Abstract
Natural killer (NK) cells control antiviral adaptive immune responses in mice during some virus infections, but the universality of this phenomenon remains unknown. Lymphocytic choriomeningitis virus (LCMV) infection of mice triggered potent cytotoxic activity of NK cells (NKLCMV) against activated CD4 T cells, tumor cells, and allogeneic lymphocytes. In contrast, NK cells activated by vaccinia virus (VACV) infection (NKVACV) exhibited weaker cytolytic activity against each of these target cells. Relative to NKLCMV cells, NKVACV cells exhibited a more immature (CD11b-CD27+) phenotype, and lower expression levels of the activation marker CD69, cytotoxic effector molecules (perforin, granzyme B), and the transcription factor IRF4. NKVACV cells expressed higher levels of the inhibitory molecule NKG2A than NKLCMV cells. Consistent with this apparent lethargy, NKVacv cells only weakly constrained VACV-specific CD4 T-cell responses. This suggests that NK cell regulation of adaptive immunity, while universal, may be limited with viruses that poorly activate NK cells.
Keywords: NK cells, CD4 T cells, LCMV, vaccinia virus, NKG2A, interferon, IRF4
Introduction
Natural killer (NK) cells have been known to be cytotoxic to T cells and T cell lines since their initial discovery (Hansson et al., 1979; Nabel et al., 1981; Rabinovich et al., 2000), but recently there has been renewed interest in the capacity of NK cells to regulate adaptive immunity by targeting T cells during infection (Cook et al., 2015; Crouse et al., 2014; Gill et al., 2016; Lang et al., 2012; Lee et al., 2009; Rydyznski et al., 2015; Schuster et al., 2014; Sepulveda et al., 2015; Soderquest et al., 2011; Waggoner et al., 2011; 2014; Xu et al., 2014). Studies with lymphocytic choriomeningitis virus (LCMV) infections in mice showed that NK cells directly attack activated CD4 T (CD4act) cells and lyse them in a perforin-dependent manner (Waggoner et al., 2011). This was shown by in vivo cytotoxicity assay analysis, wherein fluorescently-labeled splenocytes from LCMV-infected mice were transferred directly into other infected mice that were depleted, or not, of NK cells, and a selective NK cell-dependent loss of donor CD4act cells was detected 5 hours later. By virtue of this targeting of CD4act T cells, NK cells indirectly affected cytotoxic CD8 T lymphocyte (Waggoner et al., 2011) and germinal center B-cell responses (Rydyznski et al., 2015). Cytolytic NK cell regulation of T cells consequently altered the balance between viral clearance and persistence as well as that between protective immunity and damaging immune pathology (Waggoner et al., 2011).
Several studies have revealed the importance of NK-cell suppression of T cells in the LCMV (Cook et al., 2015; Cook and Whitmire, 2013; Crouse et al., 2014; Guo et al., 2016; Lang et al., 2012; Rydyznski et al., 2015; Su et al., 2001; Waggoner et al., 2011; Waggoner and Kumar, 2012; Waggoner et al., 2010; Xu et al., 2014) and murine cytomegalovirus (MCMV) systems (Andrews et al., 2010; Lee et al., 2009; Schuster et al., 2014; Su et al., 2001; Waggoner et al., 2011; Zamora et al., 2017), but work with other viruses has been more limited, such that the universality of this phenomenon is unclear. Our group previously used an in vivo cytotoxicity assay to demonstrate that activation of CD4 T cells during infection with several different viruses induced susceptibility of these cells to NK cell-mediated killing (Waggoner et al., 2011; 2010; Waggoner and Kumar, 2012). These viruses included LCMV, MCMV, mouse hepatitis virus (MHV), Pichinde virus (PICV), and vaccinia virus (VACV). Similarly, three viruses (LCMV, MHV, PICV) tested for their capability to induce NK cell killing were capable of stimulating this activity. In contrast, VACV infection failed to stimulate substantial NK cell lysis of activated CD4act cells in the in vivo assays (unpublished observations). This exception suggested that NK cell killing of CD4act cells might not be a universal phenomenon and that the explanation and possible significance of this should be examined. Here we question why VACV is a weak trigger for NK-cell killing of CD4act cells and whether NK cells have any impact on VACV-specific T cell responses. We characterize NKVACV cells as being in a reduced state of activation and diminished cytolytic function. Nevertheless, these poorly activated NK cells still had a negative impact on VACV-specific CD4 T cell responses. For the purposes of this study, NK cells are defined by their expression of NK1.1 and the lack of CD3 expression.
Materials and methods
Virus strains and poly I:C treatment
The following virus strains were used with doses indicated in plague forming units (pfu)/mouse: lymphocytic choriomeningitis virus (LCMV) [Armstrong] 5 × 104 pfu; vaccinia virus (VACV) [Western Reserve] 2 × 106 pfu; mouse hepatitis virus (MHV) [A59] 8 × 105 pfu; and Pichinde virus (PICV) [AN3739] 1.5 × 107 pfu. Poly I:C was injected at a dose of 100 μg per mouse in HBSS. All infections and treatments were delivered by intraperitoneal injection.
Cell culture
YAC-1 cells were grown in RPMI (Gibco BRL) and L929 cells were grown in MEM (Gibco BRL). RPMI and MEM each were supplemented with 10% fetal calf serum (FCS), L-Glu (5 mM), and Penn-Strep (5 U/mL) at 37° C and 5% CO2.
Antibodies
Fluorescently-labeled antibodies were purchased from: BD Biosciences - NK1.1 (PK136), CD3e (145-2C11), CD4 (RM4-5), CD8β (YTS156.7.7), CD44 (IM7), CD11b (M1/70), CD107a (H4A3), Ly49 A (A1), Ly49 C/I (5E6), Ly49 D (4E5), Ly49 F (HBF-719), Ly49 G2 (4D11); eBioscience – 2B4 (244F4), Ly49 H (3D10), NKp46 (29A1.4), NKG2D (CX5); BioLegend – CD43 (1B11), CD69 (H1.2F3), CD27 (LG.3A10) DNAM-1 (TX42.1), NKG2A (16a11), IFNAR1 (MAR1-5A3), PD-1 (J43), IRF4 (3E4); and Invitrogen - GzmB (MHGB05).
Peptides
All peptides were ordered from 21st Century Biochemicals based on previously reported sequences (Cornberg et al., 2010; Jing et al., 2005; Moutaftsi et al., 2007; Oseroff et al., 2005; 2008; Tscharke et al., 2005): A3L270-277 (KSYNYMLL); A11R198-206 (AIVNYANL); A18R49-63 (PKGFYASPSVKTSLV); A20R233-247 (DNIFIPSVITKSGKK); A47L138-146 (AAFEFINSL); B2R46-60 (VKDKYMWCYSQVNKR); B5R46-60 (FTCDQGYHSSDPNAV);B8R20-27 (TSYKFESV); D13L486-500 (PKIFFRPTTITANVS); E9L179-193 (PSVFINPISHTSYCY); F15L55-69 (TPRYIPSTSISSSNI); I1L7-21 (QLVFNSISARALKAY); and K3L6-15 (YSLPNAGDVI).
Mouse strains and tissue harvesting
C57BL/6 mice were used for all cytotoxic and phenotypic NK cell studies. BALB/c mice were used only as donors for some in vivo cytotoxicity assays (as specified). C57BL/6 and BALB/c mouse strains were purchased from Jackson Laboratories and housed in the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS). All research was done under the guidance and approval of the UMMS Institutional Animal Use and Care Committee. Spleens were harvested from infected mice into RPMI media supplemented with 10% fetal calf serum (FCS), L-Glu (5 mM, Gibco), and Penn-Strep (5 U/mL, Gibco). Cells were gently pelleted by spinning at 800 × g for all centrifugation steps. Leukocyte preparations were made by treatment of bulk splenocytes with 0.84% NH4Cl in 10 mM Tris (pH 7.2) to lyse red blood cells followed by washing with HBSS containing FCS (10%) prior to antibody staining.
Flow cytometry
Splenocytes and peritoneal exudate cells were washed and rinsed in HBSS containing 2% FCS, and surface staining antibodies were applied in this same buffer. After washing, surface-stained cells were fixed for 5 minutes with Cytofix (BD Biosciences) followed by washing and storage in HBSS with 2% FCS. Cells stained for CD107a were incubated at 37 C and 5% CO2 for 1 hour in RPMI with FCS, L-Glu, Penn-Strep and monensin (BD Bioscience). Nuclear staining of IRF4 made use of the PhosFlow (BD Biosciences) fixation and permeabilization reagents followed by washing and storage in HBSS containing FCS. Peptide stimulation of T cells for intracellular cytokine staining (ICCS) was performed using the Cytofix/CytoPerm kit (BD Biosciences) according to manufacturer's instructions. Granzyme B (GzmB) staining of NK cells was also performed using Cytofix/CytoPerm. Nuclear and cytoplasmic stains each preclude the use of the other and were always performed separately according to manufacturer's instructions, which also allow for the inclusion of surface stains.
Reagents and labeling dyes
Poly I:C (Invivogen) was dissolved in HBSS and stored in frozen aliquots at -20° C. Cell Trace Violet (Molecular Probes), DDAO far red (Molecular Probes), and CFSE were freshly dissolved in HBSS at 37° C before incubation with target leukocytes prior to in vivo cytotoxicity assays.
In vivo cytotoxicity assay
Targets were incubated with labeling dye in HBSS for 15 minutes in a 37° C water bath and thoroughly rinsed in HBSS before transferring to host mice by tail vein injection. Spleens were harvested from host mice after incubation for 5-6 hours. In assays measuring the killing of activated T cells, donors were LCMV-infected mice that had been previously depleted of NK cells by intraperitoneal injection of monoclonal antibody (mAb) against NK1.1 (PK136, Bio-XCell) at 25 μg/mouse. Donors were harvested at day 4 post-infection to ensure that CD4act and CD8act cells would be abundant among the transferred target cells. Activated T cells were identified by high CD43 and CD44 expression (Waggoner et al., 2011). In assays measuring the killing of BALB/c target leukocytes, spleens were harvested from naïve BALB/c mice (Brehm et al., 2005) and then leukocyte suspensions were prepared and labeled as described above.
Chromium release assay
YAC-1 target cells were labeled with Na251CrO4 (Perkin Elmer) for 1 hour, and then combined with lymphocytes from individual mice. Targets and effectors were incubated at 37° C and 5% CO2 for 4 hours before centrifugation at 800 × g for 5 minutes. Supernatants were dispensed into Optiphase scintillation fluid (Perkin Elmer) and incubated overnight at room temperature to allow for passive mixing and resolution of sample turbidity prior to reading.
Trogocytosis assay
Leukocyte suspensions were prepared with room temperature buffers and media from spleens collected in room temperature RPMI containing FCS (10 %), L-Glu (5 mM), and Penn-Strep (5 U/mL). YAC-1 target cells were labeled as previously described (Daubeuf et al., 2006) using a stock solution of the fluorescently-labeled lipid SpDiI18C (Molecular Probes) dissolved in dimethyl formamide (Fisher) that was freshly diluted in Diluent C (Sigma-Aldrich) before labeling.
RNA isolation and RT-PCR
NKLCMV and NKVACV cells were sorted from day 3 post-infection bulk splenocytes using antibodies against NKp46 (PE, eBioscience), NK1.1 (APC, BD Biosciences), and CD3 (eFluor 450, eBioscience) sorted on a FACS Aria II (BD Biosciences) dedicated to biosafety level 2 plus (BSL 2+) usage. Fluorophores were chosen to minimize spectral overlap. Each pre-sort mixture was prepared from the pooled splenocytes of either 5 LCMV-infected, or 5 VACV-infected, C57BL/6 mice. Total RNA was purified from sorted splenocytes using the RNeasy Micro kit (Qiagen) and reverse transcribed into cDNA using random hexamer primers (Clontech). qPCR reactions were assembled with iQ SYBR Green Supermix (BioRad). Primers were ordered from Integrated DNA Technologies for perforin (Baars et al., 2005) (F: 5′-TGTGAACCCTAGGCCAGAGG-3′; R: 5′-GTGGAGCTGTTAAAGTTGCGG-3′), granzyme B (Revell et al., 2005) (F: 5′-ATCAAGGATCAGCAGCCTGA-3′; R: 5′-TGATGTCATTGGAGAATGTCT-3′), and the housekeeping gene HPRT (Baars et al., 2005) (F: 5′-GCAGTACAGCCCCAAAATGG-3′; R: 5′-AACAAAGTCTGGCCTGTATCCAA-3′) were used in separate reactions and run in triplicate on a CFX96 Real-Time PCR Machine (BioRad).
Data acquisition
Fluorescently-labeled cells were read by a LSR-II flow cytometer (BD Biosciences) and data were recorded in the FACS Diva suite followed by analysis in FlowJo. Samples from the 51Cr-release assay were read by a Wallac Trilux 1450 MicroBeta for 1 minute/well.
Statistical analysis
Comparisons between NKLCMV and NKVACV cells used Student's t-test (unpaired, parametric) with Welch's Correction in the Prism analysis suite (GraphPad).
Results
NK cell-mediated killing of activated CD4 T cells is negligible during VACV infection
Experiments were designed to compare the cytolytic activity induced 3 days after infection of C57BL/6 mice with LCMV or VACV, where the former but not latter virus efficiently triggers NK cell killing of activated CD4 T (CD4act) cell targets in vivo. Splenocytes from NK cell-depleted, LCMV-infected donor mice at day 4 p.i. were labeled with a fluorescent intracellular dye and then transferred by tail vein injection into LCMV- or VACV-infected hosts at day. Target splenocytes were taken from NK cell-depleted donor mice at day 4 in order to have a detectable number of activated CD4 T cell targets that had not already been eliminated by NK cells. Furthermore, although LCMV-infected donors were used in this study, CD4act cells from VACV-infected donors are known to have a comparable susceptibility to killing by NK cells in this cytotoxicity assay (Waggoner et al., 2011). Harvesting splenocytes from NK cell-depleted LCMV-infected donors ensures that a sufficiently large number of vulnerable CD4act cells is obtained for use as common targets for both LCMV- and VACV-infected hosts. NK depletion of the donors also prevents killing of the desired CD4act targets by NK cells in the donor prior to harvest or a carryover of donor NK cells skewing the results of the assay.
The donor cells were transferred into infected hosts at day 3 p.i. because at that time point NK cells are highly active and are the only cytotoxic lymphocytes mounting a significant response (Welsh, 1978; Welsh et al., 1979). CD4act express high levels of CD43 and CD44, and the loss of CD4 T cells expressing these molecules was an indicator of cytotoxicity against them. This loss was previously shown to be dependent on perforin expression by the NK cells and associated with apoptosis of the T cells (Waggoner et al., 2011), thus indicating that the decrease in CD4act population size is due to the death of CD43+CD44hi CD4 T cells rather than a downregulation of CD43 and CD44 on CD4act cells. Previous studies have also demonstrated that the loss of transplanted CD4act cells in infected host spleens cannot be accounted for by altered trafficking of CD4act cells to, and subsequent sequestration in, organs such as the lungs and liver (unpublished observations). Because CD4act cells were also lost in these host tissues when NK cells were not depleted, these data suggest that NK cell-mediated killing of CD4act cells was also occurring in the lungs and liver. Staining with Annexin V, to identify dying cells with exposed phosphatidylserine on the extracellular face of their plasma membranes, previously demonstrated that the percentage of Annexin V(+) staining CD4act cells decreased in the absence of NK cells, indicating that the CD4act cells in question were indeed dying disproportionately when NK cells were present (Waggoner et al., 2011). NK cells in this in vivo cytotoxicity assay have been shown to target CD4act cells specifically, but not B cells, CD8 T cells, or naïve CD4 T cells, thus allowing these cells and other donor leukocyte subpopulations to serve as internal controls whose percentages among lymphocytes are unaffected by NK cell-mediated cytotoxicity (Waggoner et al., 2011). The differences in percentages of remaining donor CD4act cells between the infected and uninfected hosts were used to calculate the percentage of CD4act cells killed.
The loss of donor CD4act cells was greater following transfer into mice infected with LCMV (mean 28%) as compared to recipient mice infected with VACV (mean 8%) in an in vivo cytotoxicity assay (Fig. 1A). Previously, LCMV-associated loss of CD4act cells was shown to be NK cell-dependent and required perforin (Waggoner et al., 2011). Here we observe that the percentages of splenic NK cells are reduced at day 3 of either infection relative to naïve (uninfected) control mice, but that the percentage of NK cells in VACV-infected mice was higher than that in LCMV-infected mice (Fig. 1B). These results argue that the poor killing of CD4act cells in VACV-infected mice was not due to virus-induced reductions in NK-cell frequencies.
Figure 1.
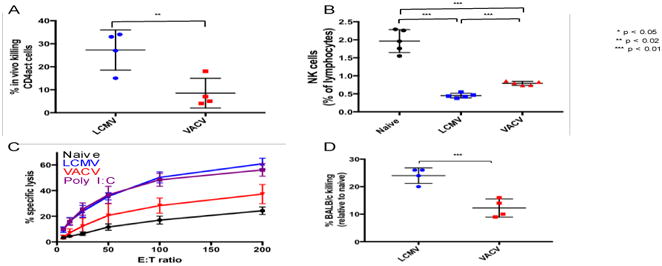
Comparison of cytolytic potential between LCMV-and VACV-induced NK cells. A. Percentage of labeled donor splenic CD4act cells from day 4 LCMV-infected mice killed in day 3 LCMV-infected and VACV-infected hosts after delivery of 6 × 107 labeled LCMV-infected (day 4) splenocytes, from NK cell-depleted donors, by tail vein injection and recovery in splenocyte preparation following 5 hour incubation. B. Splenic NK cell frequencies among naïve, LCMV- and VACV-infected mice at day 3 post-infection. C. Cytotoxicity of naïve, virally-induced, and poly I:C- activated splenic NK cells against 51Cr-labeled YAC-1 cells. D. Killing of BALB/c donor splenocytes in LCMV-and VACV-infected C57BL/6J hosts after delivery of 6 × 107 labeled naïve BALB/c splenocytes by tail vein injection and recovery in splenocyte preparations following 2-hour incubation. * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
VACV-induced NK cells demonstrate lower cytolytic activity than LCMV-induced NK cells
We first questioned whether the poor VACV-induced killing of CD4act cells reflected a selective defect or a more generalized dysfunction of NKVACV cytolytic functionality. Compared to NK cells from mice infected with LCMV or injected with the interferon-inducer, poly I:C, NK cells isolated from VACV-infected mice were poor killers of YAC-1 lymphoma target cells in an ex vivo 51Cr-release cytotoxicity assay (Fig. 1C). These results support the hypothesis that NKVACV cells exhibit a more generalized cytotoxicity deficiency. As another correlate of NK cell-mediated cytotoxicity in vivo, day 3 LCMV- or VACV- infected C57BL/6 mice were injected with CFSE-labeled allogeneic BALB/c splenocytes bearing H2d ligands that trigger the activating receptor Ly49D on C57BL/6 NK cells (Nakamura and Seaman, 2001). In these experiments, target splenocytes from BALB/c donors were labeled with CFSE and co-administered with internal control C57BL/6 splenocytes, using sufficiently different concentrations of CFSE to allow resolution of the two donor populations during flow cytometry. BALB/c splenocytes were cleared more vigorously in LCMV-infected than in VACV-infected mice (Fig. 1D). The greater abundance of NK cells in the spleens of VACV-infected mice, compared to LCMV-infected mice, was another indicator that the NKVACV cells have a lower cytotoxic capacity.
The studies comparing NK cell-mediated cytotoxicity against CD4act cells between LCMV- and VACV-infected hosts (Waggoner et al., 2011) set the parameters for how NK cells from these infections would be analyzed. Because these assays were performed in hosts at day 3 post-infection, this was chosen as the time when detailed comparisons of function and phenotype between NKLCMV and NKVACV would also be done.
The potency of NK cell-mediated cytotoxicity was compared between LCMV- and VACV-infected mice by measuring expression of the effector molecules granzyme B (GzmB) and perforin as indicators of cytotoxic potential. In this report, we define NK cells as NK1.1(+), CD3(-) lymphocytes and the term “prevalence” refers to the percentage of NK cells staining positive for a marker or receptor while “expression level” refers to the mean fluorescence intensity (MFI) associated with that marker or receptor among positive-staining cells. GzmB is closely associated with perforin-mediated cytotoxicity in NK cells (Bochan et al., 1995; Clement et al., 1990). During viral infections, NK cell-mediated cytotoxicity against CD4act cells depends on expression of the effector protein perforin (Waggoner et al., 2011). Among splenic NK cells, lower GzmB prevalence and expression levels, compared to NKlcmV cells, were measured in NKVACV cells at day 3 post-infection. These differences were apparent at both the mRNA and protein levels. Total RNA was harvested from NKp46(+) NK1.1(+) CD3(-) NK cells that were FACS-purified from the spleens of day 3 LCMV- and VACV-infected mice. Quantitative PCR (qPCR) of the cDNA synthesized from these RNA preparations revealed that in NKLCMV cells, GzmB mRNA was approximately eight times more abundant than in NKVACV cells (p < 0.0001) (Fig. 2A). The level of perforin mRNA was slightly higher in NKLCMV cells by approximately 14% (p < 0.05) compared to NKVACV cells (Fig. 2B). An overlay of histograms (representing NK cells from individual mice among the naïve, LCMV-infected, and VACV-infected cohorts) illustrates the relative distribution of GzmB-expressing NK cells and the level of GzmB expression on GzmB(+) NK cells (Fig. 2C). Statistical comparison of these three cohorts revealed that GzmB was expressed in both a smaller percentage of NK cells from VACV-infected mice than from LCMV-infected mice (Figs. 2D) and at lower levels within the GzmB(+) NKVACV cells (Fig. 2E) than for GzmB(+) NKLCMV cells.
Figure 2.
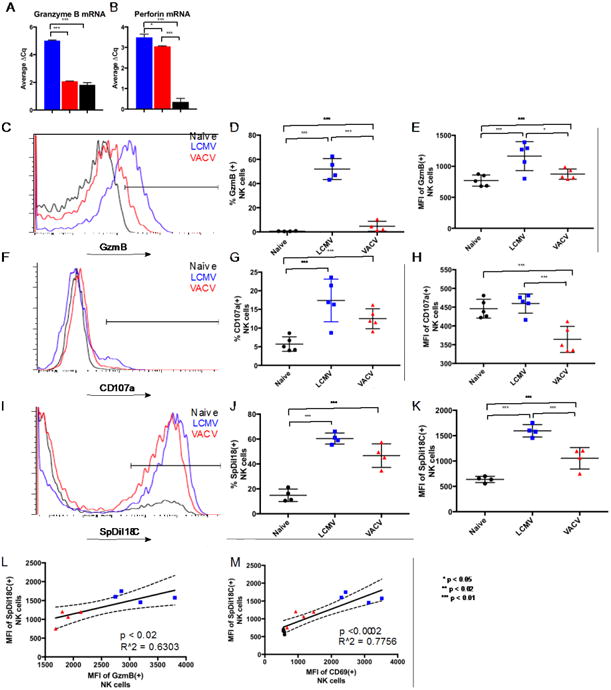
Comparison of cytolytic indicators between LCMV-and VACV-induced NK cells. A, B. GzmB and Perforin mRNA levels in day 3 purified splenic NKp46(+) NK 1.1(+) CD3(-) NKLCMV (blue bars) and NKVACV (red bars) cells compared to bulk LCMV-infected splenocytes (black bars). C. Overlaid flow cytometry profiles showing GzmB protein expression in NK cells from representative naive and infected mice (each histogram represents NK cells from a single mouse). D. Prevalence of GzmB(+) NK cells among total splenic NK cells. E. Expression level of GzmB in GzmB(+) splenic NK cells. F. Overlaid flow cytometry profiles showing steady state exposure of CD107a in the plasma membrane of splenic NK cells, after one hour ex vivo incubation with no additional stimulus, from representative naive and infected mice (each histogram represents NK cells from a single mouse) G. Percentages of splenic NK cells with detectable surface CD107a. H. Amounts of CD107a trafficked to the surface of CD107a(+) splenic NK cells. I. Comparison of trogocytosis among splenic NK cells from representative individual naïve, LCMV-, and VACV-infected mice in overlaid flow cytometry profiles. J. Frequency of membrane transfer to splenic NK cells. K. Amount of labeled YAC-1 target cell membrane absorbed by naïve and infected splenic NK cells through trogocytosis. L, M. Association of intensity of transferred SpDiI18C-labeled target membrane with GzmB and CD69 expression levels on splenic NK cells. * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
The delivery of cytotoxic granules during perforin-mediated cytolysis results in the surface exposure of CD107a, a component of lysosome-derived vesicle membranes (Olsen et al., 1990), at the surface of cytotoxic lymphocytes (Rubio et al., 2003). CD107a is required for efficient loading of perforin into lytic granules (Krzewski et al., 2013). CD107a that is deposited in the plasma membrane during the efflux of lytic granules will be endocytosed and recycled but accumulates to greater steady state surface densities on more cytolytically active NK cells and serves as a marker of degranulation (Aktas et al., 2009; Alter et al., 2004). CD107a-expressing splenic NK cells were more common during LCMV infection compared to infection with VACV (Fig. 2F) with no additional stimulus applied in vitro during the staining. Both infections induced NK cells with greater CD107a percentages than observed in uninfected mice (Fig. 2G), although variations in CD107a percentages for NKLCMV cells among LCMV-infected mice prevented statistically significant differences between the LCMV- and VACV-infected cohorts. The steady state cell surface density of CD107a was lower in NKVACV cells than in either naïve or NKLCMV cells, which indicated weaker degranulation by NKVACV cells (Fig. 2H). A previous study with human NK cells from whole blood stimulated in vitro by UV-inactivated VACV showed that VACV-stimulated NK cells contained lower percentages of CD107a(+) cells than controls receiving media without inactivated VACV (Moreira-Silva et al., 2014). Although these data appear contradictory to our own observations with regard to the percentages of CD107a(+) NK cells, they still indicate that exposure to VACV causes a decrease in the percentage of NK cells bearing CD107a on their surface. One explanation for this phenomenon could be that VACV exposure causes a decrease in the basal levels of vesicle trafficking present in naïve NK cells. The expression levels of CD107a on CD107a(+) NK cells were not provided in this study and thus could not be compared to our own results which are consistent with a VACV-mediated decrease in CD107a at the NK cell surface.
The term trogocytosis refers to the ability of cells to capture portions of surface membrane from other cells during close intercellular interactions. We used a trogocytosis assay previously developed for measuring T cell interactions with cultured dendritic cells (Daubeuf et al., 2006) and modified it to use YAC-1 cells as targets. NKVACV cells assimilated less membrane material from labeled YAC-1 target cells than did NK cells from LCMV-infected mice (Fig. 2I). Both the percentages of trogocytosing NK cells and the amount of the SpDiI-18C YAC-1 membrane marker transferred onto NK cells were lower in NKVACV cells than in NKLCMV cells (Figs. 2J, 2K). The amount of SpDiI-18C transferred from the labeled YAC-1 cells to NK cells correlated positively with the level of GzmB expression in GzmB(+) NK cells when data from NKLCMV, NKVACV, and naïve NK cells were combined (Fig. 2L). These data are consistent with the relatively low killing potential of NKVACV cells observed during in vivo and ex vivo cytotoxicity assays.
It seemed likely that the degree of trogocytosis in these NK cells might be related to their level of activation. CD69 is an activating C-lectin type receptor that serves as a reliable marker of NK cell activation. CD69 is poorly expressed on resting NK cells, but is prominently expressed on activated NK cells (Lanier et al., 1988). There was a positive correlation between the level of CD69 on CD69(+) NK cells and the amount of SpDiI-18C-labeled target membrane incorporated by NK cells (Fig. 2M).
NK cells from VACV-infected mice have a lower activation state than their LCMV-induced counterparts
The lower levels of GzmB expression and trogocytosis among NKVACV cells compared to NKLCMV cells implied that NKVACV cells might possess a lower activation state. As noted above, the level of CD69 expression varies among NK cells in accordance with the degree of SpDiI-18C absorption by NK cells from targets, and SpDiI-18C incorporation is lower among naïve NK cells and NKVACV cells. The percentage of NKVACV cells expressing CD69 was substantially lower than the corresponding percentage of NKLCMV cells (Figs. 3A, B). The CD69 expression level on CD69(+) NK cells was also much lower in NKVACV cells compared to NKLCMV cells (Fig. 3C). The percentage of CD69(+) NK cells and the CD69 expression level correlated positively with the cytolytic effector molecule GzmB (Fig. 3D, E). The weak expression of CD69 and GzmB in NKVACV cells, along with poor trogocytosis, were consistent with a lower activation state.
Figure 3.
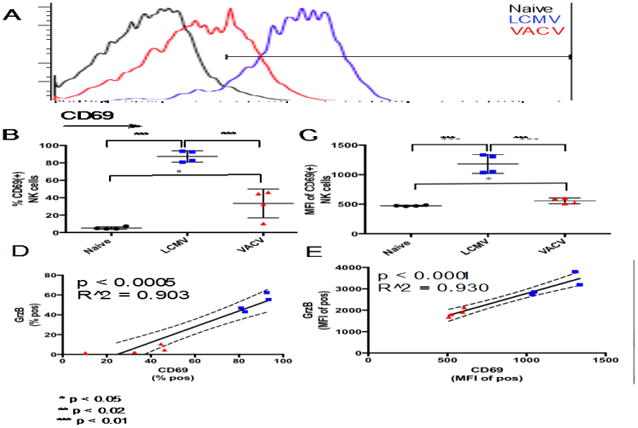
Comparison of NK cell activation between day 3 LCMV- and VACV-induced splenic NK cells. A. Overlaid flow cytometry profiles showing CD69 protein expression in NK cells from representative naive and infected mice (each histogram represents NK cells from a single mouse). B, C. CD69 prevalence and expression level in NK cells. D, E. Correlation of CD69 prevalence and expression level to GzmB. * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
VACV-induced NK cells exhibit a delayed maturation profile
Various stages of murine NK cell maturation have been identified according to CD11b and CD27 expression (Chiossone et al., 2009). Maturity follows a progression beginning with immature CD11b-CD27+ NK cells that acquire CD11b to become positive for both markers. The double positive NK cells then shed CD27 to become part of the CD11b+CD27- subset. The CD11b-CD27+ and CD11b+CD27+ subsets are associated with cytokine production while the CD11b+CD27- subset is considered the most cytolytically active. CD11b and CD27 expression have also been shown to define functionally distinct human NK cell populations (Fu et al., 2011).
Because NKVACV cells exhibited lower cytotoxicity and appeared less activated than their LCMV-induced counterparts, we tested the hypothesis that a larger percentage of NKVACV cells would be distributed among less cytolytically mature subsets. Indeed, there were significant differences between NKLCMV and NKvaCv cells in their distribution among the various maturity subsets (Fig. 4A). The most significant differences in subset frequency were for the CD11b-CD27+ and CD11b+CD27+ subsets (Fig. 4B). As hypothesized, a larger percentage of NKVACV cells possessed the early immature CD11b-CD27+ phenotype than NK cells induced by LCMV. The relatively small percentages of CD11b+CD27- NKLCMV and NKVACV cells might be explained by activity-dependent losses among this maturity subset following the destruction of multiple targets (Jewett and Bonavida, 1996; 1995), although the severity of these losses has been shown to vary according to the cytokine environment (Bhat and Watzl, 2007). The majority of NKLCMV and NKVACV cells are notably of the late immature CD11b+CD27+ phenotype that immediately precedes the cytolytically mature CD11b+CD27- phenotype. The greater percentage of CD11b+CD27+ NK cells during LCMV infection, compared to VACV infection, suggests a larger reservoir of CD11 b+CD27+ NKLCMV cells poised for progression to full cytolytic maturity.
Figure 4.
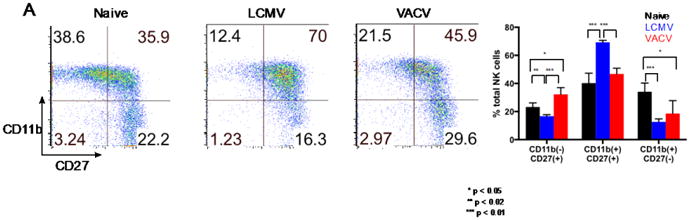
Differences in maturation between day 3 post-infection LCMV- and VACV-induced splenic NK cells. A. Expression of the maturity markers CD11b and CD27. B. Distribution of NK cells among CD11 b/CD27 subsets. Bars represent distribution of NK cells across CD11b/CD27 subsets for cohorts of naïve (black bars, n=4), LCMV-infected (blue bars, n=4), and VACV-infected (red bars, n=4) mice. * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
NK cells from LCMV- and VACV-infected mice possess distinct NKR expression profiles
The cytolytic action of NK cells is controlled by a balance of signaling inputs through stochastically-expressed activating and inhibitory receptors (Lee and Biron, 2010; Taylor et al., 2000). Activating NK cell receptors (NKRs) are a diverse group of proteins whose members recognize various ligand types, including stress ligands (Bauer et al., 1999), cellular adhesion molecules (Bottino et al., 2003), and other non-MHC surface proteins (Bottino et al., 2003; Latchman et al., 1998). Activating receptors mediate signaling through kinases that promote NK cell functions (McVicar and Burshtyn, 2001). Inhibitory receptors generally recognize MHC ligands (Kärre et al., 1986), with some exceptions (Rahim et al., 2015; Stanietsky et al., 2009), and prevent NK cells from lysing potential target cells by signaling through intracellular phosphatases (Burshtyn et al., 1996) that remove phosphate groups from activated protein substrates (Campbell, 2016). When NK cells engage a cell with few ligands to bind inhibitory receptors, any activating signals present in the NK cell will predominate and lead to cytolysis.
The prevalence of various NKRs within the populations of LCMV- and VACV-induced NK cells, and the intensity of each NKR's expression on the NK cells staining positively for that particular NKR (i.e. expression level), were examined at day 3 post-infection. Five activating receptors (Ly49D, Ly49H, NKG2D, NKp46, and DNAM-1) and six inhibitory receptors (Ly49A, Ly49C/I, Ly49F, Ly49G2, NKG2A, and PD-1) were evaluated. Expression of the SLAM family receptor 2B4, for which both stimulatory (Cannons et al., 2011) and inhibitory activity (Waggoner et al., 2010) have been reported, was also examined. Comparative analyses revealed differential expression of several NKRs between LCMV- and VACV-infected mice. Both the prevalence of a receptor among the NK cell population and its expression level on positively staining NK cells were considered. Cohorts of LCMV- and VACV-infected mice, consisting of 4-5 animals, were harvested on day 3 post-infection, and splenic NK cells were analyzed for percentage of NK cells expressing a given NKR (i.e. prevalence) and the MFI of the NKR(+) cells (i.e. expression level). The LCMV- and VACV-infected cohorts were compared using a parametric, unpaired t-test using Welch's correction, which does not assume equal standard deviations. The inhibitory receptors Ly49C/I and 2B4 were more prevalent among NKLCMV cells while NKG2A (another inhibitory receptor) was more prevalent among NKVACV cells (Supp. Table 1). With regard to differences in expression level (of receptor-positive cells) between NKLCMV and NKVACV cells, five receptors (Ly49D, Ly49F, Ly49H, NKG2A, and DNAM-1) were higher in NKVACV cells relative to NKLCMV cells, and five (Ly49C/I, Ly49G2, NKG2D, 2B4, and PD-1) were lower (Supp. Table 2). Each receptor was surveyed in a minimum of four experiments, and the reported data are from representative experiments.
The NKR expression analysis assumed that the NK cells present in the spleen were representative of circulating NK cells; however, the potential differences in receptor prevalence and expression levels between compartments were considered. The peritoneal cavity was the primary site of inoculation for both the LCMV- and VACV-infected mice. For all receptors examined, except NKp46 and PD-1, the prevalence and expression levels on NK cells from the spleen and peritoneal exudate were compared. For each NKR, ratios were computed for the percentage of NKR(+) splenic NK cells to the percentage of NKR(+) peritoneal exudate NK cells in individual mice (Supp. Table 3).
Most NKRs showed either no differences in prevalence between the spleen and peritoneal cavity, indicated by an average ratio of 1, or had similar relative differences between these two compartments for both LCMV- and VACV-infected mice. Ly49A(+) NK cells were more common in the spleens of VACV-infected mice, while there was no difference in the percentages of Ly49A(+) NK cells between the spleens and peritoneal cavities of LCMV-infected mice. This observation suggests that Ly49A is present on more NK cells in the spleen than in the peritoneal cavity during VACV infection. Ly49D(+), Ly49G2(+), and Ly49H(+) NK cells were less common in the spleens of VACV-infected mice than in the peritoneal cavity, but present at similar percentages in both compartments of LCMV-infected mice, suggesting a slight migration of these cells to the peritoneum during VACV infection.
DNAM-1 appeared to be present on fewer NK cells in the spleens of LCMV-infected mice than in the peritoneal cavity whereas there was little difference between compartments in VACV-infected mice. The lower percentage of DNAM-1(+) NK cells in the spleens of LCMV-infected mice compared to the percentage during VACV infection indicates either a mobilization of splenic DNAM-1(+) NK cells to the primary site of infection in the peritoneum or the proliferation of DNAM-1(+) NK cells already present in the peritoneum. This result is particularly interesting as it offers an explanation for the unexpected observations that expression of the activating receptor DNAM-1 exhibits a strong inverse correlation to GzmB expression (data not shown) and expression of DNAM-1 is higher in NKVACV cells than in NKLCMV cells (Supp. Tables 1, 2).
Ratios of expression levels on NKR(+) splenic NK cells to expression levels on NKR(+) peritoneal exudate NK cells were also computed (Supp. Table 4) for individual mice. For most NKRs, the average ratio of expression level on NKR(+) NK cells in the spleen to the expression level on NKR(+) peritoneal exudate NK cells was close to 1 for both LCMV- and VACV-infected mice, or exhibited similar differences between the two compartments for both LCMV- and VACV-infected mice. DNAM-1 expression level appeared similar on DNAM-1(+) NK cells between compartments for VACV-infected mice (average ratio of 0.96); however, in LCMV-infected mice, DNAM-1 expression appeared lower in splenic NK cells compared to peritoneal exudate NK cells (average ratio of 0.59). Ly49G2 expression in NKVACV cells was similar between the spleens and peritoneal cavities of VACV-infected mice, yet slightly lower in the splenic NK cells of LCMV-infected mice than in the NK cells within their peritoneal cavities.
Expression of the inhibitory NK cell receptor NKG2A is higher in VACV-induced NK cells than in LCMV-induced NK cells
The differential expression of NKG2A between VACV- and LCMV-induced NK cells, as well as the strength of this receptor's statistically significant inverse correlation with GzmB expression, made it the best potential marker of poor cytolysis against CD4act cells to emerge from the screen. The low p-value and high R-squared value for the correlation between NKG2A and GzmB indicate that there is a highly significant and strong inverse correlation between NKG2A and GzmB expression levels (Supp. Table 2). Strong correlations between NKG2A and GzmB in this system of virus-induced NK cell-mediated killing of CD4act cells were also notable because of previous reports linking NKG2A to NK cell-mediated killing of specific CD4act subsets during autoimmune pathologies (Leavenworth et al., 2011).
NKG2A displayed both increased prevalence and expression level in NKVACV cells when compared to NK cells from LCMV-infected mice (Fig. 5A-C). Both the prevalence and expression level of NKG2A exhibited a strong inverse correlation to the cytotoxic effector GzmB (Fig. 5D, E). In trogocytosis assays with SpDiI18C-labeled YAC-1 target cells, the expression level (Fig. 5G), but not prevalence (Fig. 5F), of NKG2A inversely correlated with membrane exchange between NK cells and YAC-1 targets. The prevalence and expression levels of NKG2A correlated inversely with CD69 prevalence and expression levels, respectively (Fig. 5H-I). These data are consistent with observations that CD94, the co-receptor of NKG2A, has been shown to mitigate the activity of CD69 in NK cells (Borrego et al., 1999) and decreased NKG2A expression is associated with the upregulation of CD69 expression in γδ T cells (Wang et al., 2012).
Figure 5.
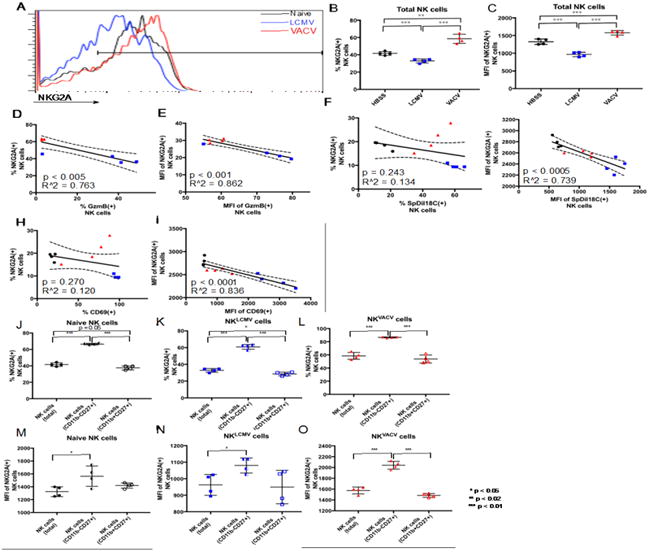
Differential expression of NKG2A between LCMV- and VACV-induced splenic NK cells. A. Overlaid flow cytometry profiles showing NKG2A protein expression in NK cells from representative naive and infected mice (each histogram represents NK cells from a single mouse). B, C. NKG2A prevalence and expression level in NK cells. D, E. Correlation of NKG2A prevalence and expression level to GzmB. F. Correlation of percent NKG2A(+) NK cells to the percentage of NK cells engaged in membrane exchange. G. Correlation of NKG2A expression level in NKG2A(+) NK cells to the degree of trogocytosis in SpDiI-18C(+) NK cells. H, I. Correlation of NKG2A prevalence and expression level to CD69. L-O. Prevalence and expression levels of NKG2A for CD11 b-CD27+ and CD11b+CD27+ NK cell maturity profiles compared to total NK cells for naïve NK cells (J, M), NKLCMV cells (K, N), and NKVACV cells (L, O). * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
The disparities between the prevalence of NKG2A(+) NKLCMV and NKVACV cells, and expression levels between these NK cells, appeared greatest in the less mature subsets. NKG2A was most prevalent among NK cells with the less mature CD11b-CD27+ phenotype (Figs. 5J-L). The greatest percentage of NKG2A expression was observed in CD11b-CD27+ NKVACV cells, of which an average of 88% express NKG2A (Fig. 5L) compared to 62% of NKLCMV cells (Fig. 5K). For NKLCMV and naïve NK cells, NKG2A prevalence in the intermediately mature CD11b+CD27+ NK cell population was slightly lower than the prevalence observed in the total NK cell populations (Fig. 5J, K), yet similar for NKVACV cells (Fig. 5L). Expression levels for NKG2A were also higher in the CD11b-CD27+ subsets, with NKVACV cells having the highest expression levels of NKG2A (Fig. 5M-O). These results suggest that that NKG2A expression is also a hallmark of immaturity among NK cells, especially among poorly activated populations.
VACV-induced NK cells express low levels of the IRF4 transcription factor
Type 1 IFN is induced at high levels in many virus infections and has been shown to be a major activator of NK cells (Gidlund et al., 1978; Oehler et al., 1978; Santoli et al., 1978a; 1978b; Trinchieri et al., 1978; Welsh, 1978). The WR strain of VACV used here is a weak type 1 IFN (IFN-I) inducer, like most orthopoxviruses. Furthermore, although IFN-I may be produced during infection with VACV, the effective IFN-I titer is suppressed by an arsenal of viral accessory proteins (Perdiguero and Esteban, 2009). Average VACV-induced serum IFN-I titers at day 3 post-infection are lower than 3 [log2] units per 50 μL of serum, in contrast to more than 10 [log2] units for LCMV-infected mice and 6 [log2] units for mice examined after treatment with the potent IFN-I inducer poly I:C (Fig 6A). NKLCMV cells had lower apparent expression of the interferon α/β receptor alpha chain (IFNAR1) than NKVACV cells (Fig. 6B), perhaps due to either a possible ligand-mediated blocking of the staining antibody or a genuine downregulation of IFN-I receptors following activation (Branca and Baglioni, 1982).
Figure 6.
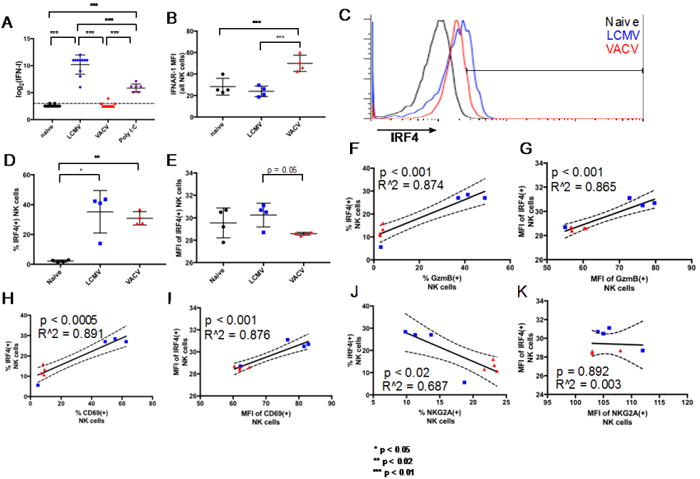
Comparison of IFN-I response and IRF4 expression between LCMV- and VACV-induced splenic NK cells. A. Serum IFN-I titers. B. Average IFNAR1 expression on all NK cells present in naïve, LCMV-, and VACV-infected mice. C. Overlaid flow cytometry profiles showing IRF4 protein expression in NK cells from representative naive and infected mice (each histogram represents NK cells from a single mouse). D, E. IRF4 prevalence and expression levels in NK cells. F-I. Correlation of IRF4 prevalence and expression levels to GzmB and CD69. J, K. Correlation of IRF4 prevalence and expression level to NKG2A. * p < 0.05, ** p < 0.02, *** p < 0.01. NK cell gating scheme: Singlets (FSC)>Lymphocytes(SSC)>NK 1.1(+) CD3(-).
Expression of IRF4 among NKVACV cells is generally lower than among NKLCMV cells (Fig. 6C) although variability among the LCMV-infected mice precludes statistical significance of the difference in IRF4 prevalence between the LCMV- and VACV-infected cohorts (Fig. 6D). IRF4(+) NK cells had lower expression levels of IRF4 (Fig. 6E) in NKVACV cells than in NKlCmv cells. IRF4 is a lymphoid cell-specific transcription factor that belongs to the IRF family, whose members are defined by the ability to bind the interferon-stimulated response element (ISRE). IRF4 is induced in human NK and T cells by IFN-I (Lehtonen et al., 2003) and mitogens, often acting in concert with other transcription factors such as PU.1, phosphorylated STAT6, and Bcl6 to promote cytokine production in T cells (Ahyi et al., 2009; Hu et al., 2002). IRF4 is also required for the proper function of mature B and T cells (Mittrücker et al., 1997). There were strong positive correlations between IRF4 and GzmB with respect to both prevalence and expression levels when NKVACV and NKLCMV cells were compared (Fig. 6F, G). Similarly, strong positive correlations were observed between IRF4 and CD69 (Fig. 6H, I). CD69 expression is known to be subject to induction by IFN-I (Gerosa et al., 1991) and might be expected to coincide with IRF4 if the expression of IRF4 is IFN-driven. There was a significant inverse correlation between the prevalence of NKG2A and IRF4 (Fig. 6J) while no correlation was observed between the expression levels of NKG2A and IRF4 (Fig. 6K). It is noteworthy that, despite the variability in IRF4 prevalence among NK cells from LCMV-infected mice, these cells maintain the correlations between IRF4 prevalence and the prevalence of GzmB, CD69, and NKG2A (Fig. 6F, H, J).
NK cell-dependent suppression of VACV-specific CD4 T cell responses late in VACV infection
The poor cytolytic capacity of the NKVACV cells motivated us to question whether NK cells had as much effect on the T cell response to VACV as they have on the T cell response to LCMV and other viruses. First we questioned whether the depletion of NK cells had an effect on VACV titers. An increase in VACV titers after NK cell depletion might suggest that NK cells were directly controlling the virus. Alternatively, a decrease in viral titers would be consistent with NK cells controlling the T cells that regulate viral titer. VACV titers were measured at days 4, 6, 8, and 10 post-infection in the liver and fat pads, and found to be similar in NK cell-depleted mice and undepleted controls (Supplementary Fig. 1). Virus was cleared in the liver by day 8 and lingered longer in the fat pads, but titers were similar in both groups of mice. These data indicate that any increase in the antiviral CD4 T cell population in the absence of NK cells would not be due to marked differences in antigen load.
The failure of NKVACV cells to kill CD4act cells effectively early in infection, relative to NK cells induced by other virus infections, was likely due to the poor cytolytic capacity of the NKVACV cells during this discrete time period. However, it was unclear if adaptive immunity was completely unaffected by NK cells in the VACV system, or whether effects would be noticed after a longer time period of infection. Thus, the total number and percentages of CD4 and CD8 T cells were examined over a time course spanning day 4 to day 14 post-infection. The number and percentage of total CD4 T cells present within the spleen lymphocyte population of NK cell-depleted mice were comparable to the values observed in non-depleted control mice until day 14, when both the number and frequency of CD4 T cells appeared slightly lower in the absence of NK cells, perhaps due to resolution of the response (Fig.7A, C). The CD8 T cell numbers were not statistically different at any time point, although the percentage may have been slightly higher in the absence of NK cells (Fig. 7B, D). Because CD4act cells, identified by the expression of CD43 and high expression of CD44 (CD43+CD44hi), are the target of NK cell-mediated killing during viral infections, it was important to determine if these CD4act cells were culled by NK cells during days 4 to 14 post-infection. On most days during this time course, the presence of NK cells had no significant effect on the frequencies of CD4act cells except for on days 6 and 10 p.i. when CD4act cells were slightly more abundant in the absence of NK cells (Fig. 7E). There were no NK cell-dependent differences in the frequencies of CD8act cells on any of the days examined (Fig 7F).
Figure 7.
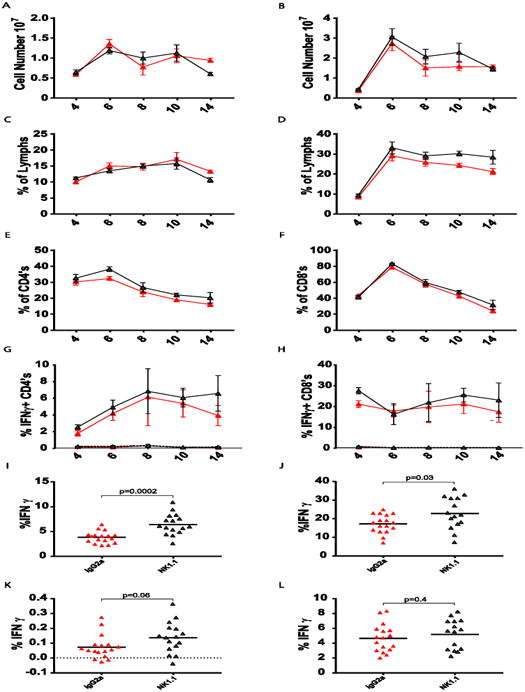
Effects of VACV-induced NK cells on T cell populations. VACV-infected mice were treated with either monoclonal antibody against NK1.1 (red) or the non-depleting isotype control antibody (grey) prior to infection. Data in panels A-H are pooled from three identical experiments, with n = 8-10 per group, except for day 14, which was one experiment in a complete time course with n = 5 per group. Day 14 data from panels I-L are pooled data from four identical experiments. A, B. Comparison of CD4 and CD8 T cell population size in the presence and absence of NK cells. C, D. CD4 and CD8 T cell percentages in the presence and absence of NK cells. E, F. CD4act and CD8act cell percentages in the presence and absence of NK cells, as indicated by co-expression of CD43 and CD44. G, H. Percentages of IFNy(+) CD4 and CD8 T cells responsive to activation by antibody to CD3ε (solid lines) or control background (dotted lines), in the presence and absence of NK cells. I, J. Percentages of anti-CD3-activated IFNγ(+) CD4 and CD8 T cells in the presence and absence of NK cells at day 14 post-infection. K. B5R-specific antiviral IFNγ (+) CD4 T cell response at day 14 p.i. with the background IFNγ (+) percentage subtracted. L. The immunodominant B8R-specific antiviral IFNγ (+) CD8 T cell subpopulation at day 14 with the background IFNγ (+) percentages subtracted. T cell pre-gating scheme: Singlets (FSC)>Lymphocytes(SSC).
As another indicator of the mobilization of the T cell response into he infection, spleen leukocytes were stimulated with antibody to CD3 and tested for IFNγ production in intracellular cytokine assays (Fig. 7G, H, solid lines). Background stimulation without anti-CD3 antibody is shown in the dotted lines. There were no significant differences in the responses of either CD4 or CD8 T cells to NK cell deletion, except perhaps for the day 14 CD4 T cells, for which the NK cell-depleted samples appeared to have higher percentages. For better statistical reliability, T cells from mice from four day 14 experiments were stimulated with anti-CD3 and tested for IFNγ production. Fig. 7I and J show modest but statistically higher frequencies of IFNγ producing both CD4 and CD8 cells, with the CD4 cells being of particularly high significance (p = 0.0002). To test for specific T cell responses, the T cell responses to 14 VACV-encoded CD4 T cell epitopes and 5 VACV-encoded CD8 T cell epitopes were evaluated during the course of infection. Responses to the CD4 epitopes were very weak and not easily distinguished from background responses during the earlier stages of infection. However, with background subtracted, weak but elevated responses to some epitopes, such as B5R, could be detected at Day 14 and were slightly higher in NK-depleted mice (Fig. 7K). The percentages of T cells responsive to the CD8 epitopes were more easy to detect but did not seem very different in the NK-depleted vs. control group, as shown with the immunodominant CD8 epitope, B8R (Fig. 7L). These data suggest that, despite their poor cytolytic activity, NKVACV cells might still have a modest effect on the virus-specific CD4 T cell response over time.
Discussion
This report addresses the question of whether the NK cell-mediated control of adaptive immunity could be a universal phenomenon in viral infections. We demonstrate that NKVACV cells, in contrast to NK cells induced by the other virus infections previously tested (Waggoner et al., 2011), including LCMV, exhibited poor capacity to lyse activated CD4 T cells in vivo. NKVACV cells were also poorly cytotoxic against YAC-1 lymphoma cells ex vivo and against allogeneic leukocytes in vivo. In comparison to NKLCMV cells, this poor cytotoxicity of NKVACV cells was associated with lower levels of mRNA encoding the cytolytic effector molecule GzmB and also lower levels of GzmB protein. The NKVacv cells skewed more dramatically towards an immature differentiation state than NKLCMV cells and expressed intermediate levels of the activation molecule CD69 and high levels of the inhibitory NKR NKG2A. This reduced differentiation and cytolytic activity correlated with a poor type 1 IFN response and relatively low expression of the IFN-associated transcription factor, IRF4. Expression of IRF4 correlated with the expression of granzyme B and other activation and maturation markers.
Despite the poor cytolytic activity at the peak of the NK cell response, NKVACV cells maintained some level of activity over and above naïve NK cells and clearly were responding to infection. This was indicated by the moderate expression of activation markers and effector molecules. Indeed, extensive analyses of five experiments at day 14 post-infection showed an elevated CD4 T cell response to the class II MHC-presented VACV-encoded epitope B5R in the absence of NK cells. There also was a significantly elevated response to CD4 T cells stimulated with anti-CD3 at this time. This is consistent with the concept that these cytolytically weak NK cells could still have a modest though weak impact on the CD4 T cell response over a sustained period of time. In contrast to the effect on CD4 T cells, there was little effect of NK cell depletion on the epitope-specific CD8 T cell epitopes, which in the LCMV system have been reported to be poor targets for activated NK cells due to elevated expression of CD48, which drives negative signals to NK cells by virtue of the receptor 2B4 (Waggoner et al., 2010).
The ability of NK cells to exert direct control over VACV in the mouse model has long been a subject of controversy and our data in this report (Supplementary Fig. 1) indicate that they do not directly control VACV in males of the C57BL/6 mouse strain. Previous work suggesting that NK cells control VACV might be accounted for by the possibility that gamma delta (γδ) T cells are the lymphocyte population behind this phenomenon. The γδ T cells express high levels of the NK cell marker asialo-GM1 and low levels of NK1.1. Our method of anti-NK1.1 mAb-mediated depletion has been carefully designed to use a dose of anti-NK1.1 sufficient for robust elimination of NK cells while leaving other known NK1.1-expressing populations, such as γδ T cells, unperturbed because they express levels of NK1.1 that are insufficient to make them vulnerable to opsonization and antibody-dependent cell cytotoxicity (ADCC). Nevertheless, our findings that VACV viral load in the liver and fat pads was unaffected by the lack of NK cells allowed us to analyze the influence of NK cells on the developing T cell response without having to worry that any differences in the absence of NK cells were being caused by differences in viral load rather than the NK cells themselves. Indeed, as mentioned above, our data indicate that, as expected, there was only a modest and barely detectable increase in the CD4 T cell response when NK cells had been depleted.
Our data demonstrate that NKVACV cells are poorly activated and ineffective at killing CD4act cells. IFN-I is a strong driver of the NK cell response (Welsh, 1978) and low levels of IFN-I or other cytokines found during VACV infection seem sufficient for the weak activation of some crucial NK cell functions. Previous work from the Yang laboratory has supported the requirement of IFN-I signaling (Martinez et al., 2008), and the STAT1 transcription factor on which it depends (Fortin et al., 2013), in the VACV system. Genetic studies with knockout mice for toll-like receptor 2 (TLR2) and myeloid differentiating factor 88 (MyD88) have suggested that signaling through the TLR2-MyD88 pathway is necessary for the activation of NK cells during VACV infection (Martinez et al., 2010). These studies suggest that VACV-induced NK cells still depend on the relatively weak IFN-I signaling present during VACV infection, yet these cells also might require other activating signals to augment their cytotoxicity.
The finding that expression of the inhibitory receptor NKG2A was higher in VACV-induced NK cells than in LCMV-induced NK cells was intriguing because of reports that NKG2A might play a role in inhibiting the NK cell-mediated control of autoreactive CD4act cells that otherwise lead to autoimmune disorders (Leavenworth et al., 2011; 2010; Lu et al., 2007). The data presented here reveal that NKG2A expression was not only higher in NKVACV cells relative to NKLCMV cells, but it also had strong inverse correlations with the activation marker CD69 and the cytotoxic effector GzmB. Our data suggest that NKG2A is a marker of a poorly activated NK cell; however, further studies are required before functional importance can be assigned to NKG2A in modulating the ability of NK cells to kill virus-induced CD4act cells. Interestingly, in human NK cells, the transcription factor Gata-3 increases transcription from the NKG2A promoter (Marusina et al., 2005), and there are possible binding sites for Gata-3 in the mouse NKG2A promoter. A hallmark of VACV infection is a weak type I interferon response, and IFN-I has been shown to suppress Gata-3 transcription from an alternate first exon in human Th2 cells (Huber et al., 2014).
In conclusion, NKVACV cells are phenotypically distinct underperformers among NK cells from viral infections with regard to their abilities to kill CD4act cells and other targets. This report reveals that the lower overall cytotoxicity of NKVACV cells is associated with lower activation and a shift of the NK cell population towards lower maturity states. A poor type 1 IFN response is likely at least partially responsible for this NKVACV cell phenotype, which is also associated with low expression of GzmB and IRF4. NKG2A was more highly expressed among NKVACV cells compared to their LCMV-induced counterparts, but we have no evidence that this NKR is functionally important other than being a marker of poorly activated cells. Overall these data show why CD4act cells are poorly killed by NKVACV cells during cytotoxicity assays in vivo. This might suggest that NK cells are poor regulators of adaptive immunity during VACV infection. However, when the long-term effects of NK cell depletion were examined, there were substantially higher percentages of CD4 T cells specific for one VACV-encoded epitope late in infection. This would argue that the role of NK cells in regulating adaptive immunity may indeed be a universal phenomenon, even with viruses like VACV that are poor inducers of NK cell activity.
Supplementary Material
Supplemental Figure 1. Effect of NK cell depletion on VACV load during the course of infection. Numbers plotted are log10 values derived from viral plaque assay on vero cells at the indicated day post infection. The dotted line represents the limit of detection for the indicated organ. Top, Liver (n=5 per group). Bottom, Fat pads (n=5 per group).
Highlights.
NKVACV cells are atypically poor killers of CD4 T cells early in infection.
NKVACV cells are less cytotoxic and mature than CD4 T cell-killing NKLCMV cells.
The inhibitory receptor NKG2A is enriched in NKVACV cells.
Poor cytotoxicity of NKVACV cells corresponds to low IFN-I and IRF-4 expression.
Nevertheless, NKVACV cells still partially reduce the CD4 response by day 14 of infection.
Acknowledgments
We thank Liisa K. Selin, Eva Szomolanyi-Tsuda, Rabinarayan Mishra, and Mauricio Calvo-Calle for helpful discussion; Laurie Kenney for VACV stock preparation; Stina Urban, Jenny Che, Barry Kriegsman, and Jian-Jyh Lai for technical expertise; and the UMMS Flow Cytometry Core for fluorescent sorting of NK cells prior to qPCR analysis. This work was supported by the Ellison Medical Foundation New Scholar Award and United States National Institutes of Health (NIH) research grant DA038017 to SNW and NIH grant AI109858 to RMW. The conclusions represent the opinions of the authors and not necessarily those of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahyi ANN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254:149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RAW. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochan MR, Goebel WS, Brahmi Z. Stably transfected antisense granzyme B and perforin constructs inhibit human granule-mediated lytic ability. Cell Immunol. 1995;164:234–239. doi: 10.1006/cimm.1995.1166. [DOI] [PubMed] [Google Scholar]
- Borrego F, Robertson MJ, Ritz J, Peña J, Solana R. CD69 is a stimulatory receptor for natural killer cell and its cytotoxic effect is blocked by CD94 inhibitory receptor. Immunology. 1999;97:159–165. doi: 10.1046/j.1365-2567.1999.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, Vitale M, Moretta L, Lopez M, Moretta A. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branca AA, Baglioni C. Down-regulation of the interferon receptor. J Biol Chem. 1982;257:13197–13200. [PubMed] [Google Scholar]
- Brehm MA, Daniels KA, Ortaldo JR, Welsh RM. Rapid conversion of effector mechanisms from NK to T cells during virus-induced lysis of allogeneic implants in vivo. J Immunol. 2005;174:6663–6671. doi: 10.4049/jimmunol.174.11.6663. [DOI] [PubMed] [Google Scholar]
- Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KS. Suppressing the killer instinct. Sci Signal. 2016;9:fs8–fs8. doi: 10.1126/scisignal.aaf6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- Clement MV, Haddad P, Soulie A, Guillet J, Sasportes M. Involvement of granzyme B and perforin gene expression in the lytic potential of human natural killer cells. Nouv Rev Fr Hematol. 1990;32:349–352. [PubMed] [Google Scholar]
- Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol. 2015;98:153–162. doi: 10.1189/jlb.4HI1214-594R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol. 2013;190:641–649. doi: 10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim SK, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol. 2010;184:2825–2838. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Daubeuf S, Puaux AL, Joly E, Hudrisier D. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nat Protoc. 2006;1:2536–2542. doi: 10.1038/nprot.2006.400. [DOI] [PubMed] [Google Scholar]
- Fortin C, Huang X, Yang Y. Both NK cell-intrinsic and -extrinsic STAT1 signaling are required for NK cell response against vaccinia virus. J Immunol. 2013;191:363–368. doi: 10.4049/jimmunol.1202714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350–359. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Scardoni M, Tommasi M, Benati C, Snelli L, Gandini G, Libonati M, Tridente G, Carra G. Interferon alpha induces expression of the CD69 activation antigen in human resting NK cells, while interferon gamma and tumor necrosis factor alpha are ineffective. Int J Cancer. 1991;48:473–475. doi: 10.1002/ijc.2910480328. [DOI] [PubMed] [Google Scholar]
- Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gill US, Peppa D, Micco L, Singh HD, Carey I, Foster GR, Maini MK, Kennedy PTF. Interferon Alpha Induces Sustained Changes in NK Cell Responsiveness to Hepatitis B Viral Load Suppression In Vivo. PLoS Pathog. 2016;12:e1005788. doi: 10.1371/journal.ppat.1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Cranert SA, Lu Y, Zhong MC, Zhang S, Chen J, Li R, Mahl SE, Wu N, Davidson D, Waggoner SN, Veillette A. Deletion of Slam locus in mice reveals inhibitory role of SLAM family in NK cell responses regulated by cytokines and LFA-1. J Exp Med. 2016;213:2187–2207. doi: 10.1084/jem.20160552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Kiessling R, Andersson B, Kärre K, Roder J. NK cell-sensitive T-cell subpopulation in thymus: inverse correlation to host NK activity. Nature. 1979;278:174–176. doi: 10.1038/278174a0. [DOI] [PubMed] [Google Scholar]
- Hu CM, Jang SY, Fanzo JC, Pernis AB. Modulation of T cell cytokine production by interferon regulatory factor-4. J Biol Chem. 2002;277:49238–49246. doi: 10.1074/jbc.M205895200. [DOI] [PubMed] [Google Scholar]
- Huber JP, Gonzales-van Horn SR, Roybal KT, Gill MA, Farrar JD. IFN-α suppresses GATA3 transcription from a distal exon and promotes H3K27 trimethylation of the CNS-1 enhancer in human Th2 cells. J Immunol. 2014;192:5687–5694. doi: 10.4049/jimmunol.1301908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156:907–915. [PubMed] [Google Scholar]
- Jewett A, Bonavida B. Target-induced anergy of natural killer cytotoxic function is restricted to the NK-target conjugate subset. Cell Immunol. 1995;160:91–97. doi: 10.1016/0008-8749(95)80013-9. [DOI] [PubMed] [Google Scholar]
- Jing L, Chong TM, McClurkan CL, Huang J, Story BT, Koelle DM. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J Immunol. 2005;175:7550–7559. doi: 10.4049/jimmunol.175.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK- cell cytotoxicity. Blood. 2013;121:4672–4683. doi: 10.1182/blood-2012-08-453738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, Dissanayake D, Rahbar R, Ghazarian M, Brüstle A, Fine J, Chen P, Weaver CT, Klose C, Diefenbach A, Häussinger D, Carlyle JR, Kaech SM, Mak TW, Ohashi PS. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, Phillips JH. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988;167:1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, McKay PF, Reiser H. Identification of the 2B4 molecule as a counter-receptor for CD48. J Immunol. 1998;161:5809–5812. [PubMed] [Google Scholar]
- Leavenworth JW, Schellack C, Kim HJ, Lu L, Spee P, Cantor H. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab')2. Proc Natl Acad Sci USA. 2010;107:2562–2567. doi: 10.1073/pnas.0914732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc Natl Acad Sci USA. 2011;108:14584–14589. doi: 10.1073/pnas.1112188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Biron CA. Here today--not gone tomorrow: roles for activating receptors in sustaining NK cells during viral infections. Eur J Immunol. 2010;40:923–932. doi: 10.1002/eji.201040304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen A, Lund R, Lahesmaa R, Julkunen I, Sareneva T, Matikainen S. IFN-alpha and IL-12 activate IFN regulatory factor 1 (IRF-1), IRF-4, and IRF-8 gene expression in human NK and T cells. Cytokine. 2003;24:81–90. doi: 10.1016/j.cyto.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Lu L, Ikizawa K, Hu D, Werneck MBF, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- Marusina AI, Kim DK, Lieto LD, Borrego F, Coligan JE. GATA-3 is an important transcription factor for regulating human NKG2A gene expression. J Immunol. 2005;174:2152–2159. doi: 10.4049/jimmunol.174.4.2152. [DOI] [PubMed] [Google Scholar]
- McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Sci STKE. 2001;2001:re1–re1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- Mittrücker HW, Matsuyama T, Grossman A, Kündig TM, Potter J, Shahinian A, Wakeham A, Patterson B, Ohashi PS, Mak TW. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275:540–543. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- Moreira-Silva EADS, Medeiros-Silva DC, Gomes JdeAS, da Fonseca FG, Correa-Oliveira R. Profile of natural killer cells after a previous natural Vaccinia virus infection in an in vitro viral re-exposure. Virus Research. 2014;184:20–29. doi: 10.1016/j.virusres.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, Pasquetto V, Crotty S, Croft M, Lefkowitz EJ, Grey H, Sette A. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J Immunol. 2007;178:6814–6820. doi: 10.4049/jimmunol.178.11.6814. [DOI] [PubMed] [Google Scholar]
- Nabel G, Bucalo LR, Allard J, Wigzell H, Cantor H. Multiple activities of a cloned cell line mediating natural killer cell function. J Exp Med. 1981;153:1582–1591. doi: 10.1084/jem.153.6.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura MC, Seaman WE. Ligand interactions by activating and inhibitory Ly-49 receptors. Immunol Rev. 2001;181:138–148. doi: 10.1034/j.1600-065x.2001.1810111.x. [DOI] [PubMed] [Google Scholar]
- Oehler JR, Lindsay LR, Nunn ME, Holden HT, Herberman RB. Natural cell-mediated cytotoxicity in rats. II In vivo augmentation of NK-cell activity. Int J Cancer. 1978;21:210–220. doi: 10.1002/ijc.2910210213. [DOI] [PubMed] [Google Scholar]
- Olsen I, Bou-Gharios G, Abraham D. The activation of resting lymphocytes is accompanied by the biogenesis of lysosomal organelles. Eur J Immunol. 1990;20:2161–2170. doi: 10.1002/eji.1830201003. [DOI] [PubMed] [Google Scholar]
- Oseroff C, Kos F, Bui HH, Peters B, Pasquetto V, Glenn J, Palmore T, Sidney J, Tscharke DC, Bennink JR, Southwood S, Grey HM, Yewdell JW, Sette A. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc Natl Acad Sci USA. 2005;102:13980–13985. doi: 10.1073/pnas.0506768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff C, Peters B, Pasquetto V, Moutaftsi M, Sidney J, Panchanathan V, Tscharke DC, Maillere B, Grey H, Sette A. Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus western reserve. J Immunol. 2008;180:7193–7202. doi: 10.4049/jimmunol.180.11.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero B, Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- Rabinovich BA, Shannon J, Su RC, Miller RG. Stress renders T cell blasts sensitive to killing by activated syngeneic NK cells. J Immunol. 2000;165:2390–2397. doi: 10.4049/jimmunol.165.5.2390. [DOI] [PubMed] [Google Scholar]
- Rahim MMA, Chen P, Mottashed AN, Mahmoud AB, Thomas MJ, Zhu Q, Brooks CG, Kartsogiannis V, Gillespie MT, Carlyle JR, Makrigiannis AP. The mouse NKR-P1B:Clr-b recognition system is a negative regulator of innate immune responses. Blood. 2015;125:2217–2227. doi: 10.1182/blood-2014-02-556142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell PA, Grossman WJ, Thomas DA, Cao X, Behl R, Ratner JA, Lu ZH, Ley TJ. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J Immunol. 2005;174:2124–2131. doi: 10.4049/jimmunol.174.4.2124. [DOI] [PubMed] [Google Scholar]
- Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Rydyznski C, Daniels KA, Karmele EP, Brooks TR, Mahl SE, Moran MT, Li C, Sutiwisesak R, Welsh RM, Waggoner SN. Generation of cellular immune memory and B-cell immunity is impaired by natural killer cells. Nat Commun. 2015;6:6375. doi: 10.1038/ncomms7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D, Trinchieri G, Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II Interferon induction and activation of natural killer cells. J Immunol. 1978a;121:532–538. [PubMed] [Google Scholar]
- Santoli D, Trinchieri G, Lief FS. Cell-mediated cytotoxicity against virus-infected target cells in humans. I Characterization of the effector lymphocyte. J Immunol. 1978b;121:526–531. [PubMed] [Google Scholar]
- Schuster IS, Wikstrom ME, Brizard G, Coudert JD, Estcourt MJ, Manzur M, O'Reilly LA, Smyth MJ, Trapani JA, Hill GR, Andoniou CE, Degli-Esposti MA. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity. 2014;41:646–656. doi: 10.1016/j.immuni.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Sepulveda FE, Maschalidi S, Vosshenrich CAJ, Garrigue A, Kurowska M, Ménasche G, Fischer A, Di Santo JP, de Saint Basile G. A novel immunoregulatory role for NK-cell cytotoxicity in protection from HLH-like immunopathology in mice. Blood. 2015;125:1427–1434. doi: 10.1182/blood-2014-09-602946. [DOI] [PubMed] [Google Scholar]
- Soderquest K, Powell N, Luci C, van Rooijen N, Hidalgo A, Geissmann F, Walzer T, Lord GM, Martín-Fontecha A. Monocytes control natural killer cell differentiation to effector phenotypes. Blood. 2011;117:4511–4518. doi: 10.1182/blood-2010-10-312264. [DOI] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Nguyen KB, Salazar-Mather TP, Ruzek MC, Dalod MY, Biron CA. NK cell functions restrain T cell responses during viral infections. Eur J Immunol. 2001;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048∷AID-IMMU3048>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Taylor LS, Paul SP, McVicar DW. Paired inhibitory and activating receptor signals. Rev Immunogenet. 2000;2:204–219. [PubMed] [Google Scholar]
- Trinchieri G, Santoli D, Koprowski H. Spontaneous cell-mediated cytotoxicity in humans: role of interferon and immunoglobulins. J Immunol. 1978;120:1849–1855. [PubMed] [Google Scholar]
- Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SMM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Daniels KA, Welsh RM. Therapeutic depletion of natural killer cells controls persistent infection. J Virol. 2014;88:1953–1960. doi: 10.1128/JVI.03002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Kumar V. Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front Immunol. 2012;3:377. doi: 10.3389/fimmu.2012.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner SN, Taniguchi RT, Mathew PA, Kumar V, Welsh RM. Absence of mouse 2B4 promotes NK cell-mediated killing of activated CD8+ T cells, leading to prolonged viral persistence and altered pathogenesis. J Clin Invest. 2010;120:1925–1938. doi: 10.1172/JCI41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kang N, Zhou J, Guo Y, Zhang X, Cui L, Ba D, He W. Downregulation of CD94/NKG2A inhibitory receptor on decreased γδ T cells in patients with systemic lupus erythematosus. Scand J Immunol. 2012;76:62–69. doi: 10.1111/j.1365-3083.2012.02705.x. [DOI] [PubMed] [Google Scholar]
- Welsh RM. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Zinkernagel RM, Hallenbeck LA. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. II “Specificities” of the natural killer cells. J Immunol. 1979;122:475–481. [PubMed] [Google Scholar]
- Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Köhrer K, Rahbar R, Diefenbach A, Gibbert K, Löhning M, Höcker L, Waibler Z, Häussinger D, Mak TW, Ohashi PS, Lang KS, Lang PA. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Zamora AE, Aguilar EG, Sungur CM, Khuat LT, Dunai C, Lochhead GR, Du J, Pomeroy C, Blazar BR, Longo DL, Venstrom JM, Baumgarth N, Murphy WJ. Licensing delineates helper and effector NK cell subsets during viral infection. JCI Insight. 2017;2 doi: 10.1172/jci.insight.87032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of NK cell depletion on VACV load during the course of infection. Numbers plotted are log10 values derived from viral plaque assay on vero cells at the indicated day post infection. The dotted line represents the limit of detection for the indicated organ. Top, Liver (n=5 per group). Bottom, Fat pads (n=5 per group).


