Short abstract
Background
Disease activity differs in young patients with multiple sclerosis (MS) compared with the overall adult MS population.
Objective
The objective of this paper is to evaluate the effect of fingolimod 0.5 mg on disease activity in young adults with MS from three randomized, double-blind Phase 3 trials.
Methods
Annualized relapse rate (ARR), number of new/newly enlarging T2 lesions (neT2), and no evidence of disease activity (NEDA-3) were estimated in the intent-to-treat population at age 20 (youngest) and 30 (young) and compared to the overall population. Models used included a negative binomial regression (ARR/neT2) and a logistic regression (NEDA), with age at baseline as a continuous covariate.
Results
ARRs were higher in younger patients (all p < 0.05), and significantly reduced with fingolimod versus placebo or interferon beta-1a (IFN β-1a), with the percentage reduction inversely proportional to age. Fingolimod was significantly associated with a lower number of neT2 lesions versus placebo/IFN in all age groups except versus IFN in the youngest patients. Regardless of age, fingolimod-treated patients were more likely to achieve NEDA-3 versus placebo/IFN β-1a, with strongest benefits in the youngest patients (all p < 0.05).
Conclusions
Young adults show higher levels of MS disease activity, and may particularly benefit from fingolimod treatment compared with the overall study population.
Keywords: Age, disability, fingolimod, multiple sclerosis, relapse rates, young adult
Introduction
Multiple sclerosis (MS) is the most common neurological disease affecting young and middle-aged adults that causes significant disability resulting in poor health-related quality of life.1 Patients with early-onset MS tend to relapse more frequently with higher magnetic resonance imaging (MRI) activity compared with older patients.2–5 Consistent with this observation, studies focusing on children found that the annualized relapse rate (ARR) in pediatric MS patients was generally higher than in adult patients with MS.6,7 Although patients with earlier disease onset accumulate irreversible disability at a slower rate compared with their adult counterparts, they reach significant disability at a younger age and develop a progressive MS course earlier.8–11 Evidence from natural history studies indeed indicates that age at clinical onset is an important predictor for poor prognosis of MS, in that the earlier the onset, the younger the age at attainment of disability milestones.12–14 In spite of the significant burden of MS in pediatric and young adult patients, in practice, controlled studies in MS generally recruit adult patients with an average age of 36–41 years,15–17 and to-date little information is available from clinical trials in patients with pediatric MS,18,19 with only one randomized controlled trial conducted.20 Thus, more systematic analyses of MS disease activity in young patients from well-controlled trials are needed.
Fingolimod 0.5 mg (Gilenya®, Novartis Pharma AG) was found to be effective on both clinical and MRI outcomes versus placebo or interferon beta-1a (IFN β-1a) intramuscular (IM) injection based on data from three Phase 3 trials.15–17 In this study, we analyzed data from FREEDOMS, FREEDOMS II, and TRANSFORMS to characterize the relationship between age and MS disease activity (including relapses and MRI lesion activity) and to evaluate the efficacy and safety of fingolimod 0.5 mg versus placebo/IFN β-1a in young adult patients compared with the overall study population.
Patients and Methods
Study Population
This post hoc analysis included patients from the pivotal fingolimod clinical trials FREEDOMS (NCT00289978), FREEDOMS II (NCT00355134), and TRANSFORMS (NCT00340834). The study designs and patient populations of all three clinical studies have been published previously,15–17 and the analysis in this article does not include new patients. FREEDOMS15 and FREEDOMS II16 were both 24-month, placebo-controlled, parallel-group studies conducted in 1272 and 1083 patients, aged 18 to 55 years, respectively. Patients were randomized in a 1:1:1 ratio to receive once-daily fingolimod 0.5 mg, fingolimod 1.25mg, or placebo. TRANSFORMS17 was a 12-month, active-controlled, parallel-group trial including 1292 patients aged 18 to 55 years, randomized in a 1:1:1 ratio to receive once-daily fingolimod 0.5 mg, fingolimod 1.25 mg, or IFN β-1a IM injection.
Statistical Analysis
For each trial, baseline information was summarized for patients aged ≤20 years, ≤30 years and for the overall population. The relationship between MS disease activity and age was evaluated based on the entire intent-to-treat (ITT) population in each trial. Disease activity was analyzed as a continuous function of age in statistical models. ARR, number of new/newly enlarging T2 (neT2) lesions and no evidence of disease activity (NEDA-3) were estimated at age 20 years and 30 years and compared with that of the overall population. ARR and neT2 lesions were both analyzed in negative binomial regression model with log-link. The models were adjusted for treatment, age, and age-by-treatment interaction. The ARR model used log time-in-study as an offset to standardize the relapse rates to one year. The relationship between age and disease activity was tested for each treatment based on the statistical model.
To test the robustness of our findings on ARR, three sensitivity analyses were conducted: (a) by excluding patients who dropped out of the study before completing three months, (b) by excluding patients who dropped out of the study before completing six months and (c) by excluding patients who had an ARR of >2.5 estimated on an individual level. NEDA-3, defined as absence of T1 gadolinium-enhancing (Gd+) and neT2 lesions, no confirmed relapse, and no three-month confirmed Expanded Disability Status Scale (EDSS) progression, was analyzed in a logistic regression model with treatment as a factor, age as a continuous explanatory variable, and an age-by-treatment interaction.
Safety in young adult patients was analyzed based on adverse events (AEs) in the subgroup of patients aged ≤30 years using the combined data of all the controlled Phase 2/3 trials of the fingolimod relapsing–remitting MS (RRMS) program. Overall AEs with fingolimod 0.5 mg (n = 1364) and placebo (n = 966) are also reported for comparison purposes. No separate safety analysis was carried out for patients aged ≤20 years because of the low sample size for an evaluation.
Results
Baseline Characteristics
The baseline demographic and disease characteristics for patients at age ≤20 and ≤30 years and for the overall population are presented in Table 1. Of the patients in the FREEDOMS, FREEDOMS II and TRANSFORMS studies, 28/1272 (2.2%), 17/1083 (1.6%), and 40/1292 (3.1%), respectively, were ≤20 years of age; and 325/1272 (25.6%), 150/1083 (13.9%) and 355/1292 (27.5%), respectively, were ≤30 years of age at baseline. The median disease duration at baseline was 2.1–2.4 years and 3.4–4.9 years in patients aged ≤20 and ≤30 years, respectively. Compared with the overall population, patients at age ≤20 and ≤30 years showed lower EDSS scores and a higher number of T1 Gd+ lesions at baseline.
Table 1.
Demographic and baseline disease characteristics of patients at age ≤20 years, ≤30 years and the overall population.
| FREEDOMS | FREEDOMS II | TRANSFORMS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age ≤20 years n = 28 |
Age ≤30 years n = 325 |
Overall population N = 1272 |
Age ≤20 years n = 17 |
Age ≤30 years n = 150 |
Overall population N = 1083 |
Age ≤20 years n = 40 |
Age ≤30 years n = 355 |
Overall population N = 1292 |
|
| Demographics | |||||||||
| Age, years | 19.0 ± 0.84 | 25.8 ± 3.33 | 37.1 ± 8.76 | 19.5 ± 0.80 | 25.9 ± 3.39 | 40.5 ± 8.58 | 19.2 ± 0.83 | 25.5 ± 3.39 | 36.2 ± 8.5 |
| Female, n (%) | 22 (78.6%) |
217 (66.8%) | 889 (69.9%) | 12 (70.6%) |
113 (75.3%) | 844 (77.9%) | 34 (85.0%) |
246 (69.3%) |
870 (67.3%) |
| Baseline disease characteristics | |||||||||
| Disease durationsince onset ofsymptoms, years,median (range) | 2.1 (0–9) |
3.4 (0–18) |
6.7 (0–37) |
2.4 (0–10) |
4.9 (0–18) |
8.9 (0–50) |
2.2 (0.3–8.4) |
3.7 (0.2–16.5) |
5.9 (0–40) |
| EDSS score | 2.0 ± 1.38 | 1.86 ± 1.22 | 2.4 ± 1.32 | 1.74 ± 1.06 | 1.88 ± 1.13 | 2.4 ± 1.32 | 1.74 ± 1.11 | 1.73 ± 1.14 | 2.2 ± 1.3 |
| Relapses in theprevious year | 1.6 ± 0.74 | 1.6 ± 0.88 | 1.5 ± 0.77 | 1.5 ± 0.72 | 1.6 ± 0.96 | 1.5 ± 0.96 | 1.7 ± 1.07 | 1.65 ± 0.94 | 1.5 ± 0.97 |
| Number of Gd+ T1lesions | 1.7 ± 3.11 | 2.7 ± 7.2 | 1.6 ± 4.53 | 5.4 ± 10.83 | 2.6 ± 5.62 | 1.3 ± 3.41 | 1.6 ± 4.63 | 2.0 ± 5.8 | 1.2 ± 3.57 |
| Volume of T2lesions, mm3 | 7432.64 ± 8961.86 |
6140.69 ± 7802.09 |
6374.6 ± 7759.71 |
4468.7 ± 3764.22 |
5470.95 ± 6391.42 |
5319.8 ± 7708.45 |
3668.3 ± 3816.07 |
4723.62 ± 6129.61 |
5059.5 ± 6116.41 |
Data presented are mean ± standard deviation unless otherwise specified.
EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing.
Annualized relapse rate
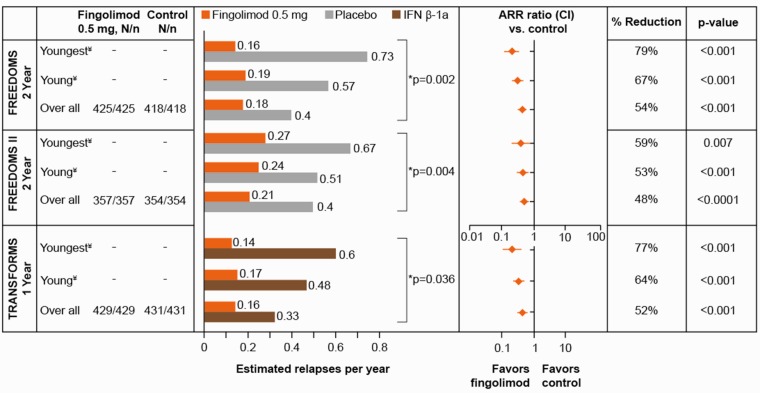
In the control arms of all three Phase 3 trials (placebo in FREEDOMS and FREEDOMS II, or IFN β-1a in TRANSFORMS), young adult patients relapsed more frequently compared with the overall population (Figure 1). For the placebo arm, patients at age 20 years, 30 years or in the overall population, the ARR in FREEDOMS was 0.73, 0.57, and 0.40, respectively and in FREEDOMS II was 0.67, 0.51, and 0.40, respectively (p = 0.002 and p = 0.004 for the relationship between age and ARR). The corresponding values for the IFN β-1a arm of TRANSFORMS were 0.60, 0.48, and 0.33 (p = 0.036 for the relationship between age and ARR). Compared with the overall population, the youngest adult patients (20 years of age) in the FREEDOMS and FREEDOMS II placebo arms had a 1.7- to 1.8-fold higher ARR.
Figure 1.
Estimated ARRs at age 20 years, age 30 years and in the overall population in the FREEDOMS, FREEDOMS II, and TRANSFORMS trials. ARR ratio <1 favors fingolimod 0.5 mg compared with controls. *P values for relationship between age and ARR in control patients. ¥Values for patients at age 20 or 30 years are based on a statistical model using all data in the ITT population. N = patients in ITT, n = patients with available data. Control refers to placebo for the FREEDOMS and FREEDOMS II trials, and IFN β-1a for the TRANSFORMS trial. Youngest and young MS patients refer to the estimates at age 20 years and 30 years, respectively, in all three studies. ARR: annualized relapse rate; CI: confidence interval; IFN β-1a: interferon beta-1a; ITT: intent-to-treat; MS: multiple sclerosis.
In fingolimod 0.5 mg-treated patients, there was no significant association between age and ARR, with reductions in ARR being consistently lower across all age groups (0.14 − 0.27) compared with placebo/IFN β-1a. The percentage reduction in ARR associated with fingolimod 0.5 mg compared with placebo or IFN β-1a was inversely proportional to the age, with the youngest patients showing the strongest reductions (versus placebo: 79% in FREEDOMS, 59% in FREEDOMS II; and versus IFN β-1a: 77% in TRANSFORMS). Sensitivity analyses performed to test the robustness of the relationship between young age and high ARR, and to test the superiority of fingolimod 0.5 mg versus placebo/IFN β-1a, based on predetermined criteria, did not change the results.
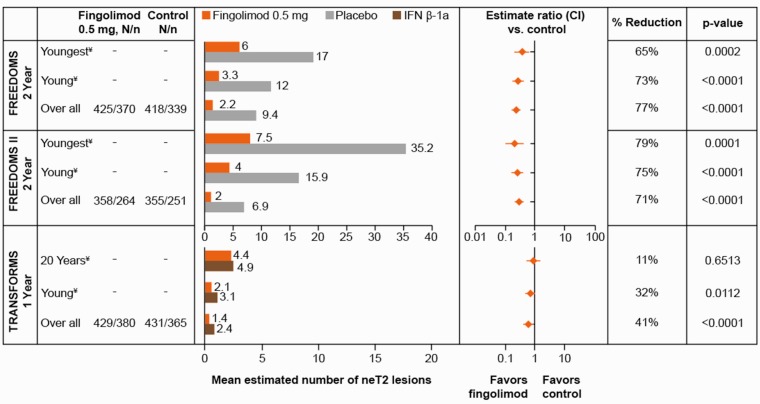
New/newly enlarging T2 Lesions
In all three trials across all treatment groups, young adult patients had 1.8- to 5.1-fold higher numbers of neT2 lesions compared with the overall population (Figure 2). Compared with placebo in FREEDOMS and FREEDOMS II, fingolimod 0.5 mg was associated with significantly lower numbers of neT2 lesions in patients at age 20 years (p = 0.0002 and 0.0001), patients at age 30 years (p < 0.0001 for both) and in the overall population (p < 0.0001 for both). Fingolimod 0.5 mg was also associated with a significant reduction of neT2 lesions compared with IFN β-1a in the overall population (41%, p < 0.0001) and in patients at age 30 years (32%, p = 0.0112). However, lesion suppression was similar with fingolimod and IFN β-1a in the youngest patient group.
Figure 2.
Estimated number of neT2 lesions at age 20 years, age 30 years and in the overall population in the FREEDOMS, FREEDOMS II and TRANSFORMS trials. A ratio <1 between fingolimod 0.5 mg and control groups signifies a treatment effect in favor of fingolimod. ¥Values for patients at age 20 or 30 years are based on a statistical model using all data in the ITT population. N = patients in ITT, n = patients with available data. Control refers to placebo for the FREEDOMS and FREEDOMS II trials, and IFN β-1a for the TRANSFORMS trial. Youngest and young MS patients refer to the estimates at age 20 and 30 years, respectively, in all three studies. CI: confidence interval; IFN β-1a: interferon beta-1a; ITT: intent-to-treat; MS: multiple sclerosis; neT2: new/newly enlarging T2.
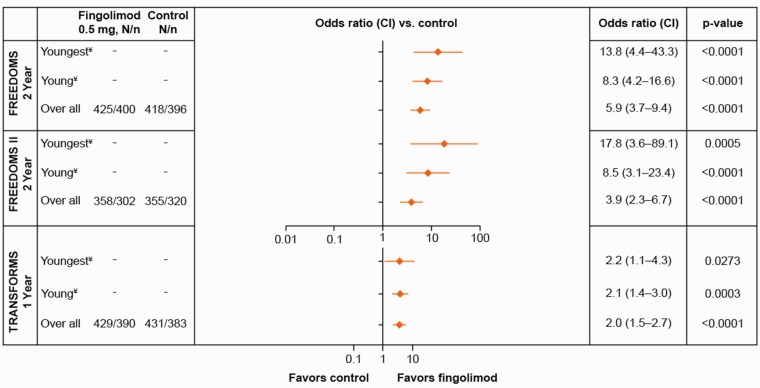
No Evidence of Disease Activity
The odds of achieving a NEDA-3 status over two years in young adult patients were 8.3- to 17.8-fold higher (p < 0.0001) in the fingolimod group compared with placebo, with the strongest treatment benefit observed in the youngest patients (Figure 3). Compared with IFN β-1a, the odds of achieving a NEDA status over one year was approximately doubled in fingolimod-treated patients irrespective of the patient’s age (odds ratio >2.0, p < 0.05).
Figure 3.
Odds of achieving a NEDA-3 status at age 20 years, age 30 years and in the overall population in the FREEDOMS, FREEDOMS II and TRANSFORMS trials. An odds ratio >1 signifies a treatment effect in favor of fingolimod. ¥Values for patients at age 20 or 30 years are based on a statistical model using all data in the ITT population. N = patients in ITT, n = patients with available data. Youngest and young MS patients refer to the estimates at age 20 and 30 years, respectively, in all three studies. Control refers to placebo for the FREEDOMS, FREEDOMS II and IFN β-1a for the TRANSFORMS. CI: confidence interval; IFN β-1a: interferon beta-1a; ITT: intent-to-treat; MS: multiple sclerosis; NEDA: no evidence of disease activity.
Safety
The AE profile of fingolimod in young adults (≤30 years) is presented in Table 2. The most common AEs in the fingolimod 0.5 mg group were nasopharyngitis (26.3%), headache (24.2%), upper respiratory tract infections (14.1%), and diarrhea (10.1%). The frequency of AEs reported in the young adults was similar to those reported in the overall population (Table 2). In young adult patients (≤30 years of age), the most common AEs, with an incidence of at least 5% in the fingolimod 0.5 mg group, included influenza, pharyngitis, bronchitis, headache, diarrhea, nausea, fatigue, back pain, cough, oropharyngeal pain, dyspnea and increased levels of alanine aminotransferase (ALT); this was similar to the AEs reported in the overall population versus placebo.
Table 2.
Most common adverse events (>5% incidence in the fingolimod group) in young adults (≤30 years) and the overall population.
| Young adults (≤30 years) |
Overall population |
|||
|---|---|---|---|---|
| AE, n (%) | Fingolimod 0.5 mg N = 1364 n = 327 |
Placebo N = 966 n = 198 |
Fingolimod 0.5 mg N = 1364 n = 1364 |
Placebo N = 966 n = 966 |
| Participants with any AE | 295 (90.2) | 179 (90.4) | 1256 (92.1) | 886 (91.7) |
| Infections and infestations | ||||
| Nasopharyngitis | 86 (26.3) | 55 (27.8) | 326 (23.9) | 240 (24.8) |
| URT infection | 46 (14.1) | 37 (18.7) | 202 (14.8) | 168 (17.4) |
| Urinary tract infection | 26 (8.0) | 15 (7.6) | 113 (8.3) | 107 (11.1) |
| Influenza | 22 (6.7) | 8 (4.0) | 120 (8.8) | 69 (7.1) |
| Pharyngitis | 20 (6.1) | 9 (4.5) | 57 (4.2) | 38 (3.9) |
| Sinusitis | 10 (3.1) | 6 (3.0) | 95 (7.0) | 66 (6.8) |
| Bronchitis | 16 (4.9) | 7 (3.5) | 86 (6.3) | 36 (3.7) |
| Nervous system disorders | ||||
| Headache | 79 (24.2) | 31 (15.7) | 314 (23.0) | 196 (20.3) |
| Dizziness | 11 (3.4) | 12 (6.1) | 100 (7.3) | 72 (7.5) |
| Gastrointestinal disorders | ||||
| Diarrhea | 33 (10.1) | 14 (7.1) | 137 (10.0) | 82 (8.5) |
| Nausea | 30 (9.2) | 19 (9.6) | 148 (10.9) | 98 (10.1) |
| General disorders and administration-site conditions | ||||
| Fatigue | 27 (8.3) | 17 (8.6) | 120 (8.8) | 85 (8.8) |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 22 (6.7) | 9 (4.5) | 106 (7.8) | 76 (7.9) |
| Pain in extremity | 14 (4.3) | 8 (4.0) | 97 (7.1) | 63 (6.5) |
| Arthralgia | 8 (2.4) | 7 (3.5) | 75 (5.5) | 78 (8.1) |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | 26 (8.0) | 15 (7.6) | 123 (9.0) | 90 (9.3) |
| Oropharyngeal pain | 20 (6.1) | 18 (9.1) | 76 (5.6) | 66 (6.8) |
| Dyspnea | 15 (4.6) | 4 (2.0) | 75 (5.5) | 54 (5.6) |
| Investigations | ||||
| ALT increase | 28 (8.6) | 6 (3.0) | 102 (7.5) | 25 (2.6) |
N = number of patients in the analysis set; n = number of patients in the subgroup.
AE: adverse event; ALT: alanine aminotransferase; URT: upper respiratory tract.
Discussion
This post hoc analysis of three Phase 3 fingolimod trials investigated the relationship between age and MS disease activity and estimated treatment effects of fingolimod 0.5 mg versus placebo or IFN β-1a in young adult patients (at age 20 and 30 years) in comparison with that of the overall study population. At baseline, young adult patients showed higher clinical and MRI disease activity compared with the overall adult study population. However, the differences in relapse activity between young adult patients and the overall population were less pronounced at baseline than on-study in the control group (placebo/IFN β-1a), because the study protocol mandated a minimum of one relapse in the last year, or two relapses in the last two years before study entry in all patients. Fingolimod 0.5 mg was associated with significant reductions in ARR (53% to 79%) and neT2 lesions (65% to 79%) versus placebo in young adult patients, with the youngest group (patients at age 20 years) showing the highest reductions in ARR. Similarly, fingolimod 0.5 mg significantly reduced the ARR compared with IFN β-1a, with the strongest relative reductions seen in the youngest patients. The odds of achieving a NEDA status over two years was strongest in the youngest patients versus placebo/IFN β-1a, and was twice as high with fingolimod compared with IFN β-1a irrespective of patient age.
Observational data from natural history studies in MS suggest that the clinical phenotype and prognosis of MS are age-dependent.9,12,21,22 The inverse relationship between age and relapse activity observed in this study supports the results reported previously.2,3 In the present analysis, the relapse rates estimated for young adult patients in the placebo arms of FREEDOMS and FREEDOMS II (at age 20, ARR = 0.73 and 0.67, respectively) are in line with those reported in the literature for younger RRMS patients,2,3 and are in between those reported for children with MS and the overall adult population.6,7 Our findings are further supported by evidence from previous analyses. A retrospective analysis including 2477 RRMS patients showed that the relapse rates were age-dependent and that patients who are younger than 40 years tend to benefit the most from drugs that can modify relapse rates.23 In a recent meta-analysis of randomized, blinded MS clinical trials involving >28,000 MS patients, the efficacy of disease-modifying therapies (DMTs) on MS disability was found to be strongly dependent on age, with younger patients showing the most benefit.24 Furthermore, the results suggested that delaying any DMT at an early stage of disease could lead to a decrease in cumulative efficacy of a DMT at later age.24 In the preparatory work of this analysis, we also analyzed the relationship between MS disease activity and disease duration (data not shown), and found the relationship between MS disease activity and age to be stronger.
The disease course in young RRMS patients is predominantly inflammatory, and accumulation of irreversible disability takes longer.9,14 In a study that used age as a categorical time-varying covariate for evaluating the relationship between age and rate of disability progression found that the median times to reach EDSS scores of 4.0 and 6.0 were significantly longer among patients aged 20 to 35 years compared with patients aged 36 to 50 and 51 to 65 years (p < 0.0001).11 A possible explanation for the slower disease worsening in young patients is the higher ability of the brains of younger patients to repair and compensate via remyelination and neuroplasticity mechanisms. Studies in experimental animal rodent models indeed indicate that, compared to observed rapid remyelination in younger animals, remyelination occurs slowly in older animals because of changes in the central nervous system environment and intrinsic epigenetic changes in oligodendrocytes.25,26 Further, age has long been linked to plasticity, with greater plasticity associated with younger age.27 As inflammation interferes with plasticity, its pharmacological modulation may restore plasticity and thereby promote patterns of functional reorganization underlying recovery in MS.28 Clinical research indeed shows that lowered capacity for brain plasticity can negatively influence functional reorganization and connectivity that is required for recovery from disease-related structural damage.29 By efficiently suppressing the higher inflammatory response observed in younger patients, fingolimod may help restore brain plasticity, thereby facilitating recovery mechanisms. We can therefore hypothesize that the mode of action of fingolimod may translate into greater benefits in younger patients in the long term.
In IFN β-1a-treated patients, there was a significant association between young age and high disease activity, similar to what was observed in the placebo arm in the FREEDOMS15 and FREEDOMS II16 studies (which contrasts with fingolimod 0.5 mg-treated patients who had a low ARR, irrespective of age). Comparing between fingolimod and IFN β-1a treatment in young patients, it is noteworthy that although both treatments have suppressed MRI activity relatively efficiently, there was still high clinical breakthrough activity in the IFN β-1a arm of TRANSFORMS. It is a consistent finding in most clinical trials in MS that treatment effects on lesion formation are stronger than those on relapse rates.2–5 Our results thus suggest that, although IFN β-1a is comparable to fingolimod 0.5 mg in reducing inflammatory MRI activity in young adults, it is suboptimal to prevent relapses, especially in younger patients with higher disease activity. It should also be noted that mean treatment duration was shorter in TRANSFORMS (one year) as compared with FREEDOMS/FREEDOMS II (two years), which may have influenced efficacy results for both MRI outcomes and NEDA-3 status in this patient population.
The safety profile of fingolimod 0.5 mg reported for patients aged 30 or less based on pooled data from the fingolimod RRMS program was similar to that of placebo and consistent with that observed in the overall adult population. Headache, influenza, diarrhea, back pain, dyspnea and increased levels of ALT reported in the fingolimod group were higher compared with placebo in young adults; similar trends were observed in the overall adult population. The overall incidence of AEs reported in the young adult patients treated with fingolimod was similar to placebo and consistent with the overall adult population.
The results from this post hoc analysis of data from adult patients of the fingolimod Phase 3 program supported sample size assumptions made for the PARADIGMS trial (NCT01892722). Recently concluded, PARADIGMS is the first prospective, double-blind, double-dummy, randomized, active-controlled trial in a pediatric MS population. This study evaluated the efficacy and safety of fingolimod 0.5 mg versus IFN β-1a in children and adolescents with MS (aged 10–17 years). Results show that oral fingolimod significantly reduced the number of relapses (ARR) in this patient population over a period of up to two years, compared with IFN β-1a injections.20
Strengths and Limitations
In this post hoc analysis, we included all ITT patients from three randomized, double-blind Phase 3 trials to quantify the relationship between age and disease activity in statistical models, and then evaluated the model for young adult patients at age 20 years and age 30 years in each study. Age was used as a continuous covariate because it is more likely that MS disease activity changes gradually with increasing age, rather than following a step function at arbitrary age cutoffs. By applying the statistical model to all ITT data, it was possible to avoid arbitrary cutoffs, and make robust inference for the youngest adult patients despite a relatively limited sample size. Although interpretation at “the edge” of the data (here the youngest) should always be performed with some caution, this analysis method was used to extrapolate to pediatric patients, which proved useful for the planning and execution of the PARADIGMS study for the fingolimod program as there are currently no data available from controlled clinical trials in pediatric MS patients. The robustness of the results was tested and confirmed in several sensitivity analyses, and by the side-by-side presentation of a similar analysis in three randomized controlled clinical trials. Excluding extreme values did not change the overall conclusion, and findings were remarkably similar in all three studies. Consistently, young patients were more susceptible to high levels of MS disease activity, and the youngest patients benefited from the greatest relative reductions in ARR compared to placebo or IFN β-1a.
The relationship between age and NEDA seems less pronounced compared to the relationship between age and MRI lesions or age and ARR. The underlying reason is that in a NEDA analysis, disease activity information is reduced to a binary yes/no response and information on recurrent lesions or recurrent relapses is lost, thereby lowering the sensitivity to detect an age-dependency. The safety analysis in this study was restricted to patients ≤30 years of age because of a too small sample size of patients ≤20 years for a reliable evaluation of safety results. Finally, the study has the inherent limitations of a post hoc analysis and, as such, the results should be interpreted cautiously.
Conclusions
Benefits of fingolimod versus placebo and IFN β-1a in young adults with MS on clinical and MRI measures of disease activity were consistent across three trials. This post hoc data analysis from randomized controlled trials involving young adult patients helps in understanding the drivers of disease activity and its relationship with age. Our findings show that the youngest patients can benefit the most from fingolimod treatment. Further, these data and the statistical analysis method used have informed the planning and design of the PARADIGMS Phase 3 study, providing useful information on a yet unexplored area of treatment effects of DMTs in pediatric patients with MS.
Acknowledgements
The authors would like to acknowledge Vimal Kumar Varma Muthyala of Novartis Healthcare Pvt Ltd, Hyderabad, India, and Marie-Catherine Mousseau of Novartis Ireland Ltd, Dublin, Ireland, for providing medical writing support, which encompassed preparing the manuscript, formatting, referencing, preparing tables and figures, incorporating authors’ revisions, finalizing and submission all under the direction of the authors. In keeping with the guidelines of the International Committee of Medical Journal Editors, all authors have contributed significantly to the study and were thoroughly involved in the critical review of the manuscript for important intellectual content. All authors have reviewed and approved the final draft for submission.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jutta Gärtner has received honoraria for lectures and consultancy from Bayer, Biogen, Teva, and Novartis, and has received research grant support from Novartis.
Tanuja Chitnis has received personal compensation from advisory board/consulting for Biogen-Idec and Novartis Pharmaceuticals and financial support for research activities from Merck-Serono and Novartis Pharmaceuticals.
Angelo Ghezzi has received honoraria for speaking from Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis, and Sanofi-Aventis; and for consultancy from Merck-Serono, Biogen-Idec, Teva, and Novartis.
Daniela Pohl has received personal compensation for activities with Bayer-Schering, Biogen-Idec, Merck-Serono, Novartis Pharmaceuticals, and Teva.
Wolfgang Brück has received honoraria for lectures by Bayer Vital, Biogen, Merck-Serono, Teva Pharma, Genzyme, Sanofi-Aventis, and Novartis and is a member of scientific advisory boards for Teva Pharma, Biogen, Novartis, and Genzyme and receives research support from Teva Pharma, Biogen, Medday, Genzyme, and Novartis. He serves on the editorial boards of Neuropathology and Applied Neurobiology, Therapeutic Advances in Neurological Diseases, and Multiple Sclerosis International and received research support by the Deutsche Forschungsgemeinschaft, SFB Transregio 43, The brain as a target of inflammatory processes, project B9, the German Ministry for Education and Research (BMBF, “German Competence Network Multiple Sclerosis” [KKNMS]), the Klaus Tschira Foundation, and the German Multiple Sclerosis Society.
Dieter A. Häring, Goeril Karlsson and Norman Putzki are all employees of Novartis Pharma AG, Basel, Switzerland.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Novartis Pharma AG, Basel, Switzerland.
References
- 1.Hunter SF. Overview and diagnosis of multiple sclerosis. Am J Manag Care 2016; 22 (6 Suppl): s141–s150. [PubMed] [Google Scholar]
- 2.Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: Subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 2012; 11: 420–428. [DOI] [PubMed] [Google Scholar]
- 3.Derfuss T, Ontaneda D, Nicholas J, et al. Relapse rates in patients with multiple sclerosis treated with fingolimod: Subgroup analyses of pooled data from three phase 3 trials. Mult Scler Relat Disord 2016; 8: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JA, Barkhof F, Comi G, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol 2013; 260: 2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JA, Khatri B, Barkhof F, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: Results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry 2016; 87: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009; 66: 54–59. [DOI] [PubMed] [Google Scholar]
- 7.Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord 2014; 3: 186–193. [DOI] [PubMed] [Google Scholar]
- 8.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: Comparison with adult-onset forms. Neurology 2002; 59: 1922–1928. [DOI] [PubMed] [Google Scholar]
- 9.Scalfari A, Lederer C, Daumer M, et al. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler 2016; 22: 1750–1758. [DOI] [PubMed] [Google Scholar]
- 10.Tremlett H, Devonshire V. Is late-onset multiple sclerosis associated with a worse outcome? Neurology 2006; 67: 954–959. [DOI] [PubMed] [Google Scholar]
- 11.Trojano M, Liguori M, Bosco Zimatore G, et al. Age-related disability in multiple sclerosis. Ann Neurol 2002; 51: 475–480. [DOI] [PubMed] [Google Scholar]
- 12.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006; 129: 595–605. [DOI] [PubMed] [Google Scholar]
- 13.Scott TF, Schramke CJ, Novero J, et al. Short-term prognosis in early relapsing–remitting multiple sclerosis. Neurology 2000; 55: 689–693. [DOI] [PubMed] [Google Scholar]
- 14.Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007; 356: 2603–2613. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 18.Rose K, Müller T. Children with multiple sclerosis should not become therapeutic hostages. Ther Adv Neurol Disord 2016; 9: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitnis T, Tardieu M, Amato MP, et al. International Pediatric MS Study Group Clinical Trials Summit: Meeting report. Neurology 2013; 80: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitnis T, Arnold DL, Banwel B, et al. PARADIGMS: A randomised double-blind study of fingolimod versus interferon β-1a in paediatric multiple sclerosis. Presented at Joint ECTRIMS-ACTRIMS 2017, Paris, France, PS16, 276.
- 21.Confavreux C, Vukusic S. Natural history of multiple sclerosis: A unifying concept. Brain 2006; 129: 606–616. [DOI] [PubMed] [Google Scholar]
- 22.Vukusic S, Confavreux C. Natural history of multiple sclerosis: Risk factors and prognostic indicators. Curr Opin Neurol 2007; 20: 269–274. [DOI] [PubMed] [Google Scholar]
- 23.Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 24.Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 2017; 8: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeifenbring S, Nessler S, Wegner C, et al. Remyelination after cuprizone-induced demyelination is accelerated in juvenile mice. J Neuropathol Exp Neurol 2015; 74: 756–766. [DOI] [PubMed] [Google Scholar]
- 26.Sim FJ, Zhao C, Penderis J, et al. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 2002; 22: 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis M, Spiegler BJ, Juranek JJ, et al. Age, plasticity, and homeostasis in childhood brain disorders. Neurosci Biobehav Rev 2013; 37: 2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomassini V, d’Ambrosio A, Petsas N, et al. The effect of inflammation and its reduction on brain plasticity in multiple sclerosis: MRI evidence. Hum Brain Mapp 2016; 37: 2431–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocca MA, Absinta M, Moiola L, et al. Functional and structural connectivity of the motor network in pediatric and adult-onset relapsing–remitting multiple sclerosis. Radiology 2010; 254: 541–550. [DOI] [PubMed] [Google Scholar]