Abstract
Background:
Inner retinal layer atrophy in patients with multiple sclerosis (MS) has been validated as a structural imaging biomarker for neurodegeneration.
Objective:
To determine how retinal layer thickness relates to high-contrast visual acuity (HCVA), low-contrast visual acuity (LCVA) and vision-related quality of life (QoL) and to investigate the effect of previous episodes on MS-associated optic neuritis (MSON).
Methods:
Spectral-domain optical coherence tomography (SD-OCT) was performed in 267 patients with MS. Images were segmented for the peripapillary retinal nerve fiber layer (pRNFL) and the macular ganglion cell inner plexiform layer (GCIPL). Ophthalmological evaluations included history of MSON, HCVA, LCVA, and vision-related QoL.
Results:
Independent of MSON, HCVA and LCVA were significantly associated with pRNFL and GCIPL thicknesses. Vision-related QoL was positively associated with pRNFL (β = 0.92, p = 0.06) and GCIPL (β = 0.93, p = 0.02) thicknesses. These associations were independent of MSON. Not only binocular but also monocular atrophy of the inner retinal layers was associated with lower vision-related QoL.
Conclusion:
This study showed that retinal atrophy has a significant impact on visual functioning in patients with MS. OCT may therefore provide useful insight to patients with visual dysfunction, and our findings support including OCT and vision-related QoL measures into optic neuritis treatment trials.
Keywords: Multiple sclerosis, optical coherence tomography, retina, neurodegeneration, visual acuity, quality of life
Introduction
Atrophy of the inner retinal layers, as observed with the use of optical coherence tomography (OCT), is a common observation in multiple sclerosis (MS) patients with a history of MS-associated optic neuritis (MSON). However, even in patients without a history of MSON, substantial thinning of the inner retinal layers is also observed.1 This retinal atrophy is thought to be caused by retrograde trans-synaptic degeneration,2,3 although other mechanisms, such as local microinflammatory processes in the optic nerve,4 have also been suggested. Retinal atrophy has shown to be associated with clinical disability,1,5,6 gray and white matter atrophy,7,8 and cognitive functioning.9 Although spectral domain (SD)-OCT has been suggested as a structural outcome measure for neuroaxonal degeneration,5 it should be noted that the clinical meaningfulness of this outcome is not always self-evident. A large proportion of patients with MS (up to 80%) will experience visual disability at some point during their course of disease, the most common being MSON, decreased high-contrast visual acuity (HCVA) and low-contrast visual acuity (LCVA), and eye movement disorders.10–12 This poor visual functioning has a major impact on quality of life (QoL) as good visual function is highly valued by patients. Importantly, Heesen et al.13 demonstrated that MS patients reported visual functioning as the second most important body function affecting QoL, after lower limb function. Despite this, the visual system is not generally included as outcome measure. Even the commonly used MS functional composite does not include an objective assessment of visual functioning.14
Previous studies have investigated the relationship between peripapillary retinal nerve fiber layer (pRNFL) thickness and visual dysfunction15–17 or general measures of QoL18 in patients with MS. The assessment of general QoL in patients with MS is however strongly influenced by the mobility of the patient. The vision-related QoL measures the specific influence of visual disability and visual symptoms on different QoL domains and is therefore not biased by ambulation or other non-visual symptoms. These vision-specific measures may provide information on the clinical meaningfulness of retinal atrophy. Therefore, the objective of this study was to investigate how inner retinal layer thickness relates to LCVA, HCVA, and vision-related QoL and to determine whether previous MSON affects this relationship.
Methods
Study design and patient population
For this observational cross-sectional study, patients were enrolled from the Amsterdam MS Cohort (MS Centre Amsterdam, VU University Medical Centre, The Netherlands). This study was approved by the Medical Ethical Committee on Human Research of the VU University Medical Center in Amsterdam, The Netherlands. Written informed consent was obtained from all subjects before study inclusion.
All included subjects were diagnosed with clinically definite MS following the revised McDonald criteria19 and were part of an ongoing observational cohort study (the Amsterdam MS Cohort) of which previous assessments have been described.20–22 All subjects were required to be between 18 and 80 years of age and had a diagnosis of a relapsing-remitting multiple sclerosis (RRMS), secondary progressive multiple sclerosis (SPMS), or primary progressive multiple sclerosis (PPMS) disease course at the time of their assessment.23 Patients were excluded if they fulfilled any of the following criteria: pregnancy, received a course of steroids or had a relapse within 6 weeks prior to inclusion, HIV or other immunodeficiency syndrome, or history of substance abuse (drug or alcohol). Patients were also excluded if they had experienced symptomatic MSON within six months preceding the OCT assessment because thickening of the pRNFL during the acute stages of MSON may confound the OCT measurement. All assessments (clinical, OCT, and questionnaires) were performed on the same day.
SD-OCT
SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) was performed in all subjects, with eye-tracking function enabled. All OCT scans were obtained at the same site, by three different experienced technicians. Room light conditions were dimmed and pupil diameter was sufficient for obtaining high-quality OCT images, such that pharmacological pupil dilation was not required in any of the cases. Data on global pRNFL thickness (µm) were obtained using a 12° ring scan, manually placed around the optic disc. Data on the mean ganglion cell inner plexiform layer (GCIPL) thickness (µm) in the macular area were acquired using a macular volume scan (20 × 20° field, 49 B-scans, vertical alignment) centered on the fovea, averaging thickness for all but the central sector of the 1-, 2.22-, and 3.4-mm grid. Automated segmentation of the pRNFL and GCIPL was performed (Heidelberg Engineering, software version 1.9.10.0). Scans were excluded from the analyses if they did not fulfill the revised quality control criteria (OSCAR-IB).24
Clinical and ophthalmological outcome measures
Disease duration was defined as the time from the first MS symptom. The Expanded Disability Status Scale (EDSS)25 was obtained by a certified examiner.
The assessment of history of symptomatic MSON was based on medical history, according to a standard protocol.26 Visual acuity (VA) was tested using Sloan letter charts (100% for HCVA, 2.5% for LCVA),27 placed on a retro-illuminated cabinet, at a 2-m distance. Each eye was tested individually on both contrast levels, with best possible correction for refractive errors. VA scores were quantified as the number of letters correctly read by the patient.
Vision-related QoL was assessed using the National Eye Institute Visual Function questionnaire (NEI-VFQ-25), which is a validated tool to assess self-reported visual disability and vision-targeted health status.28 The NEI-VFQ-25 is widely used in ophthalmological research and has shown to be sensitive and useful in patients with MS.29 The NEI-VFQ-25 consists of 25 vision-targeted questions representing 11 vision-related constructs (global vision rating, difficulty with near-vision activities, difficulty with distance vision activities, limitations in social functioning due to vision, role limitations due to vision, dependency on others due to vision, mental health symptoms due to vision, driving difficulties, limitations with peripheral vision, limitations with color vision, and ocular pain). Next, all 11 subscores are converted to a 0–100 scale, and overall composite score is calculated by averaging the weighted subscale scores. This overall score is therefore also on a scale from 0 (lowest possible score) to 100 (highest possible score).30
Statistical analyses
Linear regression analyses were used to analyze group differences and associations with clinical outcome measures assessed on patient level. When data on VA were analyzed on patient level, the mean value of both eyes was used, in accordance with the advised protocol for OCT study terminology and elements (APOSTEL) guidelines.31 Vision-related QoL was compared between groups with the non-parametric Mann–Whitney U-test. Generalized estimating equations (GEE), with an exchangeable correlation matrix and adjustments for intra-subject inter-eye dependency, were used for analyses and comparisons when the outcome was assessed on eye level (retinal thickness, VA). All linear regression and GEE analyses were additionally adjusted for relevant confounders (age, sex, disease duration, history of MSON, use of disease-modifying treatment (DMT), VA) as indicated.
In order to investigate the effect of binocular, monocular, and no retinal atrophy on vision-related QoL, patients were divided into three groups based on the level of atrophy in each eye (group 1: patients with binocular atrophy, group 2: patients with monocular atrophy, and group 3: patients with no retinal atrophy in either eye). The presence of retinal atrophy was defined as a pRNFL thickness ⩽75 µm, which was based on the findings of Costello et al.,32 who demonstrated a threshold of pRNFL thickness of 75 µm as the “point of no return” for predicting visual recovery after optic neuritis. There is no published cut-off for the GCIPL. For this reason, we decided to use the same percentile corresponding to a pRNFL of less than 75 µm, which corresponded to the 25th percentile, for the GCIPL. The 25th percentile for the GCIPL thickness resulted in a cut-off of ⩽68 µm. Patients with missing data for at least one eye were excluded from these subanalyses. Groups were compared using linear regression analyses with dummy variables. Statistical analyses were performed using SPSS V.22.0, with a two-sided statistical significance level of 0.05.
Results
Descriptives
A total of 267 patients with MS were included in this cross-sectional observational study. Demographic and clinical characteristics of all included MS patients and stratified by history of MSON are shown in Table 1. In order to avoid the introduction of noise by pooling MSON eyes and no history of MS-associated optic neuritis (MSNON) eyes within the same patient, only patients with the same history of both eyes (i.e. bilateral MSON and bilateral MSNON) were included in the right part of Table 1. Patients had a mean disease duration of 19.1 years (±7.4), but this differed between disease types, as SPMS and PPMS patients (22.6 ± 8.5 and 23.1 ± 7.7 years, respectively) had a considerable longer disease duration compared to the RRMS patients (17.7 ± 6.5). The majority of patients (68.9%) had a relapsing-remitting (RR) disease course. More than half of all patients (N = 156, 58.4%), of which 27 were PPMS, had never experienced a clinically identified episode of MSON. Of all patients with a history of MSON (N = 97, 36.4%), 64 patients had a unilateral MSON and 33 a bilateral MSON. For 5% of patients, the MSON history was ambiguous. Nearly half of the patients never received DMT (45.7%). Among the patients who were treated at the time of their assessment, the majority used β-interferon or glatiramer acetate (73%).
Table 1.
Demographic and clinical characteristics of all included MS patients and stratified by history of MSON.
| All subjects |
Subjects with MSNON |
Subjects with bilateral MSON |
|
|---|---|---|---|
| N = 267 | N = 156 | N = 33 | |
| Gender (N, female, %) | 184 (69%) | 100 (64.1%) | 25 (75.8%) |
| Age (years) | 52.3 (±10.5) | 53.1 (±10.7) | 52.1 (±9.9) |
| Disease duration (years) | 19.1 (±7.4) (range: 8.7–48.0) | 18.5 (±7.2) | 23.5 (±7.2) |
| EDSS (median (range)) | 3.5 (0–8.5) | 3.5 (0–8.5) | 4.0 (1.0–8.0) |
| Disease type | |||
| RRMS | 184 | 99 | 24 |
| SPMS | 53 | 31 | 9 |
| PPMS | 27 | 24 | 0 |
| Unclassifiable | 3 | 2 | 0 |
| Disease-modifying treatment | |||
| Current | 90 (33.7%) | 43 (27.6%) | 11 (33.3%) |
| β-interferon/glatiramer acetate | 66 | 32 | 9 |
| Natalizumab | 9 | 4 | 2 |
| Othera | 15 | 7 | 0 |
| Past | 55 (20.6%) | 27 (17.3%) | 11 (33.3%) |
| Never | 122 (45.7%) | 86 (55.1%) | 11 (33.3%) |
| HCVA (mean ODS) | 52.6 (±8.8) | 53.4 (±8.0) | 50.5 (±9.5) |
| LCVA (mean ODS) | 27.2 (±11.5) | 26.9 (±10.5) | 24.0 (±13.9) |
MSNON: no history of MS-associated optic neuritis; MSON: MS-related optic neuritis; RR: relapsing-remitting; SP: secondary progressive; PP: primary progressive; HCVA: high-contrast visual acuity; LCVA: low-contrast visual acuity; ODS: right (OD) and left (OS) eye combined.
Fingolimod, dimethylfumarate, and teriflunomide.
Furthermore, mean visual function (both HCVA and LCVA) was decreased in patients with bilateral MSON (see Table 1). Patients with no MSON history showed a difference of 2.8 letters for HCVA and 2.9 letters for LCVA compared with patients with bilateral MSON (p = 0.108 and p = 0.322, respectively).
Retinal thickness and visual functioning in MS, with and without MSON
All OCT scans were checked for quality control criteria by two independent raters (L.J.B. and D.C.), which led to a rejection rate of 14% (149/1068 scans).
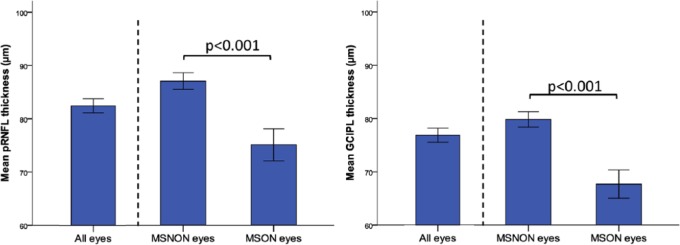
Figure 1 shows the pRNFL and GCIPL thickness for all included eyes and also stratified by history of MSON. When all eyes of MS patients were included (N = 485 eyes), the pRNFL showed a mean thickness of 84.0 µm (±15.1) and the GCIPL a thickness of 76.9 µm (±14.8). Previous episodes of MSON had a significant effect on both retinal layers, with significant thinning in eyes with a history of MSON (pRNFL: 75.1 µm (±15.6) vs 87.1 (±13.7), GCIPL: 67.7 (±14.6) vs 79.9 (±13.7), p < 0.001 for both comparisons; see Figure 1). After adjustments for disease duration, these differences between MSON and MSNON remained significant (pRNFL difference 9.6 µm, p < 0.001, GCIPL difference 10.9 µm, p < 0.001).
Figure 1.
Retinal layer thickness (with 95% CIs) in all (N = 485), MSNON (N = 343), and MSON (N = 119) eyes. Note that history of MSON was ambiguous in 23 eyes which were excluded from further analyses.
Table 2 shows the associations between retinal layer thickness and VA. Independent of MSON, VA was significantly associated with pRNFL and GCIPL thickness. Although both LCVA and HCVA showed significant associations, stronger associations were observed for LCVA. Every 10 µm reduction in pRNFL thickness corresponded with a reduction in HCVA score of 2.0 letters (p < 0.001) and 2.9 letters for LCVA (p < 0.001). Likewise, for the GCIPL, a 10-µm reduction corresponded to a lower HCVA score of 3.1 letters (p < 0.001) and 4.7 letters for LCVA (p < 0.001; for all GEE models accounting for age, history of MSON, use of DMT, and inter-eye dependency, see Table 2).
Table 2.
Association between retinal layer thickness and visual acuity.
| HCVA | LCVA | |
|---|---|---|
| pRNFL global (per 10 µm) | 2.0 (1.0–3.0, p < 0.001)a | 2.9 (1.3–4.5, p < 0.001)a |
| GCIPL (per 10 µm) | 3.1 (2.1–4.2, p < 0.001)a | 4.7 (3.4–6.1, p < 0.001)a |
CI: confidence interval; GEE: generalized estimating equations; pRNFL: peripapillary retinal nerve fiber layer; GCIPL: ganglion cell inner plexiform layer; HCVA: high-contrast visual acuity; LCVA: low-contrast visual acuity; MSON: MS-related optic neuritis; DMT: disease-modifying treatment.
Data reported as β (95% CI, p value).
GEE, adjusted for age, history of MSON, use of DMT, and inter-eye dependency.
Retinal thickness and vision-related QoL in MS
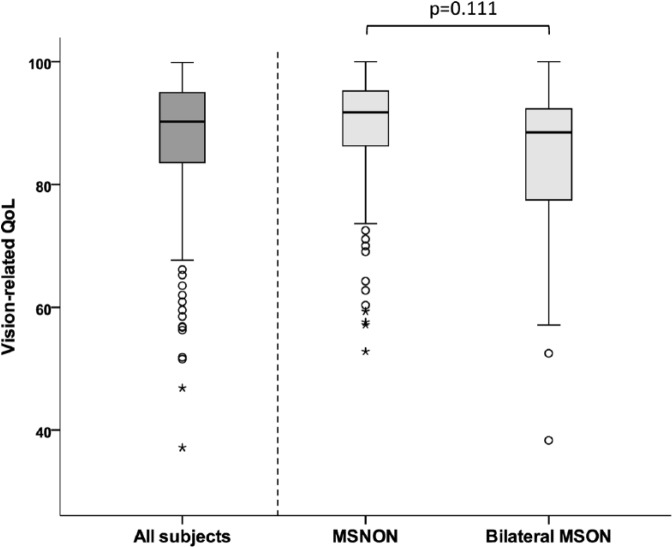
The MS patients reported a median overall vision-related QoL score of 90.4 (interquartile range (IQR): 11.9). When the effect of MSON on overall vision-related QoL was investigated, it was shown that bilateral MSON patients had a lower vision-related QoL (median: 88.5, IQR: 15.3) compared to MSNON patients (median: 91.7 (IQR: 9.3), p = 0.111; see Figure 2).
Figure 2.
Box-and-whisker plot showing that the median vision-related QoL is lower in patients with bilateral MSON (median: 88.5), compared to MSNON (median: 91.7) patients (p = 0.111).
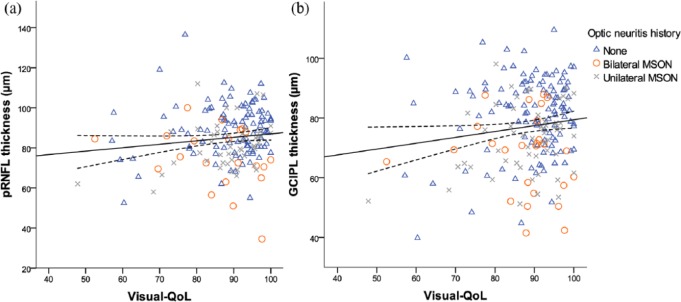
The overall vision-related QoL score was positively associated with pRNFL and GCIPL thickness, showing higher vision-related QoL scores in patients with less retinal atrophy (Figure 3(a) and (b)).
Figure 3.
Scatter plot and fitted linear regression line (with 95% confidence curves) demonstrating the association between (a) pRNFL and (b) GCIPL thickness and the overall visual quality of life score (NEI-VFQ-25). Data are shown for patients without MSON (blue triangle), bilateral MSON (red circle), and unilateral MSON (gray cross).
When this association was adjusted for age, sex, use of DMT, and history of MSON, the effect slightly decreased, but remained significant for GCIPL thickness (see Table 3). The goodness of fit (based on scale parameter) of the adjusted models was 10% for pRNFL and 9% for GCIPL.
Table 3.
GEE analyses demonstrating the association between vision-related QoL (per 5 points) and pRNFL and GCIPL thickness.
| pRNFL β (95% CI) | p value | GCIPL β (95% CI) | p value | |
|---|---|---|---|---|
| Vision-related QoL (unadjusted) | 1.03 (0.01 to 2.01) | 0.038 | 1.12 (0.26 to 1.99) | 0.011 |
| Vision-related QoL (adjusted for age, sex, and use of DMT) | 0.96 (0.01 to 1.91) | 0.048 | 1.08 (0.28 to 1.87) | 0.008 |
| Vision-related QoL (adjusted for age, sex, use of DMT, and MSON | 0.92 (−0.04 to 1.87) | 0.060 | 0.93 (0.15 to 1.71) | 0.020 |
CI: confidence interval; GEE: generalized estimating equations; pRNFL: peripapillary retinal nerve fiber layer; GCIPL: ganglion cell inner plexiform layer; QoL: quality of life; MSON: MS-associated optic neuritis; DMT: disease-modifying treatment.
Adjustments for age, sex, use of DMT, and history of MSON had minimal effect. Data shown as β (95% CI, p value).
All analyses were corrected for inter-eye dependency.
Additionally, the GEE analyses were performed for the VFQ subscales (Table 4). All analyses were adjusted for age, sex, use of DMT, and MSON. Of the 11 subscales, the largest effects were observed for “distance activities” (pRNFL: β = 0.97, p = 0.004 and GCIPL: β = 0.87, p = 0.008), “social functioning” (pRNFL: β = 1.39, p = 0.002 and GCIPL: β = 1.31, p = 0.005), and colour vision (pRNFL: β = 1.05, p = 0.043).
Table 4.
GEE analyses demonstrating the association (β (95% CI)) between the overall vision-related QoL and all 11 subscales (per 5 points) and pRNFL and GCIPL thickness.
| pRNFLa | p value | GCIPLa | p value | |
|---|---|---|---|---|
| Overall vision-related QoL | 0.92 (−0.04 to 1.87) | 0.060 | 0.93 (0.15 to 1.71) | 0.020 |
| Subscales | ||||
| General vision | 0.37 (−0.19 to 0.94) | 0.196 | 0.47 (−0.07 to 1.00) | 0.088 |
| Ocular pain | −0.35 (−1.03 to 0.34) | 0.326 | −0.18 (−0.63 to 0.28) | 0.445 |
| Near activities | 0.76 (0.11 to 1.41) | 0.022 | 0.79 (0.18 to 1.39) | 0.011 |
| Distance activities | 0.97 (0.31 to 1.63) | 0.004 | 0.87 (0.23 to 1.52) | 0.008 |
| Social functioning | 1.39 (0.54 to 2.33) | 0.002 | 1.31 (0.40 to 2.21) | 0.005 |
| Mental health | 0.33 (−0.16 to 0.81) | 0.186 | 0.21 (−0.24 to 0.65) | 0.361 |
| Role difficulties | 0.19 (−0.30 to 0.67) | 0.453 | 0.15 (−0.27 to 0.55) | 0.494 |
| Dependency | 0.33 (−0.13 to 0.78) | 0.156 | 0.22 (−0.08 to 0.52) | 0.141 |
| Driving | 0.29 (−0.52 to 1.10) | 0.484 | 0.37 (−0.29 to 1.04) | 0.271 |
| Colour vision | 1.05 (0.03 to 2.06) | 0.043 | 0.93 (−0.12 to 1.98) | 0.082 |
| Peripheral vision | 0.43 (−0.09 to 0.94) | 0.101 | 0.52 (0.03 to 1.00) | 0.038 |
CI: confidence interval; GEE: generalized estimating equations; pRNFL: peripapillary retinal nerve fiber layer; GCIPL: ganglion cell inner plexiform layer; QoL: quality of life; MSON: MS-related optic neuritis; DMT: disease-modifying treatment.
All analyses were adjusted for age, sex, use of DMT, and MSON.
Monocular versus binocular atrophy
In order to investigate the effect of monocular or binocular retinal thinning on vision-related QoL, patients were divided into three groups, based on the level of retinal atrophy (group 1: patients with binocular atrophy, group 2: patients with monocular atrophy, and group 3: patients with no retinal atrophy in either eye).
Patients with binocular atrophy of the pRNFL showed the lowest vision-related QoL score (82.7 ± 13.3) followed by patients with monocular pRNFL atrophy (85.1 ± 13.3) and patients with no pRNFL atrophy (90.0 ± 8.2). The difference in vision-related QoL between patients with monocular and binocular atrophy (2.4 points) was not statistically significant (p = 0.363; see Table 5).
Table 5.
Vision-related QoL in patients with binocular, monocular, or no inner retinal layer atrophy.
| Binocular atrophy | Monocular atrophy | No retinal atrophy |
p valuea
Binocular vs monocular |
p valuea
Binocular vs no atrophy |
p valuea
Monocular vs no atrophy |
|
|---|---|---|---|---|---|---|
| pRNFLb | ||||||
| Vision-related QoL | 82.7 (13.3) | 85.1 (12.8) | 90.0 (8.2) | 0.363 | 0.001 | 0.013 |
| GCIPLc | ||||||
| Vision-related QoL | 84.0 (11.9) | 87.3 (11.4) | 89.4 (8.5) | 0.152 | 0.005 | 0.232 |
CI: confidence interval; pRNFL: peripapillary retinal nerve fiber layer; GCIPL: ganglion cell inner plexiform layer; QoL: quality of life; MSON: MS-related optic neuritis; DMT: disease-modifying treatment.
Linear regression analyses. Adjustments for MSON did not change the results.
pRNFL atrophy cut-off at 75 µm (binocular atrophy N = 30, monocular atrophy N = 35, no atrophy N = 129).
GCIPL atrophy cut-off at 68 µm (binocular atrophy N = 35, monocular atrophy N = 44, no atrophy N = 148).
Regarding the GCIPL, a similar situation was observed as patients with binocular GCIPL atrophy showed the lowest vision-related QoL score (84.0 ± 11.9), followed by patients with monocular atrophy (87.3 ± 11.4) and patients with no retinal atrophy (89.4 ± 8.5, see Table 5).
Discussion
This study showed that retinal atrophy has a significant impact on visual functioning in patients with MS. Both VA and vision-related QoL were decreased in patients with atrophy of the inner retinal layers, independent of previous MSON.
Consistent with current literature, the present data showed significant thinning of the pRNFL and GCIPL in eyes with a history of MSON compared to unaffected eyes (difference of about 12 µm for both layers) as a result of retrograde degeneration.1 Furthermore, the presence of MSON also influenced the VA of the patient, showing lower HCVA and LCVA for patients with bilateral MSON. While the retinal thickness and VA were both affected by history of MSON, the association between the two was similar for MSON and MSNON eyes (no effect modification by MSON). The data suggest that VA reduces with decreasing pRNFL or GCIPL thickness. Every 10 µm reduction in pRNFL thickness corresponded with a reduction in HCVA score of 2.0 letters and 2.9 letters for LCVA. Likewise, for the GCIPL, a 10-µm reduction corresponded to a lower HCVA score of 3.1 letters and 4.7 letters for LCVA. The clinical meaningfulness of these reductions in VA has previously been defined as >5 letters for HCVA, and >7 letters for LCVA at 2.5%.33,34 Assuming a linear relationship, this would correspond to 24 µm (LCVA) or 25 µm (HCVA) for the pRNFL and 15 µm (LCVA) or 16 µm (HCVA) for GCIPL thickness. This suggests that although the observed effect was larger in LCVA, the clinical impact of retinal thinning on HCVA and LCVA seems to be quite similar. Our findings on the association between retinal atrophy and VA are consistent with previous studies although comparing outcomes is difficult due to methodological differences. Nevertheless, the majority of studies showed that thinning of both pRNFL and GCIPL is associated with VA,15–17,34,35 whereas some only found significant associations with LCVA.36
Overall vision-related QoL was positively associated with inner retinal layer thickness. Patients with a higher vision-related QoL score showed less atrophy of both the pRNFL and GCIPL. Adjustment for confounding factors, such as age, sex, use of DMT, and MSON, only resulted in minor changes of the effect. Importantly, the association between QoL and inner retinal layer atrophy is mediated by VA. This was supported by the strong reduction of the effect when LCVA was added to the model (pRNFL: β = 0.07 (95% CI: −1.6 to 1.85, p = 0.941) and GCIPL: β = 0.15 (95% CI: −0.95 to 1.25, p = 0.791)). Our findings build upon a previous study by Walter et al.,17 reporting similar associations between vision-related QoL and GCIPL thickness of 0.9 µm and 1.0 µm on pRNFL thickness per 5 points on the NEI-VFQ-25 scale (using GEE models accounting for age and within-patient inter-eye correlations). Furthermore, a study by Longbrake et al. showed that vision-related QoL correlated with average pRNFL thickness, but only below a critical threshold of 75 µm. Above 75 µm, no relationship between pRNFL thickness and vision-related QoL was observed.37 In contrast, the cohort study (N = 54) by Garcia-Martin et al.18 did not show any relationship between pRNFL (Spectralis) and overall MSQOL-54 score (r = 0.08), but they did report significant correlations with the physical health composite (r = 0.23, p < 0.05) and fatigue (r = 0.30, p < 0.05). It should however be noted that in this study, a non-specific QoL measure was used (MSQOL-54), which may explain the lack of correlation with other items than the physical health composite and fatigue.
Retinal atrophy is undoubtedly assessed on eye level, as both eyes are scanned individually. Although some relevant outcomes are also eye-specific (history of MSON and VA), many relevant research questions include clinical outcome measures assessed on a patient level (EDSS score, cognition, and QoL). Besides the fact that this discrepancy in level of assessment results in methodological challenges, as the suggested approach (GEE with adjustments for inter-eye dependency) is methodologically not correct in such situations, it also raises the question whether inter-eye differences are clinically relevant to a patients visual functioning. In this study, we have investigated the effect of monocular or binocular retinal atrophy on vision-related QoL and demonstrated that having atrophy of the pRNFL in only one eye resulted in a significantly lower vision-related QoL score compared to having two unaffected eyes. When both eyes showed atrophy of the pRNFL, this only further decreased the vision-related QoL score minimally (difference 2.4 points, p = 0.363). Regarding the GCIPL, a more stepwise situation was observed, with binocular GCIPL atrophy showing the lowest vision-related QoL score, followed by patients with monocular atrophy and finally patients with no retinal atrophy. These findings suggest that monocular atrophy of the inner retinal layers already has significant impact on the vision-related QoL of a patient. This phenomenon may be a result of binocular inhibition (when the best eye has better acuity than both eyes together), which is present in patients with MSON,38 but this was not further investigated as it was beyond the scope of this study and no data on binocular vision were available.
One limitation of this study is its cross-sectional design, which does not permit to hint on causality. We are therefore in the process of re-investigating all patients after two more years of follow-up.
In conclusion, this study showed that retinal atrophy has a significant impact on visual functioning in patients with MS. Both VA and vision-related QoL were decreased in patients with atrophy of the macular ganglion cells. Retinal OCT gives useful insight to patients with visual dysfunction, and our findings support including OCT and visual functioning measures into optic neuritis treatment trials.
Acknowledgments
The authors thank the patients and healthy control subjects for participating and for their substantial time commitment. They also thank the staff of the Departments of Ophthalmology and Neurology and the data unit of the MS Centre VUMC for supporting this study. L.J.B. contributed to designing the study concept and performing data collection, revision and QC assessment of OCT scans, automated and manual layer segmentation, and statistical analyses and prepared the first draft of the manuscript. D.C. performed data collection, revision and QC assessment of OCT scans, and revised the manuscript. J.A.N.B., J.K., B.M.J.U., and A.P. contributed to designing the study concept and revised the manuscript. All authors gave final approval of the version submitted.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.K. has accepted speaker and consulting fees from Merck Serono, Biogen Idec, Teva, Genzyme, and Novartis. B.M.J.U. has received personal compensation for consulting from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and TEVA. A.P. is member of the steering committee for the OCTiMS study (Novartis) and received no consulting fees. He is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. He performed OCT QC for the PASSOS study (Novartis) and received consulting fees. L.J.B., D.C., and J.A.N.B. have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The VUMC MS Centre Amsterdam received financial research support for OCT projects from TEVA.
Contributor Information
Lisanne J Balk, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Danko Coric, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Jenny A Nij Bijvank, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands/Department of Ophthalmology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Joep Killestein, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Bernard MJ Uitdehaag, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands.
Axel Petzold, Department of Neurology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands/Department of Ophthalmology, Amsterdam Neuroscience, VUmc MS Center Amsterdam, VU University Medical Center, Amsterdam, The Netherlands/UCL Institute of Neurology, University College London (UCL), London, UK/Moorfields Eye Hospital, London, UK.
References
- 1. Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol 2010; 9: 921–932. [DOI] [PubMed] [Google Scholar]
- 2. Balk LJ, Steenwijk MD, Tewarie P, et al. Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis.J Neurol Neurosurg Psychiatry 2015; 86:419–424. [DOI] [PubMed] [Google Scholar]
- 3. Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol 2014; 75: 98–107. [DOI] [PubMed] [Google Scholar]
- 4. Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013; 80: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balcer LJ, Miller DH, Reingold SC, et al. Vision and vision-related outcome measures in multiple sclerosis. Brain 2015; 138: 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol 2016; 15: 574–584. [DOI] [PubMed] [Google Scholar]
- 7. Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol 2015; 78: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmermann H, Freing A, Kaufhold F, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler 2013; 19: 443–450. [DOI] [PubMed] [Google Scholar]
- 9. Coric D, Balk LJ, Verrijp M, et al. Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler 2018; 24: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonald WI, Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatry 1992; 55: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnes D, McDonald WI. The ocular manifestations of multiple sclerosis. 2. Abnormalities of eye movements. J Neurol Neurosurg Psychiatry 1992; 55: 863–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salter AR, Tyry T, Vollmer T, et al. “Seeing” in NARCOMS: A look at vision-related quality of life in the NARCOMS registry. Mult Scler 2013; 19: 953–960. [DOI] [PubMed] [Google Scholar]
- 13. Heesen C, Bohm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: Gait and visual function are the most valuable. Mult Scler 2008; 14: 988–991. [DOI] [PubMed] [Google Scholar]
- 14. Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999; 122(Pt 5): 871–882. [DOI] [PubMed] [Google Scholar]
- 15. Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler 2011; 17: 1449–1463. [DOI] [PubMed] [Google Scholar]
- 16. Satue M, Rodrigo MJ, Otin S, et al. Relationship between visual dysfunction and retinal changes in patients with multiple sclerosis. PLoS ONE 2016; 11: e0157293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology 2012; 119: 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Martin E, Rodriguez-Mena D, Herrero R, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology 2013; 81: 76–83. [DOI] [PubMed] [Google Scholar]
- 19. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tewarie P, Schoonheim MM, Schouten DI, et al. Functional brain networks: Linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum Brain Mapp 2015; 36: 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 2016; 139: 115–126. [DOI] [PubMed] [Google Scholar]
- 22. Balk LJ, Twisk JW, Steenwijk MD, et al. A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry 2014; 85: 782–789. [DOI] [PubMed] [Google Scholar]
- 23. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 24. Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: Validation of the OSCAR-IB criteria. Mult Scler 2015; 21: 163–170. [DOI] [PubMed] [Google Scholar]
- 25. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 26. Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: A review and proposed protocol. Nat Rev Neurol 2014; 10: 447–458. [DOI] [PubMed] [Google Scholar]
- 27. Balcer LJ, Baier ML, Cohen JA, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology 2003; 61: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 28. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001; 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 29. Noble J, Forooghian F, Sproule M, et al. Utility of the National Eye Institute VFQ-25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol 2006; 142: 464–468. [DOI] [PubMed] [Google Scholar]
- 30. Mangione CM. The National Eye Institute 25-item Visual Function Questionnaire scoring algorithm (version 2000), https://nei.nih.gov/sites/default/files/nei-pdfs/vfq_sa.pdf
- 31. Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016; 86: 2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol 2006; 59: 963–969. [DOI] [PubMed] [Google Scholar]
- 33. Balcer LJ, Baier ML, Pelak VS, et al. New low-contrast vision charts: Reliability and test characteristics in patients with multiple sclerosis. Mult Scler 2000; 6: 163–171. [DOI] [PubMed] [Google Scholar]
- 34. Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol 2010; 67: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schinzel J, Zimmermann H, Paul F, et al. Relations of low contrast visual acuity, quality of life and multiple sclerosis functional composite: A cross-sectional analysis. BMC Neurol 2014; 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davies EC, Galetta KM, Sackel DJ, et al. Retinal ganglion cell layer volumetric assessment by spectral-domain optical coherence tomography in multiple sclerosis: Application of a high-precision manual estimation technique. J Neuroophthalmol 2011; 31: 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longbrake EE, Lancia S, Tutlam N, et al. Quantitative visual tests after poorly recovered optic neuritis due to multiple sclerosis. Mult Scler Relat Disord 2016; 10: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Costello F. The afferent visual pathway: Designing a structural-functional paradigm of multiple sclerosis. ISRN Neurol 2013; 2013: 134858. [DOI] [PMC free article] [PubMed] [Google Scholar]