Abstract
Aberrant cell survival plays a critical role in cancer progression and metastasis. We have previously shown that ezrin, a cAMP-dependent protein kinase A–anchoring protein (AKAP), is up-regulated in colorectal cancer (CRC) liver metastasis. Phosphorylation of ezrin at Thr-567 activates ezrin and plays an important role in CRC cell survival associated with XIAP and survivin up-regulation. In this study, we demonstrate that in FET and GEO colon cancer cells, knockdown of ezrin expression or inhibition of ezrin phosphorylation at Thr-567 increases apoptosis through protein kinase A (PKA) activation in a cAMP-independent manner. Transforming growth factor (TGF) β signaling inhibits ezrin phosphorylation in a Smad3-dependent and Smad2-independent manner and regulates pro-apoptotic function through ezrin-mediated PKA activation. On the other hand, ezrin phosphorylation at Thr-567 by insulin-like growth factor 1 receptor (IGF1R) signaling leads to cAMP-dependent PKA activation and enhances cell survival. Further studies indicate that phosphorylated ezrin forms a complex with PKA RII, and dephosphorylated ezrin dissociates from the complex and facilitates the association of PKA RII with AKAP149, both of which activate PKA yet lead to either cell survival or apoptosis. Thus, our studies reveal a novel mechanism of differential PKA activation mediated by TGFβ and IGF1R signaling through regulation of ezrin phosphorylation in CRC, resulting in different cell fates. This is of significance because TGFβ and IGF1R signaling pathways are well-characterized tumor suppressor and oncogenic pathways, respectively, with important roles in CRC tumorigenesis and metastasis. Our studies indicate that they cross-talk and antagonize each other's function through regulation of ezrin activation. Therefore, ezrin may be a potential therapeutic target in CRC.
Keywords: transforming growth factor beta (TGF-β), ezrin, A-kinase anchoring protein (AKAP), X-linked inhibitor of apoptosis protein (XIAP), survivin, colon cancer, apoptosis, metastasis, IGF1R, Smad3
Introduction
Colorectal cancer (CRC)2 is the third leading cause of cancer-related death in the United States. Stage I and II cancers that are confined within the wall of the colon are curable by surgical resection; however, the survival rate of cancer patients is drastically reduced when cancer metastasizes to distant organ sites such as liver and/or lungs (1, 2). Despite significant improvements in early diagnosis and treatment of CRC, metastasis and recurrence of the disease remain the main cause of cancer death (1, 3, 4). Genetic and epigenetic changes pivotal for metastasis are acquired at late stages of CRC during progression to advanced disease (1). Therefore, identification of these changes and understanding the underlying mechanisms are critical for the development of novel anti-metastatic therapies.
Our recent studies have demonstrated that expression of ezrin is up-regulated in liver metastases when compared with primary tumors in an orthotopic model of colon cancer and that ezrin expression is increased in primary tumors from colon cancer patients (5). Ezrin, a member of the ezrin–radixin–moesin (ERM) family (6–9), exists in two conformations: an active open form mainly localized at the plasma membrane and a dormant closed form that largely resides in the cytoplasm. Inactive ezrin forms oligomers where the C-terminal domain folds back and binds tightly to the FERM domain, masking several of its active sites (8, 10, 11). Phosphorylation at Thr-567 transitions ezrin from inactive oligomers into active monomers by unmasking the active sites through dissociation of FERM and C-terminal domains (11–15). Ezrin plays an important role in cell motility and invasion and has been implicated in metastasis of several types of cancer including CRC (16–21). In addition, ezrin plays a key role in cell survival of colon cancer cells and regulates expression of IAPs (inhibitor of apoptosis proteins), XIAP and survivin, which have been shown to be involved in cell survival and metastasis (5, 22). We have previously shown that IGF1R signaling regulates ezrin phosphorylation at Thr-567 (5). Other studies have shown that ezrin is a downstream effector of the PI3K–AKT pathway (23, 24). Nevertheless, little is known of the mechanism of ezrin-mediated cell survival.
Dransfield et al. (25) characterized ezrin as a cAMP-dependent protein kinase A–anchoring protein (AKAP). There are more than 50 AKAPs identified. Protein kinase A (PKA) consists of catalytic subunits and inhibitory regulatory subunits and plays a dominant role in the integration of multiple signal transduction networks (26). AKAPs interact with the regulatory subunits of PKA and target these supramolecular complexes to specific subcellular localizations, where they regulate phosphorylation of specific substrates and execute different functions (27, 28). For example, AKAP149–PKA contributes to the disruption of the XIAP–survivin complex through phosphorylation of survivin at serine 20, leading to proteasome-mediated degradation of XIAP (29, 30).
In this study, we demonstrate that knockdown of ezrin expression or inhibition of ezrin phosphorylation in GEO and FET cells increases apoptosis through activation of PKA in a cAMP-independent manner. AKAP149 plays an important role in this process. In addition, we show that TGFβ inhibits ezrin phosphorylation at Thr-567 in a Smad2-independent and Smad3-dependent manner, resulting in PKA activation and induction of apoptosis. On the other hand, phosphorylation of ezrin at Thr-567 by IGF1R signaling leads to cAMP-dependent PKA activation and increased cell survival. Further studies indicate that phosphorylated ezrin displays more association with PKA RII than dephosphorylated ezrin; hypophosphorylation of ezrin facilitates complex formation of PKA RII and AKAP149, whereas hyperphosphorylation of ezrin reduces their association. Therefore, our studies uncover a novel mechanism of differential activation of PKA mediated by TGFβ and IGF1R signaling through regulation of the phosphorylation status of ezrin, which leads to different cell fates. Given the importance of TGFβ and IGF1R signaling in CRC, it implies that ezrin may be a potential therapeutic target in CRC.
Results
Knockdown of ezrin expression activates PKA and induces apoptosis in colon cancer cells
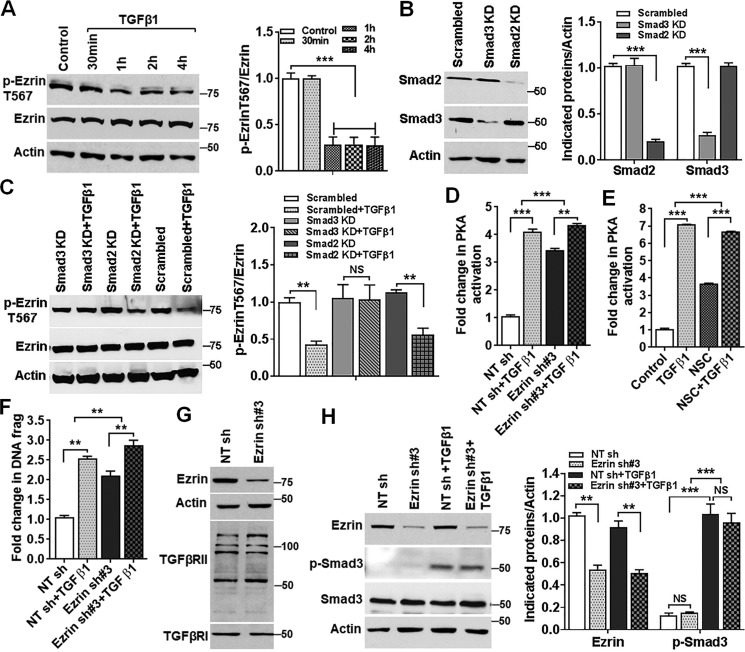
Recently, we demonstrated that transient knockdown of ezrin using siRNA leads to down-regulation of XIAP and survivin expression (5). To understand the underlying molecular mechanism, stable knockdown (KD) of ezrin expression was performed using GIFZ lentiviral shRNA#1 and #3 in GEO and FET colon cancer cells. A nontargeting shRNA (NT sh) was used as a control. Ezrin expression was significantly reduced by ezrin shRNAs in both cell lines (Fig. 1A). As a result, DNA fragmentation assays showed an ∼2-fold increase in apoptosis in ezrin KD cells when compared with the NT sh control cells (Fig. 1B). In addition, cleaved caspase 7 was higher in ezrin KD cells than in NT sh cells (Fig. 1C), confirming the results of DNA fragmentation assays. Expression of XIAP and survivin was down-regulated in both GEO and FET cells (Fig. 1C). These results indicated that inhibition of ezrin expression leads to increased apoptosis associated with reduced XIAP and survivin expression in colon cancer cells. PKA activation has been shown to disrupt XIAP–survivin complex formation, leading to degradation of XIAP and survivin and induction of apoptosis (30). We next determined whether ezrin KD had any effect on PKA activity. PKA activity assays showed that ezrin KD increased PKA activation by 2–3-fold relative to NT sh control (Fig. 1D). Phosphorylation of CREB, a direct target of PKA (31), was increased in ezrin KD cells, and pretreatment with H89, a specific PKA inhibitor that binds to the PKA catalytic α subunit to inhibit its activation, completely abrogated the ezrin KD-mediated increase in CREB phosphorylation (Fig. 1E), supporting the ability of ezrin KD to activate PKA. Further studies showed that ezrin KD dissociated the PKA catalytic α subunit from its inhibitory regulatory subunit RII (Fig. 1F). Because cAMP binds to the PKA regulatory subunits and dissociates them from the catalytic subunits to activate PKA (29, 30), we next determined whether ezrin KD increased cAMP production. cAMP levels were measured using a nonradioactive cAMP enzyme immunoassay. Forskolin, a PKA activator, was used as a positive control. Treatment with forskolin led to a significant increase in cAMP levels (Fig. 1G). In contrast, ezrin KD was unable to increase cAMP production in both GEO and FET cells (Fig. 1G). These results indicate that ezrin KD activates PKA in a cAMP-independent manner.
Figure 1.
Knockdown of ezrin expression activates PKA and induces apoptosis in colon cancer cells. A, GEO and FET colon cancer cells with stable expression of ezrin shRNA (sh#1 and sh#3) showed a significant reduction in ezrin protein expression. Nontargeting shRNA (NT sh) was used as a control (left panel). Quantification of Western blots was performed as described under “Experimental procedures” (right panel). **, p < 0.01 (n = 3). B, ezrin KD cells showed an ∼2-fold increase in apoptosis, as determined by DNA fragmentation assays. **, p < 0.01 (n = 3). C, ezrin KD led to down-regulation of XIAP and survivin and an increase in cleaved caspase 7 (left panel). Quantification of Western blots is shown on the right. **, p < 0.01; ***, p < 0.001 (n = 4). D, ezrin KD GEO and FET cells exhibited increased PKA activation compared with NT sh cells. Forskolin (10 μm) was used as a positive control. **, p < 0.01 ; ***, p < 0.001 (n = 3). E, ezrin KD-mediated increase of p-CREB is abrogated by PKA inhibitor H89 (15 μm) (left panel). Quantification of Western blots is shown on the right. **, p < 0.01; ***, p < 0.001. NS, not statistically significant (n = 2). F, IP assays showed the decreased association of PKARII with PKACatα in ezrin KD cells compared with NT sh control cells (left panel). Relative levels of PKACatα to PKARII were analyzed, and the results are shown on the right. ***, p < 0.001 (n = 2). G, production of cAMP was not increased in ezrin KD cells as determined by cAMP assay. Forskolin was used as a positive control. NS, not statistically significant (n = 3); IB, immunoblot.
To determine whether ezrin KD induces apoptosis through PKA activation, expression of the PKA catalytic α subunit was knocked down by a shRNA in FET cells (designated PKACatα KD) (Fig. 2A). As expected, PKACatα KD markedly reduced endogenous PKA activity and blocked ezrin KD-induced PKA activation (Fig. 2B). There was no difference in ezrin phosphorylation at Thr-567 and expression of total ezrin between PKACatα KD and scrambled shRNA control cells (Fig. 2A). Although knockdown of PKACatα did not affect XIAP and survivin expression (Fig. 2A), it blocked ezrin KD-mediated down-regulation of XIAP and survivin expression (Fig. 2C) Consequently, PKACatα KD did not induce apoptosis but blocked apoptosis induced by ezrin KD (Fig. 2D). These results demonstrated that ezrin KD mediates apoptosis through PKA activation.
Figure 2.
Knockdown of ezrin expression induces apoptosis through PKA activation. A, expression of PKA catalytic α subunit (PKACatα) was knocked down in FET cells, with no change in ezrin phosphorylation at Thr-567 and XIAP and survivin expression (left panel). Quantification of Western blots is shown on the right. **, p < 0.01. NS, not statistically significant (n = 3). B, PKACatα KD blocked endogenous as well as ezrin KD-mediated PKA activation. **, p < 0.01; ***, p < 0.001. NS, not statistically significant (n = 3). C, PKACatα KD abrogates ezrin KD-mediated XIAP and survivin down-regulation (left panel). Quantification of Western blots is shown on the right. **, p < 0.01. NS, not statistically significant (n = 3). D, PKACatα KD abrogates ezrin KD-induced apoptosis. ***, p < 0.001. NS, not statistically significant (n = 3).
Inhibition of ezrin phosphorylation at Thr-567 leads to PKA activation and induction of apoptosis
Ezrin is present in an inactive and closed conformation in the cytoplasm, and phosphorylation at Thr-567 activates ezrin (11). Previously, we have shown that ezrin is hyperphosphorylated at Thr-567 in CRC liver metastasis when compared with primary tumors (5). We therefore hypothesized that inhibition of ezrin phosphorylation at Thr-567 would inactivate ezrin, leading to PKA activation and induction of apoptosis. To test this hypothesis, site-directed mutagenesis was performed. An ezrin phospho-deficient mutant (designated as T567A) in which threonine 567 was replaced by alanine was generated. GFP-tagged ezrin T567A was introduced into ezrin KD cells, and GFP-tagged WT ezrin (designated as WT) was used as a control (Fig. 3A). Expression of ezrin T567A led to down-regulation of XIAP and survivin expression (Fig. 3A), increased apoptosis (Fig. 3, A and B), and enhanced PKA activation (Fig. 3C), whereas restoration of WT ezrin increased XIAP and survivin expression (Fig. 3A), decreased apoptosis (Fig. 3, A and B), and reduced PKA activation (Fig. 3C) to levels observed in control cells. Ezrin T567A-mediated PKA activation was independent of cAMP because cAMP levels remained unchanged in ezrin T567A- or WT ezrin-expressing cells (Fig. 3D). These results indicate that inhibition of ezrin phosphorylation at Thr-567 activates PKA and induces apoptosis in colon cancer cells.
Figure 3.
Dephosphorylation of ezrin at Thr-567 regulates PKA activation and apoptosis in GEO cells. A, ezrin T567A down-regulated XIAP and survivin expression and increased caspase 7 cleavage (left panel). Quantification of Western blots is shown on the right. **, p < 0.01 (n = 3). B, ezrin T567A increased apoptosis. **, p < 0.01 (n = 3). C and D, ezrin T567A activated PKA with no increase in cAMP production. Forskolin was used as a positive control. **, p < 0.01. NS, not statistically significant (n = 3).
We next determined the effects of NSC668394 (designated as NSC), a small molecule inhibitor that inhibits ezrin phosphorylation at Thr-567 (32), on PKA activation and apoptosis of colon cancer cells. Treatment of GEO and FET cells with increasing concentrations of NSC showed a dose-dependent inhibition of ezrin phosphorylation at Thr-567, with no change in the levels of total ezrin (Figs. 4A). Expression of XIAP and survivin were down-regulated by NSC in a dose-dependent manner (Fig. 4A). In addition, a 2–4- and 3–5-fold increase in apoptosis was observed after NSC treatment for 2 and 6 h, respectively (Fig. 4B). Moreover, PKA activity assays showed a significant time-dependent activation of PKA by NSC treatment in GEO and FET cells, which was abrogated by pretreatment with H89 (Fig. 4C). Forskolin was included as a positive control (Fig. 4C). As expected, NSC-mediated PKA activation was independent of cAMP (Fig. 4D). These results indicate that inhibition of phosphorylation of ezrin at Thr-567 by NSC activates PKA and induces apoptosis in colon cancer cells. Importantly, knockdown of the PKA catalytic α subunit in FET cells blocked NSC-mediated down-regulation of XIAP and survivin expression (Fig. 4E), prevented NSC-induced apoptosis (Fig. 4F), and abrogated NSC-mediated PKA activation (Fig. 4G). These results demonstrate that inhibition of phosphorylation of ezrin at Thr-567 by NSC leads to apoptosis through PKA activation.
Figure 4.
Inhibition of ezrin phosphorylation at Thr-567 by NSC668394 induces apoptosis through PKA activation. A and B, GEO and FET colon cancer cells treated with the ezrin inhibitor NSC668394 (NSC) showed a dose-dependent decrease in phosphorylation of ezrin at Thr-567, down-regulation of XIAP, and survivin expression (A, upper panel) and induction of apoptosis (B). The lower panels show quantification of Western blots. **, p < 0.01, ***, p < 0.001 (n = 3). C, PKA activity assays showed PKA activation by NSC (20 μm) treatment. PKA inhibitor H89 (15 μm) pretreatment abrogated endogenous and NSC-induced PKA activation. Forskolin was used as a positive control. ***, p < 0.001. NS, not statistically significant (n = 3). D, NSC-induced PKA activation is independent of cAMP production. NS, not statistically significant (n = 3). E, PKACatα KD blocked NSC-mediated XIAP and survivin down-regulation (upper panel). The lower panel shows quantification of Western blots. **, p < 0.01. NS, not statistically significant (n = 3). F and G, PKACatα KD abrogated NSC-mediated apoptosis (F) and PKA activation (G). **, p < 0.01. NS, not statistically significant (n = 3).
TGFβ inhibits ezrin phosphorylation at Thr-567, resulting in PKA activation and apoptosis
TGFβ signaling plays an important role in tumorigenesis and metastasis in many cancers, including CRC (33). It has been shown that abrogation of TGFβ signaling promotes cell survival under stress and increases metastatic potential of colon cancer cells (34). We have previously shown that TGFβ activates PKA in a cAMP-independent manner leading to apoptosis and that this activation depends on Smad3 (30, 35). TGFβ signaling also down-regulates XIAP and survivin expression (30). These results suggest that TGFβ may function through ezrin regulation. We therefore determined whether TGFβ signaling regulates ezrin phosphorylation and activation. Treatment of FET cells with TGFβ1 resulted in a time-dependent decrease in ezrin phosphorylation at Thr-567 (Fig. 5A). To further determine whether TGFβ-mediated inhibition of ezrin phosphorylation is Smad-dependent, expression of Smad2 and Smad3 was knocked down individually in FET cells by shRNAs specific for Smad2 or Smad3 (Fig. 5B). Knockdown of Smad2 or Smad3 had little effect on the basal levels of ezrin phosphorylation at Thr-567 (Fig. 5C). However, TGFβ-mediated inhibition of ezrin phosphorylation was prevented in Smad3 knockdown cells but not in Smad2 knockdown cells (Fig. 5C). These results indicate that TGFβ inhibits ezrin phosphorylation in a Smad3-dependent and Smad2-independent manner.
Figure 5.
TGFβ inhibits ezrin phosphorylation at Thr-567, resulting in PKA activation and inhibition of XIAP and survivin expression. A, TGFβ1 treatment of FET cells decreased ezrin phosphorylation at Thr-567 in a time-dependent manner (left panel). Relative levels of p-ezrin Thr-567 to total ezrin were analyzed, and the results are shown on the right. ***, p < 0.001 (n = 3). B, expression of Smad2 and Smad3 was knocked down in FET cells (left panel). Quantification of Western blots is shown on the right. ***, p < 0.001 (n = 2). C, Smad3 KD, but not Smad2 KD, prevented TGFβ1-mediated inhibition of ezrin phosphorylation (left panel). Relative levels of p-ezrin Thr-567 to total ezrin were determined and are shown on the right. **, p < 0.01. NS, not statistically significant (n = 3). D, TGFβ1 treatment increased PKA activation to a much lesser degree in ezrin KD cells than in NT sh control cells. **, p < 0.01; ***, p < 0.001 (n = 3). E, NSC attenuated TGFβ-induced PKA activation. ***, p < 0.001 (n = 3). F, ezrin KD reduces TGFβ1-induced apoptosis. **, p < 0.01 (n = 2). G and H, ezrin KD has no effect on TGFβRI or TGFβRII expression (G) or on Smad3 expression or TGFβ-mediated Smad3 phosphorylation (H, left). Quantification of Western blots is shown on the right. **, p < 0.01; ***, p < 0.001. NS, not statistically significant (n = 3).
Previous studies have shown that TGFβ signaling induces apoptosis in colon cancer cells in a Smad3-dependent manner (30, 35). To demonstrate that TGFβ activates PKA and induces apoptosis through inhibition of ezrin activation, ezrin expression was knocked down in FET cells (Fig. 1A). Although TGFβ markedly increased PKA activity in NT sh control cells, it activated PKA to a much lesser degree in ezrin KD cells (Fig. 5D). Consistently, inhibition of ezrin phosphorylation by NSC treatment attenuated TGFβ-induced PKA activation (Fig. 5E). Furthermore, knockdown of ezrin expression reduced TGFβ-induced apoptosis (Fig. 5F). These results indicate that TGFβ activates PKA and increases apoptosis at least partially through ezrin inhibition. Of note, knockdown of ezrin had no effect on expression of TGFβRI and TGFβRII (Fig. 5G), expression of Smad3, or TGFβ-mediated Smad3 phosphorylation (Fig. 5H), indicating that ezrin does not affect canonical TGFβ signaling.
Phosphorylation of ezrin at Thr-567 leads to PKA activation and cell survival
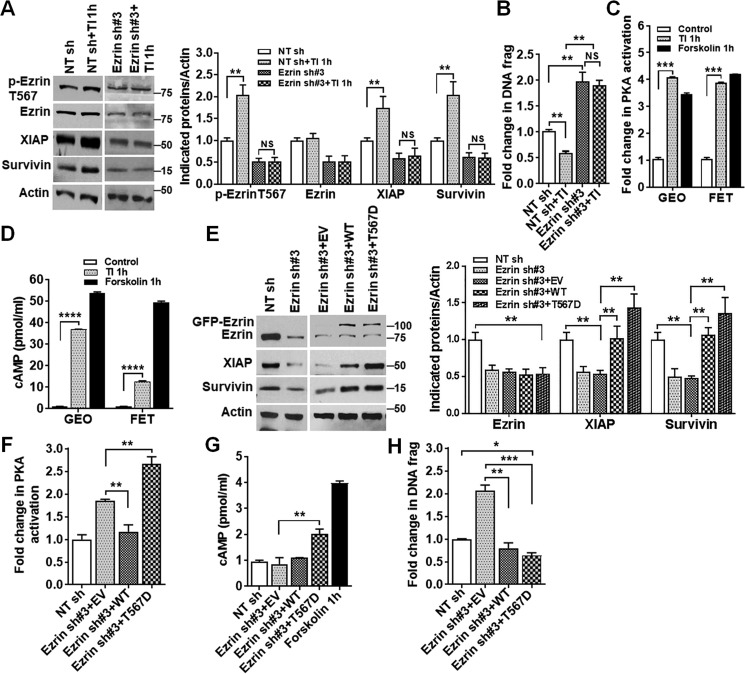
IGF1R signaling plays an important role in colon cancer cell survival (36, 37). Whereas its inhibition leads to down-regulation of XIAP and survivin and induction of apoptosis (36), its activation by transferrin and insulin (TI) increases cell survival (37). We have previously shown that inhibition of IGF1R decreases ezrin phosphorylation at Thr-567 (5), indicating that IGF1R-mediated signaling activates ezrin by increasing Thr-567 phosphorylation. Consistently, treatment of GEO cells with TI, which activates IGF1R signaling (37), increased ezrin phosphorylation at Thr-567, and concurrently enhanced expression of XIAP and survivin and reduced apoptosis (Fig. 6, A and B). Knockdown of ezrin expression abrogated TI-mediated prosurvival effects (Fig. 6, A and B). Importantly, TI treatment activated PKA and increased cAMP production (Fig. 6, C and D). These results suggest that phosphorylation of ezrin at Thr-567 activates PKA in a cAMP-dependent manner. To determine whether this is the case, an ezrin phospho-mimetic mutant (designated as T567D), in which threonine 567 was replaced by aspartic acid, was generated. GFP-tagged ezrin T567D was introduced into ezrin KD cells, and GFP-tagged WT ezrin was used as a control (Fig. 6E). Analysis of PKA activity showed that expression of WT ezrin reversed the stimulative effect of ezrin KD on PKA activation, whereas expression of ezrin T567D further increased PKA activation (Fig. 6F) and enhanced production of cAMP (Fig. 6G). It indicates that, in contrast to ezrin T567A-mediated PKA activation, which is cAMP-independent (Fig. 3D), ezrin T567D activates PKA in a cAMP-dependent manner. Moreover, overexpression of WT ezrin increased expression of XIAP and survivin (Fig. 6E) and prevented apoptosis induced by ezrin KD (Fig. 6H), whereas overexpression of ezrin T567D not only blocked ezrin KD-induced apoptosis but further increased cell survival as compared NT sh control (Fig. 6H). Taken together, these studies indicate that phosphorylation of ezrin at Thr-567 activates PKA and enhances cell survival.
Figure 6.
Hyperphosphorylation of ezrin at Thr-567 activates PKA and promotes survival. A, TI treatment increased phosphorylation of ezrin at Thr-567 and expression of XIAP and survivin together with inhibition of apoptosis in NT sh control cells. However, the increase was abrogated in ezrin KD cells (left panel). Quantification of Western blots is shown on the right. **, p < 0.01. NS, not statistically significant (n = 3). B, TI treatment reduced apoptosis in NT sh control cells, and this inhibition was abrogated in ezrin KD cells. **, p < 0.01. NS, not statistically significant (n = 2). C and D, TI activated PKA (C) and increased cAMP production (D) ***, p < 0.001; ****, p < 0.0001 (n = 3). E, ectopic expression of ezrin Thr-567 phospho-mimetic mutant (T567D) or WT ezrin increased XIAP and survivin expression in GEO cells (left panel). Quantification of Western blots is shown on the right. **, p < 0.01 (n = 3). F and G, ezrin T567D mutant enhanced PKA activation (F) concomitant with increased cAMP production (G). Forskolin is used as a positive control. **, p < 0.01 (n = 3). H, ectopic expression of ezrin T567D and ezrin WT reduced apoptosis. *, p < 0.05 and **, p < 0.01; ***, p < 0.001 (n = 3).
AKAP149 contributes to ezrin inhibition-mediated PKA activation
It has been shown that AKAP–PKA interaction is a prerequisite for targeting PKA and its substrate complexes to specific subcellular locations (27). To determine whether AKAP is involved in ezrin inhibition-mediated PKA activation, a pan-AKAP inhibitor Ht31 was utilized. Pretreatment with Ht31 abrogated NSC-mediated PKA activation in FET and GEO colon cancer cells (Fig. 7A). These results indicate that one or more AKAPs contribute to ezrin inhibition-mediated PKA activation. It has been previously shown that TGFβ–Smad3-mediated PKA activation depends upon AKAP149 (30, 35). We therefore used siRNA knockdown strategies to determine whether AKAP149 is required for PKA activation by ezrin inhibition. Knockdown of AKAP149 had little effect on ezrin expression and phosphorylation, and ezrin KD did not affect AKAP149 expression (Fig. 7B). Although AKAP149 KD had no effect on XIAP and survivin expression in the control cells, it slightly increased their expression in ezrin KD cells (Fig. 7B). In addition, AKAP149 KD reduced apoptosis in ezrin KD cells but not in the control cells (Fig. 7C). Interestingly, knockdown of AKAP149 did not affect PKA activation in the control cells; however, it completely abrogated ezrin inhibition-mediated PKA activation by NSC (Fig. 7D). These results indicate that AKAP149 is essential for PKA activation when ezrin activation is inhibited. However, it has little effect on PKA activation when ezrin is activated. Given that AKAPs bind the regulatory subunits of PKA (PKA RI or PKA RII), leading to the dissociation of regulatory subunits from the catalytic α subunit and activation of PKA, and that AKAP149 binds PKA RII (30), we next determined the complex formation of PKA RII and AKAP149 by immunoprecipitation (IP) analysis. As shown in Fig. 7E, ezrin KD increased PKA RII–AKAP149 complex formation. Although expression of ezrin T567A further increased the association of PKA RII and AKAP149, expression of ezrin T567D markedly decreased their association (Fig. 7E). These results indicate that hypophosphorylation of ezrin facilitates complex formation of PKA RII and AKAP149, whereas hyperphosphorylation of ezrin prevents their association. It suggests that phosphorylation of ezrin enhances complex formation of ezrin and PKA RII. To investigate whether this is the case, PKA RII–ezrin complex formation was determined by IP analysis. The results showed that ezrin T567D displays more association with PKARII than ezrin T567A (Fig. 7F). These studies indicate that phosphorylated ezrin forms a complex with PKA RII and dephosphorylated ezrin dissociates from the complex and facilitates the association of PKA RII with AKAP149.
Figure 7.
PKA activation induced by ezrin inhibition depends on AKAP149 expression. A, pretreatment with a pan AKAP inhibitor Ht31 (25 μm) abrogated NSC-mediated PKA activation in FET and GEO cells. **, p < 0.01; ***, p < 0.001. NS, not statistically significant (n = 3). B, AKAP149 KD had no effect on expression of ezrin, p-ezrin Thr-567, XIAP, or survivin but restored XIAP and survivin expression by ezrin KD (upper left panels). Ezrin KD had no effect on AKAP149 expression (upper right panel). Quantification of Western blots is shown in the lower panel. **, p < 0.01. NS, not statistically significant (n = 3). C, AKAP149 KD abrogated ezrin KD-mediated apoptosis. **, p < 0.01. NS, not statistically significant (n = 3). D, AKAP149 KD blocked NSC-mediated PKA activation. **, p < 0.01. NS, not statistically significant (n = 3). E, reciprocal IP analysis with AKAP149 or PKARII antibodies showed increased association of AKAP149 with PKARII in ezrin T567A cells and decreased association in ezrin T567D cells (upper panel). Relative levels of PKARII and AKAP149 were analyzed, and the results are shown in the lower panel. *, p < 0.05; **, p < 0.01 (n = 2). F, IP analysis with ezrin or PKARII antibodies indicated that there was more PKARII associated with ezrin in T567D cells than in T567A cells (upper panel). The relative levels of PKARII and ezrin are shown in the lower panel. *, p < 0.05; **, p < 0.01 (n = 2).
Taken together, our studies suggest a novel model of differential activation of PKA mediated by the TGFβ–Smad3–AKAP149 or IGF1R–ezrin signaling axis (Fig. 8). In the presence of TGFβ, TGFβ–Smad3 inhibits ezrin phosphorylation at Thr-567, leading to the association of AKAP149 with PKA RII to activate PKA in a cAMP-independent manner, suppress XIAP and survivin expression, and induce apoptosis. In the context of IGF1R activation, ezrin is hyperphosphorylated at Thr-567, which results in the association of ezrin with PKA RII and PKA activation. This activation of PKA is cAMP-dependent, increases expression of XIAP and survivin, and enhances cell survival.
Figure 8.

Proposed model of cross-talk between TGFβ–Smad3 and IGF1R signaling pathways. TGFβ–Smad3 inhibits ezrin phosphorylation at Thr-567, leading to the association of PKA RII with AKAP149 to activate PKA in a cAMP-independent manner, which suppresses XIAP and survivin expression and induces apoptosis. With IGF1R activation, ezrin is hyperphosphorylated at Thr-567, resulting in association of ezrin with PKA RII and PKA activation in a cAMP-dependent manner leading to XIAP and survivin up-regulation and cell survival. GF, growth factors; P, phosphorylation.
Discussion
We have shown in this study that ezrin mediates cell survival through PKA activation. Ezrin knockdown or inhibition leads to PKA activation in a cAMP-independent manner and induces apoptosis associated with down-regulation of XIAP and survivin expression. Further studies indicate that PKA activation by ezrin inhibition depends upon AKAP149, knockdown of which abrogated ezrin inhibition-mediated PKA activation and induction of apoptosis. In addition, activation of TGFβ signaling dephosphorylates ezrin at Thr-567 in a Smad3-dependent and Smad2-independent manner, and TGFβ–Smad3 induces its pro-apoptotic effects through inhibition of ezrin phosphorylation. On the other hand, ezrin hyperphosphorylation at Thr-567 mediated by IGF1R signaling leads to PKA activation in a cAMP-dependent manner, promoting cell survival associated with XIAP and survivin up-regulation. These findings suggest that TGFβ and IGF1R signaling antagonize each other by differentially regulating ezrin phosphorylation, which activates PKA through different mechanisms and mediates the equilibrium between cell survival and apoptosis. Therefore, PKA signaling plays an important role in the integration of multiple signal transduction pathways in colon cancer cells. Of note, serine/threonine kinases such as protein kinase B2/Akt2, Rho-kinase, protein kinase Cα, protein kinase Cθ, G protein–coupled receptor kinase 2, and Cdc42 have been implicated as mediators of ezrin phosphorylation at Thr-567 (24, 39–43). In addition, myosin phosphatase and type 2C protein phosphatase have been suggested to dephosphorylate Thr-567 in ERM proteins (12, 39, 40). Further studies are needed to determine whether TGFβ and IGF1R signaling regulates these kinases and/or phosphatases to modulate ezrin phosphorylation and activation.
Previous studies have shown that deregulation of AKAPs contributes to cancer (44, 45). We have shown that ezrin is hyperphosphorylated at Thr-567 in CRC liver metastasis when compared with primary tumors (5). Our present studies indicate that phosphorylation of ezrin at Thr-567 enhances complex formation of ezrin and PKA RII to activate PKA in favor of cell survival, whereas dephosphorylation of ezrin at Thr-567 facilitates the association of AKAP149 and PKA RII, which activates PKA and leads to induction of apoptosis. These results suggest that phosphorylation of ezrin at Thr-567 acts as a signaling node, determining differential PKA activation accomplished by differential AKAP association with PKA RII, leading to different cell fate. This adds to the complexity of the context-dependent AKAP-PKA interaction, which mediates different PKA function by directing PKA supramolecular complexes to various substrates, activating different downstream signaling. Although ezrin-PKA association may enable downstream signaling pathways that promote survival, AKAP149-PKA association likely activates the apoptotic pathways to induce apoptosis. Determining the dynamics of the context-dependent interaction between PKA and different AKAPs, and the mechanisms underlying the activation of different downstream signaling pathways will be critical for understanding the function of AKAPs in regulating cell survival and metastasis of colon cancer cells.
XIAP and survivin are anti-apoptotic proteins important for cell survival. Independent of their cytoprotective function, XIAP and survivin also play important roles in tumor cell invasion and metastasis (22). It has been shown that PKA–AKAP149 signaling contributes to the disruption of the association of XIAP–survivin through phosphorylation of survivin at serine 20, leading to proteasome-mediated degradation of XIAP and induction of apoptosis (29, 30). This regulation involves protein phosphatase 2A-mediated dephosphorylation of Akt (30). However, it is not clear how ezrin–PKA enhances expression of XIAP–survivin. In addition to regulation of its expression, it has been shown that, under stress, pro-apoptotic proteins, XIAP-associated factor 1, Smac, and Omi, interact with XIAP to inhibit its anti-apoptotic function (46). Given the importance of XIAP and survivin in cancer metastasis and relative lack of knowledge of their regulation, more studies are needed to determine the mechanisms of regulation of their expression and function.
In summary, we have made novel observations that TGFβ–Smad3 and IGF1R signaling activates PKA through differential regulation of ezrin phosphorylation and activation, leading to induction of apoptosis or promotion of cell survival (Fig. 8). Our studies uncover a novel mechanism by which TGFβ and IGF1R signals cross-talk to mediate apoptosis and cell survival. This is of significance because TGFβ and IGF1R signaling pathways are well-characterized tumor suppressor and oncogenic pathways, respectively, with important roles in CRC tumorigenesis and metastasis. Because they both function through regulation of ezrin activation, ezrin could be used as a potential therapeutic target in CRC treatment.
Experimental procedures
Cell lines
FET and GEO cell lines were originally derived from primary tumors obtained from two different colon cancer patients (47). Both cell lines harbor mutations in APC/β-catenin and K-Ras (48) and have been used by various groups to investigate colon cancer pathways (49–56). The cells were maintained at 37 °C in a humidified atmosphere, using chemically defined serum-free medium consisting of McCoy's 5A medium (Sigma–Aldrich) supplemented with amino acids, pyruvate, vitamins, antibiotics, and growth factors transferrin (4 μg/ml; Sigma–Aldrich), insulin (20 μg/ml; Sigma–Aldrich), and EGF (10 ng/ml; R & D Systems). Supplemented McCoy's medium is McCoy's 5A medium supplemented with antibiotics and nutrients but lacking growth factors. The cells were routinely subcultured with 0.25% trypsin (Invitrogen) in Joklik's medium (Invitrogen) containing 0.1% EDTA.
Antibodies
The following primary antibodies were obtained from Cell Signaling Technology (Danvers, MA): survivin rabbit polyclonal antibody (catalog no. 2803), XIAP rabbit mAb (catalog no. 2045), AKAP149 rabbit mAb (catalog no. 5203), PKACatα rabbit mAb (catalog no. 5842), TGFβRI rabbit polyclonal antibody (catalog no. 3712), Smad2 rabbit mAb (catalog no. 3122), Smad3 rabbit mAb (catalog no. 9523), p-Smad3 rabbit mAb (catalog no. 9520), CREB rabbit mAb (catalog no. 9197), p-CREB rabbit mAb (catalog no. 9198), and caspase 7 rabbit polyclonal antibody (catalog no. 9492). Ezrin mouse mAb (catalog no. sc-71082) and TGFβRII mouse mAb (catalog no. sc-17791) were obtained from Santa Cruz Biotechnology (Dallas, TX). p-EzrinT567 rabbit polyclonal antibody (catalog no. ab47293) and PKARII mouse mAb (catalog no. ab124400) were obtained from Abcam (Cambridge, MA). Actin rabbit antibody (catalog no. A2066) was obtained from Sigma–Aldrich (St. Louis, MO).
Pharmacological antagonists
Ezrin small molecule inhibitor NSC668394 (NSC) was purchased from Millipore. TGFβ1 was purchased from R & D Systems. PKA inhibitor H89 (catalog no. 9844) and forskolin (catalog no. 3828) were obtained from Cell Signaling Technology. Pan-AKAP inhibitor Ht31 (catalog no. V8211) was obtained from Promega.
Transfection studies
Ezrin GIPZ lentiviral shRNAs and on-TARGETplus SMART pool AKAP149 siRNA were purchased from Dharmacon (Thermo Fisher Scientific), and knockdowns were performed according to the manufacturer protocols. Smad2, Smad3, and PKACatα shRNAs were obtained from Santa Cruz Biotechnology, and knockdowns were performed as described previously (30, 33). Ezrin WT, T567A (phospho-deficient), and T567D (phospho-mimetic) mutants were kindly provided by the Khanna Laboratory, National Institutes of Health (16). Stable transfections were performed in GEO cells using Lipofectamine 2000 as described previously (16).
Cell lysate preparation
GEO and FET cells were harvested at 70–80% confluency. The cells were washed in cold 5% PBS and collected in lysis buffer (50 mmol/liter Tris, pH 7.4, 100 mmol/liter NaCl, 1% Nonidet P-40, 2 mmol/liter EDTA, 0.1% SDS, 50 mmol/liter NaF, 10 mmol/liter Na3VO4, 1 mmol/liter phenylmethylsulfonyl fluoride, 25 μg/ml β-glycerophosphate, and one protease inhibitor mixture tablet from Roche). Crude cell lysates were homogenized using a 21-gauge needle to shear DNA and lysed for 30 min on ice at 4 °C. The cell lysates were then cleared by centrifugation at 13,000 rpm for 20 min at 4 °C. Protein concentrations were determined by the Pierce bicinchoninic acid protein assay (Pierce Biotechnology).
Western blotting and immunoprecipitation
Protein (30–100 μg) was fractionated by SDS-PAGE and transferred onto a nitrocellulose membrane (Amersham Biosciences) by electroblotting. The membrane was blocked with 5% nonfat dry milk in 1× TBST (50 mm Tris, pH 7.5, 150 mm NaCl, 0.05% Tween 20) for 1 h at room temperature or overnight at 4 °C. The membrane was then incubated in primary antibodies for 1.5 h at room temperature or overnight at 4 °C with 5% nonfat dry milk in 1× TBST or 5% BSA in 1× TBST according to the antibody manufacturer's directions. The membrane was washed three times with 1× TBST for 10 min each and incubated with horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences) for 1 h at room temperature. This was followed by washing in 1× TBST, and proteins were detected by enhanced chemiluminescence system (Amersham Biosciences). Immunoprecipitation was performed with 500-μg protein aliquots using magnetic beads (Millipore) according to the manufacturer's instructions. Quantification of Western blots was performed using ImageJ.
Apoptosis assay
Apoptosis was measured by the cell death detection ELISA Plus kit (Roche) as described previously (57). Inhibition of cell proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described previously (38, 57).
PKA activity assay
PKA activity was measured using the PepTag nonradioactive protein kinase assay kit from Promega (catalog no. V5340) using kemptide (LRRASLG) in the absence of cAMP according to the manufacturer's protocol as described previously (30).
cAMP activity assay
For quantitative determination of cAMP, a nonradioactive Direct cAMP enzyme immunoassay kit from Enzo Life Sciences (catalog no. ADI-901-066) was utilized, and the assay was performed according to the manufacturer's protocol as described previously (30).
Statistical analysis
Statistical significance was determined using one-way analysis of variance and student t test with a p value less than 0.05 using the GraphPad Prism 7 software. All the experiments were repeated three times independently, for the analysis of variance. The results were expressed as means ± S.E. for each treatment.
Author contributions
P. D. L., M. G. B., J. D. B., and J. W. conceptualization; P. D. L. and J. W. data curation; P. D. L. and J. W. formal analysis; P. D. L., J. D. B., and J. W. validation; P. D. L. investigation; P. D. L. and J. W. visualization; P. D. L. methodology; P. D. L. and J. W. writing-original draft; P. D. L. project administration; P. D. L., J. D. B., and J. W. writing-review and editing; M. G. B., J. D. B., and J. W. supervision; M. G. B., J. D. B., and J. W. funding acquisition; J. D. B. and J. W. resources; J. D. B. and J. W. software.
Acknowledgments
We thank Dr. Chand Khanna (NCI Pediatric Oncology Branch) for providing the ezrin WT and ezrin T567A/D mutant constructs.
This work was supported by National Institutes of Health Grants R01CA038173 and R01CA054807 (to M. G. B.), R01CA208063, R01CA212241, and R01CA215389 (to J. W.), and R01CA191894 (to J. D. B.) and by Fred and Pamela Buffett Cancer Center Grant P30 CA036727. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CRC
- colorectal cancer
- AKAP
- A-kinase anchoring protein
- CREB
- cAMP response element binding protein
- ERM
- ezrin–radixin–moesin
- FERM
- four-point-one, ezrin, radixin, moesin
- IGF1R
- insulin-like growth factor 1 receptor
- XIAP
- X-linked inhibitor of apoptosis
- PKA
- protein kinase A
- KD
- knockdown
- TI
- transferrin and insulin
- IP
- immunoprecipitation.
References
- 1. Markowitz S. D., and Bertagnolli M. M. (2009) Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 361, 2449–2460 10.1056/NEJMra0804588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. André T., Boni C., Mounedji-Boudiaf L., Navarro M., Tabernero J., Hickish T., Topham C., Zaninelli M., Clingan P., Bridgewater J., Tabah-Fisch I., de Gramont A., and Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 350, 2343–2351 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 3. Mehlen P., and Puisieux A. (2006) Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458 10.1038/nrc1886 [DOI] [PubMed] [Google Scholar]
- 4. Nguyen D. X., Bos P. D., and Massagué J. (2009) Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 10.1038/nrc2622 [DOI] [PubMed] [Google Scholar]
- 5. Leiphrakpam P. D., Rajput A., Mathiesen M., Agarwal E., Lazenby A. J., Are C., Brattain M. G., and Chowdhury S. (2014) Ezrin expression and cell survival regulation in colorectal cancer. Cell Signal. 26, 868–879 10.1016/j.cellsig.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato N., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., and Tsukita S. (1992) A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J. Cell Sci. 103, 131–143 [DOI] [PubMed] [Google Scholar]
- 7. Gould K. L., Bretscher A., Esch F. S., and Hunter T. (1989) cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 8, 4133–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Algrain M., Turunen O., Vaheri A., Louvard D., and Arpin M. (1993) Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 120, 129–139 10.1083/jcb.120.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearson M. A., Reczek D., Bretscher A., and Karplus P. A. (2000) Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259–270 10.1016/S0092-8674(00)80836-3 [DOI] [PubMed] [Google Scholar]
- 10. Bretscher A., Reczek D., and Berryman M. (1997) Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 110, 3011–3018 [DOI] [PubMed] [Google Scholar]
- 11. Bretscher A., Edwards K., and Fehon R. G. (2002) ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 10.1038/nrn900,10.1038/nrn906,10.1038/nrm882 [DOI] [PubMed] [Google Scholar]
- 12. Gautreau A., Louvard D., and Arpin M. (2000) Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J. Cell Biol. 150, 193–203 10.1083/jcb.150.1.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu L., Zhou R., Mettler S., Wu T., Abbas A., Delaney J., and Forte J. G. (2007) High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am. J. Physiol. Cell Physiol. 293, C874–C884 10.1152/ajpcell.00111.2007 [DOI] [PubMed] [Google Scholar]
- 14. Fievet B. T., Gautreau A., Roy C., Del Maestro L., Mangeat P., Louvard D., and Arpin M. (2004) Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 164, 653–659 10.1083/jcb.200307032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fehon R. G., McClatchey A. I., and Bretscher A. (2010) Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren L., Hong S. H., Cassavaugh J., Osborne T., Chou A. J., Kim S. Y., Gorlick R., Hewitt S. M., and Khanna C. (2009) The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene 28, 792–802 10.1038/onc.2008.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y., Wang D., Guo Z., Zhao J., Wu B., Deng H., Zhou T., Xiang H., Gao F., Yu X., Liao J., Ward T., Xia P., Emenari C., Ding X., et al. (2011) Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer Res. 71, 1721–1729 10.1158/1538-7445.AM2011-1721,10.1158/0008-5472.CAN-09-4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elzagheid A., Korkeila E., Bendardaf R., Buhmeida A., Heikkilä S., Vaheri A., Syrjänen K., Pyrhönen S., and Carpén O. (2008) Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Hum. Pathol. 39, 1737–1743 10.1016/j.humpath.2008.04.020 [DOI] [PubMed] [Google Scholar]
- 19. Weng W. H., Ahlén J., Aström K., Lui W. O., and Larsson C. (2005) Prognostic impact of immunohistochemical expression of ezrin in highly malignant soft tissue sarcomas. Clin. Cancer Res. 11, 6198–6204 10.1158/1078-0432.CCR-05-0548 [DOI] [PubMed] [Google Scholar]
- 20. Wang H. J., Zhu J. S., Zhang Q., Sun Q., and Guo H. (2009) High level of ezrin expression in colorectal cancer tissues is closely related to tumor malignancy. World J. Gastroenterol. 15, 2016–2019 10.3748/wjg.15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tynninen O., Carpén O., Jääskeläinen J., Paavonen T., and Paetau A. (2004) Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol. Appl. Neurobiol. 30, 472–477 10.1111/j.1365-2990.2004.00562.x [DOI] [PubMed] [Google Scholar]
- 22. Mehrotra S., Languino L. R., Raskett C. M., Mercurio A. M., Dohi T., and Altieri D. C. (2010) IAP regulation of metastasis. Cancer Cell 17, 53–64 10.1016/j.ccr.2009.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiue H., Musch M. W., Wang Y., Chang E. B., and Turner J. R. (2005) Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J. Biol. Chem. 280, 1688–1695 10.1074/jbc.M409471200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agarwal E., Chaudhuri A., Leiphrakpam P. D., Haferbier K. L., Brattain M. G., and Chowdhury S. (2014) Akt inhibitor MK-2206 promotes anti-tumor activity and cell death by modulation of AIF and Ezrin in colorectal cancer. BMC Cancer 14, 145 10.1186/1471-2407-14-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dransfield D. T., Bradford A. J., Smith J., Martin M., Roy C., Mangeat P. H., and Goldenring J. R. (1997) Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 16, 35–43 10.1093/emboj/16.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor S. S., Yang J., Wu J., Haste N. M., Radzio-Andzelm E., and Anand G. (2004) PKA: a portrait of protein kinase dynamics. Biochim. Biophys. Acta 1697, 259–269 10.1016/j.bbapap.2003.11.029 [DOI] [PubMed] [Google Scholar]
- 27. Wong W., and Scott J. D. (2004) AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959–970 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- 28. Taskén K., and Aandahl E. M. (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 10.1152/physrev.00021.2003 [DOI] [PubMed] [Google Scholar]
- 29. Dohi T., Xia F., and Altieri D. C. (2007) Compartmentalized phosphorylation of IAP by protein kinase A regulates cytoprotection. Mol. Cell 27, 17–28 10.1016/j.molcel.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chowdhury S., Howell G. M., Rajput A., Teggart C. A., Brattain L. E., Weber H. R., Chowdhury A., and Brattain M. G. (2011) Identification of a novel TGFβ/PKA signaling transduceome in mediating control of cell survival and metastasis in colon cancer. PLoS One 6, e19335 10.1371/journal.pone.0019335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L., Duan C. J., Binkley C., Li G., Uhler M. D., Logsdon C. D., and Simeone D. M. (2004) A transforming growth factor β-induced Smad3/Smad4 complex directly activates protein kinase A. Mol. Cell Biol. 24, 2169–2180 10.1128/MCB.24.5.2169-2180.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bulut G., Hong S. H., Chen K., Beauchamp E. M., Rahim S., Kosturko G. W., Glasgow E., Dakshanamurthy S., Lee H. S., Daar I., Toretsky J. A., Khanna C., and Uren A. (2012) Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene 31, 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang Y., Liu X. Q., Rajput A., Geng L., Ongchin M., Zeng Q., Taylor G. S., and Wang J. (2011) Phosphatase PRL-3 is a direct regulatory target of TGFβ in colon cancer metastasis. Cancer Res. 71, 234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J., Yang L., Yang J., Kuropatwinski K., Wang W., Liu X. Q., Hauser J., and Brattain M. G. (2008) Transforming growth factor β induces apoptosis through repressing the phosphoinositide 3-kinase/AKT/survivin pathway in colon cancer cells. Cancer Res. 68, 3152–3160 10.1158/0008-5472.CAN-07-5348 [DOI] [PubMed] [Google Scholar]
- 35. Hedrick E. D., Agarwal E., Leiphrakpam P. D., Haferbier K. L., Brattain M. G., and Chowdhury S. (2013) Differential PKA activation and AKAP association determines cell fate in cancer cells. J. Mol. Signal. 8, 10 10.1186/1750-2187-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leiphrakpam P. D., Agarwal E., Mathiesen M., Haferbier K. L., Brattain M. G., and Chowdhury S. (2014) In vivo analysis of insulin-like growth factor type 1 receptor humanized monoclonal antibody MK-0646 and small molecule kinase inhibitor OSI-906 in colorectal cancer. Oncol. Rep. 31, 87–94 10.3892/or.2013.2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu Y. P., Patil S. B., Panasiewicz M., Li W., Hauser J., Humphrey L. E., and Brattain M. G. (2008) Heterogeneity of receptor function in colon carcinoma cells determined by cross-talk between type I insulin-like growth factor receptor and epidermal growth factor receptor. Cancer Res. 68, 8004–8013 10.1158/0008-5472.CAN-08-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J., Han W., Zborowska E., Liang J., Wang X., Willson J. K., Sun L., and Brattain M. G. (1996) Reduced expression of transforming growth factor β type I receptor contributes to the malignancy of human colon carcinoma cells. J. Biol. Chem. 271, 17366–17371 10.1074/jbc.271.29.17366 [DOI] [PubMed] [Google Scholar]
- 39. Fukata Y., Kimura K., Oshiro N., Saya H., Matsuura Y., and Kaibuchi K. (1998) Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J. Cell Biol. 141, 409–418 10.1083/jcb.141.2.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsui T., Maeda M., Doi Y., Yonemura S., Amano M., Kaibuchi K., Tsukita S., and Tsukita S. (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 140, 647–657 10.1083/jcb.140.3.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ng T., Parsons M., Hughes W. E., Monypenny J., Zicha D., Gautreau A., Arpin M., Gschmeissner S., Verveer P. J., Bastiaens P. I., and Parker P. J. (2001) Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 20, 2723–2741 10.1093/emboj/20.11.2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cant S. H., and Pitcher J. A. (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol. Biol. Cell 16, 3088–3099 10.1091/mbc.E04-10-0877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakamura N., Oshiro N., Fukata Y., Amano M., Fukata M., Kuroda S., Matsuura Y., Leung T., Lim L., and Kaibuchi K. (2000) Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes Cells 5, 571–581 10.1046/j.1365-2443.2000.00348.x [DOI] [PubMed] [Google Scholar]
- 44. Su B., Bu Y., Engelberg D., and Gelman I. H. (2010) SSeCKS/Gravin/AKAP12 inhibits cancer cell invasiveness and chemotaxis by suppressing a protein kinase C-Raf/MEK/ERK pathway. J. Biol. Chem. 285, 4578–4586 10.1074/jbc.M109.073494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tröger J., Moutty M. C., Skroblin P., and Klussmann E. (2012) A-kinase anchoring proteins as potential drug targets. Br. J. Pharmacol. 166, 420–433 10.1111/j.1476-5381.2011.01796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liston P., Fong W. G., and Korneluk R. G. (2003) The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene 22, 8568–8580 10.1038/sj.onc.1207101 [DOI] [PubMed] [Google Scholar]
- 47. Brattain M. G., Levine A. E., Chakrabarty S., Yeoman L. C., Willson J. K., and Long B. (1984) Heterogeneity of human colon carcinoma. Cancer Metastasis Rev. 3, 177–191 10.1007/BF00048384 [DOI] [PubMed] [Google Scholar]
- 48. Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., Janssen K.-P., Robine S., Velcich A., Augenlicht L. H., Black A. R., and Black J. D. (2009) PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 315, 1415–1428 10.1016/j.yexcr.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tabernero J., Van Cutsem E., Díaz-Rubio E., Cervantes A., Humblet Y., André T., Van Laethem J. L., Soulié P., Casado E., Verslype C., Valera J. S., Tortora G., Ciardiello F., Kisker O., and de Gramont A. (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 25, 5225–5232 10.1200/JCO.2007.13.2183 [DOI] [PubMed] [Google Scholar]
- 50. Mariotti E., Gemei M., Mirabelli P., D'Alessio F., Di Noto R., Fortunato G., and Del Vecchio L. (2010) The percentage of CD133+ cells in human colorectal cancer cell lines is influenced by Mycoplasma hyorhinis infection. BMC Cancer 10, 120 10.1186/1471-2407-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Napolitano S., Martini G., Rinaldi B., Martinelli E., Donniacuo M., Berrino L., Vitagliano D., Morgillo F., Barra G., De Palma R., Merolla F., Ciardiello F., and Troiani T. (2015) Primary and acquired resistance of colorectal cancer to anti-EGFR monoclonal antibody can be overcome by combined treatment of regorafenib with cetuximab. Clin. Cancer Res. 21, 2975–2983 10.1158/1078-0432.CCR-15-0020 [DOI] [PubMed] [Google Scholar]
- 52. Grau A. M., Datta P. K., Zi J., Halder S. K., and Beauchamp R. D. (2006) Role of Smad proteins in the regulation of NF-κB by TGF-β in colon cancer cells. Cell Signal. 18, 1041–1050 10.1016/j.cellsig.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 53. Halder S. K., Rachakonda G., Deane N. G., and Datta P. K. (2008) Smad7 induces hepatic metastasis in colorectal cancer. Br. J. Cancer 99, 957–965 10.1038/sj.bjc.6604562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang X., Chen W., Singh N., Promkan M., and Liu G. (2010) Effects of potential calcium sensing receptor inducers on promoting chemosensitivity of human colon carcinoma cells. Int. J. Oncol. 36, 1573–1580 [DOI] [PubMed] [Google Scholar]
- 55. Hogan F. S., Krishnegowda N. K., Mikhailova M., and Kahlenberg M. S. (2007) Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J. Surg. Res. 143, 58–65 10.1016/j.jss.2007.03.080 [DOI] [PubMed] [Google Scholar]
- 56. Beck S. E., Jung B. H., Del Rosario E., Gomez J., and Carethers J. M. (2007) BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 19, 1465–1472 10.1016/j.cellsig.2007.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang J., Kuropatwinski K., Hauser J., Rossi M. R., Zhou Y., Conway A., Kan J. L., Gibson N. W., Willson J. K., Cowell J. K., and Brattain M. G. (2007) Colon carcinoma cells harboring PIK3CA mutations display resistance to growth factor deprivation induced apoptosis. Mol. Cancer Ther. 6, 1143–1150 10.1158/1535-7163.MCT-06-0555,10.4161/cbt.6.7.4704 [DOI] [PubMed] [Google Scholar]