ABSTRACT
The ω subunit is the smallest subunit of bacterial RNA polymerase (RNAP). Although homologs of ω are essential in both eukaryotes and archaea, this subunit has been known to be dispensable for RNAP in Escherichia coli and in other bacteria. In this study, we characterized an indispensable role of the ω subunit in Mycobacterium tuberculosis. Unlike the well-studied E. coli RNAP, the M. tuberculosis RNAP core enzyme cannot be functionally assembled in the absence of the ω subunit. Importantly, substitution of M. tuberculosis ω with ω subunits from E. coli or Thermus thermophilus cannot restore the assembly of M. tuberculosis RNAP. Furthermore, by replacing different regions in M. tuberculosis ω with the corresponding regions from E. coli ω, we found a nonconserved loop region in M. tuberculosis ω essential for its function in RNAP assembly. From RNAP structures, we noticed that the location of the C-terminal region of the β′ subunit (β′CTD) in M. tuberculosis RNAP but not in E. coli or T. thermophilus RNAP is close to the ω loop region. Deletion of this β′CTD in M. tuberculosis RNAP destabilized the binding of M. tuberculosis ω on RNAP and compromised M. tuberculosis core assembly, suggesting that these two regions may function together to play a role in ω-dependent RNAP assembly in M. tuberculosis. Sequence alignment of the ω loop and the β′CTD regions suggests that the essential role of ω is probably restricted to mycobacteria. Together, our study characterized an essential role of M. tuberculosis ω and highlighted the importance of the ω loop region in M. tuberculosis RNAP assembly.
IMPORTANCE DNA-dependent RNA polymerase (RNAP), which consists of a multisubunit core enzyme (α2ββ′ω) and a dissociable σ subunit, is the only enzyme in charge of transcription in bacteria. As the smallest subunit, the roles of ω remain the least well studied. In Escherichia coli and some other bacteria, the ω subunit is known to be nonessential for RNAP. In this study, we revealed an essential role of the ω subunit for RNAP assembly in the human pathogen Mycobacterium tuberculosis, and a mycobacterium-specific ω loop that plays a role in this function was also characterized. Our study provides fresh insights for further characterizing the roles of bacterial ω subunit.
KEYWORDS: Mycobacterium tuberculosis, transcription, RNA polymerase, omega subunit
INTRODUCTION
DNA-dependent RNA polymerase (RNAP) carries out transcription in all organisms (1–3). In bacteria, RNAP is composed of a multisubunit core enzyme (α2ββ′ω), with an additional subunit (σ) used for promoter binding (4). Although the specific functions of each subunit have been extensively studied (5, 6), the roles of the smallest subunit (ω) remain the least well studied.
In Escherichia coli, ω has been observed to play a part in RNAP assembly by facilitating the binding of β′ to α2β to generate the RNAP core enzyme (5, 7, 8). Recent studies have showed that the stringent response alarmone molecule ppGpp directly binds to the RNAP β′-ω interface (9, 10) and regulates transcriptions from a subset of promoters (11, 12). However, deletion of ω in E. coli has no major impact on bacterial growth or RNAP activity (10, 13, 14), suggesting that ω is unnecessary for E. coli RNAP. Other studies have shown that ω is involved in either RNAP stability in Staphylococcus aureus (15) and or RNAP core-σ interaction in cyanobacteria (16), suggesting that the ω subunit may have diverse functions.
In Mycobacterium tuberculosis, the roles of the ω subunit have been poorly characterized. Multiple lines of evidence have indicated that ω plays an important role in mycobacteria. First, Mycobacterium leprae, which has lost many genes during the process of reductive evolution, still preserves the ω subunit (8, 17). Second, deletion of rpoZ, a gene encodes the ω subunit, significantly decreased the growth rate of Mycobacterium smegmatis and resulted in proteolytic cleavage of the RNAP β′ subunit (18). Third, rpoZ has been predicted to be an essential gene in M. tuberculosis by transposon mutagenesis screening (19, 20). However, how the ω subunit functions in RNAP in mycobacteria is not fully understood.
In this study, we investigated the roles of ω in M. tuberculosis RNAP. In contrast to E. coli RNAP, ω is critical for the assembly of M. tuberculosis RNAP. Using a combination of sequence alignment, structural comparison, and mutational analyses, we demonstrated that a loop region of the ω subunit plays an essential role in the ω-dependent M. tuberculosis RNAP assembly. These findings highlight the importance of ω in M. tuberculosis and offer fresh insights into the roles of the bacterial ω subunit.
RESULTS
The ω subunit is required for M. tuberculosis RNAP assembly.
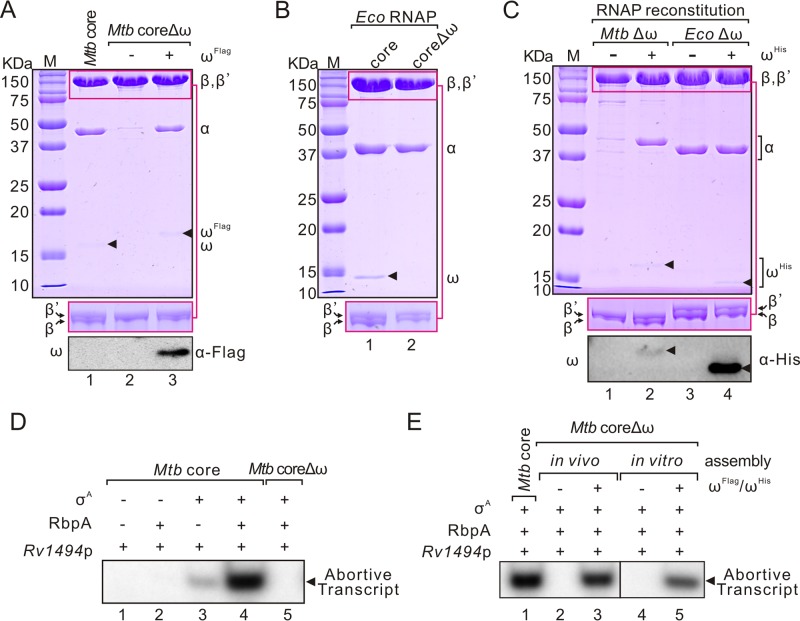
To examine the role of the M. tuberculosis ω subunit, we first purified M. tuberculosis RNAP core (abbreviated as M. tuberculosis core, containing a His6 tag at the C terminal of the β′ subunit) with or without ω using coexpression plasmids (pMtRc and pMtRcΔω, respectively). As shown in Fig. 1A, a significant loss of α and β subunits from M. tuberculosis core was observed when ω was absent. Coexpression of M. tuberculosis ωFlag protein with M. tuberculosis coreΔω in E. coli successfully restored RNAP assembly (Fig. 1A). In contrast, assembly of E. coli RNAP core (abbreviated as E. coli core) did not rely on ω (Fig. 1B). Furthermore, in vitro RNAP reconstitution using individual subunits (αHis, β, β′, and ωHis) also showed that, in contrast to E. coli core, the M. tuberculosis core required the ω subunit for assembly (Fig. 1C).
FIG 1.
Roles of ω in M. tuberculosis and E. coli RNAP core assembly. (A) SDS-PAGE analysis of purified M. tuberculosis RNAP core, coreΔω, and coreΔω coexpressed with ωFlag protein. Proteins loaded onto 4 to 20% gel to separate β and β′ subunits are shown in the middle panel. Western blots of ωFlag with a Flag tag antibody are shown in the bottom panel. (B) Purified E. coli core and coreΔω. (C) In vitro reconstitution of M. tuberculosis and E. coli core with or without ωHis. Western blots of ωHis with a His tag antibody are shown in the bottom panel. (D) In vitro abortive transcription of purified M. tuberculosis core or coreΔω at the Rv1494 promoter (Rv1494p). RbpA and σA were added as indicated to reconstitute RNAP for promoter-dependent transcription. (E) In vitro abortive transcriptional activities of in vivo or in vitro reconstituted M. tuberculosis core in the presence or absence of the ω subunit at Rv1494p. RbpA and σA were added in each reaction in promoter-dependent transcription assay. A representative result from three independent tests is shown. Mtb, M. tuberculosis; Eco, E. coli.
In vitro abortive transcription at the Rv1494 promoter (Rv1494p) (see Fig. S1A in the supplemental material) showed that M. tuberculosis coreΔω, when purified from in vivo assembly or in vitro reconstitution, was inactive when reconstituted with the principle factor σA (Fig. 1D and E), which was also confirmed by transcription assays using a different sigma factor (σB) (see Fig. S1B in the supplemental material) or at a different promoter (Rv0005p) (see Fig. S1C in the supplemental material). These data suggest that the defect in M. tuberculosis coreΔω assembly influenced the activity of RNAP core enzyme. To test this hypothesis, we applied a σ-independent transcription elongation assay (21) to compare the activities of M. tuberculosis core with or without ω and found that M. tuberculosis coreΔω was inactive in catalysis of RNA synthase (see Fig. S1D and S1E in the supplemental material). In contrast, E. coli coreΔω from in vivo or in vitro assembly was active and showed more resistance to ppGpp than wild-type E. coli core (10, 22) (see Fig. S1F and S1G in the supplemental material). The loss in the function of M. tuberculosis coreΔω did not result from the purification procedures, since M. tuberculosis coreΔω was inactive after the first step of purification (see Fig. S1H in the supplemental material). Collectively, these data suggest that ω plays a more important role in assembling a functional RNAP in M. tuberculosis compared to that in E. coli.
The ω loop is essential for M. tuberculosis RNAP assembly.
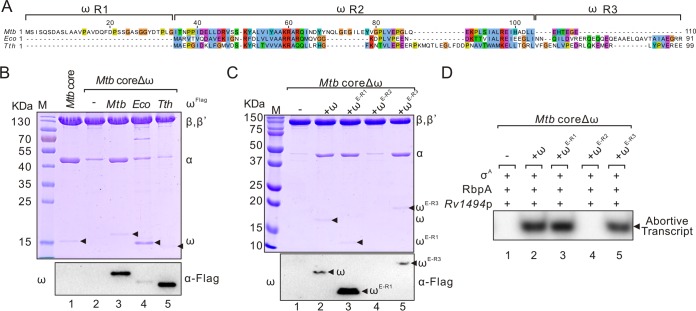
To explore the reason for the different actions of M. tuberculosis ω in RNAP assembly from that of E. coli ω, we aligned the amino acid sequences of ω from M. tuberculosis, E. coli, and T. thermophilus and found that the sequences of ω subunits were not conserved among these strains (Fig. 2A). Therefore, we tried to coexpress the ω from E. coli or T. thermophilus with M. tuberculosis coreΔω to test whether E. coli or T. thermophilus ω can complement the assembly of M. tuberculosis coreΔω. As shown in Fig. 2B, neither E. coli nor T. thermophilus ω rescued M. tuberculosis coreΔω assembly, although T. thermophilus ω was able to bind to M. tuberculosis RNAP. Based on the sequence conservation, we divided ω into three regions: the nonconserved N-terminal R1, the relatively conserved R2, and the nonconserved C-terminal R3 (Fig. 2A). To identify the specific region that is essential for the function of M. tuberculosis ω, we constructed and expressed three M. tuberculosis ω mutants (ωE-R1, ωE-R2, and ωE-R3) by replacing these three regions with the corresponding regions from E. coli ω. Western blot analysis showed that these mutant proteins were all solubly expressed (see Fig. S2A in the supplemental material). Interestingly, replacement of the relatively conserved R2 region but not the R1 or R3 region disrupted the binding of ω on RNAP and the function of M. tuberculosis core (Fig. 2C and D).
FIG 2.
The relatively conserved R2 region is essential for the function of M. tuberculosis ω. (A) Sequence alignment of the ω subunits from M. tuberculosis, E. coli, and T. thermophilus. The amino acid numbering shown on top of the this figure is for M. tuberculosis ω. (B) SDS-PAGE analysis of purified M. tuberculosis coreΔω coexpressed with Flag-tagged ω from M. tuberculosis, E. coli, or T. thermophilus. Western blots of ωFlag with a Flag tag antibody are shown in the bottom panel. (C) Purified M. tuberculosis core with a mutated ω subunit. M. tuberculosis ω mutants, in which the R1, R2, or R3 regions of M. tuberculosis ω was replaced with the corresponding regions of E. coli ω (ωE-R1, ωE-R2, and ωE-R3), respectively, were coexpressed with M. tuberculosis coreΔω in E. coli. Western blots of mutated ωFlag subunit with a Flag tag antibody are shown in the bottom panel. (D) In vitro abortive transcription of purified M. tuberculosis coreΔω derivatives at Rv1494p. RbpA and σA were added in each reaction in this promoter-dependent transcription assay. A representative result from three independent tests is shown. Mtb, M. tuberculosis; Eco, E. coli; Tth, T. thermophilus.
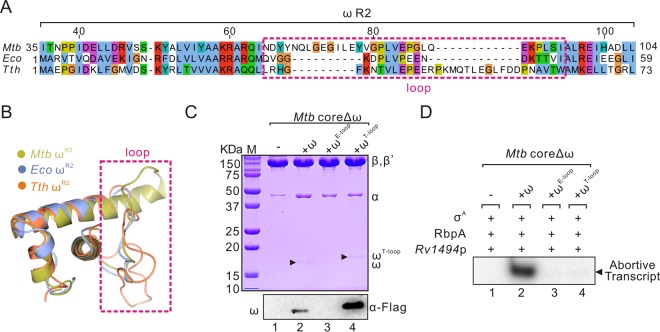
Structural superposition of the ω R2 regions from M. tuberculosis, E. coli, and T. thermophilus (23–25) showed that the loop regions differ greatly in both sequence and conformation (Fig. 3A and B), indicating that this loop region may be responsible for the different actions of ω. To test this hypothesis, we replaced the loop region of M. tuberculosis ω (amino acids [aa] 65 to 89) with the corresponding regions from E. coli (aa 31 to 44) or T. thermophilus (aa 31 to 58) to generate two mutants named as ωE-loop and ωT-loop. Although these proteins were all solubly expressed (see Fig. S2B in the supplemental material), complementation of ωE-loop or ωT-loop mutant affected the assembly and function of M. tuberculosis coreΔω, despite the fact that ωT-loop can bind to M. tuberculosis RNAP (Fig. 3C). Replacement of amino acids in the M. tuberculosis ω loop region with a flexible sequence (GSGGS) further proved that this region, especially N65-N69 amino acids, is important for RNAP assembly (see Fig. S3 in the supplemental material). These data provide further evidence that the loop region is essential for the role of M. tuberculosis ω.
FIG 3.
The ω loop is required for the assembly of M. tuberculosis core. (A) Sequence alignment of the R2 region in ω subunits from M. tuberculosis, E. coli, and T. thermophilus. The ω loop region is marked with a dashed box. The amino acid numbering in top part is for M. tuberculosis ω. (B) Structural overlay of the ω R2 regions in M. tuberculosis (aa 35 to 104, yellow, PDB 5UH8), E. coli (aa 1 to 59, blue, PDB 4YG2), and T. thermophilus (aa 1 to 73, orange, PD 1IW7). (C) Purified M. tuberculosis core with a mutated ω subunit carrying the loop regions from E. coli (aa 31 to 44) or T. thermophilus (aa 31 to 58) (ωE-loop and ωT-loop, respectively). (D) In vitro abortive transcription of purified M. tuberculosis coreΔω derivatives at Rv1494p. RbpA and σA were added in each reaction. Mtb, M. tuberculosis; Eco, E. coli; Tth, T. thermophilus.
The β′CTD plays a role in ω-dependent RNAP assembly.
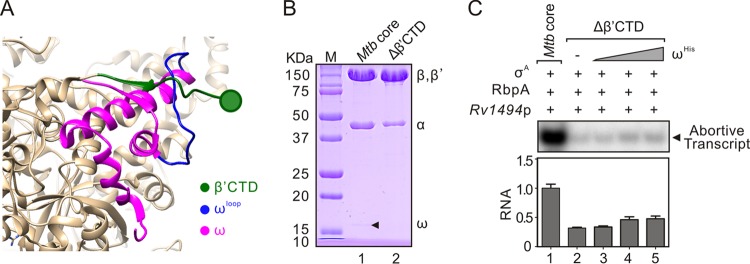
From the structure of M. tuberculosis RNAP (23, 26), we noticed that the ω loop locates on the outside surface of RNAP and probably associates with the C-terminal region of the M. tuberculosis β′ subunit (aa 1271 to 1316, β′CTD) (Fig. 4A). Therefore, we tested the importance of this observed interaction in the assembly of M. tuberculosis RNAP. For this purpose, we constructed a plasmid expressing M. tuberculosis core without the β′CTD region (pMtRcΔβ′CTD). Compared to wild-type M. tuberculosis core, the amounts of ω and α subunits were decreased in purified M. tuberculosis coreΔβ′CTD (Fig. 4B; also see Fig. S4 in the supplemental material), suggesting that the β′CTD is important for ω stability and core assembly in M. tuberculosis RNAP. In vitro abortive initiation assays also showed that the M. tuberculosis coreΔβ′CTD was defective in transcription compared to M. tuberculosis core (Fig. 4C). Interestingly, the addition of purified ω subunit to M. tuberculosis coreΔβ′CTD (ω:coreΔβ′CTD, 4:1) only slightly restored its transcription activity (Fig. 4C), suggesting that the β′CTD is essential for the function of the ω subunit in M. tuberculosis core. Based on the facts that the β′CTD and ω loop regions are located closely in M. tuberculosis RNAP structure and M. tuberculosis core with mutation of either of these two regions behaved similarly in assembly and function, we suggest that the M. tuberculosis β′CTD may associate with the ω loop region to stabilize the ω subunit in M. tuberculosis RNAP and facilitate the ω-dependent RNAP assembly.
FIG 4.
The β′CTD stabilizes the ω in M. tuberculosis core. (A) Structure of M. tuberculosis RNAP core with the magnified view showing the β′ C-terminal domain (aa 1271 to 1316, β′ CTD, green), ω subunit (magenta), and ω loop region (aa 65 to 89, blue) (PDB 5UH8). (B) SDS-PAGE of the purified M. tuberculosis core and coreΔβ′CTD. (C) In vitro abortive transcription of purified M. tuberculosis core or coreΔβ′CTD at Rv1494p. Activities of M. tuberculosis coreΔβ′CTD (200 nM) with addition of purified ωHis (200, 400, or 800 nM, respectively) were tested. RbpA and σA were added in each reaction. A representative transcription result from two independent tests is shown in each panel. Quantifications of transcripts from two independent tests are shown in each bottom section. Mtb, M. tuberculosis.
The role of ω is probably conserved in mycobacteria.
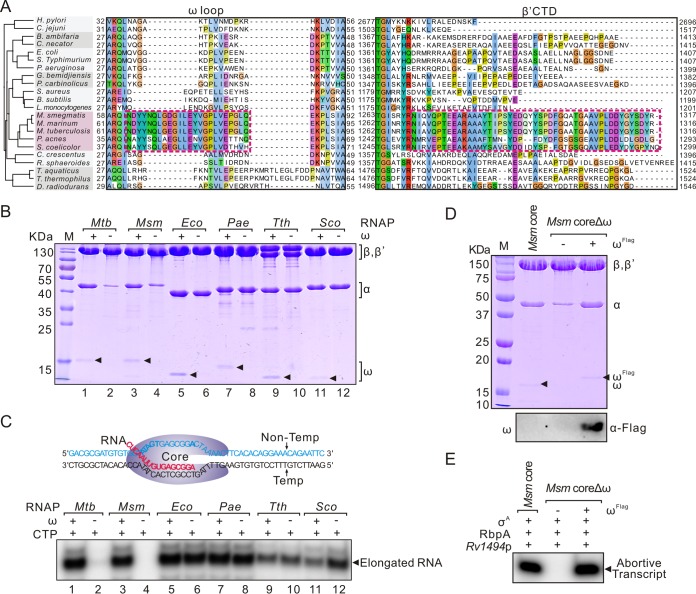
To test whether the role of ω subunit, especially the association between the ω loop and β′CTD, in RNAP assembly is also essential in other bacteria, we aligned the protein sequences of the ω and β′ subunits from different bacteria. Amino acid sequences of the ω loop and the β′CTD regions are highly conserved in mycobacteria and moderately conserved in actinobacteria (Fig. 5A). We therefore expressed and purified the RNAP core and coreΔω from M. smegmatis, Streptomyces coelicolor, Pseudomonas aeruginosa, and T. thermophilus. As expected, the absence of the ω subunit had a marked impact on the assembly and function of M. smegmatis core as observed for M. tuberculosis core but not those from P. aeruginosa, T. thermophilus, or E. coli (Fig. 5B and C). Consistently, the location of β′CTD is close to the ω loop in M. smegmatis RNAP structure (27) but not in E. coli (25) and T. thermophilus (24) RNAPs (see Fig. S5 in the supplemental material). Interestingly, although the S. coelicolor and mycobacterial core sequences are similar, ω is not required for the assembly and function of S. coelicolor RNAP (Fig. 5B and C). Complementation of M. smegmatis coreΔω with ωFlag fully restored its assembly and function (Fig. 5D and E). Collectively, these results demonstrate that the essential role of ω in RNAP assembly is probably conserved in mycobacteria.
FIG 5.
Roles of ω are conserved in mycobacteria. (A) Sequence alignments of the ω loop and β′CTD regions from different bacteria. A phylogenetic tree constructed with bacterial 16S rRNA genes is shown in the left section. Bacterial species used: Helicobacter pylori, Campylobacter jejuni, Burkholderia ambifaria, Cupriavidus necator, E. coli, Salmonella enterica serovar Typhimurium, P. aeruginosa, Geobacter bemidjiensis, Pelobacter carbinolicus, Staphylococcus aureus, Bacillus subtilis, Listeria monocytogenes, M. smegmatis, M. marinum, M. tuberculosis, Propionibacterium acnes, S. coelicolor, Caulobacter crescentus, Rhodobacter sphaeroides, Thermus aquaticus, T. thermophilus, and Deinococcus radiodurans. Regions conserved in actinobacteria are shown in a dashed box. (B) Purified M. tuberculosis, M. smegmatis, E. coli, P. aeruginosa, T. thermophilus, and S. coelicolor RNAP cores with or without the ω subunits. The ω subunits are marked with black triangles. (C) Transcriptional activities of these core enzymes in a σ-independent transcription elongation assay. A scheme for this test is shown at the upper part. The RNA primer is shown in red. [α-32P]CTP was added as the substrate for elongating RNA primer. (D) Complementation of M. smegmatis coreΔω by coexpression with M. smegmatis ωFlag. Assemblies of these RNAPs are shown in the upper section, and Western blotting of ωFlag with a Flag tag antibody is shown in the bottom panel. (E) In vitro abortive transcription of M. smegmatis core and coreΔω at Rv1494p. RbpA and σA were added in each reaction to reconstitute RNAP for promoter-dependent transcription.
DISCUSSION
As an RNAP subunit, ω and its homologs are present in all sequenced genomes of free-living organisms. Although its homologs (RpoK and RPB6) are essential in archaea and eukaryotes (28), ω has been shown to be nonessential in several bacteria (29–32). In M. tuberculosis, the roles of ω have not been clearly investigated (33–35). In this study, we showed that the ω subunit is essential for the assembly of M. tuberculosis RNAP core and have characterized a loop region in M. tuberculosis ω which is essential for its roles.
The roles of ω are diverse in bacteria. Although ω has been observed to play a part in RNAP core assembly in E. coli (7, 13), E. coli core could assemble well without ω, both in vitro and in vivo (13, 36) (Fig. 1), suggesting that the role of the ω subunit in E. coli RNAP assembly is nonessential. In this study, we provide clear evidence (in vivo and in vitro assembly) that, in contrast to E. coli, M. tuberculosis core could not be well assembled and almost lost its function in the absence of ω. In addition, a previous study has shown that deletion of the rpoZ gene in M. smegmatis resulted in proteolytic cleavage of RNAP β′ subunit and further affected the function of RNAP in vivo (18). All these data indicate that mycobacterial ω plays more important roles than that of E. coli ω. A loss of α and β subunits in RNAP was observed in the absence of the ω subunit when His-tagged β′ was used in purification, indicating that ω might facilitate the binding of α2β to β′, as was reported in E. coli (7). Regardless, the detailed contributions of ω to RNAP assembly in M. tuberculosis and E. coli may be different and require further studies.
To the best of our knowledge, the role of the loop region in the middle part of ω subunit in RNAP assembly has not been reported. In this study, we showed that mutation of this loop region in M. tuberculosis ω compromised the assembly of M. tuberculosis core and the structurally closely located C-terminal region of the β′ subunit is also involved in the roles of ω subunit in M. tuberculosis core. Although structures of both M. tuberculosis and M. smegmatis RNAP have been determined, a fraction of the β′CTD has not been fully resolved in either model (23, 26, 27, 37). Anyhow, this β′CTD region is close to the ω loop region in both M. tuberculosis and M. smegmatis RNAP structures but not in the E. coli and T. thermophilus RNAP structures (24, 25, 38), suggesting that this species-specific association between the ω loop and the β′CTD regions may contribute to the essential role of ω subunit in RNAP assembly in mycobacteria.
Our sequence alignment showed that the β′CTD and ω loop regions, which determine the dependence of M. tuberculosis RNAP for ω, are conserved in mycobacteria. Although the sequences of these two regions from S. coelicolor, a representative species of actinobacteria, are similar to those from mycobacteria, our data showed that the role of ω in S. coelicolor is not essential, which is in consistent with a previous study (30) but is different from that of mycobacteria. These differences indicate that amino acids that are distinct between mycobacteria and S. coelicolor in these conserved regions or some additional element(s) may be required to determine the specific features of RNAP.
Although we have shown a strict dependence for ω by the mycobacterial RNAP core in vitro, deletion of a region in the rpoZ gene has been successfully constructed in M. smegmatis, although the growth of this strain is strongly repressed (18). Whether the role of ω could be complemented in part by other factors in mycobacteria, such as homologs of GroEL in E. coli (32), is worthy of further investigation. The lack of a proper chaperone(s) may partly explain the defects in RNAP assembly observed in M. tuberculosis coreΔω. However, E. coli coreΔω, but not the M. tuberculosis coreΔω, is well assembled in in vitro reconstitution experiments, which suggests that the role of ω subunit is more important in M. tuberculosis than in E. coli. Nevertheless, our study provides fresh insights for further characterizing the roles of the bacterial ω subunit in transcription.
MATERIALS AND METHODS
Plasmids, oligonucleotides, and bacterial growth conditions.
The plasmids used in this study are summarized in Table S1 in the supplemental material. The oligonucleotides used in plasmid constructions are listed in Table S2 in the supplemental material. E. coli strains were grown in Luria-Bertani (LB) broth or on LB agar-solidified plates at 37°C or at other temperatures, as indicated.
Protein expression and purification.
Plasmids expressing RNAPs or associated proteins were constructed using a ClonExpress II one-step cloning kit (Vazyme, China). Mutations in genes were constructed with a QuikChange II XL site-directed mutagenesis kit (Stratagene). Bacterial RNAPs (with a His tag at the C terminal of β′ subunit) expressed in E. coli BL21(DE3) were purified as we previously described (21). For a detailed description of the methods, see the supplemental material.
RNAP reconstitution.
RNAP reconstitution was performed as previously described (36, 39). Briefly, M. tuberculosis and E. coli αHis and ωHis subunits were expressed in E. coli BL21(DE3) and subsequently purified with Ni-resin. The M. tuberculosis and E. coli β and β′ subunits were overexpressed in E. coli BL21(DE3) and isolated from inclusion bodies. The RNAP subunits were mixed at a molar ratio of 1:1.5:3:3 for β′:β:α:ω (when ω was present) in 10 ml of denaturing buffer (50 mM Tris-HCl [pH 7.9], 10 mM MgCl2, 10 μM ZnCl2, 1 mM EDTA, 10 mM dithiothreitol [DTT], 10% glycerol, 6 M guanidine hydrochloride). The mixtures were then dialyzed against refolding buffer (50 mM Tris-HCI [pH 7.9], 200 mM KCl, 10 mM MgCl2, 10 μM ZnCl2, 1 mM EDTA, 5 mM 2-mercaptoethanol, 20% glycerol) at 4°C for 16 h, with two rounds of buffer changes. Next, the reconstitution mixtures were incubated at 30°C for 60 min and centrifuged at 13,000 × g for 10 min at 4°C. RNAPs were then purified with heparin and MonoQ columns. Details regarding the protein purification methods are provided in the supplemental material.
Western blot analysis.
Samples were resolved by SDS–12% PAGE and transferred onto polyvinylidene difluoride membranes. After being blocked with 5% (wt/vol) nonfat milk powder in phosphate buffer with 0.05% Tween 20 (PBST), the membranes were incubated with anti-Flag (mouse monoclonal; Sigma-Aldrich) or anti-His (mouse monoclonal; Beyotime Biotechnology) primary antibody (diluted 1:1,000). The membranes were washed three times with PBST and then incubated with a 1:10,000 dilution of a horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma-Aldrich) for 1 h. Signals were detected using an ECL kit (Bio-Rad) according to the manufacturer's protocol.
In vitro transcription.
For abortive initiation assay, reactions were performed as previously described (21, 40). Briefly, transcription was performed in 5 μl of transcription buffer (20 mM Tris-HCl [pH 7.9], 50 mM NaCl, 5 mM MgSO4, 1 mM DTT, 0.1 mM EDTA, 5% glycerol). RNAP holoenzyme was assembled by mixing 200 nM RNAP core and 400 nM σ factor. The activator protein RbpA (21) was added at 800 nM for mycobacterial RNAP in transcription assays. A promoter fragment (30 nM) was incubated with RNAP at 37°C for 10 min. Transcription was initiated by the addition of 50 μM limited nucleoside triphosphates (GTP and UTP for Rv1494p and rrnP3; GTP and ATP for Rv0005p), together with 1 μCi of [α-32P]CTP. The reactions were carried out at 37°C for 20 min for mycobacterial RNAP and for 5 min for E. coli RNAP.
The σ-independent transcription elongation reactions were performed as described previously (21). Briefly, 80 nM RNA (5′-CUCAAUUGUGAGCGGA-3′) and 40 nM template strand DNA (5′-GAATTCTGTTTCCTGTGTGAAGTTTTAGTCCGCTCACTATACCACACATCGCGTC-3′) were first annealed and then incubated with 200 nM RNAP core at 24°C for 30 min. Next, 40 nM nontemplate DNA (5′-GACGCGATGTGTGGTATAGTGAGCGGACTAAAACTTCACACAGGAAACAGAATTC-3′) was added, followed by incubation at 37°C for 10 min. Transcription reactions were initiated by the addition of 1 μCi of [α-32P]CTP or a mixture of 50 μM ATP, UTP, and GTP and 1 μCi of [α-32P]CTP. Reactions were carried out at 24°C (for M. tuberculosis, M. smegmatis, S. coelicolor, E. coli, and P. aeruginosa RNAP) or 65°C (for T. thermophilus RNAP) for 5 min. Transcripts were analyzed on 20% denaturing PAGE (7 M urea) and detected by a cyclone storage phosphor system (Perkin-Elmer). All tests were performed at least twice independently. ImageJ software was used to quantify the products.
Statistical analysis.
The experiments were analyzed using Student t tests and repeated three times. Statistical analyses were performed using GraphPad Prism software. Quantitative data are presented as mean values with standard deviations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Konstantin Brodolin for comments during the preparation of the manuscript. We also thank Yu Zhang for providing the genomic DNA of T. thermophilus, Gang Liu for S. coelicolor, Dongru Qiu for P. aeruginosa PAO1, and Jiaoyu Deng for M. smegmatis.
This study was supported by National Natural Science Foundation of China (grant 31670134), the 135 Research Project of Wuhan Institute of Virology, and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB03). Y.H. was supported by the Youth Innovation Promotion Association CAS. Support from the Core Facility and Technical Support at the Wuhan Institute of Virology are also acknowledged. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00159-18.
REFERENCES
- 1.Werner F. 2008. Structural evolution of multisubunit RNA polymerases. Trends Microbiol 16:247–250. doi: 10.1016/j.tim.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Werner F, Grohmann D. 2011. Evolution of multisubunit RNA polymerases in the three domains of life. Nat Rev Microbiol 9:85–98. doi: 10.1038/nrmicro2507. [DOI] [PubMed] [Google Scholar]
- 3.Cramer P. 2002. Multisubunit RNA polymerases. Curr Opin Struct Biol 12:89–97. doi: 10.1016/S0959-440X(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 4.Murakami KS, Darst SA. 2003. Bacterial RNA polymerases: the wholo story. Curr Opin Struct Biol 13:31–39. doi: 10.1016/S0959-440X(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 5.Murakami KS. 2015. Structural biology of bacterial RNA polymerase. Biomolecules 5:848–864. doi: 10.3390/biom5020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borukhov S, Nudler E. 2003. RNA polymerase holoenzyme: structure, function and biological implications. Curr Opin Microbiol 6:93–100. doi: 10.1016/S1369-5274(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh P, Ishihama A, Chatterji D. 2001. Escherichia coli RNA polymerase subunit omega and its N-terminal domain bind full-length β′ to facilitate incorporation into the α2β subassembly. Eur J Biochem 268:4621–4627. doi: 10.1046/j.1432-1327.2001.02381.x. [DOI] [PubMed] [Google Scholar]
- 8.Mathew R, Chatterji D. 2006. The evolving story of the omega subunit of bacterial RNA polymerase. Trends Microbiol 14:450–455. doi: 10.1016/j.tim.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Y, Wang Y, Steitz TA. 2013. The mechanism of E. coli RNA polymerase regulation by ppGpp is suggested by the structure of their complex. Mol Cell 50:430–436. doi: 10.1016/j.molcel.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross W, Vrentas CE, Sanchez-Vazquez P, Gaal T, Gourse RL. 2013. The magic spot: a ppGpp binding site on Escherichia coli RNA polymerase responsible for regulation of transcription initiation. Mol Cell 50:420–429. doi: 10.1016/j.molcel.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnusson LU, Farewell A, Nystrom T. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee K, Chatterji D. 1997. Studies on the omega subunit of Escherichia coli RNA polymerase: its role in the recovery of denatured enzyme activity. Eur J Biochem 247:884–889. doi: 10.1111/j.1432-1033.1997.00884.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar P, Sardesai AA, Murakami KS, Chatterji D. 2013. Inactivation of the bacterial RNA polymerase due to acquisition of secondary structure by the omega subunit. J Biol Chem 288:25076–25087. doi: 10.1074/jbc.M113.468520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss A, Moore BD, Tremblay MH, Chaput D, Kremer A, Shaw LN. 2017. The omega subunit governs RNA polymerase stability and transcriptional specificity in Staphylococcus aureus. J Bacteriol 199:e00459-16. doi: 10.1128/JB.00459-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunnelius L, Hakkila K, Kurkela J, Wada H, Tyystjarvi E, Tyystjarvi T. 2014. The omega subunit of the RNA polymerase core directs transcription efficiency in cyanobacteria. Nucleic Acids Res 42:4606–4614. doi: 10.1093/nar/gku084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 18.Mathew R, Ramakanth M, Chatterji D. 2005. Deletion of the gene rpoZ, encoding the omega subunit of RNA polymerase, in Mycobacterium smegmatis results in fragmentation of the β′ subunit in the enzyme assembly. J Bacteriol 187:6565–6570. doi: 10.1128/JB.187.18.6565-6570.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high-density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 20.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Morichaud Z, Perumal AS, Roquet-Baneres F, Brodolin K. 2014. Mycobacterium RbpA cooperates with the stress-response σB subunit of RNA polymerase in promoter DNA unwinding. Nucleic Acids Res 42:10399–10408. doi: 10.1093/nar/gku742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igarashi K, Fujita N, Ishihama A. 1989. Promoter selectivity of Escherichia coli RNA polymerase: omega factor is responsible for the ppGpp sensitivity. Nucleic Acids Res 17:8755–8765. doi: 10.1093/nar/17.21.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W, Mandal S, Degen D, Liu Y, Ebright YW, Li S, Feng Y, Zhang Y, Mandal S, Jiang Y, Liu S, Gigliotti M, Talaue M, Connell N, Das K, Arnold E, Ebright RH. 2017. Structural basis of Mycobacterium tuberculosis transcription and transcription inhibition. Mol Cell 66:169–179. doi: 10.1016/j.molcel.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassylyev DG, Vassylyeva MN, Perederina A, Tahirov TH, Artsimovitch I. 2007. Structural basis for transcription elongation by bacterial RNA polymerase. Nature 448:157–162. doi: 10.1038/nature05932. [DOI] [PubMed] [Google Scholar]
- 25.Murakami KS. 2013. X-ray crystal structure of Escherichia coli RNA polymerase sigma70 holoenzyme. J Biol Chem 288:9126–9134. doi: 10.1074/jbc.M112.430900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyaci H, Chen J, Lilic M, Palka M, Mooney RA, Landick R, Darst SA, Campbell EA. 2018. Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. Elife 7:e34823. doi: 10.7554/eLife.34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubin EA, Fay A, Xu C, Bean JM, Saecker RM, Glickman MS, Darst SA, Campbell EA. 2017. Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. Elife 6:e22520. doi: 10.7554/eLife.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minakhin L, Bhagat S, Brunning A, Campbell EA, Darst SA, Ebright RH, Severinov K. 2001. Bacterial RNA polymerase subunit omega and eukaryotic RNA polymerase subunit RPB6 are sequence, structural, and functional homologs and promote RNA polymerase assembly. Proc Natl Acad Sci U S A 98:892–897. doi: 10.1073/pnas.98.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentry DR, Burgess RR. 1989. rpoZ, encoding the omega subunit of Escherichia coli RNA polymerase, is in the same operon as spoT. J Bacteriol 171:1271–1277. doi: 10.1128/jb.171.3.1271-1277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Beneit F, Barriuso-Iglesias M, Fernandez-Martinez LT, Martinez-Castro M, Sola-Landa A, Rodriguez-Garcia A, Martin JF. 2011. The RNA polymerase omega factor RpoZ is regulated by PhoP and has an important role in antibiotic biosynthesis and morphological differentiation in Streptomyces coelicolor. Appl Environ Microbiol 77:7586–7594. doi: 10.1128/AEM.00465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard CL, Tandon A, Sloan NR, Kranz RG. 2003. RNA polymerase subunit requirements for activation by the enhancer-binding protein Rhodobacter capsulatus NtrC. J Biol Chem 278:31701–31708. doi: 10.1074/jbc.M304430200. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee K, Nagai H, Shimamoto N, Chatterji D. 1999. GroEL is involved in activation of Escherichia coli RNA polymerase devoid of the omega subunit in vivo. Eur J Biochem 266:228–235. doi: 10.1046/j.1432-1327.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 33.Jacques JF, Rodrigue S, Brzezinski R, Gaudreau L. 2006. A recombinant Mycobacterium tuberculosis in vitro transcription system. FEMS Microbiol Lett 255:140–147. doi: 10.1111/j.1574-6968.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 34.Herrera-Asmat O, Lubkowska L, Kashlev M, Bustamante CJ, Guerra DG, Kireeva ML. 2017. Production and characterization of a highly pure RNA polymerase holoenzyme from Mycobacterium tuberculosis. Protein Expr Purif 134:1–10. doi: 10.1016/j.pep.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee R, Rudra P, Prajapati RK, Sengupta S, Mukhopadhyay J. 2014. Optimization of recombinant Mycobacterium tuberculosis RNA polymerase expression and purification. Tuberculosis 94:397–404. doi: 10.1016/j.tube.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Tang H, Severinov K, Goldfarb A, Ebright RH. 1995. Rapid RNA polymerase genetics: one-day, no-column preparation of reconstituted recombinant Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A 92:4902–4906. doi: 10.1073/pnas.92.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubin EA, Lilic M, Darst SA, Campbell EA. 2017. Structural insights into the mycobacteria transcription initiation complex from analysis of X-ray crystal structures. Nat Commun 8:16072. doi: 10.1038/ncomms16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuo Y, Steitz TA. 2015. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol Cell 58:534–540. doi: 10.1016/j.molcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borukhov S, Goldfarb A. 1993. Recombinant Escherichia coli RNA polymerase: purification of individually overexpressed subunits and in vitro assembly. Protein Expr Purif 4:503–511. doi: 10.1006/prep.1993.1066. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Mao C, Ge X, Wang Z, Lu P, Zhang Y, Chen S, Hu Y. 2017. Characterization of a minimal type of promoter containing the −10 element and a guanine at the −14 or −13 position in mycobacteria. J Bacteriol 199:e00385-17. doi: 10.1128/JB.00385-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.