Short abstract
Following the first report on the nucleoside phosphoramidate (ProTide) prodrug approach in 1990 by Chris McGuigan, the extensive investigation of ProTide technology has begun in many laboratories. Designed with aim to overcome limitations and the key resistance mechanisms associated with nucleoside analogues used in the clinic (poor cellular uptake, poor conversion to the 5′-monophosphate form), the ProTide approach has been successfully applied to a vast number of nucleoside analogues with antiviral and anticancer activity. ProTides consist of a 5′-nucleoside monophosphate in which the two hydroxyl groups are masked with an amino acid ester and an aryloxy component which once in the cell is enzymatically metabolized to deliver free 5′-monophosphate, which is further transformed to the active 5′-triphosphate form of the nucleoside analogue. In this review, the seminal contribution of Chris McGuigan’s research to this field is presented. His technology proved to be extremely successful in drug discovery and has led to two Food and Drug Administration-approved antiviral agents.
Keywords: Nucleoside analogues, prodrugs, phosphoramidate (ProTide), antiviral
A tribute to Chris McGuigan, “the Drug Hunter ”
In this issue, it is our immense privilege to pay tribute to Professor Chris McGuigan, an extremely dedicated and enthusiastic scientist who achieved remarkable success in the antiviral field.
Professor Chris McGuigan’s achievements in the development of ProTide technology are undoubtedly extremely significant and remarkable. ProTide technology is currently the most successful prodrug strategy applied in the antiviral field, also demonstrating promising results in other therapeutic areas.
As the remaining three members of his group, it will be our mission to continue his legacy for many years to come. We firmly believe that the best way to honor him will be to put the same efforts and passion into research for new medicines as he did.
Introduction
Nucleoside analogues
Viral infections represent a major problem to human society. Viruses are often difficult to eradicate due to the fact that they are easily spread, and are able to use the host biochemical pathways to replicate. Therefore, targeting viral machineries often presents the challenging task of reducing the viral load in the human cell without damaging it.
One of the most successful approaches to fight viral infections is using nucleoside analogues (NAs).1 NAs are synthetic compounds that exhibit structural similarities to natural nucleosides. In the cell, they can undergo the same physiological processes as the endogenous nucleosides from the uptake to the metabolism, so that in their phosphate forms they can act on cellular functions. Mono-, di-, and triphosphorylated nucleosides are therefore the active forms of these drugs. These compounds act by interfering with viral enzymes as competitive inhibitors of their natural substrates as well as by being incorporated into newly synthesized viral DNA and RNA strands. Their incorporation into nucleic acids may induce either the termination of chain elongation or the accumulation of mutations in the viral genome.2 Through these mechanisms NAs interfere with the viral genome replication and thereby work as antiviral drugs. However, human enzymes can also recognize NAs, which act as antimetabolites. Antimetabolites can have toxic effects on cells, such as halting cell growth and division and/or inducing apoptotic processes. Consequently, these NAs are specifically used as chemotherapy for cancer. NAs have been in clinical use for almost 50 years and have become cornerstones of the treatment for patients with viral infections or cancer conditions.3
In the antiviral arena, NAs are commonly used in the therapy of human immunodeficiency virus (HIV), hepatitis B and C viruses (HBV and HCV), herpes simplex virus (HSV), cytomegalovirus (CMV), and varicella zoster virus (VZV) infections. These agents are generally safe and well tolerated as they are recognized by the viral, but not human polymerases in DNA replication. The NAs used to treat HIV infections are often referred to as nucleoside reverse transcriptase inhibitors (NRTIs), a viral DNA polymerase essential for HIV replication. However, they have activity against both DNA-dependent and RNA-dependent DNA polymerases. They inhibit viral replication by several mechanisms, either by competitive inhibition of the viral polymerase or by DNA chain termination. Many of the antiviral NAs either are missing or are blocked at the 3′-hydroxyl group, which results in failure of elongation of the nascent DNA molecule. Other antiviral NAs are negative enantiomers (L-forms) of the natural (D-forms) nucleosides and interfere with replication, partially because of steric hindrance when they are taken up by the viral polymerase or added to the DNA molecule.
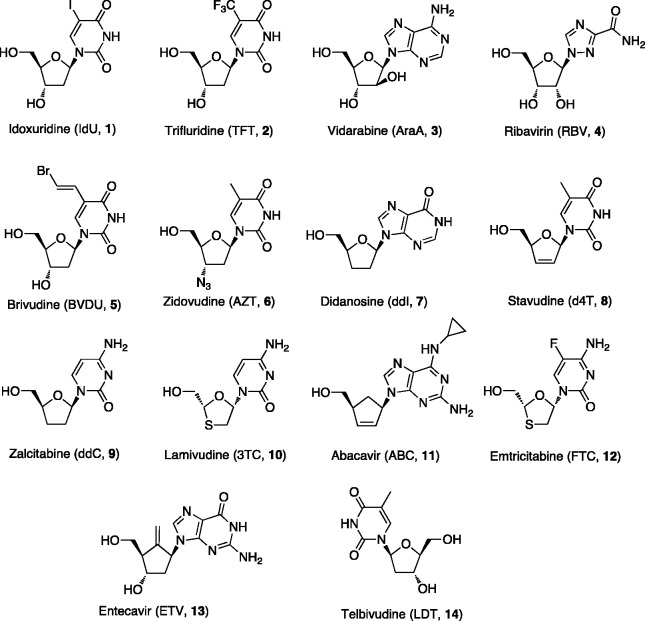
There are currently 14 approved cyclic NAs [1–14] (Figure 1) that are used as antiviral agents for several indications. The antiviral research field started with the discovery of the NA idoxuridine (IdU, [1]),4 approved in 1962 against herpes simplex keratitis,5 followed by trifluridine (TFT, [2])6 licensed in 1980 for the topical treatment of herpetic keratitis. Currently, these two NAs are used in the topical treatment of herpetic eye infections.
Figure 1.
Cyclic NAs in clinical use as antiviral agents.
Vidarabine (AraA, [3])7 was approved later on in 1986 against HSV and VZV and is now used for the treatment of acute keratoconjunctivitis, recurrent superficial keratitis caused by HSV-1 and HSV-2 and herpes zoster infections in AIDS patients. In many conditions vidarabine, due to its lower selectivity, higher inhibitory concentration and lower potency together with a low aqueous solubility, which implies intravenous dosing, has been replaced by the more powerful and effective drug acyclovir (ACV) (see infra).
The combination of pegylated interferon-α with ribavirin (RBV, [4]), approved in 1986, has for the last 10 years been the standard of care for the treatment of HCV infections.8 In addition since RBV has a broad activity spectrum including various DNA and RNA viruses, it has been used (topically, as aerosol) in the treatment of respiratory syncytial virus (RSV) infections.
Several other NAs were described as antiretroviral agents and were later approved and marketed for antiviral therapy. Among them there are brivudine (BVDU, [5]) approved in 1980 against VZV,9 zidovudine (AZT, [6]) used since 1986 to prevent and treat HIV infections,10 didanosine (ddI, 7) approved in 1991 to treat HIV infections in combination with other medications as part of highly active antiretroviral therapy (HAART)11 and stavudine (d4T, [8]) approved in 1994 against HIV.12 Zalcitabine (ddC, [9]) was the third antiretroviral drug to be approved in 1992 for the treatment of HIV infections.11 Lamivudine (3TC, [10]) is an antiretroviral medication used since 1995 to prevent and treat HIV infections and it is also used to treat chronic HBV infection when other options are not possible.13 Other NAs approved include abacavir (ABC, [11]) approved in 1998 against HIV,13 emtricitabine (FTC, [12]),14 approved in 2004 in combination with tenofovir disoproxyl fumarate (TDF) (see infra) against HIV, entecavir (ETV, [13]),15 and telbivudine (LDT, [14]),16 both approved against HBV in 2005 and in 2006, respectively (Figure 1).
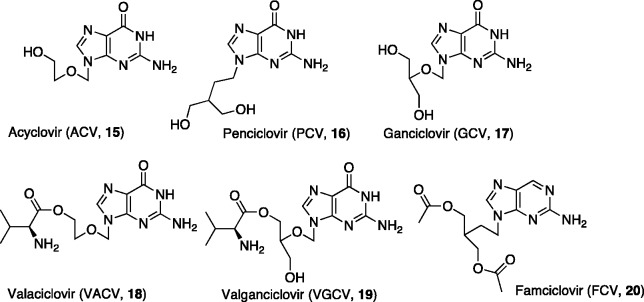
The clinical development of a second generation of antiviral NAs, including at present six approved compounds (Figure 2), started 35 years ago with the discovery of the acyclic NA ACV [15], a selective anti-herpetic agent used in the treatment of VZV and HSV-1 and HSV-2 infections.17,18 ACV possesses elevated selectivity of action, low cytotoxicity, and limited side effects. Since it was originally described, until now, ACV can still be considered as the “gold standard” for the treatment of HSV and VZV infections. In the same family is penciclovir (PCV, [16]),19,20 the guanine analogue of ACV with similar activity spectrum and mechanism of action. Currently, PCV is used as topical cream against cold sores caused by the HSV infections. Ganciclovir (GCV, [17]),21 is another guanine analogue with an extended spectrum of activity. It has been approved to treat CMV retinitis with a slow release formulation and topical ophthalmic use for acute herpes simplex keratitis. The pharmacokinetics and the oral bioavailability of this second generation of antiviral NAs were enhanced with valaciclovir (VACV, [18]) and valganciclovir (VGCV, [19]), amino acid ester derivatives of ACV and GCV, respectively and with famciclovir (FCV, [20]),22 which is the diacetyl prodrug of PCV.20 VACV, the valine ester of ACV was synthesized to increase the aqueous solubility and to increase the oral bioavailability in order to use it in eye drops or in intramuscular injections. VACV is used for preemptive prophylaxis of CMV infections after renal transplantation. FCV is used to treat shingles (caused by reactivation of VZV), genital herpes (HSV-2), and herpes labialis (HSV-1). After rapid in vivo adsorption, FCV is converted into PCV through selective deacetylation followed by final oxidation of the nucleobase at the 6-position. VGCV, used in the treatment of CMV and HSV, is the valine ester prodrug of GCV, synthesized with the aim to increase oral bioavailability and solubility in water. Following oral administration, the ester is cleaved by esterases in the intestines and in the liver to release the parent nucleoside [17].
Figure 2.
Acyclic NAs in clinical use as antiviral agents.
In order to exert their antiviral activity, NAs have to be phosphorylated (in vivo) via three consecutive phosphorylations with the first one being usually the rate-limiting step.23 However, if the first phosphorylation of the nucleoside to its 5′-monophosphate cannot take place all these drugs are inactive. This happens when the virus either does not induce a specific kinase or has developed resistance to the compound through mutations in this enzyme while the human cell fails to secure phosphorylation. Thus, to overcome this issue and improve therapeutic properties, nucleosides with a phosphate group already present in the structure have been targeted. The idea of replacing the phosphate group by an isosteric and isoelectronic phosphonate moiety was also investigated leading to the discovery of nucleoside phosphonate analogues (NPs).24 Since the CH2–P bond, unlike CO–P bond, is not susceptible to esterase and phosphatase hydrolysis, the resulting phosphonate compounds proved to be chemically and enzymatically more stable than the phosphate analogues. NPs are classified into major groups: cyclic nucleoside phosphonates (CNPs) and acyclic nucleoside phosphonates (ANPs).25 CNPs are natural-like NAs as they contain a nucleobase and a sugar moiety. Compared to the large number of the ANPs described in the literature, only a few examples of CNPs with some antiviral activity have been reported.24,26 This scarcity of examples is due the fact that in general CNPs are characterized by weak (if none) antiviral activity, which is generally explained by their poor substrate properties for cellular and viral kinases. For this reason until now, none of these compounds have reached the clinic.
On the contrary, ANPs have acquired a prominent therapeutic position.25 They exhibit a broad spectrum of antiviral activities, particularly against DNA viruses and retroviruses, which are ascribed to their ability to undergo intracellular phosphorylation to the diphosphate forms and to be incorporated in the growing nucleic acid strand. The common structural attribute of ANPs is a nucleobase attached to an aliphatic side chain containing a phosphonomethyl residue. A methylene bridge between the phosphonate moiety and the rest of the molecule excludes the possibility of enzymatic dephosphorylation; absence of the glycosidic bond in the structure of ANPs further increases their resistance to chemical and biological degradation. Flexibility in the acyclic chain is assumed to enable these compounds to adopt a conformation suitable for interaction with active sites of different enzymes involved in DNA replication.
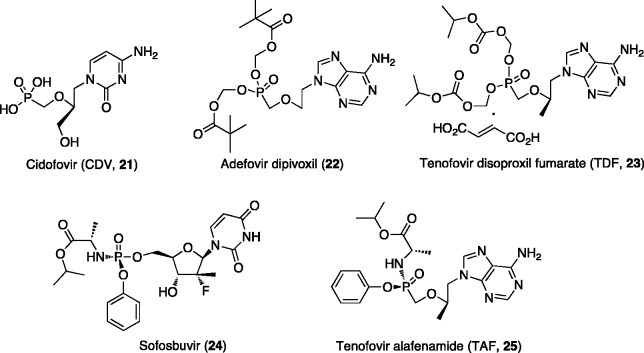
This new family of marketed antiviral drugs, includes cidofovir (CDV, [21])27 formally approved only for the treatment of human cytomegalovirus HCMV retinitis in AIDS patients, but also used successfully off-label in the treatment of various other DNA virus infections, particularly human papilloma virus (HPV)-associated lesions (Figure 3).
Figure 3.
Nucleotide analogues in clinical use as antiviral agents.
Nucleoside phosphate and phosphonate prodrugs
NAs as hydrophilic molecules do not rapidly penetrate cell membranes by non-facilitated diffusion. Instead, they permeate the cell by carrier-mediated endocytosis,28 which is an active or facilitated transport mechanism that requires energy and a specific receptor or protein on the cell surface. Unfortunately, carrier-mediated transport often requires very close structural resemblance to natural products.
As previously mentioned, nucleoside 5′-monophosphates or 5′-phosphonates bypass the slow first phosphorylation step performed by viral kinases. However, these two classes of compounds are subject to poor cell penetration as a consequence of the negative charges in the phosphate and phosphonate groups, at physiological pH. Similarly to NAs, they require active transportation to enter the cells, and might present a risk of being deactivated in vivo by several cellular enzymes. In addition, they are not ideal for oral administration, an extremely desirable requisite for the treatment of chronic diseases. To overcome these limitations, several prodrug structures of biologically active phosphate and phosphonate analogues have been developed.29–37 The rationale behind the design of such agents is to achieve temporary blockade of the free phosphonic functional group until their systemic absorption and delivery, allowing the in vivo release of the active drug only once at the target site. Such compounds have increased lipophilicity and as such are capable of altering cell and tissue distribution/elimination patterns of the parent drug.38 Passive transcellular absorption is the most general route for absorption of lipophilic molecules. Many prodrug approaches have been utilized to overcome the limitations of phosphate- and phosphonate-containing drugs. Some of these approaches are still under development and until now no clinical investigations as antiviral agents of compounds, belonging to these classes of prodrugs, have been reported. Among them, there are aryl and phenyl esters,39 cyclosaligenyl esters (CycloSal),40 bis-S-acylthioethyl esters (Bis-SATE),41 and peptidomimetic prodrugs.42–44 Nevertheless, other approaches have been more successful and include prodrugs that have reached the clinic for antiviral therapy. An example of such a well-investigated class of prodrugs is represented by the alkoxyalkyl ester prodrugs of ANPs, designed by Hostetler group.45 These include brincidofovir (CMX001, hexadecyloxypropyl-CDV), an experimental antiviral drug in clinical development by Chimerix for the treatment of CMV, adenovirus, smallpox, and Ebola virus infections46 and CMX157 (hexadecyloxypropyl-tenofovir) another novel lipid ANP that has completed a Phase I clinical trial in healthy volunteers, demonstrating a favourable safety, tolerability, and drug distribution profile.47 ContraVir Pharmaceuticals is planning further clinical development of this compound against HBV and HIV.
Cyclic 1-aryl-1,3-propanyl ester prodrugs (HepDirect), are another example of phosphate prodrugs effective as antiviral agents. This class of prodrugs features pradefovir, the 3-chlorophenyl HepDirect prodrug of adefovir,48 which has been advanced to human clinical trials for hepatitis B infection therapy. The clinical development of pradefovir, as an oral prodrug for chronic HBV infection, although discontinued in USA and Europe, is still progressing in China by Chiva Pharmaceutical.49 Currently, IDX184, a (SATE)-phosphoramidate diester prodrug of 2′-C-methylguanosine, is the only example of the successful application of this prodrug technology to reach human study. Unfortunately, the development of this antiviral agent for HCV treatment was stopped in Phase IIb in 2012.50 Another example of a successful phosphate prodrug approach is represented by the phosphonodiamidate GS-9191,51 a double-prodrug of the ANP 9–(2-phosphonylmethoxyethyl)guanine (PMEG).52 In 2011, GS-9191 has completed Phase II clinical trials by Gilead Sciences as a topical prodrug to treat external genital warts due to HPV infections.53 After that, Gilead Sciences has granted Graceway pharmaceuticals an exclusive worldwide license to GS-9191 for topical use,54 but since then no further development has been reported to date. The Pharmasset agent PSI-352938, a novel cyclic phosphate prodrug of β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methylguanosine,55 was first-in-class prodrug to be clinically evaluated for the treatment of HCV infection. It progressed up to Phase II clinical trial56 but after that its clinical development was discontinued due to observed hepatotoxicity.57
Two examples of prodrug approaches (Figure 3) applied to ANPs represented by the acyloxy and alkoxycarbonyl esters, were very effective and led to compounds that entered clinical trial studies and further obtained Food and Drug Administration (FDA) approval. Such compounds currently marketed for antiviral therapy are: adefovir dipivoxil [22],58 the bis-pivaloyloxymethyl ester of adevofir, approved in 2002 for the treatment of HBV infections; and tenofovir disoproxil fumarate [23]59 the bis-diisopropyloxycarbonyloxymethyl ester of tenofovir fumarate licensed in 2001 for the treatment of HIV infections, and in 2008 also approved to treat chronic HBV infections.
Finally, the ProTide approach, invented almost 25 years ago by Chris McGuigan has recently been proven very successful in the intracellular delivery of nucleoside monophosphate into the cell, improving the activity of the parent drug.36,60,61 During these 25 years, the ProTide technology was applied to a vast array of nucleosides and these studies have paved the way to the discovery of sofosbuvir (phosphoramidate of 2′-α-C-fluoro-2′-β-C-methyl uridine, [24])62 and tenofovir alafenamide (TAF, phosphoramidate of tenofovir, [25])63 (Figure 3), both launched in the market by Gilead Sciences for the treatment of HCV (2013) and HIV infections (2015), respectively.63 A year later, TAF was approved for the treatment of HBV infections. To further confirm the success of this phosphate prodrug approach in the antiviral arena, it is important to mention that several other phosphoramidates of NAs are in clinical or preclinical development to treat viral infections (see infra).
Given the tremendous importance of phosphor(n)amidate prodrugs in the antiviral arena and beyond, after the approval of sofosbuvir [24] and TAF [25], the application of the ProTide technology has increased considerably. We herein attempt to report the ProTide development from its discovery to the recent application in the antiviral arena. After the brief introduction on the topic in the following sections, we will focus our attention on the ProTide approach, first discussing the synthetic methodology toward phosphor(n)amidates and summarizing the studies performed to prove their metabolic activation pathway. We will then report in details on the application of this approach to antiviral nucleosides starting from McGuigan’s pioneering studies until the most recent use of his technology. We will give a full account on sofosbuvir [24] and TAF [25] and on those ProTides that are currently in clinical development.
Aryloxyphosphor(n)amidate prodrugs (ProTides)
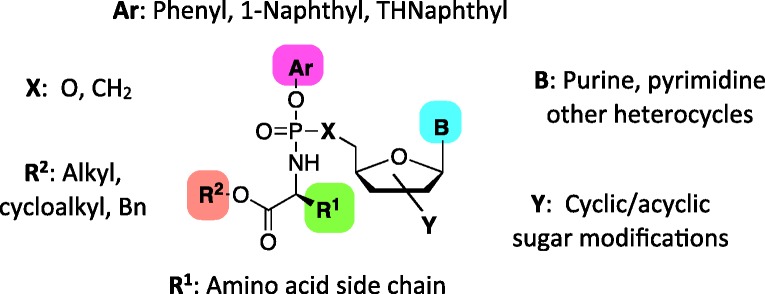
McGuigan and his team at Cardiff University researched design of novel chemically protected phosphate prodrug groups or motifs, which later became known as “ProTide” technology. A ProTide (pronucleotide) is a nucleoside aryl phosphate or phosphonate masked with an amino acid ester promoiety linked via P–N bond (Figure 4). Such a prodrug is able to enter the cell via facilitated passive diffusion through the cell membrane and when cleaved, it delivers the nucleoside monophosphate or monophosphonate releasing the two masking groups. The amino acid motif is normally selected from a range of natural and unnatural amino acids, although usually l-alanine is found to be preferred one and is featured by all ProTide drugs that have reached the clinic. In fact, in vitro study of d4T ProTides with β-amino acids as phosphoramidate moiety revealed an almost complete lack of anti-HIV activity in comparison with their α-amino acid derivatives.64 Short linear (methyl, ethyl, pentyl) or branched alkyls (isopropyl, neopentyl) and benzyl esters are usually employed. The tert-butyl is usually excluded due to its poor bioactivation.65 Phenyl and 1-naphthyl are commonly used as aryl components and are indeed those incorporated in the drugs in clinical use or development. However, the 5,6,7,8-tetrahydronaphthyl group has more recently appeared as a valid and effective moiety.66,67 Although the potency of ProTides varies with all the individual components of the phosphoramidate core, amino acid ester has been proven to drive predominantly the antiviral activity of the prodrugs, as it is closely linked with their stability and metabolic activation. Therefore, an extensive structure-activity relationship (SAR) study of amino acid ester and aryl moieties is generally performed to find an optimal combination of the promoieties for biological activity.
Figure 4.
General structure of ProTide scaffold.
The ProTide approach as a strategy to circumvent an impeded 5′-monophosphate formation was extensively applied also to anticancer NAs.66,68–80 An Edinburgh-based clinical-stage pharmaceutical company NuCana, is currently pioneering this technology in the oncology setting in collaboration with our laboratories.
The established position of the ProTide approach in the antiviral and anticancer nucleotide prodrug field provided a foundation for its further expansion into additional research area and/or non-nucleoside type compounds. These include phosphoramidates of carbohydrates such as N-acetyl-d-glucosamine for the treatment of osteoarthritis,81,82 2-fluoro-2-deoxyribose-1-phosphate,83 2,2-difluoro-2-deoxyribose-1-phosphate,83 2-deoxy-D-ribose-1-phosphate,84 and phosphonamidates of 2-deoxy-D-ribose-1-phosphonate85 as antivirals. More recently, application of the ProTide technology to N-(3–(5-(2′-deoxyuridine))prop-2-ynyl)octanamide, the Mycobacterium tuberculosis thymidylate synthase X inhibitor86 and to fingolimod, an immunomodulating drug used for treating multiple sclerosis disease87 was reported. Other research groups have also explored this prodrug approach in different areas of medicinal chemistry.88–91
Synthetic methods
Aryloxyphosphoramidates
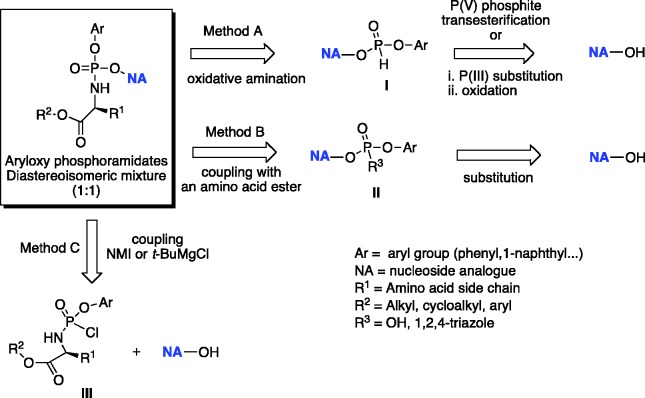
There are three methods for the preparation of aryloxyphosphoramidates as highlighted in the retrosynthetic approaches presented in Scheme 1. These procedures differ in the way the phosphoramidate moiety is introduced at the 5′-hydroxyl group of a NA. Method A is based on a coupling reaction of a NA with a diarylphosphite to form a NA-5′-monoaryl-H-phosphonate intermediate [I] suitable for subsequent oxidative amination.92 In method B, a nucleoside aryl phosphate [II] is coupled to an amino acid. Method C employs a phosphorylating agent [III] which is coupled to a NA in the presence of either N-methylimidazole (NMI) or tert-butyl magnesium chloride (Grignard reagent, t-BuMgCl).93 Over the past two decades, method C has been recognized as the most common strategy for the synthesis of aryloxyphosphoramidates and it has been applied to a wide number of antiviral and anticancer NAs. The choice of base for the coupling reaction largely depends on the presence and susceptibility of other free hydroxyl groups in the substrate. These additional groups might compete with the 5′-OH toward phosphorylation. The key phosphorylating agents [III], obtained as a pair of diastereoisomers at the phosphate centre (1:1 Rp: Sp ratio) are formed from an amino acid ester (HCl or tosyl salt) and dichlorophosphate upon the reaction with triethylamine at low temperature.94,95
Scheme 1.
General retrosynthetic approaches for the conventional synthesis of aryloxyphosphoramidates.
The Grignard reagent is not selective thus when used for the coupling reaction, the formation of undesired 3′-phosphoramidate and 3′,5′-bis-phosphoramidate is usually observed. This methodology suffers some limitations such as the need of extensive purification from a complex mixture of 3′,5′-bis-phosphorylated by-products, which is not suitable in case of large-scale synthesis.
Coupling mediated by NMI, which forms a labile imidazolium intermediate with phosphorylating agent, favors the selective phosphorylation of the primary hydroxyl group at the 5′-position of the nucleoside. However, the final outcome in terms of regioselectivity and yield with NMI or tert-BuMgCl is difficult to predict. The comprehensive review by Pradere et al.96 summarizes these results for a wide range of NAs.
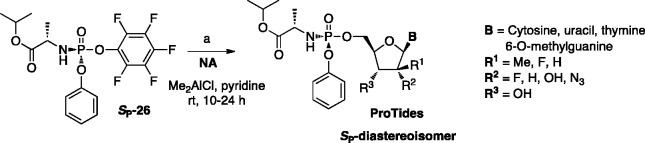
To avoid the formation of undesired 3′,5′- and 3′-phoshoramidates, usually the selective protection of the free 3′-hydroxyl group in the sugar part of a NA prior to the coupling reaction with a phosphorylating reagent [III] is often necessary. This methodology requires further deprotection of the 3′-position at the phosphoramidate stage. In the case of ribonucleoside analogues, commonly used protecting strategies include 2′,3′-diol protection with isopropylidene or cyclopentylidene moieties as reported by McGuigan and colleagues for β-2′-C-methylguanosine (2′-MeG),97 and β-2′-C-methyladenosine (2′-MeA)98 and 5-substituted uridine-based NAs such as 5-iodouridine (5-IU) and 5-bromouridine (5-BrU).99 The tert-butoxycarbonyl (Boc) and silyl-containing groups such as tert-butyldimethylsilyl (TBDMS) are often used to protect either 3′-OH100 or 2′- and 3′-OH functional groups in the 2′-deoxy-, 2′-modified-2′-deoxyribonucleoside or ribonucleoside analogues,101,102 respectively. The selective removal of these protecting groups at the phosphoramidate stage is usually performed under acidic conditions. Isopropylidene or cyclopentylidene moieties are normally removed using 60% acetic acid at 90–95°C, or 80% formic acid at room temperature.97–99 Whereas, tert-butoxycarbonyl (Boc) and silyl-containing groups are detached using a mixture of formic acid/water (1:1 v/v) or trifluoroacetic acid/water/THF (1:1:4) as described for gemcitabine and pseudoisocytidine-based ProTides.76,80 In general, the yields of deprotection under acidic conditions are rather low, despite the somewhat acid-stable nature of ProTides. Although, yields of the coupling reaction are generally significantly improved using 2′,3′-protected nucleoside, the overall yields of the protection–deprotection sequence remain moderate. The benzyloxycarbonyl (Cbz) group can be efficiently employed to protect hydroxy- and amino-groups in both sugar and nucleobase moieties as reported by Cho et al., for cytidine, uridine, adenosine, and guanosine analogues.103 The ease with which the Cbz is introduced on both the sugar and the nucleobase, coupled with its facile and clean removal via hydrogenation under neutral conditions, make this protecting group particularly attractive. To mask a competitive site such as NH2 in a nucleobase unit of NAs and to significantly improve general solubility of NAs, temporary protection of this functional group with either benzoyl or dimethylformamidine groups can be introduced prior to coupling reaction as reported for pyrimidine methylenecyclopropanes104,105 and the anti-HSV agent ACV, respectively.106 A more straightforward method toward the 5′-regioselective synthesis of phosphoramidates was recently published by Simmons et al.,107 consisting of a direct and highly selective 5′-phosphorylation reaction, mediated by dimethylaluminum chloride without employing 3′-protection manipulations. Moreover, when using the single isomer of 2,3,4,5,6-pentafluorophenyl-bearing phosphorochloridate (Sp-[26]),100 both stereoselectivity (see infra) and regioselectivity of resulting prodrugs can be achieved. This method, reported in Scheme 2, was successfully applied to a wide number of modified NAs leading to pharmaceutically relevant compounds such as anti-HCV clinical agents sofosbuvir and INX-08189 with good yields.
Scheme 2.
Regioselective synthesis of 5′-O-phosphoramidates.
When the key phosphorylating agents are used as a pair of diastereoisomers at the phosphorus centre (1:1 ratio RP and SP), formation of two diastereoisomeric aryloxyphosphoramidates in the same ratio is achieved. Such diastereomeric mixtures are often very difficult to separate by standard chromatographic methods, including reverse-phase chromatography or crystallization.108,109 Therefore, a lack of stereoselectivity represents one of the major limitations of these approaches.
Over the past 10 years, the main focus of researchers was concentrated on the development of diastereoselective strategies toward phosphoramidates obtained as a single isomer. Demand for efficient diastereoselective methods appeared to increase particularly after the discovery of a significant difference in the antiviral activity between SP and RP isomers as reported for SP-isomer of TAF [25], and Sp-isomer of sofosbuvir [24] (Figure 3), which showed a 10-fold increase in potency against HIV110,111 and an 18-fold difference in HCV activity versus the corresponding Rp isomer,62 respectively.
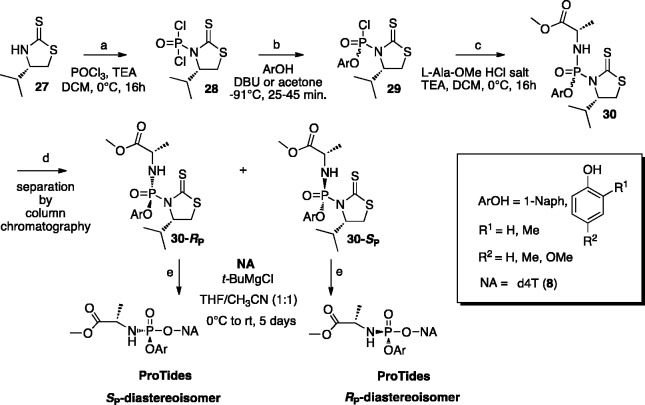
A diastereoselective method to obtain phosphoramidates using a chiral auxiliary-bearing phosphoramidating reagent [30] was developed by Meier and colleagues (Scheme 3).112 In this approach, (S)-4-isopropylthiazolidine-2-thione [27]113 acts as a chiral auxiliary and introduces the stereochemistry at the phosphorus atom in the intermediate [29], which is formed following the reaction of phosphorodichloridate [28] with aryl derivatives (1-naphthol or substituted phenols) at −91°C in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) for 25–75 min. The ratio (up to 14:1 Sp:Rp) of the two diastereoisomers Sp-[29] and Rp-[29], obtained in this step, where the chirality transfer is taking place, is dependent on the phenol derivative used in the reaction. The (SP)-configured diastereomer is assumed to be preferentially formed as the result of an addition/elimination mechanism.114 When diastereomerically enriched mixtures of phosphorochloridates [29] are reacted with l-alanine methyl ester hydrochloride, the phosphorodiamidates [30] are obtained as a mixture of chromatographically separable RP and SP diastereoisomers (de > 95%). The diastereoselectivity ratio is achieved irrespectively of the variations in the amino acids.115 In the final SN2-type reaction each of the diastereoisomer [30] (configuration of [30]-RP isomer confirmed by X-ray crystallography) was separately reacted with NAs to give phosphoramidate prodrugs as single RP and SP-isomers. In particular, this procedure was reported for d4T [8] ProTides obtained in 11–50% yield with 85–95% diastereomeric excess (Scheme 3).
Scheme 3.
Asymmetric synthesis of phosphoramidates via chiral auxiliary.
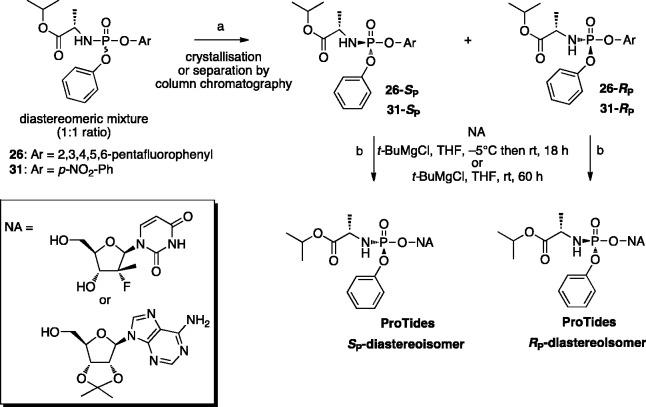
A novel approach to develop a diastereoselective synthesis of aryloxyphosphoramidates was reported more recently by Ross and colleagues.100 In this approach, a diastereomerically pure phosphoramidating agent with substituted phenolic leaving groups such as p-nitrophenyl or 2,3,4,5,6-pentafluorophenyl (Scheme 4), was isolated by crystallization with additional supercritical fluid chromatography and subsequently used in the coupling reaction with two NAs, yielding ProTides as a single isomer. Among a series of different phosphoramidating reagents investigated in these studies, the reagent with 2,3,4,5,6-pentafluorophenyl as the aryl moiety [26] was identified as the optimal reagent and its SP-isomer was used to prepare the HCV clinical agent PSI-7977 (sofosbuvir) in multi-gram scale.100
Scheme 4.
Diastereoselective synthesis of aryloxyphosphoramidates using a single isomer of 2,3,4,5,6-pentafluorophenyloxy or para-nitrophenyloxy phosphorylating agents.
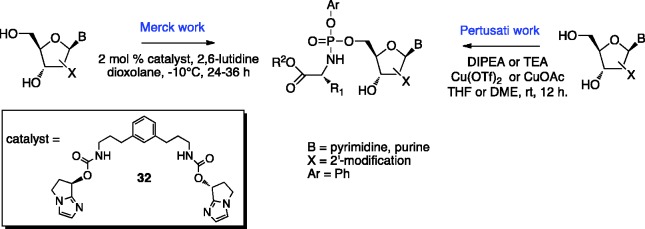
A diastereoselective method for the synthesis of P-chirogenic phosphoramidate prodrugs via copper-catalyzed reaction in the presence of a base was recently developed by Pertusati and McGuigan in the McGuigan group.116 Among several catalysts screened in this study, Cu(OTf)2 and CuOAc proved to be the most effective when used in the synthesis of purine-based and pyrimidine-based ProTides, respectively. An assessment of the effect of the base and solvent on the stereoselectivity and yield of the coupling reaction showed that diisopropylethylamine (DIPEA) and dimethoxyethane (DME) are able to provide the phosphoramidates with good diastereoselectivity and moderate yields (diastereomeric ratio 1:8.3 RP/SP; 40–60% yield) (Scheme 5).
Scheme 5.
Catalyst-mediated diastereoselective synthetic approaches to phosphoramidates.
Another catalytic stereoselective method that attains high selectivity for nucleoside phosphoramidation was reported in 2017 by researchers at Merck. In this methodology a metal free small-molecule catalyst [32] enabled the phosphoramidation of different 2′-modified nucleosides with high stereoselectivity at the phosphorus center towards RP configuration (Scheme 5).117 When using this methodology to optimize the synthetic route to the therapeutic anti-HCV agent, the desired RP-isomer MK-3682 was obtained in high diastereomeric ratio of 99:1 (RP:SP) in 92% yield.
All aryloxyphosphoramidates synthetic strategies described above are based on the phosphoramidation process performed at the level of either protected or unprotected NAs. A different synthetic approach, involving the preparation of an aryloxyphosphoramidate ribose derivative as the key building block was recently testified by Gao et al.118 This aryloxyphosphoramidate ribose bearing l-aspartic acid diisoamyl ester can be further coupled with a number of nucleobases under Vorbrüggen conditions to afford the desired products. The presence of a 2-acyloxy-group in the sugar moiety is a prerequisite for the neighbouring group participation, which allows N-glycosylation. However, the exclusive formation of the nucleotide analogue with β-configuration is obtained only in certain cases and it depends on the sugar and nucleobase (pyrimidine vs. purine) used for N-glycosylation reaction. Moreover, basic conditions required for the deacetylation (at the ProTide level) might not always be compatible with promoieties other than l-aspartic diisoamyl ester.
Aryloxyphosphonamidates
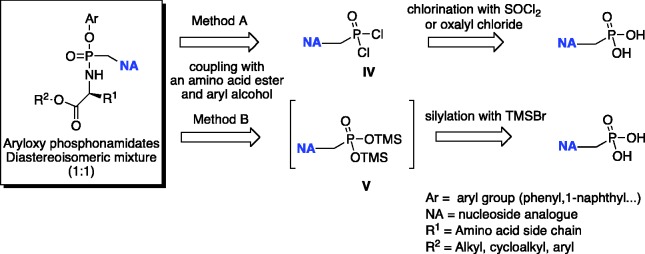
The preparation of phosphonamidate prodrugs of ANPs is generally accomplished from the corresponding phosphonic acid via two general procedures A and B (Scheme 6).
Scheme 6.
Retrosynthetic analysis for the conventional synthesis of phosphonamidates.
Method A, developed in McGuigan’s laboratory119 consists of the formation of the nucleoside phosphorodichloridate [IV] by treatment of the phosphonic acid with thionyl chloride, followed by reaction with the desired aryloxy-compound and amino acid ester to obtain the corresponding prodrug. During the first step, partial hydrolysis of the second P–Cl bond has been reported. For this reason, before the reaction with the appropriate amino acid, the intermediate must be treated again with thionyl chloride. Under these conditions, tenofovir and adefovir aryloxyphosphonamidate prodrugs were obtained in very low yields (5–10%). Modification of this method was reported by Gilead Sciences.120 In the first step, a mixture of DMF/sulfolane is replaced by dichloromethane, whereas trimethylsilyl phenolate is instead used as the nucleophile. The temperature is also increased up to 100°C. In this case, the intermediate monochloride is intentionally hydrolyzed with sodium hydroxide and isolated from the reaction mixture. Next, the amino acid ester is introduced via prior chlorination of the mono-acid. This method was further modified for the industrial synthesis of TAF [25].120–122 The introduction of the aryl alcohol is accomplished by its coupling with the free phosphonic acid using N,N'-dicyclohexylcarbodiimide (DCC) as a coupling agent in the presence of an organic base, usually triethylamine. The reaction is generally performed in N-methyl-2-pyrrolidinone (NMP) at 100°C. The aryloxy phosphonic acid is then isolated and transformed to the corresponding chloride with thionyl chloride in acetonitrile. The chloride is then reacted at low temperature (−30°C) with the appropriate amino acid ester. In comparison with method A, yields for the formation of phosphonate prodrugs improved up to 25% over two steps.
Method B is based on a modified methodology for the synthesis of symmetrical bis-amidate prodrugs of ANPs, which was reported first by Janeba and colleagues.123 This latter procedure consists of the synthesis of the silyl ester of the phosphonic acid via the reaction of a selected ANP with an excess of trimethylsilylbromide (TMSBr) in acetonitrile. The silyl ester was not isolated but immediately reacted with the desired amino acid ester in pyridine and triethylamine used as a coupling reagent with a mixture of triphenyl phosphine and 2,2′-dipyridyldisulfide (Aldrithiol-2). Phosphonodiamidates of several ANPs were obtained in high yields. This method is operatively simpler when compared to the procedures described above and offers the advantage that either the free phosphonic acids or the corresponding alkyl esters can be used as a starting material. This is of a great advantage, considering the difficulties generally encountered in the purification of free phoshonic acids. McGuigan’s group adapted this methodology to the synthesis of phosphonamidate ANPs prodrugs.66 To accomplish that, the silyl ester [V] must be treated with a 1:1 mixture of aryl alcohol and amino acid ester hydrochloride. Under these conditions, both adefovir and tenofovir phosphonamidates were isolated in moderate yields.66 Later, the same authors discovered that under this protocol for C5-pyrimidine ANPs, functionalized with a but-2-enyl-chain, only traces of the desired phosphonamidate were detected with the phosphonodiamidate being the major product. To circumvent this problem, the addition of an excess (6 equivalents) of aryl alcohol with respect to the amino acid ester (1 equivalent) was essential.67
Metabolic activation pathway
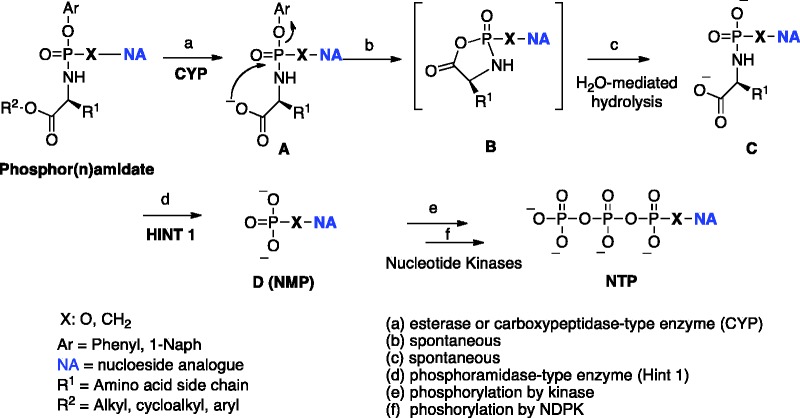
The biological activity of aryloxyphosphor(n)amidate prodrugs is expressed after their metabolic activation to the intracellularly released corresponding monophosph(on)ate nucleoside, further phosphorylated to the corresponding active di– and –triphosphate forms by nucleotide kinases.124
The early reports on the phosphoramidate activation pathway date back to the late 1990s.64,125 In these preliminary investigations, the metabolism of d4T phosphoramidates with pig liver carboxylesterase (CES) was studied125 using in situ 31P NMR analysis, a technique following that is now routinely employed in McGuigan’s laboratories as a predictive tool for the likely in vitro biological activity as well as for SAR establishment. The original protocol of this enzymatic experiment was later adapted to study the ProTide first activation step with carboxypeptidase Y enzyme, to prove the nucleoside monophosphate release in biological matrix such as cell lysate126 or to test the prodrug stability in human serum.76
Over the same period, these investigations were then extended to AZT phosphoramidates, where stability was tested with pig liver CES and different biological media such as human lymphocyte CEM cell extract, human serum, and mouse serum.64,125
The results of these studies suggested that the carboxylester group linked to the amino acid moiety has pronounced influence on the pharmacokinetics of the prodrugs and their associated stability. Introducing a tert-butyl ester was shown to lead to a significant reduction in antiviral potency due to a poor esterase-mediated activation. The metabolism of the prodrug was found to be markedly dependent on the amino acid moiety, with α-amino acids necessary for biological activity. Although compounds bearing longer amino acids (β–amino acids) showed efficient ester cleavage, they were found biologically inert and displayed no phenyl loss. L-Alanine was identified as the preferred amino acid, thus fully agreeing with the superior antiviral activity of the L-alaninyl-containing phosphoramidates of d4T seen at that time and consistently observed for other nucleosides analogous in following programmes. These results clearly suggested three key points for the metabolic activation of this class of prodrug: (1) the amino acyl liberation is necessary for biological action, (2) an α-amino acid is necessary for the phenyl cleavage (by intramolecular cyclization), and (3) phenyl loss proceeds after ester cleavage. Based on these findings, for the first time McGuigan proposed the metabolic pathway of the phosphoramidate prodrug,127 which is commonly accepted and considered valid also for phoshonamidate prodrugs. A general scheme representing metabolic activation of phosphor(n)amidate ProTides is depicted in Figure 5. The mechanism involves an initial carboxylic esterase or carboxypeptidase-mediated hydrolysis of the carboxylic ester of the amino acid leading to intermediate [A]. As evidence of this first step Gilead Sciences was able to show that cathepsin A is the primary enzyme that activates TAF [25] and GS-9131 (see infra) in human lymphatic tissues.127 The ester cleavage is followed by an internal nucleophilic attack of the acid residue on the phosphorus centre, displacing the aryloxy group and giving the transient formation of the putative five-membered cyclic intermediate [B]. This cyclic mixed anhydride is rapidly hydrolyzed to the corresponding aminoacyl phosphor(n)amidate [C]. The intermediate [C] is then believed to undergo P–N cleavage, mediated by an enzyme with phosphoramidase activity128 or may result from simple hydrolysis in a more acidic subcellular compartment, to eventually release the parent drug [D]. Phosphoramidase-type enzyme belongs to the human histidine triad nucleotide-binding protein (Hint)129 and its enzymatic efficiency and substrate specificity is believed to determine the eventual activity of ProTides.130–132 The postulated mechanism of activation of ProTides was supported by the cellular metabolism study of PSI-7851, a phosphoramidate prodrug of 2′-deoxy-2′-α-fluoro-β-C-methyluridine-5′-monophosphate, in clone A and primary human hepatocytes. Murakami and colleagues isolated and characterized the metabolites of PSI-7851 and its diastereoisomer PSI-7977 (sofosbuvir), including the intermediate metabolite [C] formed upon a stereospecific hydrolysis of the carboxyl ester (catalyzed by cathepsin A and CES1), and subsequent rapid chemical reaction (steps a–c). The succeeding cleavage of the P–N bond (catalysed by Hint1) lead to a formation of a 5′-monophosphate intermediate further phosphorylated to di- and triphosphate forms (steps d–f).133
Figure 5.
General metabolic pathway for phosphor(n)amidate ProTides.
A detailed mechanism of the hHint1-catalyzed hydrolysis of nucleoside phosphoramidates (in particular sofosbuvir) was recently proposed on the basis of crystallographic studies using a combination of more slowly hydrolyzed substrates and a catalytically inactive mutant enzyme.134 Molecular modeling of phosphoramidates and their corresponding amino acyl intermediate [C] in the catalytic site of a model of either carboxypeptidase135 or hHint128 are often employed to analyze the data.
ProTide approach application to antiviral nucleosides
Chris McGuigan’s work: From early reports to his latest investigations
The first prototype of phosphoramidates of a nucleoside, reported by McGuigan et al., in 1990, displayed two alkyl amines masking the monophosphate group on AZT.136 AZT suffers from an absolute dependence on host cell kinase-mediated activation, which can lead to poor activity, emergence of drug resistance, and clinical toxicity. In order to address AZT limitations, McGuigan designed the above-mentioned compounds as membrane-soluble prodrugs of the bioactive nucleotide, capable to bypass the first phosphorylation step. Among different compounds, terminal substituted alkyl amines showed pronounced anti-HIV effect in vitro, which was observed to decline when increasing the length of the methylene spacer. These results were considered consistent with a mechanism of action involving intracellular cleavage of the phosphoramidate P–N bond, and the release of the nucleotide, or a derivative thereof. Thereafter, phosphate triester derivatives of AZT were designed and evaluated against HIV-1 in vitro.137 During these studies, it was found that simple dialkyl phosphate derivatives of AZT as well as other NAs such as d4T, were inactive as anti-HIV agents, whereas substituted dialkyl phosphates were active. In particular, compounds bearing at the phosphorus centre a trichloro- or trifluoroethyl group and a carboxyl-protected, amino-linked amino acid displayed potent anti-HIV activity and low host toxicity.
Continuing to explore different structures, the phosphorus center was then masked with an ester-containing group in combination with either a simple alkyl moiety or a trichloroethyl group or another ester-containing group.138 The results of these investigations revealed the presence of two ester-substituted groups enhances activity relative to having only one substituted group. Furthermore, suggesting that a trihaloethyl group may substitute for an ester-containing group but with reduced potency. In several cases, these phosphate derivatives were found to be more selective in their antiviral activity than AZT due to their low toxicity in comparison to the parent nucleoside. Overall, the data supported the conclusion that these phosphate derivatives exert their biological effects via intracellular release of the nucleotide form.
In this report McGuigan stated: “If these in vitro findings could be translated into a demonstrable in vivo advantage, such phosphate pro-drugs could have merit as candidates for clinical development.”138 This was clearly an anticipation of what would have happened 25 years later. His investigations underlaid the importance of the masked phosphate approach, and had significant implications for what became the future design of chemotherapeutic NAs.
The ProTide series of AZT is the earliest example of aryloxyphosphoramidate technology reported by McGuigan’s group in the early 1990s.139,140
In vitro evaluation revealed these compounds had a pronounced, selective anti-HIV activity in CEM cells; the magnitude of the biological effect varied considerably depending on the nature of the phosphate-blocking groups. Moreover, several of the compounds retained marked antiviral activity in TK- (thymidine kinase-deficient) mutant CEM cells in which AZT was virtually inactive. Diaryl phosphate derivatives of the anti-HIV NA AZT were also investigated as potential prodrugs of the bioactive free nucleotide. The compounds were shown to be inhibitors of HIV replication in several cell lines, and show reduced cytotoxicity in vitro, by comparison to the parent nucleoside. However, in contrast to the previously reported aryloxyphosphoramidate derivatives, the diaryl phosphates of AZT showed to be poorly active in HIV-infected TK-deficient CEM cells. The results clearly pointed to the aryloxyphosphoramidate as the most promising structure for the delivery of the nucleotide and paved the way for the development of this class of prodrugs.
Thereafter, the ProTide technology was extensively and successfully applied to a high number of nucleoside phosphates with antiviral activity. In particular, following AZT studies, extensive SAR studies investigating the aryl, amino acid, and ester moieties were carried out on d4T phosphoramidates.141–145 In these reports, the preliminary results on the activation mechanism of such prodrugs were also described.125,146 The nature of the amino acid appeared to be extremely important for the eventual antiviral action. Among the amino acids studied, l-alanine was the most efficacious, whilst l-proline and glycine were particularly poor. However, an unnatural amino acid moiety, dimethylglycine, was shown to be able to substitute for l-alanine with little or no loss of activity.
As part of research project (sponsored by GlaxoSmithKline in Research Triangle Park North Carolina) devoted to discover anti-HIV and anti-HBV agents, McGuigan’s team applied the ProTide approach to 2′,3′-didehydro-2′,3′-dideoxyadenosine and other 2′,3′-dideoxy nucleosides including 2′,3′-dideoxyuridine, -adenosine, -3′-fluoroadenosine, -uridine147–153 and also to the carbocyclic nucleoside ABC [11] with significant enhancement of its antiviral activity.154 Phosphoramidates of other carbocyclic nucleosides were also explored such as carbocyclic adenosine derivatives and 2′,3′-dideoxy-2′3′-didehydro-7-deazaadenosine and they were shown to possess potent antiviral activity against HIV and HBV.155,156
The phosphoramidate technology was also applied to uridine-based NAs. Although their triphosphate forms were found to posses inhibitory activity on uridine triphosphate (UTP) incorporation into RNA of influenza virus, in general they are characterized by poor antiviral activity which may be related to their inefficient phosphorylation.99 However, in this case the ProTide approach was not very successful leading to compounds with weak antiviral activity. The slow release of the active monophosphate species of these compounds observed in cell lysate, as well as inefficient di- or triphosphorylation of 5′-monophosphate forms were considered as possible explanations for their weak antiviral activity.
A different outcome was instead obtained with ProTides of 6-modified 2′-fluoro-2′-deoxyguanosines, which showed marked antiviral activity in vitro assays proving that this class of prodrugs can be pursued for influenza virus therapy.157 Rapid metabolic activation in enzymatic assays with yeast carboxypeptidase Y or crude cell lysate supported the antiviral results. Evidence for efficient removal of the 6-substituent on the guanine part was provided by enzymatic studies with adenosine deaminase, and by molecular modeling of the nucleoside 5′-monophosphates in the catalytic site of a model of this enzyme (ADAL1), thus indicating the utility of the double prodrug concept.
No improvement or broadening of the antiviral activity of the parent nucleoside RBV was obtained with a family of ProTides.158 Again, a likely explanation for this lack of activity was attributed to their poor activation to the free 5′-monophosphate, as evidenced by cell lysate incubation studies. While enzymatic studies with carboxypeptidase Y indicated that the first step in the activation of RBV ProTides was efficient, molecular modeling data with the Hint enzyme suggested that subsequent amino acid cleavage to liberate the necessary free 5′-monophosphate was most probably impeded in this case.158 Other examples in which the ProTide technology showed a lack of significant improvement of the antiviral activity versus the parent compounds are the phosphoramidates of 2-fluoro derivatives of the bicyclic NA Cf1743, the most potent anti-VZV agent reported to date,159 and of 2′-deoxy-2′,2′-difluoro-5-halouridine.160
A collaboration between McGuigan’s group (Cardiff University, UK) and Van Calenbergh’s laboratories (Ghent University, Belgium) led to the investigation of α-l-2′-deoxythreofuranosyl nucleosides with A, T, C, and U as nucleobases.161 Unfortunately, the ProTide of the T NA, included in these studies was devoid of antiviral activity.
More recently, the phosphoramidate approach was applied by the same authors to the family of apionucleosides162 such as 2′,3′-dideoxy-β-D-apio-D-furanonucleosides (ddANs), which, synthesized in the early 1990s as potential antiviral agents, were found inactive.163,164 In 2014, Van Calenbergh and colleagues discovered that the 3′-O-phosphonomethylated adenine and thymine phosphonate exhibited promising anti-HIV properties. Since these phosphonates act as bioisosteres of the corresponding phosphorylated species, the authors reinvestigated the biological activity of such ddANs and in collaboration with McGuigan’s group designed and synthesized nucleosides and their corresponding ProTides, respectively.
While all target nucleosides failed to show significant antiviral activity, the authors demonstrated that the triphosphate of 2′,3′-deoxy-D-apio-D-furanoadenosine was readily incorporated into a DNA template by HIV reverse transcriptase to act as a DNA chain terminator. The ProTides of this nucleoside were found active against HIV-1 and HIV-2, indicating that the lack of activity of the parent nucleoside, and possibly also of other members of the D-apio-D-furanose nucleoside family needed to be sought in the inefficient cellular conversion to the 5′-monophosphate form.
Application of the ProTide approach to ACV was also extensively investigated in the McGuigan laboratories. Although ACV makes an important contribution to the therapy of herpes infections, it has some limitations such as low oral bioavailability and drug resistance caused by mutation in either the TK or DNA polymerase.17,18 Though the oral bioavailability can be increased by the amino acid prodrugs of ACV, these compounds can be cleaved in the gut and the liver by hydrolase enzymes. Interestingly, ACV was reported to inhibit HIV in human herpes virus (HHV) co-infection in tissue cultures.165 This activity was found to be correlated with the phosphorylation of the parent drug to the monophosphate form mediated by HHV-encoded kinase(s), whereas further phosphorylation steps provided the active triphosphate form of ACV able to inhibit HIV-RT. Because, HIV does not encode an enzyme that recognizes ACV as a substrate for its activation (phosphorylation) step, the HHV coinfection is needed for ACV to exhibit activity. ACV was therefore a perfect substrate for the application of ProTide technology. Based on these observations, McGuigan and colleagues presented the synthesis and initial biological evaluation (against HSV-1 and HSV-2 and against HIV-1 and -2) of a series of ACV ProTides.135,166,167 The application of this strategy was efficient to overcome two main issues associated with ACV: to bypass its poor efficiency of diffusion through intact cell membranes and the first limiting phosphorylation step. SAR studies showed that in general ester and aryl variations were well tolerated, whereas the variation of the amino acid moiety seemed to be tolerated only in the case of HSV. Regarding in vitro HIV screening, good results were obtained only for the l-alanine and l-phenylalanine derivatives. Differences in the activity demonstrated by these prodrugs may be due to different substrate specificities and/or different intracellular levels of enzyme necessary for the activation of these compounds. Although the compounds lacked any improvement in activity against HSV-1 and -2 compared to the parent, they retained activity against the TK-deficient HSV-1 strain while ACV showed loss in activity.
In the absence of HHV infection, the prodrug compounds showed antiviral activity, demonstrating their nucleoside kinase independence. These findings were also supported by a different study, where ACV phosphate prodrugs showed a full retention of antiviral activity against HSV-1 and VZV TK-deficient strains. Enzymatic and molecular modeling studies were performed to better understand the antiviral behavior of these compounds. These indicated that ProTides with diminished biolability toward carboxypeptidase translate to poor anti-HIV agents and vice versa. Given that, this enzymatic assay became a predictive tool regularly used to assess potential activity of phosphoramidate prodrugs of other NAs. To overcome the cytotoxicity observed with these prodrugs, very recently a virtual screening on a library of ACV derivatives was reported.168 Docking experiments with a database of 3600 compounds against three different enzymes encompassing HIV reverse transcriptase, adenylate or guanylate kinase, and a model of DNA polymerase γ resulted in the selection of five NAs as potentially strong RT inhibitors and weak cellular DNA polymerase inhibitors including GCV, PCV, 6-Cl-PCV, 6-OMe-PCV and 2′-SH-GCV. Several phosphoramidate prodrugs of the selected NAs were synthesized and assessed for their potency against HIV, HSV, VZV, and HCMV. Most of the compounds exhibited inhibitory activity against HIV with activity in the low micromolar range, but again some toxicity was observed.
As reported before, over the last 20 years, ANPs have emerged as a novel class of clinically effective antiviral agents.168 Explorations of various types of nucleoside phosphonate prodrugs have also led to the design and development of their aryloxyamidate prodrugs. ProTides originally designed to deliver nucleoside monophosphates, have also been successfully applied to nucleoside phosphonates. McGuigan’s group was the first to report the synthesis and biological evaluation of ProTides based on adefovir and tenofovir.119 Results of these studies indicate similar SARs for such prodrugs as earlier noted for NAs like d4T (l-alanine containing ProTides being the most potent compounds).142 In vitro enzymatic studies and structure–activity relationships indicate that the activation mechanism of phosphonamidate prodrugs may be the same as that described for the phosphoramidate triesters of NAs.
This early work was followed by investigation into the synthesis and antiviral evaluation of a broad family of phosphonamidates of adefovir and tenofovir.66 ProTides, synthesized with a more efficient methodology, showed improved in vitro anti-HIV activity, compared to previously reported data for the parent ANPs. Phosphonamidates bearing 5,6,7,8-tetrahydro-1-naphthol unit which was for the first time introduced as hydrolysable aryl unit in the ProTide motif displayed an improved antiviral activity compared to the “common”-naphthyl and phenyl ProTide units. Enzymatic studies showed that this novel aryloxy group was processed through the “standard” metabolic pathway. In this view 5,6,7,8-tetrahydro-1-naphthol was considered as a good aryl group for future improvement of the ProTide motif and thus was subsequently used by the same authors to design phosphonamidates of C5-substituted pyrimidine acyclic nucleosides functionalized with but-2-enyl-chain.67
In the search of anti-HCV agents, McGuigan’s group started a project (funded by Roche Palo Alto), involving application of the ProTide technology to the ribonucleoside analogues 4′-azidouridine,169 4′-azidoadenosine,170 and 4′-azidocytidine.171 Although 4′-azidouridine and 4′-azidoadenosine did not inhibit HCV, their triphosphate forms showed potent inhibitory activity against HCV RNA polymerase. Several phosphoramidates of these NAs were prepared, including variations in the aryl, ester, and amino acid regions. Among a number of 4′-azidouridine and -adenosine prodrugs with sub-micromolar inhibition of HCV replication in cell culture, the 1-naphthyl l-alanine benzyl ester phosphoramidates of both of the two NAs were the most active compounds in the replicon assay without detectable cytotoxicity. On the contrary, no significant improvement in the activity was observed with the 4′-azido cytidine family versus the parent nucleoside. Phosphoramidate ProTides derived from 4′-azidoinosine were also reported to be active at low micromolar levels in the replicon assay against HCV, whereas the parent NA was inactive in this assay.172 These results confirmed that the ProTide technology allows delivery of ribonucleoside 5′- monophosphate, suggesting a potential path to the generation of novel antiviral agents against HCV infections. As a continuation of this anti-HCV programme, the ProTide approach was also applied by McGuigan’s team to β-2′-C-methylpurine.98 For this type of NAs, the antiviral activity is usually expressed through their corresponding intracellular triphosphate forms that are potent and competitive inhibitors of NS5B viral RNA polymerase. Although, the phosphoramidate of β-2′-methylguanosine containing l-alanine benzyl ester and 1-naphthyl group was found to be the most active, it suffered from rodent plasma instability. The variation of amino acids with the lead benzyl ester moiety led to enhanced stability in rodents, however this resulted in significant reduction in HCV replicon activity. Extensive modification of the ester functionality demonstrated no significant improvement in HCV potency.97 Following these results modifications at the C-6 of the purine base as a means of potentially affecting potency without changing the inherent plasma stability of phosphoramidates were carried out.173 These investigations led to the discovery of the aryloxyphosphoramidate double-prodrug of O-6-methyl-2′-C-methyl-guanosine bearing l-alanine neopentyl and 1-naphthyl as an amino acid ester and aryloxy moiety (INX-08189, BMS-986094, [33], Figure 6). This prodrug was licensed out to Inhibitex, the start-up company in Atlanta, and later acquired by Bristol Myers Squibb to continue the development of this compound and its analogues.
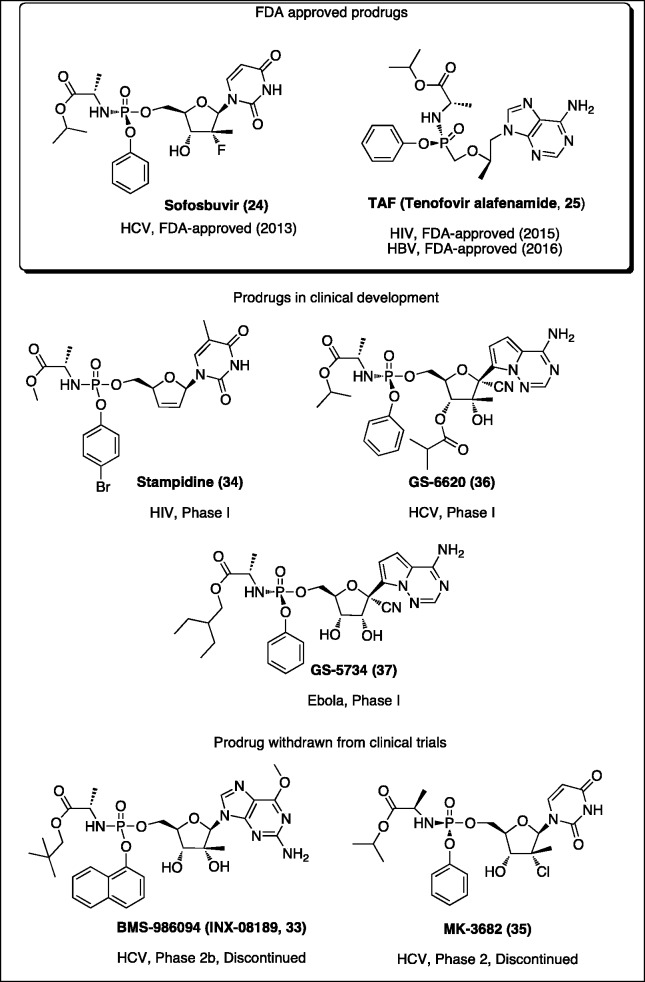
Figure 6.
Antiviral phosphor(n)amidates in clinical use or in clinical development.
INX-08189 exhibited nanomolar activity in vitro in HCV replicon assay, with EC50s of 10 nM against genotype 1b, 12 nM against genotype 1a, and 0.9 nM against genotype 2a after 72 h of exposure.174 It was also tested in the S282T mutant replicon and showed reduced activity with a 10-fold change in the EC50 yet still being capable to complete inhibition of HCV replication with EC90 value of 344 nM. In the replicon inhibition studies in which INX-08189 was used in combination with RBV, a high degree of synergy against the wild-type (WT) and S282T mutant replicons was observed. Intracellular metabolism of INX-08189 and its conversion to the active 5′-triphosphate form was investigated in the HCV genotype 1b replicon assay, showing an intracellular concentration of the triphosphate form (2′-C-MeGTP) of 0.84 ± 0.36 and 2.43 ± 0.42 pmol/1 × 106 cells were able to achieve 50% and 90% inhibition of viral application, respectively. The assessment of the mitochondrial toxicity in 14-day tissue culture studies demonstrated INX-08189 to be devoid of any mitochondria-specific toxicity in a liver-derived HepG2 and lymphocyte CEM human cell lines. Based on these advantageous properties, INX-08189 was advanced into in vivo studies supporting its further selection as a clinical candidate for the treatment of HCV infections. Pharmacokinetics and pharmacodynamics properties of INX-08189 were investigated in rats and cynomolgus monkeys by measuring the generation of the parent nucleoside 2′-MeG, along with the active triphosphate form (2′-C-MeGTP). The data for rats and monkeys were consistent in the linear relationship between the 2′-MeG AUC0–24 values and the concentration of 2′-C-MeGTP in the liver at 24 h. In addition, in vivo results indicated also that 2′-C-MeGTP concentrations in the liver equivalent to the EC90 could be reached after a single dose of 3 mg/kg and 25 mg/kg in rats and monkeys, respectively.174 The overall in vitro and in vivo data led to further progression and clinical development of INX-08189 as a highly potent HCV inhibitor. A safety and pharmacokinetics Phase Ia study in healthy volunteers, revealed INX-08189 administrated in a range of doses (3–100 mg) to be well tolerated at all doses with a lack of drug-associated serious adverse events.175,176 In a following Phase Ib study in the treatment-naive genotype 1 HCV patients, a mean HCV RNA reduction of −0.71 and −1.03 log10 IU/mL was observed when patients were dosed once daily with 9 mg or 25 mg, respectively. However, due to severe adverse events including heart failure and acute kidney injury, a further clinical development of INX-08189 was halted.177 A broad bioanalysis assays for the active nucleoside triphosphate, the prodrug INX-08189 and its metabolites in multiple target (diaphragm, heart, kidney, liver), and nontarget (lung) tissue were performed in order to evaluate the potential mechanism of the toxicity observed for INX-08189 in a Phase II clinical study. The triphosphate form (2′-C-MeGTP) was persistent in the heart and kidney in high levels after the treatment-free period (week 6 in 3 week + 3 week recover study) and thus appeared to be correlated with potential toxicities in these two organs.178
ProTides in clinical use or in clinical development as antiviral agents
The research undertaken in McGuigan’s laboratories during the last 25 years was of great inspiration for many scientists all over the world. Nowadays ProTide technology is recognized as a prodrug strategy with proven capacity to generate new drug candidates for nucleoside-based antiviral indications. In fact, a potential of this prodrug approach was confirmed with the discovery of agents that are currently in clinical use (sofosbuvir and TAF), or in clinical development as antiviral drugs including stampidine [34], MK-3682 [35], GS-6620 [36], and GS-5734 [37] (Figure 6). Furthermore, the indisputable potential of the ProTide approach in the antiviral field continuously encourages many research groups to design and develop novel phosphoramidate-type agents based on various structurally modified NAs. Currently, several academic groups and pharmaceutical companies have more ProTide compounds in the pipeline undergoing preclinical studies for the treatment of viral infections.55,179–185
Sofosbuvir
During the time of McGuigan’s research on HCV, phosphoramidate prodrugs of the 5′-phosphate β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleoside were developed by Sofia et al., (Pharmasset)62 leading to the discovery of the clinical antiviral agent sofosbuvir, which was launched in the market by Gilead Sciences. Sofosbuvir (Sovaldi®, GS-7977, PSI-7977) represents the first-in-class phosphoramidate-type inhibitor of NS5B RNA polymerase with FDA approval for the treatment of chronic HCV in patients infected with multiple HCV genotypes. Sofosbuvir (in 2009 entry to first-in-man Phase I trial) expresses high barrier of resistance186 and currently is recognized as the gold standard of care for HCV-infected patients. Moreover, sofosbuvir is also FDA-approved for HIV coinfected patients and those awaiting liver transplant. Although originally synthesized via conventional method as a 1:1 diastereomeric mixture (PSI-7851), sofosbuvir was further clinically developed as a single isomer with SP stereochemistry (PSI-7977) at the phosphorus atom as confirmed by X-ray structure determination. Biological evaluation of two individual isomers following separation by high-performance liquid chromatography (HPLC chromatography), was reported in the WT replicon cells and shown ∼18-fold difference in anti-HCV activity between PSI-7977 (Sp isomer EC90: 0.42 µM) and PSI-7976 (Rp isomer, EC90: 7.5 µM). When the two isomers were tested against replicons containing known nucleoside resistant mutants S282T and S96T, the ∼13-fold activity difference was observed for PSI-7977 (EC90 7.8 µM in S282T) versus PSI-7976 (EC90: >100 µM in S282T). In the replicon cells containing S96T NS5B polymerase mutation, the two inhibitors PSI-7977 and PSI-7976 showed similar antiviral activity (EC90 0.23 µM vs. 3.3 µM in WT and 0.11 µM vs. 1.3 µM in S96T), with no cross-resistance detected for both isomers.62 Additional studies for the ability to generate intracellular levels of the active 5′-triphosphate shown that SP isomer (PSI-7977) produced 10-fold greater levels of triphosphate form in comparison with RP isomer (PSI-7976) in clone A replicon cell lines, and 1.1-fold in human hepatocytes, respectively. An in vitro study confirmed that sofosbuvir undergoes the common metabolic pathway suggested for other phosphoramidate prodrugs to form the 5′-monophosphate metabolite which is further phosphorylated subsequently to the corresponding 5′-triphosphate active form first by uridine-monophosphate-cytidine-monophosphate kinase (UMP-CMP) and second by nucleoside diphosphate kinase. Mimicking the natural substrate of NS5B RNA-dependent RNA polymerase, sofosbuvir induces a chain termination process by being incorporated into the growing RNA. In vivo preclinical pharmacokinetics study shown that sofosbuvir was well-absorbed and metabolized to its intermediate metabolite (corresponding to the achiral intermediate [C] in Figure 5) and NA.187 A comprehensive characterization of pharmacokinetic, pharmacodynamics, and drug-interaction profile of sofosbuvir was reported by Gilead Sciences.188 The pharmacokinetics of single and multiple ascending doses of this agent administrated to HCV-infected patients revealed, similarly to single-doses studies in healthy subjects, that sofosbuvir and its parent nucleoside exhibited time-independent, near-linear pharmacokinetics within all evaluated doses.188 Following several clinical trials,186 a global Phase III clinical trial study with sofosbuvir/RBV therapy was initiated. This trial indicated, that 12 weeks treatment was efficient for patients with HCV genotype 2 and 3-infection (without previous interferon-based therapy) and resulted in high rates of sustained virologic response (>90% and >60% for patients infected with genotype-2 and 3, respectively). However, for patients with HCV genotype 3-infection (associated with possibly a greater risk of hepatocellular carcinoma), treatment extended up to 16 weeks was more beneficial than 12 weeks.189–191 On the basis of these results, an additional descriptive study was designed in which 250 patients with genotype 3-infection underwent treatment with sofosbuvir/RBV regimen for 24 weeks. The response rates in this subgroup were 91% and 68% among those patients without and with cirrhosis, respectively. Among total of 73 patients with genotype 2-infection treated for 12 weeks with sofosbuvir/RBV, 68 patients had a sustained virologic response 12 weeks after closure of study. Although with some limitations, the overall results of this study shown that the oral sofosbuvir/RBV regimen can be effective for both genotype 2 and 3-infected patients and also can offer an alternative to a pegylated interferon-based regimen for HCV patients.192 Following these results sofosbuvir was approved in December 2013 to treat chronic hepatitis C193 and in April 2017 for the same infections in paediatric patients 12 years and older.194
Tenofovir alafenamide (TAF)
As mentioned above, one of the most successful example of ANP prodrug is represented by TDF ([23], Figure 3), sold under the trade name Viread, and used in the treatment of chronic hepatitis B and to prevent and treat HIV/AIDS. Viread was found to have considerably improved cell permeability and anti-HIV activity in in vitro screening,195 increased oral bioavailability in animals,196 and more efficient loading of peripheral blood mononuclear cell (PBMC) relative to parenteral tenofovir observed in vivo.197 However, while TDF therapy is generally well tolerated it has negative effects on the renal function and on the bone mineral density. Different studies have demonstrated greater loss of kidney function and a higher risk of acute renal failure in patients receiving TDF-based therapies versus non-TDF regimens.198 Renal adverse events have been associated with the higher levels of tenofovir in the plasma observed when TDF was given with HIV protease inhibitors or pharmaco-enhancers, inhibitors of the intestinal efflux of TDF199,200 suggesting a link between plasma tenofovir exposure and the effect on proximal tubule function.201 In addition to renal disfunction patients receiving TDF, antiretroviral therapy showed a larger reduction in bone mineral density than regimens without TDF.202
To circumvent these major problems, Gilead Sciences developed TAF (TAF, [25]) fumarate, the isopropylalaninyl monoamidate phenyl monoester prodrug of tenofovir.203 TAF was initially synthesized as 1:1 mixture of diastereoisomers (GS-7171), which were then separated by chromatography. The antiviral activities of the diastereomeric mixture (GS-7171), as well as those of the individual diastereomers (RP isomer GS-7339 and SP isomer GS-7340), were evaluated in vitro in MT-2 cells infected with HIV-1 virus. When compared to the activity of tenofovir (EC50: 5.0 ± 2.6 µM) in the same test, the two single isomers GS-7339 (RP isomer, EC50: 0.06 ± 004 µM) and GS-7340 (SP isomer, EC50: 0.005 ± 002 µM) were found to be 83- and 1000-fold more active. Again, also in this case it is worth to highlight that the SP-diastereoisomer is much more active that the Rp-diastereoisomer suggesting that intracellular metabolism is sensitive to stereochemistry at the phosphorus.203 In addition, when the d-alanine was incorporated as promoiety, the resulting prodrug (GS-7485), showed similar activity to tenofovir. Given that, the two prodrugs (GS-7171 and GS-7485) release the same pharmacologically active metabolite, the considerably reduced activity of the d-alaninyl analogue (GS-7485), versus the l-alaninyl analogue (GS-7171) might be explained by a strong metabolic preference inside the cells for the natural amino acid. TAF (GS-7340) was also found to show a greater selectivity index than TDF. The higher initial intracellular concentration of tenofovir achieved with TAF, relative to TDF, was considered as to be able to differentially affect antiviral potency and cytotoxicity.203 The key properties that made TAF so successful were its stability in biological matrices, including plasma, and its selective intracellular cleavage. In vitro metabolism and accumulation in PBMCs studied in tissue culture showed that in MT-2 cell extract, GS-7340 was metabolized three times faster than in plasma, whereas TDF was metabolized 170-fold faster in plasma.203 This stability accounts for prolonged systemic exposure to intact prodrug and the accumulation of higher intracellular levels of the pharmacologically active metabolite tenofovir diphosphate relative to TDF. In MT-2 cells incubated with 10 μM GS-7340, the formation of the diphosphate metabolite was linear for 24 h with a concentration inside the MT-2 cells at 24 h exceeding the initial extracellular concentration of GS-7340 by 250-fold.203
In preclinical animal studies, TAF exhibits enhanced distribution of tenofovir into PBMCs and the lymphatic organs after oral administration, in comparison to tenofovir disoproxil fumarate. Twenty-four hours after a single dose of TAF in dogs, the concentration of tenofovir in lymphatic organs is between 5- and 15-fold greater than an equivalent dose of TDF. Intracellular tenofovir concentrations, measured by AUC0–24, in PBMCs after a single oral dose of TAF in dogs are ∼38-fold greater after an equivalent oral dose of TDF and ∼100-fold greater than those observed after subcutaneous administration of tenofovir. These in vivo pharmacokinetics studies showed also that both (GS-7340 and GS-7339) were rapidly eliminated in plasma relative to tenofovir with the SP-isomer cleared more rapidly than its Rp-counterpart.203 Pharmacokinetic study in dogs demonstrated that TAF is taken up efficiently by the liver. Further in vitro studies showed that TAF is a substrate for the hepatic transporters OATP1B1 and OATP1B3. Although this might explain the high concentration of TAF in the liver, it is more likely that the high passive permeability of the phosphonamidate prodrug is the major vehicle of the drug into the liver.204
Moreover, intracellular activation and antiviral activity of TAF are adversely non-affected by other medications (often administered in combination) such as HIV and HCV protease inhibitors,205 except for telaprevir and boceprevir that non-specifically inhibit cathepsin A, the key enzyme responsible for the activation of the prodrug.127
Furthermore, the rapid intracellular cleavage step catalyzed by cathepsin A in HIV- and HBV target cells, coupled with the formation of poorly permeable metabolites (the charged phosphates) effectively trapped in cells, accounts for the substantial accumulation of the pharmacologically active metabolite tenofovir diphosphate and increased therapeutic efficacy of TAF with respect to other prodrugs. Because of this accumulation inside the infected cells, TAF can be administered in lower therapeutic doses in comparison to TDF. Phase I/II clinical study to asses the pharmacokinetics, safety and anti-HIV activity of TAF showed that administration of 40 mg of TAF for 14 days in HIV-infected patient resulted in lower tenofovir Cmax and lower systemic exposures compared with subjects who received TDF. Higher intracellular concentrations of tenofovir in PBMCs were detected with two doses (40 mg and 120 mg) of TAF then with 300 mg TDF. TAF had the same resistance profile as tenofovir and TDF (in vitro) with no resistance mutations detected for both of them.206 Phase III studies were then undertaken to confirm the following observations and to further define the safety and efficacy profile of TAF. Results of these studies revealed TAF to be as effective as TDF in much lower dose and with lower occurrence of adverse side effects such as impaired kidney function.207 In November 2015, the TAF-based regimen (elvitegravir/cobicistat/emtricitabine/TAF) was FDA-approved for treatment of HIV-1.208
TAF was also investigated for the treatment of HBV infections. In particular, Phase III clinical trials studies evaluating investigational use of once-daily 25 mg dose of TAF in treatment-naive and treatment-experienced adults with HBeAg-negative and HBeAg-positive chronic HBV infections, demonstrated again that TAF was non-inferior to TDF based on the percentage of patients having low HBV DNA levels after 48 weeks of therapy. In addition to high efficacy, the results of these studies reflect improved renal and bone safety parameters similar to those seen in clinical studies evaluating TAF-based regimens for HIV. In November 2016, TAF received FDA approval for the treatment of chronic HBV.209
Stampidine
Stampidine [34] is a rationally designed phosphoramidate derivative of stavudine (d4T, [8]). developed as an anti-HIV agent to overcome the dependence of [8] on intracellular nucleoside kinase-mediated activation to the nucleoside 5′-monophosphate. Stampidine was shown to inhibit the replication of HIV-1 strain human T-lymphotrophic virus (HTLV)-IIIB in human PBMCs at nanomolar 50% inhibitory concentration with IC50 value being 20 times lower than its parent nucleoside [8] and selectivity index >100,000. Stampidine was found to be active also against phenotypically and/or genotypically NRTI-resistant HIV strains, including 20 primary clinical HIV-1 isolates resistant to AZT. A similar nanomolar inhibitory activity was demonstrated by stampidine against primary clinical HIV-1 isolates with non-nucleoside RT inhibitor (NNRTI) binding site mutations and/or phenotypic NNRTI-resistant profiles. Moreover, among 12 stavudine substituted-phenyl phosphoramidates, stampidine was found to be the most effective in the inhibition of adenovirus-induced plaque formation in human foreskin fibroblast with no cytotoxicity up to 100 mM and selectivity index >4000.210 In the in vivo pharmacokinetics and metabolism studies reported by Chen et al.,211 stampidine demonstrated favorable pharmacokinetics and rapid hydrolysis to l-ala-d4T-MP (the key precursor of the d4T triphosphate metabolite) as well as its parent nucleoside d4T following i.v. injection and oral administration in mice, rats, dogs, and cats.212 In fact, the unique ability of stampidine to quick hydrolysis is correlated with the presence of p-Br substituted-phenyl part of the phosphoramidate prodrug, which in turn translates into rapid formation of L-ala-d4T-MP.212,213 l-Ala-d4T-MP is considered as an intra- and/or extracellular source of d4T and/or d4T-MP, which might explain the superior antiretroviral activity of stampidine over d4T in cell culture.214 In the postulated activation pathway for ProTide [34] (as well as other RP or SP phosphoramidates of stavudine), lipases are also involved in the hydrolysis of ester moiety in the amino acid side chain showing clear preference for the l-amino acid configuration as reported in the experimental and modelling studies.215–217 Phase I randomized, placebo controlled study performed with the aim to investigate early safety, tolerability and activity data in 30 therapy-naive HIV-infected patients showed that stampidine did not cause dose-limiting toxicity at single dose levels ranging from 5 to 25 mg/kg.218 Pre-exposure prophylactic therapy studies for stampidine are ongoing.219 Despite the encouraging data, there are no reports available on further progression of this agent in clinical trials.
Uprifosbuvir
Uprifosbuvir (MK-3682, IDX-21437, [35]) is the RP-isomer of d-amino acid–based phosphoramidate prodrug of 2′-C-methyl-2′-chloro-uridine-based NA117 that reached clinical trial as anti-HCV agent. In the in vitro Huh-7 cell based HCV replicon assay, MK-3682 demonstrated weak activity, however when tested in vivo very high levels of nucleoside triphosphate were observed in mouse liver (6200 and 3750 pmol h/g for MK-3682 and its SP counterpart isomer, respectively). Interestingly, this may indicate that MK-3682 undergoes different metabolic activation or differentiated kinetics of the ester hydrolysis between two diastereoisomers as suggested for the 2′-β-C-Me-2′-α-C-F-uridine series.134 In fact, in vitro metabolic study performed ffor MK-3682 and its SP analogue and sofosbuvir by purified CES1 and cathepsin A (CatA) showed clear substrate preference of CES1 for RP isomer (94% of MK-3682 hydrolysed whereas only 4.5% of SP isomer hydrolysed over 21 h). Incubation of these three compounds with CatA for 18 h resulted with no hydrolysis of MK-3682 and SP isomer and complete metabolism of sofosbuvir.220 The most recent data from the two Phase II studies on efficacy and safety of uprifosbuvir together with grazoprevir and ruzasvir, a HCV nonstructural protein 5A inhibitor, either with or without RBV for 16 and 24 weeks, respectively in patients who had previously failed an NS5A inhibitor-containing treatment, showed that this triple-combination therapy was safe and highly effective.221 Several other clinical trials Phase I/II studies for MK-3682 were on-going in a combination therapy with RBV or non-nucleoside agents such as grazoprevir, elbasvir, and ruzasvir.222–224 In 2017, further clinical evaluation of uprifosbuvir in a combination therapy was discontinued.225
GS-6620
A single SP-isomer GS-6620 [36] represents a family of phosphoramidate prodrugs based on 2′-C-methyl-7,9-dideaza-4-aza-adenosine containing 1′-cyano and 2′-C-Me groups with inhibitory activity against NS5B polymerase.226 The presence of the 2′-C-Me group in natural ribonucleoside analogues once incorporated to the primer position was reported to arrest an incoming nucleoside triphosphate from binding to active site of NS5B polymerase and therefore causing inhibition of further RNA elongation and viral replication.227 GS-6620 is a double-prodrug with l-alanine-isopropyl and phenyl moieties attached at the 5′-hydroxyl as a phosphoramidate part, and isobutyryl ester added into the 3′-postion on the ribose ring with the aim to improve its permeability and oral bioavailability. Like other phosphoramidate agents, GS-6620 was initially synthesized via conventional method and obtained as a part of diastereomeric mixture further separated by chiral column chromatography.226 The preliminary in vitro results revealed the SP-diastereoisomer to be 6-fold more potent, and two times more efficient in the formation of triphosphate in primary human hepatocytes than its RP analogue. The antiviral activity in the panel of HCV replicon assay including stable replicons (GT1b, 1a, 2a) and chimeric replicons (2b, 3a, 4a, 5a, and 6a) was also demonstrated by GS-6620 with EC50 ranging between 0.068 and 0.43 µM. In the additional cytotoxicity studies, GS-6620 showed detectable toxicity in erythroid marrow progenitor cells (CC50 15 µM), a minimal cytotoxicity in cell lines such as Huh-7 (CC50 67 µM), HepG2 (CC50 66 µM), and PC-3 (CC50 40 µM), and no cytotoxicity in the primary hepatocytes, PBMCs (quiescent and stimulated) at the highest concentration tested (100 µM).228 In the mitochondrial toxicity study, no inhibition of mitochondrial DNA level at the highest concentration tested (20 µM) in HepG2 and PC-3 cells, as well as no inhibition of mitochondrial DNA content was observed. Moreover, no specific inhibition of mitochondrial protein synthesis and no inhibition of host DNA or RNA polymerases were detected at the highest concentration (up to 500 µM). In the cross-resistance studies GS-6620 showed reduced activity only against genotype 1b (GT1b) replicons with the S282T NS5B mutation. In order to inhibit HCV RNA polymerase in hepatocytes, GS-6620 needs to be first activated to the free 5′-monophosphate that is subsequently phosphorylated to its 5′-triphosphate derivative. The postulated activation pathway is aligned with the conventional metabolic activation route for ProTides (Figure 5) and consists of an additional 3′-isobutyryl ester hydrolysis catalysed by CES2 at first, followed by the hydrolysis of the isopropyl ester group on the 5′-ProTide moiety catalysed by CatA or CES1.229 The compound was clinically developed and evaluated for its antiviral activity, safety and tolerability in first-in-human Phase I clinical study in the treatment-naive patients chronically infected with HCV genotype 1.230 GS-6620 was well tolerated up to 900 mg when given twice a day for five days and shown the greatest antiviral activity with median viral load reductions of 1.73 and 1.63 log10 IU/mL from baseline with 900 mg (administrated as a tablet) and 450 mg (administrated as a solution), when given twice daily, respectively. Although, GS-6620 demonstrated potent antiviral activity, its clinical efficacy and utility turned to be limited due to observed high levels of substantial intra- and intersubject pharmacokinetic and pharmacodynamic variability.231
Table 1.
Prodrugs in clinic and clinical development.
| Drug name | Originator/developer | Phase | Disease | Viral Target Mechanism of action | Ref | |
|---|---|---|---|---|---|---|
| Sofosbuvir (Sovaldi, [24]) | Pharmasset/Gilead Sciences | Approved 2013 | HCV | NS5B RNA-dependent RNA polymerase inhibition | 62 | |
| Tenofovir alafenamide(TAF, [25]) | Gilead Sciences | Approved 2016Approved 2015 | HBVHIV | HBV reverse transcriptase inhibitionHIV reverse transcriptase inhibition | 63 | |
| INX‐08189 [33] | Inhibitex/BMS | Discontinued | HCV | NS5B RNA-dependent RNA polymerase inhibition | 175 | |
| Stampidine [34] | Paradigm Pharmaceuticals, Parker Hughes Institute | I | HIV | RNA-directed DNA polymerase inhibition | 213 | |
| MK‐3682 [35] | Merck | Discontinued | HCV | NS5B RNA-dependent RNA polymerase inhibition | 223 | |
| GS‐6620 [36] | Gilead Sciences | I | HCV | NS5B RNA-dependent RNA polymerase inhibition | 231 | |
| GS‐5734 [37] | Gilead Sciences | II | Ebola V | Viral RNA-dependent RNA polymerase inhibition | 235 | |
GS-5734
GS-5734 [37] is the single SP isomer of the 2-ethylbutyl-l-alaninate monophosphate prodrug of 1′-cyano-substituted adenosine C-NA with enhanced activity against a number of filoviruses including Marburg virus and several variant of ebola virus EBOV in cell-based assay.232 A comprehensive report on structure–activity relationship, lead optimization, and robust diastereoselective synthesis of GS-5734 was recently published by Gilead Sciences following their focused library screening of ∼1000 diverse nucleos(t)ide analogues with antiviral properties.233 The outcome of this work and the discovery of GS-5734 coincided with the most recent Ebola outbreak in West Africa. The prodrug GS-5734 expressed high selectivity for the viral polymerase as compared to host polymerases. In vivo efficacy evaluation of GS-5734 (conducted in rhesus monkeys as the most relevant animal model of EVD) demonstrated its rapid elimination with appearance of parent nucleoside in systemic circulation. The triphosphate levels in PBMCs were elevated to a maximum within 2 h. A maximally efficacious dose of the prodrug was determined in the preclinical in vivo efficacy study performed on an EBOV-infected rhesus challenge model.232 Further safety and pharmacokinetics study of GS-5734 (Phase I, once-daily i.v. infusion in single and multiple dose) have shown no serious adverse effects associated with the drug. GS-5734 was administered under compassionate use to two Ebola patients including a newborn infant with ebola virus disease (EVD).234 Currently, Phase II clinical study (PREVAIL IV) is ongoing.235
Conclusion
The idea of phosph(on)ates prodrugs with aryloxyphosphoramidates (ProTides) in particular, was extensively investigated over the last three decades. Designed and pioneered by McGuigan and his group, the ProTide approach was successfully applied to a great variety of modified NAs with antiviral and anticancer activity. In this review, we presented a summary of the “ProTide technology” development commencing from its discovery to the most recent applications in the antiviral field. We outlined a discovery and clinical development of recently FDA-approved sofosbuvir (Sovaldi®), one of the best-selling drugs for treatment of hepatitis C infection. We highlighted all synthetic methodologies towards aryloxphosphor(n)amidates including the latest, more sophisticated regio- and stereoselective procedures. Given its increasing interest in the scientific community, as well as a significant commercial impact, the ProTide technology will continue to be used as a strategy to improve the efficacy profile of nucleoside and also non-nucleoside existing and/or novel therapeutics. Certainly, McGuigan’s discovery and extensive research on the phosphoramidate approach has provided an inspiration and the solid foundation for bringing the ProTide concept into the clinical success.
Acknowledgement
We would like to acknowledge all the scientists from both pharmaceutical companies and academic institutions, who for many years believed in and supported Chris’s research, driving ProTide technology to success. We particularly wish to note the valuable contribution of Jan Balzarini, who was both a long-term collaborator and a dear friend of Chris. The joint efforts of Chris and Jan, coupled with their passion and dedication to investigate new medicines were undoubtedly crucial to the development of ProTide technology.
We also wish to express our sincere thanks to the work of the over 100 postdocs and PhD students who together formed part of Chris’s group for over 25 years. Their contribution was invaluable to the successful results achieved.
We also wish to extend a special thanks to Helen Murphy and Julie Hayward, for their assistance to Chris’s group, as his PA during these 25 years.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: McGuigan's laboratory actively collaborated with Inhibitex inc., and BMS (whose agent INX-08189 is discussed herein) received financial support for this work.
References
- 1.De Clercq E. Milestones in the discovery of antiviral agents: nucleosides and nucleotides. Acta Pharm Sin B 2012; 2: 535–548. [Google Scholar]
- 2.De Clercq E, Neyts J. Antiviral agents acting as DNA or RNA chain terminators. Handb Exp Pharmacol 2009; 189: 53–84. [DOI] [PubMed] [Google Scholar]
- 3.Jordheim LP, Durantel D, Zoulim F, et al. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 2013; 12: 447–464. [DOI] [PubMed] [Google Scholar]
- 4.Prusoff WH. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim Biophys Acta 1959; 32: 295–296. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman HE. Clinical cure of herpes simplex keratitis by 5-iodo-2’-deoxyuridine. Proc Soc Exp Biol Med 1962; 109: 251–253. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HE, Heidelberger C. Therapeutic antiviral action of 5-trifluoromethyl-2’-deoxyuridine in herpes simplex keratitis. Science 1964; 145: 585–586. [DOI] [PubMed] [Google Scholar]
- 7.Schabel JFM. The antiviral activity of 9-β-D-arabinofuranosyladenine (Ara-A). Chemotherapy 1968; 13: 321–338. [DOI] [PubMed] [Google Scholar]
- 8.Sidwell RW, Huffman JH, Khare GP, et al. Broad-spectrum antiviral activity of Virazole: 1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972; 177: 705–706. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq E. Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem Pharmacol 2004; 68: 2301–2315. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuya H, Weinhold KJ, Furman PA, et al. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA 1985; 82: 7096–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuya H, Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A 1986; 83: 1911–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba M, Pauwels R, Herdewijn P, et al. 3’-dideoxythymidine and its 2’,3’-unsaturated derivative (2’,3’-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem Biophys Res Commun 1987; 142: 128–134. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents 2009; 33: 307–320. [DOI] [PubMed] [Google Scholar]
- 14.Schinazi RF, McMillan A, Cannon D, et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother 1992; 36: 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honkoop P, de Man RA. Entecavir: a potent new antiviral drug for hepatitis B. Expert Opin Investig Drugs 2003; 12: 683–688. [DOI] [PubMed] [Google Scholar]
- 16.Matthews SJ. Telbivudine for the management of chronic hepatitis B virus infection. ClinTher 2007; 29: 2635–2653. [DOI] [PubMed] [Google Scholar]
- 17.Elion GB, Furman PA, Fyfe JA, et al. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A 1977; 74: 5716–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaeffer HJ, Beauchamp L, de Miranda P, et al. ( 2-Hydroxyethoxymethyl)guanine activity against viruses of the herpes group. Nature 1978; 272: 583–585. [DOI] [PubMed] [Google Scholar]
- 19.Mondal DP. Xpharm: the comprehensive pharmacology reference. New York: Elsevier, 2007, pp. 1–4. [Google Scholar]
- 20.De Clercq E, Field HJ. Antiviral prodrugs—the development of successful prodrug strategies for antiviral chemotherapy. Br J Pharmacol 2006; 147: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis 1988; 10 Suppl 3: S490–S494. [DOI] [PubMed] [Google Scholar]
- 22.Harnden MR, Jarvest RL, Boyd MR, et al. Prodrugs of the selective antiherpesvirus agent 9-[4-hydroxy-3-(hydroxymethyl)but-1-yl]guanine (BRL 39123) with improved gastrointestinal absorption properties. J Med Chem 1989; 32: 1738–1743. [DOI] [PubMed] [Google Scholar]
- 23.Varga A, Lionne C, Roy B. Intracellular metabolism of nucleoside/nucleotide analogues: a bottleneck to reach active drugs on HIV reverse transcriptase. CDM 2016; 17: 237–252. [DOI] [PubMed] [Google Scholar]
- 24.De Clercq E. The clinical potential of the acyclic (and cyclic) nucleoside phosphonates. The magic of the phosphonate bond. Biochem Pharmacol 2011; 82: 99–109. [DOI] [PubMed] [Google Scholar]
- 25.Clercq ED, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discov 2005; 4: 928–940. [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Froeyen M, Kempeneers V, et al. Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents. J Am Chem Soc 2005; 127: 5056–5065. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E, Sakuma T, Baba M, et al. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res 1987; 8: 261–272. [DOI] [PubMed] [Google Scholar]
- 28.Balimane PV, Sinko PJ. Involvement of multiple transporters in the oral absorption of nucleoside analogues. Delivery Anti-HIV Drugs 1999; 39: 183–209. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Maag H, Alfredson T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J Pharm Sci 2008; 97: 1109–1134. [DOI] [PubMed] [Google Scholar]
- 30.Wiemer AJ, Wiemer DF. Prodrugs of phosphonates and phosphates: crossing the membrane barrier. Top Curr Chem 2015; 360: 115–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecker SJ, Erion MD. Prodrugs of phosphates and phosphonates. J Med Chem 2008; 51: 2328–2345. [DOI] [PubMed] [Google Scholar]
- 32.He G-X, Krise JP, Oliyai R. Prodrugs of phosphonates, phosphinates, and phosphates In: VJBRT Stella, MJ Hageman, R Oliyai, et al. (ed.) Prodrugs biotechnology: Pharmaceutical aspects, vol V. New York, NY: Springer, 2007, pp. 923–964. [Google Scholar]
- 33.Schultz C. Prodrugs of biologically active phosphate esters. Bioorg Med Chem 2003; 11: 885–898. [DOI] [PubMed] [Google Scholar]
- 34.Maria EA. Current prodrug strategies for the delivery of nucleotides into cells. DDRO 2005; 2: 373–387. [Google Scholar]
- 35.Peterson LW, McKenna CE. Prodrug approaches to improving the oral absorption of antiviral nucleotide analogues. Expert Opin Drug Deliv 2009; 6: 405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner CR, Iyer VV, McIntee EJ. Pronucleotides: toward the in vivo delivery of antiviral and anticancer nucleotides. Med Res Rev 2000; 20: 417–451. [DOI] [PubMed] [Google Scholar]
- 37.Pertusati F, Serpi M, McGuigan C. Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antiviral Chem Chemother 2012; 22: 181–203. [DOI] [PubMed] [Google Scholar]
- 38.Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov 2008; 7: 255–270. [DOI] [PubMed] [Google Scholar]
- 39.Oliyai R, Shaw Jp Fau, Sueoka-Lennen CM, et al. Aryl ester prodrugs of cyclic HPMPC. I: physicochemical characterization and in vitro biological stability. Pharm Res 1999; 16: 1687–1693. [DOI] [PubMed] [Google Scholar]
- 40.Meier C. cycloSal phosphates as chemical Trojan horses for intracellular nucleotide and glycosylmonophosphate delivery-chemistry meets biology. Eur J Org Chem 2006; 2006: 1081–1102. [Google Scholar]
- 41.Périgaud C, Gosselin G, Lefebvre I, et al. Rational design for cytosolic delivery of nucleoside monphosphates: “SATE” and “DTE” as enzyme-labile transient phosphate protecting groups. Bioorg Med Chem Lett 1993; 3: 2521–2526. [Google Scholar]
- 42.Peterson LW, Sala-Rabanal M, Krylov IS, et al. Serine side chain-linked peptidomimetic conjugates of cyclic HPMPC and HPMPA: synthesis and interaction with hPEPT1. Mol Pharmaceut 2010; 7: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krylov IS, Kashemirov BA, Hilfinger JM, et al. Evolution of an amino acid based prodrug approach: stay tuned. Mol Pharm 2013; 10: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zakharova VM, Serpi M, Krylov IS, et al. Tyrosine-based 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine and -adenine ((S)-HPMPC and (S)-HPMPA) prodrugs: synthesis, stability, antiviral activity, and in vivo transport studies. J Med Chem 2011; 54: 5680–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: Current state of the art. Antiviral Res 2009; 82: A84–A98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.https://clinicaltrials.gov/ct2/results?cond=&term= CMX001&cntry1=&state1= &Search=Search (accessed 18 October 2017).
- 47.https://clinicaltrials.gov/ct2/results?cond=&term= CMX157&cntry1=&state1=&Search=Search (accessed 18 October 2017).
- 48.Reddy KR, Matelich MC, Ugarkar BG, et al. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J Med Chem 2008; 51: 666–676. [DOI] [PubMed] [Google Scholar]
- 49.http://adisinsight.springer.com/drugs/800016246http://adisinsight.springer.com/drugs/800016246 (accessed 18 October 2017).
- 50.Zhou X-J, Pietropaolo K, Chen J, et al. Safety and pharmacokinetics of IDX184, a liver-targeted nucleotide polymerase inhibitor of hepatitis C virus, in healthy subjects. Antimicrob Agents Chemother 2011; 55: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfgang GHI, Shibata R, Wang J, et al. GS-9191 is a novel topical prodrug of the nucleotide analog 9-(2-phosphonylmethoxyethyl)guanine with antiproliferative activity and possible utility in the treatment of human papillomavirus lesions. Antimicrob Agents Chemother 2009; 53: 2777–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreider JW, Balogh K, Olson RO, et al. Treatment of latent rabbit and human papillomavirus infections with 9-(2-phosphonylmethoxy)ethylguanine (PMEG). Antiviral Res 1990; 14: 51–58. [DOI] [PubMed] [Google Scholar]
- 53.https://clinicaltrials.gov/ct2/results?term=gs9191 (accessed 18 October 2017).
- 54.http://bciq.biocentury.com/products/GS_9191 (accessed 16 October 2017).
- 55.Lam AM, Espiritu C, Bansal S, et al. Hepatitis C virus nucleotide inhibitors PSI-352938 and PSI-353661 exhibit a novel mechanism of resistance requiring multiple mutations within replicon RNA. J Virol 2011; 85: 12334–12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.https://clinicaltrials.gov/ct2/show/NCT01435044 (accessed 16 October 2017).
- 57.Gentile I, Buonomo AR, Zappulo E, et al. Discontinued drugs in 2012 - 2013: hepatitis C virus infection. Expert Opin Investig Drugs 2015; 24: 239–251. [DOI] [PubMed] [Google Scholar]
- 58.Starrett JE, Jr., Tortolani DR, Russell J, et al. Synthesis, oral bioavailability determination, and in vitro evaluation of prodrugs of the antiviral agent 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). J Med Chem 1994; 37: 1857–1864. [DOI] [PubMed] [Google Scholar]
- 59.Murphy RA, Valentovic MA. Factors contributing to the antiviral effectiveness of tenofovir. J Pharmacol Exp Ther 2017; 362: 156–163. [DOI] [PubMed] [Google Scholar]
- 60.Mehellou Y, Balzarini J, McGuigan C. Aryloxy phosphoramidate triesters: a technology for delivering monophosphorylated nucleosides and sugars into cells. ChemMedChem 2009; 4: 1779–1791. [DOI] [PubMed] [Google Scholar]
- 61.Cahard D, McGuigan C, Balzarini J. Aryloxy phosphoramidate triesters as pro-tides. MRMC 2004; 4: 371–381. [DOI] [PubMed] [Google Scholar]
- 62.Sofia MJ, Bao D, Chang W, et al. Discovery of a β-D-2-deoxy-2-α-fluoro-2-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem 2010; 53: 7202–7218. [DOI] [PubMed] [Google Scholar]
- 63.Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016; 125: 63–70. [DOI] [PubMed] [Google Scholar]
- 64.McGuigan C, Tsang HW, Sutton PW, et al. Synthesis and anti-HIV activity of some novel chain-extended phosphoramidate derivatives of d4T (stavudine): esterase hydrolysis as a rapid predictive test for antiviral potency. Antiviral Chem Chemother 1998; 9: 109–115. [DOI] [PubMed] [Google Scholar]
- 65.McGuigan C, Sutton PW, Cahard D, et al. Synthesis, anti-human immunodeficiency virus activity and esterase lability of some novel carboxylic ester-modified phosphoramidate derivatives of stavudine (d4T). Antiviral Chem Chemother 1998; 9: 473–479. [DOI] [PubMed] [Google Scholar]
- 66.Pertusati F, Hinsinger K, Flynn ÁS, et al. PMPA and PMEA prodrugs for the treatment of HIV infections and human papillomavirus (HPV) associated neoplasia and cancer. Eur J Med Chem 2014; 78: 259–268. [DOI] [PubMed] [Google Scholar]
- 67.Pertusati F, Serafini S, Albadry N, et al. Phosphonoamidate prodrugs of C5-substituted pyrimidine acyclic nucleosides for antiviral therapy. Antiviral Res 2017; 143: 262–268. [DOI] [PubMed] [Google Scholar]
- 68.Congiatu C, Brancale A, Mason MD, et al. Novel potential anticancer naphthyl phosphoramidates of BVdU: separation of diastereoisomers and assignment of the absolute configuration of the phosphorus center. J Med Chem 2006; 49: 452–455. [DOI] [PubMed] [Google Scholar]
- 69.McGuigan C, Thiery J-C, Daverio F, et al. Anti-cancer ProTides: tuning the activity of BVDU phosphoramidates related to thymectacin. Bioorg Med Chem 2005; 13: 3219–3227. [DOI] [PubMed] [Google Scholar]
- 70.Yoo CB, Valente R, Congiatu C, et al. Activation of p16 gene silenced by DNA methylation in cancer cells by phosphoramidate derivatives of 2’-deoxyzebularine. J Med Chem 2008; 51: 7593–75601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehellou Y, Valente R, Mottram H, et al. Phosphoramidates of 2'-β-D-arabinouridine (AraU) as phosphate prodrugs; design, synthesis, in vitro activity and metabolism. Bioorg Med Chem 2010; 18: 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGuigan C, Habib NA, Wasan HS, et al. A phosphoramidate protide (NUC-1031) and acquired and intrinsic resistance to gemcitabine. JCO 2011; 29: e13540. [Google Scholar]
- 73.McGuigan C, Murziani P, Slusarczyk M, et al. Phosphoramidate ProTides of the anticancer agent FUDR successfully deliver the preformed bioactive monophosphate in cells and confer advantage over the parent nucleoside. J Med Chem 2011; 54: 7247–7258. [DOI] [PubMed] [Google Scholar]
- 74.Vande Voorde J, Liekens S, McGuigan C, et al. The cytostatic activity of NUC-3073, a phosphoramidate prodrug of 5-fluoro-2'-deoxyuridine, is independent of activation by thymidine kinase and insensitive to degradation by phosphorolytic enzymes. Biochem Pharmacol 2011; 82: 441–452. [DOI] [PubMed] [Google Scholar]
- 75.Ghazaly EA, Slusarczyk M, Mason M, et al. NUC-1031: a novel protide that overcomes the key cancer resistance mechanisms associated with poor survival. Cancer Res 2014; 74: CT401. [Google Scholar]
- 76.Slusarczyk M, Lopez MH, Balzarini J, et al. Application of protide technology to gemcitabine: a successful approach to overcome the key cancer resistance mechanisms leads to a new agent (NUC-1031) in clinical development. J Med Chem 2014; 57: 1531–1542. [DOI] [PubMed] [Google Scholar]
- 77.Blagden SP, Rizzuto I, Stavraka C, et al. Final results of ProGem1, the first in-human phase I/II study of NUC-1031 in patients with solid malignancies. J Clin Oncol 2015; 33: 2514. [Google Scholar]
- 78.Ghazaly EA, Slusarczyk M, McGuigan C, et al. NUC-3373: a novel pyrimidine nucleotide analogue that overcomes key cancer drug resistance limiting patient survival. Mol Cancer Ther 2015; 14: B46. [Google Scholar]
- 79.Kandil S, Balzarini J, Rat S, et al. ProTides of BVdU as potential anticancer agents upon efficient intracellular delivery of their activated metabolites. Bioorg Med Chem Lett 2016; 26: 5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serpi M, De Biasi R, Pertusati F, et al. Synthetic approaches for the preparation of phosphoramidate prodrugs of 2'-deoxypseudoisocytidine. ChemistryOpen 2017; 6: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGuigan C, Serpi M, Bibbo R, et al. Phosphate prodrugs derived from N-acetylglucosamine have enhanced chondroprotective activity in explant cultures and represent a new lead in antiosteoarthritis drug discovery. J Med Chem 2008; 51: 5807–5812. [DOI] [PubMed] [Google Scholar]
- 82.Serpi M, Bibbo R, Rat S, et al. Novel phosphoramidate prodrugs of N-acetyl-(d)-glucosamine with antidegenerative activity on bovine and human cartilage explants. J Med Chem 2012; 55: 4629–4639. [DOI] [PubMed] [Google Scholar]
- 83.Hamon N, Quintiliani M, Balzarini J, et al. Synthesis and biological evaluation of prodrugs of 2-fluoro-2-deoxyribose-1-phosphate and 2,2-difluoro-2-deoxyribose-1-phosphate. Bioorg Med Chem Lett 2013; 23: 2555–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamon N, Slusarczyk M, Serpi M, et al. Synthesis and biological evaluation of phosphoramidate prodrugs of two analogues of 2-deoxy-D-ribose-1-phosphate directed to the discovery of two carbasugars as new potential anti-HIV leads. Bioorg Med Chem 2015; 23: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quintiliani M, Balzarini J, McGuigan C. Design, synthesis, and biological evaluation of phosphonamidate analogs of 2-deoxy-D-ribose-1-phosphate. Tetrahedron 2013; 69: 9111–9119. [Google Scholar]
- 86.McGuigan C, Derudas M, Gonczy B, et al. ProTides of N-(3-(5-(2'-deoxyuridine))prop-2-ynyl)octanamide as potential anti-tubercular and anti-viral agents. Bioorg Med Chem 2014; 22: 2816–2824. [DOI] [PubMed] [Google Scholar]
- 87.James E, Pertusati F, Brancale A, et al. Kinase-independent phosphoramidate S1P1 receptor agonist benzyl ether derivatives. Bioorg Med Chem Lett 2017; 27: 1371–1378. [DOI] [PubMed] [Google Scholar]
- 88.Ruda GF, Alibu VP, Mitsos C, et al. Synthesis and biological evaluation of phosphate prodrugs of 4-phospho-D-erythronohydroxamic acid, an inhibitor of 6-phosphogluconate dehydrogenase. ChemMedChem 2007; 2: 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruda GF, Wong PE, Alibu VP, et al. Aryl phosphoramidates of 5-phospho erythronohydroxamic acid, a new class of potent trypanocidal compounds. J Med Chem 2010; 53: 6071–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zano SP, Pate C, Frank M, et al. Correction of a genetic deficiency in pantothenate kinase 1 using phosphopantothenate replacement therapy. Mol Genetics Metab 2015; 116: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osgerby L, Lai Y-C, Thornton PJ, et al. Kinetin riboside and its ProTides activate the Parkinson’s disease associated PTEN-induced putative kinase 1 (PINK1) independent of mitochondrial depolarization. J Med Chem 2017; 60: 3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kers A, Kers I, Stawiski J, et al. Studies on aryl H-phosphonates; part 2: a general method for the preparation of alkyl H-phosphonate monoesters. Synthesis 1995; 1995: 427–430. [Google Scholar]
- 93.Van Boom JH, Burgers PMJ, Crea R, et al. Phosphorylation of nucleoside derivatives with aryl phosphoramidochloridates. Tetrahedron 1975; 31: 2953–2959. [Google Scholar]
- 94.Uchiyama M, Aso Y, Noyori R, et al. O-Selective phosphorylation of nucleosides without N-protection. J Org Chem 1993; 58: 373–379. [Google Scholar]
- 95.Serpi M, Madela K, Pertusati F, et al. Synthesis of phosphoramidate prodrugs: ProTide approach. Curr Protoc in Nucleic Acid Chem John Wiley, 2013; 53: 15.5.1–15.5.15. [DOI] [PubMed]
- 96.Pradere U, Garnier-Amblard EC, Coats SJ, et al. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem Rev 2014; 114: 9154–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McGuigan C, Gilles A, Madela K, et al. Phosphoramidate ProTides of 2'-C-methylguanosine as highly potent inhibitors of hepatitis C virus. Study of their in vitro and in vivo properties. J Med Chem 2010; 53: 4949–4957. [DOI] [PubMed] [Google Scholar]
- 98.McGuigan C, Perrone P, Madela K, et al. The phosphoramidate ProTide approach greatly enhances the activity of β-2'-C-methylguanosine against hepatitis C virus. Bioorg Med Chem Lett 2009; 19: 4316–4320. [DOI] [PubMed] [Google Scholar]
- 99.Meneghesso S, Vanderlinden E, Stevaert A, et al. Synthesis and biological evaluation of pyrimidine nucleoside monophosphate prodrugs targeted against influenza virus. Antiviral Res 2012; 94: 35–43. [DOI] [PubMed] [Google Scholar]
- 100.Ross BS, Ganapati Reddy P, Zhang H-R, et al. Synthesis of diastereomerically pure nucleotide phosphoramidates. J Org Chem 2011; 76: 8311–8319. [DOI] [PubMed] [Google Scholar]
- 101.Ogilvie KK, Schifman AL, Penney CL. The synthesis of oligoribonucleotides. III. The use of silyl protecting groups in nucleoside and nucleotide chemistry. VIII. Can J Chem 1979; 57: 2230–2238. [Google Scholar]
- 102.Zhu X-F, Williams HJ, Scott AI. Facile and highly selective 5’-desilylation of multisilylated nucleosides. J Chem Soc Perkin Trans 1 2000; 15: 2305–2306. [Google Scholar]
- 103.Cho JH, Amblard F, Coats SJ, et al. Efficient synthesis of nucleoside aryloxy phosphoramidate prodrugs utilizing benzyloxycarbonyl protection. Tetrahedron 2011; 67: 5487–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ambrose A, Zemlicka J, Kern ER, et al. Phosphoralaninate pronucleotides of pyrimidine methylenecyclopropane analogues of nucleosides: synthesis and antiviral activity. Nucleos Nucleot Nucl 2005; 24: 1763–1774. [DOI] [PubMed] [Google Scholar]
- 105.Qiu Y-L, Ptak R, Breitenbach J, et al. (Z)- and (E)-2-(Hydroxymethylcyclopropylidene)-methylpurines and pyrimidines as antiviral agents. Antivir Chem Chemother 1998; 9: 57–68. [PubMed] [Google Scholar]
- 106.McGuigan C, Derudas M, Bugert JJ, et al. Successful kinase bypass with new acyclovir phosphoramidate prodrugs. Bioorg Med Chem Lett 2008; 18: 4364–4367. [DOI] [PubMed] [Google Scholar]
- 107.Simmons B, Liu Z, Klapars A, et al. Mechanism-based solution to the ProTide synthesis problem: selective access to Sofosbuvir, Acelarin, and INX-08189. Org Lett 2017; 19: 2218–2221. [DOI] [PubMed] [Google Scholar]
- 108.Mesplet N, Saito Y, Morin P, et al. Liquid chromatographic separation of phosphoramidate diastereomers on a polysaccharide-type chiral stationary phase. J Chromatogr A 2003; 983: 115–124. [DOI] [PubMed] [Google Scholar]
- 109.Allender CJ, Brain KR, Ballatore C, et al. Separation of individual antiviral nucleotide prodrugs from synthetic mixtures using cross-reactivity of a molecularly imprinted stationary phase. Anal Chim Acta 2001; 435: 107–113. [Google Scholar]
- 110.Chapman H, Kernan M, Prisbe E, et al. Practical synthesis, separation, and stereochemical assignment of the PMPA pro-drug GS-7340. Nucleosides Nucleotides Nucleic Acids 2001; 20: 621. [DOI] [PubMed] [Google Scholar]
- 111.Chapman H, Kernan M, Rohloff J, et al. Purification of PMPA amidate prodrugs by SMB chromatography and x-ray crystallography of the diastereomerically pure GS-7340. Nucleosides Nucleotides Nucleic Acids 2001; 20: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 112.Roman CA, Balzarini J, Meier C. Diastereoselective synthesis of aryloxy phosphoramidate prodrugs of 3’-deoxy-2’,3’-didehydrothymidine monophosphate. J Med Chem 2010; 53: 7675–7681. [DOI] [PubMed] [Google Scholar]
- 113.Delaunay D, Toupet L, Corre ML. Reactivity of beta-amino alcohols with carbon disulfide study on the synthesis of 2-oxazolidinethiones and 2-thiazolidinethiones. J Org Chem 1995; 60: 6604–6607. [Google Scholar]
- 114.Rios Morales EH, Arbelo Román C, Thomann JO, et al. Linear synthesis of chiral cycloSal-pronucleotides. Eur J Org Chem. 2011; 23: 4397–4408. [Google Scholar]
- 115.Arbelo RC, Wasserthal P, Balzarini J, et al. Diastereoselective synthesis of (aryloxy)phosphoramidate prodrugs. Eur J Org Chem 2011; 25: 4899–4909. [Google Scholar]
- 116.Pertusati F, McGuigan C. Diastereoselective synthesis of P-chirogenic phosphoramidate prodrugs of nucleoside analogues (ProTides) via copper catalysed reaction. Chem Commun 2015; 51: 8070–8073. [DOI] [PubMed] [Google Scholar]
- 117.DiRocco DA, Ji Y, Sherer EC, et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science 2017; 356: 426–430. [DOI] [PubMed] [Google Scholar]
- 118.Gao L-J, Jonghe SD, Herdewijn P. Synthesis of a nucleobase-modified ProTide library. Org Lett 2016; 18: 5816–5819. [DOI] [PubMed] [Google Scholar]
- 119.Ballatore C, McGuigan C, De Clercq E, et al. Synthesis and evaluation of novel amidate prodrugs of PMEA and PMPA. Bioorg Med Chem Lett 2001; 11: 1053–1056. [DOI] [PubMed] [Google Scholar]
- 120.Chapman H, Kernan M, Prisbe E, et al. Practical synthesis, separation, and stereochemical assignment of the Pmpa pro-drug Gs-7340. Nucleos Nucleot Nucl 2001; 20: 621–628. [DOI] [PubMed] [Google Scholar]
- 121.Thatipally S, Dammalapati VLNR, Kallam VSRR, et al. An improved process for the preparation of tenofovir alafenamide or pharmaceutically acceptable salts thereof. WO2015040640 A3; PCT/IN2014/000614, 2015.
- 122.Thatipally S, Udutha KS, Kotala MB, et al. , A recycling process for preparing tenofovir alafenamide diastereomers. WO2015079455 A3; PCT/IN2014/000734, 2015.
- 123.Jansa P, Baszczynski O, Dracinsky M, et al. A novel and efficient one-pot synthesis of symmetrical diamide (bis-amidate) prodrugs of acyclic nucleoside phosphonates and evaluation of their biological activities. Eur J Med Chem 2011; 46: 3748–3754. [DOI] [PubMed] [Google Scholar]
- 124.Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther 2000; 87: 189–198. [DOI] [PubMed] [Google Scholar]
- 125.Saboulard D, Naesens L, Cahard D, et al. Characterization of the activation pathway of phosphoramidate triester prodrugs of stavudine and zidovudine. Mol Pharmacol 1999; 56: 693–704. [PubMed] [Google Scholar]
- 126.Bourdin C, McGuigan C, Brancale A, et al. Synthesis and evaluation against hepatitis C virus of 7-deaza analogues of 2'-C-methyl-6-O-methyl guanosine nucleoside and L-alanine ester phosphoramidates. Bioorg Med Chem Lett 2013; 23: 2260–2264. [DOI] [PubMed] [Google Scholar]
- 127.Birkus G, Wang R, Fau-Liu X, et al. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob Agents Chemother 2007; 51: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Congiatu C, Brancale A, McGuigan C. Molecular modelling studies on the binding of some protides to the putative human phosphoramidase Hint1. Nucleos Nucleot Nucl 2007; 26: 1121–1124. [DOI] [PubMed] [Google Scholar]
- 129.Brenner C. Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases. Biochem 2002; 41: 9003–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lima CD, Klein MG, Hendrickson WA. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science 1997; 278: 286–290. [DOI] [PubMed] [Google Scholar]
- 131.Ozga M, Dolot R, Janicka M, et al. Histidine triad nucleotide-binding protein 1 (HINT-1) phosphoramidase transforms nucleoside 5′-O-phosphorothioates to nucleoside 5’-O-phosphates. J Biol Chem 2010; 285: 40809–40818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chou T-F, Baraniak J, Kaczmarek R, et al. Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and Escherichia coli histidine triad nucleotide binding proteins. Mol Pharmaceut 2007; 4: 208–217. [DOI] [PubMed] [Google Scholar]
- 133.Murakami E, Tolstykh T, Bao H, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem 2010; 258: 34337–34347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shah R, Maize KM, Zhou X, et al. Caught before released: structural mapping of the reaction trajectory for the sofosbuvir activating enzyme, human histidine triad nucleotide binding protein 1 (hHint1). Biochem 2017; 56: 3559–3570. [DOI] [PubMed] [Google Scholar]
- 135.Derudas M, Carta D, Brancale A, et al. The application of phosphoramidate protide technology to acyclovir confers anti-HIV inhibition. J Med Chem 2009; 52: 5520–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Curley D, McGuigan C, Devine KG, et al. Synthesis and anti-HIV evaluation of some phosphoramidate derivatives of AZT: studies on the effect of chain elongation on biological activity. Antiviral Research 1990; 14: 345–356. [DOI] [PubMed] [Google Scholar]
- 137.McGuigan C, Devine KG, O’Connor TJ, et al. Synthesis and anti-HIV activity of some haloalkyl phosphoramidate derivatives of 3’-azido-3’-deoxythymidine (AZT): potent activity of the trichloroethyl methoxyalaninyl compound. Antiviral Res 1991; 15: 255–263. [DOI] [PubMed] [Google Scholar]
- 138.McGuigan C, Nickson C, Petrik J, et al. Phosphate derivatives of AZT display enhanced selectivity of action against HIV1 by comparison to the parent nucleoside. FEBS Lett 1992; 310: 171–174. [DOI] [PubMed] [Google Scholar]
- 139.McGuigan C, Pathirana RN, Mahmood N, et al. Aryl phosphates derivatives of AZT retain activity against HIV1 in cell lines which are resistant to the action of AZT. Antiviral Res 1992; 17: 311–321. [DOI] [PubMed] [Google Scholar]
- 140.McGuigan C, Pathirana RN, Mahmood N, et al. Aryl phosphate derivatives of AZT inhibit HIV replication in cells where the nucleoside is poorly active. Bioorg Med Chem Lett 1992; 2: 701–704. [Google Scholar]
- 141.McGuigan C, Cahard D, Sheeka HM, et al. Phosphoramidate derivatives of d4T with improved anti-HIV efficacy retain full activity in thymidine kinase-deficient cells. Bioorg Med Chem Lett 1996; 6: 1183–1186. [Google Scholar]
- 142.McGuigan C, Tsang H-W, Cahard D, et al. Phosphoramidate derivatives of d4T as inhibitors of HIV: the effect of amino acid variation. Antiviral Res 1997; 35: 195–204. [DOI] [PubMed] [Google Scholar]
- 143.McGuigan C, Cahard D, Sheeka HM, et al. Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J Med Chem 1996; 39: 1748–1753. [DOI] [PubMed] [Google Scholar]
- 144.Siddiqui AQ, Ballatore C, McGuigan C, et al. The presence of substituents on the aryl moiety of the aryl phosphoramidate derivative of d4T enhances anti-HIV efficacy in cell culture: a structure−activity relationship. J Med Chem 1999; 42: 393–399. [DOI] [PubMed] [Google Scholar]
- 145.Siddiqui AQ, McGuigan C, Ballatore C, et al. Design and synthesis of lipophilic phosphoramidate d4T-MP prodrugs expressing high potency against HIV in cell culture: structural determinants for in vitro activity and QSAR. J Med Chem 1999; 42: 4122–4128. [DOI] [PubMed] [Google Scholar]
- 146.Ballatore C, McGuigan C, De Clercq E, et al. Pig liver esterase assay as a useful predictive tool for the likely in vitro antiviral activity of phosphoramidate pro-drugs. Nucleos Nucleot Nuc 1999; 18: 967–969. [DOI] [PubMed] [Google Scholar]
- 147.Balzarini J, Kruining J, Wedgwood O, et al. Conversion of 2’,3’-dideoxyadenosine (ddA) and 2’,3’-didehydro-2’,3’-dideoxyadenosine (d4A) to their corresponding aryloxyphosphoramidate derivatives markedly potentiates their activity against human immunodeficiency virus and hepatitis B virus. FEBS Lett. 1997; 410: 324–328. [DOI] [PubMed] [Google Scholar]
- 148.Siddiqui AQ, McGuigan C, Ballatore C, et al. Simple mono-derivatisation of the aryl moiety of D4A and DDA-based phosphoramidate prodrugs significantly enhances their anti-HIV potency in cell culture. Bioorg Med Chem Lett 1999; 9: 2555–2560. [DOI] [PubMed] [Google Scholar]
- 149.Aquarq S, Wedgwood O, Yarnold C, et al. Activities of masked 2',3'-dideoxynucleoside monophosphate derivatives against human immunodeficiency virus in resting macrophages. Antimicrob Agents Chemother 2000; 44: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gudmundsson KS, Daluge SM, Johnson LC, et al. Phosphoramidate protides of 2',3'-dideoxy-3'-fluoroadenosine and related nucleosides with potent activity against HIV and HBV. Nucleotides Nucleic Acids 2003; 22: 1953–1961. [DOI] [PubMed] [Google Scholar]
- 151.McGuigan C, Bellevergue P, Sheeka H, et al. Certain phosphoramidate derivatives of dideoxy uridine (ddU) are active against HIV and successfully bypass thymidine kinase. FEBS Lett 1994; 351: 11–14. [DOI] [PubMed] [Google Scholar]
- 152.Mehellou Y, McGuigan C, Brancale A, et al. Design, synthesis, and anti-HIV activity of 2',3'-didehydro-2',3'-dideoxyuridine (d4U), 2',3'-dideoxyuridine (ddU) phosphoramidate ‘ProTide’ derivatives. Bioorg Med Chem Lett 2007; 17: 3666–3669. [DOI] [PubMed] [Google Scholar]
- 153.Mehellou Y, Balzarini J, McGuigan C. An investigation into the anti-HIV activity of 2',3'-didehydro-2',3'-dideoxyuridine (d4U) and 2',3'-dideoxyuridine (ddU) phosphoramidate ProTide derivatives. Org Biomol Chem 2009; 7: 2548–2553. [DOI] [PubMed] [Google Scholar]
- 154.McGuigan C, Harris SA, Daluge SM, et al. Application of phosphoramidate pronucleotide technology to abacavir leads to a significant enhancement of antiviral potency. J Med Chem 2005; 48: 3504–3515. [DOI] [PubMed] [Google Scholar]
- 155.Gudmundsson KS, Wang Z, Daluge SM, et al. Phosphoramidate protides of carbocyclic 2',3'-dideoxy-2',3'-didehydro-7-deazaadenosine with potent activity against HIV and HBV. Nucleosides Nucleotides Nucleic Acids 2004; 23: 1929–1937. [DOI] [PubMed] [Google Scholar]
- 156.McGuigan C, Hassan-Abdallah A, Srinivasan S, et al. Application of phosphoramidate ProTide technology significantly improves antiviral potency of carbocyclic adenosine derivatives. J Med Chem 2006; 49: 7215–7226. [DOI] [PubMed] [Google Scholar]
- 157.Meneghesso S, Vanderlinden E, Brancale A, et al. Synthesis and biological evaluation of purine 2'-fluoro-2'-deoxyriboside ProTides as anti-influenza virus agents. ChemMedChem 2013; 8: 415–425. [DOI] [PubMed] [Google Scholar]
- 158.Derudas M, Brancale A, Naesens L, et al. Application of the phosphoramidate ProTide approach to the antiviral drug ribavirin. Bioorg Med Chem 2010; 18: 2748–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Derudas M, Quintiliani M, Brancale A, et al. Evaluation of novel phosphoramidate ProTides of the 2'-fluoro derivatives of a potent anti-varicella zoster virus bicyclic nucleoside analogue. Antiviral Chem Chemother 2010; 21: 15–31. [DOI] [PubMed] [Google Scholar]
- 160.Quintiliani M, Persoons L, Solaroli N, et al. Design, synthesis and biological evaluation of 2'-deoxy-2',2'-difluoro-5-halouridine phosphoramidate ProTides. Bioorg Med Chem 2011; 19: 4338–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Toti KS, Derudas M, McGuigan C, et al. Synthesis and antiviral evaluation of α-L-2'-deoxythreofuranosyl nucleosides. Eur J Med Chem 2011; 46: 3704–3713. [DOI] [PubMed] [Google Scholar]
- 162.Toti KS, Derudas M, Pertusati F, et al. Synthesis of an apionucleoside family and discovery of a prodrug with anti-HIV activity. J Org Chem 2014; 79: 5097–5112. [DOI] [PubMed] [Google Scholar]
- 163.Bamford MJ, Humber DC, Storer R. Synthesis of (±)-2’-oxa-carbocyclic-2’,3’-dideoxynucleosides as potential anti-HIV agents. Tetrahedron Lett 1991; 32: 271–274. [Google Scholar]
- 164.Nair V, Jahnke TS. Antiviral activities of isometric dideoxynucleosides of D- and L-related stereochemistry. Antimicrob Agents Chemother 1995; 39: 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe 2008; 4: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McGuigan C, Slater MJ, Parry NR, et al. Synthesis and antiviral activity of acyclovir-5'-(phenylmethoxyalaninyl) phosphate as a possible membrane-soluble nucleotide prodrug. Bioorg Med Chem Lett 2000; 10: 645–647. [DOI] [PubMed] [Google Scholar]
- 167.Vanpouille C, Lisco A, Derudas M, et al. A new class of dual-targeted antivirals: monophosphorylated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2. J Infect Dis 2010; 201: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Derudas M, Vanpouille C, Carta D, et al. Virtual screening of acyclovir derivatives as potential antiviral agents: design, synthesis, and biological evaluation of new acyclic nucleoside ProTides. J Med Chem 2017; 60: 7876–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perrone P, Luoni GM, Kelleher MR, et al. Application of the phosphoramidate ProTide approach to 4’-azidouridine confers sub-micromolar potency versus hepatitis C virus on an inactive nucleoside. J Med Chem 2007; 50: 1840–1849. [DOI] [PubMed] [Google Scholar]
- 170.Perrone P, Daverio F, Valente R, et al. First example of phosphoramidate approach applied to a 4’-substituted purine nucleoside (4-azidoadenosine): conversion of an inactive nucleoside to a submicromolar compound versus hepatitis C virus. J Med Chem 2007; 50: 5463–5470. [DOI] [PubMed] [Google Scholar]
- 171.McGuigan C, Kelleher MR, Perrone P, et al. The application of phosphoramidate ProTide technology to the potent anti-HCV compound 4’-azidocytidine (R1479). Bioorg Med Chem Lett 2009; 19: 4250–4254. [DOI] [PubMed] [Google Scholar]
- 172.McGuigan C, Daverio F, Najera I, et al. The application of the phosphoramidate ProTide approach confers micromolar potency against hepatitis C virus on inactive agent 4'-azidoinosine: kinase bypass on a dual base/sugar modified nucleoside. Bioorg Med Chem Lett 2009; 19: 3122–3124. [DOI] [PubMed] [Google Scholar]
- 173.McGuigan C, Madela K, Aljarah M, et al. Design, synthesis and evaluation of a novel double prodrug: INX-08189. A new clinical candidate for hepatitis C virus. Bioorg Med Chem Lett 2010; 20: 4850–4854. [DOI] [PubMed] [Google Scholar]
- 174.Vernachio JH, Bleiman B, Bryant KD, et al. INX-08189, a phosphoramidate prodrug of 6-O-methyl-2'-C-methyl guanosine, is a potent inhibitor of hepatitis C virus replication with excellent pharmacokinetic and pharmacodynamic properties. Antimicrob Agents Chemother 2011; 55: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Barry A, Patti J, Matson M, et al. A study of the safety and pharmacokinetics of single ascending oral doses of INX-08189, a nucleotide of polymerase inhibitor, in healthy subjects. ID 460. 46th Annual meeting of the European Association of the Study of the Liver MA, 2011, Berlin, Germany.
- 176.Patti J, Matson M, Boehlecke B, et al. 460 A study of the safety and pharmacokinetics of single ascending oral doses of INX-08189, a nucleotide polymerase inhibitor, in healthy subjects. . J Hepatol 2011. 54: S187. [Google Scholar]
- 177.http://www.businesswire.com/news/home/20120823006303/en/Bristol-Myers-Squibb-Discontinues-Development-BMS-986094-Investigational-NS5B (accessed 16 October 2017).
- 178.Liu A, Lute J, Gu H, et al. Challenges and solutions in the bioanalysis of BMS-986094 and its metabolites including a highly polar, active nucleoside triphosphate in plasma and tissues using LC–MS/MS. J Chromatogr B 2015; 1000: 29–40. [DOI] [PubMed] [Google Scholar]
- 179.Chang W, Bao D, Chun B-K, et al. Discovery of PSI-353661, a novel purine nucleotide prodrug for the treatment of HCV infection. ACS Med Chem Lett 2011; 2: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Furman PA, Murakami E, Niu C, et al. Activity and the metabolic activation pathway of the potent and selective hepatitis C virus pronucleotide inhibitor PSI-353661. Antiviral Res 2011; 91: 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jonckers THM, Lin T-I, Buyck C, et al. Deoxy-2’-spirocyclopropylcytidine revisited: a new and selective inhibitor of the hepatitis C virus NS5B polymerase. J Med Chem 2010; 53: 8150–8160. [DOI] [PubMed] [Google Scholar]
- 182.Du J, Chun B-K, Mosley RT, et al. Use of 2’-spirocyclic ethers in HCV nucleoside design. J Med Chem 2014; 57: 1826–1835. [DOI] [PubMed] [Google Scholar]
- 183.Jonckers THM, Vandyck K, Vandekerckhove L, et al. Nucleotide prodrugs of 2’-deoxy-2’-spirooxetane ribonucleosides as novel inhibitors of the HCV NS5B polymerase. J Med Chem 2014; 57: 1836–1844. [DOI] [PubMed] [Google Scholar]
- 184.Jonckers THM, Tahri A, Vijgen L, et al. Discovery of 1-((2R,4aR,6R,7R,7aR)-2-isopropoxy-2-oxidodihydro-4H,6H-spiro[furo[3,2-d][1,3,2]dioxaphosphinine-7,2’-oxetan]-6-yl)pyrimidine-2,4(1H,3H)-dione (JNJ-54257099), a 3’,5’-cyclic phosphate ester prodrug of 2’-deoxy-2’-spirooxetane uridine triphosphate useful for HCV inhibition. J Med Chem 2016; 59: 5790–5798. [DOI] [PubMed] [Google Scholar]
- 185.Mackman RL, Ray AS, Hui HC, et al. Discovery of GS-9131: design, synthesis and optimization of amidate prodrugs of the novel nucleoside phosphonate HIV reverse transcriptase (RT) inhibitor GS-9148. Bioorg Med Chem 2010; 18: 3606–3617. [DOI] [PubMed] [Google Scholar]
- 186.Cha A, Budovich A. Sofosbuvir: a new oral once-daily agent for the treatment of hepatitis C virus infection. Pharmacy Therapeut 2014; 39: 345–352. [PMC free article] [PubMed] [Google Scholar]
- 187.Babusis D, Denning JM, Wang T, et al. Translational studies to understand the mechanism of liver delivery by sofosbuvir. 15th Internationl workshop on clinical pharmacology of HIV and hepatitis therapy, 19–21 May 2014, Washington, DC. [Mismatch]
- 188.Kirby BJ, Symonds WT, Kearney BP, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin Pharmacokinet 2015; 54: 677–690. [DOI] [PubMed] [Google Scholar]
- 189.Rodriguez-Torres M, Lawitz E, Fau-Kowdley KV, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naive patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol 2013; 58: 663–668. [DOI] [PubMed] [Google Scholar]
- 190.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368: 1878–1887. [DOI] [PubMed] [Google Scholar]
- 191.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368: 1867–1877. [DOI] [PubMed] [Google Scholar]
- 192.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370: 1993–2001. [DOI] [PubMed] [Google Scholar]
- 193.https://www.drugs.com/newdrugs/fda-approves-sovaldi-chronic-hepatitis-c-3986.htmlhttps://www.drugs.com/newdrugs/fda-approves-sovaldi-chronic-hepatitis-c-3986.html (accessed 16 October 2017).
- 194.https://www.drugs.com/newdrugs/fda-approves-sovaldi-sofosbuvir-chronic-hepatitis-c-infection-pediatric-patients-12-years-older-4515.html (accessed 16 October 2017).
- 195.Robbins BL, Srinivas RV, Kim C, et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 1998; 42: 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shaw J-P, Sueoka CM, Oliyai R, et al. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm Res 1997; 14: 1824–1829. [DOI] [PubMed] [Google Scholar]
- 197.Durand-Gasselin L, Van Rompay KKA, Vela JE, et al. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol Pharmaceut 2009; 6: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010; 51: 496–505. [DOI] [PubMed] [Google Scholar]
- 199.Tong L, Phan TK, Robinson KL, et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother 2007; 51: 3498–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS 2009; 23: 1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir df vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292: 191–201. [DOI] [PubMed] [Google Scholar]
- 202.Mateo L, Holgado S, Mariñoso ML, et al. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin Rheumatol 2016; 35: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 203.Lee WA, He G-X, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005; 49: 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Murakami E, Wang T, Park Y, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother 2015; 59: 3563–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Birkus G, Bam RA, Willkom M, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother 2016; 60: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Markowitz M, Zolopa A, Squires K, et al. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother 2014; 69: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 207.Gupta S, Pozniak A, Arribas J.et al. Subjects with renal impairment switching from tenofovir disoproxil fumarate to tenofovir alafenamide have improved renal and bone safety through 48 weeks. J Int Aids Soc 2015; 18: 35–36, TUAB0103, 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention, 19-22 July, 2015, Vancouver, Canada.
- 208.http://www.gilead.com/new/press-releases/2015/11/us-food-and drug-administration-approves-gileads-single-tablet-regimen-genvoya-elvitegravir-cobicistat-emtricitabine-and-tenofovir-alafenamide-for-treatment-of-hiv1-infection (accessed 16 October 2017).
- 209.http://www.gilead.com/news/press-releases/2016/11/us-food-and-drug-administration-approves-gileads-vemlidy-tenofovir-alafenamide-for-the-treatment-of-chronic-hepatitis-B-virus-infection (accessed 16 October 2017).
- 210.D’Cruz OJ, Uckun FM. Stampidine: a selective oculo-genital microbicide. J Antimicrob Chemother 2005; 56: 10–19. [DOI] [PubMed] [Google Scholar]
- 211.Chen C-L, Venkatachalam TK, Zhu Z-H, et al. In vivo pharmacokinetics and metabolism of anti-human immunodeficiency virus agent d4T-5’-[p-bromophenyl methoxyalaninyl phosphate] (SAMPIDINE) in mice. Drug Metabol Disposit 2001; 29: 1035–1041. [PubMed] [Google Scholar]
- 212.Vig R, Venkatachalam TK, Uckun FM. D4T-5'-[p-bromophenyl methoxyalaninyl phosphate] as a potent and non-toxic anti-human immunodeficiency virus agent. Antiviral Chem Chemother 1998; 9: 445–448. [PubMed] [Google Scholar]
- 213.Venkatachalam TK, Tai HL, Vig R, et al. Enhancing effects of a mono-bromo substitution at the para position of the phenyl moiety on the metabolism and anti-HIV activity of d4T-phenyl methoxyalaninyl phosphate derivatives. Bioorg Med Chem Lett 1998; 8: 3121–3126. [DOI] [PubMed] [Google Scholar]
- 214.Balzarini J, Karlsson A, Aquaro S, et al. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc Natl Acad Sci U S A 1996; 93: 7295–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Venkatachalam TK, Samuel P, Uckun FM. Stereochemical influence on lipase-mediated hydrolysis and biological activity of stampidine and other stavudine phosphoramidates. Bioorg Med Chem 2005; 13: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 216.Venkatachalam TK, Samuel P, Li G, et al. Lipase-mediated stereoselective hydrolysis of stampidine and other phosphoramidate derivatives of stavudine. Bioorg Med Chem 2004; 12: 3371–3781. [DOI] [PubMed] [Google Scholar]
- 217.Venkatachalam TK, Yu G, Samuel P, et al. A comparative study of the hydrolysis pathways of substituted aryl phosphoramidate versus aryl thiophosphoramidate derivatives of stavudine. Eur J Med Chem 2004; 39: 665–683. [DOI] [PubMed] [Google Scholar]
- 218.Cahn P, Rolon MJ, Gun AM, et al. Preclinical and first-in-human phase I clinical evaluation of stampidine, a potent anti-HIV pharmaceutical drug candidate. J AIDS Clin Res 2012; 3: 1. [Google Scholar]
- 219.Uckun FM, Cahn P, Qazi S, et al. Stampidine as a promising antiretroviral drug candidate for pre-exposure prophylaxis against sexually transmitted HIV/AIDS. Expert Opin Investig Drugs 2012; 21: 489–500. [DOI] [PubMed] [Google Scholar]
- 220.Alexandre FR, Badaroux E, Bilello JP, et al. The discovery of IDX21437: design, synthesis and antiviral evaluation of 2'-α-chloro-2'-β-C-methyl branched uridine pronucleotides as potent liver-targeted HCV polymerase inhibitors. Bioorg Med Chem Lett 2017; 27: 4323–4330. [DOI] [PubMed] [Google Scholar]
- 221.Wyles D, Wedemeyer H, Ben-Ari Z.et al. Grazoprevir, ruzasvir, and uprifosbuvir for HCV after NS5A treatment failure. Hepatology 2017; 66: 1794–1804. [DOI] [PubMed]
- 222.https://clinicaltrials.gov/ct2/show/NCT02332720https://clinicaltrials.gov/ct2/show/NCT02332720 (accessed 12 October 2017).
- 223.https://clinicaltrials.gov/ct2/show/NCT02759315 (accessed 12 October 2017).
- 224.https://clinicaltrials.gov/ct2/show/NCT02613403 (accessed 12 October 2017).
- 225.http://investors.merck.com/news/press-release-details/2017/Merck-Discontinues-MK-3682B-and-MK-3682C-Development-Programs/default.aspxhttp://investors.merck.com/news/press-release-details/2017/Merck-Discontinues-MK-3682B-and-MK-3682C-Development-Programs/default.aspx (accessed 19 October 2017).
- 226.Cho A, Zhang L, Xu J, et al. Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients. J Med Chem 2014; 57: 1812–1825. [DOI] [PubMed] [Google Scholar]
- 227.Migliaccio G, Tomassini JE, Carroll SS, et al. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J Biol Chem 2003; 278: 49164–49170. [DOI] [PubMed] [Google Scholar]
- 228.Feng JY, Cheng G, Perry J, et al. Inhibition of hepatitis C virus replication by GS-6620, a potent C-nucleoside monophosphate prodrug. Antimicrob Agents Chemother 2014; 58: 1930–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Murakami E, Wang T, Babusis D, et al. Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620. Antimicrob Agents Chemother 2014; 58: 1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.https://clinicaltrials.gov/ct2/show/NCT01316237https://clinicaltrials.gov/ct2/show/NCT01316237 (accessed 19 October 2017).
- 231.Lawitz E, Hill J, Marbury T, et al. GS-6620, a liver-targeted nucleotide prodrug, exhibits antiviral activity and favorable safety profile over 5 days in treatment naïve chronic HCV genotype 1 subjects. EASL 1188, Journal of Hepatology. 2012; 56: S389–S548. [Google Scholar]
- 232.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against ebola virus in rhesus monkeys. Nature 2016; 531: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Siegel D, Hui HC, Doerffler E, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of ebola and emerging viruses. J Med Chem 2017; 60: 1648–1661. [DOI] [PubMed] [Google Scholar]
- 234.Lo MK, Jordan R, Arvey A, et al. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci Rep 2017; 7: 43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.https://clinicaltrials.gov/ct2/show/NCT02818582https://clinicaltrials.gov/ct2/show/NCT02818582 (accessed 19 October 2017).