Abstract
Background
Despite well-documented associations of socio-economic status (SES) with incident heart failure (HF) hospitalization, little information exists on the relationship of SES with HF diagnosed in the outpatient (OP) setting.
Methods
We used Poisson models to examine the association of area-level indicators of educational attainment, poverty, living situation, and density of primary care physicians with incident HF diagnosed in the inpatient and outpatient settings among a cohort of Medicare beneficiaries (n=109,756; 2001-2013).
Results
The age-standardized rate of HF incidence was 35.8 (95% confidence interval (CI) 35.1, 36.5) and 13.9 (95% CI 13.5, 14.4) cases per 1,000 person-years in inpatient and outpatient settings, respectively. The incidence rate differences (IRD) per 1,000 person-years in both settings suggested greater incidence of HF in high compared to low poverty areas (IP IRD=4.47 (95% CI 3.29, 5.65), OP IRD=1.41 (95% CI 0.61, 2.22)) and in low compared to high education areas (IP IRD=3.73 (95% CI 2.63, 4.82), OP IRD=1.72 (95% CI 0.97, 2.47)).
Conclusions
Our results highlight the role of area-level social determinants of health in the incidence of HF in both the inpatient and outpatient setting. These findings may have implications for HF prevention policies.
Introduction
Heart failure (HF) is a clinical syndrome characterized by breathlessness, fatigue and edema due to an inability of the heart to provide adequate cardiac output to the body at rest or with exertion, or to do so only in the setting of elevated cardiac filling pressures.1 Patients diagnosed with HF have a poor prognosis characterized by frequent hospital admissions and readmissions, they suffer from multiple comorbidities,2 and have a median survival of 5 years following the initial diagnosis.1 Geographic variation in hospitalizations among patients with HF has been observed3,4 and may be due to regional differences in access to care and socio-economic factors.
Socio-economic status (SES), often operationalized with measures of income, poverty, wealth, education, or occupational class,5,6 is an independent predictor of poor health7,8 and cardiovascular disease (CVD).5 Residence in socioeconomically disadvantaged neighborhoods is linked to greater incidence of coronary heart disease,9-11 and a greater prevalence of CVD risk factors, such as diabetes,12 hypertension,13 and obesity.14 It also imparts a greater risk of HF-related hospitalizations,15-17 re-admissions,17,18 and mortality.17,18 Disparities by SES may be due to lack of regular health care, poor access to care, less knowledge about managing CVD risk factors, or environmental factors that affect diet and physical activity participation.9,19,20
Despite well documented associations of SES with incident HF hospitalization,5,15,16 data on the relationship of SES with HF diagnosed in the outpatient setting are rare. Results from a handful of studies, which included diagnoses of HF in the outpatient setting, suggest a lower comorbidity burden,21,22 fewer hospitalizations,21 and lower case fatality21-23 for HF patients diagnosed in the outpatient as compared to the inpatient setting. However, none of these studies examined variation of incidence rates by SES or access to care. Therefore, we sought to examine the associations of area-level SES and access to care factors with incidence of HF diagnosed in the inpatient and outpatient settings among the Centers for Medicare and Medicaid Services (CMS) Medicare beneficiaries residing in four geographically distinct regions of the United States.
Methods
Study Design
We constructed an open cohort of white and African American Medicare fee-for-service (FFS) beneficiaries residing in four geographic regions in which the Atherosclerosis Risk in Communities Study (ARIC) conducts surveillance for cardiovascular disease events (Washington County, MD; Minneapolis, MN; Jackson, MS; Forsyth County, NC). We used a 100% Medicare sample from these regions. We included inpatient and outpatient Medicare claims from the longest continuous FFS enrollment period that was 2 years or longer from January 1, 2001 to December 31, 2013. The first two years of enrollment were used as a baseline period to identify prevalent HF and measure beneficiary characteristics. Follow-up to ascertain incident heart failure events started in year 3 of the enrollment period (Figure 1).
Figure 1.
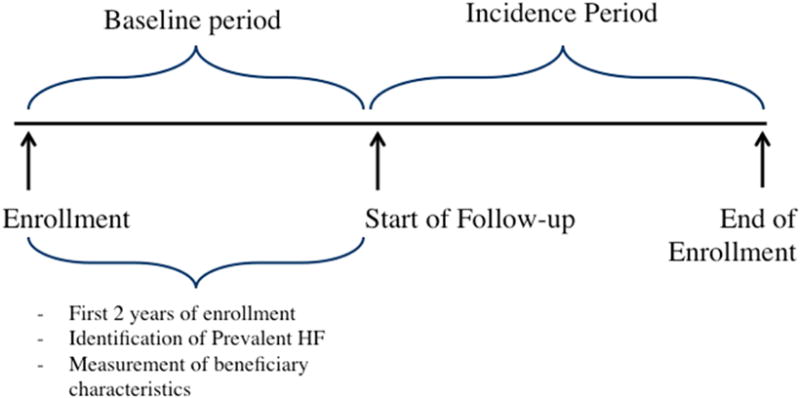
Study design for estimating incidence of heart failure diagnosis in the inpatient and outpatient setting among CMS Medicare FFS beneficiaries (2001 – 2013).
Footnotes: Abbreviations- FFS (fee-for-service), HF (heart failure)
Beneficiary sample and eligibility
Inpatient and outpatient Medicare claims for all beneficiaries were obtained and linked by a unique beneficiary identifier. Records for inpatient encounters were selected from annual Medicare Provider Analysis and Review (MedPAR) files. Outpatient claims were identified from the Carrier and Outpatient files using the following Evaluation and Management Healthcare Common Procedure Coding System codes: 99201–99205, 99211–99215, 99241–99245, 99385–99387, 99395–99397. Outpatient events that occurred in federally qualified health centers were identified from annual outpatient files as claims with revenue center codes 521 and 522.
Heart Failure Ascertainment
A diagnosis of incident HF in the inpatient setting was defined as the first hospitalization in the incidence period (Figure 1) with International Classification of Disease, 9th Revision, Clinical Modification (ICD-9) codes in any position included in the Chronic Conditions Data Warehouse (CCW) HF algorithm (ICD-9 codes 398.91, 402.01, 402.11, 402.91, 404.01, 404.11, 404.94, 404.03, 404.13, 404.93, and 428.x). A diagnosis of incident HF in the outpatient setting was defined as two consecutive claims with CCW HF algorithm codes within 365 days. The date of the second claim was defined as the date of the HF diagnosis. For a beneficiary with claims identifying HF diagnoses in both the inpatient and outpatient setting, the first diagnosis that occurred was used. If both diagnoses occurred on the same day, then beneficiaries were classified as having incident HF diagnosis in the inpatient setting (N=45). A two year baseline period of the first two years of enrollment was used to identify prevalent inpatient and outpatient HF (Figure 1).24,25
SES and Medical Care Access Factors
Area-level SES and medical care access factors were assessed at the zip code tabulation area (ZCTA) level. Area-level SES was operationalized with the following Census 2000 variables specific to the 65 years and older population accessed from the RTI Spatial Impact Factor website: proportion in poverty, proportion with more than a high school education, and proportion that live alone.26 The number of clinically active primary care physicians per 100,000 population for the year 2006 within study geographic areas was obtained from the Health Resources and Service Administration website.27 All area-level variables were categorized as tertiles. We used a cross-walk28 of ZCTA to zip code to join the area-level data to the Medicare beneficiary cohort data. In our study population we had 85 ZCTAs.
Covariates
Age at entry into the cohort, sex, race, and geographic region were considered as confounders in multivariable analyses and were identified from the annual Master Beneficiary Summary Files. Due to a small number of beneficiaries over the age of 95, we recoded ages 96 – 106 as age 95. Age was centered at 75 years and race was categorized as white and African American. We obtained the zip codes of the four geographic regions and classified beneficiaries into community by the zip code obtained from the Master Beneficiary Summary File.
To further describe beneficiary characteristics we calculated the number of inpatient and outpatient encounters, the number of visits to primary care providers (general practice, family practice, internal medicine, nurse practitioner, and multi-specialty clinic providers), and total number of visits to cardiologists during the two year baseline period prior to beginning of study follow-up (Figure 1). We also estimated prevalence of comorbidities (Supplemental Table 1 for ICD-9 codes) identified from MedPAR records and ambulatory care claims during the two year baseline period (Figure 1).
Exclusions
Excluded from analysis were beneficiaries who had less than two years of enrollment (N=20,901), those considered to have prevalent HF within the two-year baseline period (N=8,279), those missing all covariate information (N=19), and those younger than 65 years (N=5,937). This provided an analytic sample of 109,756 beneficiaries (Supplemental Figure 1). After identifying which HF diagnosis occurred first (inpatient or outpatient) two analytic samples were created for each setting. The inpatient HF sample included beneficiaries diagnosed with inpatient HF and those who did not develop HF during the period of observation (2003-2013). Similarly the outpatient sample consisted of beneficiaries diagnosed with outpatient HF and those who did not develop HF during the period of observation.
Statistical Analysis
Crude estimates of incidence rates (per 1,000 person-years) of HF diagnosis in the inpatient and outpatient setting were age-standardized to reflect the age distribution of the national 2003 CMS Medicare population age 67 and older - the earliest age of the cohort given the two year lookback period. Person-time was calculated as time from start of follow-up (after completion of first 2 years of enrollment) to an incident HF event (inpatient or outpatient setting), death, or end of enrollment, whichever came first (Figure 1). To estimate associations between area-level SES and access to care factors with incident HF diagnosis, we used Poisson models with generalized estimating equations to estimate incidence rates (IR), incidence rate ratios (IRR), incidence rate differences (IRD) per 1,000 person-years, and 95% confidence intervals (95% CI). We used an unstructured correlation matrix to adjust for clustering of beneficiaries within zip code. All models were adjusted for age, race, sex, and community.
This study has been approved by the Institutional Review Boards of participating ARIC study centers.
Results
Following exclusions, the study sample consisted of 109,756 beneficiaries (Supplemental Figure 1). At time of entry into the cohort, the average age of beneficiaries was 72 years (SD 7.4 years), 60% were female, and 85% were white (Table 1). The median number of primary care physicians per 100,000 population was similar by HF diagnosis setting, as were the mean proportion in poverty, proportion with more than a high school education, and proportion living alone (Table 1). From 2003-2013 over a median follow-up time of 3.9 years, 11.4% of beneficiaries were diagnosed with incident HF in the inpatient setting and 4.3% were diagnosed in the outpatient setting. The baseline prevalence of comorbidities was similar across diagnostic settings for beneficiaries diagnosed with HF and it was greater in comparison with beneficiaries free of HF (Table 1). The average age at diagnosis for beneficiaries diagnosed in the inpatient and outpatient setting was 82 (SD 7.6 years) and 80 years (SD 7.5 years).
Table 1.
CMS Medicare FFS beneficiary participant characteristics by setting of incident HF diagnosis (2001 – 2013) (n=109,756).
| Inpatient HF Diagnosis (N=12,461) |
Outpatient HF Diagnosis (N=4,743) |
No HF (N=92,552) |
|
|---|---|---|---|
| Age at entry to cohort, mean years (SD) | 76.2 (7.6) | 75.0 (7.3) | 71.6 (7.2) |
| Age at diagnosis, mean years (SD) | 81.7 (7.6) | 80.3 (7.5) | NA |
| Male, N (%) | 4,735 (38.0) | 2,122 (44.7) | 36,512 (39.5) |
| African American, N (%) | 2,111 (16.9) | 700 (14.8) | 15,124 (16.3) |
| Comorbidities*, N (%) | |||
| Hypertension | 7,951 (63.8) | 2,963 (62.5) | 51,635 (55.8) |
| Diabetes | 3,141 (25.2) | 1,172 (24.7) | 16,033 (17.3) |
| Coronary atherosclerosis | 3,265 (26.2) | 1,478 (31.2) | 12,227 (13.2) |
| Atrial fibrillation/Atrial flutter | 1,420 (11.4) | 654 (13.8) | 4,190 (4.5) |
| Cardiac dysrhythmias | 1,627 (13.1) | 674 (14.2) | 8,728 (9.4) |
| Heart valve disorder | 1,007 (8.1) | 431 (9.1) | 3,918 (4.2) |
| Acute myocardial infarction | 146 (1.2) | 79 (1.7) | 588 (0.6) |
| Conduction disorder | 638 (5.1) | 260 (5.5) | 2,256 (2.4) |
| Pneumonia | 871 (7.0) | 277 (5.8) | 3,868 (4.2) |
| COPD exacerbations | 437 (3.5) | 135 (2.9) | 1,621 (1.8) |
| Anemia | 1,322 (10.6) | 409 (8.6) | 6,249 (6.8) |
| Total number of comorbidities, mean (SD) | 2 (0,3) | 2 (1,3) | 1 (0,1) |
| Healthcare encounters*, †, median number of visits (P25, P75) | |||
| Inpatient setting | 0 (0,1) | 0 (0,1) | 0 (0,0) |
| Outpatient setting | 13 (8,21) | 14 (9,22) | 11 (6,18) |
| To primary care providers§ | 7 (3,11) | 8 (4,11) | 5 (2,9) |
| To cardiologists | 0 (0,0) | 0 (0,1) | 0 (0,0) |
| Area-level characteristics, mean (SD) | |||
| Number of primary care physicians║ | 52.9 (24.6, 88.2) | 53.1 (24.6, 88.2) | 47.1 (24.6, 88.2) |
| Proportion in poverty# | 0.09 (0.06) | 0.09 (0.06) | 0.09 (0.06) |
| Proportion > high school education# | 0.35 (0.16) | 0.34 (0.16) | 0.36 (0.17) |
| Proportion live alone# | 0.30 (0.06) | 0.29 (0.06) | 0.29 (0.06) |
Measured during the baseline period (first two years of enrollment)
Number of visits over two years
Includes general practice, family practice, internal medicine, nurse practitioner, and multi-specialty clinic providers
Per 100,000 population
For 65 years and older population
Abbreviations: COPD (chronic obstructive pulmonary disease), FFS (fee-for-service), HF (heart failure), IP (inpatient), OP (outpatient), P25 (25th percentile), P75 (75th percentile), SD (standard deviation)
The standardized rate of HF incidence in the inpatient setting was 35.8 (95% CI 35.1, 36.5) cases per 1,000 person years and 13.9 (95% CI 13.5, 14.4) cases per 1,000 person-years in the outpatient setting (Table 2). Across both settings, the HF incidence rate was higher for older beneficiaries than younger and for males than females (Table 2). In the inpatient setting, slightly higher HF diagnosis rates were observed for African American beneficiaries as compared to whites (40.9 vs. 35.0), with little difference by race observed for HF diagnosed in the outpatient setting.
Table 2.
Standardized HF incidence rates in the inpatient and outpatient setting by age, gender, and race, CMS Medicare FFS beneficiaries (2001 – 2013).
| N | Number of events | Person-years | Standardized IR*, † (95% CI) | |
|---|---|---|---|---|
| Inpatient Heart Failure | ||||
| Overall | 105,013 | 12,461 | 504,504 | 35.8 (35.1, 36.5) |
| Age group | ||||
| 65 – 74 | 70,344 | 5,438 | 356,893 | 18.2 (17.7, 18.8) |
| 75 and older | 34,669 | 7,023 | 147,611 | 50.2 (49.0, 51.4) |
| Gender | ||||
| Male | 41,247 | 4,735 | 193,578 | 37.8 (36.5, 39.1) |
| Female | 63,766 | 7,726 | 310,926 | 34.7 (33.8, 35.5) |
| Race | ||||
| White | 87,778 | 10,350 | 433,685 | 35.0 (34.3, 35.8) |
| African | 17,235 | 2,111 | 70,818 | 40.9 (38.9, 42.8) |
| American | ||||
| Outpatient Heart Failure | ||||
| Overall | 97,295 | 4,743 | 476,526 | 13.9 (13.5, 14.4) |
| Age group | ||||
| 65 – 74 | 67,279 | 2,373 | 343,882 | 8.12 (7.70, 8.50) |
| 75 and older | 30,016 | 2,370 | 132,644 | 18.7 (17.9, 19.5) |
| Gender | ||||
| Male | 38,634 | 2,122 | 183,771 | 17.1 (16.2, 18.0) |
| Female | 58,661 | 2,621 | 292,755 | 12.3 (11.8, 12.8) |
| Race | ||||
| White | 81,471 | 4,043 | 410,385 | 14.0 (13.5, 14.5) |
| African | 15,824 | 700 | 66,141 | 13.7 (12.5, 14.9) |
| American |
per 1,000 person-years
Standardized to the 2003 National CMS Medicare population
Abbreviations: FFS (fee-for-service), HF (heart failure), IR (incidence rate), CI (confidence interval)
Area-level associations with HF
Across both healthcare settings, the HF incidence rate differed by area-level indicators of proportion in poverty and proportion with more than a high school education (Figure 2). Compared to those living in low poverty areas beneficiaries in high poverty areas had 4.47 (95% CI 3.29, 5.65) more incident inpatient HF diagnoses per 1,000 person-years and 1.41 (95% CI 0.61, 2.22) more incident outpatient HF diagnoses per 1,000 person-years. Similarly, beneficiaries in geographic areas with low as compared to high educational attainment had 3.73 (95% CI 2.63, 4.82) more incident inpatient HF diagnoses per 1,000 person-years and 1.72 (95% CI 0.97, 2.47) more incident outpatient HF diagnoses per 1,000 person-years. Across both settings few differences in HF incidence were observed by number of primary care physicians per 100,000 population and by the proportion of persons living alone. Similar associations of area-level factors with incidence of HF across diagnostic setting were observed with incidence rate ratios as the measure of association (Supplemental Figure 2).
Figure 2.
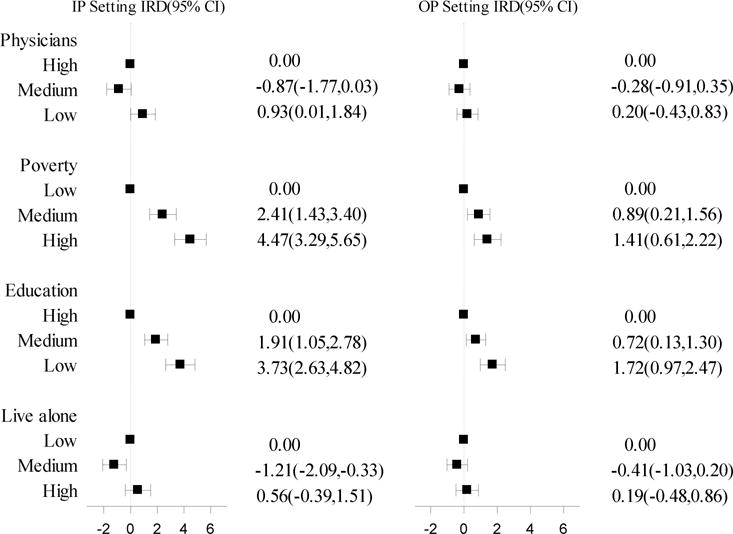
Incidence rate differences (95% CI) per 1,000 person-years of diagnosis of HF in the inpatient and outpatient setting by area-level SES and access to care factors, CMS Medicare FFS Beneficiaries (2001 – 2013) (IP Setting n=105,013, OP Setting n=97,295).
Footnotes: abbreviations- CI (Confidence Interval), FFS (fee-for-service), HF(Heart Failure), IRD (Incidence Rate Difference), IP (Inpatient), OP (Outpatient), SES (Socio-Economic Status). Category boundaries: physicians (number of primary care physicians per 100,000 population), low (0-29.4), medium (29.5-78.6), high (78.7-400); poverty (proportion > 65 in poverty) low (0 - 0.054), medium (0.055–0.088), high (0.089-0.370); education (proportion > 65 with > high school education) low (0-0.25), medium (0.25-0.42), high (0.43-0.78); live alone (proportion > 65 that live alone) low (0-0.27), medium (0.28-0.31), high (0.32-0.60).
Discussion
To our knowledge, this is one of few studies in the United States to present HF incidence estimates separately by diagnostic setting23,29 and to compare the association of area-level socioeconomic and access to care factors with the incidence of an inpatient and outpatient HF diagnosis. In this open cohort of Medicare beneficiaries, we observed the highest rates of incident inpatient and outpatient HF diagnosis among beneficiaries living in areas with low levels of educational attainment and high poverty. Overall, in this population of Medicare beneficiaries, incidence of HF was much higher in the inpatient, as compared to the outpatient setting.
Our observation of the association of high poverty with greater frequency of HF diagnosis extends findings from two extant studies that examined the association of area-level poverty measures with incident HF hospitalization15,16 to the outpatient setting. Underlying social gradients in CVD risk factor distributions may partially explain why higher rates of HF diagnosis are observed among beneficiaries in more impoverished areas. A greater burden of diabetes,12 hypertension,13 obesity,14 and coronary heart disease,9-11 conditions that often precede a HF diagnosis, is observed among individuals living in low as compared to high SES neighborhoods. Individuals with limited socioeconomic resources tend to make fewer ambulatory care visits,19 are less likely to have a regular medical provider,16,20 and more likely to delay care20 in comparison with those from less deprived areas.30
We observed a gradient in rates of HF diagnosis in both the inpatient and outpatient setting across levels of educational attainment in beneficiaries’ areas of residence. Our findings extend to the outpatient setting previous research on the association of education with HF incidence obtained from hospitalization-only data.5,31 Education may be associated with diagnosis of HF as it relates to health literacy, the ability to access health services, a person’s cognitive capacity to modify risk factors and behaviors early to prevent HF, and availability of income and the resources to manage health.32
We found a lack of association between area-level proportion of individuals 65 years and older living alone and HF incidence. Our results are not consistent with previous research, which underscores the importance of social support in the lowering HF incidence through help with medication management, emotional support, and symptom recognition.33,34 However, healthcare claims data did not allow us the direct assessment of beneficiaries’ living status.
It is possible that the observed lack of an association of area-level density of primary care providers with incident inpatient or outpatient HF diagnosis is due to the relatively greater importance of continuity of care among the elderly, independent of clinician type.35 Patients who have a large proportion of their medical care provided by one healthcare practitioner have low rates of hospitalizations, emergency department visits, and fewer complications.36 Further, findings from the National Hospital Discharge Survey suggest an association between density of primary care physicians with HF hospitalizations among non-elderly but not among those 65 years of age and older.4
The focus on HF diagnosis in the outpatient setting is a strength of this study. We are one of the few studies to provide incidence rates of HF in both the inpatient and outpatient settings and to demonstrate how those rates vary by area-level SES characteristics. Since not all individuals diagnosed with HF have a HF-related hospitalization,21,37 by including HF diagnosed in either the inpatient or outpatient setting we address patient characteristics and SES findings for those who have been overlooked in past research. Our findings also point to the greater incidence of HF diagnosed in the inpatient as opposed to the outpatient setting. Almost three-fourths of incident HF diagnoses in our cohort occurred in the inpatient setting. This finding is similar to results based on a 5% national Medicare sample23 in which the authors observed that 65% incident HF diagnoses occurred in the inpatient compared to 35% in the outpatient setting. Further, we observed that the incidence rate for HF diagnosed in the inpatient setting was 2.5 times the rate of HF diagnosed in the outpatient setting. Additionally, some of our coauthors recently published a study conducted in a 20% national Medicare sample that suggested the rate of inpatient HF diagnosis is almost twice that of the rate of outpatient HF diagnosis.29 These findings underscore opportunities for earlier recognition of incident HF in the outpatient setting and greater outpatient management of at risk populations in the prevention of HF hospitalizations.
Study limitations
The magnitude of our findings regarding the incidence of outpatient HF diagnosis may be subject to different diagnosis definitions. No agreed upon claims-based definition of outpatient HF exists, although we used a definition similar to that of other studies.22,29 By using the CCW HF algorithm based on ICD-9 codes in any position for the identification of HF, we relied on a comprehensive definition to identify beneficiaries who were recognized to have any aspect of HF. For example, this approach identifies beneficiaries who may be admitted to the hospital for other acute conditions that were coded as the primary diagnosis that may have been accompanied by a secondary diagnosis of decompensated HF.38 In a recent study, a high concordance was observed between hospitalizations with CCW HF ICD-9 codes in any position and events classified as definite or possible acute decompensated HF and chronic stable HF, determined from review of medical records of ARIC cohort participants.39 Further, this study and others observed a higher sensitivity for an ICD-9 code for HF in any position compared to the first position.39-41
We employed a 2-year baseline period to limit inclusion of prevalent HF cases. Shorter baseline periods (also known as look-back, washout, or prevalence periods) potentially misclassify incident HF events and over estimate the incidence rate when compared to longer periods.24,25 In an effort to reduce misclassification we may have selected a healthy cohort of beneficiaries who had to survive at least 2 years to be in our analysis.
Although analyses were limited to four distinct geographic regions, included were all Medicare beneficiaries residing within these regions. We thus avoided the bias which may occur when small area analyses of health disparities are conducted in randomly selected populations, such as with the 5% Medicare sample.42 Our choice of the zip code as the geographic unit was dictated by the limited availability of geographic identifiers in Medicare data. Smaller geographic areas, such as participant-defined neighborhoods or Census block groups, may have had higher validity for measuring the association of area-level SES with development of HF.43 The primary care physician density data at the zip code level were only available for the year 2006, but we anticipate the number of primary care physicians to be similar across the years covered by our analysis. We acknowledge that our results may be subject to the modifiable areal unit problem such that the use of different geographic boundaries may have led to different results.44 While we lack individual-level data on SES and access to health care, previous research suggests an independent association of area-level measures with health outcomes that is above and beyond individual-level characteristics.45
Conclusion
This is one of the few studies to present HF diagnosis incidence rates separately for the inpatient and outpatient setting in the United States. Associations of modest magnitude were observed between area-level socio-economic factors and incident HF diagnosis across both diagnosis settings. Our findings suggest that among Medicare beneficiaries, the majority of HF diagnoses occur in the inpatient setting. An emphasis on prevention of HF and outpatient HF management of at risk populations, may lead to earlier recognition of HF and reduction of avoidable hospitalization for acute decompensated HF.
Supplementary Material
Acknowledgments
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021).
List of abbreviations and acronyms
- ARIC
Atherosclerosis Risk in Communities Study
- CVD
Cardiovascular disease
- CMS
Centers for Medicare and Medicaid Services
- CCW
Chronic Conditions Data Warehouse
- 95% CI
Confidence interval
- FFS
Fee-for-service
- HF
Heart failure
- ICD-9
International Classification of Disease, 9th Revision, Clinical Modification
- IR
Incidence rates
- IRR
Incidence rate ratios
- IRD
Incidence rate differences
- MedPAR
Medicare Provider Analysis and Review
- SES
Socio-economic status
- ZCTA
Zip code tabulation area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
Contributor Information
Carmen C. Cuthbertson, Department of Epidemiology, University of North Carolina at Chapel Hill, 137 E. Franklin St., Suite 303A, Chapel Hill, NC, USA 27514.
Gerardo Heiss, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Jacqueline D. Wright, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD.
Ricky Camplain, Center for Health Equity Research, Northern Arizona University, Flagstaff, AZ.
Mehul D. Patel, Department of Emergency Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Randi E. Foraker, School of Medicine, Washington University in St. Louis, St. Louis, MO.
Kunihiro Matsushita, Department of Epidemiology, Johns Hopkins University, Baltimore, MD.
Nicole Puccinelli-Ortega, Wake Forest Baptist Health, Winston-Salem, NC.
Amil M. Shah, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA.
Anna M. Kucharska-Newton, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776/-/DC1. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013 Aug 30;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casper M, Nwaise I, Croft JB, Hong Y, Fang J, Greer S. Geographic disparities in heart failure hospitalization rates among Medicare beneficiaries. J Am Coll Cardiol. 2010 Jan 26;55(4):294–299. doi: 10.1016/j.jacc.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Watanabe-Galloway S. Ten-year secular trends for congestive heart failure hospitalizations: an analysis of regional differences in the United States. Congest Heart Fail. 2008 Sep-Oct;14(5):266–271. doi: 10.1111/j.1751-7133.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail. 2012 Feb;14(2):138–146. doi: 10.1093/eurjhf/hfr168. [DOI] [PubMed] [Google Scholar]
- 6.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 7.Marmot MG, Kogevinas M, Elston MA. Social/economic status and disease. Annu Rev Public Health. 1987;8:111–135. doi: 10.1146/annurev.pu.08.050187.000551. [DOI] [PubMed] [Google Scholar]
- 8.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconommic disparities in health in the US: what the patterns tell us. Am J Public Health. 2009;100:S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diez Roux AV. Residential Environments and Cardiovascular Risk. J Urban Health. 2003;80(4):569–589. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose KM, Suchindran CM, Foraker RE, Whitsel EA, Rosamond WD, Heiss G, Wood JL. Neighborhood disparities in incident hospitalized myocardial infarction in four U.S. communities: the ARIC surveillance study. Ann Epidemiol. 2009 Dec;19(12):867–874. doi: 10.1016/j.annepidem.2009.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foraker RE, Rose KM, Kucharska-Newton AM, Ni H, Suchindran CM, Whitsel EA. Variation in rates of fatal coronary heart disease by neighborhood socioeconomic status: the atherosclerosis risk in communities surveillance (1992-2002) Ann Epidemiol. 2011 Aug;21(8):580–588. doi: 10.1016/j.annepidem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study. Am J Epidemiol. 2010 Mar 1;171(5):564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grotto I, Huerta M, Sharabi Y. Hypertension and socioeconomic status. Curr Opin Cardiol. 2008;23:335–339. doi: 10.1097/HCO.0b013e3283021c70. [DOI] [PubMed] [Google Scholar]
- 14.Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev. 2008 Jan;66(1):2–20. doi: 10.1111/j.1753-4887.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Stewart S, Murphy NF, McMurray JJ, Jhund P, Hart CL, Hole D. Effect of socioeconomic deprivation on the population risk of incident heart failure hospitalisation: an analysis of the Renfrew/Paisley Study. Eur J Heart Fail. 2006 Dec;8(8):856–863. doi: 10.1016/j.ejheart.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.McAlister FA, Murphy NF, Simpson CR, Stewart S, MacIntyre K, Kirkpatrick M, Chalmers J, Redpath A, Capewell S, McMurray JJ. Influence of socioeconomic deprivation on the primary care burden and treatment of patients with a diagnosis of heart failure in general practice in Scotland: population based study. BMJ. 2004 May 8;328(7448):1110. doi: 10.1136/bmj.38043.414074.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: Atherosclerosis Risk in Communities cohort (1987 to 2004) Circ Heart Fail. 2011 May;4(3):308–316. doi: 10.1161/CIRCHEARTFAILURE.110.959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathore SS, Masoudi FA, Wang Y, Curtis JP, Foody JM, Havranek EP, Krumholz HM. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: findings from the National Heart Failure Project. Am Heart J. 2006 Aug;152(2):371–378. doi: 10.1016/j.ahj.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gornick ME, Eggers PW, Reilly TW, Mentnech RM, Fitterman LK, Kucken LE, Vladeck BC. Effects of race and income on mortality and use of services among Medicare beneficiaries. N Engl J Med. 1996;335:791–799. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 20.Rask KJ, Williams MV, Parker RM, McNagny SE. Obstacles predicting lack of a regular provider and delays in seeking care for patients at an urban public hospital. JAMA. 1994;271:1931–1988. doi: 10.1001/jama.1994.03510480055034. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2011 Feb;13(2):142–147. doi: 10.1093/eurjhf/hfq185. [DOI] [PubMed] [Google Scholar]
- 22.Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184(14):E765–E773. doi: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168(4):418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths RI, O’Malley CD, Herbert RJ, Danese MD. Misclassification of incident conditions using claims data: impact of varying the period used to exclude pre-existing disease. BMC Med Res Methodol. 2013;13:32. doi: 10.1186/1471-2288-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camplain R, Kucharska-Newton A, Cuthbertson CC, Wright JD, Alonso A, Heiss G. Misclassification of incident hospitalized and outpatient heart failure in administrative claims data: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacoepidemiol Drug Saf. 2017 Jan 25; doi: 10.1002/pds.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spatial Impact Factor Data Version 5. Research Triangle Institute; 2012. https://rtispatialdata.rti.org/. Accessed January 22, 2016. [Google Scholar]
- 27.Primary Care Service Areas Data Download – 2005-2006 (ZIP Code Basis) Health Resources and Service Administration; 2008. http://datawarehouse.hrsa.gov/data/dataDownload/pcsa2006Download.aspx. Accessed January 22, 2016. [Google Scholar]
- 28.ZIP Code to ZCTA Crosswalk. 2015 http://udsmapper.org/zcta-crosswalk.cfm. Accessed January 22, 2016.
- 29.Camplain R, Kucharska-Newton A, Keyserling TC, Layton B, Loehr L, Heiss G. Incidence of Heart Failure Observed in Emergency Departments and Ambulatory Clinics. American Journal of Cardiology. doi: 10.1016/j.amjcard.2018.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latour-Perez J, Gutierrez-Vicen T, Lopez-Camps V, Bonastre-Mora J, Giner-Boix JS, Rodriguez-Serra M, Rosado-Breton L. Socioeconomic status and severity of illness on admission in acute myocardial infarction patients. Soc Sci Med. 1996;43(6):1025–1029. doi: 10.1016/0277-9536(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 31.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 32.Oakes JM, Kaufman JS. Methods in Social Epidemiology. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- 33.Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: an integrative review. Int J Nurs Stud. 2014 Feb;51(2):320–333. doi: 10.1016/j.ijnurstu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Strachan PH, Currie K, Harkness K, Spaling M, Clark AM. Context matters in heart failure self-care: a qualitative systematic review. J Card Fail. 2014 Jun;20(6):448–455. doi: 10.1016/j.cardfail.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for perfomance. N Engl J Med. 2007;356:1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 36.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014 May;174(5):742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal SK, Wruck L, Quibrera M, Matsushita K, Loehr LR, Chang PP, Rosamond WD, Wright J, Heiss G, Coresh J. Temporal Trends in Hospitalization for Acute Decompensated Heart Failure in the United States, 1998-2011. Am J Epidemiol. 2016 Mar 1;183(5):462–470. doi: 10.1093/aje/kwv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucharska-Newton AM, Heiss G, Ni H, Stearns SC, Puccinelli-Ortega N, Wruck LM, Chambless L. Identification of Heart Failure Events in Medicare Claims: The Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2016 Jan;22(1):48–55. doi: 10.1016/j.cardfail.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012 Jan;21(Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012 Mar 1;5(2):152–159. doi: 10.1161/circheartfailure.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mobley L. Spatial sufficiency of 5% Medicare standard analytic files. Spatial Demography. 2012;1(2):202–218. doi: 10.1007/BF03354899. doi, http://spatialdemography.org/spatial-data-spatial-sufficiency-of-5-medicare-standard-analytic-files/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002 Sep 1;156(5):471–482. doi: 10.1093/aje/kwf068. doi. [DOI] [PubMed] [Google Scholar]
- 44.Cromley EK, McLafferty SL. GIS and Public Health. 2nd. New York, NY: The Guildford Press; 2012. [Google Scholar]
- 45.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010 Feb;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


