Abstract
Liver tumor–initiating cells (TICs) form small subsets of cells in hepatocellular tumors and account for tumorigenesis, metastasis, recurrence, and drug resistance. Recently, we found that the transcription factor Zic family member 2 (ZIC2) is highly expressed in liver TICs and required for their self-renewal. However, the molecular mechanisms underlying self-renewal of liver TICs remain unclear. Here, using expression profiling and CRISPR-interference assays with clinical samples of human liver cancers, we identified a long noncoding RNA (lncRNA), lncZic2, that is located near the ZIC2 locus and was highly expressed in liver cancer and liver TICs. We found that lncZic2 is required for the self-renewal of liver TICs in a ZIC2-independent manner. lncZic2 drove the expression of myristoylated alanine-rich protein kinase C substrate (MARCKS) and MARCKS-like 1 (MARCKSL1), whose expression levels were increased during liver tumorigenesis and liver TIC self-renewal. Mechanistically, lncZic2 interacted with BRM/SWI2-related gene 1 (BRG1) and recruited this transcriptional regulator to the promoters of the MARCKS and MARCKSL1 gene, which activated expression of these genes. Moreover, we noted that depletion of lncZic2 and BRG1 decreases MARCKS and MARCKSL1 expression and diminishes liver TIC levels. In conclusion, lncZic2 is required for the self-renewal of liver TICs by up-regulating MARCKS and MARCKSL1 gene expression via the transcription factor BRG1. Our findings suggest that the lncZic2–BRG1–MARCKS/MARCKSL1 signaling cascade might be a potential target for eliminating liver TICs in the management of liver cancer.
Keywords: cancer stem cells; long noncoding RNA (long ncRNA, lncRNA); liver cancer; chromatin regulation; tumor promoter; tumor therapy; myristoylated alanine-rich protein kinase C substrate; protein kinase C signaling; self-renewal; tumor targeting; Zic family member 2
Introduction
Liver cancer is one of the most serious cancer in the world and has two major tumor types (hepatocellular carcinoma and cholangiocarcinoma), and hepatocellular carcinoma (HCC)3 is the most common tumor type (about 90%) (1). For a long time, it was recognized that all cells within the tumor bulk do not harbor carcinogenicity and that only a small subset cells can generate tumors (2). These cells are considered liver TICs. Actually, there are many kinds of cells within the HCC tumor bulk, which are generated from a small subset of cells termed liver TICs. Liver TICs are considered the origin of liver tumorigenesis, metastasis, drug resistance, and relapse (3). The most well-known characteristics of liver TICs are self-renewal and tumor-initiating capacities, which are examined by sphere formation and tumor initiation assays, respectively (4, 5). Recently, more and more surface markers of liver TICs have been identified (including CD133, CD13, EPCAM, CD24 and so on), and CD133 is a widely accepted liver TIC marker (6–8). CD133+ cells can efficiently form new tumors and self-renew. On the contrary, CD133− cells have impaired liver TIC properties. The importance of liver TICs is widely accepted, but their biological characteristics are still largely unknown.
The self-renewal of liver TICs is under precise regulation. The NF-κB, Wnt/β-catenin, Notch, Hedgehog, and PKC signaling pathways are the most important pathways in liver TICs (9–11). Recently, we found that the transcription factor ZIC2 is highly expressed in liver cancer and liver TICs (12). ZIC2 is required for the self-renewal of liver TICs through OCT4 expression. ZIC2 interacts with the NURF complex and is recruited it to the promoter of OCT4, thus driving the expression of OCT4. ZIC2 and OCT4 can be targeted for liver TIC elimination. However, the molecular mechanism of ZIC2 overexpression in liver TIC remains unclear. Here we focused on the ZIC2 locus and found a long noncoding RNA (LINC00554, termed lncZic2).
Long noncoding RNAs (lncRNAs) are emerging as critical modulators in many biological processes, including development (13), immunology (14), neurology (15), tumorigenesis (16), and so on. lncRNAs exert their roles in cis or in trans. lncRNAs may regulate the expression of their neighboring genes in cis or regulate the expression of distant genes in trans (17). Mechanistically, lncRNAs can recruit chromatin remodeling complexes to the promoter of target genes and participate in transcription initiation (18, 19). lncRNAs also interact with some key proteins to modulate their stability or activity (20–22). Recently, we identified that a long noncoding RNA, lncSox4, is highly expressed in liver cancer and liver TICs (23). LncSox4 targets Sox4 expression and interacts with Stat3. LncSox4 is required for the binding of Stat3 to the Sox4 promoter and subsequent Sox4 expression.
It is common that lncRNAs are co-expressed with their neighboring genes (24). Here we focused on lncZic2 and found that lncZic2 was also highly expressed in liver cancer and liver TICs. Interestingly, lncZic2 is not involved in the transcription regulation of ZIC2 but regulates the expression of MARCKS and MARCKSL1. Through interacting with BRG1, lncZic2 is required for the interaction between BRG1 and the MARCKS/MARCKSL1 promoter. lncZic2–BRG1–MARCKS/MARCKSL1 can also be targeted for liver TIC clearance.
Results
lncZic2 was highly expressed in liver cancer and liver TICs
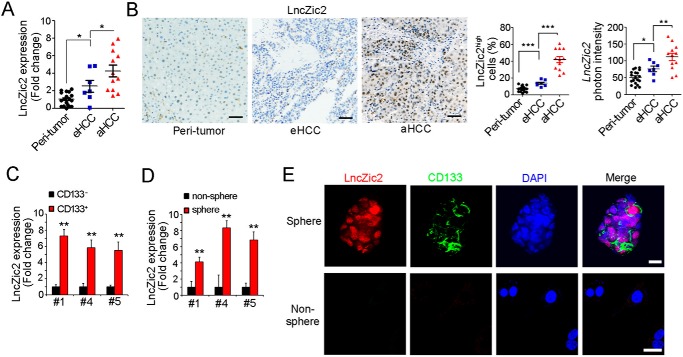
Liver TICs play critical roles in liver tumorigenesis, and the self-renewal of liver TIC is finely regulated. In previous work, we found that ZIC2 is highly expressed in liver cancer and liver TICs. Here we found a long noncoding RNA (LINC00554, hereafter termed lncZic2) near the ZIC2 locus. lncZic2 was highly expressed in liver cancer, especially in advanced liver cancer (Fig. 1, A and B). We then detected the expression profiles of lncZic2 in liver TICs and found that lncZic2 was highly expressed in CD133+ liver TICs (Fig. 1C) and stem-like oncospheres (Fig. 1D). Through fluorescence in situ hybridization, lncZic2 was overexpressed in CD133+ liver TICs (Fig. 1E). Altogether, lncZic2 was highly expressed in liver cancer and liver TICs.
Figure 1.
lncZic2 was highly expressed in liver cancer and liver TICs. A, total RNA from 19 peritumor, seven early HCC (eHCC), and 12 advanced HCC (aHCC) samples was extracted, and lncZic2 expression levels were examined by real-time PCR. B, in situ hybridization of lncZic2. 19 peritumor, seven early HCC, and 12 advanced HCC samples were used for lncZic2 hybridization. Typical images are shown in the three left panels, lncZic2high cell ratios are shown in the center panel, and lncZic2 photon intensities are shown in the right panel. C and D, lncZic2 expression levels in CD133+ liver TICs (C) and stem-like oncospheres (D) were examined through real-time PCR. lncZic2 average expression levels in liver non-TICs and nonspheres were normalized as 1. E, FISH analysis for lncZic2 expression and localization in spheres and nonspheres. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars = 10 μm. Experiments were repeated at least three times. *, p < 0.05; **, p < 0.01; ***, p < 0.001, by t test.
lncZic2 was required for the self-renewal of liver TICs
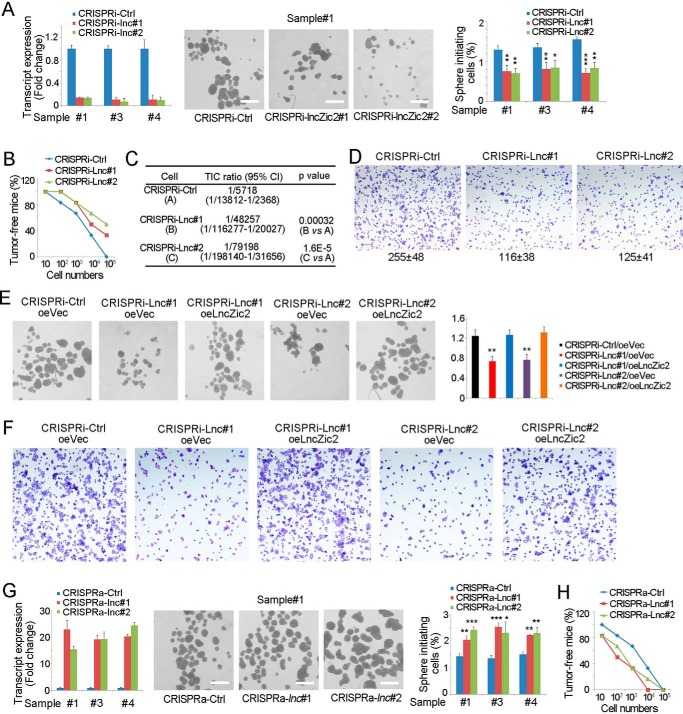
We then examined the role of lncZic2 in liver TICs. We generated lncZic2-depleted cells through the CRISPRi strategy and confirmed the depletion efficiency by real-time PCR. lncZic2 depletion largely impaired the sphere formation capacity, indicating a critical role of lncZic2 in liver TIC self-renewal (Fig. 2A). A tumor initiation assay was also performed using lncZic2-depleted cells. lncZic2-depleted cells showed impaired tumorigenesis and contained fewer liver TICs (Fig. 2, B and C). Tumor invasion capacity is another standard model for liver TICs. We then performed tumor invasion and found impaired invasion capacity upon lncZic2 depletion (Fig. 2D). These results confirmed lncZic2 as a necessary lncRNA in liver TIC self-renewal. To further confirm the role of lncZic2, we rescued the expression levels of lncZic2 and found that lncZic2 rescue restored the sphere formation capacity (Fig. 2E) and tumor invasion capacity (Fig. 2F).
Figure 2.
lncZic2 played an essential role in liver TIC self-renewal. A, lncZic2-depleted cells were depleted through the CRISPRi strategy, followed by a sphere formation assay. Typical sphere photos and calculated TIC ratios are shown. Ctrl, control. B and C, 10, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 lncZic2-depleted and control cells were subcutaneously injected into BALB/c nude mice, followed by 3 months of tumor initiation. The ratios of tumor-free mice are shown in B; liver TIC ratios were calculated through extreme limiting dilution analysis (C). D, 3 × 105 lncZic2-depleted and control cells were seeded onto a Matrigel-coated membrane, and tumor invasion was observed 36 h later. E, lncZic2 was rescued in lncZic2-depleted cells, followed by a sphere formation assay. Typical images are shown in the three left panels, and sphere initiating ratios are shown in the right panel. Lnc#1, lncZic2#1; oe, overexpression. Vec, Vector. F, the indicated cells were used for the tumor invasion assay, and typical images are shown. G, lncZic2 was highly expressed in primary cells through the CRISPRa strategy, and a sphere formation assay was performed. Typical images and sphere-initiating ratios are shown. H, 10, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 lncZic2-overexpressing cells were subcutaneously injected into BALB/c nude mice. 3 months later, tumor formation was observed; the ratios of tumor-free mice are shown. Experiments were repeated four times. *, p < 0.05; **, p < 0.01; ***, p < 0.001, by t test.
We then generated cells highly expressing lncZic2 through CRISPRa strategy and found that lncZic2 overexpression enhanced sphere formation (Fig. 2G). A gradient tumor formation assay also found that lncZic2 overexpression drove liver tumor initiation (Fig. 2H). Taken together, lncZic2 played a necessary and sufficient role in liver TIC self-renewal.
lncZic2 targeted MARCKS and MARCKSL1 expression
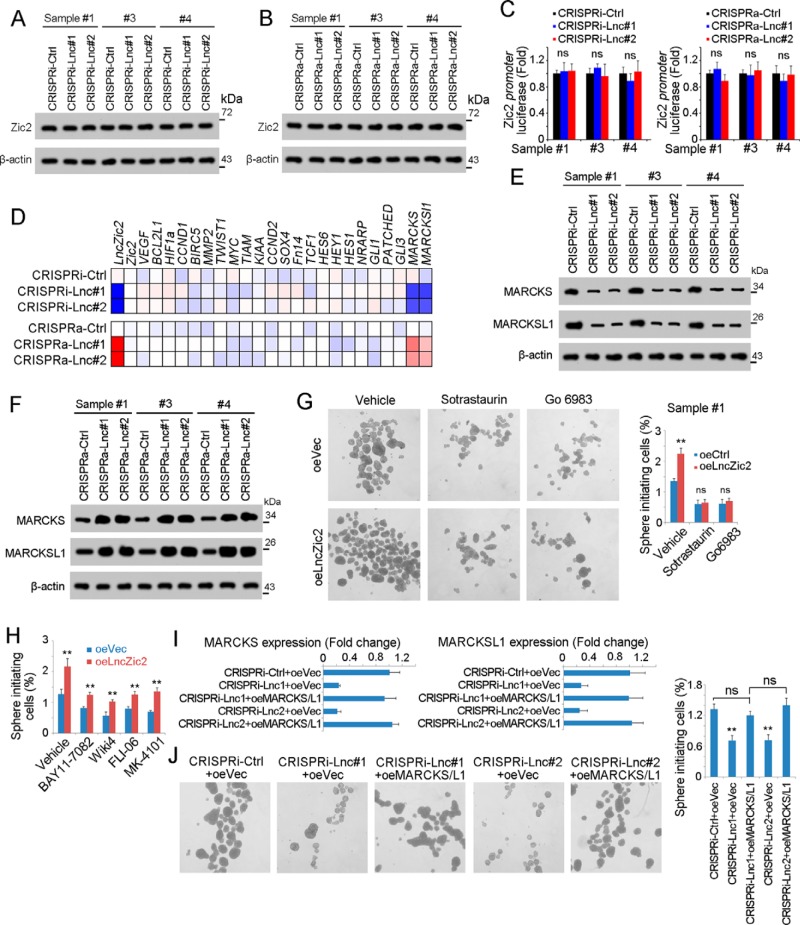
We then identified the target genes of lncZic2 in liver TIC self-renewal. First we explored whether lncZic2 regulated ZIC2 expression to drive liver tumorigenesis. We examined ZIC2 expression in lncZic2-depleted and lncZic2-overexpressed cells and found comparable expression levels of ZIC2 (Fig. 3, A and B). We then constructed a ZIC2 promoter luciferase assay and found that neither lncZic2 depletion nor overexpression participated in the activation of the ZIC2 promoter (Fig. 3C). These results confirmed that lncZic2 did not regulate ZIC2 expression.
Figure 3.
lncZic2 drove the expression of MARCKS and MARCKSL1. A and B, ZIC2 expression levels in lncZic2-depleted cells (A) or lncZic2-overexpressing cells (B) were examined by Western blotting. β-Actin served as a loading control. Ctrl, control. C, the ZIC2 promoter (−5000 bp ∼ 0) was cloned into the PGL3 luciferase reporter plasmid and transfected into lncZic2-depleted (left panel) or lncZic2-overexpressing (right panel) cells, and the activation of the ZIC2 promoter was examined by luciferase assay. ns, not significant. D, the expression levels of the indicated genes are shown as a heat map. Blue and red represent low expression and high expression, respectively. E and F, lncZic2-depleted (E) or -overexpressing (F) cells were generated, followed by Western blotting for MARCKS and MARCKSL1 expression. β-Actin served as a loading control. G, lncZic2-overexpressing (oe) and control cells were treated with PKC inhibitors (sotrastaurin and Go6983), followed by a sphere formation assay. Typical images are shown in the left panels, and liver TIC ratios are shown in the right panel. H, the indicated treated lncZic2-overexpressing or control cells were used for a sphere formation assay, and sphere initiating capacity is shown. I, lncZic2-depleted cells were used for overexpression of MARCKS/MARCKSL1, which was examined by real-time PCR. J, MARCKS and MARCKSL1 were rescued in lncZic2-depleted cells, followed by a sphere formation assay, and typical images are shown. Experiments were repeated three times. **, p < 0.01, by t test.
Then we examined the target genes of the NF-κB, Wnt/β-catenin, Notch, Hedgehog, and PKC pathways and found that the expression levels of MARCKS and MARCKSL1 were decreased upon lncZic2 depletion. On the contrary, MARCKS and MARCKSL1 were overexpressed upon lncZic2 overexpression (Fig. 3D). We then confirmed the regulation of lncZic2 in MARCKS and MARCKSL1 expression through Western blotting. lncZic2 depletion impaired the expression of MARCKS and MARCKSL1, whereas lncZic2 overexpression promoted their expression (Fig. 3, E and F).
We treated lncZic2-overexpressing cells with two PKC inhibitors, sotrastaurin and Go6983, and found that lncZic2 overexpression had an impaired effect on liver TIC self-renewal when the PKC signaling pathway was blocked (Fig. 3G). On the contrary, inhibition of other signaling pathways could not abolish the role of lncZic2 (Fig. 3H). These data indicated a critical role of PKC signaling in lncZic2 function. We then rescued the expression of MARCKS/MARCKSL1 and found that MARCKS/MARCKSL1 overexpression could restore the self-renewal of liver TICs, indicating that lncZic2 drove self-renewal mainly through MARCKS/MARCKSL1 (Fig. 3, I and J). Taken together, lncZic2 promoted the expression of MARCKS and MARCKSL1 to promote liver TIC self-renewal.
MARCKS and MARCKSL1 were required for liver TIC self-renewal
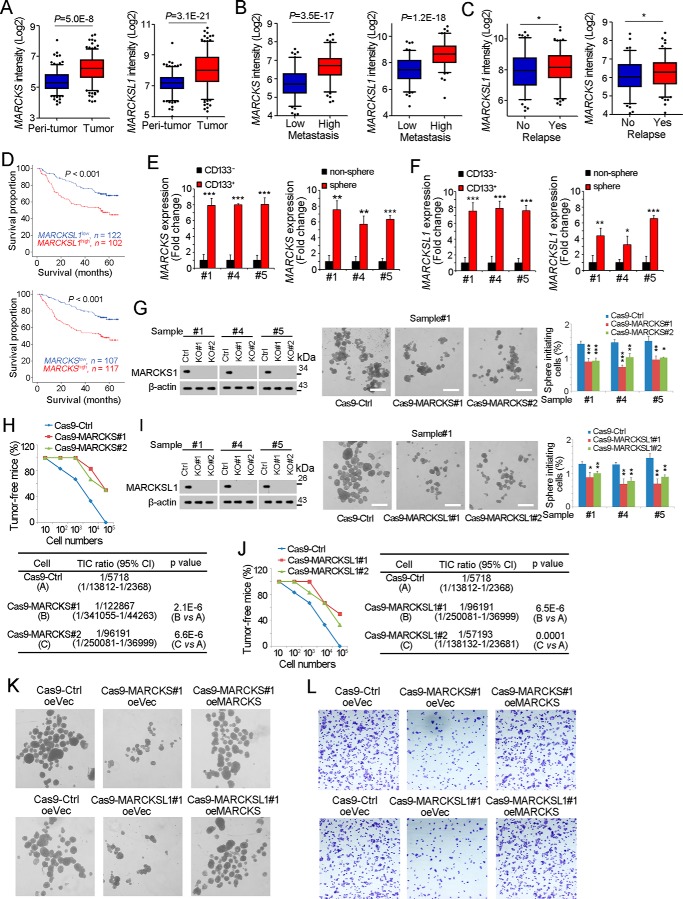
We then examined the expression profiles of MARCKS and MARCKSL1 in liver cancer and liver TICs. Analyses of a dataset available online (GSE14520 (25, 26)) revealed high expression of MARCKS and MARCKSL1 in liver tumor samples (Fig. 4A). Also, the expression levels of MARCKS and MARCKSL1 are associated with metastasis (Fig. 4B), relapse (Fig. 4C), and survival (Fig. 4D). We then isolated liver TICs through surface marker CD133 and oncosphere formation and examined the expression levels of MARCKS and MARCKSL1. As expected, MARCKS and MARCKSL1 were highly expressed in CD133+ liver TICs and stem-like spheres (Fig. 4, E and F).
Figure 4.
MARCKS and MARCKSL1 are required for liver TIC self-renewal. A, MARCKS and MARCKSL1 intensities of peritumor and tumor samples in GSE14520 are shown as box and whisker plots. Horizontal lines, median; boxes, interquartile range; whiskers, 5–95 percentile; OE, overexpression. B and C, MARCKS and MARCKSL1 expression intensity in metastasis (B) and relapse (C) samples. D, HCC samples were divided into two groups according to MARCKS (top panel) or MARCKSL1 (bottom panel) expression, followed by Kaplan-Meier survival analyses. E and F, total RNA was extracted from CD133+ liver TICs and spheres, followed by real-time PCR analyses for MARCKS (E) or MARCKSL1 (F) expression. G, MARCKS knockout primary cells were generated through the CRISPR/Cas9 approach, followed by a sphere formation assay. MARCKS knockout efficiency was examined by Western blotting; typical sphere images are shown in the center panels, and sphere initiating ratios are shown in the right panel. Ctrl, control. H, 10, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 MARCKS knockout and control cells were subcutaneously injected into BALB/c nude mice, followed by 3 months of tumor initiation. The ratios of tumor-free mice are shown in the top panel, and liver TIC ratios were calculated through extreme limiting dilution analysis (bottom panel). I, MARCKSL1 knockout cells were generated through the CRISPR/Cas9 approach, followed by a sphere formation assay. J, gradient MARCKSL1 knockout and control cells were used for a tumor initiation assay. Tumor-free ratios and liver TIC ratios are shown. K and L, MARCKS/MARCKSL1 were rescued in MARCKS/MARCKSL1 knockout cells, followed by sphere formation (K) and transwell invasion assays (L). Typical images are shown. Experiments were repeated at least three times. *, p < 0.05; **, p < 0.01; ***, p < 0.001, by t test.
We then examined the role of MARCKS and MARCKSL1 in the self-renewal of liver TICs. We established MARCKS knockout cells through the CRISPR-Cas9 approach and found that MARCKS knockout impaired sphere formation (Fig. 4G). A tumor initiation assay also revealed the importance of MARCKS in liver tumorigenesis (Fig. 4H). MARCKSL1 knockout also showed impaired self-renewal and tumorigenesis (Fig. 4, I and J). We also rescued the expression of MARCKS/MARCKSL1 and found restored sphere formation (Fig. 4K) and invasion ability (Fig. 4L). These results indicated the important and nonredundant role of MARCKS and MARCKSL1 in liver TIC self-renewal.
lncZic2 recruited BRG1 to the promoter of MARCKS and MARCKSL1
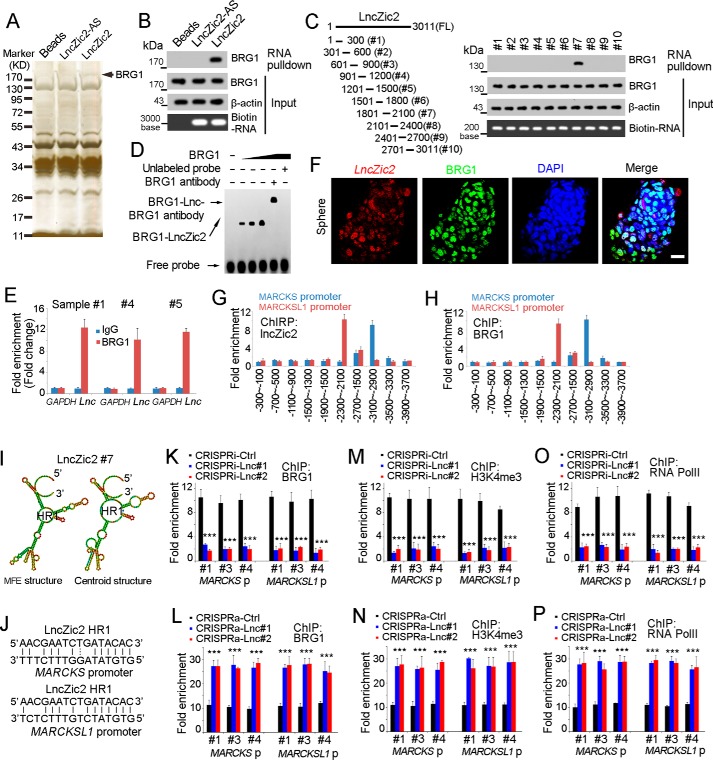
To explore the molecular mechanism of lncZic2, we performed an RNA pulldown assay, and a specific band was identified as BRG1, indicating that lncZic2 interacted with BRG1 (Fig. 5A). Western blotting confirmed the interaction between BRG1 and lncZic2 (Fig. 5B). Domain mapping identified that the #7 region of lncZic2 was required for the binding between lncZic2 and BRG1, which was confirmed by RNA EMSA (Fig. 5, C and D). Meanwhile, RNA immunoprecipitation confirmed the interaction between lncZic2 and BRG1 (Fig. 5E). Fluorescence in situ hybridization (FISH) also revealed co-localization of lncZic2 and BRG1 (Fig. 5F). Through chromatin isolation by RNA purification (ChIRP) and ChIP assays, we found lncZic2 and BRG1 combined with the same region of MARCKS and MARCKSL1 promoters (Fig. 5, G and H). In a previous work (23), we found that a complementary sequence between lncRNAs and the promoter of target genes was important for specific recruiting. Here we analyzed the binding region of MARCKS/MARCKSL1 promoters and found obvious complementary sequences between lncZic2 and MARCKS/MARCKSL1 promoters (Fig. 5, I and J). It is likely that the binding of lncZic2 and MARCKS/MARCKSL1 promoters occurs during cell replication or transcription, when the DNA double strands separate into single strands. DNA repair and other mechanisms that can trigger the accessibility of double strands are also possible to drive the sequence complement.
Figure 5.
lncZic2 recruited BRG1 to the MARCKS/MARCKSL1 promoter. A, biotin-labeled lncZic2 was generated through in vitro transcription and incubated with oncosphere lysate for RNA pulldown, and the specific band of lncZic2 was identified as BRG1 through mass spectrometry. B, RNA pulldown was performed, and the interaction between lncZic2 and BRG1 was confirmed by Western blotting. C, lncZic2 truncates were constructed (left panel), and an RNA pulldown assay was performed for BRG1 examination (right panel). FL, full length. HR, hairpin region. D, RNA EMSA confirmed the interaction between BRG1 and lncZic2. The #7 region of lncZic2 was used for RNA ESMA. E, RNA immunoprecipitation assays were performed with BRG1 antibody and control antibody, and the enrichment of lncZic2 was examined through real-time PCR. F, lncZic2 and BRG1 subcellular location was examined through FISH. Oncospheres were used for FISH, and co-localization of BRG1 and lncZic2 was observed. Scale bar = 10 μm. DAPI, 4′,6-diamidino-2-phenylindole. G and H, oncospheres derived from sample #1 were used for a ChIRP assay (G) or a ChIP assay (H), and the enrichment of the MARCKS/MARCKSL1 promoter was examined by real-time PCR. The indicated regions of the MARCKS/MARCKSL1 promoter were examined, and the data were normalized to lncZic2-AS probes (G) or IgG control antibody (H). I, the structure of the indicated region of lncZic2 was predicted through a tool available online (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party–hosted site.) MFE, minimum free energy. J, the sequence complementary between lncZic2 and MARCKS/MARCKSL1 promoter is shown. K and L, BRG1 ChIP assays were performed, and MARCKS/MARCKSL1 promoter enrichment was examined through real-time PCR. lncZic2-depleted cells (K) and lncZic2-overexpressing cells (L) were used for ChIP assays. Ctrl, control. M and N, lncZic2-depleted cells (M) and lncZic2-overexpressing cells (N) were used for H3K4Me3 ChIP, and the enrichment of the MARCKS/MARCKSL1 promoter was examined through real-time PCR. O and P, RNA polymerase II (RNA PolII) ChIP assays were performed using lncZic2-depleted cells (O) and lncZic2-overexpressing cells (P), followed by real-time PCR for enrichment of the MARCKS/MARCKSL1 promoter. Experiments were repeated at least three times. ***, p < 0.001 by t test.
We then explored the role of lncZic2–BRG1 interaction in the expression regulation of MARCKS and MARCKSL1. Impaired interaction between BRG1 and the MARCKS/MARCKSL1 promoter was found upon lncZic2 depletion, indicating that lncZic2 was required for the binding between BRG1 and the MARCKS/MARCKSL1 promoter (Fig. 5K). On the contrary, lncZic2 overexpression promoted the interaction between BRG1 and the MARCKS/MARCKSL1 promoter, further confirming that lncZic2 recruited BRG1 to the MARCKS/MARCKSL1 promoter (Fig. 5L). BRG1 is the core component of the SWI–SNF complex and plays a critical role in chromatin remodeling. We then examined chromatin remodeling with H3K4me3, a well-known marker of chromatin activation and transcription initiation. The H3K4me3 ChIP assay also confirmed the importance of lncZic2 in the activation of the MARCKS/MARCKSL1 promoter (Fig. 5, M and N). We also performed an RNA polymerase II ChIP assay and confirmed the critical role of lncZic2 in MARCKS/MARCKSL1 transcription activation (Fig. 5, O and P). Taken together, lncZic2 recruited BRG1 to the MARCKS/MARCKSL1 promoter to initiate their transcription.
lncZic2–BRG1–MARCKS/MARCKSL1 can be targeted for liver TIC elimination
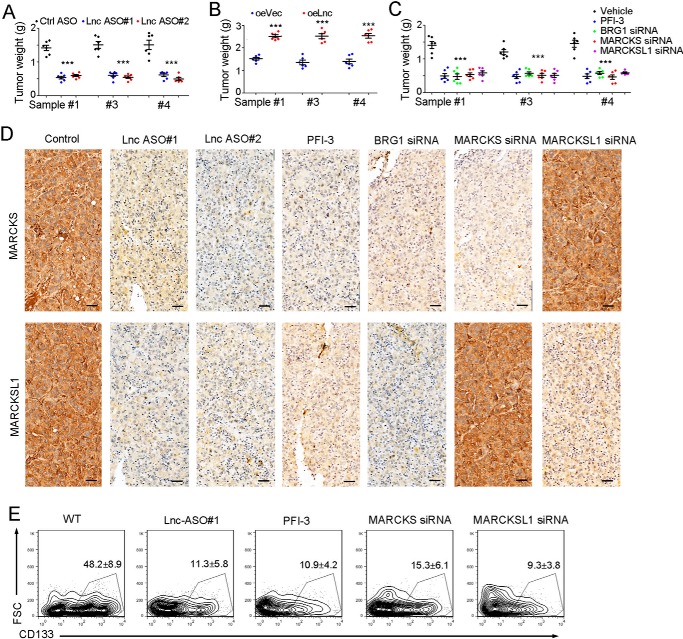
We then examined the role of the lncZic2 pathway in liver TIC targeting. We found that lncZic2-depleted cells showed impaired tumor propagation and that lncZic2-overexpressing cells formed larger tumors (Fig. 6, A and B). Cells treated with PFI-3 (a BRG1 inhibitor) or siRNA-treated cells also showed impaired tumor propagation (Fig. 6C). These results indicated that lncZic2 pathway can be targeted for tumor propagation.
Figure 6.
lncZic2–BRG1–MARCKS/MARCKSL1 can be targeted for liver TIC elimination. A, 1 × 106 primary cells were subcutaneously injected into BALB/c nude mice. When tumors were established (about 250 mm3), lncZic2 antisense oligo (ASO) and control antisense oligo were intratumorally injected every 2 days. One month later, tumors were obtained, and tumor weight was observed. Ctrl, control. B, 1 × 106 lncZic2-overexpressing (oe) cells and control cells were subcutaneously injected into BALB/c nude mice, and tumor weight was examined 1 month later. C, 1 × 106 primary cells were used for tumor propagation, and the indicated reagents were used for intratumoral injection. The tumors were weighted 1 month later. D, the indicated tumors were obtained, and MARCKS/MARCKSL1 expression levels were examined through immunohistochemistry. Scale bars = 100 μm. E, the indicated tumors were obtained, and CD133+ liver TICs were examined through FACS. n = 6 for each group, and the ratios of liver TICs were shown as means ± SD. FSC, forward scatter. Experiments were repeated at least three times. ***, p < 0.001 by t test.
We then confirmed the expression of MARCKS/MARCKSL1 in established tumors and found that lncZic2/BRG1 depletion largely impaired the expression levels of MARCKS/MARCKSL1 (Fig. 6D). We then detected liver TICs in established tumors and found that inhibition of the lncZic2 pathway largely decreased the ratios of liver TICs (Fig. 6E). Therefore, the lncZic2 signaling pathway can be targeted for liver TIC elimination.
Discussion
Liver TICs are important for liver tumorigenesis, and the self-renewal of liver TICs is finely regulated (10). Liver TICs highly express drug pump molecules (ABCG2, etc.) and can pump drugs out of cells to escape drug-induced cell death (27). When the drug is removed, TIC can repopulate to form a new tumor (28). Thus, targeting liver TICs has potential for clinical applications (5, 29, 30). We found that ZIC2 and lncSox4 were highly expressed in liver TICs (12, 23). Here we identified a long noncoding RNA, lncZic2, that is highly expressed in liver cancer and liver TICs. lncZic2 is required for the self-renewal of liver TICs in a ZIC2-independent manner. lncZic2 recruited BRG1 to the MARCKS/MARCKSL1 promoter to drive their expression. lncZic2–BRG1–MARCKS/MARCKSL1 can also be targeted for liver TIC elimination.
lncRNAs regulate the expression of nearby or distant genes in cis or in trans (17, 18). Most recently, some researches revealed that the transcription process of lncRNAs could influence the transcription of nearby genes (13). Actually, lncZic2 is located near the ZIC2 locus. ZIC2 and lncZic2 were both highly expressed in liver TIC, but lncZic2 did not regulate ZIC2 expression. Because our depletion strategy was CRISPRi-based, CRISPRi single-guide RNAs binding to their targeting sequences may impair ZIC2 expression (31), but it did not occur. These data indicated that the lncZic2 binding locus did not influence ZIC2 expression, nor did the lncZic2 transcription process. Even so, the mutual interactions of lncZic2 and ZIC2 transcription are still interesting because they were located near the chromatin. Whether ZIC2 regulates lncZic2 expression is also interesting.
MARCKS and MARCKSL1 are both substrates of PKC (32). PKC signaling is important for tumorigenesis and “stemness,” and many PKC molecules are involved in the self-renewal of TICs (33–35). However, the function of MARCKS and MARCKSL1 in liver TICs is elusive. Through analyses of datasets available online and clinical sample confirmation, we found that MARCKS and MARCKSL1 were highly expressed in liver cancer and liver TICs. MARCKS and MARCKSL1 individual knockout impaired the self-renewal of liver TICs and liver tumorigenesis, indicating that the roles of MARCKS and MARCKSL1 are nonredundant. lncZic2 and BRG1 targeted MARCKS and MARCKSL1 simultaneously, revealing that MARCKS and MARCKSL1 are regulated through a similar mechanism. The regulation mechanisms and biological roles of MARCKS and MARCKSL1 in liver tumorigenesis and liver TICs need further investigation.
BRG1 is a core component of the SWI–SNF complex (36). Actually, SWI–SNF can be grouped into two types, BRG1-embedded and BRM-embedded (37). In our previous work, we found that BRM was down-regulated in liver tumorigenesis and BRG1 was up-regulated and that BRG1 itself served as an oncogene (38). Here we found that BRG1 targeted the MARCKS and MARCKSL1 promoter, promoted the expression of MARCKS/MARCKSL1, and drove the self-renewal of liver TICs. Actually, key components of several chromatin remodeling complexes, including BPTF (a NURF component (12)), EZH2 (a PRC2 component (39)), and HDAC1/2 (a NURD component (40)), are dysregulated in liver tumorigenesis. These observations confirmed the critical role of chromatin remodeling in liver tumorigenesis. Here we found that BRG1 targeted MARCKS/MARCKSL1 to drive liver TIC self-renewal and that it can be targeted for liver TIC elimination. The exact mechanisms of BRG1 in liver tumorigenesis and liver TICs need further investigation.
Experimental procedures
Samples and reagents
Human liver cancer clinical samples were obtained with informed consent from the First Affiliated Hospital of Zhengzhou University according to institutional review board approval. HCC samples were ranked according to arrival time, and several samples with sphere formation capacity (#1, #4, and #5) were used for sphere formation. The details of these sample were as follows: #1, advanced hepatocellular carcinoma, 58 years old, male, tumor size 7.8 × 5.2 × 4.9 mm, nonmetastasis; #3, advanced hepatocellular carcinoma, 71 years old, male, tumor size 8.2 × 4.3 × 3.2 mm, nonmetastasis; #4, advanced hepatocellular carcinoma, 65 years old, female, tumor size 5.8 × 5.2 × 4.6 mm, nonmetastasis.
Anti-β-actin (catalog no. A1978) and 4′,6-diamidino-2-phenylindole (catalog no. 28718-90-3) were obtained from Sigma-Aldrich. Anti-MARCKS (10004-2-Ig) and anti-MARCKSL1 (10002-2-AP) antibodies were purchased from Proteintech Co. Anti-BRG1 (catalog no. 49360) antibody was from Cell Signaling Technology. Anti-Zic2 (ARP35821_P050) antibody was purchased from Aviva Systems Biology. Alexa 594–conjugated donkey anti-rabbit IgG and Alexa 488–conjugated donkey anti-mouse IgG antibodies were purchased from Molecular Probes. Phycoerythrin -conjugated CD133 (catalog no. 130098826) was obtained from Miltenyi Biotec. The LightShift chemiluminescent RNA EMSA kit (catalog no. 20158) and chemiluminescent nucleic acid detection module (catalog no. 89880) were purchased from Thermo Scientific. T7 RNA polymerase (catalog no. 10881767001) and biotin RNA labeling mixture (catalog no. 11685597910) were obtained from Roche Life Science.
Sphere formation
For the sphere formation assay, 5000 HCC cells were seeded into ultralow attachment 6-well plates (Corning, catalog no. 3471) and cultured in Dulbecco's modified Eagle's medium/F12 medium supplemented with B27 (catalog no. 17504-044), N2 (catalog no. 17502-048), 20 ng/ml epidermal growth factor (catalog no. E5036-200UG), and 20 ng/ml basic fibroblast growth factor (catalog no. GF446-50UG). Two weeks later, photos were taken, and sphere numbers were counted. Sphere initiating ratios (percent) = spheres/5000. B27, N2, and EGF were obtained from Life Technologies, and basic fibroblast growth factor was purchased from Millipore.
Tumor propagation and initiating assay
For the tumor propagation assay, 1 × 106 lncZic2-depleted and -overexpressing cells were subcutaneously injected into 6-week-old BALB/c nude mice for 1 month of growth. For the tumor initiation assay, 10, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 lncZic2-depleted and control cells were subcutaneously injected into 6-week-old BALB/c nude mice. 3 months later, tumor formation was observed, and the ratios of tumor-free mice were recorded.
Transwell invasion assay
For transwell invasion assays, 3 × 105 lncZic2-depleted or -overexpressing HCC cells were plated in the top chamber with a Matrigel-coated membrane and incubated in medium without fetal bovine serum. Medium containing 10% fetal bovine serum was added to the lower chamber. 36 h later, the cells that did invade through the membrane were removed with a cotton swab, and the cells on the lower surface of the membrane were stained with crystal violet. The images were taken with a Nikon EclipseTi microscope.
Immunohistochemistry
Formalin-fixed sections were deparaffinized with xylene and graded alcohols and incubated with 3% H2O2 for 15 min, followed by 15 min of boiling in citrate buffer for antigen retrieval. The samples were treated with primary antibodies (MARCKS and MARCKSL1) and horseradish peroxidase–conjugated secondary antibodies. Finally, the samples were counterstained with hematoxylin, dehydrated in graded alcohols, and observed with a Nikon EclipseTi microscope.
RNA pulldown
For RNA pulldown assays, biotin-labeled lncZic2 and control RNA were obtained with biotin RNA labeling mixture (Roche). Then the labeled RNA transcript was incubated with cell lysate derived from liver TICs or oncospheres. Components enriched with streptavidin-conjugated beads were subjected to SDS-PAGE, followed by Western blotting or mass spectrometry analysis.
RNA extraction and real-time PCR
Total RNA was extracted from primary samples or spheres by the TRIzol method according to the manufacturer's manual. Briefly, 1 ml of TRIzol was added to samples for dissolution, followed by 5-min incubation. 200 μl of chloroform was added to samples and mixed vigorously, followed by centrifugation for separation. The supernatant was precipitated with isopropanol (1:1) and then washed with 75% ethanol. Finally, the RNA pellet was dissolved with RNase-free H2O. An RT-PCR kit from Promega was used to generate complementary DNA from RNA, and a real-time PCR reaction was performed according to standard procedures (41).
Western blotting
HCC primary samples or oncospheres were crushed with radioimmune precipitation assay buffer (150 mm NaCl, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 1 mm EDTA, and 50 mm Tris (pH 8.0)) and subjected to SDS-PAGE. The samples were transferred to an nitrocellulose membrane (Beyotime Biotechnology) and incubated with primary antibodies, and then horseradish peroxidase–conjugated secondary antibodies were used for visualization.
Author contributions
Z. C., L. Y., S. G., and Y. G. data curation; Z. C., Y. L., L. Y., Y. G., and P. Z. investigation; Z. C. and P. Z. writing-original draft; Y. G. formal analysis; Y. G. and P. Z. funding acquisition; Y. G. methodology; P. Z. conceptualization.
This work was supported by the National Natural Science Foundation of China (U1604286, U1704174, 81601450, and 81601448) and the Development Fund for Outstanding Young Teachers of Zhengzhou University (1521311059). The authors declare that they have no conflicts of interest with the contents of this article.
- HCC
- hepatocellular carcinoma
- TIC
- tumor-initiating cell
- lncRNA
- long non-coding RNA
- CRISPRi
- CRISPR-interference
- PKC
- protein kinase C
- MARCKS
- myristoylated alanine-rich protein kinase C substrate
- EMSA
- electrophoretic mobility shift assay
- FISH
- fluorescence in situ hybridization.
References
- 1. Bruix J., Gores G. J., and Mazzaferro V. (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63, 844–855 10.1136/gutjnl-2013-306627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kreso A., and Dick J. E. (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14, 275–291 10.1016/j.stem.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 3. Ma S., Chan K. W., Hu L., Lee T. K., Wo J. Y., Ng I. O., Zheng B. J., and Guan X. Y. (2007) Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 132, 2542–2556 10.1053/j.gastro.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 4. Pastrana E., Silva-Vargas V., and Doetsch F. (2011) Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8, 486–498 10.1016/j.stem.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu P. P., and Fan Z. S. (2017) Cancer stem cell niches and targeted interventions. Prog. Biochem. Biophys. 44, 697–708 [Google Scholar]
- 6. Ma S., Chan K. W., Lee T. K., Tang K. H., Wo J. Y., Zheng B. J., and Guan X. Y. (2008) Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol. Cancer Res. 6, 1146–1153 10.1158/1541-7786.MCR-08-0035 [DOI] [PubMed] [Google Scholar]
- 7. Feng D. F., Peng G., Li W., Xu H. M., Zhang T. Q., and Wang N. Y. (2015) Identification and characterization of tumorigenic liver cancer stem cells by CD133 and CD24. J. Biomater. Tiss. Eng. 5, 635–646 10.1166/jbt.2015.1363 [DOI] [Google Scholar]
- 8. Yang Z. F., Ho D. W., Ng M. N., Lau C. K., Yu W. C., Ngai P., Chu P. W., Lam C. T., Poon R. T., and Fan S. T. (2008) Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13, 153–166 10.1016/j.ccr.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 9. Justilien V., Walsh M. P., Ali S. A., Thompson E. A., Murray N. R., and Fields A. P. (2014) The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 25, 139–151 10.1016/j.ccr.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takebe N., Miele L., Harris P. J., Jeong W., Bando H., Kahn M., Yang S. X., and Ivy S. P. (2015) Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 12, 445–464 10.1038/nrclinonc.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu P., Wang Y., Du Y., He L., Huang G., Zhang G., Yan X., and Fan Z. (2015) C8orf4 negatively regulates self-renewal of liver cancer stem cells via suppression of NOTCH2 signalling. Nat. Commun. 6, 7122 10.1038/ncomms8122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu P., Wang Y., He L., Huang G., Du Y., Zhang G., Yan X., Xia P., Ye B., Wang S., Hao L., Wu J., and Fan Z. (2015) ZIC2-dependent OCT4 activation drives self-renewal of human liver cancer stem cells. J. Clin. Invest. 125, 3795–3808 10.1172/JCI81979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson K. M., Anderson D. M., McAnally J. R., Shelton J. M., Bassel-Duby R., and Olson E. N. (2016) Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 10.1038/nature20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu B., Ye B., Yang L., Zhu X., Huang G., Zhu P., Du Y., Wu J., Qin X., Chen R., Tian Y., and Fan Z. (2017) Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat. Immunol. 18, 499–508 10.1038/ni.3712 [DOI] [PubMed] [Google Scholar]
- 15. Ramos A. D., Diaz A., Nellore A., Delgado R. N., Park K. Y., Gonzales-Roybal G., Oldham M. C., Song J. S., and Lim D. A. (2013) Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell 12, 616–628 10.1016/j.stem.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batista P. J., and Chang H. Y. (2013) Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307 10.1016/j.cell.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang K. C., and Chang H. Y. (2011) Molecular mechanisms of long noncoding RNAs. Mol. Cell 43, 904–914 10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponting C. P., Oliver P. L., and Reik W. (2009) Evolution and functions of long noncoding RNAs. Cell 136, 629–641 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y., He L., Du Y., Zhu P., Huang G., Luo J., Yan X., Ye B., Li C., Xia P., Zhang G., Tian Y., Chen R., and Fan Z. (2015) The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell 16, 413–425 10.1016/j.stem.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 20. Yuan J. H., Yang F., Wang F., Ma J. Z., Guo Y. J., Tao Q. F., Liu F., Pan W., Wang T. T., Zhou C. C., Wang S. B., Wang Y. Z., Yang Y., Yang N., Zhou W. P., et al. (2014) A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 25, 666–681 10.1016/j.ccr.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 21. Wang P., Xue Y., Han Y., Lin L., Wu C., Xu S., Jiang Z., Xu J., Liu Q., and Cao X. (2014) The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 344, 310–313 10.1126/science.1251456 [DOI] [PubMed] [Google Scholar]
- 22. Liu B., Sun L., Liu Q., Gong C., Yao Y., Lv X., Lin L., Yao H., Su F., Li D., Zeng M., and Song E. (2015) A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell 27, 370–381 10.1016/j.ccell.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 23. Chen Z. Z., Huang L., Wu Y. H., Zhai W. J., Zhu P. P., and Gao Y. F. (2016) LncSox4 promotes the self-renewal of liver tumour-initiating cells through Stat3-mediated Sox4 expression. Nat. Commun. 7, 12598 10.1038/ncomms12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo S., Lu J. Y., Liu L., Yin Y., Chen C., Han X., Wu B., Xu R., Liu W., Yan P., Shao W., Lu Z., Li H., Na J., Tang F., et al. (2016) Divergent lncRNAs Regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell 18, 637–652 10.1016/j.stem.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 25. Roessler S., Jia H. L., Budhu A., Forgues M., Ye Q. H., Lee J. S., Thorgeirsson S. S., Sun Z., Tang Z. Y., Qin L. X., and Wang X. W. (2010) A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 70, 10202–10212 10.1158/0008-5472.CAN-10-2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roessler S., Long E. L., Budhu A., Chen Y., Zhao X., Ji J., Walker R., Jia H. L., Ye Q. H., Qin L. X., Tang Z. Y., He P., Hunter K. W., Thorgeirsson S. S., Meltzer P. S., and Wang X. W. (2012) Integrative genomic identification of genes on 8p associated with hepatocellular carcinoma progression and patient survival. Gastroenterology 142, 957–966.e12 10.1053/j.gastro.2011.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Z., Zhang L., He Y., Shen Y., and Li Y. (2015) Enhanced shRNA delivery and ABCG2 silencing by charge-reversible layered nanocarriers. Small 11, 952–962 10.1002/smll.201401397 [DOI] [PubMed] [Google Scholar]
- 28. Kurtova A. V., Xiao J., Mo Q., Pazhanisamy S., Krasnow R., Lerner S. P., Chen F., Roh T. T., Lay E., Ho P. L., and Chan K. S. (2015) Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517, 209–213 10.1038/nature14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimokawa M., Ohta Y., Nishikori S., Matano M., Takano A., Fujii M., Date S., Sugimoto S., Kanai T., and Sato T. (2017) Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 545, 187–192 10.1038/nature22081 [DOI] [PubMed] [Google Scholar]
- 30. Chen Z., Zhu P., Zhang Y., Liu Y., He Y., Zhang L., and Gao Y. (2016) Enhanced sensitivity of cancer stem cells to chemotherapy using functionalized mesoporous silica nanoparticles. Mol. Pharm. 13, 2749–2759 10.1021/acs.molpharmaceut.6b00352 [DOI] [PubMed] [Google Scholar]
- 31. Gilbert L. A., Horlbeck M. A., Adamson B., Villalta J. E., Chen Y., Whitehead E. H., Guimaraes C., Panning B., Ploegh H. L., Bassik M. C., Qi L. S., Kampmann M., and Weissman J. S. (2014) Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 10.1016/j.cell.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aderem A. (1992) The MARCKS brothers: a family of protein kinase C substrates. Cell 71, 713–716 10.1016/0092-8674(92)90546-O [DOI] [PubMed] [Google Scholar]
- 33. Tam W. L., Lu H., Buikhuisen J., Soh B. S., Lim E., Reinhardt F., Wu Z. J., Krall J. A., Bierie B., Guo W., Chen X., Liu X. S., Brown M., Lim B., and Weinberg R. A. (2013) Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell 24, 347–364 10.1016/j.ccr.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riobo N. A., Haines G. M., and Emerson C. P. (2006) Protein kinase C-δ and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 66, 839–845 10.1158/0008-5472.CAN-05-2539 [DOI] [PubMed] [Google Scholar]
- 35. Chen Z., Forman L. W., Williams R. M., and Faller D. V. (2014) Protein kinase C-δ inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer 14, 90 10.1186/1471-2407-14-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson B. G., and Roberts C. W. (2011) SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 11, 481–492 10.1038/nrc3068 [DOI] [PubMed] [Google Scholar]
- 37. Kadam S., and Emerson B. M. (2003) Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11, 377–389 10.1016/S1097-2765(03)00034-0 [DOI] [PubMed] [Google Scholar]
- 38. Zhu P., Wang Y., Wu J., Huang G., Liu B., Ye B., Du Y., Gao G., Tian Y., He L., and Fan Z. (2016) LncBRM initiates YAP1 signalling activation to drive self-renewal of liver cancer stem cells. Nat. Commun. 7, 13608 10.1038/ncomms13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu P., Wang Y., Huang G., Ye B., Liu B., Wu J., Du Y., He L., and Fan Z. (2016) lnc-β-Catm elicits EZH2-dependent β-catenin stabilization and sustains liver CSC self-renewal. Nat. Struct. Mol. Biol. 23, 631–639 10.1038/nsmb.3235 [DOI] [PubMed] [Google Scholar]
- 40. Minucci S., and Pelicci P. G. (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer 6, 38–51 10.1038/nrc1779 [DOI] [PubMed] [Google Scholar]
- 41. Chen Z., Gao Y., Yao L., Liu Y., Huang L., Yan Z., Zhao W., Zhu P., and Weng H. (2018) LncFZD6 initiates Wnt/beta-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene 37 10.1038/s41388-018-0203-6 [DOI] [PMC free article] [PubMed] [Google Scholar]