Abstract
Background:
Natalizumab is efficacious in the treatment of relapsing-remitting multiple sclerosis. All patients receive the same treatment regimen of 300 mg every 4 weeks, despite differences in pharmacokinetics between individual patients.
Objective:
To give neurologists insight into natalizumab concentrations at time of re-dosing, we investigated longitudinal natalizumab concentrations in 80 patients in relation to disease activity, with possible influencing factors.
Methods:
In a prospective observational cohort study, natalizumab trough serum concentrations were measured in 80 patients. Data on demographics, duration of treatment, Expanded Disability Status Scale, clinical exacerbations, brain magnetic resonance imaging (MRI), and body weight were collected.
Results:
We measured high (≥10 µg/mL) natalizumab trough concentrations in 94% of patients. Intra-individual concentrations were stable. The spread in concentrations was substantial and did not correlate with disease activity. We found a negative association between natalizumab concentration and body weight (β = −0.30, p = 0.010).
Interpretation:
The majority of patients showed high natalizumab serum concentrations at time of re-dosing. Alternative treatment regimens could lead to more efficient use of natalizumab, but caution is warranted regarding the possibility of recurrence of disease activity. Prospective clinical trials are needed to establish the safety of extended dose intervals in natalizumab treatment.
Keywords: Multiple sclerosis, natalizumab, concentration
Introduction
Natalizumab (NTZ), targeting the α-4 integrin receptor, is an efficacious treatment for relapsing-remitting multiple sclerosis (RRMS).1 In a phase-I trial, NTZ stayed detectable in the serum for 3−8 weeks after infusion with dosing of 1−3 mg/kg.2 Based on the different therapeutic dosages of 3−6 mg/kg in phase-II trials, a fixed dose of 300 mg once in 4 weeks was chosen for phase-III trials so the majority of patients (with weights ranging between 50 and 100 kg) would fall between a dose of 3 and 6 mg/kg.3 Nowadays, a dose of 300 mg every 4 weeks has been approved by the European Medicines Agency (EMA)/Food and Drug Administration (FDA) for the treatment of RRMS. In this treatment regimen, NTZ concentrations may stay detectable in serum up to 200 days after cessation of therapy.4
Serum NTZ concentration corresponds with the percentage of α-4 integrin receptor saturation.5 Desaturation of the α-4 integrin receptor occurs when the serum NTZ concentration falls under 1−2 µg/mL.5 Above this threshold of 2 µg/mL, NTZ receptor saturation will roughly fall between 70% and 100%.5 An adequate receptor saturation is estimated as et al⩾70%−80% saturation, although prospective data confirming this assumption are lacking.6,7 Based on a model with results from a large phase-II trial, approximately 90% of patients showed NTZ trough concentrations largely exceeding 2.5 µg/mL. Levels exceeding 2.5 µg/mL could indicate that the approved treatment regimen of NTZ for RRMS results in a relative over-treatment, that is, the patient receives more NTZ than necessary for optimal drug efficacy.3,8 Furthermore, it is suggested that higher NTZ receptor saturation could increase the risk of progressive multifocal leukoencephalopathy (PML), the feared complication of NTZ treatment.9 This unconfirmed hypothesis leads to clinicians extending dose intervals in NTZ treatment with the aim of reducing the PML risk by decreasing NTZ exposure.9–11
The aim of our study was to measure NTZ serum trough concentrations and correlate concentrations with disease activity and possible influencing factors.
Methods
Patients
In 2006, we initiated a prospective observational cohort study to monitor different aspects of NTZ treatment at the MS Centre of the VU University Medical Centre in Amsterdam, The Netherlands. All patients (nearly 220) starting NTZ have been included in this observational cohort. Patients in this cohort are annually subjected to a brain magnetic resonance imaging (MRI) and clinical testing including the Expanded Disability Status Scale (EDSS). For this study, we included all patients of the cohort who are currently treated with NTZ. Because NTZ concentration can fluctuate in the first year, mainly because of transient NTZ antibodies, we excluded patients with a NTZ treatment duration of less than 12 months.12 All the patients included in this study received a strict treatment regimen of 300 mg NTZ every 4 weeks.
Measurement of NTZ concentration
Of all participants, blood samples were routinely obtained every 3 months before NTZ infusion. Serum was subsequently stored at −80°C at the biobank of the VU Medical Centre. For this study, we cross-sectionally measured NTZ concentrations of selected samples, using a cross-linking assay using polyclonal rabbit anti-NTZ F(ab)2 fragments for capture and a mouse anti-IgG4 monoclonal antibody for detection. This method, performed at Sanquin Laboratory, has recently been described in more detail.13 The detection limit of the assay is approximately 0.01 µg/mL.13 To investigate the stability of trough concentrations, we tested a second sample, with an interval of 3–7 months. Receptor desaturation can occur with serum concentrations of ⩽2 µg/mL, which is our appointed cut-off point for an inadequate concentration.5 Taking into account the differences of individual pharmacodynamics, we assumed that serum concentrations of 2–10 µg/mL result in adequate receptor saturation. Concentrations exceeding 10 µg/mL do not result in higher receptor saturation and is therefore labeled as the cut-off for high-trough concentration.5
Data collection
Demographic data, number of NTZ infusions, annual relapse rate before NTZ treatment, number of gadolinium enhancing lesions on the baseline scan, EDSS at start of NTZ treatment, John Cunningham virus (JCV) status at time of the measured concentration, clinical exacerbations during NTZ treatment, and body weight were assessed. The body weight was measured within 3 months of the blood sampling date. A clinical exacerbation was defined as new neurological symptoms lasting for more than 24 h and accompanied by new neurological signs found by a neurologist at the examination. All patients were subjected to a yearly MRI scan of the brain, including three-dimensional (3D) fluid-attenuated inversion recovery, axial proton density/T2-weighted sequences, and gadolinium-enhanced T1-weighted sequences. Those patients at higher risk for PML (JCV positive and >12 months on NTZ) were subjected to three monthly scans (without gadolinium except for the annual MRI scan) as is current recommended protocol.14,15 All MRI scans were evaluated by an experienced neuro-radiologist. The 2013 criteria of Lublin et al.16 were used when referred to “active MS.” According to these criteria, when referring to active MS, the patient experiences clinical relapses and/or occurrence of contrast-enhancing T1 or new/enlarging T2 lesions on brain MRI. In this study, we assessed MS activity starting 12 months after the start of NTZ treatment. If the patient experienced any clinical and/or radiological disease activity in the follow-up period, they were classified as “active MS.”
The local institutional review board approved the observational study and written informed consent was obtained from all participants.
Statistics
Continuous variables are expressed as mean and standard deviation if normally distributed or as median and interquartile range if not normally distributed. NTZ concentrations were normally distributed. For associating NTZ concentrations with different variables, we used the mean of the intra-individual longitudinal NTZ concentrations, except for the association with the number of NTZ infusions, in which we used the first measured NTZ concentration. For calculating the influence of different variables (body weight, NTZ infusions, age, and gender) on NTZ concentration, we used a linear regression model. For calculating the influence of NTZ concentration on disease activity, we used a logistic regression model. Additional adjustments were made for confounding factors such as body weight, NTZ infusions, age, and gender.
All reported p-values are based on statistic tests, with a significance level set at <0.05. The statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Approximately 220 patients have started NTZ treatment at the VU Medical Centre. At time of the start of this study, 101 patients were treated with NTZ. The main reason for discontinuing NTZ was the risk of PML, and other reasons were pregnancy-related reasons, allergic reactions, and progression to the secondary progressive phase. Of the 101 currently treated patients, 21 were excluded because of the treatment duration of less than 12 months. In the remaining 80 patients, 155 blood samples were tested for NTZ trough concentrations. Age of the patients ranged from 20 to 60 years. Patient characteristics are described in Table 1.
Table 1.
Demographic characteristics.
| Total, n = 80 | |
|---|---|
| Age, mean (SD) | 40.6 (10.1) |
| Gender, female, n (%) | 55 (68.6) |
| Number of NTZ infusions, mean (SD)a | 64.7 (32.2) |
| JCV positive, n (%) | 29 (36.3) |
| EDSS at baseline NTZ, median (IQR) | 3.3 (2.5) |
| ARR before NTZ start, mean (SD) | 1.4 (0.9) |
| Number of gadolinium enhancing lesions at baseline, median (IQR) | 1.5 (4) |
SD: standard deviation; NTZ: natalizumab; EDSS: Expanded Disability Status Scale; IQR: interquartile range; ARR: annual relapse rate; JCV: John Cunningham virus.
Number of NTZ infusions at the time of the first measured concentration.
NTZ serum trough concentrations ranged from 0.1 to 80.0 µg/mL with a mean of 26.1 ± 14.1 µg/mL. In all, 1 patient (1.3%) had an inadequate concentration (<2 µg/mL), 4 patients (5%) had an adequate concentration (2–10 µg/mL), and 75 patients (93.8%) had a high concentration (et al⩾10 µg/mL) at the time of re-dosing. The patient showing the lowest concentration (0.1 µg/mL at two measurements) appeared to have persistent high (>9000 AE/mL) NTZ antibodies.
Of 75 patients (93.8%), we measured a follow-up trough concentration. The mean concentration of all the cross-sectional samples did not differ (both 26.1 µg/mL) at the two different time points, that is, at group level, no rise or fall in concentration was observed. Longitudinal concentrations per patient fluctuated with a median difference of 3.0 µg/mL. In nine patients, the two samples differed more than 10 µg/mL; all these patients showed very high concentrations above 30 µg/mL.
The body weight of the patients ranged from 49.1 to 109.0 kg with a mean weight of 75.1 ± 13.9 kg. An inverse association was found between body weight and NTZ concentration (see Figure 1, β = −0.30, 95% confidence interval (CI) = −0.52 to −0.07; p = 0.010; r2 = 0.084). We corrected body weight for potential confounders, but none of these variables appeared to be relevant. Patients weighing up to 75 kg showed a mean concentration of 29.2 ± 15.6 µg/mL, whereas the mean concentration of patients weighing 75 kg or more was 22.7 ± 11.9 µg/mL (β = −6.6, 95% CI = −12.8 to −0.34; p = 0.039).
Figure 1.
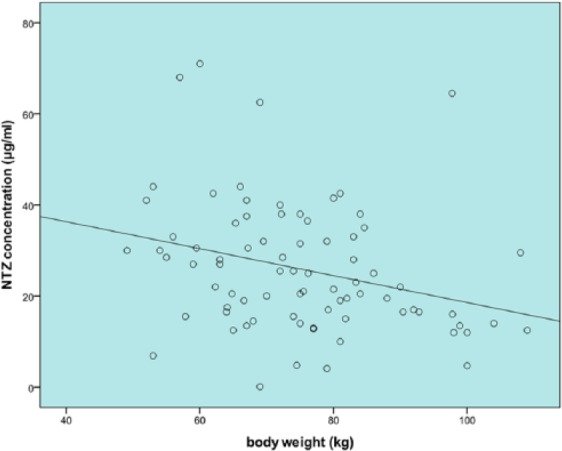
Body weight and NTZ trough concentration plot. An inverse association is found (β = −0.30, 95% CI = −0.52 to −0.07; p = 0.010; r2 = 0.084).
There was no association found between the number of NTZ infusions and trough NTZ serum concentrations, with a substantial spread in concentrations regardless the duration of treatment (see Figure 2, β = 0.022, 95% CI = −0.092 to 0.113; p = 0.84).
Figure 2.

Number of NTZ infusions (duration of treatment) and NTZ trough concentration plot. NTZ concentration of the first measured sample is displayed with associated number of NTZ infusions. No association is found (β = 0.022, 95% CI = −0.092 to 0.113; p = 0.84). We do not see a rise in concentrations in long-term (5−10 years) NTZ users.
The NTZ trough concentration was comparable between males and females, with a mean concentration of 25.0 ± 13.4 µg/mL and 26.9 ± 14.1 µg/mL, respectively (β = −1.46, 95% CI = −8.27 to 5.35; p = 0.67). The concentration was not significantly associated with age (β = −0.011, 95% CI = −0.37 to 0.30; p = 0.94).
Mean duration of NTZ treatment was 5.0 ± 2.5 years. A total of 15 patients (17.7%) had active disease under NTZ treatment (11 patients with new T2 lesions, 5 patients with a clinical exacerbation). Patients who had active disease under treatment received a median treatment duration of 5.1 years, and patients with non-active disease received a median treatment duration of 4.9 years. Mean concentrations were similar between patients with active and non-active disease with a mean concentration of 26.4 and 24.7 µg/mL, respectively (see Figure 3). When adjusting for body weight, concentrations were not statistically different for the active disease group versus the non-active disease group (odds ratio (OR) = 0.98, 95% CI = 0.94 to 1.03; p = 0.41).
Figure 3.
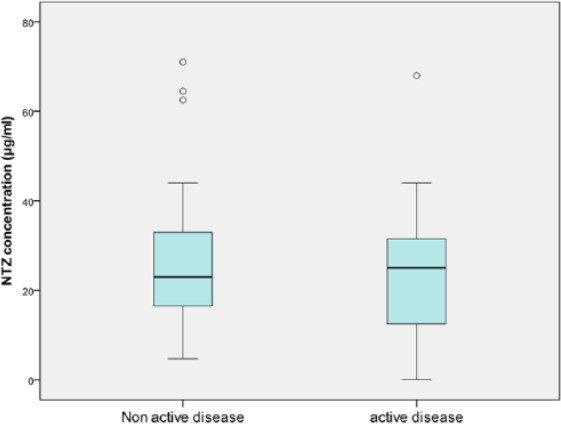
Active disease (n = 15) versus non-active disease (n = 65) (according to the 2013 Lublin criteria) and NTZ trough concentrations (OR = 0.98; 95% CI = 0.94 to 1.03; p = 0.41).
Discussion
NTZ is proven to be efficacious in the treatment of RRMS in a dosing schedule of 300 mg every 4 weeks. Despite large variations in patient pharmacokinetics, all NTZ-treated patients receive the same treatment regimen, where a personalized approach to the treatment schedule might be more appropriate.1 Some neurologists are exploring extended dose intervals in order to reduce the risk of PML, although it is not confirmed that higher NTZ concentrations increase the risk of PML.9,11 Obviously, modified treatment schedules should not interfere with drug efficacy. Our study addresses two important questions: (1) What is the proportion of high NTZ trough concentration in long-term-treated MS patients? and (2) Can we explain individual differences of NTZ concentrations?
In our study of 80 patients, 99% of patients showed adequate- to high-trough NTZ concentrations (et al⩾2 µg/mL), with 94% having high (et al⩾10 µg/mL) NTZ concentrations. The mean trough NTZ serum concentration in our cohort was above 20 µg/mL which is in agreement with recently presented data.17 The mean concentration was not lower in patients with active versus non-active disease, which suggests that high concentrations do not result in an increase in treatment efficacy in comparison with lower but still adequate concentrations. Considering this and the large proportion of high NTZ concentrations, NTZ could perhaps be administered less frequently (or with a lower dose) to reach NTZ concentrations that are lower but still cause adequate receptor saturation and consequently, optimal drug efficacy.5 Caution is advised though, because of a large spread in concentrations and the well-established rebound effect which occurs after cessation of NTZ treatment.18
In the RESTORE trial, 19% of patients (n = 23) stopping NTZ, of whom the majority switched to another therapy, experienced a relapse within 28 weeks.19 The rebound effect showed an increase over time, although 8% of relapses occurred within 4−8 weeks of NTZ withdrawal. A large retrospective study, however, showed no increase in disease activity with extending intervals up to 8 weeks and 5 days.9 In our study, 6.25% of patients showed either inadequate or adequate NTZ concentrations at time of re-dosing. For this group, extending intervals might result in rapidly falling concentrations under the therapeutic level and consequently an early rebound effect.
The large spread in NTZ trough concentrations could be explained by the variation in patient pharmacokinetics and characteristics. We associated age, sex, body weight, duration of treatment, and disease activity with NTZ concentrations. The only factor associated with NTZ concentration was body weight. This is in agreement with earlier reports, where some studies suggested a dose modification based on patients’ body weight.20,21 Our results indeed confirmed an inverse association but this correlation was weak, only accounting for less than 10% of the variability in NTZ concentration. Therefore, body weight is an unreliable predictor for NTZ concentration.
NTZ can be found in serum up to 6 months after cessation of therapy.4 It has been suggested that NTZ concentrations increase over time in individual patients.20 In our study, in 75 patients with a second measurement of NTZ trough concentration, the intra-individual concentrations were stable. Although we do not present long-term longitudinal follow-up trough NTZ serum concentrations, we did not find a correlation between duration of treatment and NTZ concentration. These data highlight that we should not expect very high concentrations in long-term NTZ-treated patients (> 5 years).
A limitation of this study is that measurements of saturation of the α-4 integrin receptor are lacking. Previous studies show that NTZ concentration is correlated with the α-4 integrin receptor saturation; however, above a certain concentration threshold, the receptor will be fully saturated (75%−100%). Based on available literature, we estimated this threshold to be 10 µg/mL NTZ serum concentration, but this cut-off point needs to be confirmed in larger trials. Furthermore, if measuring NTZ levels, it is of importance to realize that the drug is a wild-type IgG4 antibody that becomes monovalent in vivo via “Fab arm exchange.” This will affect concentration measurements to various degrees.13,22 Comparative studies between assays will be necessary to eliminate potential discrepancies between studies.
In conclusion, the large majority (94%) of NTZ patients have high NTZ trough concentrations which could be an indication that most patients receive a “relative over-treatment.” Extended dose intervals could help reduce costs of medication and increase quality of life for the patient with fewer hospital visits, but further studies are needed to establish the safety of alternative treatment regimens. We are now conducting a prospective clinical trial with concentration-based extended dose intervals in completely stable NTZ-treated patients, to assess whether concentration-based extended treatment regimens do not result in recurrence of disease activity. Results of such trials will hopefully give a decisive answer to the question whether extending dose intervals in NTZ treatment is feasible without losing drug efficacy.
Acknowledgments
The authors wish to thank all patients included in the study for agreeing to the use of their data for research and education purposes. They thank Ms A. Kalei for her help in the logistics regarding the measurement of sample concentrations, and they also thank Ms L. Balk for her advice regarding the statistical analyses.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Z.L.E.K., C.E.L., B.I.W., and A.V. have nothing to report; M.P.W. has received speaking fees from Janssen-Cilag, Gayer-Schering, and Biogen Idec; T.R. has received speaking fees from Pfizer and AbbVie; J.K. has received speaker and consulting fees from Merck Serono, Biogen Idec, TEVA, Genzyme, Roche, and Novartis. VUmc MS Centre Amsterdam has received financial support for research activities from Bayer Schering Pharma, Biogen Idec, Roche, GlaxoSmithKline, Merck Serono, Novartis, and TEVA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was support by the Brain Foundation Netherlands (HA2015.01.05).
Contributor Information
Zoé LE van Kempen, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Cyra E Leurs, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Birgit I Witte, Department of Epidemiology and Biostatistics, VU University Medical Center, Amsterdam, The Netherlands.
Annick de Vries, Biologicals Lab, Sanquin Diagnostic Services, Sanquin, Amsterdam, The Netherlands.
Mike P Wattjes, Department of Radiology & Nuclear Medicine, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Theo Rispens, Department of Immunology, Sanquin Research and Landsteiner Laboratory, Amsterdam, The Netherlands.
Joep Killestein, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
References
- 1. Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 2. Sheremata WA, Vollmer TL, Stone LA, et al. A safety and pharmacokinetic study of intravenous natalizumab in patients with MS. Neurology 1999; 52: 1072–1074. [DOI] [PubMed] [Google Scholar]
- 3. Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. New Engl J Med 2003; 348: 15–23. [DOI] [PubMed] [Google Scholar]
- 4. Rispens T, Vennegoor A, Wolbink GJ, et al. Natalizumab remains detectable in patients with multiple sclerosis long after treatment is stopped. Mult Scler 2012; 18: 899–901. [DOI] [PubMed] [Google Scholar]
- 5. Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009; 72: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Connor P. Natalizumab and the role of alpha 4-integrin antagonism in the treatment of multiple sclerosis. Expert Opin Biol Ther 2007; 7: 123–136. [DOI] [PubMed] [Google Scholar]
- 7. European Medicines Agency. Tysabri: EPAR: Scientific discussion. London: European Medicines Agency, 2007. [Google Scholar]
- 8. US Food and Drug Administration (US FDA). Natalizumab: Clinical and pharmacology and biopharmaceutical reviews. Silver Spring, MD: US FDA, 2004. [Google Scholar]
- 9. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 885–889. [DOI] [PubMed] [Google Scholar]
- 10. Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: A two-center, 7-year experience. Ther Adv Neurol Disord 2014; 7: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Kempen ZL, Leurs CE, Vennegoor A, et al. Natalizumab-associated progressive multifocal leukoencephalopathy is not preceded by elevated drug concentrations. Mult Scler 2017; 23: 772–789. [DOI] [PubMed] [Google Scholar]
- 12. Vennegoor A, Rispens T, Strijbis EM, et al. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler 2013; 19: 593–600. [DOI] [PubMed] [Google Scholar]
- 13. Rispens T, Leeuwen A, Vennegoor A, et al. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem 2011; 411: 271–276. [DOI] [PubMed] [Google Scholar]
- 14. Wattjes MP, Rovira A, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis–establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015; 11: 597–606. [DOI] [PubMed] [Google Scholar]
- 15. McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: Recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016; 87: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foley JF, Hoyt T, Christensen A, et al. Natalizumab extended interval dosing reduces serum trough natalizumab concentrations and a4 integrin receptor occupancy. Poster Sessions, ECTRIMS 2016. [Google Scholar]
- 18. Killestein J, Vennegoor A, Strijbis EM, et al. Natalizumab drug holiday in multiple sclerosis: Poorly tolerated. Ann Neurol 2010; 68: 392–395. [DOI] [PubMed] [Google Scholar]
- 19. Fox RJ, Cree BA, De Seze J, et al. MS disease activity in RESTORE: A randomized 24-week natalizumab treatment interruption study. Neurology 2014; 82: 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foley JF, Metzger R, Hoyt T, et al. The effect of dosing interval extension and patient weight on long term natalizumab pharmacokinetics. Neurology 2015; 84(Suppl 14): 3.258. [Google Scholar]
- 21. Tanaka M, Kinoshita M, Foley JF, et al. Body weight-based natalizumab treatment in adult patients with multiple sclerosis. J Neurol 2015; 262: 781–782. [DOI] [PubMed] [Google Scholar]
- 22. Labrijn AF, Buijsse AO, van den Bremer ET, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27: 767–771. [DOI] [PubMed] [Google Scholar]


