‡, median (Q1–Q3) |
|---|---|---|---|---|
| General surgery | ||||
| Appendectomy (lap) | 156 | 87 | 3 | 160 (120–240) |
| Cholecystectomy (lap) | 331 | 90 | 3 | 160 (120–240) |
| Colectomy, partial (lap or open) | 179 | 80 | 5 | 225 (113–300) |
| Hernia (inguinal, ventral, incisional) | 445 | 90 | 3 | 160 (96–225) |
| Mastectomy, partial | 198 | 74 | 1 | 96 (0–160) |
| Gynecology | ||||
| Hysterectomy (lap or vaginal) | 217 | 98 | <1 | 240 (225–300) |
| Hysterectomy (open) | 65 | 98 | 0 | 300 (240–320) |
| Hysteroscopy | 246 | 23 | 0 | 0 (0–0) |
| Urethral sling procedure | 94 | 82 | 0 | 75 (40–113) |
| Orthopaedic surgery | ||||
| Carpal tunnel release | 320 | 65 | 5 | 75 (0–119) |
| Hip arthroplasty | 320 | 84 | 78 | 300 (150–480) |
| Knee arthroplasty | 341 | 79 | 65 | 300 (113–480) |
| Knee arthroscopy | 273 | 92 | 5 | 225 (150–240) |
| Lumbar arthrodesis | 101 | 79 | 61 | 375 (113–630) |
| Rotator cuff repair (arthroscopic) | 52 | 90 | 0 | 503 (450–600) |
| Trigger finger release | 103 | 42 | 3 | 0 (0–75) |
| Urology | ||||
| Cystoscopy (± stent, biopsy, tumor fulguration or resection) | 633 | 60 | 1 | 64 (0–113) |
| Prostatectomy (lap) | 88 | 97 | 2 | 160 (160–160) |
| Transurethral resection or laser vaporization of prostate | 80 | 65 | 0 | 75 (0–113) |
Average number of procedures performed per year
Proportion of annual procedures that received an opioid or tramadol prescription
For non-tramadol opioid prescriptions
Lap, laparoscopic; MME, morphine milligram equivalents; Q1, 1st quartile; Q3, 3rd quartile.
Prospective telephone questionnaire and chart review data
Of the 539 patients who were eligible for the telephone questionnaire, 180 were either not contacted, unable to communicate by telephone, or declined to participate. Data were successfully collected on 359 (67% participation rate). Table 4 highlights certain characteristics of these patients who were eligible and agreed to participate in the study.
Table 4.
Characteristics of Eligible Participants for Evaluation of Opioid Prescribing and Use: Data from a 2016–2017 Prospective Dataset Questionnaire and Chart Review
| Characteristic | Data |
|---|---|
| All patients, n | 359 |
| Age, y, median (Q1–Q3) | 58 (44–68) |
| Sex, n (%) | |
| Female | 170 (47) |
| Male | 189 (53) |
| Procedure, n (%) | |
| General surgery | |
| Mastectomy, partial | 24 (7) |
| Appendectomy (lap) | 18 (5) |
| Umbilical hernia repair (open) | 19 (5) |
| Inguinal hernia repair (open) | 26 (7) |
| Cholecystectomy (lap) | 40 (11) |
| Orthopaedic surgery | |
| Carpal tunnel release | 28 (8) |
| Knee arthroscopy/repair | 32 (9) |
| Shoulder arthroscopy/repair | 25 (7) |
| Knee arthroplasty | 32 (9) |
| Hip arthroplasty | 44 (12) |
| Urology | |
| Vasectomy | 12 (3) |
| Endoscopy | 42 (12) |
| RALP | 17 (5) |
Lap, laparoscopic; Q1, 1st quartile; Q3, 3rd quartile; RALP, robotic-assisted laparoscopic prostatectomy.
Of the patients in our final sample of 359, 53% were male and the median age was 58. We found that 84% (n=300) of patients reported receiving instructions on non-opioid pain management including acetaminophen or non-steroidal anti-inflammatory drug (NSAID) use, heat or cold therapy, or exercise. Most patients (92%, n=330) reported receiving an opioid after their surgery. In contrast, 34% of all patients (n=122) did not receive, did not fill, or did not use an opioid prescription.
For those patients who were prescribed an opioid (n=330), the most commonly prescribed medications were hydromorphone (47%, n=148) and oxycodone (41%, n=128). Combination hydrocodone/acetaminophen made up 9% of prescriptions (n=27). Tramadol was prescribed along with a more potent opioid medication in 17% of patients (n=55) and prescribed alone in 3% (n=8). Only 18% of patients (n=44) recalled receiving instructions on safe opioid disposal.
To better understand the true distribution of opioid prescribing and use across all patients, we included patients who did not receive an opioid prescription after surgery and extrapolated their opioid use to be 0 MME when running our analyses. We found that 332 patients (including those who did not receive an opioid prescription) had the necessary data to calculate MME prescribed, MME used, and proportion of opioid used by procedure (Table 5). Across all patients and procedures, the median prescription size was 120 MME with a range of 0 to 648 MME. The median proportion of opioid used was 27% of the prescribed amount, or 24 MME. We found that 75% of patients used 80% or less of the amount prescribed, or 96 MME. The MME used across all patients and procedures ranged from 0 to 528.
Table 5.
Quantity of Opioid Prescribed and Used after Common Surgical Procedures: Data from a 2016–2017 Prospective Dataset Questionnaire and Chart Review
| Surgical procedure | MME prescribed* | MME used | Proportion used, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Q1 | Median | Q3 | Range | Q1 | Median | Q3 | Range | Median | Q3 | |
| All patients | 332 | 75 | 120 | 300 | (0–648) | 0 | 24 | 96 | (0–528) | 27 | 80 |
| General surgery | |||||||||||
| Mastectomy, partial | 21 | 40 | 40 | 80 | (0–120) | 0 | 0 | 16 | (0–80) | 0 | 40 |
| Umbilical hernia repair (open) | 16 | 68 | 80 | 113 | (40–160) | 9 | 39 | (0–136) | 20 | 70 | |
| Appendectomy (lap) | 17 | 80 | 96 | 128 | (0–200) | 0 | 23 | 48 | (0–160) | 18 | 71 |
| Cholecystectomy (lap) | 40 | 80 | 120 | 155 | (50–320) | 0 | 25 | 80 | (0–173) | 20 | 68 |
| Inguinal hernia repair (open) | 25 | 80 | 120 | 200 | (0–480) | 8 | 64 | 96 | (0–240) | 50 | 100 |
| Orthopaedic surgery | |||||||||||
| Carpal tunnel release | 28 | 0 | 50 | 100 | (0–300) | 0 | 0 | 15 | (0–70) | 7 | 60 |
| Knee arthroscopy/repair | 30 | 80 | 155 | 300 | (0–450) | 0 | 86 | 150 | (0–450) | 50 | 100 |
| Shoulder arthroscopy/repair | 23 | 375 | 450 | 563 | (150–648) | 32 | 122 | 300 | (0–525) | 25 | 65 |
| Hip arthroplasty | 45 | 174 | 338 | 450 | (0–600) | 0 | 68 | 300 | (0–528) | 31 | 90 |
| Knee arthroplasty | 31 | 225 | 320 | 450 | (75–600) | 8 | 135 | 347 | (0–480) | 50 | 100 |
| Urology | |||||||||||
| Vasectomy | 11 | 60 | 60 | 60 | (0–60) | 0 | 0 | 0 | (0–10) | 0 | 0 |
| Endoscopy | 29 | 0 | 40 | 64 | (0–75) | 0 | 0 | 40 | (0–75) | 75 | 100 |
| RALP | 17 | 160 | 160 | 160 | (120–160) | 16 | 32 | 80 | (0–144) | 20 | 50 |
Includes patients who were not prescribed an opioid after their procedure.
Lap, laparoscopic; MME, morphine milligram equivalents; Q1, 1st quartile; Q3, 3rd quartile; RALP, robotic-assisted laparoscopic prostatectomy.
A side by side comparison of the MME prescribed and MME used for each procedure is illustrated in a violin plot in Figure 1.
Figure 1.
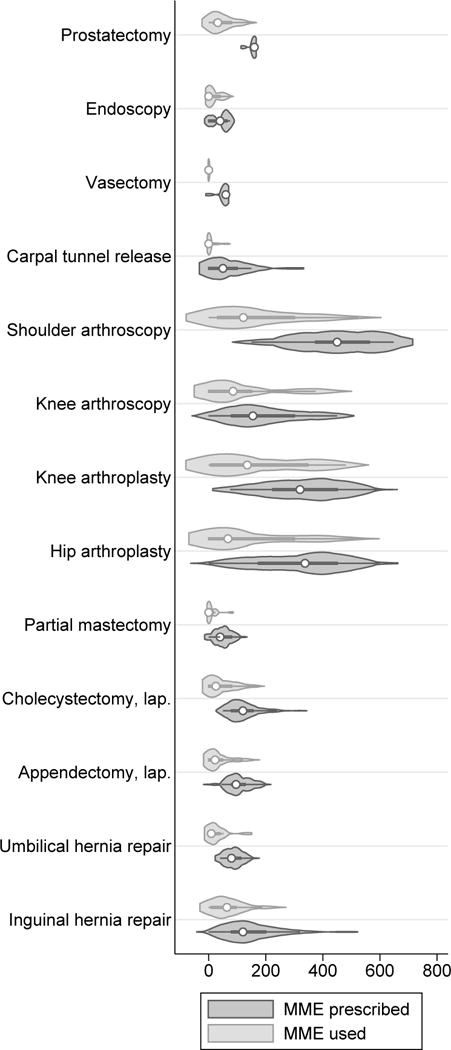
Violin plot of morphine milligram equivalents (MME) prescribed and used after common surgical procedures—2016–2017 (prospective dataset). Interior markings represent the median (white dot), interquartile range (thick line), and the adjacent values (thin line, i.e. the typical whiskers on a box plot). Lap, laparoscopic.
Discussion
A unique feature of our study is the prospective collection of data on both opioid prescribing and use across multiple surgical specialties in the immediate post-operative period. We found a wide range in the amount of opioid prescribed after common surgical procedures (median prescription size 120 MME, range 0 to 648 MME), which is consistent with prior studies (13–15). This variability is likely influenced by a number of factors, including patient education, surgeon-specific practices, and certain patient-specific differences in post-operative pain. Furthermore, our procedure groupings (e.g. “endoscopy” for urology and “shoulder arthroscopy/repair” for orthopaedic surgery) each included a range of procedures with varying degrees of expected postoperative pain, which may also have contributed to opioid prescription variability.
Our data demonstrated that the median amount of opioid used by patients was only 27% of the prescribed amount, which further highlights a concerning pattern of over-prescribing across multiple surgical specialties seen elsewhere in the literature (15–20). With 73% of medication remaining unused and at risk for diversion or misuse in the community, our finding that 82% of patients did not recall being instructed in the safe disposal of unused opioid medication has prompted the development of more uniform discharge instructions at our institution, including links within the electronic medical record to the Vermont Department of Health and medication disposal resources. Interestingly, over one-third of all patients we contacted did not receive, fill, or use an opioid prescription after their procedure. Similar findings have been noted in other studies (15, 18) and serve as a reminder that opioid pain management is not always necessary, even after major procedures such as joint arthroplasty.
Many state legislatures and health departments are adopting stricter rules governing opioid prescription practices, risk assessment of patients, and prescription drug monitoring programs (26). As of July 1, 2017, there are new requirements in the state of Vermont for providers prescribing opioids for acute and chronic pain (27). These include checking the Vermont Prescription Monitoring System (VPMS) for first-time opioid prescriptions greater than 10 pills, documenting patient informed consent for opioid use, and MME limits on certain opioid prescriptions according to the severity of the condition being treated or type of procedure performed.
Because of this trend, it will be increasingly incumbent on all prescribers to be familiar with MME dosing. Also unique to our study is the conversion of all opioid quantities into MME, which facilitates a more direct comparison across opioid type and prescription strength. We expect our findings, as well as those at other institutions, will be a useful reference as prescribing standards evolve. It will also be important for surgeons to engage with health departments and legislators in this process, in order to facilitate safe and effective prescribing, and minimize the unintended consequence of under treating post-operative pain.
In some cases, we found that the amount of medication use by patients was significantly more or less than expected. For example, the median opioid used by partial mastectomy, carpal tunnel and vasectomy patients was 0 MME (maximum 10–80 MME), while the median amount prescribed was a high as 40–60 MME, or the equivalent of 4–8 pills of 2 mg hydromorphone. These data would suggest that pre-operative discussions with patients about their pain tolerance, risk of opioid abuse, willingness to take opioids, or alternative methods of pain control could lead to fewer unused prescriptions. Our institutional opioid informed consent form, which complies with the 2017 Vermont Department of Health Rule, reviews these topics and facilitates such a discussion with patients.
In contrast, our data on hip and knee arthroplasty demonstrated that the median proportion used ranged from 31–50%. There was a much wider range of opioid use in these procedures (0–528 MME and 0–480 MME, respectively), including those patients who used no opioid at all. We suspect that pre-operative pain, and perhaps prior use of opioids, may have influenced post-operative usage. Identifying the factors that influence post-operative opioid use was beyond the scope of this study, however other studies have suggested patient age, insurance type, non-opioid adjunct therapy and prior opioid use to be contributing factors (17, 20).
We also noted that in both datasets the concurrent prescribing of tramadol in addition to a more potent opioid was taking place more often than anticipated, the majority of this practice occurring after orthopaedic surgeries. As this practice was an unexpected finding, our prospective questionnaire was designed to capture only the amount of the most potent opioid used, thus limiting our ability to assess whether concurrent tramadol use influenced the amount of opioid used.
While our findings reinforce the general perception that excess opioids are being prescribed after surgery, they also reflect the experience of a single academic medical center and are likely influenced by institution-specific factors. They also reflect practices and medication use specific to the specialties and procedures included in the study. While this may limit generalizability to some extent, the similarity of our findings to other recent studies in the literature suggests they may be more broadly applicable.
An inherent limitation of telephone questionnaire data is the reliance on patient recall and the inability to confirm the number of pills that patients reported using. This was felt to be acceptable given the effort it would have required to coordinate in-person interviews within the immediate post-operative timeframe. Our contact window was intended to occur at a time when patients were no longer requiring opioid medication and could still recall their pain management regimen and the amount of medication they used. The number of pills a patient was prescribed after surgery was confirmed by chart review in most cases.
Finally, it is possible that our exclusion criteria, especially regarding hospital length of stay and malignancy, were excessively stringent, thus limiting our sample size and the analyses that could be performed, including the detection of differences in opioid use in patients taking an opioid prior to surgery or receiving non-opioid pain management instructions.
Ultimately the question remains, how much should we be prescribing after surgery? There are some procedures for which the use of non-opioid pain management strategies should suffice with the addition of an opioid prescription on a case-by-case basis. This approach has been advocated for in the oral surgery literature for their most common procedure, extraction of wisdom teeth (28). Using data like ours, percentiles of MMEs prescribed and used among all patients who undergo specific procedures could be used to guide prescribing in subsequent patients undergoing the same procedures. If we set a policy of prescribing at the median level of use reported, then half of all patients would have adequate coverage of their pain, while half would require a refill. Alternatively, targeting prescriptions for the 75th percentile of opioid use for each procedure would result in a 20% reduction in prescription size across all procedures, while ensuring that the vast majority of patients achieve sufficient pain control with a single opioid prescription.
It has been shown that procedure-specific recommendations for opioid prescribing at discharge reduces the amount of opioid medication prescribed (29, 30). Our institution has developed a built-in decision support tool in the electronic medical record system to guide prescribers to the recommended MME to prescribe for certain common surgical procedures in conjunction with prescribing requirements outlined in the 2017 Vermont Department of Health Rule. This tool incorporates the patient usage data described here into a table grouping common surgical procedures to different pain categories and indicating the appropriate dosing and frequency of commonly prescribed opioid medications for mild, moderate, and severe pain. The goals of this effort are to minimize the quantity of excess opioids prescribed and familiarize prescribers with the concept of prescribing based on MME, particularly the residents and APPs who are doing the majority of opioid prescribing.
Conclusions
Our study shows that opioids are frequently being prescribed in excess after common surgical procedures and patients are not adequately instructed on how to dispose of unused medication. It also highlights considerable variability in the quantity of opioids prescribed and used after common procedures. Further investigation into the factors that influence opioid use after surgery is indicated.
Acknowledgments
The authors thank Kathleen Olson and Theodore Cisu for assistance with patient enrollment and data collection, as well as the Jeffords Center for Quality and Operational Effectiveness for assistance in obtaining the retrospective prescription data.
Support: Dr. Ahern was supported by a grant from the National Institute of General Medicine at the National Institutes of Health [P20 GM103644].
Support for this study: This work was supported by a grant from the National Institute of General Medicine at the National Institutes of Health [P20 GM103644].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the 98th Annual Meeting of the New England Surgical Society, Bretton Woods, NH, September 2017, and at the 86th Annual Meeting of the New England American Urological Association, Montreal, Canada, September 2017.
References
- 1.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in Drug and Opioid Overdose Deaths – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 2.Johnson H, Paulozzi L, Porucznik C, et al. Decline in drug overdose deaths after state policy changes – Florida, 2010–2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):569–574. [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 4.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use – United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam A, Gomes T, Zheng H, et al. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425–430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 6.Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ (Clinical research ed) 2014;348:g1251. doi: 10.1136/bmj.g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soneji N, Clarke HA, Ko DT, et al. Risks of Developing Persistent Opioid Use After Major Surgery. JAMA Surgery. 2016;151(11):1083–1084. doi: 10.1001/jamasurg.2016.1681. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surgery. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37–44. doi: 10.1016/j.drugpo.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132(1–2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Martins SS, Sarvet A, Santaella-Tenorio J, et al. Changes in US Lifetime Heroin Use and Heroin Use Disorder: Prevalence From the 2001–2002 to 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(5):445–455. doi: 10.1001/jamapsychiatry.2017.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker JA, Avorn J, Levin R, Bateman BT. Opioid Prescribing After Surgical Extraction of Teeth in Medicaid Patients, 2000–2010. JAMA. 2016;315(15):1653–1654. doi: 10.1001/jama.2015.19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wunsch H, Wijeysundera DN, Passarella MA, et al. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. JAMA. 2016;315(15):1654–1657. doi: 10.1001/jama.2016.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill MV, McMahon ML, Stucke RS, et al. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg. 2017;265(4):709–714. doi: 10.1097/SLA.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 16.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185(2):551–555. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers J, Cunningham K, Fitzgerald K, et al. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645–650. doi: 10.1016/j.jhsa.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Bartels K, Mayes LM, Dingmann C, et al. Opioid Use and Storage Patterns by Patients after Hospital Discharge following Surgery. PLoS One. 2016;11(1):e0147972. doi: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Matzon JL, Abboudi J, et al. A Prospective Evaluation of Opioid Utilization After Upper-Extremity Surgical Procedures: Identifying Consumption Patterns and Determining Prescribing Guidelines. J Bone Joint Surg Am. 2016;98(20):e89. doi: 10.2106/JBJS.15.00614. [DOI] [PubMed] [Google Scholar]
- 20.Lou I, Chennell TB, Schaefer SC, et al. Optimizing Outpatient Pain Management After Thyroid and Parathyroid Surgery: A Two-Institution Experience. Ann Surg Oncol. 2017;24(7):1951–1957. doi: 10.1245/s10434-017-5781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicaid and Medicare Services. Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors. 2017 Available at: http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-April-2017.pdf. Accessed May 18, 2017.
- 23.Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. 2015 Available at: http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed August 12, 2017.
- 24.Hintze JL, Nelson RD. Violin plots: A box plot-density trace synergism. Am Stat. 1998;52(2):181–184. [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 26.Centers for Disease Control and Prevention. Integrating & Expanding Prescription Drug Monitoring Program Data: Lessons from Nine States. 2017 Available at: http://www.cdc.gov/drugoverdose/pdf/pehriie_report-a.pdf. Accessed August 21, 2017.
- 27.Vermont Department of Health. Rule Governing the Prescribing of Opioids for Pain: 2017. Available at: http://www.healthvermont.gov/sites/default/files/documents/2016/12/REG_opioids-prescribing-for-pain.pdf. Accessed May 18, 2017. [Google Scholar]
- 28.Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 2013;144(8):898–908. doi: 10.14219/jada.archive.2013.0207. [DOI] [PubMed] [Google Scholar]
- 29.Stanek JJ, Renslow MA, Kalliainen LK. The Effect of an Educational Program on Opioid Prescription Patterns in Hand Surgery: A Quality Improvement Program. J Hand Surg Am. 2015;40(2):341–346. doi: 10.1016/j.jhsa.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 30.Hill MV, Stucke RS, Mcmahon ML, et al. An Educational Intervention Decreases Opioid Prescribing After General Surgical Operations. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002198. In press. [DOI] [PubMed] [Google Scholar]


