Abstract
Objective
To investigate the efficacy and safety of ondansetron during cesarean section under spinal anesthesia.
Methods
We sought randomized controlled trials (RCTs) on ondansetron during spinal anesthesia for cesarean section in The Cochrane Library, PubMed, MEDLINE, and Web of Science from their inception to September 2016.
Results
Altogether, 21 RCTs were included in this study. Meta-analysis showed that the ondansetron group had a lower incidence of nausea/vomiting and bradycardia than the placebo group during cesarean section under spinal anesthesia [relative risk (RR) = 0.43, 95% confidence interval (CI) (0.36, 0.51) and RR = 0.45, 95% CI (0.26, 0.80), respectively]. There were no significant differences in the incidences of pruritus, hypotension, or shivering during cesarean section under spinal anesthesia [RR = 0.92, 95% CI (0.83, 1.02); RR = 0.72 (0.50, 1.06), 95% CI (0.50, 1.06); and RR = 0.89, 95% CI (0.71, 1.11), respectively].
Conclusion
Ondansetron effectively reduces the incidences of nausea/vomiting and bradycardia under spinal anesthesia during cesarean section.
Keywords: Ondansetron, spinal anesthesia, cesarean section, meta-analysis, RCT, shivering, nausea/vomiting
Introduction
The focus of obstetric anesthesia is to ensure the safety of mother and child. Therefore, it is essential to select the anesthesia and its administration carefully. Spinal anesthesia, because it is a simple medication that has little impact on the fetus, has become a preferred choice for cesarean section. The Apgar score of the fetus under spinal anesthesia for cesarean section was higher than that under general anesthesia.1,2 Although spinal anesthesia is ideal for cesarean section, it also causes adverse reactions. Spinal anesthesia can lead to severe bradycardia or hypotension in puerperae who display unstable hemodynamics.3 Yeh et al.4 found that, because of the special physiological characteristics of obstetrics, the postoperative incidence of pruritus may be as high as 85% in patients who have epidural analgesia with morphine after cesarean section. In addition, according to Teresa and Cartoon,5 the incidence of shivering during cesarean section is as high as 57%. Patient- controlled intravenous analgesia is commonly used after cesarean section. As most analgesic drugs are opioids, however, they often trigger nausea, vomiting, and other adverse puerperal reactions after cesarean section.
Currently, ondansetron is widely used during cesarean section, and numerous related high-quality studies have been published. To date, however, there has been no meta-analysis of ondansetron used during cesarean section under spinal anesthesia. We therefore conducted a meta-analysis to investigate the efficacy and safety of ondansetron during cesarean section under spinal anesthesia.
Materials and methods
Inclusion criteria
- Study design: randomized controlled trials (RCTs), regardless of whether allocation concealment and blinding were used.
- Study subjects: patients given ondansetron during cesarean section under spinal anesthesia.
- Interventions: (1) ondansetron administration (experimental group); (2) administration of a placebo (control group).
- Outcome measures: Main: incidence of nausea/vomiting. Secondary: incidence of pruritus, bradycardia, shivering, hypotension.
Exclusion criterion
Literature had no specific data or full text.
Search strategy
The Cochrane Library, PubMed, MEDLINE, and Web of Science were searched for RCTs studying ondansetron given under spinal anesthesia for cesarean section from the database inception to September 2016. English search terms included “randomized controlled trial,” “controlled clinical trial,” “cesarean section,” “ondansetron,” “epidural,” “spinal,” among others. For example, a specific search strategy in PubMed is described in Box 1.
#1 epidural
#2 subarachnoid space
#3 spinal
#4 ondansetron
#5 cesarean section
#6 randomized controlled trial
#7 #1 OR #2 OR #3
#8 #4 AND #7 AND #5 AND #6
Literature screening, data extraction, and quality evaluation
Two reviewers independently selected the literature, extracted the data, and assessed the quality according to the inclusion and exclusion criteria. When there was a disagreement, it was resolved by further discussion. The contents of the data extraction included the title, author, publication year, study objects and characteristics, sample size, interventions, outcome measures and measurement results, quality evaluation, and other related contents. Jadad scores were performed in terms of the randomization method employed and if there was allocation concealment. Also considered were the presence of blinding, withdrawal, or dropouts.
Statistical analysis
A meta-analysis was performed via using RevMan 5.2 provided by the Cochrane Collaboration. Enumeration data were presented as relative risk (RR) or odds ratio (OR) with a 95% confidence interval (CI). Measurement data were expressed as the mean difference (MD) with a 95% CI. The heterogeneity among included studies was tested by the χ2 test. If homogeneity was found (P > 0.10, I2 < 50%), a fixed-effects model was employed for the meta-analysis. If P < 0.1 and I2 ≥ 50%, we further analyzed the source of heterogeneity. A random-effects model for the meta-analysis was used in the absence of significant clinical heterogeneity, and a subgroup analysis or descriptive analysis was used in the presence of significant clinical heterogeneity.
Results
Literature search results
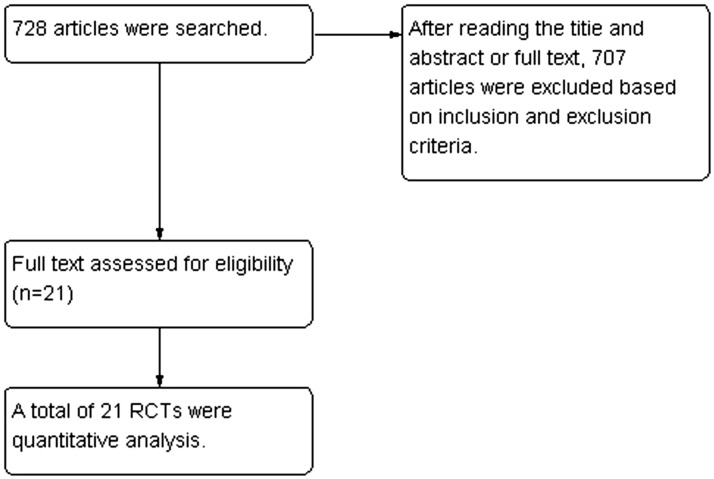
Initially, 728 related articles were detected, with 21 RCTs finally enrolled after step-by-step screening.6–26 The literature screening process and results are shown in Figure 1.
Figure 1.
Flow diagram for the study. RCTs, randomized controlled trials.
For the basic characteristics of the included studies see Table 1. The methodological quality assessment of the included studies is also shown in Table 1 (Jadad score).
Table 1.
Characteristics and Jadad scores of the included studies in the meta-analysis.
| Study | Country | Head count (E/P) | Ondansetron treatment targeta | Jadad score |
|---|---|---|---|---|
| Abouleish 1999 | USA | 74 (36/38) | 2,3 | 6 |
| Browning 2013 | Australia | 116 (56/60) | 4 | 5 |
| Charuluxananan 2003 | Thailand | 120 (60/60) | 1,2 | 5 |
| El-Deeb 2011 | Egypt | 300 (150/150) | 2 | 5 |
| Fattahi 2015 | Iran | 212 (106/106) | 2 | 6 |
| Koju 2015 | Nepal | 50 (25/25) | 2,4 | 5 |
| Marciniak 2015 | Poland | 70 (36/34) | 1,2,5 | 6 |
| Moustafa 2016 | Egypt | 60 (24/28) | 1,2,4 | 6 |
| Ortiz-Gómez 2014 | Spain | 64 (32/32) | 1,2 | 5 |
| Pan 1996 | Virginia | 32 (16/16) | 2 | 5 |
| Pan 2001 | Canada | 105 (54/51) | 2 | 5 |
| Rashad 2013 | Egypt | 40 (20/20) | 2,4,5 | 5 |
| Sahoo 2012 | India | 52 (26/26) | 2,4 | 5 |
| Sarvela 2006 | Finland | 59 (30/29) | 1,2 | 6 |
| Siddik-Sayyid 2007 | Lebanon | 87 (42/45) | 1,2 | 5 |
| Terkawi 2015 | USA | 86 (44/42) | 1,2,3 | 5 |
| Trabelsi 2015 | Tunisia | 80 (40/40) | 2,3,5 | 6 |
| Wang M 2014 | China | 60 (30/30) | 2,3,5 | 5 |
| Wang Q 2014 | China | 65 (33/32) | 2,3,5 | 5 |
| Yazigi 2002 | Lebanon | 100 (50/50) | 1,2 | 5 |
| Yeh 2000 | Taiwan | 40 (20/20) | 1 | 5 |
E/P: intervention group (ondansetron)/placebo (saline) group
1, Pruritus; 2, nausea/vomiting; 3, hypotension; 4, shivering; 5, bradycardia
Meta-analysis results
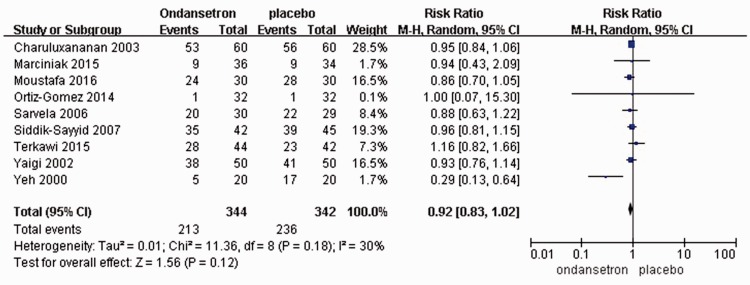
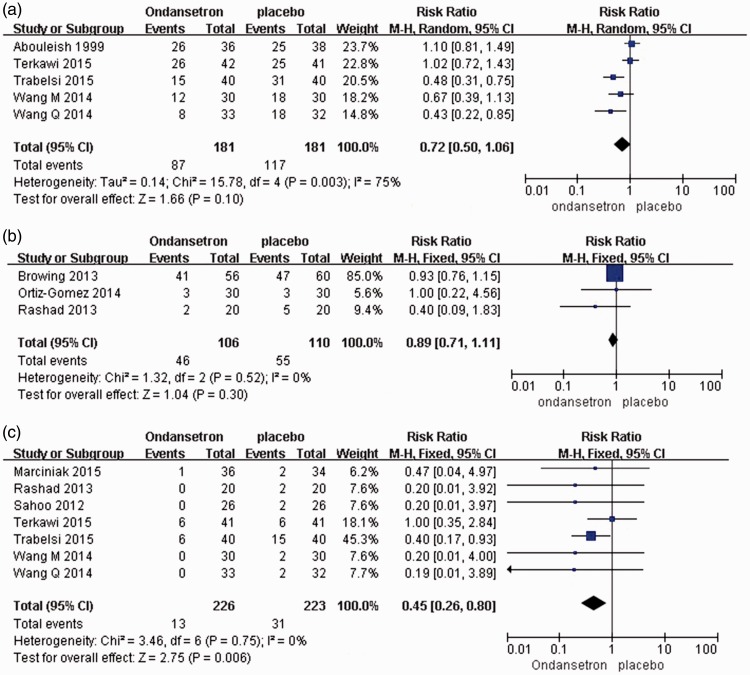
Maternal side effects, including hypotension, nausea/vomiting, and shivering, were compared between the ondansetron and placebo groups. There were no significant differences in the incidences of pruritus, hypotension, or shivering during cesarean section under spinal anesthesia [RR = 0.92, 95% CI ([0.83, 1.02); RR = 0.72, 95% CI (0.50, 1.06); and RR = 0.89, 95% CI (0.71, 1.11), respectively] (Figure 2, Figure 3).
Figure 2.
Studies reporting pruritus with ondansetron administration.
Figure 3.
Studies reporting the incidence of (a) hypotension, (b) shivering, and (c) bradycardia with ondansetron administration.
Nausea/vomiting
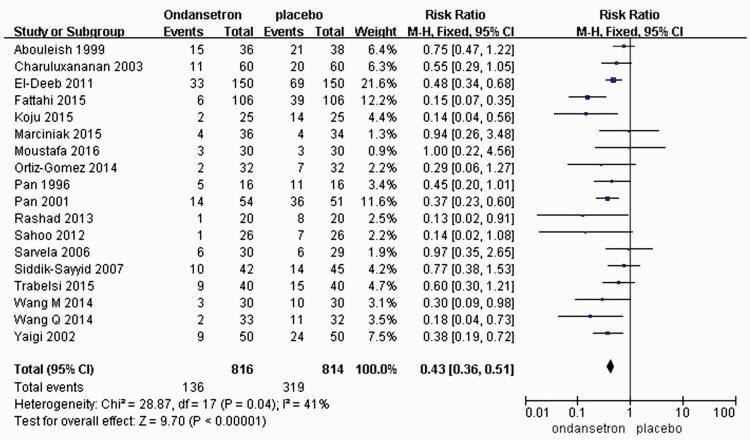
A total of 18 RCTs including 1630 patients were enrolled in this study. The incidences of nausea/vomiting caused by spinal anesthesia during cesarean section were reported. The meta-analysis results of the fixed-effects model showed that the incidence of nausea/vomiting was significantly lower in the ondansetron group than in the placebo group [RR = 0.43, 95% CI (0.36, 0.51), P < 0.00001] (Figure 4).
Figure 4.
Studies reporting nausea and vomiting with ondansetron administration.
Bradycardia
A total of 7 RCTs, with 449 people, were included in the study. Bradycardia triggered by spinal anesthesia during cesarean section was reported. The meta-analysis results of the fixed-effects model showed that the incidence of bradycardia in the ondansetron group was statistically significantly lower than that in the placebo group [RR = 0.45, 95% CI (0.26, 0.80), P = 0.006] (Figure 4(c)).
Discussion
We conducted subgroup analyses on the 21 included studies according to the outcome indicators of the control group. The results showed that the ondansetron group experienced significantly lower incidences of bradycardia and nausea/vomiting than the placebo group under spinal anesthesia during cesarean section. The two groups, however, differed little in their incidences of pruritus, hypotension, or shivering.
Ondansetron is a potent, highly selective serotonin (5-HT3) receptor antagonist. It can prevent the combination of 5-HT released by activated platelets with 5-HT3 receptors in the vagal nerve endings of the left ventricle, attenuate Bezold–Jarisch reflexes produced by left ventricular mechanoreceptors stimulated by 5-HT, inhibit further expansion of peripheral blood vessels, and increase venous return, thereby reducing the incidence of hypotension.27,28
Owczuk et al.29 observed that intravenously injecting 8 mg ondansetron 5 min before spinal anesthesia can curb the reduction of systolic blood pressure without affecting the diastolic blood pressure or heart rate. Sahoo et al.14 reported that intravenous injection of 8 mg ondansetron 5 min before spinal anesthesia can significantly reduce the incidences of hypotension, nausea, and vomiting in puerperae undergoing spinal anesthesia and reduce the use of vasoconstrictor drugs. Ondansetron is structurally similar to 5-HT3 and has a high selectivity of dense region in the 5-HT3 receptor. It can block vomiting reflexes caused by the 5-HT3 receptor-induced vagal stimulation and inhibit 5-HT release in the fourth ventricle caused by vagal excitement, effectively controlling vomiting. Several studies have demonstrated that ondansetron can significantly reduce the incidence of postoperative nausea/vomiting.30
Giving ondansetron to prevent pruritus and shivering is still debatable. A possible mechanism for initiating pruritus is opioid spread via cerebrospinal fluid to the head, where it acts on the medulla oblongata and spinal L receptors or 5- HT3 receptors. Ondansetron, a selective 5-HT3 receptor antagonist, is commonly used to prevent or treat nausea/vomiting after surgery and chemotherapy. Several clinical studies have confirmed8,31 that it can effectively control pruritus due to intrathecal injection of morphine. In the present study, ondansetron did not effectively prevent skin pruritus caused by intrathecal injection of sufentanil, possibly because the ondansetron had not yet reached the location to make the difference. In addition to medulla oblongata and 5-HT3 receptors, opioids have many other ways to produce pruritus. Ondansetron can effectively prevent morphine- or fentanyl-induced skin pruritus. Yazigi et al.32 believed that ondansetron’s antagonism cannot be used effectively as sufentanil has higher fat solubility than the former two drugs, thus acting on the medulla oblongata and spinal cord more rapidly.
Shivering is a common complication of anesthesia. Currently, the mechanism of postoperative shivering is not entirely clear. It may relate to dysfunctional temperature regulation, or it may be associated with the recovery sequence of the nerve center after anesthesia. One study showed that 5-HT secreted by the hypothalamus plays an important role in thermoregulation.33 In animal models, intravenous injection of 5-HT into mice can induce hemangiectasis, causing shivering,34 suggesting that the 5-HT system plays an important role in controlling postoperative shivering. Studies have shown that 5-HT3 antagonists play a part in preventing postoperative shivering, and its mechanism may be associated with inhibition of 5-HT in the preoptic anterior hypothalamus.35
The adverse reactions of ondansetron often present as neurological symptoms (e.g., headache, dizziness) or digestive symptoms (e.g., abdominal discomfort, abnormally elevated alanine aminotransferase), but the overall incidences are relatively low.
There are some limitations of this systematic review. (1) The included studies differ in regard to the patient's position, the anesthesia puncture points, measurement indicators, and use of drugs—each of which could affect the conclusions of this study. (2) The heterogeneity of the included studies is distinct, which may influence the reliability of the meta-analysis. (3) RCTs included in some subgroup analyses were not enough. (4) Some RCTs did not give enough information to judge the scientific rationality of the trial, and there was a possibility of implementation biases and measurement biases. Meanwhile, as we only covered the published literature, the search strategy and publication bias could also affect the results of this study.
In summary, ondansetron can effectively reduce the incidences of nausea, vomiting, and bradycardia during spinal anesthesia for cesarean section, and its safety is relatively good. Because of the small sample size of this study, this conclusion remains to be confirmed by studies with a larger sample size and multi-center studies.
Acknowledgements
The authors are grateful to You-Jing Luo, MD for her extensive support throughout the article development, which substantially improved the quality of the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Solangi SA, Siddiqui SM, Khaskheli MS, et al. Comparison of the effects of general vs. spinal anesthesia on neonatal outcome. Anaesth Intens Care 2012; 16: 18–23. [Google Scholar]
- 2.Kolatat T, Somboonnanonda A, Lertakyamanee J, et al. Effects of general and regional anesthesia on the neonate (a prospective, randomized trial). J Med Assoc Th ailand 1999; 82: 40–45. [PubMed] [Google Scholar]
- 3.Lyons G. Saving mothers, lives: confidential enquiry into maternal and child health: 2003-5. Int J Obstet Anesth 2008; 17: 103–103. [DOI] [PubMed] [Google Scholar]
- 4.Yeh HM, Chen LK, Lin CJ, et al. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg 2000; 9: 172–172. [DOI] [PubMed] [Google Scholar]
- 5.Teresa VG, Cartoon RF. Shivering post epidural anesthesia different doses of intravenous clonine. Can J Anesth 2002; 49: 54–54. [Google Scholar]
- 6.Abouleish EI, Rashid S, Haque S, et al. Ondansetron versus placebo for the control of nausea and vomiting during Caesarean section under spinal anaesthesia. Anaesthesia 1999; 54: 479–482. [DOI] [PubMed] [Google Scholar]
- 7.Browning RM, Fellingham WH, O’Loughlin EJ, et al. Prophylactic ondansetron does not prevent shivering or decrease shivering severity during cesarean delivery under combined spinal epidural anesthesia: a randomized trial. Reg Anesth Pain Med 2013; 38: 39–43. [DOI] [PubMed] [Google Scholar]
- 8.Charuluxananan S, Kyokong O, Somboonviboon W, et al. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg 2003; 96: 1789–1793. [DOI] [PubMed] [Google Scholar]
- 9.El-Deeb AM, Ahmady MS. Effect of acupuncture on nausea and/or vomiting during and after cesarean section in comparison with ondansetron. J Anesth 2011; 25: 698–703. [DOI] [PubMed] [Google Scholar]
- 10.Moustafa AA, Baaror AS, Abdelazim IA. Comparative study between nalbuphine and ondansetron in prevention of intrathecal morphine-induced pruritus in women undergoing cesarean section. Anesth Essays Res 2016; 10: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz-Gomez JR, Palacio-Abizanda FJ, Morillas-Ramirez F, et al. The effect of intravenous ondansetron on maternal haemodynamics during elective caesarean delivery under spinal anaesthesia: a double-blind, randomised, placebo-controlled trial. Int J Obstet Anesth 2014; 23: 138–143. [DOI] [PubMed] [Google Scholar]
- 12.Pan PH, Moore CH. Comparing the efficacy of prophylactic metoclopramide, ondansetron, and placebo in cesarean section patients given epidural anesthesia. J Clin Anesth 2001; 13: 430–435. [DOI] [PubMed] [Google Scholar]
- 13.Pan PH, Moore CH. Intraoperative antiemetic efficacy of prophylactic ondansetron versus droperidol for cesarean section patients under epidural anesthesia. Anesth Analg 1996; 83: 982–986. [DOI] [PubMed] [Google Scholar]
- 14.Sahoo T, Sendasgupta C, Goswami A, et al. Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: a double-blind randomised, placebo-controlled study. Int J Obstet Anesth 2012; 21: 24–28. [DOI] [PubMed] [Google Scholar]
- 15.Sarvela PJ, Halonen PM, Soikkeli AI, et al. Ondansetron and tropisetron do not prevent intraspinal morphine- and fentanyl-induced pruritus in elective cesarean delivery. Acta Anaesthesiol Scand 2006; 50: 239–244. [DOI] [PubMed] [Google Scholar]
- 16.Siddik-Sayyid SM, Aouad MT, Taha SK, et al. Does ondansetron or granisetron prevent subarachnoid morphine-induced pruritus after cesarean delivery? Anesth Analg 2007; 104: 421–424. [DOI] [PubMed] [Google Scholar]
- 17.Terkawi AS, Tiouririne M, Mehta SH, et al. Ondansetron does not attenuate hemodynamic changes in patients undergoing elective cesarean delivery using subarachnoid anesthesia: a double-blind, placebo-controlled, randomized trial. Reg Anesth Pain Med 2015; 40: 344–348. [DOI] [PubMed] [Google Scholar]
- 18.Trabelsi W, Romdhani C, Elaskri H, et al. Effect of Ondansetron on the occurrence of hypotension and on neonatal parameters during spinal anesthesia for elective caesarean section: a prospective, randomized, controlled, double-blind study. Anesthesiol Res Pract 2015; 2015: 158061–158061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Zhuo L, Wang Q, et al. Efficacy of prophylactic intravenous ondansetron on the prevention of hypotension during cesarean delivery: a dose-dependent study. Int J Clin Exp Med 2014; 7: 5210–5216. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Zhuo L, Shen MK, et al. Ondansetron preloading with crystalloid infusion reduces maternal hypotension during cesarean delivery. Am J Perinatol 2014; 31: 913–922. [DOI] [PubMed] [Google Scholar]
- 21.Yazigi A, Chalhoub V, Madi-Jebara S, et al. Prophylactic ondansetron is effective in the treatment of nausea and vomiting but not on pruritus after cesarean delivery with intrathecal sufentanil-morphine. J Clin Anesth 2002; 14: 183–186. [DOI] [PubMed] [Google Scholar]
- 22.Yeh HM, Chen LK, Lin CJ, et al. Prophylactic intravenous ondansetron reduces the incidence of intrathecal morphine-induced pruritus in patients undergoing cesarean delivery. Anesth Analg 2000; 91: 172–175. [DOI] [PubMed] [Google Scholar]
- 23.Fattahi Z, Hadavi SM, Sahmeddini MA. Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. J Anesth 2015; 29: 702–707. [DOI] [PubMed] [Google Scholar]
- 24.Rashad MM, Farmawy MS. Effects of intravenous ondansetron and granisetron on hemodynamic changes and motor and sensory blockade induced by spinal anesthesia in parturients undergoing cesarean section. Eg J Anaesth 2013; 29: 369–374. [Google Scholar]
- 25.Marciniak A, Owczuk R, Wujtewicz M, et al. The influence of intravenous ondansetron on maternal blood haemodynamics after spinal anaesthesia for caesarean section: a double-blind, placebo-controlled study. Ginekol Polska 2015; 86: 461–467. [DOI] [PubMed] [Google Scholar]
- 26.Koju RB, Gurung BS, Dongol Y. Prophylactic administration of ondansetron in prevention of intrathecal morphine-induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. BMC Anesthesiol 2015; 15: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashad MM, Farmawy MS. Effects of intravenous ondansetron and granisetron on hemodynamic changes and motor and sensory blockade induced by spinal anesthesia in parturients undergoing cesarean section. Eg J Anaesth 2013; 29: 369–374. [Google Scholar]
- 28.Martinek RM. Witnessed asystole during spinal anesthesia treated with atropine and ondansetron: a case report. Can J Anaesth 2004; 51: 226–226. [DOI] [PubMed] [Google Scholar]
- 29.Owczuk R, Wenski W, Polak-Krze minska A, et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: a double-blind, placebo-controlled study. Reg Anesth Pain Med 2008; 33: 332–332. [DOI] [PubMed] [Google Scholar]
- 30.Alghanem SM, Massad IM, Rashed EM, et al. Optimization of anesthesia antiemetic measures versus combination therapy usine dexamethasone or ondansetron for the prevention of postoperative nausea and vomiting. Surg Endosc 2010; 24: 353–358. [DOI] [PubMed] [Google Scholar]
- 31.Charuluxananan S, Somboonviboon W, Kyokong O, et al. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. RegAnesth PainMed 2000; 25: 535–539. [DOI] [PubMed] [Google Scholar]
- 32.Yazigi A, Chalhoub V, Madi-Jebara S, et al. Ondansetron for prevention of intrathecal opioids-induced pruritus, nausea and vomiting after cesarean delivery. Anesth Analg 2004; 98: 264–264. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh MT, Chueh FY, Lin MT. Magnolol decreases body temperature by reducing 5-hydroxytryptamine release in the rat hypothalamus. Clin Exp Pharmacol Physiol 1998; 25: 813–817. [DOI] [PubMed] [Google Scholar]
- 34.Dawson NJ, Malcolm JL. Initiation and inhibition of shivering in the rat: interaction between peripheral and central factors. Clin Exp Pharmacol Physiol 1982; 9: 89–93. [DOI] [PubMed] [Google Scholar]
- 35.Alfonsi P. Postanaesthetic shivering epidemiology, pathophysiology and approaches to prevention and management. Drugs 2001; 61: 2193–2205. [DOI] [PubMed] [Google Scholar]