Abstract
Background:
The infections caused by drug resistant strains of Klebsiella pneumoniae are becoming an important health problem worldwide. There are several reports on antimicrobial resistant status of K. pneumoniae in Iran. However, a comprehensive analysis on drug-resistant K. pneumoniae from different parts of Iran has not yet been performed.
Methods:
The searches were done according to several English and Persian databases including PubMed, Scopus, Iranmedex, and SID to identify studies addressing antibiotic resistant K. pneumoniae in Iran from Jan 1998 to Nov 2014. Comprehensive Meta-Analysis (V2.2, Biostat) software was used to analyze the data.
Results:
The incidence rate of imipenem and ceftazidime resistance in K. pneumoniae isolates was 3.2% (95% confidence interval [CI], 1.5–6.5) and 55.7% (95% CI, 46.9–64.1), respectively. The highest rate of resistance in isolates of K. pneumoniae was seen against ampicillin (82.2%), aztreonam (55.4%) and nitrofurantoin (54.5%).
Conclusion:
There is a relatively high prevalence of drug resistant K. pneumoniae isolates in Iran. Thus, a high degree of awareness among physicians and microbiologists, active infection control committee, appropriate antimicrobial therapy, improvement of hygiene condition and monitoring of drug resistant isolates are urgently needed in order to better control the emergence and spread of drug-resistant K. pneumoniae isolates in hospital settings.
Keywords: Klebsiella pneumoniae, Drug resistance, Iran
Introduction
Klebsiella pneumoniae is an important causative agent of hospital-acquired infections, including severe pneumonia, urinary tract infection as well as septicemia and wound infections (1, 2). This bacterium can survive in hospitals, persist on environmental surface and colonize different parts of human body. Therefore, transmission of this opportunistic pathogen can easily occur among patients via the hands of healthcare personnel. Furthermore, the increased use of antibiotics and persistent exposure of K. pneumoniae to a number of antimicrobial agents, facilitating the emergence of multidrug-resistant strains, which has further intensified the infection control strategies in many health care settings (3).
The most important resistant isolates of K. pneumoniae are carbapenem and cephalosporin resistant strains (4). These strains can cause serious infections in immunocompromised patients, in association with prolonged hospital, stays, limited therapeutic options and increased mortality rates, ranging from 12% to as high as 72%, depending on the study population (5–9). In these regards, a reliable estimate of the extent of drug resistant isolates of K. pneumoniae is needed for the programmatic management of drug resistant strains within the context of national infection control programs.
This study was designed to determine the prevalence of drug resistant strains of K. pneumoniae in Iran according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (10, 11).
Methods
Search strategies
We conducted the search using PubMed, Web of Science, Cochrane library and Scopus for all studies addressing the prevalence of drug resistant strains of K. pneumoniae in Iran, from Jan 1998 to Nov 2014. The applied keywords include Klebsiella, Klebsiella pneumoniae, antibiotic resistance, antibiotic susceptibility, and Iran. Iranian databases including Iranmedex and Scientific Information Database (SID) were also searched (with Persian keywords).
Inclusion and exclusion criteria
We considered all the original articles about the incidence rate of drug resistant strains of K. pneumoniae from hospital-acquired infections in Iran. These articles should reference to the standard method, which recommended by clinical and laboratory standards Institute (CLSI) for drug susceptibility testing of K. pneumoniae against; carbapenems, cephalosporins and the other most used antimicrobial agents. Due to the following reasons, some studies were excluded from our analysis. Articles have focused only on community acquired K. pneumoniae or focused only on non-K. pneuomoniae stains, and studies not used CLSI recommended drug susceptibility testing methods. Furthermore, case reports, meta-analyses or systematic reviews, letters to editor, review articles, non-English or Persian studies, and duplicate publication, were also excluded.
Data extraction and definitions
The extracted data in current study include the first author’s name, the publication time, year of study, number of samples, and prevalence of drug resistant strains of K. pneumoniae. Two authors extracted data from all of the included studies independently and a third investigator reviewed results.
Statistical analysis
The comprehensive meta-analysis software (ver. 2.0) was used to analyse the data. Because of the heterogeneity between studies, random effects models were used and tested with the Cochrane Q test. Moreover, Egger weighted regression and Begg rank correlation tests were performed to assess possible publication bias.
Results
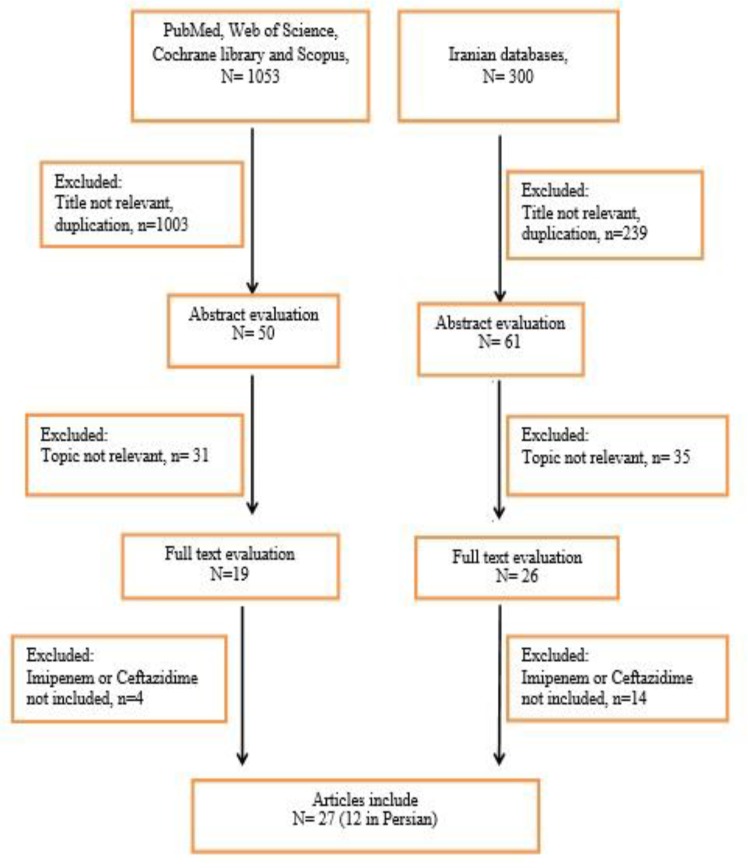
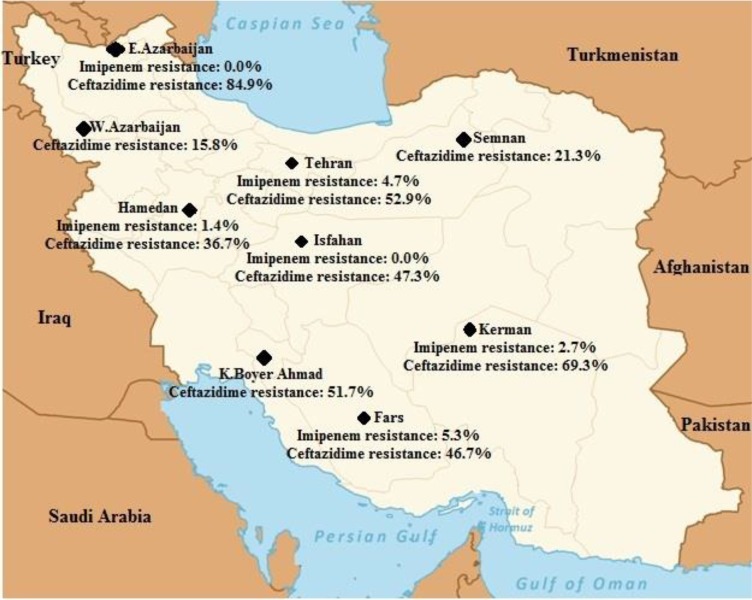
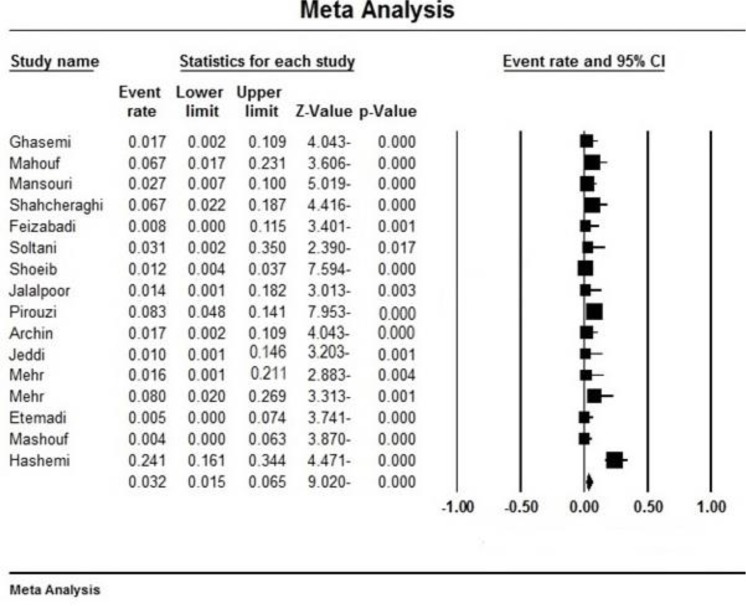
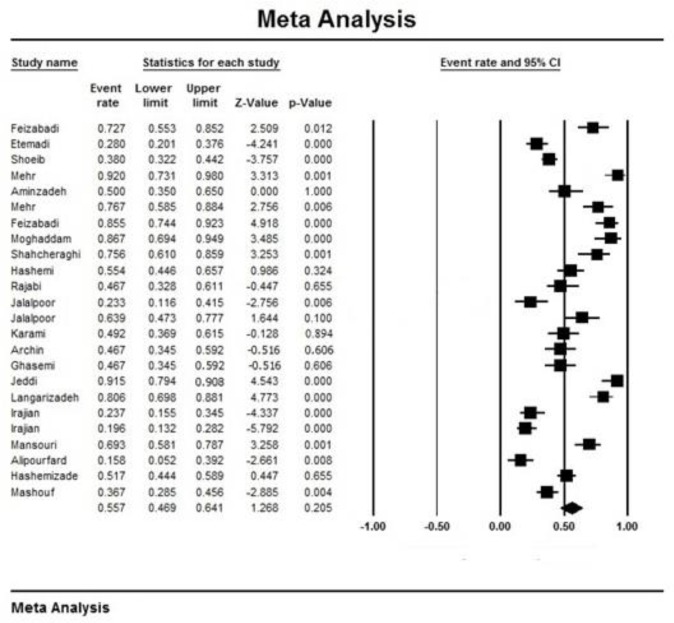
Initially, 1353 articles were collected (Fig. 1). However, in a secondary screening, 1308 of them were excluded according to duplication, title, and abstract evaluation, and full-text of 45 papers was evaluated. Finally, 27 articles describing the prevalence of the ceftazidime- and/or imipenem-resistant strains of K. pneumoniae were selected for meta-analysis (Table 1). In all included studies, antimicrobial susceptibility testing had been performed using disc diffusion method as recommended by CLSI guidelines. Most of the studies were done in Tehran (n=11) compared with Isfahan (n=4), Fars (n=3), East Azerbaijan (n=2), Semnan (n=2), Hamadan (n=2), K. Boyer Ahmad (n=1), West Azerbaijan (n=1) and Kerman (n=1). Fig. 2 shows the distribution of drug-resistant strains of K. pneumoniae in different regions of Iran. The prevalence of imipenem and ceftazidime resistance was found to be 3.2% (95% CI, 1.5–6.5) and 55.7% (95% CI, 46.9–64.1), respectively (Table 2). Fig. 3 and 4 show the forest plot of the Meta-analysis of imipenem and ceftazidime resistant K. pneumoniae.
Fig. 1:
Summary of the literature search and study selection
Table 1:
Included studies after full-text evaluation
| References | Published time | Enrollment time | Province | Total number of samples | Isolates of Klebsiella penomoniae | Number of Ceftazidime (%) | Resistance to Imipenem (%) |
|---|---|---|---|---|---|---|---|
| 12 | 2007 | 2002–2005 | Tehran | 200 | 33 | 24(73) | - |
| 13 | 2005 | 2003–2004 | Tehran | 115 | 100 | 28(28) | 0(0) |
| 14 | 2011 | 2006–2009 | Tehran | 250 | 250 | 95(38) | 3(1) |
| 15 | 2010 | 2007–2008 | Tehran | 101 | 25 | 23(92) | 2(8) |
| 16 | 2008 | 2007–2008 | Tehran | 164 | 40 | 20(50) | - |
| 17 | 2009 | 2007–2008 | Tehran | 65 | 30 | 23(77) | 0(0%) |
| 18 | 2010 | 2008–2009 | Tehran | 81 | 62 | 53(85) | 0(0%) |
| 19 | 2014 | 2009–2010 | Tehran | 50 | 30 | 26(87) | - |
| 20 | 2013 | 2009–2011 | Tehran | 360 | 45 | 34(76) | 3(7) |
| 21 | 2014 | 2011–2012 | Tehran | 83 | 83 | 46(55) | 20(24) |
| 22 | 2012 | 2011–2012 | Tehran | 120 | 45 | 21(47) | - |
| 23 | 2011 | 2009–2010 | Isfahan | 211 | 30 | 7(23) | - |
| 24 | 2014 | 2013–2014 | Isfahan | 123 | 15 | - | 0(0) |
| 25 | 2011 | 2009–2010 | Isfahan | 167 | 36 | 23(64) | 0(0) |
| 26 | 2013 | 2010–2011 | Isfahan | 61 | 61 | 30(49) | - |
| 27 | 2013 | 2009–2010 | Fars | 571 | 60 | 28(47) | 1(2) |
| 28 | 2012 | 2009–2010 | Fars | 328 | 144 | - | 12(8) |
| 29 | 2013 | 2009–2010 | Fars | 60 | 60 | 28(47) | 1(2) |
| 30 | 2008 | 2007–2008 | East Azarbaijan | 88 | 47 | 43(91) | 0(0) |
| 31 | 2010 | 2008–2009 | East Azarbaijan | 72 | 72 | 58(81) | - |
| 32 | 2010 | 2007–2008 | Semnan | 310 | 76 | 18(24) | - |
| 33 | 2009 | 2007–2008 | Semnan | 382 | 107 | 21(20) | - |
| 34 | 2014 | 2007–2008 | Kerman | 413 | 75 | 52(69) | 2(3) |
| 35 | 2005 | 1999–2001 | West Azarbaijan | 251 | 19 | 3(16) | - |
| 36 | 2013 | 2010–2012 | Kohgiluyeh and Boyer Ahmad | 202 | 180 | 93(52) | - |
| 37 | 2013 | 2011–2012 | Hamedan | 120 | 120 | 44(37) | 0(0) |
| 38 | 2009 | 2004–2006 | Hamedan | 209 | 30 | - | 2(7) |
Fig. 2:
Distribution of drug-resistant Klebsiella pneumoniae in different regions of Iran
Table 2:
The prevalence of imipenem and ceftazidime resistance among Klebsiella pneumoniae
| Subgroups | No. of study | Prevalence of drug resistance (95% CI) | n/N* | Heterogeneity Test | Egger’s test for publication bias | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | t | P-value | ||||
| Overall effects of resistant to imipenem | 16 | 3.2 (1.5–6.5) | 46/1182 | 75.9 | <.001 | 5.1 | 0.00016 |
| Overall effects of resistant to ceftazidime | 24 | 55.7 (46.9–64.1) | 841/1686 | 92.2 | <.001 | 2.4 | 0.02454 |
CI, confidence interval; n, number of events (drug resistance); N, total number of Klebsiella pneumoniae from the included studies
Fig. 3:
Forest plot of the meta-analysis on imipenem resistance. CI, confidence interval
Fig. 4:
Forest plot of the meta-analysis on ceftazidime resistance. CI, confidence interval
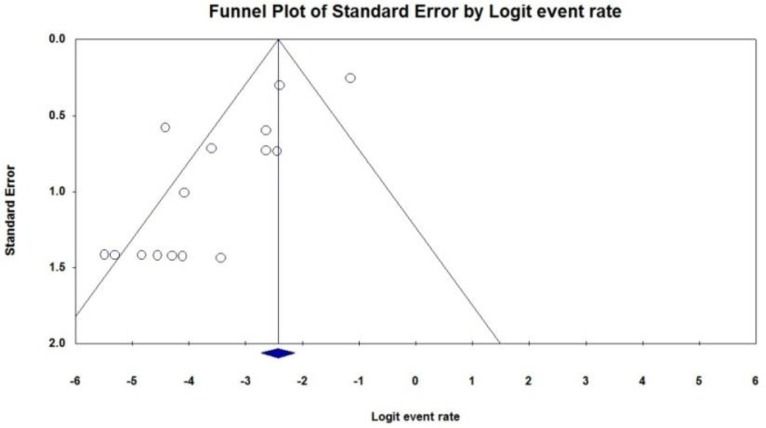
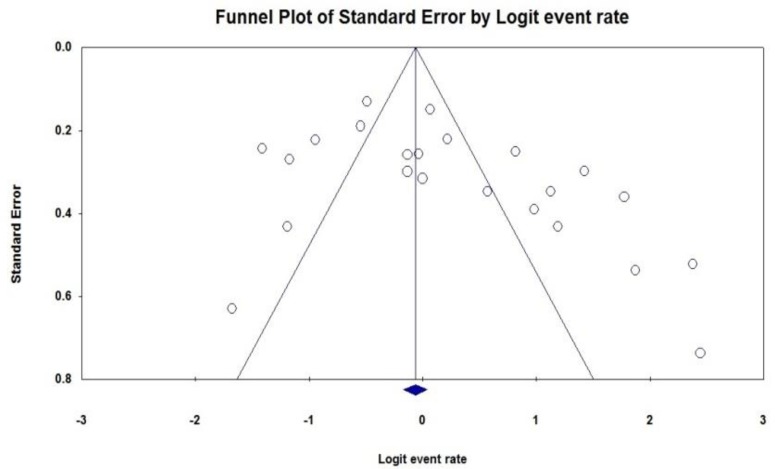
Some evidence for publication bias for imipenem and ceftazidime was observed (P<0.05 for Begg rank correlation analysis; P<0.05 for Egger weighted regression analysis) (Fig. 5, 6). The resistance of K. pneumoniae to other important antimicrobial agents is shown in Table 3.
Fig. 5:
Funnel plot of the meta-analysis on imipenem resistance
Fig. 6:
Funnel plot of the meta-analysis on ceftazidime resistance
Table 3:
Drug resistance status in Klebsiella pneomoniae
| References | Enrollment time | Case number | Carbapenem | Cephalosporins | Aminogly-cosides | Fluoroquinolones | Monobactam | Penicillins | Macrolid | Cotrimoxazole | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMP1 | MEM2 | CAZ3 | CTX4 | CRO5 | CPM6 | AMK7 | GM8 | CIP9 | AZT10 | AMP11 | NF12 | TMP/SXT13 | |||
| 12 | 2002–2005 | 33 | - | - | 24 | 19 | 19 | - | 14 | 21 | 15 | 26 | - | 14 | - |
| 13 | 2003–2004 | 100 | 0 | - | 28 | - | 20 | - | 9 | 30 | 20 | - | - | 31 | - |
| 14 | 2006–2009 | 250 | 3 | - | 95 | 91 | 86 | 100 | 53 | 82 | 85 | - | - | - | - |
| 15 | 2007–2008 | 25 | 2 | - | 23 | 22 | 23 | 22 | 24 | - | 17 | - | 24 | 13 | 18 |
| 16 | 2007–2008 | 40 | - | - | 20 | - | 19 | - | 8 | 14 | 12 | - | 40 | 16 | 20 |
| 17 | 2007–2008 | 30 | 0 | - | 23 | 6 | 20 | 25 | 20 | 16 | 18 | - | 30 | 18 | 18 |
| 18 | 2008–2009 | 62 | 0 | - | 53 | 56 | 47 | 44 | 14 | 30 | 32 | 59 | - | 16 | 47 |
| 19 | 2009–2010 | 30 | - | - | 26 | 25 | - | - | 16 | 17 | 26 | - | 30 | - | - |
| 20 | 2009–2011 | 45 | 3 | 13 | 34 | 37 | - | 33 | 11 | - | 32 | 32 | - | - | 38 |
| 21 | 2011–2012 | 83 | 20 | 20 | 46 | 50 | 49 | 30 | 12 | 29 | 46 | 49 | 65 | - | - |
| 22 | 2011–2012 | 45 | - | - | 21 | - | - | - | - | - | 43 | - | - | 23 | 31 |
| 23 | 2009–2010 | 30 | - | - | 7 | 5 | - | - | 0 | 7 | 6 | - | 21 | 10 | 8 |
| 24 | 2013–2014 | 15 | 0 | 0 | - | 15 | - | 15 | 8 | - | 12 | - | 15 | 10 | - |
| 25 | 2009–2010 | 36 | 0 | - | 23 | 21 | - | 22 | 12 | - | 13 | - | 33 | 7 | 28 |
| 26 | 2010–2011 | 61 | - | - | 30 | 49 | 37 | - | - | - | - | - | - | - | - |
| 27 | 2009–2010 | 60 | 1 | - | 28 | 34 | - | 29 | 5 | 8 | 13 | 19 | 60 | - | 26 |
| 28 | 2009–2010 | 144 | 12 | - | - | - | - | - | 61 | 65 | 42 | - | 23 | - | 43 |
| 29 | 2009–2010 | 60 | 1 | - | 28 | 34 | - | 29 | 5 | 8 | 13 | 19 | 60 | - | 26 |
| 30 | 2007–2008 | 47 | 0 | - | 43 | 42 | 44 | 39 | 5 | - | - | 41 | - | - | - |
| 31 | 2008–2009 | 72 | - | - | 58 | - | - | - | 31 | 53 | 31 | - | - | 68 | 69 |
| 32 | 2007–2008 | 76 | - | - | 18 | 19 | - | - | - | 19 | 35 | - | 73 | - | 41 |
| 33 | 2007–2008 | 107 | - | - | 21 | 24 | - | - | - | 19 | 21 | - | 97 | - | 27 |
| 34 | 2007–2008 | 75 | 2 | - | 52 | 25 | - | 27 | - | 48 | 21 | - | - | - | 35 |
| 35 | 1999–2001 | 19 | - | - | 3 | - | - | - | 0 | 5 | 3 | - | 14 | - | 3 |
| 36 | 2010–2012 | 180 | - | 41 | 93 | 87 | 81 | - | 40 | 65 | 31 | 83 | - | 138 | 108 |
| 37 | 2011–2012 | 120 | 0 | - | 44 | 50 | 52 | 30 | - | 32 | 20 | 52 | - | - | 49 |
| 38 | 2004–2006 | 30 | 2 | - | - | - | 3 | 3 | 11 | 13 | 7 | 19 | 60 | - | - |
| Mean | - | - | 46 | 74 | 841 | 711 | 500 | 448 | 359 | 581 | 613 | 399 | 645 | 364 | 635 |
| Rate | (3.2) | (18.9) | (55.7) | (49.9) | (47.1) | (47.8) | (25.8) | (36.3) | (34.8) | (55.4) | (82.2) | (54.5) | (51.8) | ||
Abbreviations: 1. IMP, imipenem; 2. MEM, meropenem; 3.CAZ, ceftazidime; 4. CTX, cefotaxime; 5. CRO, ceftrixone; 6. CPM, cefepime; 7. AMK, amikacin; 8. GM, gentamycin; 9.CIP, ciprofloxacin; 10. AZT, aztreonam; 11. AMP, ampicillin; 12. NF, nitrofurantoin; 13.SXT/TMP, trimethoprim/sulfamethoxazole
Discussion
The emergence and spread of carbapenem and cephalosporin resistant strains of K. pneumoniae are a considerable threat to public health (2). The major goal of this systematic review was to evaluate the current situation and distribution of drug-resistant K. pneumoniae in Iran.
This analysis showed that 3.2% K. pneumoniae isolates from Iran was resistant to imipenem and 55.7% to ceftazidime. Thereby despite ceftazidime, the imipenem remains as a powerful weapon against K. pneumoniae isolates in Iran. In the current study more than half of K. pneumoniae isolates were resistant to other important antimicrobial agents such as aztreonam (55.4 %), nitrofurantoin (54.5%) and cotrimoxazole (51.8%), we highly recommend that antimicrobial test should be performed prior to any antibiotic prescription in K. pneumonia infections. Very low number of K. pneumonia population (17.8%) were sensitive to ampicillin suggesting ampicillin is not effective drug for empiric treatment of K. pneumonia infections unless we use it in combination with other relevant drugs.
The relatively high rates of drug resistant isolates of K. pneumoniae observed in this study may have several negative effects on public health issues (39). For example, this could cause difficulty in treating K. pneumoniae associated infections since fewer effective drugs are available for treating those highly drug-resistant strains. Unfortunately, these microorganisms are even showing rising rates of resistance to new expensive antibiotics subsequently considered the treatment of choice (40). This is due to the widespread use of broad-spectrum antibiotics in health care settings for empiric treatment of infections. Furthermore, patients infected with these pathogens require prolonged antimicrobial therapy that has considerable implications for the individual patient and for the health care settings. Finally, infections due to these highly resistant strains are reported to be associated with higher morbidity and mortality rates (41). In Iran, 50000 people die each year because of multidrug-resistant bacterial infections and that this costs Iranian economy 2.5 million dollars annually (4).
Some important reasons for the increasing rates of drug resistant isolates in Iran include limited infection surveillance programs, the lack of communication between physicians and microbiologists, lack of standardized or accepted criteria to determine drug resistant isolates, limited laboratory facilities, and poor sanitation. Therefore, active infection control committee, appropriate antimicrobial therapy, and improvement of hygiene condition will prevent or lower the emergence of antimicrobial-resistant pathogens (42).
Current review was carried out according to provinces of Iran and the published time. Because of many hospitals and health care centers in Tehran Province, Iran, patients from other provinces come to Tehran for better treatment. Therefore, most of the studies in this analysis belonged to Tehran, where the ceftazidime- and/or imipenem-resistant strains of K. pneumoniae mostly reported by researchers.
Conclusion
There is a relatively high prevalence of drug resistant K. pneumoniae isolates in Iran. Thus, a high degree of awareness among physicians and microbiologists, active infection control committee, appropriate antimicrobial therapy, improvement of hygiene condition and monitoring of drug resistant isolates are urgently needed in order to better control the emergence and spread of drug-resistant K. pneumoniae isolates in hospital settings.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.Pages JM, Lavigne JP, Leflon Guibout V, et al. (2009). Efflux pump, the masked side of ß-Lactam resistance in Klebsiella pneumoniae clinical isolates. PLoS One, 4(3):e4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidary M, Goudarzi H, Hashemi A, et al. (2016). The prevalence of genes that encode quinolone resistance in Klebsiella pneumoniae strains isolated from hospitalized patients during 2013–2014. Arch Pediatr Infect Dis, (In press). [Google Scholar]
- 3.Heidary M, Bahramian A, Goudarzi H, et al. (2016). To study the association between AcrAB and Qep A efflux pumps and ciprofloxacin resistance among Escherichia coli and Klebsiella pneumoniae clinical strains. Arak Med Univ J, 19(4):1–10. [Google Scholar]
- 4.World Health Organization (2014). Antimicrobial resistance: global report on surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1
- 5.Vardakas KZ, Matthaiou DK, Falagas ME, et al. (2015). Characteristics, risk factors and outcomes of carbapenem resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect, 70(6):592–9. [DOI] [PubMed] [Google Scholar]
- 6.Maatallah M, Vading M, Kabir MH, et al. (2014). Klebsiella variicola is a frequent cause of bloodstream infection in the Stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One, 9(11):e113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouloudi E, Massa E, Piperidou M, et al. (2014). Tigecycline for treatment of carbapenem-resistant Klebsiella pneumoniae infections after liver transplantation in the intensive care unit: a 3-year study. Transplant Proc, 46(9):3219–21. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui NU, Qamar FN, Jurair H, Haque A. (2014). Multi-drug resistant gram negative infections and use of intravenous polymyxin B in critically ill children of developing country: retrospective cohort study. BMC Infect Dis, 14:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura Y, Tanaka M, Yamamoto M, et al. (2015). High prevalence of carbapenem resistance among plasmid-mediated AmpC β-lactamase-producing Klebsiella pneumoniae during outbreaks in liver transplantation units. Int J Antimicrob Agents, 45(1):33–40. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med, 151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 11.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, et al. (2014). Prevalence of drug-resistant tuberculosis in Iran: Systematic review and meta-analysis. Am J Infect Control, 42(11):1212–8. [DOI] [PubMed] [Google Scholar]
- 12.Feizabadi MM, Etemadi G, Rahmati M, et al. (2007). Antibiotic resistance patterns and genetic analysis of Klebsiella pneumoniae isolates from the respiratory tract. Tanaffos, 6(3):20–25. [Google Scholar]
- 13.Etemadi G, Sadeghian S, Amirkhani A, et al. (2005). Drug resistance patterns and prevalence of extended spectrum betalactamase producers among isolates of Klebsiella pneumonia cultured from patients at Tehran hospitals during 2003–2004. Tehran Univ Med J, 63(7):543–50. [Google Scholar]
- 14.Nematzadeh S, Shahcheraghi F, Feizabadi MM, et al. (2011). Molecular characterization of CTX-M β-lactamases among Klebsiella pneumoniae isolated from patients at Tehran hospitals. Indian J Med Microbiol, 29(3):254–7. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadimehr M, Feizabadi M, Bahadori A. (2011). Antibiotic resistance pattern of gram negative bacilli caused nosocomial infections in ICUs in khanevadeh and golestan hospital in Tehran-2007. J Army Univ, 8(4):283–90. [Google Scholar]
- 16.Aminzadeh Z, Kashi MS, Sha’bani M. (2008). Bacteriuria by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Iran J Kidney Dis, 2(4):197–200. [PubMed] [Google Scholar]
- 17.Mohammadimehr M, Feizabadi MM, Bahadori O, et al. (2009). Study of prevalence of gram-negative bacteria caused nosocomial infections in ICU in Besat hospital in Tehran and detection of their antibiotic resistance pattern-year 2007. Iran J Med Microbiol, 3(2):47–54. [Google Scholar]
- 18.Feizabadi MM, Delfani S, Raji N, et al. (2010). Distribution of bla TEM, bla SHV, bla CTXM genes among clinical isolates of Klebsiella pneumoniae at Labbafinejad Hospital, Tehran, Iran. Microb Drug Resist, 16(1):49–53. [DOI] [PubMed] [Google Scholar]
- 19.Moghaddam MM, Barjini KA, Ramandi MF, Amani J. (2014). Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol, 30(5):1533–40. [DOI] [PubMed] [Google Scholar]
- 20.Shahcheraghi F, Nobari S, Rahmati Ghezelgeh F, et al. (2013). First report of New Delhi metallo-beta-lactamase-1-producing Klebsiella pneumoniae in Iran. Microb Drug Resist, 19(1):30–6. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi A, Fallah F, Erfanimanesh S, et al. (2014). Detection of β-lactamases and outer membrane porins among Klebsiella pneumoniae strains isolated in Iran. Scientifica, 2014:726179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajabi Z, Akbari N, Mardane J, Soltan DM. (2012). Antibiotic susceptibility of the strains isolated from blood and urinary tract infections in neonatal intensive care units of Imam Hossein Hospital. Microb Biotechnol, 4(12):53–9. [Google Scholar]
- 23.Jalalpoor S, Mobasherizadeh S. (2011). Frequency of ESBLs in Escherichia coli and Klebsella pneumoniae strains isolated from hospitalized and out-patients with urinary tract infection in selective centers in Esfahan (2009–2010). Razi J Med Sci, 18(85):7–16. [Google Scholar]
- 24.Soltani R, Ehsanpoor M, Khorvash F, Shokri D. (2014). Antimicrobial susceptibility pattern of extended-spectrum β-lactamase-producing bacteria causing nosocomial urinary tract infections in an Iranian referral teaching hospital. J Res Pharm Pract, 3(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalalpoor S. (2011). Antibiotic resistant pattern in ESBLs producer Klebsiella pneumoniae strains isolated of hospitalized and out patients acquired urinary tract infection. J Isfahan Med Sch, 29(142): 695–705. [Google Scholar]
- 26.Karami M, Amirmozafari N, Doudi M. (2013). Effects of silver nanoparticles on ESBL-producing Klebsiellae. Qom Univ Med Sci J, 7(3):28–34. [Google Scholar]
- 27.Archin T, Afzalian E, Kargar M, Ghasemi Y. (2014). Molecular identification of SHV, TEM, CTX-M β-lactamases genes and antibiotics resistance pattern of K. peneumoniae isolates collected from ICU patients of Namazi Hospital, Shiraz, Iran. Armaghane Danesh, 18(10): 816–25. [Google Scholar]
- 28.Pirouzi A, Jafari M, Kargar M, et al. R (2012). Molecular detection of simultaneous occurrence of antibiotic-and heavy metal-resistance in Klebsiella pneumoniae isolated from urinary tract infection. J Isfahan Med Sch, 30(186): 1–12 [Google Scholar]
- 29.Ghasemi Y, Archin T, Kargar M, Mohkam M. (2013). A simple multiplex PCR for assessing prevalence of extended-spectrum β-lactamases producing Klebsiella pneumoniae in intensive care units of a referral hospital in Shiraz, Iran. Asian Pac J Trop Med, 6(9):703–8. [DOI] [PubMed] [Google Scholar]
- 30.Mobasher Kare Jeddi A, Nahaei M, et al. (2009). Molecular study of extended-spectrum beta-lactamase (SHVtype) in Esherichia coli and Klebsiella pneumonia isolated from medical centers of Tabriz. Iran J Med Microbiol, 2(3–4):9–17. [Google Scholar]
- 31.Langari ZM, Ahangarzade RM, Aghazade M, Hasani A. (2010). Comparison the prevalence of multidrug-resistant Klebsiella pneumoniae in adults and children with urinary tract infection. J Animal Physiol Develop, 4(1): 9–17. [Google Scholar]
- 32.Irajian G, Jazayeri Moghadas A. (2010). Frequency of extended-spectrum beta lactamase positive and multidrug resistance pattern in Gram-negative urinary isolates, Semnan, Iran. Jundishapur J Microbiol, 3(3):107–13. [Google Scholar]
- 33.Moghadas AJ. (2009). Prevalence of extended-spectrum beta lactamase positive and multi-drug resistance pattern of Escherichia coli and Klebsiella pneumoniae isolates, Semnan, Iran. Iran J Microbiol, 1(1):49–53. [Google Scholar]
- 34.Mansouri S, Neyestanaki DK, Shokoohi M, et al. (2014). Characterization of AmpC, CTX-M and MBLs types of β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli producing extended spectrum β-lactamases in Kerman, Iran. Jundishapur J Microbiol, 7(2): e8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahbar M, Gra Agaji R, Hashemi S. (2005). Nosocomial blood stream infections in Imam Khomeini Hospital, Urmia, Islamic Republic of Iran, 1999–2001. East Mediterr Health J, 11(3):478–84. [PubMed] [Google Scholar]
- 36.Hashemizadeh FS, Zamanzad B, Jahandideh S, et al. (2013). Identification of KPC-producing Klebsiella pneumoniae in clinical samples in Iran. Yafteh, 15(1):105–14. [Google Scholar]
- 37.Mashouf RY, Alijani P, Saidijam M, et al. (2013). Study of antibiotic resistance pattern and phenotypic detection of ESBLs in Klebsiella pneumoniae strains isolated from clinical samples and determination of minimum inhibitory concentrations of imipenem and ceftazidim antibiotics. Sci J Hamadan Univ Med Sci, 20(4): 295–302. [Google Scholar]
- 38.Mashouf RY, Babalhavaeji H, Yousef J. (2009). Urinary tract infections: bacteriology and antibiotic resistance patterns. Indian Pediatr, 46(7):617–20. [PubMed] [Google Scholar]
- 39.Van der Steen M, Leenstra T, Kluytmans JA, et al. (2015). Trends in expanded-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae among dutch clinical isolates, from 2008 to 2012. PLoS One, 10(9):e0138088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji S, Lv F, Du X, et al. (2015). Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing K. pneumoniae infection. Int J Infect Dis, 38:108–14. [DOI] [PubMed] [Google Scholar]
- 41.Joseph NM, Bhanupriya B, Shewade DG, Harish BN. (2015). Relationship between antimicrobial consumption and the incidence of antimicrobial resistance in Escherichia coli and Klebsiella pneumoniae isolates. J Clin Diagn Res, 9(2):DC08–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Ma Y, Ye L, et al. (2014). Prevalence and antimicrobial susceptibility of hypervirulent Klebsiella pneumoniae isolates in China. Clin Infect Dis, 58(10):1493–94. [DOI] [PubMed] [Google Scholar]