Abstract
Objectives
Universal neonatal hearing screening (UNHS) started late in some underdeveloped areas in China, with relatively scarce screening resources and a wide regional distribution. This study aimed to compare the screening performance between rural and urban populations, and to examine the characteristics and problems of UNHS in underdeveloped regions in China.
Methods
A two-step hearing screening program was used in neonates born in Liuzhou Maternal and Child Health Hospital and in patients who were born in other hospitals, but admitted to the neonatal intensive care unit. This program involved distortion product otoacoustic emission and automated auditory brainstem response. Characteristics of each newborn, as well as the screening outcomes and performance were compared between rural and urban populations.
Results
A total of 19,098 newborns were screened with a referral rate of 17.9% at the first step. Sixty-three (0.33%) newborns had hearing loss. The prevalence of permanent hearing loss was 2.25‰. The average screening age was significantly older in the rural population than in the urban population in the first (P < 0.01) and second steps of screening (P < 0.05). The rural population had a higher referral rate in both steps than the urban population (P < 0.01). The follow-up rate was much lower in the rural population than in the urban population (P < 0.05), but dramatically increased in 2014 compared with the previous 2 years.
Conclusions
A low follow-up rate is a critical issue when carrying out UNHS in developing countries, such as China, especially for rural populations. The government should establish more hearing referral centres to increase service coverage and supply financial assistance for low-income populations.
Keywords: Neonatal hearing screening, distortion product otoacoustic emission, automated auditory brainstem response, developing country
Abbreviations
- NICU
neonatal intensive care unit
- UNHS
universal neonatal hearing screening
- DPOAE
distortion product otoacoustic emission
- AABR
automated auditory brainstem response
- dB HL
decibels hearing level
Introduction
Congenital hearing impairment is one of the most common birth defects in neonates, with an incidence of 1‰ to 3‰.1–3 Auditory abnormalities can affect speech and language development, and other social cognitive functions in childhood, resulting in long-term learning difficulties and permanent disability.4 Early detection and intervention by use of a hearing aid and cochlear implant are essential for minimizing the effect of deficits caused by hearing impairment in children.5,6
Neonatal hearing screening has been carried out in some developed cities in China for almost 10 years. The costs and effectiveness of different hearing screening protocols were analysed between several provinces of China.7,8 Screening methods and strategies have been gradually improved and standardized. However, as the largest developing country in the world, there are still many underdeveloped areas in China, where neonatal hearing screening started late. There are relatively scarce screening resources and a wide regional distribution combining urban and rural populations in China.
Liuzhou, located in the southwest of China, is the largest industrial city in the less developed Province of Guangxi, covering an area of over 18,000 square kilometres. Health care resources in this area are relatively scarce, and comprehensive medical facilities and faculties of Obstetrics and Neonatology are concentrated only in one hospital, Liuzhou Maternal and Child Health Hospital. This hospital has the only licensed hearing diagnostic centre in this region, and is the first health institution to implement neonatal hearing screening in Guangxi.
This study aimed to analyse the screening results of Liuzhou Maternal and Child Health Hospital, including the referral rate, follow-up rate of urban and rural populations, and the false-negative rates of the distortion product otoacoustic emission (DPOAE) and automated auditory brainstem response (AABR) techniques. This study also aimed to examine the characteristics and problems in Guangxi, an underdeveloped region in China, to determine how to improve the universal neonatal hearing screening work in developing countries, such as China.
Subjects and methods
Subjects
Hearing screening was offered to all neonates who were born in Liuzhou Maternal and Child Health Hospital between 1st January 2012 and 31st May 2014. This screening was also offered to those who were born in other hospitals, but admitted to the neonatal intensive care unit (NICU) during that period of time. A total of 19,098 newborns were included in this study, of which 6964 were from the NICU and 12,134 were healthy neonates from the Department of Obstetrics. Written informed consent was obtained from the parents of the children who participated in the bedside universal neonatal hearing screening (UNHS) program.
Screening procedures
We applied a two-step screening protocol using distortion product otoacoustic emission (DPOAE) and automated auditory brainstem response (AABR). The screening procedure was in accordance with the universal hearing screening technical specifications issued by the Chinese Ministry of Health. These specifications were based on the goals set by the Joint Committee on Infant Hearing as follows: neonates should receive universal hearing screening before discharge; those who do not pass the initial screening should be screened before 42 days after birth; and all neonates who fail the rescreening should be diagnosed by medicinal and audiological evaluation within 3 months. Screening was performed by four nurses after specific training in neonatal screening techniques. Newborns were screened at the bedside without induced sleeping. Single DPOAE (OtoRead) was performed as initial screening for healthy neonates in the Department of Obstetrics before discharge. Considering the higher number of neonates with risk factors for hearing loss and auditory neuropathy in the NICU, DPOAE and AABR (AccuScreen) were conducted for the first screening in the NICU when the patients’ general condition was stable.
DPOAE was considered to be present at any frequency if emissions were at least 5 dB sound pressure level (SPL) above the mean noise floor. The device used in-ear calibration before screening commenced. Two simultaneous pure-tone signals with a frequency ratio of 1.22 at 60 dB SPL and 65 dB SPL were presented at 1500, 2000, 2500, 3000, 3500, and 4000 Hz. The clicks of AABR were delivered at a fixed intensity of 35 dB normal hearing level (nHL). Pass was defined as negative results in both ears for both methods. These devices were fixed to detect a hearing loss of 35 dB HL or higher. In each screening, a maximum of three tests was permitted per ear. All of the children who were referred in the initial screening were required to receive rescreening by DPOAE and AABR at the Department of Audiology within 6 weeks. Similarly, a successful pass for DPOAE and AABR tests in both sides was needed to prevent referral to the next step. After rescreening, children who needed diagnostic tests were referred to the hearing centre within 3 months of age.
Assessment procedures for hearing loss
Neonates who were referred to the Audiology Centre (Liuzhou Maternal and Child Health Hospital) had received an otological examination and audiological evaluation, including otoscopy, tympanometry with 226- and 1000 Hz-tone probes (GSI Tympstar), DPOAEs (Smart OAE; f2:f1 = 1.22, L2/L1 = 55/65 dB SPL), and auditory brainstem response (ABR, Intelligent Hearing Systems). Hearing loss was confirmed when the ABR threshold by air conduction was higher than 30 dB HL in either ear. A bone conduction test of the ABR was performed according to the tympanometry result to confirm the situation of conductive hearing loss.
Data analysis
SPSS software (version 17.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Descriptive statistics show the frequency distribution. The two-sample t test was used to compare the average screening age between rural and urban populations. The χ2 test was used to investigate correspondence between test outcomes by different screening techniques in urban and rural populations. For all of the statistical analyses, a probability value of P < 0.05 was considered statistically significant.
Ethics
This study was approved by the Medical Ethics Committee of the Xinhua Hospital affiliated with Shanghai Jiaotong University School of Medicine and the Medical Ethics Committee of Guangxi Province Liuzhou City Maternal and Child Health Hospital. The universal hearing screening program was promoted and recognized by the local government. Parents were aware of the necessity of the UNHS before delivery, similar to other neonatal screenings. Written informed consent from all of the neonates’ parents was also obtained prior to the screening.
Results
Overall results of the screening process
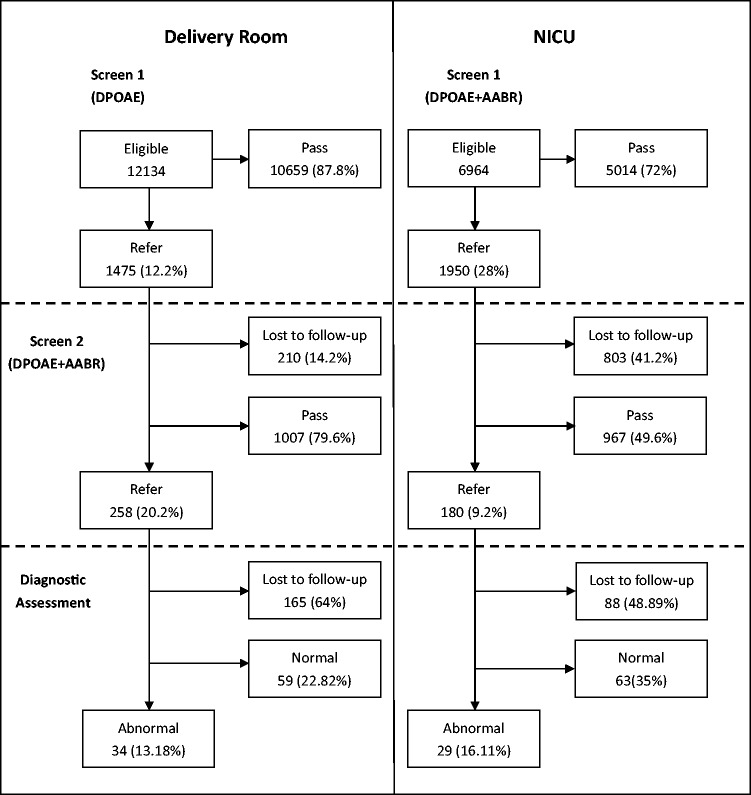
A total of 19,098 newborns were screened with a capture rate of 97.94% in Liuzhou City Maternal and Child Health Hospital. Among these newborns, 6964 were from the NICU and followed a different first screening protocol to that for healthy newborns. The referral rate at the first step was 17.9%, where the referral rate of newborns from the NICU (28%) was much higher than that of healthy newborns (12.2%). Of 3425 newborns who were referred, 2412 (70.4%) had rescreening and 438 (18.2%) failed in the second stage. Only 185 (42.2%) newborns received diagnostic tests and 63 (0.33%) of the 19,098 newborns had hearing loss, including conductive, sensorineural, and mixed hearing impairment (Figure 1). The prevalence of permanent hearing loss, including sensorineural and mixed hearing loss, of all screened newborns was 2.25/1000. The incidence of PCHL in newborns from the NICU (2.73/1000) was higher than that in healthy newborns (1.98/1000).
Figure 1.
Flow diagram of participants from the delivery room and the NICU throughout the study.
Comparison of screening between urban and rural populations
In this study, the urban population was 8427 and the rural population was 10,671. The percentages of newborns in the rural population in the NICU and healthy newborns were 59.8% and 53.6%, respectively. The average screening age of the rural population (3.81, 0–189 days) was significantly older than that in the urban population (3.24, 0–180 days) in the first (P < 0.01) and second steps (P < 0.05) of screening, but not in the diagnostic step (P > 0.05). We was also found that the rural population had a higher positive rate in two steps of hearing screening than did the urban population (P < 0.01). However, the follow-up rate of the rural population was much lower than that of the urban population for the rescreening step and the diagnostic test. The incidence of hearing loss was similar between the rural and urban populations (Table 1).
Table 1.
Distribution of screening performance in urban and rural populations.
| Rural population | Urban population | Statistical analysis | |
|---|---|---|---|
| Total number | 10,671 | 8427 | |
| First screening | |||
| Age (days) | 3.81 (SD, 8.210) | 3.24 (SD, 7.073) | T = −5.167, P < 0.01 |
| Referred | 2017 (18.9%) | 1408 (16.7%) | χ2 = 15.393, P < 0.01 |
| Second screening | |||
| Attendance | 1361 (67.5%) | 1051 (74.6%) | |
| Age (days) | 45.47 (SD, 21.364) | 43.78 (SD, 12.673) | T = −2.417, P < 0.05 |
| Referred | 272 (20.0%) | 166 (15.8%) | χ2 = 7.008, P < 0.01 |
| DPOAE | 250 (18.4%) | 154 (14.7%) | χ2 = 5.873, P < 0.05 |
| AABR | 122 (9.0%) | 71 (6.8%) | χ2 = 3.929, P < 0.05 |
| Diagnostic tests | |||
| Attendance | 106 (39.0%) | 79 (47.6%) | |
| Age (days) | 99.25 (SD, 49.047) | 105.59 (SD, 46.789) | T = 0.849, P > 0.05 |
| Hearing loss | 37 (3.5‰) | 26 (3.1‰) | χ2 = 1.764, P > 0.05 |
| Sensorineural | 21 (2.0‰) | 18 (2.1%) | |
| Conductive | 14 (1.3‰) | 6 (0.7‰) | |
| Mixed | 2 (0.2‰) | 2 (0.2‰) |
Follow-up rate of different populations across the years
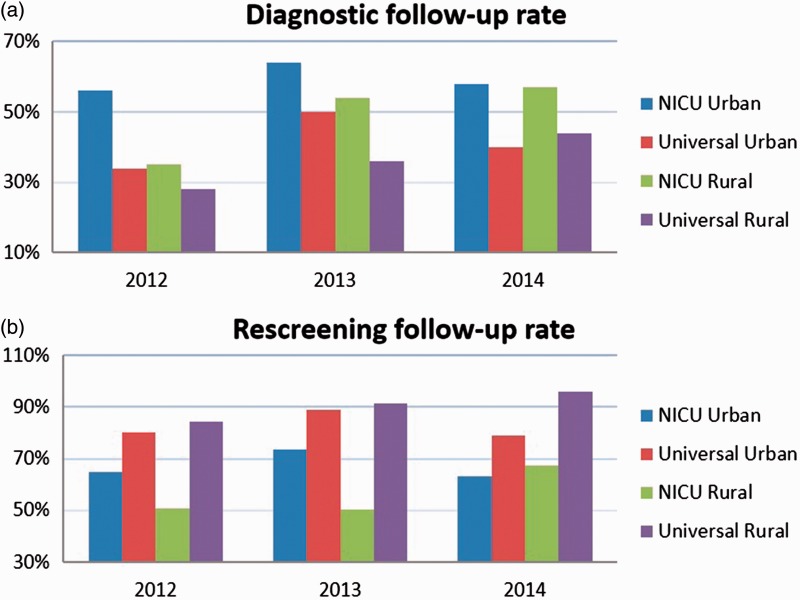
The follow-up rate in each group showed an upward trend through the years. The follow-up rate of newborns in the NICU was generally lower than that in healthy newborns for the rescreening step, but the rate of the diagnostic step was higher in newborns in the NICU than in healthy newborns. The rural population of newborns in the NICU showed a much lower follow-up rate for both steps than did the urban population in the first 2 years, but this rate was similar between the groups in 2014. Similar results were observed in the diagnostic follow-up rate of healthy newborns (Figure 2).
Figure 2.
Distribution of follow-up rate in different population over years. (a) Distribution of follow-up rate for diagnosis over years; (b) Distribution of follow-up rate for rescreening (second step) over years.
Comparative study on the positive rate of initial screening
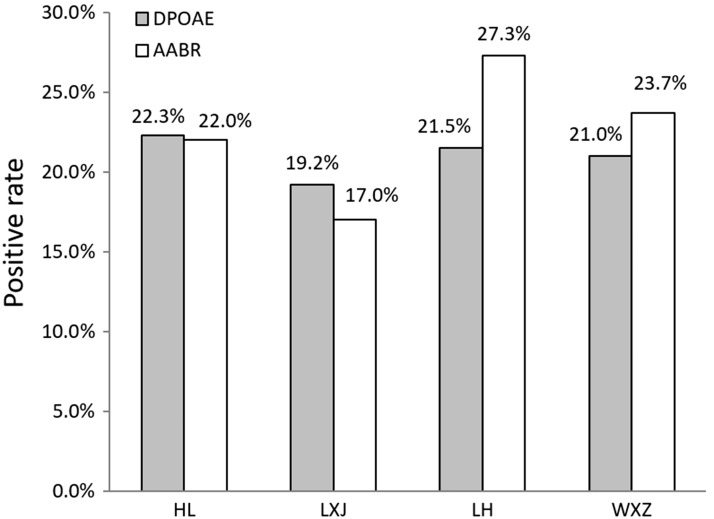
In the DPOAE and AABR tests, a higher rate of referral was observed in the left ear and in boys than in the right ear and in girls (P < 0.01) (Table 2). A predominance of the left ears (57.1%) and male sex (64.3%) were found in 14 patients who were diagnosed with unilateral sensorineural and mixed hearing loss. We also found that the positive rate of initial screening in the NICU was much higher than that in the Department of Obstetrics. For NICU neonates, the referral rate of AABR was slightly higher than that of DPOAE. We compared the screening results of NICU neonates according to the categories of four nurses who performed the screening. We found that differences in the positive rate of AABR screening were significant across the four specialists (χ2 = 34.813, P < 0.01), but this was not shown for DPOAE (χ2 = 1.972, P > 0.05) (Figure 3).
Table 2.
Distribution of initial screening results according to method and sex.
| NICU |
Department of Obstetrics | ||
|---|---|---|---|
| Results | DPOAE | AABR | DPOAE |
| Total | N = 6964 | N = 12,134 | |
| Passed | 5488 (78.8%) | 5228 (75.1%) | 10,659 (87.8%) |
| Referred on both sides | 387 (5.6%) | 433 (6.2%) | 556 (4.6%) |
| Referred on left only | 616 (8.8%) | 789 (11.3%) | 552 (4.5%) |
| Referred on right only | 473 (6.8%) | 514 (7.4%) | 367 (3.0%) |
| Referred cases | M = 3806 | F = 3158 | M = 6397 F = 5737 |
| Male | 870 (22.9%) | 1001 (26.3%) | 888 (13.9%) |
| Female | 606 (19.2%) | 735 (23.3%) | 587 (10.2%) |
| Statistical | χ2 = 13.912 | χ2 = 8.447 | χ2 = 37.728 |
| Analysis | P < 0.01 | P < 0.01 | P < 0.01 |
Figure 3.
Positive rate of two screening methods by different testers.
False-negative results of DPOAE and AABR
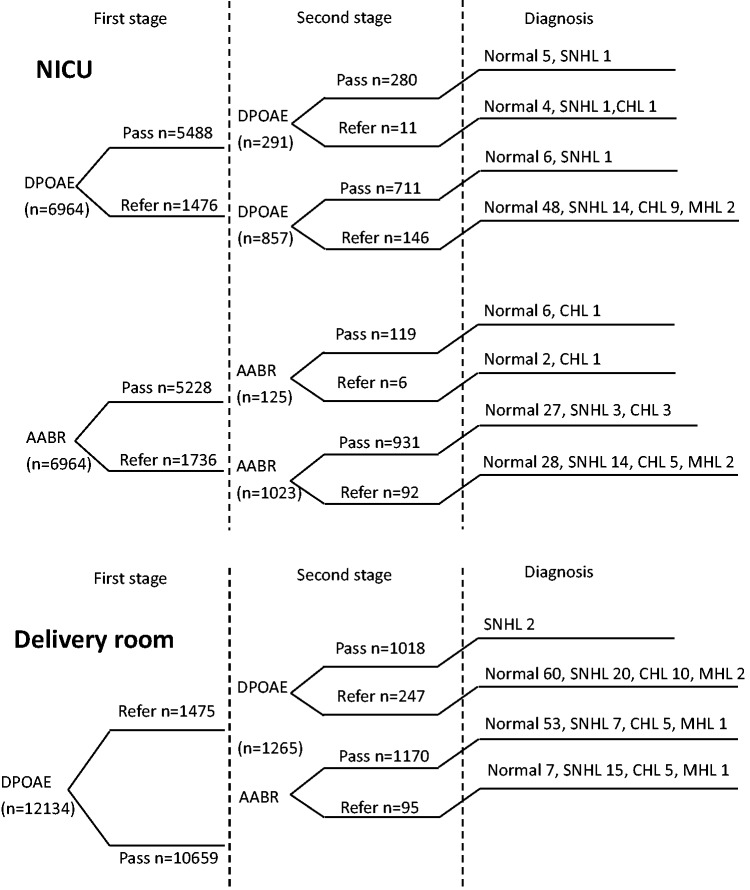
According to the screening results in the NICU, two neonates who were found to have permanent hearing loss (one with unilateral mild hearing loss, one with bilateral profound hearing loss) passed the initial screening of DPOAE. Another neonate who was diagnosed with bilateral mild hearing loss was referred in the first screening, but passed in the second step of DPOAE. The initial screening of AABR identified all of the patients who had permanent hearing loss in the NICU. However, three of the patients (two with unilateral mild hearing loss, one with bilateral mild hearing loss) passed in the second step of AABR, but were referred with DPOAE. In screening in the Department of Obstetrics, the number of false-negative cases for the second step of AABR was seven (three with unilateral mild hearing loss, two with bilateral mild hearing loss, two with bilateral moderate hearing loss), which was much higher than that of DPOAE (one with unilateral moderate hearing loss and one with bilateral moderate hearing loss) (Figure 4).
Figure 4.
Flow chart of screening performance at each stage according to different screening rules.
Risk factors of neonates in the NICU
Approximately 55% of neonates in the NICU had risk factors related to permanent hearing loss (Table 3), according to the 13 high risk factors mentioned in the Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.9 There was no significant difference in the positive rate of screening between the two groups of neonates with or without risk factors in this study (P > 0.05). However, the majority of neonates who were diagnosed with permanent hearing loss had one or more risk factors.
Table 3.
Characteristics of neonates confirmed with permanent hearing loss in the NICU.
| No. | Sex | Screening (referred at 1 and 2) | Degree of hearing loss | Threshold (dB HL) |
||
|---|---|---|---|---|---|---|
| Left | Right | Risk | ||||
| SNHL | ||||||
| 1 | M | Both, both | Uni, profound | 90 | 30 | Preterm |
| 2 | M | Both, both | Uni, mild | 30 | 40 | Preterm |
| 3 | M | Both, DPOAE | Bi, moderate | 40 | 50 | Hematosepsis |
| 4 | M | Both, AABR | Bi, mild | 40 | 40 | |
| 5 | M | Both, both | Bi, moderate | 50 | 50 | Low birth weight |
| 6 | M | Both, both | Bi, severe | 70 | 70 | Low birth weight |
| 7 | M | Both, DPOAE | Uni, mild | 40 | 30 | |
| 8 | M | Both, both | Uni, moderate | 50 | 30 | NICU > 5 days |
| 9 | F | AABR, DPOAE | Uni, mild | 40 | 30 | Preterm |
| 10 | M | Both, both | Bi, moderate | 50 | 40 | NICU > 5 days |
| 11 | M | AABR, AABR | Bi, profound | >100 | >100 | Hyperbilirubinaemia |
| 12 | M | Both, both | Bi, moderate | 50 | 50 | Hyperbilirubinaemia |
| 13 | M | Both, both | Bi, severe | 90 | 80 | Preterm, asphyxia |
| 14 | M | Both, both | Bi, moderate | 40 | 60 | Preterm, asphyxia |
| 15 | F | Both, both | Bi, moderate | 50 | 50 | Preterm |
| 16 | M | Both, both | Bi, moderate | 50 | 60 | |
| 17 | M | Both, both | Bi, moderate | 50 | 50 | |
| MHL | ||||||
| 18 | F | Both, both | Left, severe Right, mild | 80 | 40 | Hyperbilirubinaemia |
| 19 | F | Both, both | Bi, severe | 70 | 70 | Low birth weight Hyperbilirubinaemia |
M, male; F, female; Bi, bilateral; uni, unilateral.
Screening (referred at 1 and 2) indicates which screening methods neonates were referred by in the first and second steps.
Discussion
UNHS has been carried out by a two-step protocol using DPOAE and AABR in Liuzhou Maternal and Child Health Hospital for almost 3 years. The DPOAE and AABR tests are easily performed in neonates and infants. Both of these tests have been successfully used for UNHS, providing noninvasive recordings of physiological activity underlying normal auditory function.9 OAE records cochlear responses to acoustic stimuli by using a sensitive microphone within a probe assembly, reflecting the status of the peripheral auditory system to the cochlear outer hair cells. AABR records neural activity generated in the cochlea, auditory nerve, and brainstem in response to acoustic stimuli delivered via an earphone. This activity reflects the status of the peripheral auditory system, the eighth nerve, and the brainstem auditory pathway. The sensitivity and specificity of the two screening methods greatly differ in the literature. The sensitivity and specificity are 90%–100% and 80%–100% for OAE,10–12 while they are 90%–100% and 93%–96% for AABR, respectively.13,14 Before this study was performed, early use of OAE alone for UNHS was widely applied in developing countries. Currently, the UNHS service covers all neonates who are born in Liuzhou City Maternal and Child Health Hospital and also those who are admitted to the NICU, but are born in other hospitals. In view of the limited human resources and equipment, we only performed the DPOAE test for healthy neonates in the Department of Obstetrics in initial hearing screening. Neonates who were in the NICU received both of the DPOAE and AABR tests at the first screening step because of the higher possibility of having risk factors related to hearing loss and auditory neuropathy.9,15 Instead of applying single AABR screening, our study also aimed to compare the performance of the two screening methods in the NICU within remote areas. All of the neonates who failed in the initial screening were required to undergo rescreening of the DPOAE and AABR tests within 6 weeks after birth. Most parents chose to have rescreening for their children when they received a routine health examination after the first month.
In this study, the positive rate of initial screening for NICU neonates was much higher than that of healthy neonates from the Department of Obstetrics. This rate is also higher than that in other reports on hearing screening in the NICU,16 but it is similar to results from an Australian study.12 In addition to the finding that the incidence of hearing loss in NICU neonates is higher than that for healthy newborns,18 a low screening pass rate in newborns with prematurity and low birth weight,19,20 and external noise interference to AccuScreen caused by monitors may be two other important factors. A higher referral rate can place additional burden on the hearing screening program and increase the difficulties in follow-up of referred infants,21 which were found in this study. Diagnostic results in our study showed that the incidence of PCHL in NICU neonates (2.73/1000) was higher than that in healthy neonates (1.98/1000), but this incidence is not similar to other domestic and international reports.16,17 The actual prevalence of PCHL should be much higher owing to the extremely low follow-up rate of the rescreening and diagnostic tests for neonates in the NICU.
We compared the screening results between rural and urban populations. The positive rates for initial screening and rescreening of the rural population were higher than those in the urban population. This finding indicates that rural neonates might be more likely to have a hearing problem than urban neonates. In this study, there were significant, but not obvious, differences in the screening age at the first and second steps between the rural and urban populations. For the initial screening, the age disparity was mainly because some rural neonates who stayed in the NICU were referred from other hospitals, leading to a slightly older age at screening. For neonates born in Liuzhou City Maternal and Child Health Hospital, screening was usually performed 48 hours after birth. Unexpectedly, the age at the diagnostic test of rural neonates was younger than that in urban neonates, but this difference was not significant. However, the follow-up rate of the rural population appeared to be lower than that in the urban population, even though the rates of both groups were far below the recommended benchmarks.8 This finding demonstrated the poorer compliance of rural parents, leading to more loss of cases. The diagnostic age of the rural population should have been delayed, but many parents did not detect hearing problems of their children in this 3-year study. Additionally, considering the similar incidence of hearing loss between the two groups in our study, the lower follow-up rate of the rural population suggests that the actual incidence of hearing loss in rural neonates should be higher than that in urban neonates. Therefore, increasing the rural follow-up rate is important.
A low follow-up rate of neonatal hearing screening is a major issue worldwide, particularly in developing countries and remote areas.22,23 In our study, the total follow-up rate of rescreening reached 70%, but the percentage of infants who received a diagnostic test was only 42.2%. Expenses for the rescreening and diagnostic tests that the neonates underwent, borne by their parents irrespective of their economic background, would have been a major cause for dropouts. The cost of a diagnostic test is approximately 50 US dollars, which is much higher than the cost of a rescreening test, which is approximately 15 US dollars. Therefore, there is an additional economic burden, especially for rural families living in remote areas. We also found that the rescreening follow-up rate of NICU neonates was lower than that of neonates from the Department of Obstetrics. Olusanya showed similar findings that newborns who were admitted to the Special Care Baby Unit (SCB) were more likely to miss the second-stage screening before discharge.24 However, in contrast to Olusanya’s24 explanation that parents from the SCBU were eager to leave the hospital because of the financial burden, second-stage screening in our study was after discharge. Therefore, our results were due to some other reasons. The majority of healthy newborns from the Department of Obstetrics in our study, including many rural patients, lived relatively closer to the hospital than did NICU neonates. Liuzhou City Maternal and Child Health Hospital is the largest maternal and children’s medical institution with advanced facilities and excellent faculties in this area. However, the NICU receives patients from the whole city, including suburban areas and affiliated counties, as well as a small amount of patients who are transferred from neighbouring cities. Therefore, follow-up of these newborns after discharge was challenging. Currently, the hearing centre of this hospital is the only referral institution for diagnostic assessment, and it does not appear to be sufficient for such a large population in this wide area. For those families living far away from the Centre, the cost of rescreening and diagnostic tests also includes expenses of transportation and accommodation, besides fees of tests. The reason why some families did not participate in hearing diagnostic tests was because they could not afford interventions, such as hearing aid fitting and follow-up cochlear implantation. Some parents might believe that nothing would change even if their child had a hearing problem, and thus there was no need for further examinations. The follow-up rate of screening programs is expected to increase by establishing more diagnostic centres for remote areas, supplying convenient transportation and finance for people with a low income, and building a network of hearing screening information across the regions.
Another large challenge is setting up an effective tracking system in China,24 especially for the rural population who live in remote regions, and have inferior health conditions, poor economic incomes, and a lack of awareness about health care. We compared the follow-up rate over time in rural and urban population separately. We found an increasing trend across the years, in spite of the low rescreening and diagnostic follow-up rates overall. Surprisingly, the follow-up rate of the rural population exceeded that of the urban population in the final year. All of these improvements were due to implementation of policies that were supported by the local government. In 2013, Liuzhou City Maternal and Child Health Hospital started fully using a web-based data collection system for neonatal hearing screening instead of traditional manual statistics. Information related to neonatal hearing screening is available from the network database, including results of initial screening, rescreening, diagnostic tests, follow-up recording, contact methods, and other personal information. At present, the system is undergoing detailed adjustments and modifications, and collecting data of neonatal hearing screening throughout the city. Additionally, at the end of 2013, the government implemented a financial aid policy covering most costs of screening and diagnostic tests for the rural population, which boosted the initiative of rural parents’ to participate in the program and follow-up visits.
However, economic issues are not the only reason for parents’ refusal to return.25 Through repeated phone invitations, many rural parents and some urban parents of the newborns who were lost to follow-up expressed their distrust in the hearing screening program and insisted that their children did not have a hearing impairment. In some remote areas, people even believed that a child not learning to talk until 2 years old is normal. Parents of our subjects did not show disparities in religious beliefs. However, sex discrimination was found to be a new issue in this study. The follow-up rate in girls was lower than that in boys, especially in rural areas. The follow-up rates of the first and second steps of screening for girls from urban areas were 73.69% and 46.97% respectively, while those for boys from urban areas were 75.34% and 48%, respectively. However, in the rural population, the follow-up rates of the two steps were 66.79% and 34.58% for girls and 67.95% and 41.82% for boys, respectively. This could have resulted in the male predominance in the number of cases that were confirmed with hearing impairment in this study. This male predominance is due to the Chinese traditional view of preferring boys to girls, particularly in rural areas. Therefore, to increase the follow-up rate in underdeveloped regions, raising awareness of the importance of hearing screening programs, as well as breaking feudal ideology should be top priorities.
A higher positive rate of initial DPOAE and AABR screening was shown in the left ear and in boys than in the right ear and in girls, which is consistent with other previous studies.26 The positive rate of AABR is vulnerable to the influence of the testers’ operation compared with DPOAE.27 The AABR test operated by one person might lead to a higher or lower positive rate than by others. The underlying reason for this finding needs further study. The next task of AABR screening should pay more attention to enhancing the training of practitioners and monitor screening qualities, especially in underdeveloped areas with numerous subjects, but limited human resources. Consistent training and appraisals should be implemented by hearing screening institutions. Periodic review is also needed to detect relative problems in time during screening. A high positive rate of initial DPOAE and AABR screening not only increases the burden of the screening program, but also makes follow-up work more difficult. Further research is required to determine what type of characteristics are required for qualified AABR screening testers, and which factors affect the screening results and increase bias.
There are false-negative questions in the methods of DPOAE and AABR. In this study, the false-negative rate in rescreening of AABR might have been even higher than that in rescreening of DPOAE. However, we also found that most of the missed cases of hearing impairment through AABR were mild hearing loss, while DPOAE had a higher risk of missing cases of severe hearing loss. With regard to the NICU, neonates with positive initial screening results should receive detailed diagnostic audiological assessment. Many neonates who fail an initial screening would be expected to pass a second screening, and some of them had hearing loss in our study. All of the three confirmed cases of hearing loss had a risk factor related to hearing loss. Therefore, neonates in the NICU with a positive result of initial AABR screening might need diagnostic tests, especially those who with one or more risk factors of hearing impairment.
Conclusion
In developing countries, more attention should be paid to improve the referral follow-up rate and to monitor screening qualities when carrying out a neonatal hearing screening program, especially in rural areas. People’s awareness needs to be improved for the importance of this screening program, especially for rural populations. Establishment of a web-based database and financial support from the government play active roles in promoting neonatal hearing screening. Building more referral and diagnostic centres is the next step for further improving this screening program in remote areas.
Contributors
Wu Wenjin: conceived and designed the study, drafted the manuscript and analysed data; Tang Xiangrong: supervised all of the screening tests in Liuzhou; Li Yun, Lü Jingrong, and Chen Jianyong: collected the data; Wang Xueling: participated in data analysis; Huang Zhiwu: supervised hearing assessments; Wu Hao: conceived the study; Wu Wenjin wrote the first draft of the manuscript, and no payment was provided to anyone to produce the manuscript.
Acknowledgements
We are grateful to the doctors and nurses from Liuzhou City Maternal and Child Health Hospital in Guangxi for their assistance throughout this study. We also thank all of the participating subjects and their parents.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a grant from Shanghai Science and Technology Committee, China (grant number: 14DZ2260300).
References
- 1.Fortum HM, Summerfield AQ, Marshall DH, et al. Incidence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based assessment study. BMJ 2001; 323: 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotby MN, Tawfik S, Aziz A, et al. Public health impact of hearing impairment and disability. Folia Phoniatr Logop 2008; 60: 58–63. [DOI] [PubMed] [Google Scholar]
- 3.Hutt N, Rhodes C. Post-natal hearing loss in universal neonatal hearing screening communities: current limitations and future directions. J Paediatr Child Health 2008; 44: 87–91. [DOI] [PubMed] [Google Scholar]
- 4.Vohr B, Jodoin-Krauzyk J, Tucker R, et al. Early language outcomes of early-identified infants with permanent hearing loss at 12 to 16 months of age. Pediatrics 2008; 122: 535–544. [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga-Itano C, Sedey AL, Coulter DK, et al. Language of early and late identified children with hearing loss. Paediatrics 1998; 102: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 6.Valencia DM, Rimell FL, Friedman BJ, et al. Cochlear implantation in infants less than 12 months of age. Int J Pediatr Otorhinolaryngol 2008; 72: 767–773. [DOI] [PubMed] [Google Scholar]
- 7.Tobe RG, Mori R, Huang L, et al. Cost-effectiveness analysis of a national neonatal hearing screening program in China: conditions for the scale-up. PloS One 2013; 8: e51990–e51990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang LH, Zhang L, Tobe RY, et al. Cost-effectiveness analysis of neonatal hearing screening program in china: should universal screening be prioritized? BMC Health Serv Res 2012; 12: 97–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007; 120: 898–921. [DOI] [PubMed] [Google Scholar]
- 10.Davis A, Bamford J, Wilson I, et al. A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment. Health Technol Assess 1997; 1: 1–176. [PubMed] [Google Scholar]
- 11.Agence Nationale d’Accreditation et d’Evaluation en Sante.1999. Evaluation Clinique et economique du depistage neonatal de la surdite permanente par les otoemissions acoustiques. Available: http://www.has-sante.fr/portail/jcms/c_ 464137/evaluation-clinique-et-economique-du-depistage-neonatal-de-lasurdite-permanente-par-les-otoemissions-acoustiques. Accessed 2010 May 16.
- 12.Kennedy CR, McCann DC, Campbell MJ, et al. Universal newborn screening for permanent childhood hearing impairment: an 8-year follw-up of a controlled trial. Lancet 2005; 366: 660–662. [DOI] [PubMed] [Google Scholar]
- 13.Mason S, Davis A, Wood S. Field sensitivity of targeted neonatal hearing screening using the Nottingham ABR screener. Ear Hear 1998; 19: 91–102. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson JT, Jacobson CA, Spahr RC. Automated and conventional ABR screening techniques in high-risk infant. J Am Acad Audiol 1990; 1: 187–195. [PubMed] [Google Scholar]
- 15.Jakubíková J, Kabátová Z, Pavlovcinová G, et al. Newborn hearing screening and strategy for early detection of hearing loss in infants. Int J Pediatr Otorhinolaryngol 2009; 73: 607–612. [DOI] [PubMed] [Google Scholar]
- 16.Xu ZM, Cheng WX, Yang XL. Performance of two hearing screening protocols in NICU in Shanghai. Int J Pediatr Otorhinolaryngol 2011; 75: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 17.Barker MJ, Hughes EK, Wake M. NICU-only versus universal screening for newborn hearing loss: Population audit. J Paediatr Child Health 2013; 49: E74–79. [DOI] [PubMed] [Google Scholar]
- 18.van Straaten HL, Hille ET, Kok JH, Verkerk PH, et al. Implementation of a nation-wide automated auditory brainstem response hearing screening programme in neonatal intensive care units. Acta Paediatr 2003; 92: 332–338. [PubMed] [Google Scholar]
- 19.Gabbard SA, Northern JL, Yoshinaga-Itano C. Hearing screening in newborns under 24 hours of age. Semin Hear 1999; 4: 291–307. [Google Scholar]
- 20.Lupoli Lda M, Garcia L, Anastasio AR, et al. Time after birth in relation to failure rate in newborn hearing screening. Int J Pediatr Otorhinolaryngol 2013; 77: 932–935. [DOI] [PubMed] [Google Scholar]
- 21.Yoshinaga-Itano C. Achieving optimal outcomes from EHDI. The ASHA Leader 2011; 14–17.
- 22.Bush ML, Bianchi K, Lester C, et al. Delays in diagnosis of congenital hearing loss in rural children. J Pediatr 2014; 164: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olusanya BO, Akinyemi OO. Community-based infant hearing screening in a developing country: parental uptake of follow-up services. BMC Public Health 2009; 9: 66–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olusanya BO. Follow-up default in a hospital-based universal newborn hearing screening programme in a low-income country. Child Care Health Dev 2009; 35: 190–198. [DOI] [PubMed] [Google Scholar]
- 25.Scheepers LJ, Swanepoel de W, Roux TI. Why parents refuse newborn hearing screening and default on follow-up rescreening – a South African perspective. Int J Pediatr Otorhinolaryngol 2014; 78: 652–658. [DOI] [PubMed] [Google Scholar]
- 26.Berninger E, Westling B. Outcome of a universal newborn hearing-screening programme based on multiple transient-evoked otoacoustic emissions and clinical brainstem response audiometry. Acta Otolaryngol 2011; 131: 728–739. [DOI] [PubMed] [Google Scholar]
- 27.Guastini L, Mora R, Dellepiane M, et al. Evaluation of an automated auditory brainstem response in a multi-stage infant hearing screening. Eur Arch Otorhinolaryngol 2010; 267: 1199–1205. [DOI] [PubMed] [Google Scholar]