Abstract
Vegetables and fruits contain non-provitamin A (lycopene, lutein, and zeaxanthin) and provitamin A (β-carotene, β-cryptoxanthin, and α-carotene) carotenoids. Within these compounds, β-carotene has been extensively studied for its health benefits, but its supplementation at doses higher than recommended intakes induces adverse effects. β-Carotene is converted to retinoic acid (RA), a well-known immunomodulatory molecule. Human interventions suggest that β-carotene and lycopene at pharmacological doses affect immune functions after a depletion period of low carotenoid diet. However, these effects appear unrelated to carotenoids and retinol levels in plasma. Local production of RA in the gut-associated lymphoid tissue, as well as the dependency of RA-induced effects on local inflammation, suggests that personalized nutrition/supplementation should be considered in the future. On the other hand, the differential effect of RA and lycopene on transforming growth factor-beta suggests that lycopene supplementation could improve immune functions without increasing risk for cancers. However, such preclinical evidence must be confirmed in human interventions before any recommendations can be made.
1. Introduction
Major dietary non-provitamin A (lycopene, lutein, and zeaxanthin) and provitamin A (β-carotene, β-cryptoxanthin, and α-carotene) carotenoids have different biological activities and efficacy, depending on their food content, dietary intake, bioavailability, and bioconversion [1]. The intestine and liver are crucial organs for vitamin A uptake and liver accounts for the majority of retinoid stores [2, 3]. The provitamin A carotenoid, β-carotene, is a significant source of vitamin A in the diet. β-Carotene ′ oxygenase-1 (BCO1) and β-carotene 9′,10′ oxygenase-2 (BCO2) are the two known carotenoid cleavage enzymes in humans [4]. In rats, both BCO1 and BCO2 are highly expressed in the liver and intestine, localized in hepatocytes and mucosal epithelium, and BCO1 is also expressed in hepatic stellate cells [4]. Both enzymes have provitamin A and non-provitamin A as preferential substrates, respectively, and genetic variations of these enzymes have been suggested within the factors affecting carotenoid status in humans [5, 6].
β-Carotene is known as an antioxidant, but its prooxidant activity in some conditions accounts for its adverse effects [6]. In particular, β-carotene failed to prevent cancer in two large clinical trials: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC Study; α-tocopherol 50 mg and β-carotene 20 mg/d) [7] and the Beta-Carotene and Retinol Efficacy Trial (CARET; β-carotene 30 mg/d and retinyl palmitate 25,000 IU) [8]. Moreover, β-carotene supplementation increased lung cancer risk in smokers [9, 10] and the overall mortality [11, 12]. On the other hand, a safer profile for non-provitamin A carotenoids (up to 20 mg/d for lutein and 75 mg/d for lycopene) has been suggested [13]. Lycopene has been extensively studied [14], and encapsulation has been suggested to improve bioavailability for therapeutic use in many conditions, including immune-mediated diseases [15].
Retinol bound to the retinol-binding protein (RBP) is a source of retinoic acid (RA) [2, 16], and the latter is metabolized by cytochrome P450 26 (CYP26) [3]. After uptake, retinol can be oxidized by ubiquitously expressed alcohol dehydrogenases (ADH) to form retinaldehyde (retinal) which is then metabolized into RA by retinaldehyde/aldehyde dehydrogenases (ALDH) in the liver [3, 17, 18]. ALDH are also expressed in the gut-associated lymphoid tissue (GALT) [3]. Although RA is the major active metabolite affecting the immune system, non-provitamin A carotenoids are active in immune modulation [19]. Furthermore, it has been reported that BCO1 could yield acycloretinal from lycopene [20] and that lycopene-derived BCO2 metabolites could mediate in some circumstance signals similar to that induced by retinoic acid receptor (RAR) ligands [21].
In this review, we aim to discuss the potential role of carotenoids as immunomodulators, on the light of their intake and safety.
2. Carotenoid Sources
The major carotenoids present in food products are β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin [22] (Table 1). With the exception of egg yolk rich in lutein, the main sources of these compounds in human diet are of plant origin; they are widely distributed in the plastids of flowers, leaves, seeds, and roots. Orange, yellow- and green-colored vegetables are the rich sources; lycopene is found abundantly in tomatoes and their related products and is also present in fruits, such as watermelon and pink grapefruit [23]. Citrus fruits, papaya, and peaches contain significant levels of β-cryptoxanthin. The xanthophylls lutein and zeaxanthin are mainly found in leafy green vegetables, such as spinach or broccoli [24]. Likewise, an emerging source of carotenoids is the by-products of industry processing of fruits and vegetables [25].
Table 1.
Carotenoid food/spice content.
| Range mg/100 g | α-Carotene | β-Carotene | β-Cryptoxanthin | Lutein + zeaxanthin | Lycopene |
|---|---|---|---|---|---|
| 20–50 | Carrot, paprika, peppers red | Tomatoes | |||
|
| |||||
| 10–20 | Carrot | Carrots, peppers red | Chard, chicory greens, kale, paprika, pepper, spinach, turnip greens, | Catsup, tomatoes | |
|
| |||||
| 5–10 | Peppers red, pumpkin, carrot juice | Acai berry drink, carrot juice, carrots, chili powder, kale, parsley, pumpkin, spinach, turnip greens | Pepper, red or cayenne paprika | Basil, parsley, radicchio, watercress | Guavas, tomato juice, tomato soup |
|
| |||||
| 1–5 | Carrot, chili powder, pepper | Apricots, broccoli, cabbage Chinese, cherries, chicory greens, endive, lettuce (green and red leaf), melons, oregano, parsley, peas green, peppers green, plums, pumpkin, sweet potato, thyme, watercress | Chili powder, squash | Broccoli, brussels sprouts, carrot, fava, lettuce (green and red leaf), oregano, parsley, peas green, pistachio, pumpkin, thyme, tomatoes, zucchini | Grapefruit (pink and red), papayas, watermelon |
From: United States Department of Agriculture Agricultural Research Service (USDA) Food Composition Databases (https://ndb.nal.usda.gov/ndb/).
Contents of carotenoids vary widely because their syntheses are greatly influenced by a wide variety of factors, including climate, soil, cultivar, and cultivation [26]. Further, their profile in berries changes with ripening stage, with higher levels of α-carotene and lycopene in advanced ripening [27]. In addition to preharvest factors, their contents can be affected by all treatments during postharvest because their highly unsaturated structures with conjugated double bonds make them very susceptible to oxidative reactions and dimerization. For example, cutting of vegetables increases the exposure to oxygen and releases enzymes from the cell vacuoles of plant parenchyma, which further promote their degradation. Excessive exposure to sunlight also decreases the content of carotenoids in harvested products [28]. Degradation of carotenoids can be diminished by storage at low temperatures, protection from light (packaged in dark containers), or package under modified atmospheres. However, the impact of thermal treatments on carotenoids appeared mixed. For example, nonthermally treated tomatoes had higher amounts of carotenoids compared to thermally treated ones and similar results were observed with carrot [29]. However, home culinary techniques, such as boiling in hot water, cause partial degradation and isomerization of both β-carotene and lycopene. Current industrial processing techniques as high-pressure treatment tend to preserve or even increase the content of carotenoids [30].
3. Dietary Intake, RDA, and Retinol Equivalents
Dietary data on consumption of carotenoids were in the past usually expressed as β-carotene, β-carotene equivalents, or retinol equivalents, and only more recently, carotenoid food composition databases have been developed. There is a general consensus regarding that the contribution of dietary carotenoids from food sources depends not only on their contents in foods but also on the frequency of their consumptions. Estimated intakes of carotenoids vary widely on individual, regional, and national levels, and significant seasonal variations have also been reported in some countries [31]. Furthermore, assessment of carotenoid intake is a complex matter mainly because of the high variability within and between subjects, the degree of imprecision in data collection, and discrepancies in carotenoid food composition databases, which reflect in different intakes of carotenoids in the literature.
Studies on dietary carotenoids are few, and the main results of one of the few comparative studies are presented in Table 2 [32], where the assessment of carotenoid intakes was carried out by a Food Frequency Questionnaire (FFQ) at the individual level of five countries. It should be noticed that the population in this study was a group in a determined area of each of the five participant countries (ca. per country). Thus, subjects might not necessarily be representative of the overall population although it was assumed that they followed a typical dietary pattern of their countries. Moreover, it should not be ignored that FFQ overestimates carotenoid intake [33], especially of lutein and zeaxanthin when comparing with 3-day food records. Table 2 summarizes carotenoid intake in some countries from the representative literature with a larger sample size. The total carotenoid intakes range between 5.42 and 15.44 mg/d; however, comparisons should be considered with caution since, as shown, sample size and methodology differ between studies.
Table 2.
Comparison of carotenoid intake (mg/d) in adults reported in several countries.
| Population (subjects) | α-Carotene | β-Carotene | β-Cryptoxanthin | Lutein + zeaxanthin | Lycopene | Dietary methods | Ref. |
|---|---|---|---|---|---|---|---|
| Australia, N = 3100 | 1.25/1.13 (m/w) | 5.14/5.27 (m/w) | 0.32/0.35 (m/w) | 1.62/1.70 (m/w) | 7.11/6.26 (m/w) | FFQ | [39] |
| Costa Rica, N = 459 | 0.45/0.73 (m/w) | 3.41/4.67 (m/w) | 0.38/0.55 (m/w) | 2.41/2.89 (m/w) | 5.45/5.77 (m/w) | FFQ and 7-day diary | [40] |
| France, N = 76 | 0.74 | 5.84 | 0.45 | 2.50 | 4.75 | FFQ | [32] |
| France, N = 12,741 | — | 3.14/3.79 (m/w) | — | — | — | 6-day food diary | [41] |
| Ireland, N = 828 | — | — | — | 1.60 | — | 166-item FFQ | [42] |
| Italy (INRAN-SCAI study), N = 2313 | 0.15/0.18 (m/w) | 3.07/3.01 (m/w) | — | 3.79/3.73 (m/w) | 7.10/5.64 (m/w) | 3-day food diary | [43, 44] |
| Japan JACC Study Group, N = 3095 | — | 2.11 (m) | — | — | — | 35-item FFQ | [45] |
| Korea National Health and Nutrition Examination Survey, N = 24,377 | 0.56 | 3.62 | 0.55 | 2.300 | 2.22 | 1-day 24 h recall | [46] |
| Spain, N = 70 | 0.29 | 2.96 | 1.36 | 3.25 | 1.64 | FFQ | [32] |
| Spain (EPIC cohort), N = 41,446 | 0.27 | 1.31 | 0.22 | 0.84 | 3.0 | Dietary history questionnaire | [47] |
| Rep Ireland, N = 76 | 1.23 | 5.16 | 0.78 | 1.56 | 4.43 | FFQ | [32] |
| The Netherlands, N = 75 | 0.68 | 4.35 | 0.97 | 2.01 | 4.86 | FFQ | [32] |
| USA, N = 584 | 0.69/0.79 (m/w∗) | 3.28/0.63 (m/w∗) | 0.15/0.17 (m/w∗) | 1.47/1.56 (m/w∗) | 6.07/5.35 (m/w∗) | 118-items FFQ | [48] |
| 0.98/0.91 (m/w∗∗) | 4.09/3.82 (m/w∗∗) | 0.16/0.13 (m/w∗∗) | 2.88/2.25 (m/w∗∗) | 5.79/4.64 (m/w∗∗) | |||
| USA, N = 2787 | 0.78 (w) | 4.40 (w) | 0.18 (w) | 0.30 (w) | 6.34 (w) | FFQ | [49] |
| UK, N = 71 | 1.04 | 5.55 | 0.99 | 1.59 | 5.01 | FFQ | [32] |
| UK (EPIC Norfolk cohort), N = 14,803 | 0.41/0.40 (m/w) | 2.07/2.04 (m/w) | 0.41/0.46 (m/w) | 1.10/1.14 (m/w) | 1.43/1.29 (m/w) | 7-day diary | [50] |
FFQ: food frequency questionnaire; m/w: men/women; JACC: Japan Collaborative Cohort; EPIC: European Prospective Investigation into Cancer and Nutrition. ∗Hispanics and ∗∗Non-Hispanics.
In a review from Maiani et al. [1], a calculation of the relative contribution of each carotenoid to total carotenoid intake, according to FAO Food Balance Sheet data from several European countries, was performed. Lutein + zeaxanthin and β-carotene were those most frequently found in European diet (48% and 33%, respectively, on a total carotenoid intake of 11.8 mg/d). No formal dietary recommendation for carotenoids has yet been established, and the European Food Safety Authority (2006) had decided that the existing evidence was insufficient to establish a recommended dietary allowance (RDA) or adequate intake (AI) for β-carotene and other carotenoids [34]. In most European countries, the recommended intake was established based on the assumption that 4.8 mg β-carotene is needed to meet the requirement of 800 micrograms of vitamin A (conversion factor 6). In other countries, for example in USA, a conversion factor of 12 for β-carotene and 24 for other carotenoids such as β-cryptoxanthin was applied [35]. For very complex matrices (i.e., spinach), human studies have revealed an even higher conversion factor for β-carotene such as 1 : 21 for a fruit/vegetable mix or 1 : 26 for vegetables [36]. Conclusions of many epidemiological studies revealed that a plasma level of 0.4 μmol/L β-carotene should be aimed at in order to benefit from the preventive health potential. This concentration can be achieved with consumption of 2–4 mg/d β-carotene [37], far below the supplemented dose used in the ATBC study [7] and the CARET study [8], in which an increased risk of lung cancer was noted in heavy smokers taking high doses (5 to 10 times the dose previously indicated of 2–4 mg/d) of β-carotene for long periods.
Consumption of foods rich in β-carotene is highly recommended since it is associated with a lower risk of chronic diseases and to ensure the intake of a sufficient amount of antioxidants. Healthy diet, which realistically contains 100–500 g/d of fruit and vegetables, shall contain a high proportion of carotenoid-rich food. On the other hand, proposed intake recommendations for some non-provitamin A carotenoids are 10–20 mg/d for lutein and 5.7–15 mg/d for lycopene [38].
4. Bioavailability and Accessibility
Bioavailability of dietary xanthophylls is varied widely between individuals and subject to the influence of many intrinsic and extrinsic factors [51]. Bioavailability is defined as the portion of the ingested nutrients that are absorbed in the small intestine, enter in the circulation, and become available for utilization or storage in organs [52–54]. Before nutrients in foods, beverages, or nutraceuticals are absorbed in the intestine, they must be made themselves ready for the transportation from the chyme in the lumen to enterocytes, a process defined as bioaccessibility. In the case of lipid-soluble carotenoids, ingested carotenoids must be first released from the food matrix, transferred into lipid emulsion, incorporated into the micelles containing pancreatic lipases and bile salts, and then available for transport into enterocytes [54–56]. The micelles act as a polar carrier from the hydrophilic chyme to the mucosal cell surface for the uptake through passive diffusion [57]. The factors influencing carotenoid bioaccessibility and bioavailability can be categorized to carotenoid-related and unrelated groups. The carotenoid-related includes dosage, chemical structure (isomeric forms), and interactions between carotenoids, and the unrelated includes cooking, nutrient composition of co-consumed foods, particle size of digested foods, biometrics of consumers, efficiency of micellarization, and transport from the enterocytes to the lymph system [36, 57–61]. Thus, carotenoid contents in foods may not be well correlated with their bioavailability and the ultimate bioefficacy because of the interference of negative effectors [62]. Among the unrelated factors, presence of dietary fat, heat treatment, and reduced particle size have a noticeable positive effect whereas dietary fibers and proteins have a negative effect [62]. Mechanical processing, including chopping and chewing, help reduce particle size and release carotenoids from chloroplasts and tissue for the bioaccessibility [63–65]. The amounts of naturally occurring lipids are rather low in most carotenoid-rich fruits and vegetables so that 3–5 g of fat intake per day is essential for the optimal absorption of carotenoids [66, 67]. Further, the presence of dietary fats, particularly long-chain fatty acids, for example, oleic acid, is more beneficial for the absorption of nonpolar carotenoids (carotenes) than that of polar ones (xanthophylls) [62, 68–70] because polar carotenoids can be more easily transferred from emulsified lipid to micelles [71]. Dietary fibers, the principle components of plant foods, compromise carotenoid release from food matrixes, and both fibers and proteins inhibit the incorporation of carotenoids into the micelles [60, 72]. While heating during cooking can degrade most nutrients in foods, such a treatment increases the bioavailability of certain nutrients, such as lycopene [73]. Therefore, understanding factors influencing bioaccessibility and bioavailability of carotenoids is crucial to achieving their ultimate bioefficacy.
5. Encapsulation
Nutrient bioavailability precedes its bioactivity at target tissues. In order to obtain the maximum bioefficacy of any given nutrients whose bioaccessibility and bioavailability are not satisfactory, a number of strategies are sought for their improvements. For example, encapsulation with food grade or related Generally Recognized As Safe (GRAS) materials has emerged as a novel strategy to improve the bioavailability and bioactivity of phytonutrients, including carotenoids. This encapsulation technology can include, but not limited to, microemulsions, matrix systems, solid dispersions, reassembled proteins, cross-linked polysaccharides, and liposomes [74–81]. The encapsulation, such as liposomes and emulsions, can stabilize carotenoids from possible degradation in the harsh gastrointestinal environment [82]. Nanoencapsulation is defined as a technology involving the formation of active loaded particles with diameters ranging from 1 to 1000 nm [83]. Particularly, polymeric nanoencapsulation has been adopted as one of preferred methods due to its higher loading capacity and better stability [84–86] and has been proven effective to augment bioavailability of carotenoids. For example, in a feeding study with male Swiss albino mice, Arunkumar et al. [87] reported that lutein nanoencapsulated by chitosan triphosphate was accumulated in a larger concentration in plasma, liver, and eyes as compared to the control. Furthermore, using an in vitro Caco-2 cell model, Yi et al. [88] found that solid lipid nanoentrapment significantly improved cellular uptake of β-carotene. Vishwanathan et al. [89] found in a small clinical trial that lutein supplemented in a stable hydrophilic nanoemulsion was 1.3-fold more bioavailable as evidenced in its serum status compared to lutein delivered in a pill. Thus, encapsulation can be a promising technology to enhance carotenoid bioaccessibility and bioavailability and to navigate precise delivery to target tissues such as eyes, brain, or/and skin for the maximum health benefits. However, clinical data supporting their applications remain largely lacking.
6. Safety and Efficacy of Carotenoids
It is well known that an excess of retinoids induces teratogenic effects [90, 91] and affects xenobiotic metabolism [92]. Although β-carotene is not teratogenic [9], high doses of β-carotene and vitamin E can be prooxidant and toxic [93, 94] and increase cancer risk. In particular, despite that high intake of β-carotene reduces the risk of many cancers (Table 3), the effect on breast cancer risk depends on estrogen receptor (ER) and progesterone receptor (PR) statuses [95] (Table 3). In general, the relationships between carotenoids and cancer risk depend on type of carotenoids and site of cancer, but the supplementation never confirms the suggestions from intake data (Table 3). Moreover, the increased risk of lung cancer after β-carotene supplementation had been reported in smokers and people drinking ≥11 g ethanol/d (ATBC study) [7]. The ATBC (20 mg/d) and CARET (30 mg/d) studies also showed increased risk for intracerebral hemorrhage [96], cardiovascular diseases [97, 98], and hyperlipidemia (in asbestos-exposed subjects) [98]. On the contrary, lycopene supplementation decreased LDL cholesterol [99] and blood pressure [100], at doses of ≥25 and > 12 mg/d, respectively, and lycopene has been suggested for preventing the toxic effects of antineoplastic drugs [101].
Table 3.
Carotenoids and cancer risk.
| β-Carotene | α-Carotene | β-Cryptoxanthin | Lycopene | Lutein + zeaxanthin | |
|---|---|---|---|---|---|
| High intake | Ovarian (postmenopausal) ↓ [116] | ||||
| High intake | Bladder ↓ [117] | Bladder ↓ [117] | Bladder ↓ [117] | Bladder ↔ [117] | Bladder ↔ [117] |
| Supplement | Bladder ↑ [118] | ||||
| High intake | Breast (ER+, ER+/PR+) ↑ [95] | Breast ↓ [95] | Breast ↓ [95] | Breast (ER−/PR+ or ER−/PR−) ↓ [95] | Breast (ER−/PR+ or ER−/PR−) ↓ [95] |
| (ER−/PR+ or ER−/PR−) ↓ [95] | |||||
| Supplement | Gut (colorectal) ≈↑ [119] | ||||
| High intake | Gut (esophageal) ↓ [120] | Gut (esophageal) ↓ [120] | Gut (esophageal) ↓ [120] | Gut (esophageal) ↓ [120] | Gut (esophageal) ↓ [120] |
| High intake | Gut (gastric) ↓ [121, 122] | Gut (gastric) ↓ [121, 122] | Gut (gastric) ↔ [121, 123] | Gut (gastric) (lutein) ↔ [121] | |
| Supplement | Gut (liver) ↔ [124] | ||||
| High intake | Gut (pancreatic) ↓ [125] | Gut (pancreatic) ↔ [125] | Gut (pancreatic) ↓ [125] | Gut (pancreatic) ≈↓ [125] | Gut (pancreatic) ↔ [125] |
| Supplement | Gut gastric ≈↑ [126] | ||||
| Supplement | Gut intestinal ↑ [126] | ||||
| High intake | Hodgkin lymphoma ↓ [127] | Hodgkin lymphoma ↓ [127] | Hodgkin lymphoma ↔ [127] | Hodgkin lymphoma ↔ [127] | Hodgkin lymphoma ↓ [127] |
| High intake | Lung ↓ [128] | ||||
| Supplement | Lung ↑ [7, 129] | ||||
| High intake | melanoma ≈↓ [130] | ||||
| Supplement | Oral ↔ [131] | ||||
| High intake | Oral ↓ [132] | Oral ↓ [132] | Oral ↓ [132] | Oral ↓ [132] | |
| High intake | Prostate ↔ [133] | Prostate ↓ [133] | Prostate ↓ [112, 133] | ||
| Supplement | Prostate ↔ [134] |
≈ ns. increase or decrease; ↓: decrease; ↑: increase; ↔: no change; ER: estrogen receptor; PR: progesterone receptor.
The overall mortality increased after β-carotene supplementation [102–104] at a dose of >9.6 mg/d [104]. On the contrary, for non-provitamin A carotenoids, an Observed Safe Level (OSL) of 20 mg/d for lutein and 75 mg/d for lycopene [13] has been suggested and an acceptable daily intake (ADI) of 53 mg/d has been proposed for zeaxanthin [105]. The positive effect of lutein and zeaxanthin on age-related macular degeneration is well known [106].
In the ATBC study, an induction of cytochrome P450 enzymes (CYP450) in male smokers supplemented with β-carotene has been reported [10]. Since CYP450 is the primary metabolizer of xenobiotics in humans, interactions between medication use and dietary supplements can occur. In this context, β-carotene supplementation (25,000 IU twice daily, 28 days) did not affect pharmacokinetics of nelfinavir and its active metabolite M8 in HIV-1-infected individuals [107], whereas a mixed supplement (400 IU/d of vitamin E, 500 mg/d of vitamin C, and 6 mg/d of β-carotene twice daily, 6 months) decreased cyclosporine A in renal transplant recipients [108]. Therefore, potential nutraceutical-drug interactions must be evaluated on the basis of the pharmacokinetics. Furthermore, interactions between alcohol and RA precursors are well documented and the combination of β-carotene with ethanol results in hepatotoxicity [109].
In particular, competitive inhibition of ADH could account for this adversity [110] and for the less adverse effects of non-provitamin A carotenoids (Table 3 and Table 4).
Table 4.
Effects of lycopene and β-carotene supplementation on cardiometabolic outcomes.
| Lycopene | Lutein | β-Carotene | |
|---|---|---|---|
| Blood lipids | ↓ Cholesterol [99] | ↔ [135] | ↑ Cholesterol and triglycerides (asbestos-exposed) [98] |
| ↔ Cholesterol [136] | |||
| Diabetes/insulin resistance | ↔ Insulin resistance [135] | ↔ Type 2 diabetes [137] | |
| Diabetic macrovascular disease | ↔ [138] | ||
| Metabolic syndrome | ↓ [135] | ||
| Blood pressure | ↓ [100, 136] | ↔ [135] | |
| CVD and nonfatal myocardial infarction | ↑ [97, 98] | ||
| Stroke | ↓ [113] | ||
| Intracerebral hemorrhage | ↑ [96] | ||
| CV death | ↑ [103] |
↓: decrease; ↑: increase; ↔: no change; CVD: cardiovascular disease; CV: cardiovascular.
In the CARET study, β-carotene increased from 17 to 210 μg/dL after 4 months of supplementation [111], whereas circulating lycopene concentrations between 2.17 and 85 μg/dL were inversely associated with prostate cancer risk [112]. It shall be noted that such an association did not exist at concentrations greater than 85 μg/dL [112]. It has been reported that circulating lycopene, rather than dietary lycopene, decreases stroke risk [113]. In this context, dietary guidance should consider upper limits for food-derived bioactive substances [114]. Also, efficacy should be determined in order to establish a therapeutic index of non-nutrient phytochemicals in foods and beverages [115].
7. Carotenoids and the Immune System
It is widely recognized that vitamin A deficiency decreases both humoral and cellular immune responses [16, 139] and that RA regulates innate immune response [140]. Vitamin A deficiency was associated with incidence of tuberculosis in human immunodeficiency virus- (HIV-) negative subjects [141] and in HIV-infected patients after antiretroviral therapy [142]. In addition, carotenoid concentrations were lower in tuberculosis cases before antiretroviral therapy [142]. However, in the ATBC study, β-carotene (20 mg/d) increased the risk of pneumonia in those who had initiated smoking at 21 years or later age [143] and the incidence of common cold in people undertaking strenuous exercise [144]. On the other hand, vitamins (vitamin C 120 mg, β-carotene 6 mg, and α-tocopherol 15 mg) with zinc (20 mg) and selenium (100 μg) decreased the infectious events in elderly subjects [145]. However, low levels of vitamin A and carotenoids are associated not only with immunodeficiency but also with inflammation and autoimmunity and both systemic and GALT immune dysfunctions [18]. Patients with rheumatoid arthritis [146, 147], systemic lupus erythematosus [146], celiac disease [148], and/or Crohn's disease [149] had lower serum concentrations of carotenoids [149], β-carotene [146, 147], and/or retinol [146, 148]. Concerning non-provitamin A carotenoids, in the Third National Health and Nutrition Examination Survey (NHANES III), high serum lycopene concentrations were associated with lower mortality in patients with systemic lupus erythematosus [150].
Despite the potential concerted modulation of redox and inflammatory status, in a review of studies that investigated the effect of supplementation with antioxidant-rich foods or nutraceuticals on combined markers of redox and inflammatory status in humans, overall improvement in both markers of redox and inflammatory status was observed only in 27 studies of the 88 studies analyzed and only 28.6% (2/7) of the interventions with carrot, tomato, or lycopene-derived tomato (Lyc-O-mato) improved at least one marker of redox or inflammatory status [151]. Some serum inflammatory cytokines, such as tumor necrosis factor- (TNF-) α and interleukin- (IL-) 6, are also called adipomyokines [152] and are not specific markers of immune function, whereas their ex vivo production from peripheral blood mononuclear cells can be an index of immune response.
Table 5 describes major findings of human intervention studies [153–173] that investigated the effect of β-carotene, lycopene, mixed supplements, or carotenoid-rich juices and diet (fruits/vegetables) on immune function assays, including the in vivo test of cell-mediated immune response delayed-type hypersensitivity (DTH) and/or ex vivo assays of innate (i.e., natural killer (NK) activity and oxidative burst) and adaptive immunity (i.e., lymphocyte proliferation and cytokine production).
Table 5.
Effects of carotenoid and carotenoid-rich food and beverages on test of immune function.
| Subjects (study) | Treatment | Outcomes [ref.] |
|---|---|---|
| Healthy (RCT) | β-Carotene (15–120 mg), 4–7 wk | ↔ lymphocyte proliferation [153], ROS production [154] |
| ↑ DTH (30 mg) versus control (↓ after UV exposure response only in the placebo group) [155] | ||
|
| ||
| Elderly (RCT) | β-Carotene (8.2, 30, 50, and 90 mg), 3–6 wk to 10–12 y | ↑ DTH (30 mg) versus control (↓ after UV exposure response only in the placebo group) [156] |
| ↑ NK activity [157] | ||
| ↔ production of IL-12 and IFN-γ (50, 90 mg) [157] | ||
| ↔ DTH (50 and 90 mg), production of IL-2 [158], and lymphocyte proliferation [158, 159] | ||
|
| ||
| Smokers (RCT) | β-Carotene (40 mg), 4 and 6 wk | ↓ ROS production [160] |
|
| ||
| Type 2 diabetes (RCT) | Lycopene (10 mg/d), 8 wk | ↔ DHT [161, 162] |
|
| ||
| Elderly (RCT) | Lycopene (13.3 mg), 12 wk | ↔ lymphocyte proliferation [159] |
|
| ||
| Elderly (RCT) | Mixed supplement | |
| β-Carotene (0.75 mg), vitamin C (90 mg), and vitamin E (20 mg), 1 y | ↑ DHT [163] | |
| β-Carotene (6 mg), vitamin C (120 mg), and vitamin E (15 mg), 1–2 y | ↔ DHT [164], lymphocyte proliferation [165] | |
|
| ||
| Healthy (RCT) | Mixed supplement | |
| β-Carotene (12 mg), vitamin E (288 mg), and vitamin C (375 mg), 6 and 10 wk | ↑ DTH [166] | |
| ↔ lymphocyte proliferation, ROS production [166] | ||
| ↔ DHT [167] | ||
| β-Carotene (30 mg), lycopene (15 mg), and lutein (9 mg), 5 wk | ↓ IL-2 [167] and ROS [167, 168] production versus depletion (↑) | |
|
| ||
| Healthy (RCT/longitudinal) | Carrot juice (330 mL, 21.6–27.1 mg β-carotene, and 13.1–15.7 mg α-carotene), 2 wk | ↑ TNF-α versus depletion (arm carrot juice-tomato juice, arm tomato juice-carrot juice) [169] |
| ↑ IL-2 versus depletion (arm carrot juice-tomato juice) [169] | ||
| ↔ lymphocyte proliferation and IL-4 production [169, 170] | ||
| ↑ NK activity [169] | ||
|
| ||
| Healthy (longitudinal) | Dried spinach powder 10 g (11.3 mg lutein and 3.1 mg β-carotene), 2 wk | ↔ lymphocyte proliferation, IL-2 and IL-4 production [170] |
|
| ||
| Healthy (RCT/longitudinal) | Tomato-based drink (Lyc-o-Mato) (5.7 mg lycopene, 1 mg β-carotene, and 1.8 mg α-tocopherol), 26 days Tomato juice (330 mL, 37.0–40 mg lycopene and 1.5 mg β-carotene), 2 wk | ↓ TNF-α production [171] |
| ↔ IFN-γ production (versus baseline, ↑ in placebo versus baseline) [171] | ||
| ↔ lymphocyte proliferation [169, 170], IL-2 and IL-4 production [169] | ||
| ↑ TNF-α versus depletion (arm tomato juice-carrot juice) [169] | ||
| ↑ IL-2 and IL-4 production versus depletion (↑) [170], | ||
| ↑ NK activity [169] | ||
|
| ||
| Elderly (RCT) | Tomato juice (330 mL, 47.1 mg lycopene), 8 wk | ↔ DTH, lymphocyte proliferation [172] |
| ↓ IL-2 production (versus baseline, ns versus water) [172] | ||
| ↑ activity of NK, IL-4, and TNF-α production (versus baseline, ns versus water) [172] | ||
|
| ||
| Healthy (RCT) | Vegetables and fruit: 2, 5, or 8 servings/d, 4 wk | ↔ NK activity, IL-2, IL-12, IFN-γ, TNF-α production, lymphocyte proliferation [173] |
↓: decrease; ↑: increase; ↔: no change; d: days; DTH: delayed-type hypersensitivity; IFN: interferon; IL: interleukin; mo: months; NK: natural killer cells; RCT: randomized controlled trials; TNF: tumor necrosis factor; UV: ultraviolet light; wk: weeks; y: years.
Increased levels of β-carotene [155, 156, 158–160, 163–165, 167–169, 172], lycopene [159, 161, 162, 167–169, 172], and lutein [167, 168] as well as of antioxidant vitamins (vitamin E and/or C) in the case of mixed supplements (Table 5) were found in response to treatment. Furthermore, increases in plasma carotenoid from 2.03 to 3.05 μM were reported after 8 weeks of a consumption of 8 servings/d of vegetables and fruits, including carrots, green beans, peas, broccoli, zucchini, tomatoes, kohlrabi, Brussels sprouts, red cabbage, cauliflower, spinach, lettuce, radishes, cucumbers, fennel, apples, pears, kiwis, bananas, peaches, nectarines, cherries, strawberries, and red currants [173].
β-Carotene inhibited the ultraviolet light- (UV-) induced immunosuppression, evaluated with a DHT test in both healthy and elderly subjects, whereas contrasting results were reported on DHT when lycopene, β-carotene, or mixed supplements were used without UV irradiation (Table 5).
Data from ex vivo markers of adaptive immunity do not support an effect of lymphocytes' proliferation, whereas results concerning cytokine production are of difficult interpretation due to the differences in the dosage and duration of carotenoid supplementation and the use of carotenoid depletion periods (Table 5). In a longitudinal study of four periods, each lasting 2 weeks (weeks 1–2: low-carotenoid period; weeks 3–4: 330 mL tomato juice; weeks 5–6: 330 mL carrot juice; weeks 7–8 : 10 g dried spinach powder), tomato juice consumption increased IL-2 and IL-4 secretion compared with that at the end of the depletion period, whereas no effects were observed after carrot juice and spinach powder [170] (Table 5).
The same group [169] observed, in a crossover design, that ex vivo IL-2 production increased after carrot juice only in the arm depletion-carrot juice-depletion-tomato juice. TNF-α increased after the first supplementation (both juices) but only with carrot juice after the second supplementation [169]. Moreover, IL-2 further increased after supplementation and lymphocyte proliferation increased in both groups after the end of the first juice supplementation period despite that it did not change after carrot or tomato juice consumption compared with that at the end of the first low-carotenoid period [170]. Authors reported that this immunomodulation could not be explained by changes in the plasma carotenoid concentrations [170] and that provitamin A effect can be excluded because plasma retinol levels did not change after juice supplementation.
Concerning innate immunity, conflicting results were reported for oxidative burst-induced reactive oxygen species (ROS) production, whereas NK activity resulted to be increased in the majority of the studies (Table 5). However, the maximal increase in NK activity has been observed 1 week after juice supplementations had been stopped and the increase in NK cell activity is not associated to increase in NK percentage [157].
Accordingly, results on lymphocyte subsets are conflicting. Despite that in older subjects β-carotene (30 mg/d, 2 months) increased plasma β-carotene and the percentage of NK, without affecting plasma retinol [174], many studies did not observe any effect on lymphocyte subsets after β-carotene supplementation [153, 158, 159, 165–167, 172, 173, 175]. Moreover, in a randomized controlled trial (RCT), β-carotene (30 mg/d) supplementation for 3 months in subjects with colonic polyps or colon cancers increased CD4 count only in cancer patients who had a lower percentage of CD4 than in patients with polyps and in controls [176]. On the other hand, β-carotene (60 mg/d) increased CD4+ cell counts only in patients with AIDS who have greater than 10 cells/microliters [177]. In HIV patients, β-carotene (60 mg/d, 3 months) increased NK, but not CD4 [178]. On the contrary, others reported that in HIV patients, β-carotene (60 mg/d orally three times daily and at 1 month and 3 months) did not change T cell subsets and NK, despite the increase in serum β-carotene [175]. Contrasting results came from supplementation with β-carotene in doses ranging from 60 mg/d to 180 mg/d on CD4 count in HIV patients [175, 177, 179–183], and data from a recent meta-analysis does not support β-carotene supplementation for increased CD4 cell count in patients with HIV [184]. However, GALT resulted to be depleted of CD4 also after restoration of blood CD4 by combined antiretroviral therapy (cART) [185]. In particular, it has been reported that HIV patients had defective gut homing of C-C chemokine receptor 9 (CCR9) and gut-homing β7 integrin on T helper cells producing IL-17 (Th17) [185]. In this context, it is well known that RA induces the gut-homing molecules α4β7 integrin and CCR9 in B and T (CD4 and CD8) cells [2, 3, 139] (Figure 1). RA can also induce α4β7 integrin and CCR9 on type 1 and 3 innate lymphoid cells (ILCs), but does not lead to CCR9 expression on type 2 ILCs [3, 18]. In terms of cytokine production, ILC1, ILC2, and ILC3 cells are Th1-like, Th2-like, and Th17-like cells, respectively [186] (Figure 1). Although plasticity has been suggested between ILC2/ILC1 and between ILC3/ILC1, ILC2 has been involved in asthma, lung fibrosis, esophagitis, and atopic dermatitis; ILC1 in chronic obstructive pulmonary disease and Crohn's disease; and ILC3 in psoriasis and obesity-associated inflammation [187]. Furthermore, ILC1 and ILC3 induce the polarization of inflammatory macrophages M1 [139]. Therefore, innate immunity can affect local inflammation.
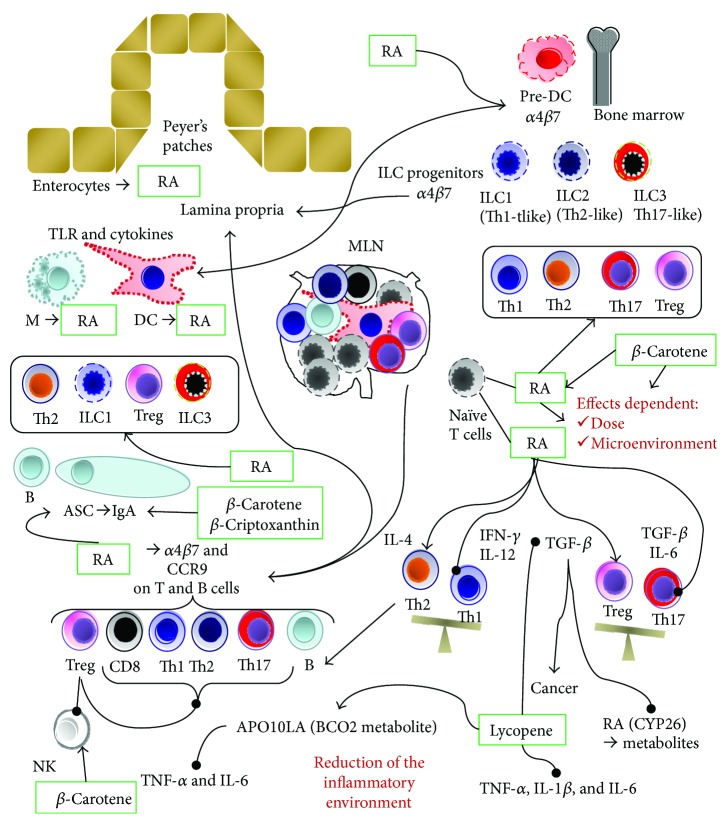
Figure 1.
Immunomodulatory effects of carotenoids and retinoic acid. α4β7: α4β7 integrin; APO10LA: Apo-10′-lycopenoic acid; ASC: antibody-secreting cells; BCO2: β-carotene 9′,10′ oxygenase-2; CCR9: C-C chemokine receptor 9; CYP26: cytochrome P450 26; DC: dendritic cells; IFN: interferon; Ig: immunoglobulin; IL: interleukin; ILC: innate lymphoid cells; M: macrophages; MLN: mesenteric lymph nodes; NK: natural killer; RA: retinoic acid; TGF: transforming growth factor; Th: T helper; TLR: Toll-like receptor; TNF: tumor necrosis factor; Treg: regulatory T. →: homing and improvement; −•: inhibition.
In addition to the enterocytes' production, RA is also produced by stromal cells in the lamina propria (LP) and mesenteric lymph nodes (MLN), as well as by dendritic cells (DC) and macrophages [3] in the GALT. DC are major RA producers in LP, Peyer's patch, and MLN [188] (Figure 1). Preclinical studies suggest that the expression of gut-homing molecules by DC precursors in marrow is regulated by RA [18] (Figure 1). These cells migrate in the gut and induce oral tolerance by inducing regulatory T cells (Treg) [18]. RA induces also RA-producing CCR7+ DC that migrate to the MLN and induce gut homing in T cells [18] (Figure 1). RA production by DC is regulated by many local signals. Microbial-derived signals, by Toll-like receptor (TLR) 2 and TLR5, as well as butyrate produced by commensal bacteria, induce ALDH expression in DC [3, 18]. Besides IL-4 from ILC2 and Th2 cells, transforming growth factor beta (TGF-β) may also induce ALDH expression [3, 18]. The effects of RA on Th subsets depend on the local microenvironment [2, 3, 139].
In physiological conditions, RA produced by DC inhibits the differentiation of naïve T cells to Th17 cells by blocking IL-6, IL-21, and IL-23 signaling in naïve T cells [3]. RA-primed DC induce the production of the anti-inflammatory cytokine IL-10 in Tregs [3], and RA itself promotes TGF-β-mediated Treg conversion of naïve T cells [2, 3] (Figure 1). TGF-β is also involved in IgA class switching [189], and RA induces the expression of α4β7-integrin and CCR9 on B cells and antibody-secreting cells (ASC) [2, 189] (Figure 1). Furthermore, DC-derived RA, plus IL-5, IL-6, or TLR signals, has a primary role in the polarization of B cells in favor of IgA-producing ASC, by inducing IgA class switching in B cells [3, 18, 139, 189], and it has been suggested that oral RA administration before vaccine can increase the secretion of IgA into gut secretions [91]. Concerning provitamin A carotenoids, some preclinical studies suggest an effect on humoral immunity (Figure 1). In mice, 50 mg/kg β-carotene for 21 d increased the concentrations of IgA and the numbers of ASC in the jejunum [190]. Also, β-cryptoxanthin (5–10 mg/kg, 14 and 21 d) in rabbit increased the blood CD4, IL-4, and humoral immunity (IgG, IgM, and IgA) [191].
During inflammation, IL-1 enhances an IL-6-induced shift of the Treg/Th17 balance towards Th17 cells [3], and RA promotes, in the presence of IL-15, the secretion of IL-12 and IL-23 by DC, inducing the IFN-γ-producing Th1 and Th17 cells, and enhances the IL-4-mediated induction of Th2 [3, 18, 140]. On the other hand, in deficiency state, there are marked increases of ILC2 cell proliferation and cytokine (IL-4, IL-5, IL-6, IL-9, and IL-13) production, and, at the same time, the proliferation and function of ILC3 subset are suppressed [139].
It has been also suggested that RA has a dose-dependent effect: at pharmacological or high doses (10 nM and higher), RA inhibits Th17 and Th1 cells and induces Treg, whereas at physiological low doses (1 nM), RA favors Th17 cell differentiation [3, 16] (Figure 1). Th17 is involved in Crohn's disease [192], and the anti-α4β7 integrin therapeutic antibody (vedolizumab) targets gut-homing Th17 [193]. Although a reduced Treg/Th17 balance is often associated with inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis, the potential role of vitamin A or RA treatments is controversial [3].
IL-6 has a primary role in Th17 induction (Figure 1), and a recent meta-analysis reported that tomato supplementation was associated with significant reductions in IL-6 [136]. In a study using an animal model of ulcerative colitis (dextran sulfate sodium), β-carotene decreased colon IL-6 (5, 10, and 20 mg/kg), TNF-α (10 and 20 mg/kg), and IL-17 (20 mg/kg) and reduced plasma lipopolysaccharide [194]. On the other hand, intragastric lycopene administration (5 mg/kg [195]; 1, 2, and 4 mg/kg [196]) reduced TNF-α, IL-1β, IL-6, and/or TGF-β in a rat model of Alzheimer's disease and inhibited the β-amyloid-induced upregulation of TLR4 in the choroid plexus [195]. The effect on TGF-β has implication also in cancer (Figure 1). Lycopene inhibited TGF-β-induced migration, invasion, and adhesion activity of human liver adenocarcinoma SK-Hep-1 cells (2.5 μM) [197] and decreased TGF-β1 mRNA levels in fibroblasts [198]. On the contrary, the role of RA in cancer is controversial.
Despite that RA is required for the expansion of tumor-reactive CD8 T cells, the induction of the TGF-β-producing Treg may inhibit tumor immunosurveillance [188]. In this context, TGF-β reduced the expression of CYP26, inhibiting the breakdown of RA [3] (Figure 1). Therefore, non-provitamin A carotenoids could have anti-inflammatory properties without compromising cancer immunosurveillance and could not increase cancer risk as observed after β-carotene supplementation (Table 3). However, although the activity of β-carotene on immune function could be due to its conversion to vitamin A and RA [19], it has been suggested that apo-10′-lycopenoic acid (apo10LA), a BCO2 metabolite of lycopene, activates the RAR, reducing IL-6 and IL-1β [199]. In mice, APO10LA at 10 mg/kg diet for 24 weeks reduced diethylnitrosamine-initiated, high-fat diet- (HFD-) promoted hepatic tumorigenesis, lung tumor incidence, and hepatic TNF-α and IL-6 concentrations [200]. Data from BCO2-knockout (BCO2-KO) and wild-type mice suggest that IL-6 inhibition and chemoprevention could depend on BCO2 expression [201]. Therefore, the role of metabolites from non-provitamin A carotenoids deserves future investigation.
8. Conclusion
From the reviewed data, the total carotenoid intakes range from 5.42 to 15.44 mg/d (Table 2) and the suggested recommended intake range are 2–4.8 mg/d for β-carotene [34, 37], 10–20 mg/d for lutein, and 5.7–15 mg/d for lycopene [38]. Higher intakes from foods rather than supplementation with β-carotene have been associated with healthy effects (Table 3 and Table 4), whereas more promising results came from lycopene supplementations (Table 4). However, the majority of the available data came from epidemiological studies and meta-analysis that include few RCT (<15) [99, 100, 136], with a small sample size (<100), and no supplementation data on cancer risk is available. Therefore, large-scale intervention studies are warranted to substantiate the health effects of lycopene.
Despite the antioxidant activity of β-carotene, the major provitamin A carotenoid, its prooxidant activity in smokers and alcohol drinkers justifies its adverse effects in doses ranging from 20 mg/d to 30 mg/d [96–98, 143]. The overall mortality increased after β-carotene supplementation at doses >9.6 mg/d [104], and potential food/drug or supplements/alcohol interactions can be also taken into account due to competition for and/or induction of metabolism enzymes [10, 108–110].
On the contrary, non-provitamin A carotenoids could have a safer profile (20 mg/d for lutein, 75 mg/d for lycopene, and 53 mg/d for zeaxanthin) [13, 105] than β-carotene. The latter is converted to RA with immunomodulatory effects (Figure 1).
Human intervention studies that investigated the effects of carotenoids on immune function involve β-carotene, lycopene, or food sources and suggest that carotenoids affect immune function only after a depletion period and at doses (≥30 mg/d β-carotene and lycopene) (Table 5) higher than recommended intakes. Some effects, unrelated to carotenoids and retinol plasma levels, have been observed after the end of the supplementation period. Furthermore, results on lymphocyte subsets are conflicting. In this context, local production of RA can affect the GALT and lymphocyte gut homing. The effect of RA on T-helper subsets depends on local microenvironment and inflammatory status. In this context, although RA is the major active metabolite affecting the immune system, preclinical data suggest that lycopene metabolites derived from BCO2 can modulate immune function by reducing the inflammatory cytokine IL-6 (Figure 1). In this context, there is a growing interest in BCO2 metabolites [202] and it is well known that based on genetic polymorphisms of BCO1 it is possible to cluster subjects as strong responders or weak responders to carotenoids [203, 204]. BCO1 polymorphisms also affect non-provitamin A carotenoids, such as lutein [205, 206] and lycopene [206]. This body of evidence suggests that personalized nutrition/supplementation should be considered in the future.
On the other hand, preclinical studies suggest that the differential effect of RA and lycopene on TGF-β can account for the safer profile of lycopene in the context of cancer incidence (Figure 1).
However, on the light of the different effects of RA at physiological and pharmacological doses [3, 16] (Figure 1), more studies are needed in order to establish the therapeutic index for lycopene and caution must be taken to extrapolate preclinical data to clinical uses. Furthermore, the majority of human interventions report the effects of lycopene on immune function administering mixed supplements or tomato products with lycopene ranging from 15 to 47.1 mg (Table 5). These doses are near or over the higher value of the suggested recommended intake (5.7–15 mg/d) [38], raising a safety concern.
In conclusion, although lycopene supplementation for immune-regulation seems more promising than β-carotene, human studies with adequate power and duration are needed in order to confirm this hypothesis.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Maiani G., Periago Castón M. J., Catasta G., et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. 2009;53(Supplement S2):S194–S218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 2.Mora J. R., Iwata M., Von Andrian U. H. Vitamin effects on the immune system: vitamins A and D take centre stage. 2008;8(9):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkelens M. N., Mebius R. E. Retinoic acid and immune homeostasis: a balancing act. 2017;38(3):168–180. doi: 10.1016/j.it.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Raghuvanshi S., Reed V., Blaner W. S., Harrison E. H. Cellular localization of β-carotene 15,15′ oxygenase-1 (BCO1) and β-carotene 9′,10′ oxygenase-2 (BCO2) in rat liver and intestine. 2015;572:19–27. doi: 10.1016/j.abb.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borel P. Genetic variations involved in interindividual variability in carotenoid status. 2012;56(2):228–240. doi: 10.1002/mnfr.201100322. [DOI] [PubMed] [Google Scholar]
- 6.Von Lintig J. Provitamin A metabolism and functions in mammalian biology. 2012;96(5):1234S–1244S. doi: 10.3945/ajcn.112.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albanes D., Heinonen O. P., Taylor P. R., et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. 1996;88(21):1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 8.Redlich C. A., Blaner W. S., Van Bennekum A. M., et al. Effect of supplementation with beta-carotene and vitamin A on lung nutrient levels. 1998;7(3):211–214. [PubMed] [Google Scholar]
- 9.Woutersen R. A., Wolterbeek A. P. M., Appel M. J., van den Berg H., Goldbohm R. A., Feron V. J. Safety evaluation of synthetic β-carotene. 1999;29(6):515–542. doi: 10.1080/10408449991349267. [DOI] [PubMed] [Google Scholar]
- 10.Mondul A. M., Sampson J. N., Moore S. C., et al. Metabolomic profile of response to supplementation with β-carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. 2013;98(2):488–493. doi: 10.3945/ajcn.113.062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. 2014;34:e478–e486. doi: 10.14694/EdBook_AM.2014.34.e478. [DOI] [PubMed] [Google Scholar]
- 12.Bjelakovic G., Nikolova D., Gluud L. L., Simonetti R. G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. 2012;(3, article CD007176) doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao A., Hathcock J. N. Risk assessment for the carotenoids lutein and lycopene. 2006;45(3):289–298. doi: 10.1016/j.yrtph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Lindshield B. L., Canene-Adams K., Erdman J. W., Jr Lycopenoids: are lycopene metabolites bioactive? 2007;458(2):136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Souto E. B., Severino P., Basso R., Santana M. H. A. Encapsulation of antioxidants in gastrointestinal-resistant nanoparticulate carriers. In: Armstrong D., Bharali D., editors. Vol. 1028. Totowa, NJ: Humana Press; 2013. pp. 37–46. (Methods in Molecular Biology (Methods and Protocols)). [DOI] [PubMed] [Google Scholar]
- 16.Bono M. R., Tejon G., Flores-Santibanez F., Fernandez D., Rosemblatt M., Sauma D. Retinoic acid as a modulator of T cell immunity. 2016;8(6):p. 349. doi: 10.3390/nu8060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigmundsdottir H., Butcher E. C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. 2008;9(9):981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czarnewski P., Das S., Parigi S. M., Villablanca E. J. Retinoic acid and its role in modulating intestinal innate immunity. 2017;9(1):p. 68. doi: 10.3390/nu9010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chew B. P., Park J. S. Carotenoid action on the immune response. 2004;134(1):257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 20.dela Seña C., Narayanasamy S., Riedl K. M., Curley R. W., Jr., Schwartz S. J., Harrison E. H. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1) 2013;288(52):37094–37103. doi: 10.1074/jbc.M113.507160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydemir G., Kasiri Y., Birta E., et al. Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene’s anti-cancer potential. 2013;57(5):739–747. doi: 10.1002/mnfr.201200548. [DOI] [PubMed] [Google Scholar]
- 22.Amorim-Carrilho K. T., Cepeda A., Fente C., Regal P. Review of methods for analysis of carotenoids. 2014;56:49–73. doi: 10.1016/j.trac.2013.12.011. [DOI] [Google Scholar]
- 23.Estévez-Santiago R., Beltrán-de-Miguel B., Olmedilla-Alonso B. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish survey of dietary intake (2009–2010) 2016;67(3):305–313. doi: 10.3109/09637486.2016.1147020. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer B., Natoli S., Liew G., Flood V. M. Lutein and zeaxanthin—food sources, bioavailability and dietary variety in age‐related macular degeneration protection. 2017;9(2) doi: 10.3390/nu9020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galanakis C. M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. 2012;26(2):68–87. doi: 10.1016/j.tifs.2012.03.003. [DOI] [Google Scholar]
- 26.Prado J., Veggi P., Meireles M. Extraction methods for obtaining carotenoids from vegetables - review. 2014;10(1):29–66. doi: 10.2174/1573411011410010005. [DOI] [Google Scholar]
- 27.N’Dri D., Calani L., Mazzeo T., et al. Effects of different maturity stages on antioxidant content of Ivorian Gnagnan (Solanum indicum L.) berries. 2010;15(10):7125–7138. doi: 10.3390/molecules15107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Hernández G. B., Boluda-Aguilar M., Taboada-Rodríguez A., Soto-Jover S., Marín-Iniesta F., López-Gómez A. Processing, packaging and storage of tomato products: influence on the lycopene content. 2016;8(1):52–75. doi: 10.1007/s12393-015-9113-3. [DOI] [Google Scholar]
- 29.Palmero P., Lemmens L., Hendrickx M., Van Loey A. Role of carotenoid type on the effect of thermal processing on bioaccessibility. 2014;157:275–282. doi: 10.1016/j.foodchem.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez C., Baranda A. B., Martinez De Maranon I. The effect of high pressure and high temperature processing on carotenoids and chlorophylls content in some vegetables. 2014;163:37–45. doi: 10.1016/j.foodchem.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 31.Granado F., Blazquez S., Olmedilla B. Changes in carotenoid intake from fruit and vegetables in the Spanish population over the period 1964–2004. 2007;10(10):1018–1023. doi: 10.1017/S1368980007662314. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill M. E., Carroll Y., Corridan B., et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. 2001;85(4):499–507. doi: 10.1079/BJN2000284. [DOI] [PubMed] [Google Scholar]
- 33.Granado-Lorencio F., Olmedilla-Alonso B., Blanco-Navarro I., Botella-Romero F., Simal-Antón A. Assessment of carotenoid status and the relation to glycaemic control in type I diabetics: a follow-up study. 2006;60(8):1000–8. doi: 10.1038/sj.ejcn.1602411. [DOI] [PubMed] [Google Scholar]
- 34.SCOF European Food Safety Authority. 2006.
- 35.FaNBI Institute of Medicine. Washingto, D.C.: National Academy Press; 2000. Beta-carotene and other carotenoids; pp. 325–400. [Google Scholar]
- 36.de Pee S., West C. E., Permaesih D., Martuti S., Muhilal, Hautvast J. G. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. 1998;68(5):1058–1067. doi: 10.1093/ajcn/68.5.1058. [DOI] [PubMed] [Google Scholar]
- 37.Biesalski H. K., Böhles H., Esterbauer H., et al. Antioxidant vitamins in prevention. 1997;16(3):151–155. doi: 10.1016/S0261-5614(97)80245-2. [DOI] [PubMed] [Google Scholar]
- 38.Lupton J. R., Atkinson S. A., Chang N., et al. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. 2014;53(Supplement 1):1–9. doi: 10.1007/s00394-014-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge A. M., Simpson J. A., Fridman M., et al. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. 2009;12(12):2438–2447. doi: 10.1017/S1368980009005539. [DOI] [PubMed] [Google Scholar]
- 40.El-Sohemy A., Baylin A., Kabagambe E., Ascherio A., Spiegelman D., Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. 2002;76(1):172–179. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- 41.Faure H., Preziosi P., Roussel A. M., et al. Factors influencing blood concentration of retinol, α-tocopherol, vitamin C, and β-carotene in the French participants of the SU.VI.MAX trial. 2006;60(6):706–717. doi: 10.1038/sj.ejcn.1602372. [DOI] [PubMed] [Google Scholar]
- 42.Trieschmann M., Beatty S., Nolan J. M., et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. 2007;84(4):718–728. doi: 10.1016/j.exer.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Sette S., Le Donne C., Piccinelli R., Arcella D., Turrini A., Leclercq C. The third Italian National Food Consumption Survey, INRAN-SCAI 2005–06 – part 1: nutrient intakes in Italy. 2011;21(12):922–932. doi: 10.1016/j.numecd.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Sinu. 2014.
- 45.Umesawa M., Iso H., Mikami K., et al. Relationship between vegetable and carotene intake and risk of prostate cancer: the JACC study. 2014;110(3):792–796. doi: 10.1038/bjc.2013.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quansah D., Ha K., Jun S., et al. Associations of dietary antioxidants and risk of type 2 diabetes: data from the 2007–2012 Korea National Health and Nutrition Examination Survey. 2017;22(10, article 1664) doi: 10.3390/molecules22101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beltran-De-Miguel B., Estevez-Santiago R., Olmedilla-Alonso B. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010) 2015;66(6):706–712. doi: 10.3109/09637486.2015.1077787. [DOI] [PubMed] [Google Scholar]
- 48.Bermudez O. I., Ribaya-Mercado J. D., Talegawkar S. A., Tucker K. L. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. 2005;135(6):1496–1502. doi: 10.1093/jn/135.6.1496. [DOI] [PubMed] [Google Scholar]
- 49.Hendrickson S. J., Willett W. C., Rosner B. A., Eliassen A. H. Food predictors of plasma carotenoids. 2013;5(10):4051–4066. doi: 10.3390/nu5104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayhoe R. P. G., Lentjes M. A. H., Mulligan A. A., Luben R. N., Khaw K.-T., Welch A. A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. 2017;117(10):1439–1453. doi: 10.1017/S0007114517001180. [DOI] [PubMed] [Google Scholar]
- 51.Zaripheh S., Erdman J. W., Jr Factors that influence the bioavailablity of xanthophylls. 2002;132(3):531s–534s. doi: 10.1093/jn/132.3.531S. [DOI] [PubMed] [Google Scholar]
- 52.Holst B., Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. 2008;19(2):73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Granado-Lorencio F., Blanco-Navarro I., Pérez-Sacristán B., Hernandez-Alvarez E. Biomarkers of carotenoid bioavailability. 2017;99(Part 2):902–916. doi: 10.1016/j.foodres.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 54.Saini R. K., Nile S. H., Park S. W. Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. 2015;76(Part 3):735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 55.Marze S. Bioaccessibility of lipophilic micro-constituents from a lipid emulsion. 2015;6(10):3218–3227. doi: 10.1039/c5fo00441a. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Amaya D. B., Kimura M., Godoy H. T., Amaya-Farfan J. Updated Brazilian database on food carotenoids: factors affecting carotenoid composition. 2008;21(6):445–463. doi: 10.1016/j.jfca.2008.04.001. [DOI] [Google Scholar]
- 57.Donhowe E. G., Kong F. Beta-carotene: digestion, microencapsulation, and in vitro bioavailability. 2014;7(2):338–354. doi: 10.1007/s11947-013-1244-z. [DOI] [Google Scholar]
- 58.Rein M. J., Renouf M., Cruz-Hernandez C., Actis-Goretta L., Thakkar S. K., da Silva Pinto M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. 2013;75(3):588–602. doi: 10.1111/j.1365-2125.2012.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colle I. J. P., Lemmens L., Knockaert G., Van Loey A., Hendrickx M. Carotene degradation and isomerization during thermal processing: a review on the kinetic aspects. 2016;56(11):1844–1855. doi: 10.1080/10408398.2013.790779. [DOI] [PubMed] [Google Scholar]
- 60.Lemmens L., Colle I., Van Buggenhout S., Palmero P., Van Loey A., Hendrickx M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. 2014;38(2):125–135. doi: 10.1016/j.tifs.2014.05.005. [DOI] [Google Scholar]
- 61.Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. 2013;5(9):3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Priyadarshani A. M. B. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. 2017;57(8):1710–1717. doi: 10.1080/10408398.2015.1023431. [DOI] [PubMed] [Google Scholar]
- 63.Palafox-Carlos H., Ayala-Zavala J. F., Gonzalez-Aguilar G. A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. 2011;76(1):R6–r15. doi: 10.1111/j.1750-3841.2010.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeum K. J., Russell R. M. Carotenoid bioavailability and bioconversion. 2002;22(1):483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- 65.Goltz S. R., Campbell W. W., Chitchumroonchokchai C., Failla M. L., Ferruzzi M. G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. 2012;56(6):866–877. doi: 10.1002/mnfr.201100687. [DOI] [PubMed] [Google Scholar]
- 66.Castenmiller J. J. M., West C. E. Bioavailability and bioconversion of carotenoids. 1998;18(1):19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- 67.Roodenburg A. J. C., Leenen R., van het Hof K. H., Weststrate J. A., Tijburg L. B. M. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. 2000;71(5):1187–1193. doi: 10.1093/ajcn/71.5.1187. [DOI] [PubMed] [Google Scholar]
- 68.Victoria-Campos C. I., de Jesús Ornelas-Paz J., Yahia E. M., Failla M. L. Effect of the interaction of heat-processing style and fat type on the micellarization of lipid-soluble pigments from green and red pungent peppers (Capsicum annuum) 2013;61(15):3642–3653. doi: 10.1021/jf3054559. [DOI] [PubMed] [Google Scholar]
- 69.Lakshminarayana R., Raju M., Keshava Prakash M. N., Baskaran V. Phospholipid, oleic acid micelles and dietary olive oil influence the lutein absorption and activity of antioxidant enzymes in rats. 2009;44(9):799–806. doi: 10.1007/s11745-009-3328-0. [DOI] [PubMed] [Google Scholar]
- 70.Lakshminarayana R., Raju M., Krishnakantha T. P., Baskaran V. Lutein and zeaxanthin in leafy greens and their bioavailability: olive oil influences the absorption of dietary lutein and its accumulation in adult rats. 2007;55(15):6395–6400. doi: 10.1021/jf070482z. [DOI] [PubMed] [Google Scholar]
- 71.Borel P., Grolier P., Armand M., et al. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. 1996;37(2):250–261. [PubMed] [Google Scholar]
- 72.Soukoulis C., Bohn T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. 2018;58(1):1–36. doi: 10.1080/10408398.2014.971353. [DOI] [PubMed] [Google Scholar]
- 73.Colle I. J. P., Lemmens L., Van Buggenhout S., Met K., Van Loey A. M., Hendrickx M. E. Processing tomato pulp in the presence of lipids: the impact on lycopene bioaccessibility. 2013;51(1):32–38. doi: 10.1016/j.foodres.2012.11.024. [DOI] [Google Scholar]
- 74.Hu B., Liu X., Zhang C., Zeng X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. 2017;25(1):3–15. doi: 10.1016/j.jfda.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Speranza B., Petruzzi L., Bevilacqua A., et al. Encapsulation of active compounds in fruit and vegetable juice processing: current state and perspectives. 2017;82(6):1291–1301. doi: 10.1111/1750-3841.13727. [DOI] [PubMed] [Google Scholar]
- 76.Pralhad T., Rajendrakumar K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. 2004;34(2):333–339. doi: 10.1016/S0731-7085(03)00529-6. [DOI] [PubMed] [Google Scholar]
- 77.Zhu F. Encapsulation and delivery of food ingredients using starch based systems. 2017;229:542–552. doi: 10.1016/j.foodchem.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 78.Wani T. A., Shah A. G., Wani S. M., et al. Suitability of different food grade materials for the encapsulation of some functional foods well reported for their advantages and susceptibility. 2016;56(15):2431–2454. doi: 10.1080/10408398.2013.845814. [DOI] [PubMed] [Google Scholar]
- 79.Gonnet M., Lethuaut L., Boury F. New trends in encapsulation of liposoluble vitamins. 2010;146(3):276–290. doi: 10.1016/j.jconrel.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 80.Augustin M. A., Sanguansri L. Challenges and solutions to incorporation of nutraceuticals in foods. 2015;6(1):463–477. doi: 10.1146/annurev-food-022814-015507. [DOI] [PubMed] [Google Scholar]
- 81.Morales J. O., Valdés K., Morales J., Oyarzun-Ampuero F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. 2015;10(2):253–269. doi: 10.2217/nnm.14.159. [DOI] [PubMed] [Google Scholar]
- 82.Brannon-Peppas L. Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. 1995;116(1):1–9. doi: 10.1016/0378-5173(94)00324-x. [DOI] [Google Scholar]
- 83.Auffan M., Rose J., Bottero J.-Y., Lowry G. V., Jolivet J.-P., Wiesner M. R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. 2009;4(10):634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 84.He W., Lu Y., Qi J., Chen L., Hu F., Wu W. Nanoemulsion-templated shell-crosslinked nanocapsules as drug delivery systems. 2013;445(1-2):69–78. doi: 10.1016/j.ijpharm.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 85.Semete B., Booysen L., Lemmer Y., et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. 2010;6(5):662–671. doi: 10.1016/j.nano.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Murugeshu A., Astete C., Leonardi C., Morgan T., Sabliov C. M. Chitosan/PLGA particles for controlled release of α-tocopherol in the GI tract via oral administration. 2011;6(9):1513–1528. doi: 10.2217/nnm.11.44. [DOI] [PubMed] [Google Scholar]
- 87.Arunkumar R., Harish Prashanth K. V., Baskaran V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: characterization and bioavailability of lutein in vitro and in vivo. 2013;141(1):327–337. doi: 10.1016/j.foodchem.2013.02.108. [DOI] [PubMed] [Google Scholar]
- 88.Yi J., Lam T. I., Yokoyama W., Cheng L. W., Zhong F. Cellular uptake of β-carotene from protein stabilized solid lipid nanoparticles prepared by homogenization-evaporation method. 2014;62(5):1096–1104. doi: 10.1021/jf404073c. [DOI] [PubMed] [Google Scholar]
- 89.Vishwanathan R., Wilson T. A., Nicolosi R. J. Bioavailability of a nanoemulsion of lutein is greater than a lutein supplement. 2009;1(1):38–49. doi: 10.5101/nbe.v1i1.p38-49. [DOI] [Google Scholar]
- 90.Comptour A., Rouzaire M., Belville C., et al. Nuclear retinoid receptors and pregnancy: placental transfer, functions, and pharmacological aspects. 2016;73(20):3823–3837. doi: 10.1007/s00018-016-2332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mwanza-Lisulo M., Kelly P. Potential for use of retinoic acid as an oral vaccine adjuvant. 2015;370(1671) doi: 10.1098/rstb.2014.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shmarakov I. O. Retinoid-xenobiotic interactions: the Ying and the Yang. 2015;4(4):243–267. doi: 10.3978/j.issn.2304-3881.2015.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vrolijk M. F., Opperhuizen A., Jansen E. H. J. M., et al. The shifting perception on antioxidants: the case of vitamin E and β-carotene. 2015;4:272–278. doi: 10.1016/j.redox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Russell R. M. The vitamin A spectrum: from deficiency to toxicity. 2000;71(4):878–884. doi: 10.1093/ajcn/71.4.878. [DOI] [PubMed] [Google Scholar]
- 95.Bae J. M. Reinterpretation of the results of a pooled analysis of dietary carotenoid intake and breast cancer risk by using the interval collapsing method. 2016;38, article e2016024 doi: 10.4178/epih.e2016024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leppala J. M., Virtamo J., Fogelholm R., et al. Controlled trial of α-tocopherol and β-carotene supplements on stroke incidence and mortality in male smokers. 2000;20(1):230–235. doi: 10.1161/01.ATV.20.1.230. [DOI] [PubMed] [Google Scholar]
- 97.Tornwall M. E., Virtamo J., Korhonen P. A., et al. Effect of α-tocopherol and β-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. 2004;25(13):1171–1178. doi: 10.1016/j.ehj.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Cartmel B., Dziura J., Cullen M. R., et al. Changes in cholesterol and triglyceride concentrations in the Vanguard population of the Carotene and Retinol Efficacy Trial (CARET) 2005;59(10):1173–1180. doi: 10.1038/sj.ejcn.1602229. [DOI] [PubMed] [Google Scholar]
- 99.Ried K., Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: meta-analyses of intervention trials. 2011;68(4):299–310. doi: 10.1016/j.maturitas.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 100.Li X., Xu J. Lycopene supplement and blood pressure: an updated meta-analysis of intervention trials. 2013;5(9):3696–3712. doi: 10.3390/nu5093696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sahin K., Sahin N., Kucuk O. Lycopene and chemotherapy toxicity. 2010;62(7):988–995. doi: 10.1080/01635581.2010.509838. [DOI] [PubMed] [Google Scholar]
- 102.Schwingshackl L., Boeing H., Stelmach-Mardas M., et al. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. 2017;8(1):27–39. doi: 10.3945/an.116.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vivekananthan D. P., Penn M. S., Sapp S. K., Hsu A., Topol E. J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. 2003;361(9374):2017–2023. doi: 10.1016/s0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 104.Bjelakovic G., Nikolova D., Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? 2013;8(9, article e74558) doi: 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edwards J. A. Zeaxanthin: review of toxicological data and acceptable daily intake. 2016;2016:15. doi: 10.1155/2016/3690140.3690140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma L., Liu R., Du J. H., Liu T., Wu S. S., Liu X. H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. 2016;8(7):p. 426. doi: 10.3390/nu8070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheehan N. L., van Heeswijk R. P. G., Foster B. C., et al. The effect of β-carotene supplementation on the pharmacokinetics of nelfinavir and its active metabolite M8 in HIV-1-infected patients. 2012;17(1):688–702. doi: 10.3390/molecules17010688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blackhall M. L., Fassett R. G., Sharman J. E., Geraghty D. P., Coombes J. S. Effects of antioxidant supplementation on blood cyclosporin A and glomerular filtration rate in renal transplant recipients. 2005;20(9):1970–1975. doi: 10.1093/ndt/gfh875. [DOI] [PubMed] [Google Scholar]
- 109.Leo M. A., Lieber C. S. Alcohol, vitamin A, and β-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. 1999;69(6):1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- 110.Wolf G. Tissue-specific increases in endogenous all-trans retinoic acid: possible contributing factor in ethanol toxicity. 2010;68(11):689–692. doi: 10.1111/j.1753-4887.2010.00323.x. [DOI] [PubMed] [Google Scholar]
- 111.Goodman G. E., Omenn G. S., Thornquist M. D., Lund B., Metch B., Gylys-Colwell I. The Carotene and Retinol Efficacy Trial (CARET) to prevent lung cancer in high-risk populations: pilot study with cigarette smokers. 1993;2(4):389–396. [PubMed] [Google Scholar]
- 112.Chen P., Zhang W., Wang X., et al. Lycopene and risk of prostate cancer: a systematic review and meta-analysis. 2015;94(33, article e1260) doi: 10.1097/MD.0000000000001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li X., Xu J. Dietary and circulating lycopene and stroke risk: a meta-analysis of prospective studies. 2014;4(1, article 5031) doi: 10.1038/srep05031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yates A. A., Erdman J. W., Jr., Shao A., Dolan L. C., Griffiths J. C. Bioactive nutrients - time for tolerable upper intake levels to address safety. 2017;84:94–101. doi: 10.1016/j.yrtph.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Peluso I., Palmery M. Flavonoids at the pharma-nutrition interface: is a therapeutic index in demand? 2015;71:102–107. doi: 10.1016/j.biopha.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 116.Li X., Xu J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. 2015;4(1, article 4885) doi: 10.1038/srep04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang J. E., Wang R. J., Zhong H., Yu B., Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. 2014;12(1):p. 130. doi: 10.1186/1477-7819-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park S. J., Myung S. K., Lee Y., Lee Y. J. Effects of vitamin and antioxidant supplements in prevention of bladder cancer: a meta-analysis of randomized controlled trials. 2017;32(4):628–635. doi: 10.3346/jkms.2017.32.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pais R., Dumitrascu D. L. Do antioxidants prevent colorectal cancer? A meta-analysis. 2013;51(3-4):152–163. [PubMed] [Google Scholar]
- 120.Ge X. X., Xing M. Y., Yu L. F., Shen P. Carotenoid intake and esophageal cancer risk: a meta-analysis. 2013;14(3):1911–1918. doi: 10.7314/APJCP.2013.14.3.1911. [DOI] [PubMed] [Google Scholar]
- 121.Zhou Y., Wang T., Meng Q., Zhai S. Association of carotenoids with risk of gastric cancer: a meta-analysis. 2016;35(1):109–116. doi: 10.1016/j.clnu.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Li P., Zhang H., Chen J., et al. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. 2014;135(6):1444–1453. doi: 10.1002/ijc.28777. [DOI] [PubMed] [Google Scholar]
- 123.Yang T., Yang X., Wang X., Wang Y., Song Z. The role of tomato products and lycopene in the prevention of gastric cancer: a meta-analysis of epidemiologic studies. 2013;80(4):383–388. doi: 10.1016/j.mehy.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 124.Lai G. Y., Weinstein S. J., Taylor P. R., et al. Effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and chronic liver disease mortality in the ATBC study. 2014;111(12):2220–2223. doi: 10.1038/bjc.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen J., Jiang W., Shao L., Zhong D., Wu Y., Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. 2016;67(7):744–753. doi: 10.1080/09637486.2016.1197892. [DOI] [PubMed] [Google Scholar]
- 126.Malila N., Taylor P. R., Virtanen M. J., et al. Effects of alpha-tocopherol and beta-carotene supplementation on gastric cancer incidence in male smokers (ATBC Study, Finland) 2002;13(7):617–623. doi: 10.1023/A:1019556227014. [DOI] [PubMed] [Google Scholar]
- 127.Chen F., Hu J., Liu P., Li J., Wei Z., Liu P. Carotenoid intake and risk of non-Hodgkin lymphoma: a systematic review and dose-response meta-analysis of observational studies. 2017;96(6):957–965. doi: 10.1007/s00277-016-2898-1. [DOI] [PubMed] [Google Scholar]
- 128.Yu N., Su X., Wang Z., Dai B., Kang J. Association of dietary vitamin a and β-carotene intake with the risk of lung cancer: a meta-analysis of 19 publications. 2015;7(11):9309–9324. doi: 10.3390/nu7115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fritz H., Kennedy D., Fergusson D., et al. Vitamin A and retinoid derivatives for lung cancer: a systematic review and meta analysis. 2011;6, article e21107(6) doi: 10.1371/journal.pone.0021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y. P., Chu R. X., Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. 2014;9(7, article e102527) doi: 10.1371/journal.pone.0102527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lodi G., Franchini R., Warnakulasuriya S., et al. Interventions for treating oral leukoplakia to prevent oral cancer. 2016;7, article CD001829 doi: 10.1002/14651858.CD001829.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leoncini E., Nedovic D., Panic N., Pastorino R., Edefonti V., Boccia S. Carotenoid intake from natural sources and head and neck cancer: a systematic review and meta-analysis of epidemiological studies. 2015;24(7):1003–1011. doi: 10.1158/1055-9965.EPI-15-0053. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y., Cui R., Xiao Y., Fang J., Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and dose-response meta-analysis of observational studies. 2015;10(9, article e0137427) doi: 10.1371/journal.pone.0137427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neuhouser M. L., Barnett M. J., Kristal A. R., et al. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. 2009;18(8):2202–2206. doi: 10.1158/1055-9965.EPI-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leermakers E. T. M., Darweesh S. K. L., Baena C. P., et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. 2016;103(2):481–494. doi: 10.3945/ajcn.115.120931. [DOI] [PubMed] [Google Scholar]
- 136.Cheng H. M., Koutsidis G., Lodge J. K., Ashor A., Siervo M., Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 137.Kataja-Tuomola M., Sundell J. R., Männistö S., et al. Effect of α-tocopherol and β-carotene supplementation on the incidence of type 2 diabetes. 2007;51(1):47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 138.Kataja-Tuomola M. K., Kontto J. P., Mannisto S., Albanes D., Virtamo J. R. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: results of the ATBC Study. 2010;42(3):178–186. doi: 10.3109/07853890903508887. [DOI] [PubMed] [Google Scholar]
- 139.Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. 2015;33(2):71–89. [PubMed] [Google Scholar]
- 140.Raverdeau M., Mills K. H. G. Modulation of T cell and innate immune responses by retinoic acid. 2014;192(7):2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 141.Aibana O., Franke M. F., Huang C. C., et al. Impact of vitamin a and carotenoids on the risk of tuberculosis progression. 2017;65(6):900–909. doi: 10.1093/cid/cix476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tenforde M. W., Yadav A., Dowdy D. W., et al. Vitamin A and D deficiencies associated with incident tuberculosis in HIV-infected patients initiating antiretroviral therapy in multinational case-cohort study. 2017;75(3):e71–e79. doi: 10.1097/QAI.0000000000001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hemilä H., Virtamo J., Albanes D., Kaprio J. Vitamin E and beta-carotene supplementation and hospital-treated pneumonia incidence in male smokers. 2004;125(2):557–565. doi: 10.1378/chest.125.2.557. [DOI] [PubMed] [Google Scholar]
- 144.Hemilä H., Virtamo J., Albanes D., Kaprio J. Physical activity and the common cold in men administered vitamin E and β-carotene. 2003;35(11):1815–1820. doi: 10.1249/01.MSS.0000093616.60899.92. [DOI] [PubMed] [Google Scholar]
- 145.Girodon F., Lombard M., Galan P., et al. Effect of micronutrient supplementation on infection in institutionalized elderly subjects: a controlled trial. 1997;41(2):98–107. doi: 10.1159/000177984. [DOI] [PubMed] [Google Scholar]
- 146.Comstock G. W., Burke A. E., Hoffman S. C., et al. Serum concentrations of α tocopherol, β carotene, and retinol preceding the diagnosis of rheumatoid arthritis and systemic lupus erythematosus. 1997;56(5):323–5. doi: 10.1136/ard.56.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Profumo E., Di Franco M., Buttari B., et al. Biomarkers of subclinical atherosclerosis in patients with autoimmune disorders. 2012;2012:8. doi: 10.1155/2012/503942.503942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jamnik J., Jenkins D. J. A., El-Sohemy A. Biomarkers of cardiometabolic health and nutritional status in individuals with positive celiac disease serology. 2017;24(1):37–45. doi: 10.1177/0260106017748053. [DOI] [PubMed] [Google Scholar]
- 149.Drai J., Borel P., Faure H., et al. Fasting plasma carotenoids concentrations in Crohn’s and pancreatic cancer patients compared to control subjects. 2009;79(2):87–94. doi: 10.1024/0300-9831.79.2.87. [DOI] [PubMed] [Google Scholar]
- 150.Han G. M., Han X. F. Lycopene reduces mortality in people with systemic lupus erythematosus: a pilot study based on the third national health and nutrition examination survey. 2016;27(5):430–5. doi: 10.3109/09546634.2015.1133879. [DOI] [PubMed] [Google Scholar]
- 151.Serafini M., Peluso I. Functional foods for health: the interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. 2017;22(44):6701–6715. doi: 10.2174/1381612823666161123094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li F., Li Y., Duan Y., Hu C.-A. A., Tang Y., Yin Y. Myokines and adipokines: involvement in the crosstalk between skeletal muscle and adipose tissue. 2017;33:73–82. doi: 10.1016/j.cytogfr.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 153.Daudu P. A., Kelley D. S., Taylor P. C., Burri B. J., Wu M. M. Effect of a low β-carotene diet on the immune functions of adult women. 1994;60(6):969–972. doi: 10.1093/ajcn/60.6.969. [DOI] [PubMed] [Google Scholar]
- 154.Mobarhan S., Bowen P., Andersen B., et al. Effects of β‐carotene repletion on β‐carotene absorption, lipid peroxidation, and neutrophil superoxide formation in young men. 1990;14(3-4):195–206. doi: 10.1080/01635589009514094. [DOI] [PubMed] [Google Scholar]
- 155.Fuller C. J., Faulkner H., Bendich A., Parker R. S., Roe D. A. Effect of β -carotene supplementation on photosuppression of delayed-type hypersensitivity in normal young men. 1992;56(4):684–690. doi: 10.1093/ajcn/56.4.684. [DOI] [PubMed] [Google Scholar]
- 156.Herraiz L. A., Hsieh W. C., Parker R. S., Swanson J. E., Bendich A., Roe D. A. Effect of UV exposure and β-carotene supplementation on delayed-type hypersensitivity response in healthy older men. 1998;17(6):617–624. doi: 10.1080/07315724.1998.10718811. [DOI] [PubMed] [Google Scholar]
- 157.Santos M. S., Gaziano J. M., Leka L. S., Beharka A. A., Hennekens C. H., Meydani S. N. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: an investigation of the role of cytokines. 1998;68(1):164–170. doi: 10.1093/ajcn/68.1.164. [DOI] [PubMed] [Google Scholar]
- 158.Santos M. S., Leka L. S., Ribaya-Mercado J. D., et al. Short- and long-term beta-carotene supplementation do not influence T cell-mediated immunity in healthy elderly persons. 1997;66(4):917–924. doi: 10.1093/ajcn/66.4.917. [DOI] [PubMed] [Google Scholar]
- 159.Corridan B. M., O’Donoghue M., Hughes D. A., Morrissey P. A. Low-dose supplementation with lycopene or β-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans. 2001;55(8):627–635. doi: 10.1038/sj.ejcn.1601187. [DOI] [PubMed] [Google Scholar]
- 160.Richards G. A., Theron A. J., van Rensburg C. E. J., et al. Investigation of the effects of oral administration of vitamin E and beta-carotene on the chemiluminescence responses and the frequency of sister chromatid exchanges in circulating leukocytes from cigarette smokers. 1990;142(3):648–654. doi: 10.1164/ajrccm/142.3.648. [DOI] [PubMed] [Google Scholar]
- 161.Neyestani T. R., Shariat-Zadeh N., Gharavi A., Kalayi A., Khalaji N. The opposite associations of lycopene and body fat mass with humoral immunity in type 2 diabetes mellitus: a possible role in atherogenesis. 2007;6(2):79–87. [PubMed] [Google Scholar]
- 162.Neyestani T. R., Shariatzadeh N., Gharavi A., Kalayi A., Khalaji N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with type 2 diabetes mellitus: a possible role in the prevention of long-term complications. 2007;30(10):833–838. doi: 10.1007/BF03349224. [DOI] [PubMed] [Google Scholar]
- 163.Bogden J. D., Bendich A., Kemp F. W., et al. Daily micronutrient supplements enhance delayed-hypersensitivity skin test responses in older people. 1994;60(3):437–447. doi: 10.1093/ajcn/60.3.437. [DOI] [PubMed] [Google Scholar]
- 164.Girodon F., Galan P., Monget A. L., et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. 1999;159(7):748–754. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]
- 165.Galan P., Preziosi P., Monget A. L., et al. Effects of trace element and/or vitamin supplementation on vitamin and mineral status, free radical metabolism and immunological markers in elderly long term-hospitalized subjects. Geriatric Network MIN. VIT. AOX. 1997;67(6):450–460. [PubMed] [Google Scholar]
- 166.Wolvers D. A. W., van Herpen-Broekmans W. M. R., Logman M. H. G. M., van der Wielen R. P. J., Albers R. Effect of a mixture of micronutrients, but not of bovine colostrum concentrate, on immune function parameters in healthy volunteers: a randomized placebo-controlled study. 2006;5(1) doi: 10.1186/1475-2891-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Farges M. C., Minet-Quinard R., Walrand S., et al. Immune status is more affected by age than by carotenoid depletion–repletion in healthy human subjects. 2012;108(11):2054–2065. doi: 10.1017/S0007114512000177. [DOI] [PubMed] [Google Scholar]
- 168.Walrand S., Farges M. C., Dehaese O., et al. In vivo and in vitro evidences that carotenoids could modulate the neutrophil respiratory burst during dietary manipulation. 2005;44(2):114–120. doi: 10.1007/s00394-004-0501-3. [DOI] [PubMed] [Google Scholar]
- 169.Watzl B., Bub A., Briviba K., Rechkemmer G. Supplementation of a low-carotenoid diet with tomato or carrot juice modulates immune functions in healthy men. 2003;47(6):255–261. doi: 10.1159/000072397. [DOI] [PubMed] [Google Scholar]
- 170.Watzl B., Bub A., Brandstetter B. R., Rechkemmer G. Modulation of human T-lymphocyte functions by the consumption of carotenoid-rich vegetables. 1999;82(5):383–389. doi: 10.1017/S0007114599001634. [DOI] [PubMed] [Google Scholar]
- 171.Riso P., Visioli F., Grande S., et al. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. 2006;54(7):2563–2566. doi: 10.1021/jf053033c. [DOI] [PubMed] [Google Scholar]
- 172.Watzl B., Bub A., Blockhaus M., et al. Prolonged tomato juice consumption has no effect on cell-mediated immunity of well-nourished elderly men and women. 2000;130(7):1719–1723. doi: 10.1093/jn/130.7.1719. [DOI] [PubMed] [Google Scholar]
- 173.Watzl B., Kulling S. E., Möseneder J., Barth S. W., Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. 2005;82(5):1052–1058. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 174.Watson R. R., Prabhala R. H., Plezia P. M., Alberts D. S. Effect of β-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. 1991;53(1):90–94. doi: 10.1093/ajcn/53.1.90. [DOI] [PubMed] [Google Scholar]
- 175.Coodley G. O., Coodley M. K., Lusk R., et al. β-carotene in HIV infection: an extended evaluation. 1996;10(9):967–973. doi: 10.1097/00002030-199610090-00006. [DOI] [PubMed] [Google Scholar]
- 176.Kazi N., Radvany R., Oldham T., et al. Immunomodulatory effect of β‐carotene on T lymphocyte subsets in patients with resected colonic polyps and cancer. 1997;28(2):140–145. doi: 10.1080/01635589709514566. [DOI] [PubMed] [Google Scholar]
- 177.Fryburg D. A., Mark R. J., Griffith B. P., Askenase P. W., Patterson T. F. The effect of supplemental beta-carotene on immunologic indices in patients with AIDS: a pilot study. 1995;68(1-2):19–23. [PMC free article] [PubMed] [Google Scholar]
- 178.Garewal H. S., Ampel N. M., Watson R. R., Prabhala R. H., Dols C. L. A preliminary trial of beta-carotene in subjects infected with the human immunodeficiency virus. 1992;122(Supplement_3):728–732. doi: 10.1093/jn/122.suppl_3.728. [DOI] [PubMed] [Google Scholar]
- 179.Bianchi-Santamaria A., Fedeli S., Santamaria L. Short communication: possible activity of beta-carotene in patients with the AIDS related complex. A pilot study. 1992;9(3):151–3. doi: 10.1007/BF02987747. [DOI] [PubMed] [Google Scholar]
- 180.Coodley G. O., Nelson H. D., Loveless M. O., Folk C. Beta-carotene in HIV infection. 1993;6(3):272–276. [PubMed] [Google Scholar]
- 181.Coodley G. O., Nelson H. D., Loveless M. O., Folk C. Beta-carotene in HIV infection. 1993;691:277–278. doi: 10.1111/j.1749-6632.1993.tb26194.x. [DOI] [PubMed] [Google Scholar]
- 182.Silverman S., Jr., Kaugars G. E., Gallo J., et al. Clinical and lymphocyte responses to beta-carotene supplementation in 11 HIV-positive patients with chronic oral candidiasis. 1994;78(4):442–447. doi: 10.1016/0030-4220(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 183.Nimmagadda A. P., Burri B. J., Neidlinger T., O'Brien W. A., Goetz M. B. Effect of oral β-carotene supplementation on plasma human immunodeficiency virus (HIV) RNA levels and CD4+ cell counts in HIV-infected patients. 1998;27(5):1311–1313. doi: 10.1086/514990. [DOI] [PubMed] [Google Scholar]
- 184.Visser M. E., Durao S., Sinclair D., Irlam J. H., Siegfried N. Micronutrient supplementation in adults with HIV infection. 2017;5, article CD003650 doi: 10.1002/14651858.CD003650.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mavigner M., Cazabat M., Dubois M., et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. 2012;122(1):62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Goldberg R., Prescott N., Lord G. M., Macdonald T. T., Powell N. The unusual suspects—innate lymphoid cells as novel therapeutic targets in IBD. 2015;12(5):271–283. doi: 10.1038/nrgastro.2015.52. [DOI] [PubMed] [Google Scholar]
- 187.Ebbo M., Crinier A., Vely F., Vivier E. Innate lymphoid cells: major players in inflammatory diseases. 2017;17(11):665–678. doi: 10.1038/nri.2017.86. [DOI] [PubMed] [Google Scholar]
- 188.Guo Y., Brown C., Ortiz C., Noelle R. J. Leukocyte homing, fate, and function are controlled by retinoic acid. 2015;95(1):125–148. doi: 10.1152/physrev.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mora J. R., Von Andrian U. H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. 2009;21(1):28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nishida K., Sugimoto M., Ikeda S., Kume S. Effects of supplemental β-carotene on mucosal IgA induction in the jejunum and ileum of mice after weaning. 2014;111(2):247–253. doi: 10.1017/S0007114513002195. [DOI] [PubMed] [Google Scholar]
- 191.Ghodratizadeh S., Kanbak G., Beyramzadeh M., Dikmen Z. G., Memarzadeh S., Habibian R. Effect of carotenoid β-cryptoxanthin on cellular and humoral immune response in rabbit. 2014;38(1):59–62. doi: 10.1007/s11259-013-9584-8. [DOI] [PubMed] [Google Scholar]
- 192.Kleinschek M. A., Boniface K., Sadekova S., et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. 2009;206(3):525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Soler D., Chapman T., Yang L. L., Wyant T., Egan R., Fedyk E. R. The binding specificity and selective antagonism of vedolizumab, an anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. 2009;330(3):864–875. doi: 10.1124/jpet.109.153973. [DOI] [PubMed] [Google Scholar]
- 194.Trivedi P. P., Jena G. B. Mechanistic insight into beta-carotene-mediated protection against ulcerative colitis-associated local and systemic damage in mice. 2015;54(4):639–652. doi: 10.1007/s00394-014-0745-5. [DOI] [PubMed] [Google Scholar]
- 195.Liu C. B., Wang R., Yi Y. F., Gao Z., Chen Y. Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. 2018;53:66–71. doi: 10.1016/j.jnutbio.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 196.Sachdeva A. K., Chopra K. Lycopene abrogates Aβ(1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. 2015;26(7):736–744. doi: 10.1016/j.jnutbio.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 197.Jhou B. Y., Song T. Y., Lee I., Hu M. L., Yang N. C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. 2017;65(32):6893–6903. doi: 10.1021/acs.jafc.7b03036. [DOI] [PubMed] [Google Scholar]
- 198.Fletcher N. M., Awonuga A. O., Saed M. G., Abu-Soud H. M., Diamond M. P., Saed G. M. Lycopene, a powerful antioxidant, significantly reduces the development of the adhesion phenotype. 2014;60(1):14–20. doi: 10.3109/19396368.2013.847129. [DOI] [PubMed] [Google Scholar]
- 199.Gouranton E., Aydemir G., Reynaud E., et al. Apo-10′-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. 2011;1811(12):1105–1114. doi: 10.1016/j.bbalip.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 200.Ip B. C., Hu K.-Q., Liu C., et al. Lycopene metabolite, Apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet–promoted hepatic inflammation and tumorigenesis in mice. 2013;6(12):1304–1316. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ip B. C., Liu C., Ausman L. M., von Lintig J., Wang X.-D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. 2014;7(12):1219–1227. doi: 10.1158/1940-6207.CAPR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lietz G., Oxley A., Boesch-Saadatmandi C., Kobayashi D. Importance of β,β-carotene 15,15′-monooxygenase 1 (BCMO1) and β,β-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. 2012;56(2):241–250. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 203.Wang T. T. Y., Edwards A. J., Clevidence B. A. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to beta-carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms. 2013;24(8):1538–1546. doi: 10.1016/j.jnutbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 204.Hendrickson S. J., Hazra A., Chen C., et al. β-Carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. 2012;96(6):1379–1389. doi: 10.3945/ajcn.112.034934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Borel P., de Edelenyi F. S., Vincent-Baudry S., et al. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. 2011;43(1):47–59. doi: 10.3109/07853890.2010.531757. [DOI] [PubMed] [Google Scholar]
- 206.Ferrucci L., Perry J. R. B., Matteini A., et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. 2009;84(2):123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]