Abstract
Background:
Noroviruses and rotaviruses are important viral etiologies of severe gastroenteritis. Noroviruses are the primary cause of nonbacterial diarrheal outbreaks in humans, whilst rotaviruses are a major cause of childhood diarrhea. Although both enteric pathogens substantially impact human health and economies, there are no approved drugs against noroviruses and rotaviruses so far. On the other hand, whilst the currently licensed rotavirus vaccines have been successfully implemented in over 100 countries, the most advanced norovirus vaccine has recently completed phase-I and II trials.
Methods:
We performed a structured search of bibliographic databases for peer-reviewed research litera-ture on advances in the fields of norovirus and rotavirus therapeutics and immunoprophylaxis.
Results:
Technological advances coupled with a proper understanding of viral morphology and replication over the past decade has facilitated pioneering research on therapeutics and immunoprophylaxis against noroviruses and rotaviruses, with promising outcomes in human clinical trials of some of the drugs and vaccines. This review focuses on the various developments in the fields of norovirus and rotavirus thera-peutics and immunoprophylaxis, such as potential antiviral drug molecules, passive immunotherapies (oral human immunoglobulins, egg yolk and bovine colostral antibodies, llama-derived nanobodies, and anti-bodies expressed in probiotics, plants, rice grains and insect larvae), immune system modulators, probiot-ics, phytochemicals and other biological substances such as bovine milk proteins, therapeutic nanoparti-cles, hydrogels and viscogens, conventional viral vaccines (live and inactivated whole virus vaccines), and genetically engineered viral vaccines (reassortant viral particles, virus-like particles (VLPs) and other sub-unit recombinant vaccines including multi-valent viral vaccines, edible plant vaccines, and encapsulated viral particles).
Conclusions:
This review provides important insights into the various approaches to therapeutics and im-munoprophylaxis against noroviruses and rotaviruses..
Keywords: Gastroenteritis, norovirus, rotavirus, antiviral molecules, passive immunotherapy, other therapeutic approaches, vaccines
1. INTRODUCTION
1.1. Norovirus
Noroviruses, a member of the family Caliciviridae, are one of the leading causes of viral gastroenteritis in humans, accounting for approximately 18% of all cases of diarrhea worldwide [1, 2]. In developed countries, noroviruses have been recognized as the most common cause of non-bacterial diarrheal outbreaks in both children and adults, responsible for around 19-21 million illnesses, 56,000-71,000 hospitalizations and 570-800 deaths annually in the US alone [1-4]. Although rotaviruses are regarded as the major cause of childhood diarrhea in developing countries, noroviruses are now fast emerging as the most prevalent etiological agent of severe gastroenteritis in children in countries that have implemented rotavirus vaccines in their immunization programs [4]. A recent review attributed over 200,000 childhood deaths in developing countries to noroviruses [5].
Noroviruses are transmitted directly by the fecal-oral route, or through exposure to aerosolized vomitus, and indirectly through contaminated food, water and fomites [2, 6]. Clinical signs of norovirus infection in humans include diarrhea, abdominal cramps, nausea and vomiting, which may be uncontrolled, profuse and projectile, resulting in severe dehydration [2, 6]. In healthy individuals, norovirus infection runs a self-limiting course with clinical signs usually lasting for 2-4 days. On the other hand, the disease has been shown to be prolonged and/or more severe, sometimes associated with life threatening complications in young children, older people, immunocompromised individuals and patients with underlying health conditions, such as chronic heart, or kidney ailments [2, 6].
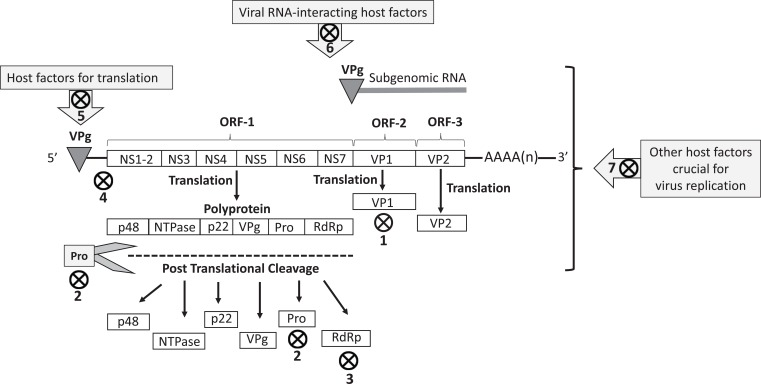
Morphologically, noroviruses possess a single-stranded positive sense RNA genome (~7-8 kb) enclosed in a non-enveloped icosahedral capsid [7]. The RNA is covalently linked to the nonstructural protein VPg (viral protein, genome-linked) at the 5-’end and has a polyA tail at the 3-’ end [8]. Human norovirus RNA contains three open reading frames (ORFs), designated as ORF1-3 [8]. ORF-1 encodes a polyprotein which is post-translationally cleaved into six or seven nonstructural proteins (p48 [NS1/2], NTPase [NS3], p22 [NS4], VPg (NS5], a viral protease [Pro, 3C-like, NS6] and a viral RNA-dependent RNA polymerase [RdRp, NS7]) by the virus-encoded 3C-like cysteine protease (3CLpro) (Fig. 1) [7-9].
Fig. (1).
The norovirus genomic RNA, subgenomic RNA, and structural (VP1 and VP2) and nonstructural (p48, NTPase, p22, VPg, 3CLpro and RdRp] viral proteins. The VPg protein covalently binds to the 5’- end of the viral genomic and subgenomic RNA, whilst the 3’- end of the viral RNA is polyadenylated. Anti-noroviral targets, including potential targets, have been shown with symbol. 1. Virus capsid (VP1)-host cell receptor binding blockers: carbohydrate analogs of fucose (citrates, glucomimetics), heparan sulfate analogs (Suramin), soluble histones, tannic acid, and HBGA-blocking monoclonal antibodies. 2. 3C-like cysteine protease (3CLpro) inhibitors: peptidyl transition state (TS) inhibitors, latent peptidyl TS inhibitors, peptidyl TS mimics, Macrocyclic peptide inhibitors, and Rupintrivir. 3. RNA-dependent RNA-polymerase inhibitors: nucleoside (Ribavirin, Favipiravir and 2'-C-methyl-cytidine) and non-nucleoside (Suramin and NF023) analogs. 4. Targeting viral RNA: Peptide-conjugated phosphorodiamidate morpholine oligomers (PMO), and siRNA. 5. Targeting VPg-host factors interactions: Hippuristanol. 6. Targeting viral RNA-interacting host factors: potential inhibitors of factors, such as La, PTB, DDX3, PCPB2, and hnRNPs. 7. Targeting other host factors/pathways crucial to virus replication, such as inhibitors of cellular deubiquitinases (WP1130 and 2-Cyano-3-Acrylamide Compound-6), molecular chaperone hsp90, and cholesterol pathways.
On the other hand, ORF-2 and ORF-3 encode the structural proteins VP1 and VP2, respectively (Fig. 1) [8]. The VP1 is the major capsid protein and contains antigenically significant epitopes [10]. Ninety dimers of the VP1 constitute the norovirus icosahedral capsid. Structurally, the norovirus VP1 consists of an internal shell (S) domain and the protruding (P) domain. Whilst the S domain tends to be conserved, the P domain is more variable and has been further subdivided into P1 and P2 subdomains [7, 8, 11]. The P2 subdomain is positioned on the external surface of the viral capsid and consists of a hypervariable region that interacts with Histo-Blood Group Antigen (HBGA) receptors on host cells [12-14]. Noroviruses encode only a few copies of the VP2. The VP2 is located at the inner surface of the viral capsid, in association with the VP1 S domain, and is believed to enhance the stability of VP1 [7, 8, 15].
The replication of noroviruses has been exhaustively reviewed elsewhere [7, 8, 16], and will be discussed briefly here, as many of the approaches to designing anti-norovirus molecules target various stages of the virus replication process. Noroviruses attach to host cells through the interactions between the VP1 and the host HBGA oligosaccharide receptors [10, 17]. However, recent studies have provided in vivo evidence for HBGA-independent binding and internalization of human norovirus particles, pointing towards the involvement of other or additional host cell surface receptor/s [18]. Although the virus internalization events remain to be clearly elucidated, murine noroviruses have been shown to rely on cholesterol and dynamin in a clathrin- and caveolae-independent pathway [19, 20].
Norovirus replication has been shown to occur in close association with rearranged intracellular membranes, such as those derived from the Golgi apparatus, or endoplasmic reticulum [21]. Following uncoating and disassembly, transcription and translation of viral RNA occurs in the cytoplasm of infected host cells [7, 8, 16]. The VPg protein recruits host translational factors that mediate the translational process of viral RNA which acts as the mRNA. ORF-1 encodes the polyprotein that is cleaved post-translationally into 6 or 7 nonstructural proteins by 3CLpro. Subgenomic +RNA synthesized during virus replication are utilized for translation of viral VP1 and VP2 proteins. The processes involved in norovirus assembly and release remain to be clearly elucidated [2, 6-8, 16].
Noroviruses exhibit high levels of genetic diversity [2]. Important mechanisms of genetic diversity of noroviruses include point mutations that are attributed to the error-prone activity of RdRp, and recombination events. Based on phylogenetic analyses of the VP1- encoding gene, noroviruses are classified into at least six genogroups (GI-VI) and a tentative 7th genogroup [22]. Genogroups are further subdivided into over 30 genotypes [22]. Human noroviruses primarily belong to GI, GII, or GIV. Majority of the clinical cases of norovirus infection have been associated with GII.4 strains [22-25]. However, recently, GII.P17-GII.17 strains were found to be predominant in some parts of Asia [26, 27].
1.2. Rotavirus
Rotaviruses, a member of the family Reoviridae, are a major cause of diarrhea in young of humans and animals [28]. Although recent global, regional, and country estimates on rotavirus mortality rates revealed an overall decline in rotavirus-associated childhood (aged < 5 year) deaths from 528,000 in 2000 to 215,000 in 2013, attributed to the introduction of rotavirus vaccines in immunization programs, the number of deaths from rotavirus disease still remains very high, especially in developing countries, such as India, Nigeria, Pakistan, and Democratic Republic of Congo [29]. Rotaviruses are responsible for 114 million episodes of diarrhea that require home care, 24 million visits to the clinics, and 2.4 million hospitalizations, accounting for ~36% of all hospitalizations with diarrhea [30, 31].
Rotaviruses are commonly transmitted by fecal-oral route, directly, or indirectly through contaminated food, water, or fomites [28, 32]. Depending on the complex interactions between the host and viral factors, rotavirus infection can be symptomatic or asymptomatic [33]. Clinical signs in symptomatic cases include self-limiting diarrhea with defection of watery and non-bloody stool, low grade fever, vomiting and abdominal pain [32]. Rotavirus disease can be severe and/or prolonged in children with immunodeficiencies, or following organ transplantation [32]. Rotaviruses are zoonotic, as evident from increasing accumulation of conclusive data on animal-to-human interspecies transmission and reassortment events [34, 35].
Rotaviruses are non-enveloped viruses with a three layered capsid that contains 11 segments of double-stranded RNA (dsRNA) [36]. The rotavirus genome encodes six structural proteins, designated as VP1-VP4, VP6 and VP7, and five or six nonstructural proteins, designated as NSP1-NSP5/6. The VP7 protein forms the outer layer of the capsid. The VP4 spike proteins project outward from the capsid. The outer capsid VP7 and VP4 proteins are antigenically significant, eliciting neutralizing antibodies, and form the basis of the currently licensed rotavirus vaccines [36]. The VP6 constitutes the middle capsid layer, whilst VP2 is the inner core protein. The VP1 is the viral RNA-dependent RNA-polymerase. VP3 functions as the capping enzyme for viral RNA. The rotavirus nonstructural proteins coordinate and regulate virus replication, antagonizes host immune responses, subvert important cellular mechanisms to facilitate successful virus replication, and are associated directly and indirectly with viral pathogenesis [28, 33, 36, 37].
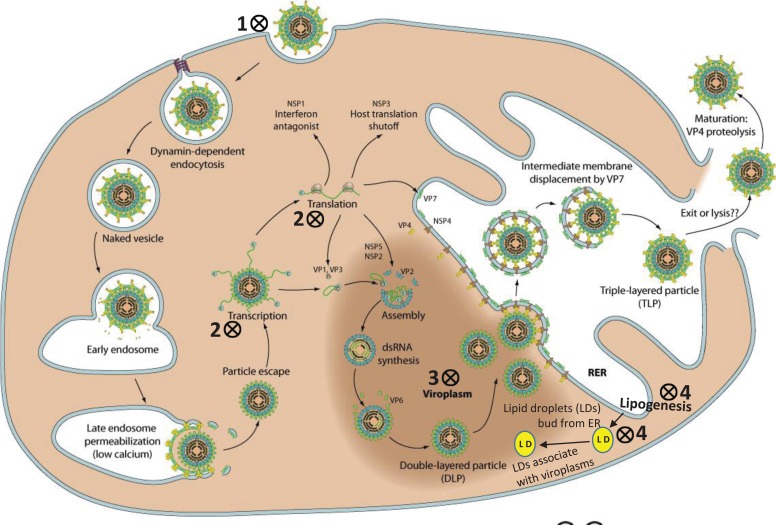
The rotavirus replication process (Fig. 2) has been exhaustively reviewed elsewhere [36], and will not be discussed in details here. Briefly, the virus primarily infects the enterocytes of the intestinal villus. Following virus attachment and entry into host cells, which is a multistep process that involves sialic acid receptors and integrins, the virus is localized into endosomes. Within the endosome, the virus loses its outer capsid layer, and the resultant double-layered particles (DLPs) actively transcribe mRNA from viral RNA that are released into the cytoplasm. These mRNAs serve as templates for dsRNA synthesis for progeny virions, or are translated into the structural and nonstructural viral proteins [36]. The initial stages of packaging (early stage DLPs) and replication occur in cytoplasmic inclusion bodies known as viroplasms. Thereafter, DLPs interact with endoplasmic reticular membrane, acquire the outer capsid protein, and progeny virions are eventually released by budding [36].
Fig. (2).
Replication of rotavirus in host cell. Potential anti-rotaviral targets have been shown with symbol. 1. Blocking virus attachment and entry into host cells: sialylmimetics, lactadherin-derived peptides, neoglycolipid receptor mimetics, and membrane-impermeant thiol/disulfide-blockers. 2. Inhibition of viral RNA and/or protein synthesis: genistein, phosphonoformic acid [foscarnet, PFA], ribavirin and other nucleoside analogs, 3-deazaguanine (3-DG), neomycin and other aminoglycosides, actinomycin D, mycophenolic acid, isoprinosine, viscogens (glycerol), and siRNA. 3. Inhibition of viroplasm formation: nitazoxanide. 4. Suppression of virus replication, and/or virus maturation through inhibition of host cell lipid metabolism pathways and/or homeostasis of lipid droplets (LD): bile acids and farnesoid X receptor (FXR) agonists, 5-(tetradecyloxy)-2-furoic acid (TOFA), triacsin C, isobutylmethylxanthine (IBMX) + isoproterenol, stilbenoids, lovastatin, and cyclooxygenase inhibitors. Lipogenesis and its role in rotavirus replication has been excellently reviewed by Lever and Desselberger, 2016 [169]. This image has been modified with permission from the original source: ViralZone www.expasy.org/viralzone, © SIB Swiss Institute of Bioinformatics [297]. RER, rough endoplasmic reticulum.
Based on differences in the VP6 protein and/or gene, rotaviruses have been classified into at least 8 groups/species and a tentative 9th species [38, 39]. Among them, rotavirus-A (RVA) are the major cause of viral diarrhea in infants and children. RVAs are further classified into G- and P- genotypes on the basis of variations in the antigenically significant outer capsid protein VP7- and VP4- encoding gene, respectively [36]. To date, at least 32 G and 47 P genotypes have been reported [40]. Although multiple G-P genotype combinations have been reported in humans so far, G1-G4, G9 and G12 in conjunction with p [4], p [6], or p [8] are considered as the common human genotypes [28, 35, 38, 40]. A whole genome-based genotyping scheme that assigns genotype to all the 11 gene segments is also available [38].
2. NOROVIRUS THERAPEUTICS AND IMMUNOPROPHYLAXIS
Although human noroviruses are a major public health concern, small animal models and cell culture systems were until now lacking, hindering developments on therapeutics and prophylaxis of norovirus infection [1, 2, 41]. As a result, there is no licensed norovirus vaccine for humans so far, although the most advanced vaccine candidate has successfully demonstrated proof-of-concept in two human challenge studies [42]. On the other hand, there are only a few reports on antiviral treatment in humans. However, on the brighter side, technological advances over the past decade has facilitated pioneering research on norovirus therapeutics and immunoprophylaxis, resulting in the identification of several potential antiviral drug molecules (Fig. 1, Table 1) and some vaccine candidates (Table 2). In the following two sections, we shall be focusing on the numerous advances made in the fields of norovirus therapeutics and immunoprophylaxis.
Table 1.
Therapeutic approaches against norovirus infection.
| Antiviral Approach | Molecule/Formulation |
|---|---|
| Targeting virus binding to host cell surface | • Carbohydrate analogs with structures resembling fucose (citrate and other glucomimetics, milk oligosaccharides). • Heparan sulfate analogs (heparin and suramin). • Soluble histones. • Tannic acid (Chinese herbs). • HBGA-blocking monoclonal antibodies. |
| Targeting the virus-encoded cysteine protease, 3CLpro | • Peptidyl TS inhibitors (aldehyde, α-ketoamide, or α-ketoheterocycle moiety as war head). • Latent TS inhibitors (bisulfite adducts). • TS mimics (α-hydroxyphosphonates and α-hydroxyesters). • Macrocyclic peptide inhibitors. • 3C protease inhibitors possessing a Michael acceptor (Rupintrivir). |
| Targeting the virus-encoded RNA-dependent RNA polymerase | • Nucleoside analogs (Ribavirin, Favipiravir and 2'-C-methyl-cytidine). • Non-nucleoside analog (Suramin and NF023). |
| Other approaches targeting the virus/viral replication | • Heterocyclic carboxamide derivatives. • Cyclosulfamide- and acyclic sulfamide derivatives. • Synthetic (E)-2-styrylchromones. • Piperazine derivatives. • Hippuristanol. • NTPase (NS3) inhibitors. • Peptide-conjugated phosphorodiamidatemorpholine oligomers. • siRNA. |
| Targeting host cell factors/pathways crucial to virus replication | • Inhibitors of virus RNA-interacting host proteins. • Cellular deubiquitinase inhibitors (WP1130 and 2-Cyano-3-Acrylamide Compound-6). • hsp90 inhibitors. • Inhibitors of cholesterol pathways (ACAT inhibitors). |
| Passive immunotherapies | • Oral human immunoglobulins. • Egg yolk antibodies. • Conventional monoclonal antibodies. • Recombinant monoclonal llama-derived nanobodies. |
| Nanoparticles | • Silver nanoparticles. • Gold/Copper Sulfide Core/Shell Nanoparticles. • Copper iodide nanoparticles. |
| Natural phytochemicals and other biological substances | • Flavonoids (black raspberries, cranberries, grape seeds, green tea extracts, mulberries, persimmons and pomegranates). • Catechins (grape seeds and green tea). • Polymeric tannins (berries and persimmons). • Anthocyanidins and polyphenols (Black berries, cranberries, mulberries and pomegranates). • Saponins (red ginseng). • Chitosan (chitins from crustacean exoskeleton). • Oregano oil. • Citric acid. |
| Probiotics | • Bifidobacterium adolescentis • Lactococcus lactis • Lactobacillus paracasei |
| Others | • Nitazoxanide (Drug under clinical development, limited data available on antiviral actions). • HBGAs incorporated hydrogels. • Bismuth subsalicylate. |
ACAT, acyl-CoA:cholesterol acyltransferase; HBGA,histo-blood group antigen; hsp90, heat shock protein 90; siRNA, small interfering RNA; TS, transition state.
Table 2.
Features of norovirus vaccine candidates.
| Vaccine Candidate | VLP Formulation | VLP (Based on Viral Vectors) Formulation | P Particle Formulation |
|---|---|---|---|
| Contains VP1 capsid domain(s) | P and S | P and S | P |
| Expression systems | Baculovirus (Eukaryotic), potato, tobacco, tomato, yeast | Adenovirus, Newcastle disease virus, Venezuelan equine encephalitis virus, Vesicular stomatitis virus. |
E. coli, yeast |
| Platform for foreign antigens | Yes (Rotavirus) | Not evaluated | Yes (Astrovirus, Hepatitis E virus, Influenza virus, Rotavirus) |
| Animal studies | Yes | Yes | Yes |
| Human clinical trials | Several [Takeda IM bivalent (GI.1 + GII.4) vaccine, proof of concept] | No | No |
| Route (Humans) | Oral Nasal Intramuscular (IM) |
Not studied | Not studied |
2.1. Norovirus Therapeutics
In typical outbreak situations, the primary line of treatment of clinical cases of norovirus infection has been rehydration therapy, with a lot of emphasis on decontamination procedures [6, 7]. Depending on the severity of dehydration and frequency of vomition, treatment may comprise oral intake of fluids, or intravenous administration of fluids.
2.1.1. Drugs Under Clinical Development
Among the norovirus drugs in clinical development, Nitazoxanide, initially developed and marketed as an antiprotozoal drug, was shown to reduce the duration of norovirus illness in a double-blind, placebo-controlled clinical trial [43]. In another study, Nitazoxanide alleviated the clinical signs in an immunosuppressed patient, although he kept on asymptomatically shedding the virus for one more month [44]. Although Nitazoxanide has shown promise in the treatment of norovirus and rotavirus gastroenteritis, limited information is available on its antiviral actions, necessitating further studies [45].
2.1.2. Targeting Virus Binding to Host Cell Surface
Using state-of-art tools, such as X-ray crystallography, Mass spectrometry, STD-NMR and surface plasma resonance technology, it has been possible to gain vital and detailed insights into the norovirus capsid-host cell HBGA receptor/s interactions, presenting attractive targets for designing antiviral molecules [7, 46-49]. Since the capsid binding sites that interact with fucose residues of HBGA receptors are conserved among human noroviruses, several carbohydrate analogs with structures resembling that of fucose are being considered as potential norovirus binding blockers, such as citrate and other glucomimetics [46, 47, 49-52]. For example, in a recent study, two oligosaccharides present in human milk, 2’-fucosyllactose (2’FL) and 3-fucosyllactose (3FL), were shown to block GII.10 norovirus Virus-Like Particles (VLPs) from binding to HBGAs [53]. GII noroviruses have also been found to bind to cell surface heparan sulfate proteoglycan, and therefore, the role of heparan sulfate analogs, such as heparin and suramin as binding blockers are being explored [54]. Soluble histones, such as histone H1, a heterologous histone molecule, appear to associate with both VLPs and cell surfaces, thereby preventing the binding of viruses to intestinal cells [55]. In a study based on 50 Chinese medicinal herbs, tannic acid was shown to inhibit the interaction between noroviruses and HBGA receptors [56]. The prospects of generating HBGA-blocking monoclonal antibodies that would act in a similar fashion as the hemagglutination inhibiting or neutralizing antibodies of influenza viruses are also being considered [57].
2.1.3. Targeting the Virus-encoded Cysteine Protease, 3CLpro
One of the attractive targets for designing anti-norovirus molecules is the virus encoded enzyme, 3CLpro. As mentioned earlier, 3CLpro catalyzes the cleavage of the viral polyprotein into nonstructural proteins during virus replication. The high-resolution structure of 3CLpro with a detailed map of the active site has been obtained [58-61]. The active site of the enzyme contains a catalytic triad which consists of a cysteine (nucleophile) residue, a histidine (general base catalyst) residue, and a glutamic acid that aligns the base and facilitates the deprotonation of the nucleophile [61, 62]. Using Fluorescence Resonance Energy Transfer (FRET)-based assays [63] and a cell-based norovirus replicon system [64], it has been possible to identify several 3CLpro inhibiters. These include peptidyl Transition State (TS) inhibitors, latent TS inhibitors and TS mimics [65-71]. The TS inhibitors are competitive inhibitors with an aldehyde, α-ketoamide, or α-ketoheterocycle moiety as the war head, and form a reversible-covalent bond with the enzyme [72]. The latent TS inhibitors are bisulfite adducts derived from TS inhibitors that revert back to the aldehyde warhead [70]. On the other hand, the peptidyl TS mimics, such as α-hydroxyphosphonates and α-hydroxyesters, bind non-covalently to the enzyme [68, 72]. Peptidyl inhibitors have been shown to suppress replication of murine noroviruses (strain MNV-1) in the small intestine of mice [66].
In addition to TS inhibitors, 3C protease inhibitors possessing a Michael acceptor, such as Rupintrivir (α, β-unsaturated ester as the Michael acceptor moiety), are being considered as anti-norovirus agents [73]. Rupintrivir interacts in an irreversible-covalent manner with the active site of 3CLpro. Although originally used for the treatment of enterovirus-71 infections, Rupintrivir was found to demonstrate cross-genotypic anti-norovirus activity, including human and murine noroviruses, and holds promise as a broad-spectrum anti-norovirus agent [73].
Macrocyclic peptides have been found to be less susceptible to proteolysis and have a more rigid drug-like structure compared to the linear peptidyl TS inhibitors [72]. Moreover, conformational constrained macrocyclic peptides exhibited enhanced membrane permeability compared to linear TS inhibitors [72]. Therefore, these molecules are promising candidates with regards to drug stability and oral bioavailability. Two recent studies have demonstrated the inhibitory activities of oxadiazole-based and triazole-based macrocyclic peptides on the norovirus 3CLpro [66, 74].
2.1.4. Targeting the Virus-encoded RNA-Dependent RNA Polymerase, RdRp
Another attractive target for designing anti-norovirus molecules is the RdRp, as this enzyme is crucial to replication of the norovirus genome [7]. Inhibitors of norovirus RdRp include nucleoside and non-nucleoside analogs. The nucleoside inhibitors are phosphorylated to triphosphates by host cell enzymes, following which they exert antiviral effects [75, 76]. Ribavirin (RBV) is a purine analog that was shown to be effective against human and murine noroviruses in cell culture [77]. In a recent study, RBV therapy resulted in complete symptomatic and histological recovery of 3 persistently infected human patients with common variable immunodeficiency [78]. Although RBV is believed to exert antiviral effects through multiple mechanisms, the anti-norovirus activity of RBV is believed to be primarily associated with depletion of GTP levels in host cells [77, 79]. Murine noroviruses were found to exhibit high quasispecies diversity following in vitro exposure to RBV [80].
Two other nucleoside analogs, Favipiravir (T-705) and 2'-C-methyl-cytidine, have been shown to inhibit human and murine noroviruses [81-86]. 2'-C-methyl-cytidine acts as a classic chain terminator, whilst Favipiravir is believed to exert antiviral actions through multiple mechanisms. Favipiravir has been shown to compete mostly with ATP and GTP at the initiation and elongation steps [86]. Favipiravir was also found to elicit norovirus mutagenesis in vivo, which may sometimes lead to virus extinction [87]. In cell-free enzymatic assays, Favipiravir and 2'-C-methyl-cytidine were shown to be more potent inhibitors of human norovirus RdRp than RBV [86].
The non-nucleoside analogs, such as Suramin and NF023, induce a conformational change in RdRp by binding to the allosteric sites of the enzyme, thereby inhibiting the replication process [88, 89]. Suramin also binds to heparan sulfate, and its potential role as a norovirus binding-blocker is also been explored [54].
2.1.5. Other Studies Targeting the Virus/Viral Replication
In addition to the compounds discussed in previous sections, several other molecules have been screened for anti-norovirus properties by various research groups, although the exact mechanism/s of action/s of these agents remain to be more clearly elucidated. In a recent study, heterocyclic carboxamide derivatives appeared to exhibit antiviral effects on murine noroviruses in cell-based screening systems, possibly by acting on intracellular viral replication, or during virus assembly and release [90]. Cyclosulfamide- and acyclic sulfamide derivatives have been found to inhibit norovirus replication in cell-based systems [91, 92]. In another study, synthetic (E)-2-styrylchromones showed potential anti-norovirus activity against murine noroviruses [93]. The prospects of designing anti-noroviral agents based on the piperazine scaffold that can bind to multiple receptors with high affinity are also being investigated [94].
Other innovative approaches targeting directly the virus that are being explored include (i) disruption of the recruitment of host factors by the VPg protein. The role of hippuristanol is being investigated in this regard, as it has been shown to inhibit translation of murine norovirus and feline calicivirus, and is less toxic to cells [95]; (ii) Inhibition of NTPase (NS3) activity; (iii) Peptide-conjugated Phosphorodiamidate Morpholine Oligomers (PMO) that are directed against the first AUG of the viral ORF1 coding sequence; and (iv) Small interfering RNA (siRNA)-mediated targeting of the norovirus genome [75, 96, 97].
2.1.6. Targeting Host Cell Factors/Pathways Crucial to Virus Replication
Since the successful replication and translation of noroviruses rely on several host factors, an alternative strategy has been to target these factors, with the advantage that such inhibitors are less susceptible to drug resistance. Viral RNA-interacting host factors, such as La, PTB, DDX3, PCPB2, and hnRNPs, have been shown to interact with the secondary structures formed by norovirus RNA, although the role/s of these factors in the viral replication cycle remain to be clearly elucidated [98]. Inhibitors of these viral RNA-interacting proteins may be explored as potential anti-noroviral drugs [98, 99].
WP1130, a small molecule inhibitor of cellular Deubiquitinases (DUBs), was found to inhibit replication of murine noroviruses in murine macrophages, and human norovirus in a replicon system by targeting the cellular DUB USP14 [100]. Recently, 2-Cyano-3-Acrylamide DUB inhibitors, such as compound C6, that are structurally related to WP1130 have been identified [101]. Compound C6 was found to display similar levels of inhibitory effects on murine noroviruses in murine macrophages, but exhibited lower toxicity in cell culture and improved pharmacokinetic properties compared to WP1130 [101]. In another recent study, the stability of capsid proteins of human and murine noroviruses were shown to be linked to the activity of the molecular chaperone hsp90 (heat shock protein 90), and inhibition of hsp90 in vivo reduced the production of infectious norovirus particles [102].
Cholesterol pathways are crucial for norovirus replication [103]. Inhibition of Acyl-CoA: cholesterol acyltransferase (ACAT) was found to reduce the levels of norovirus infection, and expression levels of Low Density Lipoprotein Receptors (LDLRs) [103]. LDLRs are hypothesized to facilitate viral replication by acting as a co-factor in viral replication complexes [103]. Interestingly, statins, a commonly prescribed cholesterol-lowering drug, were shown to increase the expression of LDLRs, and may be a risk factor in severe cases of norovirus infection [103].
2.1.7. Interferons
Interferons (IFNs) are a class of signaling peptides that have antiviral activities, and are used to treat certain viral infections. Both type-I and II IFNs inhibited translation of murine noroviruses in vitro [104]. An additive inhibitory effect on norovirus replication has been observed following the usage of RBV with IFNs in cell-based replicon system [77]. In a recent study, IFN-γ (type-III) was shown to clear murine noroviruses from a persistently infected mice in absence of adaptive immunity [105]. Synthetic nanogels based on cross-linked Polyethyleneimine (PEI)-Polyethylenglycol (PEG) are being developed for oral delivery of INFs in norovirus infection [106]. Although studies on murine noroviruses have revealed the importance of INFs in host control of viral infection, a recent study provided strong evidence that human noroviruses RNA replication does not induce an IFN response, suggesting a limited role of IFNs in host control of human norovirus replication [107].
2.1.8. Passive Immunotherapies
Oral Human Immunoglobulin (OHIG) therapy has been shown to be effective in several, but not all chronic shedders of norovirus, and found to be clinically useful in transplant and immunocompromised patients [108-110]. Passive immunization using anti-norovirus antibodies prepared in poultry eggs has also been explored [111, 112]. In one study, chickens immunized with norovirus P particles were found to continuously produce high titers of antibodies in eggs for at least 3 months [111]. The egg yolk antibodies not only blocked binding of norovirus VLPs and P particles to HBGA receptors, but also strongly reacted with norovirus P particles by both ELISA and Western blot. In another study, eggs containing dual antibodies against both rotavirus and norovirus were produced by vaccinating chickens with a divalent vaccine composed of norovirus P particle (as carrier) and rotavirus VP8* protein (as surface insertion) [112]. Since the immunogenicity of egg yolk antibodies, and the associated risk of allergic reactions need to be evaluated before its large-scale application in humans, development of anti-norovirus monoclonal antibodies that are more similar to human IgGs are being considered. Conventional monoclonal antibodies with blockade properties against GII noroviruses have been developed that have potential for humanization [113, 114]. Nanobodies are recombinant, single-domain, camelid heavy-chain antibody fragments that can bind with high affinities to a specific antigen [115]. Recombinant monoclonal llama-derived nanobodies (VHH) have been generated against GI and GII noroviruses, displaying promising results in surrogate neutralizing assays [116]. In a recent study, two nanobodies, Nano-25 and Nano-85, derived from alpaca, were shown to bind to the lower region of the P domain of GII noroviruses [117]. Nano-25 was found to be specific for GII.10, whilst Nano-85 was broadly reactive against several different GII genotypes. Binding of Nano-85 also caused the intact viral particles to disassemble in vitro. Nanobodies are nonimmunogenic when administered orally, and are resistant to protease, which makes them an ideal therapeutic approach for enteric viruses [116]. They can also be engineered into any human immunoglobulin scaffold [116].
2.1.9. Nanoparticles
Silver nanoparticles (AgNPs) have been used as antibacterial agents in consumer products. In a study by Bekele et al. (2016), the anti-noroviral properties of silver nanoparticles were investigated against Feline Caliciviruses (FCV), a surrogate for human noroviruses [118]. 10 nm AgNP particles were shown to achieve significant reduction of FCV titers, which also depended on the time and dose. The authors proposed that the antiviral action of AgNPs might be due to interactions with the VP1 of FCVs. In another study, Gold/Copper Sulfide Core/Shell Nanoparticles (Au/CuS core/shell NPs) were found to inactivate norovirus GI.1 VLPs [119]. Evidence for capsid protein degradation and capsid damage were obtained by immunoblotting, dynamic light scattering, and transmission electron microscopy results. The effects of copper iodide nanoparticles (CuI NPs) on FCV has been studied in Crandell-Rees feline kidney (CRFK) cells [120]. The antiviral effects of CuI Nps was attributed to generation of Cu2+ ions which subsequently caused viral capsid protein oxidation.
2.1.10. Plants and Other Natural Biological Substances
The anti-noroviral activities of natural phytochemicals and biological substances have been exhaustively reviewed by Ryu et al. (2015) [121]. In general, natural phytochemicals and biological substances have been shown to exhibit milder antiviral activities compared to conventional chemical antivirals [121]. However, many of these natural products are safe to consume with minimal, or no side effects, and are a part of the daily diet [121]. Natural compounds with anti-norovirus activity include catechins, flavonoids, anthocyanidins, proanthocyanidins, polyphenols and polymeric tannins [121]. Black raspberries, cranberries, grape seeds, green tea extracts, mulberries, persimmons and pomegranates contain flavonoids that exhibit anti-norovirus activity. Grape seeds and green tea are rich in catechins. Polymeric tannins can be found in berries and persimmons. Black berries, cranberries, mulberries and pomegranates contain large amounts of anthocyanidins and polyphenols. Red ginseng contains saponins, also known as ginsenosides, such as Rb1 and Rg1 that have been shown to reduce FCV and murine norovirus titers [122]. Chitosan, a biopolymer derived from chitin, a component of exoskeletons of crustaceans, has been proposed to interact with the norovirus capsid, thereby inhibiting virus attachment and entry [123, 124]. Herbal essential oils, such as oregano oil and its major volatile compound carvacrol have been shown to cause expansion of the capsid of murine noroviruses, resulting in degradation of the viral capsid [125]. Although noroviruses are resistant to low pH, citric acid has been found to cause virus inhibition [46, 121]. In the gut, citrate reacts with water to form a structure similar to fucose, which may act as a competitive inhibitor and prevent noroviruses from binding to HBGA receptors.
2.1.11. Probiotics
Probiotics are living microorganisms, which when consumed in certain numbers, exert beneficial effects on health, beyond that obtained from inherent basic nutrition [126]. A lactic acid bacteria, Bifidobacterium adolescentis, was shown to inhibit murine norovirus replication in RAW 264.7 cells within 48 h of co-incubation [126]. Bifidobacterium adolescentis also reduced the binding of human NoVGI.1VLPs to both Caco-2cells and HT-29cells, but did not decrease in the binding of human NoV GII.4 VLPs to Caco-2 cells [126]. Pretreatment of FCV with Lactococcus lactis resulted in reduction of viral titers in CRFK cells [127]. Another probiotic, Lactobacillus paracasei, was engineered to secrete a 3D8 single-chain variable fragment that prevented induction of apoptosis by murine norovirus infection, and also reduced messenger RNA (mRNA) expression of the viral VP1 protein [128].
2.1.12. Other Therapeutic Approaches
An interesting therapeutic approach to treat norovirus infection has been the incorporation of HBGAs in hydrogels [129]. In the gut, noroviruses will bind to these receptor molecules, get trapped in the hydrogel which swells following imbibition of water, and eventually excreted from the body. Bismuth Subsalicylate (BSS) is an insoluble salt with antimicrobial properties [130]. Following ingestion, BSS is hydrolyzed in stomach, and the bismuth form insoluble salts including Bismuth Oxychloride (BiOCl) in the gut [130]. BSS and BiOCL have been shown to reduce the infectivity of murine noroviruses, possibly by preventing the attachment of viruses to host cells, and also caused a slight decrease in viral RNA levels [130].
2.2. Norovirus Immunoprophylaxis
Host immune response to norovirus infection has been comprehensively reviewed elsewhere [2, 131, 132]. Briefly, the three key players of the adaptive immune system (B cells and antibodies, CD4+ T cells and CD8+ T cells) as well as innate immune responses, such as INFs, appear to play roles in clearance of infection, reduced pathogenicity, and decreased duration of shedding of viruses. However, a recent study provided evidence that human noroviruses RNA replication may not induce an IFN response [107].
2.2.1. Limitations to Vaccine Development
Although the need for an effective and safe norovirus vaccine has been felt for a long time, research aimed at development of vaccines faced serious challenges. For long periods, human noroviruses could not be propagated in cell-culture, and no small animal models were available. It was only recently that cell culture systems for studying human norovirus replication in B-cells and stem cell-derived human enteroids, and an immunocompromised mice model for human norovirus infection have been established [41, 133, 134]. Previous studies suggested that protective immunity against noroviruses lasts for short periods of time [135], which questioned the feasibility of developing norovirus vaccines, given the fact that vaccine manufacturing and clinical trials are an expensive process. However, a recent exhaustive epidemiological modeling study provided evidence for long-term immunity, lasting between 4 to 8 years [136]. Human noroviruses are highly genetically and antigenically diverse, with cross-reactivity of <5% between GI and GII genotypes [2, 6, 137]. The plasticity of the norovirus genome has raised concerns on the protection spectrum of human norovirus vaccines against diverse strains [6].
2.2.2. Current Vaccine Strategies
Since the establishment of a tissue-culture system for propagating noroviruses is a recent development, most studies on norovirus vaccines rely on VLPs containing recombinantly expressed VP1 capsid proteins. VLPs are non-replicating particles that lack viral RNA [6]. Because of the VP1 protein(s), VLPs possess strain- and genogroup- specific antigenic determinants of the wild norovirus strains, and have been shown to elicit both humoral and cell-mediated immune responses in the host [10, 138]. VLPs can be produced in large quantities using different expression systems, such as baculovirus, potato, tomato, tobacco and yeast [139-142]. Viral vectors, such as adenovirus, paramyxovirus (Newcastle disease virus) and vesicular stomatitis virus have also been used to express VLPs [143-145]. This approach seems to be more appealing than VLPs from traditional expression systems, as only a single dose may be required and higher concentration of VLPs is achieved in the host [132]. However, concerns related to biosafety and interference from prior immunity may hinder development of such vectored vaccines [132]. Norovirus VLPs generated using either of the systems possess both S and P domains of VP1, and exhibit the same HBAG binding patterns as the wild virions [132]. Another new strategy has been the expression of P particles containing only the P domain of VP1 [146]. P particles induce similar levels of immune response as VLPs, and can also be used to express other viral antigens, such as rotavirus and hepatitis virus [146].
2.2.3. Human Clinical Trials
To date, only VLP-based norovirus vaccines have been subjected to human clinical trials [131]. Having completed phase 1 and 2 human clinical trials, a bivalent Intramuscular (IM) norovirus vaccine (Takeda Pharmaceutical) is the most advanced VLP-based candidate so far [147-149]. This aluminium hydroxide adjuvant-based vaccine formulation contains GI.1, and the current major norovirus genotype, GII.4. The GI.1 VLP of the vaccine is based on the prototype Norwalk virus strain, whist the GII.4 was based on a consensus sequence from three GII.4 outbreak variants (2006a_Yerseke, 2006b_Den Haag and 2002_Houston) [147]. After oral challenge with a heterovariant GII.4 strain, the bivalent vaccine offered protection against symptomatic illness, which was higher against severe vomiting and diarrhea, but did not significantly reduce the incidence of protocol-defined illness [138, 147, 148]. Prior to the bivalent vaccine, Takeda (LigoCyte) also had developed an intranasal monovalent vaccine with a GI.1 component that is identical to GI of the bivalent vaccine [147, 150]. Following challenge, the intranasal vaccine was shown to induce moderate levels of protection in human volunteers [151]. A limitation of these vaccine clinical trials was that they did not recruit elderly adults and children, age groups in which vaccine outcomes may be different.
2.2.4. Other Recent Approaches to Vaccine Development
A trivalent VLP-based vaccine (University of Tampere, Finland and UMN Pharma, Japan) composed of a recombinant VP6 of rotavirus and norovirus GI.3 and GII.4 VLPs has been studied in mice [152]. Some of the other norovirus vaccine candidates that are currently under development include an intranasal dry powder VLP vaccine, production of large amounts of P particles using E. coli expression systems, combined norovirus P particle and rotavirus VP8* antigen, combined VLPs of norovirus GII.4 and enterovirus 71 (cause of Hand, foot and mouth disease), P particle-based trivalent vaccine against norovirus, astrovirus and hepatitis E virus, and a chimeric norovirus P particle vaccine candidate expressing influenza A virus subtype antigens [153-158].
3. ROTAVIRUS THERAPEUTICS AND IMMUNOPROPHYLAXIS
The ease and early adaptation of rotaviruses to different tissue culture systems coupled with the availability of small animal models has greatly facilitated the development of human rotavirus vaccines, with the first vaccine licensed for use in 1998 [159]. On the other hand, there is no approved antiviral drug for treatment of rotavirus disease so far. Although various aspects of rotavirus vaccines including their long term impacts on strain diversity have been periodically reviewed, information on anti-rotaviral drugs, or other strategies to rotavirus therapeutics are limited and scattered. In the following section, we have attempted to compile and discuss the various approaches to therapeutics against rotavirus infection (Fig. 2, Table 3). Rotavirus vaccines have been reviewed in the subsequent section, both from the perspective of current advances, and historical significance. Alternate vaccine strategies have also been discussed.
Table 3.
Therapeutic approaches against rotavirus infection.
| Antiviral Approach | Molecule/Formulation |
|---|---|
| Blocking virus attachment and entry into host cells | • Sialylmimetics. • Lactadherin-derived peptides. • Neoglycolipid receptor mimetics. • Membrane-impermeant thiol/disulfide-blockers (DTNB and bacitracin). |
| Suppression of virus replication, and/or virus maturation through inhibition of host cell lipid metabolism pathways and/or homeostasis of lipid droplets (LD) | • Bile acids and FXR agonists. • TOFA. • Triacsin C. • IBMX + isoproterenol. • Stilbenoids. • Lovastatin. • Cyclooxygenase inhibitors. |
| Inhibition of viroplasm formation | Thiazolides (Nitazoxanide). |
| Inhibition of viral RNA and/or protein synthesis | • Genistein. • foscarnet, PFA. • Ribavirin and other nucleoside analogs. • 3-DG. • Neomycin and other aminoglycosides. • Actinomycin D. • Mycophenolic acid. • Isoprinosine. • Viscogens (glycerol). • siRNAs. |
| Passive immunotherapy | • Oral immunoglobulin derived from bovine colostrum, egg yolk, and pooled human plasma. • Llama-derived heavy chain antibody fragment (VHHs ARP1 and ARP3). • Transgenic rice expressing rotavirus-specific llama heavy-chain antibody fragment (MucoRice-ARP1). • Lactobacillus paracasei, yeast, and baculovirus-infected insect larvae expressing VHHs. • Transgenic purple tomatoes expressing neutralizing antibodies. |
| Modulating immune system | • Probiotics. • Interferon therapy. • Cyclosporin. • Meglumine acridonacetate. • Norkurarinol. • Bacterial flagellin. |
| Others | • Plant extracts. • Bovine milk proteins (κ-casein and collectins). • Racecadotril. • Gut microbiota. |
3-DG, 3-deazaguanine; ARP, anti-rotavirus protein; DTNB, 5,5-dithio-bis-(2-nitrobenzoic acid); FXR, farnesoid X receptor; TOFA, 5-(tetradecyloxy)-2-furoic acid; IBMX, Isobutylmethylxanthine; PFA, phosphonoformic acid; siRNA, small interfering RNA;VHH, camelid-derived heavy chain antibody fragment.
3.1. Rotavirus Therapeutics
Whilst giant strides have been made in the development and implementation of rotavirus vaccines, there are no approved antiviral drugs for treating rotavirus infections so far. Treatment is primarily aimed at restoring fluid levels and electrolyte balance by rehydration therapy [28, 36].
3.1.1. Targeting Virus Attachment and Entry into Host Cells
Rotavirus entry into host cells is a multistep process in which the virus initially binds to sialic acid containing host cell-surface glycoconjugates, followed by interactions in which integrins play a crucial role [36, 160]. Considering the role of sialic acid in virus-host cell attachment, some of the sialylmimetics were biologically evaluated as anti-rotavirus agents in vitro [161]. None of the sialylmimetics demonstrated any significant inhibition of the rhesus rotavirus strain (strain RRV), whilst modest levels of inhibition were observed against human RVA strain Wa. In another in vitro study using MA-104 cell culture system, a novel sialylphospholipid, a sialic acid derivative, was found to inhibit simian (strain SA-11) and human (strain MO) rotaviruses in a dose dependent manner [162].
Integrin α2β1 is pivotal to adhesion of rotaviruses to host cell surface [36, 160, 163]. In a recent study, donkey lactadherin-derived peptides containing both DGE and RGD motifs were found to bind to integrin α2β1, thereby inhibiting rotavirus attachment to host cells [163]. Intestinal ganglioside receptors have been demonstrated to play a major role in sialic acid-dependent recognition of porcine host cells by rotaviruses [164]. Applying this knowledge, neoglycolipid receptor mimetics (SPLE and LPE), mimicking the activity of the natural ganglioside receptor, have been synthesized, and shown to significantly reduce rotavirus binding and infectivity in both in vivo and in vitro (piglets) studies [165].
Protein Disulfide Isomerase (PDI) is a family of oxido-reductase that acts as a reductase on the surface of cell membranes [166]. A recent review proposed that rotavirus entry into host cells was dependent on the reductase activity of cell surface PDIs [167]. Cell surface PDIs appeared to generate thiol groups in rotavirus VP5* and/or VP6 proteins, which either primed the proteins to interact with other cell surface receptors, or contributed to virus disassembly [167]. The redox activity of PDI can be inhibited by membrane-impermeant thiol/disulfide-blockers, such as DTNB [5,5-dithio-bis-(2-nitrobenzoic acid)] and bacitracin [168]. It has been shown that treatment of MA104 cells with DTNB and bacitracin resulted in decreased rotavirus infectivity, whilst post-entry treatment of cells with these inhibitors did not influence virus infectivity [168].
3.1.2. Targeting Host Cell Lipid Metabolic Pathways Crucial to Virus Replication
Rotavirus replication is intricately linked to host cell lipid metabolic pathways [169]. Increased levels of triglycerides have been observed in host cells following rotavirus infection [169, 170]. Bile acids and Farnesoid X Receptor (FXR) agonists were shown to downregulate this process of lipid metabolism, thereby suppressing rotavirus replication in vivo and in vitro, and reduced viral shedding in mice [170]. Defective rotavirus particles have been observed in MA-104 cells following treatment with inhibitors of cholesterol biosynthesis, such as lovastatin [171]. Stilbenoids, a group of phenolic compounds obtained from grapes, peanuts and some berries, have been shown to exert anti-rotavirus effects in host cells, possibly through modulation of the cannabinoid receptors and subsequent interference with lipid metabolism [172].
A part of rotavirus replication occur in viroplasms, which are intracytoplasmic inclusion bodies that appear temporarily in virus infected host cells [169]. Viroplasms interact physically and functionally with Lipid Droplets (LD) of host cellular organelles. Therefore, compounds that interfere with cellular lipid metabolism and homeostasis of LD, such as 5-(tetradecyloxy)-2-furoic acid (TOFA), Triacsin C, and a combination of Isobutylmethylxanthine (IBMX) and isoproterenol exert inhibitory effects on replication of rotaviruses in cell culture systems [169, 173, 174]. Inhibition of fatty acid synthesis is also believed to negatively influence the later stages of rotavirus maturation in the endoplasmic reticulum [169].
Cyclooxygenases (COXs) are enzymes that are required for the biosynthesis of prostaglandins (PGs) [175]. Elevated levels of prostaglandins PGE2 have been observed in the fecal of children infected with rotaviruses, pointing towards the involvement of COX and PG in rotavirus pathogenesis [176]. PGE2 is most likely thought to be involved in synthesis of rotaviral proteins and production of virus progeny [176]. COX inhibitors have been shown to block human rotavirus strain Wa infection as well as simian rotavirus strain SA11 infection of Caco-2 cells [175]. Interestingly, the reduction in the duration of rotavirus illness in children following oral administration of aspirin might be attributed to the non-specific inhibition of COX by the drug [175, 177].
3.1.3. Inhibition of Viroplasm Formation
Thiazolides, such as nitazoxanide and its active circulating metabolite, tizoxanide, were shown to inhibit viroplasm formation and interfere with morphogenesis of human (strain Wa) and simian (strain SA11) rotaviruses in different cell-culture systems [178]. Thiazolides are believed to exert their antiviral action by hindering the interaction between the RVA nonstructural proteins NSP5 and NSP2. A double-blind placebo-controlled trial conducted in Egypt revealed a significantly reduction in the duration of rotavirus disease in children (aged <12 years) following a 3-day course of nitazoxanide (drug Alinia) [179]. In another study, nitazoxanide and probiotics were shown to decrease the duration of rotaviral diarrhea in a randomized, single-blind, controlled trial [180].
3.1.4. Targeting Viral RNA and Protein Synthesis
In a recent in vitro study, an isoflavone, genistein, was shown to repress rotaviral RNA transcripts, and possibly viral protein synthesis. Genistein also upregulated the aquaporin water channel (AQP4) mRNA and protein expression, which are downregulated in rotavirus-infected cells [181]. Phosphonoformic acid (foscarnet, PFA), a non-nucleoside pyrophosphate analogue inhibitor of viral polymerases, was demonstrated to inhibit both plus- and minus-strand RNA synthesis in a dose dependent manner [182]. In another study, a series of nucleoside analogs were demonstrated to inhibit synthesis of either plus strand or minus strand rotavirus RNA [183]. Interestingly, Neomycin and other aminoglycosides have also been shown to exhibit inhibitory effects on rotavirus plus- and minus-strand RNA synthesis [184]. Actinomycin D, an antineoplastic antibiotic, has been proposed to complex with viral RNA and prevent newly synthesized mRNA from undergoing translation [185].
One of earlier studies using antiviral drugs demonstrated that RBV is inactive against murine rotaviral disease in mice, but inhibited cytopathic effect induced by simian (SA11) rotavirus in vitro [186]. The authors attributed the ineffectiveness of RBV in vivo to the presence of guanosine inhibitors in the murine gut. In a subsequent study, the effects of RBV and three other antiviral agents (3-deazaguanine [3-DG], 3-deazauridine, and 9-(S)-(2,3-dihydroxy- propyl)adenine [(S)-DHPA]) were evaluated against simian RVA strain SA-11 in tissue culture, and murine RVAs in mice inoculation studies [187]. None of the agents were found to inhibit RdRp activity of SA11, although evidence for inhibition of viral polypeptide synthesis was obtained for RBV and 3-DG. On the other hand, weak to moderate [with (S)-DHPA)] antiviral effects were observed in mice inoculated with murine RVAs.
Mycophenolic acid, an immunosuppressant drug, has been shown to inhibit rotavirus infection in Caco-2 cells and organoids, possibly via inhibiting the inosine-5′-monophosphate dehydrogenase (IMPDH) enzyme and depleting intracellular guanosine nucleotides [188]. Isoprinosine have both immunomodulating and antiviral properties [189]. The antiviral properties of isoprinosine was studied on simian rotavirus strain SA-11 replication in MA-104 cells. Isoprinosine was shown to inhibit synthesis of rotaviral protein as well as double-stranded nucleic acid [189].
Viscosity appears to play a vital role in transcriptionally active dsRNA viral particles [190]. Exploiting this approach, a recent study showed that viscogens, such as glycerol, can inhibit rotavirus transcription in DLPs in a dose dependent manner. The major target of inhibition appeared to be the elongation step of transcription [190].
3.1.5. Passive Immunotherapy
Oral immunoglobulin therapy using immunoglobulins derived from bovine colostrum, egg yolk of immunized hens, or human pooled plasma has been shown to confer passive immunity against rotavirus infection, and reduce duration of viral diarrhea, stool output and/or virus excretion in trials involving children, but not neonates [191-194]. On the other hand, in a small trial involving hospitalized low birthweight babies, no significant differences in rotavirus infection rates were observed after oral gammaglobulin therapy vs the placebo group [194]. Immunoglobulins sourced from bovine colostrum were found to have the highest titer of neutralizing anti-rotavirus antibodies, followed by egg yolk and human pooled plasma [195]. Furthermore, a combination treatment of Hyper-Immune Bovine Colostrum (HBC antibodies) and probiotics, such as Lactobacillus rhamnosus strain GG, has been shown to provide an effective therapeutic effect against rotavirus diarrhea [196].
Llama-derived heavy chain antibody fragments (VHHs), designated as ARP1 and ARP3 (ARP: anti-rotavirus protein), have been derived from an adult llama immunized with rhesus monkey RVA strain RRV (G3p [3]I2) [197, 198]. In a recent placebo-controlled trial, ARP1 was shown to significantly reduce stool output in severe cases of infantile rotaviral diarrhea [199]. Although the mechanism of action of ARP1 is not clear, it may involve binding to VP6 that might result in conformational changes in the outer capsid proteins VP4 and VP7, thereby preventing virus entry, and virus neutralization by immune exclusion [197-199]. The advantages of passive immunotherapy using transgenic rice expressing a water soluble, heat resistant rotavirus-specific llama heavy-chain antibody fragment (termed MucoRice-ARP1) are also being evaluated [200]. To benefit from the synergistic effects of VHH and probiotics, probiotics, such as Lactobacillus paracasei and yeast, expressing anti-RVA VHHs (ARP-1 in secreted and anchored forms, or anchored ARP1/ARP-3) have been constructed [198, 201, 202]. In another recent study, two other VHHs, designated as 3B2 and 2KD1, that are specific for the inner capsid protein VP6 of RVAs, were expressed in baculovirus-infected insect larvae [203]. Neutralizing antibodies against rotavirus have also been produced in vegetables, such as transgenic purple tomatoes [204].
3.1.6. Modulating the Immune System
Both type I and type III IFNs have been shown to confer antiviral protection of sucking mice against simian rotavirus strain RRV, and also restrict extra-intestinal viral replication in other tissues [205]. Meglumine acridonacetate, a synthetic INF inducer, was shown to reduce clinical signs of rotavirus gastroenteritis in children [206]. Probiotics, such as Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 have been found to inhibit replication of human RVA strain Wa in infected Caco-2 cells and also in infected neonatal mouse model [207]. The antiviral effect of these probiotics was attributed to modulation of the immune system through promotion of type I IFNs. In another recent study, cyclosporin, an antiviral agent, was shown to reduce rotavirus diarrhea and viral shedding in sucking mice, possibly through increasing expression of IFN-β mRNA and down regulation of mRNA expression levels of inflammatory cytokines [208]. Norkurarinol, a lavandulylated flavanone, isolated from the roots of Sophora flavescens, was found to modulate rotavirus replication and toll-like receptor 3 (TLR3)-mediated inflammatory pathways, thereby inhibiting rotavirus-induced cytopathic effects [209].
3.1.7. Probiotics
The use of probiotics in combination with other therapeutic agents have been discussed in previous sections. In a recent study, Lactobacillus rhamnosus GG was shown to modulate the innate signaling pathway and cytokine responses to rotavirus vaccine in neonatal human gut microbiota transplanted pigs [210]. Soluble factors released by Lactobacillus casei DN-114 001 were found to block rotavirus infection of cultured human mucus-secreting HT29-MTX cells [211]. In rotavirus infected rodent models, probiotics, such as Lactobacillus rhamnosus GG, Lactobacillus casei DN-114 001 and Lactobacillus reuteri DSM17938 have been shown to reduce the duration of diarrhea as well as severity of intestinal lesions [212]. Randomized Controlled Trials (RCTs) investigating the therapeutic effects of human probiotic Lactobacillus strains on childhood rotavirus diarrhea have been summarized in an excellent review by Liévin-Le Moal and Servin (2014) [212]. Another study performed a meta-analysis study on efficacy of probiotics in children with rotavirus diarrhea, concluding that probiotics have a positive significant effect in reduction of rotavirus diarrhea [213].
3.1.8. Plants and Herbs
The role of plant extracts as anti-rotaviral agents has been evaluated by some researchers. In a Brazilian study, 4 species of medicinal plants were found to exhibit in vitro activity against rotaviruses [214]. In another in vitro study, fractions obtained from Achyrocline bogotensis were shown to exert a virucidal effect on rotaviruses and astroviruses, resulting in reduction of infectious viral particles [215]. Nutritional extracts of plants, such as Nelumbo nucifera Gaertn, Aspalathus linearis (Burm.f.) R. Dahlgren, Urtica dioica L., Glycyrrhiza glabra L. and Olea europaea L. have also been demonstrated to possess anti-rotaviral activity [216]. A combination of Sophora Flavescens Extract (SFE) and Stevioside (SV) from Stevia rebaudiana Bertoni has been shown to alleviate diarrhea, decrease viral shedding in feces, and reduce severity of intestinal lesions in piglets [217]. Persimmon extracts, which are rich in tannins, were found to exhibit antiviral activities against rotaviruses, possibly by aggregating viral proteins [218]. Saponin extracts from Quillaja saponaria Molina have been proposed to cause disruption of virus receptors on cell membranes, thereby inhibiting attachment of rotavirus to host cells [219]. Pectic polysaccharides, derived from Panax ginseng, have also been found to inhibit rotavirus attachment to host cells [220]. Commercially available cranberry fruit juice drink has been demonstrated to inhibit rotavirus replication in MA-104 cells [221]. Extracts from Glycyrrhiza uralensis were found to alleviate rotaviral diarrhea and decrease small intestinal lesions in piglets, possibly by exerting antiviral as well as anti-inflammatory effects [222]. The anti-rotaviral activity of 60 different flavones and flavonols have been studied against human rotavirus strain Wa in vitro [223]. Krisanaklan, a herbal extract used in Thailand, was found to exert antidiarrheal effects by inhibiting the luminal cAMP-dependent chloride channel and Ca(2+)-activated Cl(-) channels in enterocytes of neonatal mice infected with rotaviruses [224]. Another herbal remedy, the Qiwei Baizhu powder, was demonstrated to alleviate rotavirus diarrhea and improve small intestinal absorption in neonatal mice [225].
3.1.9. Other Therapeutic Approaches
The bovine milk protein, κ-casein, was found to inhibit human rotavirus infection by directly binding to viral particles via glycan residues [226]. Purified bovine collections have been proposed to neutralize rotaviruses by various mechanisms, such as hemagglutinin inhibition, blocking virus-integrin interactions and virus aggregation [227]. Racecadotril, an antisecretory drug, has been demonstrated to reduce stool output in small scale drug vs placebo studies, and is being explored as an adjunct to rehydration therapy in children hospitalized with severe rotavirus diarrhea [228].
Some other recent approaches to rotavirus therapeutics include (i) treatment with bacterial flagellin [229]. Flagellin has been shown to exert anti-rotaviral effect through TLR5/NLRC4-mediated production of IL-22 and IL-18; (ii) screening libraries for siRNAs that may prevent rotavirus replication [230], (iii) using 3D cultures of primary intestinal organoids to explore potential antiviral agents [231]; and (iv) assessing the inhibitory effects of gut microbiota on virus infection [232].
3.2. Rotavirus Immunoprophylaxis
Since majority of human cases of rotaviral diarrhea are caused by RVA strains, rotavirus vaccine candidates developed so far are designed to confer immunity against RVAs. Live-attenuated human RVA vaccines have been broadly classified into (i) vaccines based on common or atypical human RVA strains, (ii) vaccines containing genetically engineered animal-human reassortant RVAs, and (iii) vaccines based on bovine or simian RVAs (Table 4). In a recent systemic review, vaccine efficacy against severe rotavirus diarrhea was shown to be the highest (90.6%) in developed countries, followed by Eastern/Southeastern Asia (88.4%), Latin America and the Caribbean (79.6%), Southern Asia (50%) and sub Saharan Africa (46.1%) [233]. Following accumulation of data from additional clinical trials that addressed some of the concerns round the performance of RVA vaccines in developing countries, the World Health Organization (WHO) issued a recommendation for worldwide use of RVA vaccines in 2009 [234]. The efficacies of the currently licensed RVA vaccines against homotypic and heterotypic strains have been excellently reviewed by Leshem et al. (2014) [235], and are summarized in Table 5.
Table 4.
Live-attenuated whole virus vaccines against rotavirus-A (RVA).
| Approach | Vaccine | Make | Currently in Use |
|---|---|---|---|
| Monovalent, common human RVA strain | RotarixTM | Live attenuated G1P1A[8] | Worldwide |
| Rotavin-M1TM | Live attenuated G1P1A[8] | No a | |
| Monovalent, atypical human RVA strain | ROTAVAC TM | Live-attenuated human-bovine reassortant G9P8[11] strain | India |
| I321 | Live-attenuated human-bovine reassortant G10P[11] strain | No b | |
| RV3-BB | Live-attenuated human G3P2A[6] strain | No a | |
| Multivalent, animal-human reassortant Strains | RotaTeqTM | Pentavalent, contains 5 reassortant RVA strains possessing G1, G2, G3, G4, or P[8] on bovine RVA genetic backbone. | Worldwide |
| RotaShieldTM | Tetravalent, contains 3 reassortant strains possessing G1, G2, or G4 on simian RVA (rhesus G3P7[5] strain RRV) genetic backbone, and strain RRV. | No c | |
| Human-bovine reassortant vaccine (NIAID, NIH, USA) | Tetravalent, G1, G2, G3, or G4 genotypes on bovine RVA (UK) genetic backbone | No a | |
| Human-bovine reassortant vaccine (Serum Institute, India-NIAID, NIH, USA) | Pentavalent, G1, G2, G3, G4, or G9 genotypes on bovine RVA (UK) genetic backbone | No a | |
| Monovalent, animal RVA strain | RIT 4237 | Bovine RVA G6P6[1] strain RIT 4237 | No b |
| WC3 | Bovine RVA G6P7[5] strain Wistar Calf 3 | No b | |
| MMU 1006 | Simian (rhesus) RVA G3P5B[3] strain MMU 1006 | No b | |
| LLR | Lamb RVA G10P[12] strain LLR | China |
a Undergoing/completed clinical studies/trials.
b Low efficacy/inconsistent results/not pursued further.
c Withdrawn due to risk of intussusception.
Table 5.
Features of the currently licensed RVA vaccines RotarixTM and RotaTeqTM.
| RotarixTM | RotaTeqTM | |
|---|---|---|
| Manufacturer | GlaxoSmithKline | Merck |
| Dosage schedulea | Middle- & upper-income countries: 2 and 4 months of age. Low-income countries: 6 and 10 weeks of age. |
Middle- & upper-income countries: 2, 4 and 6 months of age. Low-income countries: 6, 10 and 14 weeks of age. |
| Route of administration | Oral | Oral |
| Formulation | Lypholized-reconstituted, and Liquid b | Liquid |
| Pooled efficacy c |
High-income countries: 94% against homotypic strains. 71% against partly heterotypic strains. 87% against fully heterotypic strains. Middle-income countries: 59% against homotypic strains. 72% against partly heterotypic strains. 47% against fully heterotypic strains. |
High-income countries: 83% against homotypic strains. 82% against single-antigen vaccine type strains. 82% against partly heterotypic strains. 75% against single-antigen non-vaccine type strains. Middle-income countries: 70% against single-antigen vaccine type strains. 37% against partly heterotypic strains. 87% against single-antigen non-vaccine type strains. |
3.2.1. Rotavirus Vaccines Based on Common Human RVA Strains
RotarixTM is an oral monovalent human G1 rotavirus vaccine that contains a live-attenuated human G1P1a [8] rotavirus strain (strain 89-12) [236]. The foundation of this vaccine is based on the observation that G1 and P1a [8] are the most common human RVA VP7- and VP4- genotypes/antigens worldwide [28]. Based on results of phase-III clinical trials involving over 77,000 infants in Asia, Europe and the Americas, RotarixTM was found to exhibit high efficacies (81%-96%, combined 2 year follow-up) against severe rotavirus diarrhea, and posed a reduced risk of intussusception, an issue that lead to the withdrawal of a previous RVA vaccine [236]. Following a 2-dose schedule (oral doses at 6 and 10 weeks of age), efficacy rates of 83%-85%, 81%-82%, and 75%-78%, respectively, have been observed with RotarixTM against hospitalizations with diarrhea, wild-type G1 strains, and non-G1 serotypes/strains [237]. However, when compared to the high efficacies observed in developed countries, RotarixTM was found to exhibit lower vaccine efficiency against severe RVA diarrhea in developing countries [234]. To date, Rotarix has been licensed in more than 100 countries worldwide, and is a part of national immunization programs in many of these countries [28]. The presentation of the vaccine has also undergone modifications, with the development of a liquid formulation in addition to the original lyophilized version [236].
Another oral live-attenuated G1p [8] RVA vaccine candidate, known as Rotavin-M1TM, has been tested in Vietnamese children [238]. Rotavin-M1TM was shown to exhibit safety and immunogenicity profiles that were comparable to RotarixTM. The vaccine is currently undergoing a multi-center trial.
3.2.2. Rotavirus Vaccines Based on Atypical Human RVA Strains
A G9P8[11] RVA strain, 116E, isolated from an outbreak of asymptomatic rotavirus infection in India, has been developed into a live-attenuated vaccine candidate, known as ROTAVAC® [239]. Strain 116E was shown to be a human-bovine reassortant strain, possessing a bovine RVA-like VP4 on a human RVA genetic backbone [240]. The vaccine completed phase-III trials, and was found to exhibit 55.1% efficacy against severe rotavirus diarrhea in children aged up to 2 years [241].
Another human-bovine G10p [11] reassortant RVA strain, I321, detected in a nosocomial outbreak of rotavirus infection at a maternity center in Southern India, was also considered as a vaccine candidate [239]. Strain I321 possessed human RVA-like NSP1- and
NSP3- encoding genes on a bovine RVA genetic backbone [242]. The vaccine was eventually withdrawn because of poor immunogenicity [243]. Moreover, later studies detected I321-like human G10p [11] strains in symptomatic infections from the same geographical region where the vaccine strain was detected [244].
RV3 is a G3P2a [6] strain that was isolated from asymptomatic infants in Australia [245]. By whole genome sequencing, RV3 was found to be a human Wa-like RVA strain with atypical VP7-VP4 genotypic combination [240]. Follow-up studies had shown that RV-infected infants did not suffer from severe RVA diarrhea later in childhood when compared to uninfected children, leading to the consideration of this strain as a potential vaccine candidate [246]. Results obtained from 3-dose clinical trials found the naturally attenuated RV3 vaccine to be safe and well tolerated [247, 248]. However, only partial protection (vaccine efficacy of 54%) against rotavirus disease was obtained [248]. Subsequently, a high titer RV3 vaccine, known as RV3-BB [Bishop-Barnes] vaccine was developed [249]. In a recent double-blind, three-arm, placebo-controlled safety and immunogenicity trial based on 3 doses of vaccine in a neonatal or infant schedule, RV3-BB was shown to be immunogenic and well tolerated [250].
3.2.3. Multivalent Vaccines Containing Genetically Engineered Animal-Human Reassortant Rotavirus Strains
RotaTeqTM is a genetically engineered pentavalent vaccine comprising five bovine-human reassortant RVA strains [251]. Each of these reassortant vaccine strains expresses one of the major human VP7 (G1-G4), or VP4 (P1a [8]) antigens on a bovine RVA (strain WC3) genetic backbone. In addition, the vaccine also contains bovine VP7-G6 and VP4-P7[5] antigens. RotaTeqTM confers both homotypic and heterotypic immunity [252]. In the REST (Rotavirus Efficacy and Safety Trial) study involving several countries and a large study population, RotaTeqTM exhibited vaccine efficacy of 98% against severe G1-G4 rotavirus gastroenteritis, whilst efficacy of 74% was observed against G1-G4 rotavirus gastroenteritis of any severity [253]. These efficacy results were corroborated by subsequent clinical trials conducted in European countries and Japan [254, 255]. On the other hand, low vaccine efficacies (39.3% and 48.3%, respectively) have been observed against severe rotavirus gastroenteritis in some developing countries in Sub-Saharan Africa (Ghana, Kenya, and Mali) and Asia (Bangladesh and Vietnam) [256, 257]. RotaTeqTM has been approved for use in more than 100 countries [28]. Although post-licensure studies revealed approximately 1.5 cases of intussusceptions per 100,000 recipients of the 1st dose of the vaccine, there was no evidence for increased risk levels of intussusception in the vaccine group when compared to the placebo group [258, 259]. Rare cases of Kawasaki disease has also been reported in infants who received the 1st dose of vaccine [260]. However, no association was found between RotaTeqTM and increased risk of intussusception, or Kawasaki disease [261].
RotaShieldTM, a tetravalent live attenuated vaccine, was the first oral human rotavirus vaccine to be approved for use in infants in the US [159]. RotaShieldTM contains three human-rhesus reassortant strains encoding a G1, G2, or G4 VP7, and a rhesus rotavirus strain (strain RRV) with human serotype G3 [262]. RotaShieldTM exhibited a vaccine efficacy of 91% against severe rotavirus disease [263]. During clinical trials with RotaShieldTM, 5 intussusception events were observed among 10,054 vaccinated infants, resulting in the Food and Drug Administration of USA licensing the vaccine in 1998 with a mention of intussusception as a possible adverse effect in the package [159, 264]. However, 9 months after its introduction, data accumulated by the Vaccine Adverse Event Reporting System (VAERS) revealed a higher-than-expected number of intussusception events among vaccinated infants, resulting in withdrawal of RotaShieldTM from the market [265].
A tetravalent-human bovine reassortant rotavirus vaccine containing RVAs possessing human G1, G2, G3, or G4 genotypes on the genetic backbone of bovine RVA strain UK has been developed by the National Institute of Allergy and Infectious Diseases (NIAID) [266]. In a study conducted in USA, the vaccine exhibited satisfactory levels of safety and immunogenicity [266]. A new 5-valent human-bovine (UK) reassortant vaccine that contains G9 in addition to G1-G4 was shown to be safe and immunogenic in a small sample of adult volunteers [267]. Another pentavalent bovine (UK)-human reassortant vaccine candidate containing the major human G1-G4 and G9 antigens has been developed by the Serum Institute, India in collaboration with NIAID [268]. The Indian vaccine was found to be safe and immunogenic in phase I and II studies.
3.2.4. Vaccines Based on Animal RVA Strains
The idea of using of animal RVA strains as vaccines stemmed from the ‘Jennerian’ concept, whereby viruses from a heterologous host can induce immunity, but not disease [262]. Bovine strains RIT 4237 and Wistar Calf 3 (WC3), and reassortant rhesus rotavirus vaccine strain MMU18006 have been studied as human rotavirus vaccine candidates, with inconsistent and disappointing results [269-271]. In China, a lamb rotavirus strain derived vaccine, known as the Lanzhou lamb rotavirus (LLR) vaccine, has been approved for use in children [272]. The efficacies of one dose of the LLR vaccine against rotavirus infection ranged from 44.3%-51.8% in children aged between 9-35 months [272]. A more recent study reported an overall protection of 35% against all forms of RVA diarrhea, with 52% protection against moderate diarrhea caused by G3 strains [273]. Although the efficacy of the LLR vaccine remains to be properly evaluated using a randomized, placebo-controlled phase-III trial and this vaccine is not included in the national immunization program of China, around 30,000,000 doses of the LLR vaccine has been administered between 2000 and 2012 [274].
3.2.5. Alternative Vaccine Strategies
3.2.5.1. Subunit and VLP Vaccines
Rotavirus-like Particles (RLPs) are synthetic mimics of the virus that are composed of rotavirus inner capsid proteins [275]. RLPs containing VP2 and VP6 with or without VP4 and VP7, or those presenting an 18 kDa VP8* antigen have been shown to be immunogenic in mice, rabbits and/or gnotobiotic pigs [275, 276]. Although RLPs are considered as a safe alternative to oral live-attenuated rotavirus vaccines, they may not achieve the same levels of vaccine efficacy as live vaccines [275, 277]. Moreover, their production costs may be high, as other molecules may be required to complement the central immunogenic protein [275].
Other approaches to developing subunit vaccines are also being explored. Some of these advances include (i) an intramuscular P2-VP8 subunit vaccine containing a truncated human RVA VP8* subunit protein fused to the tetanus toxin P2 epitope that was shown to elicit promising immune responses in healthy adults [278], (ii) a subunit vaccine composed of truncated VP8* protein (VP8-1, amino acids 26-231) fused to the nontoxic B subunit of cholera toxin with higher immunogenicity and protective efficacy in murine models [279], and (iii) two subunit vaccine candidates containing antigens of astrovirus, hepatitis E virus, and rotavirus that were demonstrated to evoke significantly higher antibody responses in mouse inoculation experiments [280].
3.2.5.2. Inactivated Vaccines
Oral live-attenuated RVA vaccines have been shown to exhibit low immunogenicity in developing countries [234]. Moreover, they have been associated with adverse events, such as intussusception [265]. To overcome these drawbacks, as well as develop a vaccine that would be less costly to manufacture, easy to administer (parenteral vs oral route), and can be used in combination with other vaccine candidates, the potential of Inactivated Rotavirus Vaccines (IRV) are being evaluated [281]. Preclinical testing of different intramuscular IRV candidates in mice, guinea pigs, rabbits and piglets revealed varying levels of protection, ranging from none, partial, nearly complete to complete, with evidence for both homotypic and heterotypic immunity [281-283].
3.2.5.3. Encapsulated Rotavirus Particles
Encapsulation of viral particles in biodegradable non-toxic polymers, such as polylactide (PLA) or poly (lactic-co-glycolic acid) (PLGA) allow controlled delivery of viral antigens to desired locations at predetermined rates [275, 284]. The variable hydrophobicity of these polymers facilitate better interactions with antigen-presenting cells, with improved adjuvant properties for the encapsulated antigens [275]. PLA-encapsulated RVA particles have been shown to evoke significant antibody responses that persisted for longer time periods than free-rotavirus particles [285].
3.2.5.4. Edible Plant Vaccines
Plants are considered as attractive means for production of recombinant antigens, as it is less costly, and does not carry the risk of contamination by animal pathogens [286]. In a recent study, human IgA1 against rotavirus VP8* antigen was expressed in glyco-engineered ▲XT/FT Nicotiana benthamiana plants [287]. Another study reported development of VLP vaccines based on expression of rotavirus proteins of a human G1p [8] RVA strain in Nicotiana benthamiana [288]. High titers of serum IgG and mucosal IgA have been reported in mice following oral inoculation with transgenic potatoes expressing the rotavirus VP7 protein [289]. Transgenic potatoes expressing rotavirus VP7 fused with the ricin toxin B subunit, or cholera toxin B subunit have also been genetically engineered [290, 291]. Detectable levels of antibodies were found in mice following intraperitoneal inoculation with extracts of transgenic tomatoes expressing rotavirus VP2 and VP6 free proteins, and VLPs [292].
Some other lesser explored approaches to development of rotavirus vaccines include cold-adapted mutants [293], DNA-based vaccines [294], and vaccines based on isolated rotavirus proteins, such as VP4, VP6, VP7 and NSP4 [295]. Computational studies aimed at identifying surface accessible VP7 sequences that are conserved across diverse rotavirus strains and can serve as potential targets for peptide vaccines or antivirals are also being performed [296, 297].
Consent for Publication
Not applicable.
Acknowledgements
This review article was funded by the One Health Center for Zoonoses and Tropical Veterinary Medicine, Ross University School of Veterinary Medicine, St. Kitts.
list of ABBREVIATIONS
- 3CLpro
3C-like Cysteine Protease
- DTNB
5,5-dithio-bis-(2-nitrobenzoic acid)
- ACAT
Acyl-CoA: Cholesterol Acyltransferase
- AgNP
Silver Nanoparticles
- BiOCl
Bismuth Oxychloride
- DUBs
Cellular Deubiquitinases
- COX
Cyclooxygenase
- DLPs
Double-layered Particles
- dsRNA
Double-stranded RNA
- FCV
Feline Calicivirus
- HBGA
Histo-blood Group Antigen
- IFNs
Interferons
- IM
Intramuscular
- LLR
Lanzhou Lamb Rotavirus
- VHH
Llama-derived Nanobodies
- LDLRs
Low Density Lipoprotein Receptors
- mRNA
Messenger RNA
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health
- ORF
Open Reading Frame
- PLA
Polylactide
- PGs
Prostaglandins
- PDI
Protein Disulfide Isomerase
- RBV
Ribavirin
- RdRp
RNA-dependent RNA Polymerase
- RVA
Rotavirus-A
- siRNA
Small Interfering RNA
- TS inhibitors
Transition State Inhibitors
- VLPs
Virus-like Particles
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lopman B.A., Steele D., Kirkwood C.D., Parashar U.D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Med. 2016;13(4):e1001999. doi: 10.1371/journal.pmed.1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Graaf M., Van Beek J., Koopmans M.P. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016;14(7):421–433. doi: 10.1038/nrmicro.2016.48. [DOI] [PubMed] [Google Scholar]
- 3.Hall A.J., Lopman B.A., Payne D.C., Patel M.M., Gastañaduy P.A., Vinjé J., Parashar U.D. Norovirus disease in the united states. Emerg. Infect. Dis. 2013;19(8):1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayukekbong J.A., Mesumbe H.N., Oyero O.G., Lindh M., Bergström T. Role of noroviruses as aetiological agents of diarrhoea in developing countries. J. Gen. Virol. 2015;96(8):1983–1999. doi: 10.1099/vir.0.000194. [DOI] [PubMed] [Google Scholar]
- 5.Patel M.M., Hall A.J., Vinjé J., Parashar U.D. Noroviruses: A comprehensive review. J. Clin. Virol. 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Robilotti E., Deresinski S., Pinsky B.A. Norovirus. Clin. Microbiol. Rev. 2015;28(1):134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green K.Y. 2013. [Google Scholar]
- 8.Thorne L.G., Goodfellow I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014;95(Pt 2):278–291. doi: 10.1099/vir.0.059634-0. [DOI] [PubMed] [Google Scholar]
- 9.Belliot G., Sosnovtsev S.V., Mitra T., Hammer C., Garfield M., Green K.Y. In vitro proteolytic processing of the md145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J. Virol. 2003;77(20):10957–10974. doi: 10.1128/JVI.77.20.10957-10974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson E.F., Lindesmith L.C., Lobue A.D., Baric R.S. Viral shape-shifting: Norovirus evasion of the human immune system. Nat. Rev. Microbiol. 2010;8(3):231–241. doi: 10.1038/nrmicro2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocha-Pereira J., Neyts J., Jochmans D. Norovirus: Targets and tools in antiviral drug discovery. Biochem. Pharmacol. 2014;91(1):1–11. doi: 10.1016/j.bcp.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan M., Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6(8):3–4. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W., Chen Y., Jiang X., Xia M., Yang Y., Tan M., Li X., Rao Z. A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface. PLoS Pathog. 2015;11(7):e1005025. doi: 10.1371/journal.ppat.1005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han L., Kitova E.N., Tan M., Jiang X., Pluvinage B., Boraston A.B., Klassen J.S. Affinities of human histo-blood group antigens for norovirus capsid protein complexes. Glycobiology. 2015;25(2):170–180. doi: 10.1093/glycob/cwu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vongpunsawad S., Venkataram Prasad B.V., Estes M.K. Norwalk virus minor capsid protein VP2 associates within the VP1 shell domain. J. Virol. 2013;87(9):4818–4825. doi: 10.1128/JVI.03508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarvestani S.T., Cotton B., Fritzlar S., O’Donnell T.B., Mackenzie J.M. Norovirus infection: Replication, manipulation of host, and interaction with the host immune response. J. Interferon Cytokine Res. 2016;36(4):215–225. doi: 10.1089/jir.2015.0124. [DOI] [PubMed] [Google Scholar]
- 17.Schroten H., Hanisch F-G., Hansman G.S. Human norovirus interactions with histo-blood group antigens and human milk oligosaccharides. J. Virol. 2016;90(13):5855–5859. doi: 10.1128/JVI.00317-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami K., Kurihara C., Oka T., Shimoike T., Fujii Y., Takai-Todaka R., Park Y., Wakita T., Matsuda T., Hokari R. Norovirus binding to intestinal epithelial cells is independent of histo-blood group antigens. PLoS One. 2013;8(6):e66534. doi: 10.1371/journal.pone.0066534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerondopoulos A., Jackson T., Monaghan P., Doyle N., Roberts L.O. Murine norovirus-1 cell entry is mediated through a non-clathrin-, non-caveolae-, dynamin- and cholesterol-dependent pathway. J. Gen. Virol. 2010;91(6):1428–1438. doi: 10.1099/vir.0.016717-0. [DOI] [PubMed] [Google Scholar]
- 20.Perry J.W., Wobus C.E. Endocytosis of murine norovirus 1 into murine macrophages is dependent on dynamin II and cholesterol. J. Virol. 2010;84(12):6163–6176. doi: 10.1128/JVI.00331-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyde J.L., Sosnovtsev S.V., Green K.Y., Wobus C., Virgin H.W., Mackenzie J.M. Mouse norovirus replication is associated with virus-induced vesicle clusters originating from membranes derived from the secretory pathway. J. Virol. 2009;83(19):9709–9719. doi: 10.1128/JVI.00600-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J. Clin. Microbiol. 2014;53(2):373–381. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siebenga J.J., Vennema H., Zheng D-P., Vinjé J., Lee B.E., Pang X-L., Ho E.C.M., Lim W., Choudekar A., Broor S. Norovirus illness is a global problem: Emergence and spread of norovirus GII.4 variants, 2001-2007. J. Infect. Dis. 2009;200(5):802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 24.Silva A., Poló T. Peiró, J. R.; Mendes, L. C. N.; Ludwig, L. F.; de Oliveira-Filho, E. F.; Bucardo, F.; Huynen, P.; Melin, P.; Thiry, E.; Mauroy, A. Human norovirus infection in latin america. J. Clin. Virol. 2016;78:111–119. doi: 10.1016/j.jcv.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Kroneman A., Verhoef L., Harris J., Vennema H., Duizer E., Van Duynhoven Y., Gray J., Iturriza M., Böttiger B., Falkenhorst G. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the foodborne viruses in europe network from 1 July 2001 to 30 June 2006. J. Clin. Microbiol. 2008;46(9):2959–2965. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Graaf M., Van Beek J., Vennema H., Podkolzin A.T., Hewitt J., Bucardo F., Templeton K., Mans J., Nordgren J., Reuter G. Emergence of a novel GII.17 norovirus- end of the GII.4 era? Euro Surveill. 2015;20(26):1–8. doi: 10.2807/1560-7917.es2015.20.26.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan M.C., Lee N., Hung T.N., Kwok K., Cheung K., Tin E.K., Lai R.W., Nelson E.A., Leung T.F., Chan P.K. Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat. Commun. 2015;6:10061. doi: 10.1038/ncomms10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esona M.D., Gautam R. Rotavirus. Clin. Lab. Med. 2015;35(2):363–391. doi: 10.1016/j.cll.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Tate J.E., Burton A.H., Boschi-Pinto C., Parashar U.D. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin. Infect. Dis. 2016;62(Suppl. 2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass R.I., Bresee J., Jiang B., Parashar U., Yee E., Gentsch J. Rotavirus and rotavirus vaccines. Adv. Exp. Med. Biol. 2006;582:45–54. doi: 10.1007/0-387-33026-7_5. [DOI] [PubMed] [Google Scholar]
- 31.Frieden T.R., Jaffe H.W., Stephens J.W., Thacker S.B., Zaza S. Centers for disease control and prevention. rotavirus surveillance - worldwide, 2009 rotavirus. MMWR Morb. Mortal. Wkly. Rep. 2011;60(16):514–516. [PubMed] [Google Scholar]
- 32.Dennehy P.H. Rotavirus infection: A disease of the past? Infect. Dis. Clin. North Am. 2015;29:617–635. doi: 10.1016/j.idc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg H.B., Estes M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology. 2009;136(6):1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martella V., Banyai K., Matthijnssens J., Buonavoglia C., Ciarlet M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010;140(3-4):246–255. doi: 10.1016/j.vetmic.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh S., Kobayashi N. Whole-genomic analysis of rotavirus strains: Current status and future prospects. Future Microbiol. 2011;6(9):1049–1065. doi: 10.2217/fmb.11.90. [DOI] [PubMed] [Google Scholar]
- 36.Estes M.K., Greenberg H.B. 2013. [Google Scholar]
- 37.Hu L., Crawford S.E., Hyser J.M., Estes M.K., Prasad B.V.V. Rotavirus non-structural proteins: Structure and function. Curr. Opin. Virol. 2012;2(4):380–388. doi: 10.1016/j.coviro.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthijnssens J., Ciarlet M., McDonald S.M., Attoui H., Banyai K., Brister J.R., Buesa J., Esona M.D., Estes M.K., Gentsch J.R. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011;156(8):1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mihalov-Kovacs E., Gellert A., Marton S., Farkas S.L., Feher E., Oldal M., Jakab F., Martella V., Banyai K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infect. Dis. 2015;21(4):660–663. doi: 10.3201/eid2104.141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotavirus Classification Working Group(RCWG) Newly assigned genotypes. 2016 http://rega.Kuleuven.be/cev/viralmetagenomics/
- 41.Ettayebi K., Crawford S.E., Murakami K., Broughman J.R., Karandikar U., Tenge V.R., Neill F.H., Blutt S.E., Zeng X.L., Qu L., Kou B., Opekun A.R., Burrin D., Graham D.Y., Ramani S., Atmar R.L., Estes M.K. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddle M.S., Walker R.I. Status of vaccine research and development for norovirus. Vaccine. 2015;34(26):2895–2899. doi: 10.1016/j.vaccine.2016.03.077. [DOI] [PubMed] [Google Scholar]
- 43.Rossignol J-F., El-Gohary Y.M. Nitazoxanide in the treatment of viral gastroenteritis: A randomized double-blind placebo-controlled clinical trial. Aliment. Pharmacol. Ther. 2006;24(10):1423–1430. doi: 10.1111/j.1365-2036.2006.03128.x. [DOI] [PubMed] [Google Scholar]
- 44.Siddiq D.M., Koo H.L., Adachi J.A., Viola G.M. Norovirus gastroenteritis successfully treated with nitazoxanide. J. Infect. 2011;63(5):394–397. doi: 10.1016/j.jinf.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossignol J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansman G.S. Shahzad-ul-Hussan, S.; McLellan, J. S.; Chuang, G.-Y.; Georgiev, I.; Shimoike, T.; Katayama, K.; Bewley, C.; Kwong, P. D. Structural basis for norovirus inhibition and fucose mimicry by citrate. J. Virol. 2012;86(1):284–292. doi: 10.1128/JVI.05909-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X.F., Tan M., Chhabra M., Dai Y.C., Meller J., Jiang X. Inhibition of histo-blood group antigen binding as a novel strategy to block norovirus infections. PLoS One. 2013;8(7):e69379. doi: 10.1371/journal.pone.0069379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han L., Kitova E.N., Tan M., Jiang X., Klassen J.S. Identifying carbohydrate ligands of a norovirus particle using a catch and release electrospray ionization mass spectrometry assay. J. Am. Soc. Mass Spectrom. 2014;25(1):111–119. doi: 10.1007/s13361-013-0752-4. [DOI] [PubMed] [Google Scholar]
- 49.Rademacher C., Guiard J., Kitov P.I., Fiege B., Dalton K.P., Parra F., Bundle D.R., Peters T. Targeting norovirus infection-multivalent entry inhibitor design based on NMR experiments. Chemistry (Easton) 2011;17(27):7442–7453. doi: 10.1002/chem.201003432. [DOI] [PubMed] [Google Scholar]
- 50.El-Hawiet A., Shoemaker G.K., Daneshfar R., Kitova E.N., Klassen J.S. Applications of a catch and release electrospray ionization mass spectrometry assay for carbohydrate library screening. Anal. Chem. 2012;84(1):50–58. doi: 10.1021/ac202760e. [DOI] [PubMed] [Google Scholar]
- 51.Feng X., Jiang X. Library Screen for inhibitors targeting norovirus binding to histo-blood group antigen receptors. Antimicrob. Agents Chemother. 2007;51(1):324–331. doi: 10.1128/AAC.00627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan M., Jiang X. Norovirus-host interaction: Multi-selections by human histo-blood group antigens. Trends Microbiol. 2011;19(8):382–388. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weichert S., Koromyslova A., Singh B.K., Hansman S., Jennewein S., Schroten H., Hansman G.S. Structural basis for norovirus inhibition by human milk oligosaccharides. J. Virol. 2016;90(9):4843–4848. doi: 10.1128/JVI.03223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamura M., Natori K., Kobayashi M., Miyamura T., Takeda N. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 2004;78(8):3817–3826. doi: 10.1128/JVI.78.8.3817-3826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura M., Natori K., Kobayashi M., Miyamura T., Takeda N. Inhibition of attachment of virions of norwalk virus to mammalian cells by soluble histone molecules. Arch. Virol. 2003;148(9):1659–1670. doi: 10.1007/s00705-003-0143-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X.F., Dai Y.C., Zhong W., Tan M., Lv Z.P., Zhou Y.C., Jiang X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 chinese medicinal herbs. Bioorg. Med. Chem. 2012;20(4):1616–1623. doi: 10.1016/j.bmc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasad B.V., Shanker S., Muhaxhiri Z., Deng L., Choi J.M., Estes M.K., Song Y., Palzkill T., Atmar R.L. Antiviral targets of human noroviruses. Curr. Opin. Virol. 2016;18:117–125. doi: 10.1016/j.coviro.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussey R.J., Coates L., Gill R.S., Erskine P.T., Coker S.F., Mitchell E., Cooper J.B., Wood S., Broadbridge R., Clarke I.N. A structural study of norovirus 3c protease specificity: Binding of a designed active site-directed peptide inhibitor. Biochemistry. 2011;50(2):240–249. doi: 10.1021/bi1008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muhaxhiri Z., Deng L., Shanker S., Sankaran B., Estes M.K., Palzkill T., Song Y., Prasad B.V.V. Structural basis of substrate specificity and protease inhibition in norwalk virus. J. Virol. 2013;87(8):4281–4292. doi: 10.1128/JVI.02869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi D., Kim Y., Lovell S., Prakash O., Groutas W.C., Chang K.O. Structural and Inhibitor Studies of Norovirus 3C-like Proteases. Virus Res. 2013;178(2):437–444. doi: 10.1016/j.virusres.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeitler C.E., Estes M.K., Venkataram Prasad B.V. X-ray crystallographic structure of the norwalk virus protease at 1.5-a resolution. J. Virol. 2006;80(10):5050–5058. doi: 10.1128/JVI.80.10.5050-5058.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng L.S., Muhaxhiri Z., Estes M.K., Palzkill T., Prasad B.V.V., Song Y.C. Synthesis, activity and structure-activity relationship of noroviral protease inhibitors. MedChemComm. 2013;4(10):1354–1359. doi: 10.1039/C3MD00219E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang K.O., Takahashi D., Prakash O., Kim Y. Characterization and inhibition of norovirus proteases of genogroups I and II using a fluorescence resonance energy transfer assay. Virology. 2012;423(2):125–133. doi: 10.1016/j.virol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang K.O., Sosnovtsev S.V., Belliot G., King A.D., Green K.Y. Stable expression of a norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353(2):463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Tiew K.C., He G., Aravapalli S., Mandadapu S.R., Gunnam M.R., Alliston K.R., Lushington G.H., Kim Y., Chang K.O., Groutas W.C. Design, synthesis, and evaluation of inhibitors of norwalk virus 3C protease. Bioorg. Med. Chem. Lett. 2011;21(18):5315–5319. doi: 10.1016/j.bmcl.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galasiti-Kankanamalage A.C., Kim Y., Weerawarna P.M., Uy R.A.Z., Damalanka V.C., Mandadapu S.R., Alliston K.R., Mehzabeen N., Battaile K.P., Lovell S. Structure-guided design and optimization of dipeptidyl inhibitors of norovirus 3cl protease. structure-activity relationships and biochemical, x-ray crystallographic, cell-based, and in vivo studies. J. Med. Chem. 2015;58(7):3144–3155. doi: 10.1021/jm5019934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandadapu S.R., Weerawarna P.M., Gunnam M.R., Alliston K.R., Lushington G.H., Kim Y., Chang K.O., Groutas W.C. Potent inhibition of norovirus 3CL protease by peptidyl α-Ketoamides and α-Ketoheterocycles. Bioorg. Med. Chem. Lett. 2012;22(14):4820–4826. doi: 10.1016/j.bmcl.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y., Lovell S., Tiew K-C., Mandadapu S.R., Alliston K.R., Battaile K.P., Groutas W.C., Chang K-O. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 2012;86(21):11754–11762. doi: 10.1128/JVI.01348-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prior A.M., Kim Y., Weerasekara S., Moroze M., Alliston K.R., Uy R.A.Z., Groutas W.C., Chang K.O., Hua D.H. Design, synthesis, and bioevaluation of viral 3C and 3C-like protease inhibitors. Bioorg. Med. Chem. Lett. 2013;23(23):6317–6320. doi: 10.1016/j.bmcl.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandadapu S.R., Gunnam M.R., Tiew K.C., Uy R.A.Z., Prior A.M., Alliston K.R., Hua D.H., Kim Y., Chang K.O., Groutas W.C. Inhibition of norovirus 3CL protease by bisulfite adducts of transition state inhibitors. Bioorg. Med. Chem. Lett. 2013;23(1):62–65. doi: 10.1016/j.bmcl.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandadapu S.R., Gunnam M.R., Galasiti Kankanamalage A.C., Uy R.A.Z., Alliston K.R., Lushington G.H., Kim Y., Chang K.O., Groutas W.C. Potent inhibition of norovirus by dipeptidyl α-hydroxyphosphonate transition state mimics. Bioorg. Med. Chem. Lett. 2013;23(21):5941–5944. doi: 10.1016/j.bmcl.2013.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim Y., Galasiti-Kankanamalage A.C., Chang K.O., Groutas W.C. Recent advances in the discovery of norovirus therapeutics. J. Med. Chem. 2015;58(24):9438–9450. doi: 10.1021/acs.jmedchem.5b00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rocha-Pereira J., Nascimento M.S.J., Ma Q., Hilgenfeld R., Neyts J., Jochmansb D. The enterovirus protease inhibitor rupintrivir exerts cross-genotypic anti-norovirus activity and clears cells from the norovirus replicon. Antimicrob. Agents Chemother. 2014;58(8):4675–4681. doi: 10.1128/AAC.02546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Damalanka V.C., Kim Y., Alliston K.R., Weerawarna P.M., Galasiti-Kankanamalage A.C., Lushington G.H., Mehzabeen N., Battaile K.P., Lovell S., Chang K.O. Oxadiazole-based cell permeable macrocyclic transition state inhibitors of norovirus 3CL protease. J. Med. Chem. 2016;59(5):1899–1913. doi: 10.1021/acs.jmedchem.5b01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohayem J., Bergmann M., Gebhardt J., Gould E., Tucker P., Mattevi A., Unge T., Hilgenfeld R., Neyts J. Antiviral strategies to control calicivirus infections. Antiviral Res. 2010;87:162–178. doi: 10.1016/j.antiviral.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sofia M.J., Chang W., Furman P.A., Mosley R.T., Ross B.S. Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem. 2012;55:2481–2531. doi: 10.1021/jm201384j. [DOI] [PubMed] [Google Scholar]
- 77.Chang K-O., George D.W. Interferons and ribavirin effectively inhibit norwalk virus replication in replicon-bearing cells. J. Virol. 2007;81(22):12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodward J.M., Gkrania-Klotsas E., Cordero-Ng A.Y., Aravinthan A., Bandoh B.N., Liu H., Davies S., Zhang H., Stevenson P., Curran M.D. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. Am. J. Gastroenterol. 2015;110(2):320–327. doi: 10.1038/ajg.2014.432. [DOI] [PubMed] [Google Scholar]
- 79.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006;16(1):37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Julian T.R., Baugher J.D., Rippinger C.M., Pinekenstein R., Kolawole A.O., Mehoke T.S., Wobus C.E., Feldman A.B., Pineda F.J., Schwab K.J. Murine Norovirus (MNV-1) exposure in vitro to the purine nucleoside analog ribavirin increases quasispecies diversity. Virus Res. 2016;211:165–173. doi: 10.1016/j.virusres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Rocha-Pereira J., Jochmans D., Debing Y., Verbeken E., Nascimento M.S.J., Neyts J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J. Virol. 2013;87(21):11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rocha-Pereira J., Jochmans D., Neyts J. Prophylactic treatment with the nucleoside analogue 2′-C-methylcytidine completely prevents transmission of norovirus. J. Antimicrob. Chemother. 2015;70(1):190–197. doi: 10.1093/jac/dku363. [DOI] [PubMed] [Google Scholar]
- 83.Kolawole A.O., Rocha-Pereira J., Elftman M.D., Neyts J., Wobus C.E. Inhibition of human norovirus by a viral polymerase inhibitor in the B cell culture system and in the mouse model. Antiviral Res. 2016;132:46–49. doi: 10.1016/j.antiviral.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Nascimento M.S.J., Neyts J. Favipiravir(T-705) Inhibits in vitro norovirus replication. Biochem. Biophys. Res. Commun. 2012;424(4):777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 85.Costantini V.P., Whitaker T., Barclay L., Lee D., McBrayer T.R., Schinazi R.F., Vinjé J. Antiviral activity of nucleoside analogues against norovirus. Antivir. Ther. 2012;17(6):981–991. doi: 10.3851/IMP2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin Z., Tucker K., Lin X., Kao C.C., Shaw K., Tan H., Symons J., Behera I., Rajwanshi V.K., Dyatkina N. Biochemical evaluation of the inhibition properties of favipiravir and 2=-C-methyl-cytidine triphosphates against human and mouse norovirus RNA polymerases. Antimicrob. Agents Chemother. 2015;59(12):7504–7516. doi: 10.1128/AAC.01391-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arias A., Thorne L., Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. eLife. 2014;3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mastrangelo E., Pezzullo M., Tarantino D., Petazzi R., Germani F., Kramer D., Robel I., Rohayem J., Bolognesi M., Milani M. Structure-based inhibition of norovirus RNA-dependent RNA Polymerases. J. Mol. Biol. 2012;419(3-4):198–210. doi: 10.1016/j.jmb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Croci R., Pezzullo M., Tarantino D., Milani M., Tsay S.C., Sureshbabu R., Tsai Y.J., Mastrangelo E., Rohayem J., Bolognesi M. Structural bases of norovirus RNA dependent RNA polymerase inhibition by novel suramin-related compounds. PLoS One. 2014;9(3):e91765. doi: 10.1371/journal.pone.0091765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohba M., Oka T., Ando T., Arahata S., Ikegaya A., Takagi H., Ogo N., Owada K., Kawamori F., Wang Q., Saif L.J.A. A discovery and synthesis of heterocyclic carboxamide derivatives as potent anti-norovirus agents. Chem. Pharm. Bull. (Tokyo) 2016;64(5):465–475. doi: 10.1248/cpb.c16-00001. [DOI] [PubMed] [Google Scholar]
- 91.Dou D., Mandadapu S.R., Alliston K.R., Kim Y., Chang K.O., Groutas W.C. Cyclosulfamide-based derivatives as inhibitors of noroviruses. Eur. J. Med. Chem. 2012;47(1):59–64. doi: 10.1016/j.ejmech.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dou D., Tiew K.C., Mandadapu S.R., Gunnam M.R., Alliston K.R., Kim Y., Chang K.O., Groutas W.C. Potent norovirus inhibitors based on the acyclic sulfamide scaffold. Bioorg. Med. Chem. 2012;20(6):2111–2118. doi: 10.1016/j.bmc.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rocha-Pereira J., Cunha R., Pinto D.C.G.A., Silva A.M.S., Nascimento M.S.J. (E)-2-styrylchromones as potential anti-norovirus agents. Bioorg. Med. Chem. 2010;18(12):4195–4201. doi: 10.1016/j.bmc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Dou D., He G., Mandadapu S.R., Aravapalli S., Kim Y., Chang K.O., Groutas W.C. Inhibition of noroviruses by piperazine derivatives. Bioorg. Med. Chem. Lett. 2012;22(1):377–379. doi: 10.1016/j.bmcl.2011.10.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaudhry Y., Nayak A., Bordeleau M.E., Tanaka J., Pelletier J., Belsham G.J., Roberts L.O., Goodfellow I.G. Caliciviruses differ in their functional requirements for eIF4F components. J. Biol. Chem. 2006;281(35):25315–25325. doi: 10.1074/jbc.M602230200. [DOI] [PubMed] [Google Scholar]
- 96.Bok K., Cavanaugh V.J., Matson D.O., González-Molleda L., Chang K.O., Zintz C., Smith A.W., Iversen P., Green K.Y., Campbell A.E. Inhibition of norovirus replication by morpholino oligomers targeting the 5′-end of the genome. Virology. 2008;380(2):328–337. doi: 10.1016/j.virol.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cann R.M., McConkey S., O’Brien F. siRNA-mediated targeting of the RNA-dependent RNA polymerase in the Norovirus genome. BMC Proc. 2015;9(Suppl. 7):A11. [Google Scholar]
- 98.Gutiérrez-Escolano A.L., Vázquez-Ochoa M., Escobar-Herrera J., La Hernández-Acosta J. PTB, and PAB proteins bind to the 3′ untranslated region of norwalk virus genomic RNA. Biochem. Biophys. Res. Commun. 2003;311(3):759–766. doi: 10.1016/j.bbrc.2003.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vashist S., Urena L., Chaudhry Y., Goodfellow I. Identification of RNA-protein interaction networks involved in the norovirus life cycle. J. Virol. 2012;86(22):11977–11990. doi: 10.1128/JVI.00432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perry J.W., Ahmed M., Chang K.O., Donato N.J., Showalter H.D., Wobus C.E. Antiviral activity of a small molecule deubiquitinase inhibitor occurs via induction of the unfolded protein response. PLoS Pathog. 2012;8(7):43. doi: 10.1371/journal.ppat.1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Passalacqua K.D., Charbonneau M.E., Donato N.J., Showalter H.D., Sun D., Wen B., He M., Sun H., O’Riordan M.X., Wobus C.E. Anti-infective activity of 2-cyano-3-acrylamide inhibitors with improved drug-like properties against two intracellular pathogens. Antimicrob. Agents Chemother. 2016;60(7):4183–4196. doi: 10.1128/AAC.03021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vashist S., Urena L., Gonzalez-Hernandez M.B., Choi J., de Rougemont A., Rocha-Pereira J., Neyts J., Hwang S., Wobus C.E., Goodfellow I. The molecular chaperone hsp90 is a therapeutic target for noroviruses. J. Virol. 2015;89:6352–6363. doi: 10.1128/JVI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang K-O. Role of cholesterol pathways in norovirus replication. J. Virol. 2009;83(17):8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Changotra H., Jia Y., Moore T.N., Liu G., Kahan S.M., Sosnovtsev S.V., Karst S.M. Type I and Type II interferons inhibit the translation of murine norovirus proteins. J. Virol. 2009;83(11):5683–5692. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nice T.J., Baldridge M.T., McCune B.T., Norman J.M., Lazear H.M., Artyomov M., Diamond M.S., Virgin H.W. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science. 2015;347(6219):269–273. doi: 10.1126/science.1258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y., Thapa M., Hua D.H., Chang K.O. Biodegradable nanogels for oral delivery of interferon for norovirus infection. Antiviral Res. 2011;89(2):165–173. doi: 10.1016/j.antiviral.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qu L., Murakami K., Broughman J.R., Lay M.K., Guix S., Tenge V.R., Atmar R.L., Estes M.K. Replication of human norovirus RNA in mammalian cells reveals lack of interferon response. J. Virol. 2016;90(19):8906–8923. doi: 10.1128/JVI.01425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gairard-Dory A.C., Dégot T., Hirschi S., Schuller A., Leclercq A., Renaud-Picard B., Gourieux B., Kessler R. Clinical usefulness of oral immunoglobulins in lung transplant recipients with norovirus gastroenteritis: A case series. Transplant. Proc. 2014;46(10):3603–3605. doi: 10.1016/j.transproceed.2014.09.095. [DOI] [PubMed] [Google Scholar]
- 109.Chagla Z., Quirt J., Woodward K., Neary J., Rutherford C. Chronic norovirus infection in a transplant patient successfully treated with enterally administered immune globulin. J. Clin. Virol. 2013;58(1):306–308. doi: 10.1016/j.jcv.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 110.Florescu D.F., Hermsen E.D., Kwon J.Y., Gumeel D., Grant W.J., Mercer D.F., Kalil A.C. Is there a role for oral human immunoglobulin in the treatment for norovirus enteritis in immunocompromised patients? Pediatr. Transplant. 2011;15(7):718–721. doi: 10.1111/j.1399-3046.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 111.Dai Y.C., Wang Y.Y., Zhang X.F., Tan M., Xia M., Wu X.B., Jiang X., Nie J. Evaluation of anti-norovirus IgY from egg yolk of chickens immunized with norovirus P particles. J. Virol. Methods. 2012;186(1-2):126–131. doi: 10.1016/j.jviromet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai Y.C., Zhang X.F., Tan M., Huang P., Lei W., Fang H., Zhong W., Jiang X. A dual chicken IgY against rotavirus and norovirus. Antiviral Res. 2013;97(3):293–300. doi: 10.1016/j.antiviral.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lindesmith L.C., Beltramello M., Donaldson E.F., Corti D., Swanstrom J., Debbink K., Lanzavecchia A., Baric R.S. Immunogenetic mechanisms driving norovirus gii.4 antigenic variation. PLoS Pathog. 2012;8(5):e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindesmith L.C., Debbink K., Swanstrom J., Vinjé J., Costantini V., Baric R.S., Donaldson E.F. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J. Virol. 2012;86(2):873–883. doi: 10.1128/JVI.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P., Vincke C., Muyldermans S. Nanobodies and their potential applications. Nanomedicine (Lond.) 2013;8(6):1013–1026. doi: 10.2217/nnm.13.86. [DOI] [PubMed] [Google Scholar]
- 116.Garaicoechea L., Aguilar A., Parra G.I., Bok M., Sosnovtsev S.V., Canziani G., Green K.Y., Bok K., Parreno V. Llama nanoantibodies with therapeutic potential against human norovirus diarrhea. PLoS One. 2015;10(8):e0133665. doi: 10.1371/journal.pone.0133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koromyslova A.D., Hansman G.S. Nanobody binding to a conserved epitope promotes norovirus particle disassembly. J. Virol. 2015;89(5):2718–2730. doi: 10.1128/JVI.03176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bekele A. Z., Gokulan K., Williams K. M., Khare S. Dose and size-dependent antiviral effects of silver nanoparticles on feline calicivirus, a human norovirus surrogate. 2016. [DOI] [PubMed]
- 119.Broglie J.J., Alston B., Yang C., Ma L., Adcock A.F., Chen W., Yang L. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS One. 2015;10(10):e0141050. doi: 10.1371/journal.pone.0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shionoiri N., Sato T., Fujimori Y., Nakayama T., Nemoto M., Matsunaga T., Tanaka T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012;113(5):580–586. doi: 10.1016/j.jbiosc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 121.Ryu S., You H.J., Kim Y.W., Lee A., Ko G.P., Lee S.J., Song M.J. Inactivation of norovirus and surrogates by natural phytochemicals and bioactive substances. Mol. Nutr. Food Res. 2015;59(1):65–74. doi: 10.1002/mnfr.201400549. [DOI] [PubMed] [Google Scholar]
- 122.Lee M.H., Lee B.H., Jung J.Y., Cheon D.S., Kim K.T., Choi C. Antiviral effect of Korean red ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J. Ginseng Res. 2011;35(4):429–435. doi: 10.5142/jgr.2011.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su X., Zivanovic S., D’Souza D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009;72(12):2623–2628. doi: 10.4315/0362-028x-72.12.2623. [DOI] [PubMed] [Google Scholar]
- 124.Davis R., Zivanovic S., D’Souza D.H., Davidson P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012;32(1):57–62. doi: 10.1016/j.fm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 125.Gilling D.H., Kitajima M., Torrey J.R., Bright K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014;116(5):1149–1163. doi: 10.1111/jam.12453. [DOI] [PubMed] [Google Scholar]
- 126.Li D., Breiman A., Le Pendu J., Uyttendaele M. Anti-viral effect of bifidobacterium adolescentis against noroviruses. Front. Microbiol. 2016;7:864. doi: 10.3389/fmicb.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aboubakr H., El-Banna A., Youssef M.M., Al-Sohaimy S., Goyal S.M. Antiviral effects of Lactococcus lactis on feline calicivirus, a human norovirus surrogate. Food Environ. Virol. 2014;6(4):282–289. doi: 10.1007/s12560-014-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoang P.M., Cho S., Kim K.E., Byun S.J., Lee T.K., Lee S. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl. Microbiol. Biotechnol. 2015;99(6):2793–2803. doi: 10.1007/s00253-014-6257-7. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Yao Q., Xia C., Jiang X., Wang P.G. Trapping norovirus by glycosylated hydrogels: a potential oral antiviral drug. ChemMedChem. 2006;1(12):1361–1366. doi: 10.1002/cmdc.200600135. [DOI] [PubMed] [Google Scholar]
- 130.Pitz A.M., Park G.W., Lee D., Boissy Y.L. Vinj??e, J. Antimicrobial activity of Bismuth subsalicylate on Clostridium difficile, Escherichia coli O157:H7, norovirus, and other common enteric pathogens. Gut Microbes. 2015;6(2):93–100. doi: 10.1080/19490976.2015.1008336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Melhem N.M. Norovirus vaccines: Correlates of protection, challenges and limitations. Hum. Vaccin. Immunother. 2016;2:1–17. doi: 10.1080/21645515.2015.1125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kocher J., Yuan L. Norovirus vaccines and potential antinorovirus drugs: Recent advances and future perspectives. Future Virol. 2015;10(7):899–913. doi: 10.2217/fvl.15.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones M.K., Watanabe M., Zhu S., Graves C.L., Keyes L.R., Grau K.R., Gonzalez-Hernandez M.B., Iovine N.M., Wobus C.E., Vinje J., Tibbetts S.A., Wallet S.M., Karst S.M. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taube S., Kolawole A.O., Höhne M., Wilkinson J.E., Handley S.A., Perry J.W., Thackray L.B., Akkina R., Wobus C.E. A mouse model for human norovirus. MBio. 2013;4(4):e00450–e13. doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parrino T.A., Schreiber D.S.S., Trier J.S.S., Kapikian A.Z.Z., Blacklow N.R.R. Clinical immunity in acute gastroenteritis caused by norwalk agent. N. Engl. J. Med. 1977;297(2):86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 136.Simmons K., Gambhir M., Leon J., Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg. Infect. Dis. 2013;19(8):1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.LoBue A.D., Lindesmith L., Yount B., Harrington P.R., Thompson J.M., Johnston R.E., Moe C.L., Baric R.S. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24(24):5220–5234. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 138.Vesikari T., Blazevic V. Norovirus vaccine: One step closer. J. Infect. Dis. 2015;211(6):853–855. doi: 10.1093/infdis/jiu498. [DOI] [PubMed] [Google Scholar]
- 139.Jiang X., Wang M., Graham D.Y., Estes M.K. Expression, self-assembly, and antigenicity of the norwalk virus capsid protein. J. Virol. 1992;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xia M., Farkas T., Jiang X. Norovirus capsid protein expressed in yeast forms virus-like particles and stimulates systemic and mucosal immunity in mice following an oral administration of raw yeast extracts. J. Med. Virol. 2007;79(1):74–83. doi: 10.1002/jmv.20762. [DOI] [PubMed] [Google Scholar]
- 141.Zhang X., Buehner N.A., Hutson A.M., Estes M.K., Mason H.S. Tomato is a highly effective vehicle for expression and oral immunization with norwalk virus capsid protein. Plant Biotechnol. J. 2006;4(4):419–432. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 142.Mason H.S., Ballt J.M., Shi J-J. Jiang4, X.; Estes4, M. K.; Arntzen, C. J. Expression of norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Immunology. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma Y., Li J. Vesicular stomatitis virus as a vector to deliver virus-like particles of human norovirus: A new vaccine candidate against an important noncultivable virus. J. Virol. 2011;85(6):2942–2952. doi: 10.1128/JVI.02332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim S-H., Chen S., Jiang X., Green K.Y., Samal S.K. Newcastle disease virus vector producing human norovirus-like particles induces serum, cellular, and mucosal immune responses in mice. J. Virol. 2014;88(17):9718–9727. doi: 10.1128/JVI.01570-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Guo L., Wang J., Zhou H., Si H., Wang M., Song J., Han B., Shu Y., Ren L., Qu J. Intranasal Administration of a recombinant adenovirus expressing the norovirus capsid protein stimulates specific humoral, mucosal, and cellular immune responses in mice. Vaccine. 2008;26(4):460–468. doi: 10.1016/j.vaccine.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 146.Tan M., Jiang X. Vaccine against norovirus. Hum. Vaccin. Immunother. 2014;10(6):1449–1456. doi: 10.4161/hv.28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baehner F., Bogaerts H., Goodwin R. Vaccines against norovirus: State of the art trials in children and adults. Clin. Microbiol. Infect. 2016;22(5):S136–S139. doi: 10.1016/j.cmi.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 148.Bernstein D.I., Atmar R.L., Lyon G.M., Treanor J.J., Chen W.H., Jiang X., Vinje J., Gregoricus N., Frenck R.W., Moe C.L. Norovirus vaccine against experimental human GII.4 virus Illness: A challenge study in healthy adults. J. Infect. Dis. 2015;211(6):870–878. doi: 10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Treanor J.J., Atmar R.L., Frey S.E., Gormley R., Chen W.H., Ferreira J., Goodwin R., Borkowski A., Clemens R., Mendelman P.M. A novel intramuscular bivalent norovirus VLP vaccine candidate-reactogenicity, safety and immunogenicity in a phase I trial in healthy adults. J. Infect. Dis. 2014;210(11):1763–1771. doi: 10.1093/infdis/jiu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J., Ferreira J., Chen W.H., Sublett R., Richardson C. Adjuvanted intranasal norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 2010;202(11):1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Atmar R.L., Bernstein D.I., Harro C.D., Al-Ibrahim M.S., Chen W.H., Ferreira J., Estes M.K., Graham D.Y., Opekun A.R., Richardson C., Mendelman P.M. Norovirus vaccine against experimental human norwalk virus illness. N. Engl. J. Med. 2011;365(23):2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tamminen K., Lappalainen S., Huhti L., Vesikari T., Blazevic V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One. 2013;8(7):e70409. doi: 10.1371/journal.pone.0070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Velasquez L.S., Shira S., Berta A.N., Kilbourne J., Medi B.M., Tizard I., Ni Y., Arntzen C.J., Herbst-Kralovetz M.M. Intranasal delivery of norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29(32):5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kocher J., Bui T., Giri-Rachman E., Wen K., Li G., Yang X., Liu F., Tan M., Xia M., Zhong W. Intranasal P particle vaccine provided partial cross-variant protection against human gii.4 norovirus diarrhea in gnotobiotic pigs. J. Virol. 2014;88(17):9728–9743. doi: 10.1128/JVI.01249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X., Ku Z., Dai W., Chen T., Ye X., Zhang C., Zhang Y., Liu Q., Jin X., Huang Z. A bivalent virus-like particle based vaccine induces a balanced antibody response against both enterovirus 71 and norovirus in mice. Vaccine. 2015;33(43):5779–5785. doi: 10.1016/j.vaccine.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 156.Tan M., Huang P., Xia M., Fang P-A., Zhong W., McNeal M., Wei C., Jiang W., Jiang X. Norovirus P particle, a novel platform for vaccine development and antibody production. J. Virol. 2011;85(2):753–764. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xia M., Wei C., Wang L., Cao D., Meng X.J., Jiang X., Tan M. A trivalent vaccine candidate against hepatitis E virus, norovirus, and astrovirus. Vaccine. 2016;34(7):905–913. doi: 10.1016/j.vaccine.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gong X., Yin H., Shi Y., He X., Yu Y., Guan S., Kuai Z., Haji N.M., Haji N.M., Kong W., Shan Y. Evaluation of the immunogenicity and protective effects of a trivalent chimeric norovirus P particle immunogen displaying influenza HA2 from subtypes H1, H3 and B. Emerg. Microbes Infect. 2016;5:e51. doi: 10.1038/emi.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Simonsen L., Viboud C., Elixhauser A., Taylor R.J. Kapikian, a Z. More on rotashield and intussusception: the role of age at the time of vaccination. J. Infect. Dis. 2005;192(Suppl. 1):S36–S43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 160.Coulson B.S., Londrigan S.L., Lee D.J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA. 1997;94(10):5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liakatos A., Kiefel M.J., Fleming F., Coulson B., Von Itzstein M. The synthesis and biological evaluation of lactose-based sialylmimetics as inhibitors of rotaviral infection. Bioorg. Med. Chem. 2006;14(3):739–757. doi: 10.1016/j.bmc.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 162.Koketsu M. Synthesis of a novel sialic acid derivative (sialylphospholipid) as an antirotaviral agent. J. Med. Chem. 1997;40(21):3332–3335. doi: 10.1021/jm9701280. [DOI] [PubMed] [Google Scholar]
- 163.Civra A., Giuffrida M.G., Donalisio M., Napolitano L., Takada Y., Coulson B.S., Conti A., Lembo D. Identification of equine lactadherin-derived peptides that inhibit rotavirus infection via integrin receptor competition. J. Biol. Chem. 2015;290(19):12403–12414. doi: 10.1074/jbc.M114.620500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rolsma M.D., Kuhlenschmidt T.B., Gelberg H.B., Kuhlenschmidt M.S. Structure and function of a ganglioside receptor for porcine rotavirus. J. Virol. 1998;72(11):9079–9091. doi: 10.1128/jvi.72.11.9079-9091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bergner D.W., Kuhlenschmidt T.B., Hanafin W.P., Firkins L.D., Kuhlenschmidt M.S. Inhibition of rotavirus infectivity by a neoglycolipid receptor mimetic. Nutrients. 2011;3(2):228–244. doi: 10.3390/nu3020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jordan P.A., Gibbins J.M. Extracellular disulfide exchange and the regulation of cellular function. Antioxid. Redox Signal. 2006;8(3-4):312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 167.Moreno L.Y., Guerrero C.A., Acosta O. Protein disulfide isomerase and heat shock cognate protein 70 interactions with rotavirus structural proteins using their purified recombinant versions. Rev. Colomb. Biotecnologia. 2016;18(1):33–48. [Google Scholar]
- 168.Calderon M.N., Guerrero C.A., Acosta O., Lopez S., Arias C.F. Inhibiting rotavirus infection by membrane-impermeant thiol/disulfide exchange blockers and antibodies against protein disulfide isomerase. Intervirology. 2012;55(6):451–464. doi: 10.1159/000335262. [DOI] [PubMed] [Google Scholar]
- 169.Lever A., Desselberger U. Rotavirus replication and the role of cellular lipid droplets: New therapeutic targets? J. Formos. Med. Assoc. 2016;115(6):389–394. doi: 10.1016/j.jfma.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 170.Kim Y., Chang K-O. Inhibitory effects of bile acids and synthetic farnesoid X receptor agonists on rotavirus replication. J. Virol. 2011;85(23):12570–12577. doi: 10.1128/JVI.05839-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohan K.V., Muller J., Atreya C.D. Defective rotavirus particle assembly in lovastatin-treated MA104 cells. Arch. Virol. 2008;153(12):2283–2290. doi: 10.1007/s00705-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ball J.M., Medina-Bolivar F., Defrates K., Hambleton E., Hurlburt M.E., Fang L., Yang T., Nopo-Olazabal L., Atwill R.L., Ghai P. Investigation of stilbenoids as potential therapeutic agents for rotavirus gastroenteritis. Adv. Virol. 2015;2015:293524. doi: 10.1155/2015/293524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cheung W., Gill M., Esposito A., Kaminski C.F., Courousse N., Chwetzoff S., Trugnan G., Keshavan N., Lever A., Desselberger U. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 2010;84(13):6782–6798. doi: 10.1128/JVI.01757-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim Y., George D., Prior A.M., Prasain K., Hao S., Le D.D., Hua D.H., Chang K.O. Novel triacsin C analogs as potential antivirals against rotavirus infections. Eur. J. Med. Chem. 2012;50:311–318. doi: 10.1016/j.ejmech.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rossen J.W.A., Bouma J., Raatgeep R.H.C., Büller H.A., Einerhand A.W.C. Inhibition of cyclooxygenase activity reduces rotavirus infection at a postbinding step. J. Virol. 2004;78(18):9721–9730. doi: 10.1128/JVI.78.18.9721-9730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yamashiro Y., Shimizu T., Oguchi T., Sato M. Prostaglandins in the plasma and stool of children with rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1989;9:322–327. doi: 10.1097/00005176-198910000-00010. [DOI] [PubMed] [Google Scholar]
- 177.Gracey M., Phadke M.A., Burke V., Raut S.K., Singh B. Aspirin in acute gastroenteritis: A clinical and microbiological study. J. Pediatr. Gastroenterol. Nutr. 1984;3(5):692–695. doi: 10.1097/00005176-198411000-00009. [DOI] [PubMed] [Google Scholar]
- 178.La Frazia S., Ciucci A., Arnoldi F., Coira M., Gianferretti P., Angelini M., Belardo G., Burrone O.R., Rossignol J-F., Santoro M.G. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J. Virol. 2013;87:11096–11106. doi: 10.1128/JVI.01213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rossignol J.F., Abu-Zekry M., Hussein A., Santoro M.G. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet. 2006;368(9530):124–129. doi: 10.1016/S0140-6736(06)68852-1. [DOI] [PubMed] [Google Scholar]
- 180.Teran C.G., Teran-Escalera C.N., Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: A randomized, single-blind, controlled trial in bolivian children. Int. J. Infect. Dis. 2009;13(4):518–523. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 181.Huang H., Liao D., Liang L., Song L., Zhao W. Genistein inhibits rotavirus replication and upregulates AQP4 expression in rotavirus-infected caco-2 cells. Arch. Virol. 2015;160(6):1421–1433. doi: 10.1007/s00705-015-2404-4. [DOI] [PubMed] [Google Scholar]
- 182.Rios M., Munoz M., Spencer E. Antiviral activity of phosphonoformate on rotavirus transcription and replication. Antiviral Res. 1995;27(1-2):71–83. doi: 10.1016/0166-3542(94)00085-m. [DOI] [PubMed] [Google Scholar]
- 183.Pizarro J.M., Pizarro J.L., Fernández J., Sandino A.M., Spencer E. Effect of nucleotide analogues on rotavirus transcription and replication. Virology. 1991;184:768–772. doi: 10.1016/0042-6822(91)90449-l. [DOI] [PubMed] [Google Scholar]
- 184.Manchego A., Spencer E. Effect of neomycin B on rotavirus plus- and minus-strand RNA synthesis. Arch. Virol. 2003;148(6):1071–1084. doi: 10.1007/s00705-003-0004-1. [DOI] [PubMed] [Google Scholar]
- 185.Stefanelli C.C., Castilho J.G., Botelho M.V., Linhares R.E., Nozawa C.M. Effect of actinomycin D on simian rotavirus (SA11) replication in cell culture. Braz. J. Med. Biol. Res. 2002;35(4):445–449. doi: 10.1590/s0100-879x2002000400006. [DOI] [PubMed] [Google Scholar]
- 186.Schoub B.D., Prozesky O.W. Antiviral activity of ribavirin in rotavirus gastroenteritis of mice. Antimicrob. Agents Chemother. 1977;12(4):543–544. doi: 10.1128/aac.12.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smee D.F., Sidwell R.W., Clark S.M., Barnett B.B., Spendlove R.S. Inhibition of rotaviruses by selected antiviral substances: Mechanisms of viral inhibition and in vivo activity. Antimicrob. Agents Chemother. 1982;21(1):66–73. doi: 10.1128/aac.21.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yin Y., Wang Y., Dang W., Xu L., Su J., Zhou X., Wang W., Felczak K., Van der Laan L.J., Pankiewicz K.W., Van der Eijk A.A., Bijvelds M., Sprengers D., de Jonge H., Koopmans M.P., Metselaar H.J., Peppelenbosch M.P., Pan Q. Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antiviral Res. 2016;133:41–49. doi: 10.1016/j.antiviral.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 189.Linhares R.E., Rebello M.A., Nozawa C.M. Effect of isoprinosine on rotavirus replication in vitro. Braz. J. Med. Biol. Res. 1996;29(2):219–222. [PubMed] [Google Scholar]
- 190.Demidenko A.A., Lee J., Powers T.R., Nibert M.L. Effects of viscogens on RNA transcription inside reovirus particles. J. Biol. Chem. 2011;286(34):29521–29530. doi: 10.1074/jbc.M111.241703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sarker S., Casswall T.H., Juneja L.R., Hoq E., Hossain I., Fuchs G.J., Hammarström L. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001;32:19–25. doi: 10.1097/00005176-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 192.Sarker S.A., Casswall T.H., Mahalanabis D., Alam N.H., Albert M.J., Brüssow H., Fuchs G.J., Hammerström L. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr. Infect. Dis. J. 1998;17(12):1149–1154. doi: 10.1097/00006454-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 193.Guarino A., Canani R.B., Russo S., Albano F., Canani M.B., Ruggeri F.M., Donelli G., Rubino A. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics. 1994;93(1):12–16. [PubMed] [Google Scholar]
- 194.Pammi M., Haque K.N. Oral immunoglobulin for the treatment of rotavirus diarrhea in low birth weight infants. Cochrane Database Syst. Rev. 2011;10:CD003742. doi: 10.1002/14651858.CD003742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bogstedt A.K., Johansen K., Hatta H., Kim M., Casswall T., Svensson L., Hammarström L. Passive immunity against diarrhoea. Acta Paediatr. 1996;85(2):125–128. doi: 10.1111/j.1651-2227.1996.tb13975.x. [DOI] [PubMed] [Google Scholar]
- 196.Pant N., Marcotte H., Brüssow H., Svensson L., Hammarström L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007;7:86. doi: 10.1186/1471-2180-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aladin F., Einerhand A.W.C., Bouma J., Bezemer S., Hermans P., Wolvers D., Bellamy K., Frenken L.G.J., Gray J., Iturriza-Gómara M. In vitro neutralisation of rotavirus infection by two broadlyspecific recombinant monovalent llama-derived antibody fragments. PLoS One. 2012;7:329–349. doi: 10.1371/journal.pone.0032949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van der Vaart J.M., Pant N., Wolvers D., Bezemer S., Hermans P.W., Bellamy K., Sarker S.A., van der Logt C.P.E., Svensson L., Verrips C.T., Hammarstrom L., Van Klinken B.J.W. Reduction inmorbidity of rotavirus induced diarrhoea in mice by yeast produced monovalent llama-derived antibody fragments. Vaccine. 2006;24:4130–4137. doi: 10.1016/j.vaccine.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 199.Sarker S.A., Jakel M., Sultana S., Alam N.H., Bardhan P.K., Chisti M.J., Salam M.A., Theis W., Hammarstrom L., Frenken L.G.J. Anti-rotavirus protein reduces stool output in infants with diarrhea: A randomized placebo-controlled trial. Gastroenterology. 2013;145(4):740–748. doi: 10.1053/j.gastro.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 200.Tokuhara D., Alvarez B., Mejima M., Hiroiwa T., Takahashi Y., Kurokawa S., Kuroda M., Oyama M., Kozuka-Hata H., Nochi T. Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J. Clin. Invest. 2013;123(9):3829–3838. doi: 10.1172/JCI70266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pant N., Hultberg A., Zhao Y., Svensson L., Pan-Hammarstrom Q., Johansen K., Pouwels P.H., Ruggeri F.M., Hermans P., Frenken L., Boren T., Marcotte H., Hammarstrom L. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lacto bodies) confer protection against rotavirus-induced diarrhea. J. Infect. Dis. 2006;194:1580–1588. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- 202.Pant N., Marcotte H., Hermans P., Bezemer S., Frenken L., Johansen K. Lactobacilli producing bispecific llama-derived anti-rotavirus proteins in vivo for rotavirus-induced diarrhea. Future Microbiol. 2011;6:583–593. doi: 10.2217/fmb.11.32. [DOI] [PubMed] [Google Scholar]
- 203.Gómez-Sebastián S., Nuñez M.C., Garaicoechea L., Alvarado C., Mozgovoj M., Lasa R., Kahl A., Wigdorovitz A., Parreño V., Escribano J.M. Rotavirus A-specific single-domain antibodies produced in baculovirus-infected insect larvae are protective in vivo. BMC Biotechnol. 2012:7–, 12, 59. doi: 10.1186/1472-6750-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Juárez P., Presa S., Espí J., Pineda B., Antón M.T., Moreno V., Buesa J., Granell A., Orzaez D. Neutralizing antibodies against rotavirus produced in transgenically labelled purple tomatoes. Plant Biotechnol. J. 2012;10(3):341–352. doi: 10.1111/j.1467-7652.2011.00666.x. [DOI] [PubMed] [Google Scholar]
- 205.Lin J., Feng N., Sen A., Balan M., Tseng H., McElrath C., Smirnov S., Peng J., Yasukawa L., Durbin R.K. Distinct roles of type I and type III interferons in intestinal immunity to homologous and heterologous rotavirus infections. PLoS Pathog. 2016;12(4):e1005600. doi: 10.1371/journal.ppat.1005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vasiutenko E.B., Petrova A.G. Using meglumine acridonacetate for the treatment of gastroenteritis caused by rotavirus in children. Eksp. Klin. Farmakol. 2013;76(10):16–19. [PubMed] [Google Scholar]
- 207.Kang J.Y., Lee D.K., Ha N.J., Shin H.S. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected caco-2 cells and a neonatal mouse model. J. Microbiol. 2015;53(11):796–803. doi: 10.1007/s12275-015-5302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shen Z., Tian Z., He H., Zhang J., Li J., Wu Y. Antiviral effects of cyclosporine A in neonatal mice with rotavirus-induced diarrhea. J. Pediatr. Gastroenterol. Nutr. 2015;60(1):11–17. doi: 10.1097/MPG.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 209.Oh H.M., Lee S.W., Park M.H., Kim M.H., Ryu Y.B., Kim M.S., Kim H.H., Park K.H., Lee W.S., Park S.J., Rho M.C. Norkurarinol inhibits toll-like receptor 3(TLR3)-mediated pro-inflammatory signaling pathway and rotavirus replication. J. Pharmacol. Sci. 2012;118(2):161–170. doi: 10.1254/jphs.11077FP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang H., Gao K., Wen K., Allen I.C., Li G., Zhang W., Kocher J., Yang X., Giri-Rachman E., Li G.H., Clark-Deener S., Yuan L. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol. 2016;16(1):109. doi: 10.1186/s12866-016-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Varyukhina S., Freitas M., Bardin S., Robillard E., Tavan E., Sapin C., Grill J.P., Trugnan G. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes Infect. 2012;14(3):273–278. doi: 10.1016/j.micinf.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 212.Liévin-Le Moal V., Servin A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014;27(2):167–199. doi: 10.1128/CMR.00080-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ahmadi E., Alizadeh-Navaei R., Rezai M.S. Efficacy of probiotic use in acute rotavirus diarrhea in children: A systematic review and meta-analysis. Caspian J. Intern. Med. 2015;6(4):187–195. [PMC free article] [PubMed] [Google Scholar]
- 214.Cecílio A.B., De Faria D.B., Oliveira P.D.C., Caldas S., De Oliveira D.A., Sobral M.E.G., Duarte M.G.R., Moreira C.P.D.S., Silva C.G., De Almeida V.L. Screening of Brazilian medicinal plants for antiviral activity against rotavirus. J. Ethnopharmacol. 2012;141(3):975–981. doi: 10.1016/j.jep.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 215.Téllez M.A., Téllez A.N., Vélez F., Ulloa J.C. In vitro antiviral activity against rotavirus and astrovirus infection exerted by substances obtained from achyrocline bogotensis (Kunth) DC. (Compositae). BMC Complement. Altern. Med. 2015;15(1):428. doi: 10.1186/s12906-015-0949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Knipping K., Garssen J., Van’t Land B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol. J. 2012;9(1):137. doi: 10.1186/1743-422X-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alfajaro M.M., Rho M.C., Kim H.J., Park J.G., Kim D.S., Hosmillo M., Son K.Y., Lee J.H., Park S.I., Kang M.I. Anti-rotavirus effects by combination therapy of stevioside and sophora flavescens extract. Res. Vet. Sci. 2014;96(3):567–575. doi: 10.1016/j.rvsc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 218.Ueda K., Kawabata R., Irie T., Nakai Y., Tohya Y., Sakaguchi T. Inactivation of pathogenic viruses by plant-derived tannins: Strong effects of extracts from persimmon (Diospyros Kaki) on a broad range of viruses. PLoS One. 2013;8(1):e55343. doi: 10.1371/journal.pone.0055343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tam K.I., Roner M.R. Characterization of in vivo anti-rotavirus activities of saponin extracts from Quillaja saponaria molina. Antiviral Res. 2011;90(3):231–241. doi: 10.1016/j.antiviral.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baek S.H., Lee J.G., Park S.Y., Bae O.N., Kim D.H., Park J.H. Pectic Polysaccharides from Panax Ginseng as the Antirotavirus Principals in Ginseng. Biomacromolecules. 2010;11(8):2044–2052. doi: 10.1021/bm100397p. [DOI] [PubMed] [Google Scholar]
- 221.Lipson S.M., Sethi L., Cohen P., Gordon R.E., Tan I.P., Burdowski A., Stotzky G. Antiviral effects on bacteriophages and rotavirus by cranberry juice. Phytomedicine. 2007;14(1):23–30. doi: 10.1016/j.phymed.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Alfajaro M.M., Kim H-J., Park J-G., Ryu E-H., Kim J-Y., Jeong Y-J., Kim D-S., Hosmillo M., Son K-Y., Lee J-H. Anti-rotaviral effects of Glycyrrhiza uralensis extract in piglets with rotavirus diarrhea. Virol. J. 2012;9(1):310. doi: 10.1186/1743-422X-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Savi L.A., Caon T., De Oliveira A.P., Sobottka A.M., Werner W., Reginatto F.H., Schenkel E.P., Barardi C.R.M., Simões C.M.O. Evaluation of antirotavirus activity of flavonoids. Fitoterapia. 2010;81(8):1142–1146. doi: 10.1016/j.fitote.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tradtrantip L., Ko E.A., Verkman A.S. Antidiarrheal efficacy and cellular mechanisms of a Thai herbal remedy. PLoS Negl. Trop. Dis. 2014;8(2):e2674. doi: 10.1371/journal.pntd.0002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.He S.T., He F.Z., Wu C.R., Li S.X., Liu W.X., Yang Y.F., Jiang S.S., He G. Treatment of rotaviral gastroenteritis with Qiwei baizhu powder. World J. Gastroenterol. 2001;7(5):735–740. doi: 10.3748/wjg.v7.i5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Inagaki M., Muranishi H., Yamada K., Kakehi K., Uchida K., Suzuki T., Yabe T., Nakagomi T., Nakagomi O., Kanamaru Y. Bovine κ-casein inhibits Human Rotavirus (HRV) Infection via direct binding of glycans to HRV. J. Dairy Sci. 2014;97(5):2653–2661. doi: 10.3168/jds.2013-7792. [DOI] [PubMed] [Google Scholar]
- 227.Reading P.C., Holmskov U., Anders E.M. Antiviral activity of bovine collectins against rotaviruses. J. Gen. Virol. 1998;79(9):2255–2263. doi: 10.1099/0022-1317-79-9-2255. [DOI] [PubMed] [Google Scholar]
- 228.Lehert P., Chéron G., Calatayud G.A., Cézard J.P. Castrell? P. G.; Garcia, J. M. M.; Santos, M.; Savitha, M. R. Racecadotril for childhood gastroenteritis: An individual patient data meta-analysis. Dig. Liver Dis. 2011;43(9):707–713. doi: 10.1016/j.dld.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 229.Zhang B., Chassaing B., Shi Z., Uchiyama R., Zhang Z., Denning T.L., Crawford S.E., Pruijssers A.J., Iskarpatyoti J.A., Estes M.K. Viral Infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346(6211):861–865. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lopez T., Silva-Ayala D., Lopez S., Arias C.F. Methods suitable for high-throughput screening of siRNAs and other chemical compounds with the potential to inhibit rotavirus replication. J. Virol. Methods. 2012;179(1):242–249. doi: 10.1016/j.jviromet.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 231.Yin Y., Bijvelds M., Dang W., Xu L., Van Der Eijk A.A., Knipping K., Tuysuz N., Dekkers J.F., Wang Y., De Jonge J. Modeling rotavirus infection and antiviral therapy using primary intestinal organoids. Antiviral Res. 2015;123:120–131. doi: 10.1016/j.antiviral.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 232.Robinson C.M., Pfeiffer J.K. Viruses and the microbiota. Annu. Rev. Virol. 2014;1(1):55–69. doi: 10.1146/annurev-virology-031413-085550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lamberti L.M., Ashraf S., Walker C.L., Black R.E. Systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatr. Infect. Dis. J. 2016;35(9):992–998. doi: 10.1097/INF.0000000000001232. [DOI] [PubMed] [Google Scholar]
- 234.Parashar U.D., Johnson H., Steele A.D., Tate J.E. Health impact of rotavirus vaccination in developing countries: Progress and way forward. Clin. Infect. Dis. 2016;62(Suppl. 2):S91–S95. doi: 10.1093/cid/civ1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Leshem E., Lopman B., Glass R., Gentsch J., Banyai K., Parashar U., Patel M. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: A systematic review and meta-analysis. Lancet Infect. Dis. 2014;14(9):847–856. doi: 10.1016/S1473-3099(14)70832-1. [DOI] [PubMed] [Google Scholar]
- 236.Pawinski R., Debrus S., Delem A., Smolenov I., Suryakiran P.V., Han H.H. Rotarix in developing countries: Paving the way for inclusion in national childhood immunization programs in africa. J. Infect. Dis. 2010;202(Suppl. 1):S80–S86. doi: 10.1086/653547. [DOI] [PubMed] [Google Scholar]
- 237.Wang C-M., Chen S-C., Chen K-T. Current status of rotavirus vaccines. World J. Pediatr. 2015;11(4):300–308. doi: 10.1007/s12519-015-0038-y. [DOI] [PubMed] [Google Scholar]
- 238.Anh D.D., Van Trang N., Thiem V.D., Anh N.T.H., Mao N.D., Wang Y., Jiang B., Hien N.D., Luan L.T. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine. 2012;30(Suppl. 1):A114–A121. doi: 10.1016/j.vaccine.2011.07.118. [DOI] [PubMed] [Google Scholar]
- 239.Glass R.I., Bhan M.K., Ray P., Bahl R., Parashar U.D., Greenberg H., Rao C.D., Bhandari N., Maldonado Y., Ward R.L. Development of candidate rotavirus vaccines derived from neonatal strains in India. J. Infect. Dis. 2005;192(Suppl. 1):S30–S35. doi: 10.1086/431498. [DOI] [PubMed] [Google Scholar]
- 240.Rippinger C.M., Patton J.T., McDonald S.M. Complete genome sequence analysis of candidate human rotavirus vaccine strains RV3 and 116E. Virology. 2010;405(1):201–213. doi: 10.1016/j.virol.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bhandari N., Rongsen-Chandola T., Bavdekar A., John J., Antony K., Taneja S., Goyal N., Kawade A., Kang G., Rathore S.S. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: A randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dunn S.J., Greenberg H.B., Ward R.L., Nakagomi O., Burns J.W., Vo P.T., Pax K.A., Das M., Gowda K., Rao C.D. Serotypic and genotypic characterization of human serotype 10 rotaviruses from asymptomatic neonates. J. Clin. Microbiol. 1993;31(1):165–169. doi: 10.1128/jcm.31.1.165-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bhandari N., Sharma P., Glass R.I., Ray P., Greenberg H., Taneja S., Saksena M., Rao C.D., Gentsch J.R., Parashar U. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: Results of a randomised controlled trial. Vaccine. 2006;24(31-32):5817–5823. doi: 10.1016/j.vaccine.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 244.Ramani S., Iturriza-Gomara M., Jana A.K., Kuruvilla K.A., Gray J.J., Brown D.W., Kang G. whole genome characterization of reassortant G10P[11] Strain(N155) from a neonate with symptomatic rotavirus infection: Identification of genes of human and animal rotavirus origin. J. Clin. Virol. 2009;45(3):237–244. doi: 10.1016/j.jcv.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Albert M.J., Unicomb L.E., Barnes G.L., Bishop R.F. Cultivation and characterization of rotavirus strains infecting newborn babies in Melbourne, Australia, from 1975 to 1979. J. Clin. Microbiol. 1987;25(9):1635–1640. doi: 10.1128/jcm.25.9.1635-1640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bishop R.F., Barnes G.L., Cipriani E., Lund J.S. Clinical immunity after neonatal rotavirus infection. a prospective longitudinal study in young children. N. Engl. J. Med. 1983;309(2):72–76. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 247.Barnes G.L., Lund J.S., Adams L., Mora A., Mitchell S.V., Caples A., Bishop R.F. Phase 1 trial of a candidate rotavirus vaccine(RV3) derived from a human neonate. J. Paediatr. Child Health. 1997;33(4):300–304. doi: 10.1111/j.1440-1754.1997.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 248.Barnes G.L., Lund J.S., Mitchell S.V., De Bruyn L., Piggford L., Smith A.L., Furmedge J., Masendycz P.J., Bugg H.C., Bogdanovic-Sakran N. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine. 2002;20(23-24):2950–2956. doi: 10.1016/s0264-410x(02)00235-9. [DOI] [PubMed] [Google Scholar]
- 249.Danchin M., Kirkwood C.D., Lee K.J., Bishop R.F., Watts E., Justice F.A., Clifford V., Cowley D., Buttery J.P., Bines J.E. Phase I trial of RV3-BB rotavirus vaccine: A human neonatal rotavirus vaccine. Vaccine. 2013;31(23):2610–2616. doi: 10.1016/j.vaccine.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 250.Bines J.E., Danchin M., Jackson P., Handley A., Watts E., Lee K.J., West A., Cowley D., Chen M.Y., Barnes G.L. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2015;15(12):1389–1397. doi: 10.1016/S1473-3099(15)00227-3. [DOI] [PubMed] [Google Scholar]
- 251.Clark H.F., Offit P., Ellis R.W., Eiden J.J., Krah D., Shaw R., Pichichero M., Treanor J.J., Borian F.E., Bell L.M. The development of multivalent bovine rotavirus (Strain WC3) reassortant vaccine for infants. J. Infect. Dis. 1996;174:S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 252.Velasquez D.E., Parashar U.D., Jiang B. Strain diversity plays no major role in the varying efficacy of rotavirus vaccines: An overview. Infect. Genet. Evol. 2014;28:561–571. doi: 10.1016/j.meegid.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 253.Vesikari T., Matson D.O., Dennehy P., Van Damme P., Santosham M., Rodriguez Z., Dallas M.J., Heyse J.F., Goveia M.G., Black S.B. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 254.Karafillakis E., Hassounah S., Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097–2107. doi: 10.1016/j.vaccine.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 255.Iwata S., Nakata S., Ukae S., Koizumi Y., Morita Y., Kuroki H., Tanaka Y., Shizuya T., Schödel F., Brown M.L. Efficacy and safety of pentavalent rotavirus vaccine in Japan: A randomized, double-blind, placebo-controlled, multicenter trial. Hum. Vaccin. Immunother. 2013;9(8):1–8. doi: 10.4161/hv.24846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Armah G.E., Sow S.O., Breiman R.F., Dallas M.J., Tapia M.D., Feikin D.R., Binka F.N., Steele A.D., Laserson K.F., Ansah N.A. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-saharan africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 257.Zaman K., Anh D.D., Victor J.C., Shin S., Yunus M., Dallas M.J., Podder G., Thiem V.D., Mai L.T.P., Luby S.P. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 258.Yih W.K., Lieu T., Kulldorff M., Martin D., McMahill-Walraven C.N., Platt R., Selvam N., Selvan M., Lee G.M., Nguyen M. Intussusception risk after rotavirus vaccination in U.S. infants. N. Engl. J. Med. 2014;370(6):503–512. doi: 10.1056/NEJMoa1303164. [DOI] [PubMed] [Google Scholar]
- 259.Soares-Weiser K., MacLehose H., Ben-Aharon I., Goldberg E., Pitan F., Cunliffe N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2010;5:CD008521. doi: 10.1002/14651858.CD008521. [DOI] [PubMed] [Google Scholar]
- 260.Geier D.A., King P.G., Sykes L.K., Geier M.R. RotaTeq vaccine adverse events and policy considerations. Med. Sci. Monit. 2008;14(3):PH9–PH16. [PubMed] [Google Scholar]
- 261.Loughlin J., Mast T.C., Doherty M.C., Wang F.T., Wong J., Seeger J.D. Postmarketing evaluation of the short-term safety of the pentavalent rotavirus vaccine. Pediatr. Infect. Dis. J. 2012;31(3):292–296. doi: 10.1097/INF.0b013e3182421390. [DOI] [PubMed] [Google Scholar]
- 262.Midthun K., Kapikian A.Z. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 1996;9(3):423–434. doi: 10.1128/cmr.9.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Joensuu J., Koskenniemi E., Pang X.L., Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350(9086):1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 264.Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control (CDC). Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 1999;48(RR-2):1–20. [PubMed] [Google Scholar]
- 265.Advisory Committee on Immunization Practices (ACIP) Centers for disease control and prevention. Intussusception among recipients of rotavirus vaccine-united states, 1998-1999. MMWR Morb. Mortal. Wkly. Rep. 1999;48(27):577–581. [PubMed] [Google Scholar]
- 266.Clements-Mann M. Lou; Makhene, M. K.; Mrukowicz, J.; Wright, P. F.; Hoshino, Y.; Midthun, K.; Sperber, E.; Karron, R.; Kapikian, A. Z. Safety and immunogenicity of live attenuated human-bovine(UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine. 1999;17(20-21):2715–2725. doi: 10.1016/s0264-410x(98)00497-6. [DOI] [PubMed] [Google Scholar]
- 267.Luna E.J.A., Frazatti-Gallina N.M., Timenetsky M.C.S.T., Cardoso M.R.A., Veras M.A.S.M., Miraglia J.L., Escobar A.M.U., Grisi S.J.F.E., Raw I., Precioso A.R. A phase I clinical trial of a new 5-valent rotavirus vaccine. Vaccine. 2013;31(7):1100–1105. doi: 10.1016/j.vaccine.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 268.Zade J.K., Kulkarni P.S., Desai S.A., Sabale R.N., Naik S.P., Dhere R.M. Bovine rotavirus pentavalent vaccine development in India. Vaccine. 2014;32(S1):A124–A128. doi: 10.1016/j.vaccine.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 269.Bishop R. The present status of rotavirus vaccine development. Southeast Asian J. Trop. Med. Public Health. 1988;19(3):429–435. [PubMed] [Google Scholar]
- 270.Bernstein D.I. Rotavirus vaccine: Current status and future prospects. BioDrugs. 2000;14(5):275–281. doi: 10.2165/00063030-200014050-00001. [DOI] [PubMed] [Google Scholar]
- 271.Santosham M., Letson G.W., Wolff M., Reid R., Gahagan S., Adams R., Callahan C., Sack R.B., Kapikian A.Z. A field study of the safety and efficacy of two candidate rotavirus vaccines in a native american population. J. Infect. Dis. 1991;163(3):483–487. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- 272.Fu C., Wang M., Liang J., He T., Wang D., Xu J. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization: A matched case-control study. Vaccine. 2007;25(52):8756–8761. doi: 10.1016/j.vaccine.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 273.Zhen S-S., Li Y., Wang S-M., Zhang X-J., Hao Z-Y., Chen Y., Wang D., Zhang Y-H., Zhang Z-Y., Ma J-C. Effectiveness of the live attenuated rotavirus vaccine produced by a domestic manufacturer in China studied using a population-based case-control design. Emerg. Microbes Infect. 2015;4(10):e64. doi: 10.1038/emi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fu C., He Q., Xu J., Xie H., Ding P., Hu W., Dong Z., Liu X., Wang M. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31(1):154–158. doi: 10.1016/j.vaccine.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 275.Kuate-Defo Z., Lee B. New approaches in oral rotavirus vaccines. Crit. Rev. Microbiol. 2016;42(3):495–505. doi: 10.3109/1040841X.2014.962479. [DOI] [PubMed] [Google Scholar]
- 276.Tekewe A., Fan Y., Tan E., Middelberg A.P., Lua L.H. Integrated molecular and bioprocess engineering for bacterially produced immunogenic modular virus-like particle vaccine displaying 18 kDa rotavirus antigen. Biotechnol. Bioeng. 2016 doi: 10.1002/bit.26068. [DOI] [PubMed] [Google Scholar]
- 277.El-Attar L., Oliver S.L., Mackie A., Charpilienne A., Poncet D., Cohen J., Bridger J.C. Comparison of the efficacy of rotavirus VLP vaccines to a live homologous rotavirus vaccine in a pig model of rotavirus disease. Vaccine. 2009;27(24):3201–3208. doi: 10.1016/j.vaccine.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 278.Fix A.D., Harro C., McNeal M., Dally L., Flores J., Robertson G., Boslego J.W., Cryz S. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine. 2015;33(31):3766–3772. doi: 10.1016/j.vaccine.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 279.Xue M., Yu L., Jia L., Li Y., Zeng Y., Li T., Ge S., Xia N. Immunogenicity and protective efficacy of rotavirus VP8* fused to cholera toxin B subunit in a mouse model. Hum. Vaccin. Immunother. 2016;19:1–10. doi: 10.1080/21645515.2016.1204501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Xia M., Wei C., Wang L., Cao D., Meng X.J., Jiang X., Tan M. Development and evaluation of two subunit vaccine candidates containing antigens of hepatitis E virus, rotavirus, and astrovirus. Sci. Rep. 2016;6:25735. doi: 10.1038/srep25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jiang B., Gentsch J.R., Glass R.I. Inactivated rotavirus vaccines: A priority for accelerated vaccine development. Vaccine. 2008;26(52):6754–6758. doi: 10.1016/j.vaccine.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 282.Jiang B., Wang Y., Glass R.I. Does a monovalent inactivated human rotavirus vaccine induce heterotypic immunity? Evidence from animal studies. Hum. Vaccin. Immunother. 2013;9(8):1634–1637. doi: 10.4161/hv.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Velasquez D.E., Wang Y., Jiang B. Inactivated human rotavirus vaccine induces heterotypic antibody response: Correction and development of Igg avidity assay. Hum. Vaccin. Immunother. 2015;11(2):531–533. doi: 10.4161/21645515.2014.988553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.O’Hagan D.T., Singh M., Gupta R.K. Poly(lactide-Co-Glycolide) microparticles for the development of single- dose controlled-release vaccines. Adv. Drug Deliv. Rev. 1998;32(3):225–246. [PubMed] [Google Scholar]
- 285.Nayak B., Panda A.K., Ray P., Ray A.R. Formulation, characterization and evaluation of rotavirus encapsulated PLA and PLGA particles for oral vaccination. J. Microencapsul. 2009;26(2):154–165. doi: 10.1080/02652040802211709. [DOI] [PubMed] [Google Scholar]
- 286.Rybicki E.P. Plant-made vaccines for humans and animals. Plant Biotechnol. J. 2010;8(5):620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Dicker M., Tschofen M., Maresch D., König J., Juarez P., Orzaez D., Altmann F., Steinkellner H., Strasser R. Transient glyco-engineering to produce recombinant IgA1 with defined N- and O-glycans in plants. Front. Plant Sci. 2016;7:18. doi: 10.3389/fpls.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pera F.F.P.G., Mutepfa D.L.R., Khan A.M., Els J.H., Mbewana S., Van Dijk A.A.A., Rybicki E.P., Hitzeroth I.I. Engineering and expression of a human rotavirus candidate vaccine in Nicotiana benthamiana. Virol. J. 2015;12:205. doi: 10.1186/s12985-015-0436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wu Y.Z., Li J.T., Mou Z.R., Fei L., Ni B., Geng M., Jia Z.C., Zhou W., Zou L.Y., Tang Y. Oral immunization with rotavirus VP7 expressed in transgenic potatoes induced high titers of mucosal neutralizing IgA. Virology. 2003;313(2):337–342. doi: 10.1016/s0042-6822(03)00280-0. [DOI] [PubMed] [Google Scholar]
- 290.Choi N-W., Estes M.K., Langridge W.H.R. Synthesis of a ricin toxin b subunit-rotavirus VP7 fusion protein in potato. Mol. Biotechnol. 2006;32(2):117–128. doi: 10.1385/MB:32:2:117. [DOI] [PubMed] [Google Scholar]
- 291.Choi N-W., Estes M.K., Langridge W.H.R. Synthesis and assembly of a cholera toxin b subunit-rotavirus VP7 fusion protein in transgenic potato. Mol. Biotechnol. 2005;31(3):193–202. doi: 10.1385/MB:31:3:193. [DOI] [PubMed] [Google Scholar]
- 292.Saldaña S., Esquivel-Guadarrama F., Olivera Flores T.D.J., Arias N., López S., Arias C., Ruiz-Medrano R., Mason H., Mor T., Richter L. Production of rotavirus-like particles in tomato (Lycopersicon esculentum L.) fruit by expression of capsid proteins VP2 and VP6 and immunological studies. Viral Immunol. 2006;19(1):42–53. doi: 10.1089/vim.2006.19.42. [DOI] [PubMed] [Google Scholar]
- 293.Xie L., Mi K., Ye J., Niu X., Sun X., Yi S., Li H., Sun M. [Selection and identification of the biological characteristics of a cold-adapted genotype G1P[8] ZTR-68 rotavirus by serial cold-adapted passaging]. Bing Du Xue Bao. 2015;31(5):548–553. [PubMed] [Google Scholar]
- 294.Yuan L., Azevedo M.S.P., Gonzalez A.M., Jeong K. Il; Van Nguyen, T.; Lewis, P.; Iosef, C.; Herrmann, J. E.; Saif, L. J. Mucosal and systemic antibody responses and protection induced by a prime/boost rotavirus-DNA vaccine in a gnotobiotic pig model. Vaccine. 2005;23(30):3925–3936. doi: 10.1016/j.vaccine.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 295.Glass R.I., Parashar U.D., Bresee J.S., Turcios R., Fischer T.K., Widdowson M-A., Jiang B., Gentsch J.R. Rotavirus vaccines: Current prospects and future challenges. Lance. 2006;368(9532):323–332. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 296.Ghosh A., Chattopadhyay S., Chawla-Sarkar M., Nandy P., Nandy A. In silico study of rotavirus VP7 surface accessible conserved regions for antiviral drug/vaccine design. PLoS One. 2012;7(7):e40749. doi: 10.1371/journal.pone.0040749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Hulo C., De Castro E., Masson P., Bougueleret L., Bairoch A., Xenarios I., Le Mercier P. ViralZone: A knowledge resource to understand virus diversity. Nucleic Acids Res. 2011;39:D576–D582. doi: 10.1093/nar/gkq901. [DOI] [PMC free article] [PubMed] [Google Scholar]