Abstract
Background and Aim
Rectal indomethacin was reported to be effective for postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) prophylaxis. However, the preventive effect of indomethacin for average-risk patients remains unclear. Recently, some conflicting evidence was addressed by recent articles. We aimed to determine the protective role of indomethacin in PEP based on the latest available literature.
Methods
A systematic literature search was conducted using PubMed, Embase, Web of Science, and the Cochrane Library to identify related articles published before October 2016. Studies that evaluated the administration of indomethacin in the prevention of PEP were included in the analysis. We adopted a random-effects model to calculate the overall relative risk (RR) and 95% confidence interval (CI).
Results
Ten trials from an initial search were finally included in the meta-analysis. The administration of rectal indomethacin significantly reduced the incidence of PEP in consecutive ERCP population (RR, 0.63; 95% CI, 0.50–0.77). There was no significant heterogeneity across included studies (I 2 = 14.2%, P = 0.31). Further subgroup analyses also revealed that rectal indomethacin could protect the individuals at high and average risks and reduced severity of PEP. Pre-ERCP administration of indomethacin seemed to be better than the post-ERCP given. There was no evidence of significant publication bias.
Conclusions
Rectal administration of indomethacin is an effective approach to prevent the incidence of PEP in both high- and average-risk populations undergoing ERCP. However, more high-quality RCTs are needed to further investigate the optimal timing for the administration of indomethacin.
1. Introduction
Post-ERCP pancreatitis (PEP) is a serious adverse event after ERCP, with a reported incidence of 9.7% in unselected patients [1]. Several risk factors were identified for PEP, such as “suspected sphincter of Oddi dysfunction” and “female gender” [2]. Given a huge economic and clinical burden, effective approaches for post-ERCP pancreatitis prophylaxis remain a major priority for research.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are reported to be effective in PEP prophylaxis so far [3]. Several prospective RCTs and meta-analysis have well demonstrated that the rectal administration of indomethacin significantly decreased the rate of PEP [4–6]. Based on the above evidence, the European Society for Gastrointestinal Endoscopy (ESGE) guideline (2014) recommended the administration of 100 mg of rectal indomethacin for PEP prophylaxis in patients undergoing ERCP with no contraindication [3]. Subsequently, the Japanese Society of Hepato-Biliary-Pancreatic Surgery (2015) [7] also published similar guidelines. Indomethacin, therefore, as an effective pharmacologic prophylaxis, seemed to be appealing. In this context, some conflicting findings emerged recently. A recent prospective, double-blind, controlled trial conducted by Levenick et al. [8] in the USA showed that the reduction in PEP using indomethacin was not as significant as previously reported in multiple RCTs [4, 5, 9]. In fact, even more cases of pancreatitis occurred in the indomethacin group compared with the placebo group. Subsequently, a high-quality meta-analysis also concluded that there is no prophylaxis for the prevention of PEP among average-risk patients [10]. These findings raised the question of whether the administration of rectal indomethacin should be recommended in average-risk patients. However, a recent RCT with a large number of patients was performed in China and concluded that rectal indomethacin should be administrated across patients without contraindication prior to ERCP [11].
Therefore, the benefit of rectal indomethacin needs to be well demonstrated in the majority of patients (average-risk) undergoing ERCP in practice. Confronted with the above conflicting results, we performed a meta-analysis to assess the role of rectal indomethacin for PEP prophylaxis in average-risk individuals.
2. Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12].
2.1. Literature Search
A comprehensive electric search was conducted across PubMed, Embase, and the Cochrane Library for relevant articles with no language limitations from database inception to October 2016. The following Medical Subject Heading (MeSH) terms and text words were adopted in the research: (‘indomethacin') AND (‘cholangiopancreatography endoscopic retrograde' OR ‘pancreatitis' OR ‘post-ERCP pancreatitis'). References of the included articles and reviews were also manually scrutinized.
2.2. Eligibility Criteria
The included criteria were based on the patients, intervention, comparator, outcomes, and study design (PICOS) criteria and were as follows[13]: (1) population: adults undergoing endoscopic retrograde cholangiopancreatography (ERCP); (2) intervention: assessment of rectal indomethacin prior to or post ERCP; (3) comparator: indomethacin exposure compared with placebo exposure or unexposed; (4) outcomes: risk of PEP, presented as relative risks (RR) with 95% confidence intervals (CI); and (5) study design: randomized controlled trial (RCT). Reviews, case reports, abstracts, and letters were excluded. Based on the above “PICOS” criteria, two reviewers (HXK and ZWF) independently scanned all titles and abstracts of articles after an initial search and removed apparently irrelevant studies. Afterward, we reviewed the full texts of the remaining articles in order to identify relevant studies for inclusion. When there were multiple publications from the same population, the most comprehensive article was included. Any discrepancies in the processes were discussed and resolved by agreement.
2.3. Definition of Patients and Outcomes
According to previous studies, the definition of PEP was referred by previous studies [5, 8, 9, 11, 14]. The severity was classified according to “the length of hospitalization and the degree of intervention required” [15]. The criteria to identify high- or average-risk individuals were based on the study by Elmunzer et al. [4]. Detailed information is summarized in Supplementary Data (available here) [4].
2.4. Data Extraction and Quality Assessment
Two authors (HXK and ZWF) independently extracted the related information from eligible articles regarding the author, year, study design, location, number of patients, intervention, and definition of PEP. The methodological quality of eligible studies was evaluated by two reviews using the Cochrane Collaboration's tool [16] independently. The tool included seven items for assessment, and each item was graded as low bias, high bias, or unclear. The discrepancy in data extraction and assessment was resolved by the third author (SLM).
2.5. Statistical Analysis
We adopted a random-effects model to calculate the overall estimate relative risk of PEP in relation to rectal indomethacin exposure with 95% confidence intervals (CI) considering the expected heterogeneity between studies. The heterogeneity among individual studies was assessed qualitatively by the Cochran Q statistic, with P < 0.1 indicating some heterogeneity [17]. The degree of heterogeneity was evaluated by I 2, and an I 2 > 30% suggests that there is moderate to high heterogeneity between included studies [17]. Furthermore, we carried out subgroup analyses stratified by selected population, the timing of administration, the severity of pancreatitis, and different regions. Sensitivity analyses were conducted by excluding each individual study in turn in order to ensure the robustness and consistency of overall results. The funnel plot asymmetry and Egger's test were performed to evaluate the potential publication bias. All statistical analyses were used by the Review Manager (RevMan) V.5.0 software and Stata version 13 (StataCorp, Texas, USA).
3. Results
3.1. Study Characteristics
The initial search strategy yielded 506 studies, and ten studies [4, 5, 8, 9, 11, 14, 18–21] were finally included. Figure 1 depicts the detailed selection and identification process. The characteristics of each individual study are summarized in Table 1. Overall, there was a total of 6094 patients undergoing ERCP, with 459 patients presenting with PEP. All trials adopted 100 mg rectal indomethacin suppository, whereas nine studies used placebo suppository as controls, and one study did not receive placebo. All trials adopted a similar definition of post-ERCP pancreatitis to include patients. The overall methodological quality of RCTs was generally moderate to high according to the Cochrane Collaboration's tool, and detailed information is shown in Supplementary Figures S1 and S2.
Figure 1.
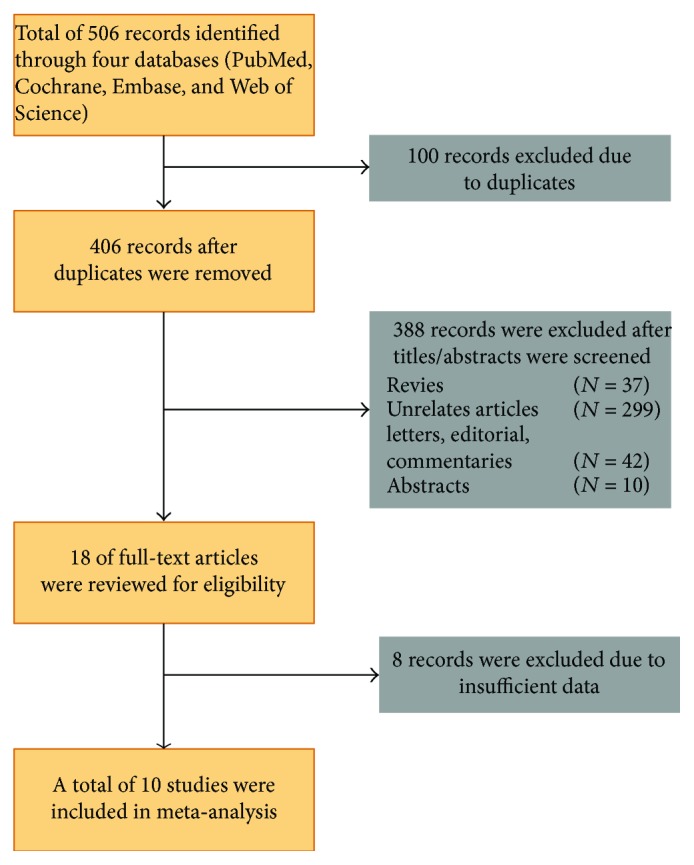
Flow diagram of included and excluded trials in this meta-analysis.
Table 1.
Characteristics of included studies in the meta-analysis.
| Study | Year | Country | Type of trial | Patients (T/C) | Intervention | Definition of PEP |
|---|---|---|---|---|---|---|
| Sotoudehmanesh et al. [14] | 2007 | Iran | Double-blind randomized trial | 245/245 | 100 mg rectal indomethacin versus inert suppository; before ERCP | Serum amylase more than 3 times the upper limit of normal associated with epigastric pain, back pain, and epigastric tenderness |
| Montaño Loza et al. [21] | 2007 | Mexico | Randomized controlled trial | 75/75 | 100 mg rectal indomethacin versus rectal glycerine; before ERCP | Amylase level 3 times the upper limit of normal and epigastric pain or throughout the abdomen radiating to back associated with nausea or vomiting |
| Döbrönte et al. [19] | 2012 | Hungary | Prospective randomized clinical trial | 130/98 | 100 mg rectal indomethacin versus inert placebo; before ERCP | Amylase level 3 times the upper limit of normal and epigastric pain or throughout the abdomen radiating to back associated with nausea or vomiting |
| Elmunzer et al. [4] | 2012 | American | Multicentre, randomized, placebo-controlled, double-blind clinical trial | 295/307 | 2 ∗ 50 mg rectal indomethacin versus placebo suppository; after ERCP | Amylase level 3 times the upper limit of normal and epigastric pain or throughout the abdomen radiating to back associated with nausea or vomiting |
| Döbrönte et al. [18] | 2014 | Hungary | Multicentre prospective, randomized, controlled trial | 347/318 | 100 mg, rectal indomethacin versus placebo suppository; before ERCP | Amylase level 3 times the upper limit of normal and epigastric pain or throughout the abdomen radiating to back associated with nausea or vomiting |
| Patai et al. [5] | 2015 | Hungary | Prospective, placebo-controlled, double-blind trial | 270/269 | 100 mg rectal indomethacin versus placebo suppository; before ERCP | Abdominal pain, extended hospitalization 2–3 days, elevation of amylase 3 times the upper limit of normal in 24 hours |
| Andrade-Dávila et al. [9] | 2015 | Mexico | Prospective randomized controlled trial | 82/84 | 100 mg rectal indomethacin versus glycerine; after ERCP | New or increased abdominal pain consistent with pancreatitis, elevated amylase or lipase greater than three times the normal upper limit until 24 hours after the procedure, and hospitalization (or prolongation of existing hospitalization) for at least 2 nights |
| Levenick et al. [8] | 2016 | America | Prospective, double-blind, placebo-controlledtrial | 223/226 | 100 mg rectal indomethacin versus placebo suppository; during the ERCP | New upper abdominal pain, an elevated lipase greater than three times the upper limit of the normal 24 hours after the onset of pain, and hospitalization for at least two nights |
| Hosseini et al. [20] | 2016 | Iran | Randomized controlled trial | 100/105 | 100 mg rectal indomethacin versus glycerine; before ERCP | New onset or worsened abdominal pain, increase in serum amylase at least 3 times above the upper limit of normal measured 24 h after the procedure, and need for more than one night of hospitalization |
| Luo et al. [11] | 2016 | China | Multicentre, single-blinded, randomized controlled trial | 1297/1303 | 100 mg rectal indomethacin versus no treatment; before ERCP | New onset of upper abdominal pain associated with an elevated serum amylase of at least three times the upper limit of normal range at 24 h after the procedure and admission to a hospital for at least 2 nights |
ERCP: endoscopic retrograde cholangiopancreatography; T/C: treatment/control.
3.2. Overall Analyses of Rectal Indomethacin for PEP Prevention
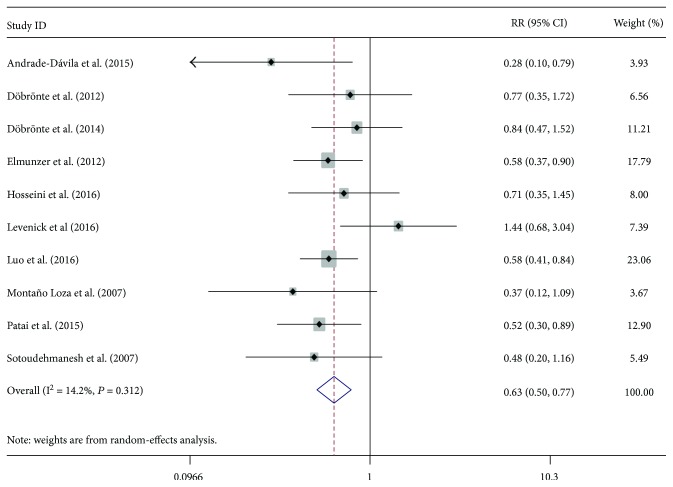
A total ten of RCTs evaluated the prophylactic effect of rectal indomethacin on the prevention of PEP, with the incidence ranging from 4.83% to 13.66% [14, 20]. The relative risk (RR) of individual studies ranged from 0.28 to 1.44, and the cumulative meta-analysis by publication year showed that the rectal administration of indomethacin before or after ERCP was associated with a reduced risk of PEP in the overall population (RR = 0.63; 95% CI, 0.50–0.77) (Figure 2). Furthermore, we also performed a cumulative meta-analysis by publication year and number of included patients. The overall results gradually became stable and tended toward becoming significant with the increase in published year and larger samples (Supplementary Figures S3 and S4). The relatively low heterogeneity (I 2 = 14.2%, P = 0.31) was observed across included studies. Furthermore, sensitivity analyses by removing each study also supported the robustness of the overall outcomes in the meta-analysis. The funnel plot (Supplementary Figure S5) and Egger's test (P = 0.59) suggested no evidence of substantial publication bias in our analysis. No trial reported a higher incidence of adverse events associated with the administration of rectal indomethacin, suggesting the safeness of indomethacin.
Figure 2.
Forest plot for the overall relative risk of post-ERCP pancreatitis with rectal indomethacin.
3.3. Subgroup Analysis
3.3.1. High-Risk versus Average-Risk Patients
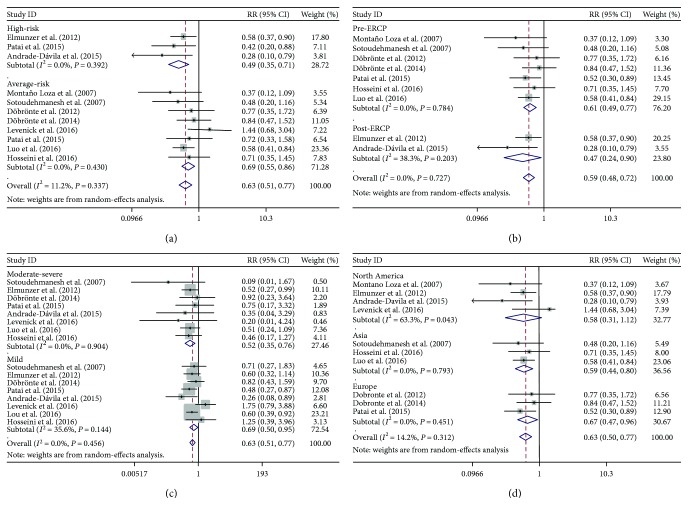
Three studies selected high-risk population, and seven studies chose average-risk patients as the targeted population. The overall rates of PEP in high- and average-risk populations were 14.1% and 6.0%, respectively. The administration of indomethacin significantly reduced the risk of PEP among high-risk patients (RR, 0.49; 95% CI, 0.35–0.71), as well as across average-risk population (RR, 0.69; 95% CI, 0.55–0.86) (Figure 3(a)). No heterogeneity for average-risk and high-risk patients was noted. Furthermore, our sensitivity analyses also showed that the overall results were not changed by a single study.
Figure 3.
Forest plots of subgroup analysis stratified by (a) high-risk and average-risk patients, (b) pre-ERCP and post-ERCP administration, (c) mild and moderate-severe post-ERCP pancreatitis, and (d) patients from different regions.
3.3.2. Pre-ERCP versus Post-ERCP Administration of Indomethacin
Most of the studies (7/10) administered indomethacin rectally prior to ERCP, two studies after ERCP, and one administered during ERCP. The pooled relative risks for pre- and post-ERCP administration were 0.61 (95% CI, 0.49–0.77) and 0.47 (95% CI, 0.24–0.90), respectively (Figure 3(b)). A low degree of heterogeneity (I 2 = 38.3%, P = 0.20) was noted among studies for post-ERCP while no heterogeneity existed in studies for pre-ERCP (I 2 = 0%, P = 0.78).
3.3.3. Mild PEP versus Moderate-Severe PEP
Six Pooled studies showed that indomethacin administration significantly decreased the risk of mild and moderate-severe PEP (RR, 0.52, 95% CI, 0.35–0.76; RR = 0.69, 95% CI, 0.50–0.95, resp.) (Figure 3(c)). A relatively low heterogeneity (I 2 = 35.6%, P = 0.14) was observed across studies for mild PEP.
3.3.4. Different Regions of Medical Centers
Among the included studies, three studies were performed in Asia (2/3 in Iran, 1/3 in China), three in Europe (3/3 in Hungary), and four in North America (2/4 in the United States, 2/4 in Mexico). The estimated pooled relative risks of PEP for Asia, Europe, and North America were 0.59 (95% CI, 0.44–0.80), 0.67 (95% CI, 0.47–0.96), and 0.58 (95% CI, 0.31–1.12), respectively. A high degree of heterogeneity (I 2 = 63.3%, P = 0.043) was noted among patients from North America.
4. Discussion
This exhaustive meta-analysis revealed a significant reduction of PEP risk (RR = 0.48, 95%, 0.26–0.87) in patients with rectal indomethacin. From 2006 to 2016, the cumulative meta-analysis by publication year showed that the overall result gradually became stable and tended toward becoming significant. In a subgroup analysis, the beneficial effect consistently favoured the administration of rectal indomethacin across most of the predefined variables. The prophylactic effect of rectal indomethacin was consistent across the average-risk and high-risk patients, and the administration of indomethacin before or after ERCP reduced the risk of mild and moderate-severe PEP. These results support the recommendation by ESGE and Japanese guidelines that individuals undergoing ERCP with no contraindications ought to be administrated indomethacin rectally to prevent post-ERCP pancreatitis [3, 7]. No increased risk of NSAID-associated adverse event was associated with the administration of indomethacin, indicating the safeness of rectal indomethacin.
Considering PEP as a serious adverse event, several pharmacologic agents were adopted for PEP prophylaxis, such as NSAIDs [22]. Several high-quality RCTs have demonstrated the effective prophylaxis of diclofenac and indomethacin for PEP, although the magnitude of benefits varied [3, 23]. In this context, it seemed that the rectal administration of NSAIDs is a sort of “panacea” for PEP prophylaxis [24]. However, discordant results from recent published RCTs and meta-analysis potentially challenge current evidence [8, 10]. The results of this trial showed that not only the effect of indomethacin for PEP was nonsignificant but also an opposite trend (higher incidence of pancreatitis in the indomethacin group than in the placebo group) was observed, although it is not significant. Levenick et al. [8] concluded that indomethacin may not prevent against PEP in ordinary population. However, we should interpret the conclusion with caution because the early termination of trial may lead to a type II statistical error [25]. Subsequently, a meta-analysis conducted by Inamdar et al. also showed that rectal indomethacin reduced the incidence of PEP in the high-risk patients, rather than in the average-risk [10] patients, which refuted the current guideline and previous meta-analysis. After that, two published RCTs also addressed this issue and supported the benefits of indomethacin in PEP prophylaxis.
Our findings are consistent with previous meta-analyses on this topic [6, 26, 27], although these are in contrast to a recent meta-analysis [10]. The advantage of the current study consists of the inclusion of enough high-quality RCTs, especially for some RCTs that have not been included in the previous studies. Relative low heterogeneity also reflects the similarity of included studies, which might further enhance the validity of results. Being different from the prior meta-analyses, our study is unique in performing cumulative meta-analyses and detailed subgroup analyses. However, this meta-analysis also has several limitations that merit further consideration. The final results and interpretations might be limited by the quantity and quality of included studies. Firstly, the significant heterogeneity across studies in North America could not be fully explained. Therefore, further trials should be conducted in the United States in order to examine the effect of indomethacin. Secondly, we were unable to identify clearly the optimal timing of administration due to lack of adequate RCT data. Finally, it was difficult to rule out the possibility of publication bias due to chance because of the limited number of included articles.
In summary, we demonstrated that rectal indomethacin significantly decreased PEP risk among high- or average-risk population undergoing ERCP and provided strong evidence for current guidelines in clinical practice. Considering its ease of administration, cost-effectiveness, and safety, indomethacin seemed to be an ideal and appealing pharmacological prophylaxis for PEP. However, optimal timing and its benefit in average-risk patients following ERCP needs to be confirmed in further larger prospective studies.
Acknowledgments
The authors thank Ms. Dong Wenjie for the revision of their manuscript. The work was funded by Zhejiang Provincial Medical Platform 2015 Specialists Class B (2015 RCB016), the Zhejiang Province Key Science and Technology Innovation Team (2013TD13), and the Zhejiang Natural Science Foundation of China (LY18H160019).
Abbreviations
- ERCP:
Endoscopic retrograde cholangiopancreatography
- PEP:
Postendoscopic retrograde cholangiopancreatography pancreatitis
- RR:
Relative risk
- CI:
Confidence interval
- RCT:
Randomized controlled trial
- ESGE:
European Society for Gastrointestinal Endoscopy
- NSAIDs:
Nonsteroidal anti-inflammatory drugs.
Contributor Information
Jianmin Si, Email: jianmin_si@zju.edu.cn.
Lei-min Sun, Email: sunlm@zju.edu.cn.
Conflicts of Interest
The authors have no potential conflicts of interest to declare.
Authors' Contributions
Lei-min Sun, Jianmin Si, and Xingkang He had the concept for the systematic review; Lei-min Sun, Xingkang He, and Wenfang Zheng designed and conducted the search strategy to identify included studies; Xingkang He, Yue Ding, and Lei-min Sun conducted the data extraction; Xingkang He, Wenfang Zheng, and Lei-min Sun conducted the statistical analysis; Xingkang He and Lei-min Sun wrote the first draft of the manuscript; and Xia Tang contributed great work to the revision of manuscript. All authors edited and critically revised the final version of the manuscript.
Supplementary Materials
Supplementary Data: major and minor criteria for patients with high risk of post-ERCP pancreatitis. Supplementary Figure S1: quality assessments for included randomized control trials in the meta-analysis. Supplementary Figure S2: overall quality assessments for included randomized control trials in the meta-analysis. Supplementary Figure S3: cumulative meta-analysis of indomethacin for post-ERCP pancreatitis prevention by publication year. Supplementary Figure S4: cumulative meta-analysis of indomethacin for post-ERCP pancreatitis prevention by the number of patients. Supplementary Figure S5: funnel plot of all studies included in the present meta-analysis for the assessment of possible publication bias.
References
- 1.Elmunzer B. J. Charleston, SC, USA: Medical University of South Carolina; 2015. [DOI] [Google Scholar]
- 2.Dumonceau J. M., Andriulli A., Deviere J., et al. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. 2010;42(6):503–515. doi: 10.1055/s-0029-1244208. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau J. M., Andriulli A., Elmunzer B. J., et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline – updated June 2014. 2014;46(9):799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 4.Elmunzer B. J., Scheiman J. M., Lehman G. A., et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. 2012;366(15):1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patai A., Solymosi N., Patai A. V. Effect of rectal indomethacin for preventing post-ERCP pancreatitis depends on difficulties of cannulation: results from a randomized study with sequential biliary intubation. 2015;49(5):429–437. doi: 10.1097/MCG.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 6.Yaghoobi M., Rolland S., Waschke K. A., et al. Meta-analysis: rectal indomethacin for the prevention of post-ERCP pancreatitis. 2013;38(9):995–1001. doi: 10.1111/apt.12488. [DOI] [PubMed] [Google Scholar]
- 7.Yokoe M., Takada T., Mayumi T., et al. Japanese guidelines for the management of acute pancreatitis: Japanese guidelines 2015. 2015;22(6):405–432. doi: 10.1002/jhbp.259. [DOI] [PubMed] [Google Scholar]
- 8.Levenick J. M., Gordon S. R., Fadden L. L., et al. Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients. 2016;150(4):911–917. doi: 10.1053/j.gastro.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade-Dávila V. F., Chávez-Tostado M., Dávalos-Cobián C., et al. Rectal indomethacin versus placebo to reduce the incidence of pancreatitis after endoscopic retrograde cholangiopancreatography: results of a controlled clinical trial. 2015;15(1):p. 85. doi: 10.1186/s12876-015-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamdar S., Han D., Passi M., Sejpal D. V., Trindade A. J. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. 2017;85(1):67–75. doi: 10.1016/j.gie.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Luo H., Zhao L., Leung J., et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. 2016;387(10035):2293–2301. doi: 10.1016/S0140-6736(16)30310-5. [DOI] [PubMed] [Google Scholar]
- 12.Stewart L. A., Clarke M., Rovers M., et al. Preferred Reporting Items for a Systematic Review and Meta-Analysis of individual participant data: the PRISMA-IPD Statement. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 13.Huang X., Lin J., Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. 2006;2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 14.Sotoudehmanesh R., Khatibian M., Kolahdoozan S., Ainechi S., Malboosbaf R., Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. 2007;102(5):978–983. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 15.Cotton P. B., Lehman G., Vennes J., et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. 1991;37(3):383–393. doi: 10.1016/S0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. 2011;343, article d5928 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Döbrönte Z., Szepes Z., Izbéki F., et al. Is rectal indomethacin effective in preventing of post-endoscopic retrograde cholangiopancreatography pancreatitis? 2014;20(29):10151–10157. doi: 10.3748/wjg.v20.i29.10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Döbrönte Z., Toldy E., Márk L., Sarang K., Lakner L. Effects of rectal indomethacin in the prevention of post-ERCP pancreatitis. 2012;153(25):990–996. doi: 10.1556/OH.2012.29403. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini M., Shalchiantabrizi P., Yektaroudy K., Dadgarmoghaddam M., Salari M. Prophylactic effect of rectal indomethacin administration, with and without intravenous hydration, on development of endoscopic retrograde cholangiopancreatography pancreatitis episodes: a randomized clinical trial. 2016;19(8):538–543. [PubMed] [Google Scholar]
- 21.Montaño Loza A., Rodríguez Lomelí X., García Correa J. E., et al. Effect of the administration of rectal indomethacin on amylase serum levels after endoscopic retrograde cholangiopancreatography, and its impact on the development of secondary pancreatitis episodes. 2007;99(6):330–336. doi: 10.4321/s1130-01082007000600005. [DOI] [PubMed] [Google Scholar]
- 22.Gu W. J., Liu J. C. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a complementary meta-analysis. 2013;77(4):672–673. doi: 10.1016/j.gie.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Isaji S., Takada T., Mayumi T., et al. Revised Japanese guidelines for the management of acute pancreatitis 2015: revised concepts and updated points. 2015;22(6):433–445. doi: 10.1002/jhbp.260. [DOI] [PubMed] [Google Scholar]
- 24.Freeman M. L., Kozarek R. A. Take 2 indomethacin (suppositories) and call me in the morning? The role of nonsteroidal anti-inflammatory drugs in protection against post-endoscopic retrograde cholangiopancreatography pancreatitis. 2016;150(4):805–808. doi: 10.1053/j.gastro.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 25.Akshintala V. S., Singh V. K., Reddy D. N. Rectal indomethacin for post-ERCP pancreatitis prophylaxis in average risk patients: too early to terminate and too early to conclude. 2016;151(3):567–568. doi: 10.1053/j.gastro.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad D., Lopez K. T., Esmadi M. A., et al. The effect of indomethacin in the prevention of post–endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis. 2014;43(3):338–342. doi: 10.1097/MPA.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 27.Shi N., Deng L., Altaf K., Huang W., Xue P., Xia Q. Rectal indomethacin for the prevention of post-ERCP pancreatitis: a meta-analysis of randomized controlled trials. 2015;26(3):236–240. doi: 10.5152/tjg.2015.6000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data: major and minor criteria for patients with high risk of post-ERCP pancreatitis. Supplementary Figure S1: quality assessments for included randomized control trials in the meta-analysis. Supplementary Figure S2: overall quality assessments for included randomized control trials in the meta-analysis. Supplementary Figure S3: cumulative meta-analysis of indomethacin for post-ERCP pancreatitis prevention by publication year. Supplementary Figure S4: cumulative meta-analysis of indomethacin for post-ERCP pancreatitis prevention by the number of patients. Supplementary Figure S5: funnel plot of all studies included in the present meta-analysis for the assessment of possible publication bias.