Abstract
Objective
To demonstrate the correlation between nuclear and cytoplasmic G protein-coupled oestrogen receptor (GPR30) expression and clinicopathological features and outcome in patients with ovarian cancer.
Methods
Nuclear and cytoplasmic GPR30 expressions were determined using immunohistochemistry to identify the intracellular location in tissues from patients with ovarian cancer. Data were correlated with clinicopathological characteristics and outcomes.
Results
Tissue samples were obtained from 110 patients with epithelial ovarian cancer between 2005 and 2010. Nuclear GPR30 was significantly more frequent in the group of patients with recurrence. The presence of nuclear GPR30 predicted lower overall survival) and 5-year progression-free survival in all patients with ovarian cancer and overall survival in patients with high grade ovarian cancer. Cytoplasmic GPR30 was observed significantly more often in advanced ovarian cancer and did not predict survival.
Conclusion
This study showed that nuclear GPR30 is an independent negative prognostic indicator in patients with ovarian cancer, especially in those with a high grade malignancy.
Keywords: GPR30, epithelial ovarian cancer, prognosis, overall survival
Introduction
Ovarian epithelial carcinoma, which accounts for most ovarian cancer, is the commonest of all gynaecological malignancies.1–3 Almost all patients are diagnosed at an advanced stage as there are few distinctive symptoms and no reliable screening tests:4 this contributes to the high mortality rate of the disease. To date, only a few tumour biomarkers have been established for high grade ovarian cancer.5 Serum cancer antigen (CA) 125, identified in the 1980s, is known to be an important marker.5,6 However, it is more useful for guiding treatment rather than predicting the prognosis in patients with ovarian cancer.7 The G protein-coupled oestrogen receptor (GPR30) which was identified in 2005,7 has been found in numerous organs8–11 and in primary carcinomas of these organs.12 Although the receptor’s promotion by oestrogen has been extensively studied10,11, the potential signalling pathway of GPR30 is still unclear.
The purpose of this study was to determine the intracellular localization of GPR30 in tissue samples from women with ovarian cancer and elucidate its impact in the disease. To this end, nuclear and cytoplasmic GPR30 expressions were correlated with clinicopathological characteristics and survival outcomes in patients with ovarian cancer.
Methods
Tissue microarrays (TMAs) were created from patients with epithelial ovarian cancer who underwent surgery in the First Affiliated Hospital, Sun Yat-sen University, China between January 2005 and December 2010. Each sample was identified by the admission number of patient. The samples were fixed with formalin and embedded in paraffin. Clinicopathological data were collected before and after surgery and recorded in the patients’ medical records as were five year follow-up data. Data were extracted and recorded by two physicians (C.Z. and G.N.)
All TMAs were subjected to immunohistochemistry. Nuclear GPR30 was evaluated using rabbit polyclonal antibody SP4674P (Acris Antibodies GmbH, Germany) while cytoplasmic GPR30 was evaluated using antibody LS-A4290 (LifeSpan BioSciences, Germany). Positive controls were made using multiorganic arrays including breast tissue known to express GPR30 and negative controls consisted of GPR30-expressing ovarian cancer tissue incubated with a matched isotype antibody.
In brief, all specimens were de-paraffinized in ethanol and subsequently rehydrated in a descending graded series of alcohol concentrations.13 Epitope retrieval was accomplished by pressure cooking the samples in a 0.01 M citric acid buffer (pH, 6.0). Endogenous immunoglobulins in the tissue were blocked by incubating in fresh 3% H2O2 for 20 minutes at room temperature. Following multiple washes, all TMAs were incubated with the GPR30-specific antibodies, LS-A4290 (3.3 g/mL) or SP4674P (4 g/mL) for 60 mins at room temperature. After scanning with the Nano Zoomer digital slide scanner (2.0-RS C10730-13, Hamamatsum, Japan), the immunohistochemical staining analysis was interpreted by two independent pathologists (H.S. and H.C.) who were blinded to the clinical data. Positive GPR30 expression was defined as a staining intensity of grade 2+ or more from a scale that ranged from 0 (negative), 1+ (weak), 2+ (moderate) and 3+ (strong).
Immunofluorescence was used to determine the intracellular localization of GPR30 in the ovarian cancer cell line, SKOV-3 (American Type Culture Collection, Rockville, MD). To block non-specific binding, viable SKOV-3 cells were pre-treated with phosphate buffered saline, 1% bovine serum albumin or 0.1% saponin. The GPR30-specific antibodies, LS-A4290 (10 g/mL), or SP4674P (5 g/mL), were applied and incubated with the cells for 30 min, while isotype control was incubated and diluted to the same final antibody protein concentration.14
After blocking and washing, secondary antibodies, Alexa-488 (2 µg/ml) and 4', 6-diamidino-2-phenylindole (DAPI, 1 µg/ml), were applied. All cells were examined using a confocal laser scanning microscope (CLSM). Any blue fluorescence from the DAPI labelling suggested nuclear binding while green fluorescence from Alexa-488 labelling identified the GPR30 receptor itself.15
Statistical analyses
Statistical analyses were performed using SPSS software (version 22.0 for Windows®; (IBM SPSS, Armonk, NY: IBM Corp, USA). The χ2 and Fisher’s exact tests were used and all statistical analyses were 2-sided. A P-value < 0.05 was considered to indicate statistical significance. Five-year progression free survival and overall survival were calculated using the Kaplan-Meier methods. Uni- and multi-variate analyses were performed using intracellular GPR30 expression and clinicopathological data.
Results
Tissue samples were available from 110 patients and their clinicopathological characteristics are listed in Table 1.
Table 1.
Clinical characteristics of the ovarian cancer patients.
| Characteristic | |
|---|---|
| Patients, n | 110 |
| Age, years | |
| <60 | 41 (37.3) |
| ≥60 | 69 (62.7) |
| FIGO, grade | |
| I/II | 36 (32.7) |
| III/IV | 74 (67.3) |
| Lymph node status | |
| Negative | 59 (53.6) |
| Positive | 51 (46.4) |
| Nuclear grading | |
| Low-grade | 41 (37.3) |
| High-grade | 69 (62.8) |
| Histology | |
| Serous | 76 (69.1) |
| Mucinous | 10 (9.1) |
| Endometrioid | 6 (5.5) |
| Clear cell | 2 (1.8) |
| Others | 16 (14.5) |
| Ascites (ml) | |
| <500 | 33 (30.0) |
| ≥500 | 36 (32.7) |
| None | 38 (34.5) |
| No data | 3 (2.7) |
| Recurrence | |
| Yes | 57 (51.8) |
| No | 53 (48.2) |
Data presented as n (%)
FIGO, International Federation of Gynaecology and Obstetrics staging system (I/II = low/moderate grade tumours, III/IV = high grade/undifferentiated)
GPR30 expression
Nuclear GPR30 expression was observed in 69/110 (62.7%) of patients with ovarian cancer, while cytoplasmic GPR30 was observed in 71/110 (64.5%) of patients. (Table 2). In total, 51/76 (67.1%) of patients with serous adenocarcinoma and 4/10 (40%) of patients with mucinous adenocarcinoma expressed nuclear GPR30. Cytoplasmic GPR30 was detected in 50/76 (65.8%) patients with serous adenocarcinoma and in 4/10 (40.0%) patients with mucinous adenocarcinoma. There were no statistically significant differences in the types of adenocarcinoma (Table 2).
Table 2.
Correlation between intracellular GPR30 expression and clinicopathological characteristics in patients with ovarian cancer (n = 110).
| Variable | Nuclear PR30 positive1 | Nuclear GPR30 negative2 | Statistical significance | Cytoplasmic GPR30 positive3 | Cytoplasmic GPR30 negative4 | Statistical significance |
|---|---|---|---|---|---|---|
| FIGO | ||||||
| I/II | 19 | 17 | ns | 17 | 19 | P = 0.032 |
| III/IV | 50 | 24 | 54 | 20 | ||
| Lymph node status | ||||||
| Negative | 33 | 26 | ns | 31 | 28 | ns |
| Positive | 36 | 15 | 36 | 15 | ||
| Nuclear grading | ||||||
| Low-grade | 25 | 16 | ns | 22 | 19 | ns |
| High-grade | 44 | 25 | 45 | 24 | ||
| Histology | ||||||
| Serous | 51 | 25 | ns | 50 | 26 | ns |
| Mucinous | 4 | 6 | 4 | 6 | ||
| Others | 14 | 10 | 13 | 11 | ||
| Ascites (ml) | ||||||
| <500 | 23 | 10 | ns | 22 | 11 | ns |
| ≥500 | 23 | 13 | 24 | 12 | ||
| None | 21 | 17 | 19 | 20 | ||
| No data | 2 | 1 | 3 | 0 | ||
| Recurrence | ||||||
| Yes | 42 | 15 | P = 0.018 | 38 | 19 | ns |
| No | 27 | 26 | 29 | 24 | ||
Data presented as n
GPR30 staining positive (≥2+) with antibody SP4674P against GPR30 located in nucleus
GPR30 staining negative (<2+) with antibody SP4674P against GPR30 located in nucleus
GPR30 staining positive (≥2+) with antibody LS-A4290 against GPR30 located in cytoplasm
GPR30 staining negative (<2+) with antibody LS-A4290 against GPR30 located in cytoplasm
FIGO, International Federation of Gynaecology and Obstetrics staging system (I/II = low/moderate grade tumours, III/IV = high grade/undifferentiated)
Nuclear GPR30 was detected in 42/67 (62.7%) of patients with tumour recurrence and in 27/53 (50.9%) of patients without recurrence (P = 0.018). There were no other significant correlations between nuclear GPR30 expression and other clinicopathologic characteristics (Table 2).
Cytoplasmic GPR30 was observed more frequently in advanced ovarian cancer with 54/74 (73.0%) patients having International Federation of Gynaecology and Obstetrics (FIGO) grades III/IV and 17/36 (47.2%) of patients having grades I/II (P = 0.032). There were no other significant correlations between cytoplasmic GPR30 expression and other clinicopathologic features (Table 2).
Intracellular localization of GPR30
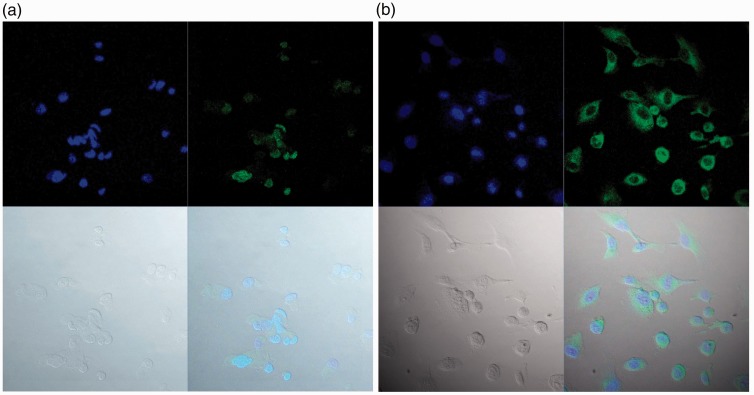
None of the viable SKOV-3 cells, including the negative controls without 0.1% saponin treatment, displayed green fluorescence. After pre-treatment with 0.1% saponin, weak blue fluorescence binding with DAPI was detected in some nuclei and green fluorescence binding with Alxea 488 indicated the presence of GPR30. GPR30 showed a granular pattern in the nucleus when the primary antibody SP4674P was used, whereas GPR30 was detected in the cytoplasm when antibody LS-A4290 was used (Figure 1).
Figure 1.
Intracellular localization of GPR30 in ovarian cancer cell line SKOV-3. (a) Nuclear GPR30 in pre-treated SKOV-3 cells. (b) Cytoplasmic GPR30 in pre-treated SKOV-3 cells.
Each figure is in four parts. Part 1 (top left) shows the blue fluorescence resulting from 4',6-diamidino-2-phenylindole (DAPI) labelling suggesting nuclear binding; Part 2 (top right) shows the green fluorescence resulting from Alexa-488 labelling identifying GPR30 receptors; Part 3 (bottom left) show the SKOV-3 cells without fluorescence; Part 4 (bottom right) shows the integrated picture with DAPI and Alxea-488 fluorescence.
Prognostic impact of GPR30 expression
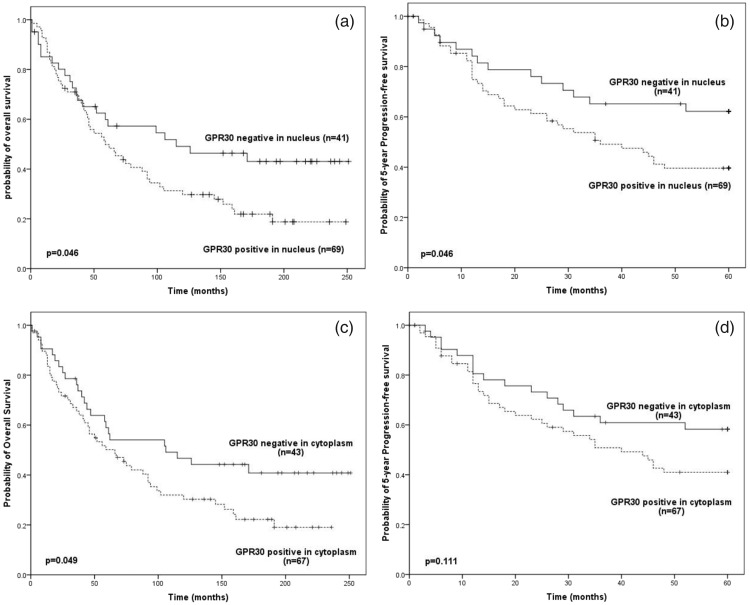
In patients with ovarian cancer, nuclear GPR30 expression predicted both a poorer overall survival (Figure 2a; P = 0.046) and 5-year progression free survival (Figure 2b: P = 0.046). However, cytoplasmic GPR30 expression was only significantly associated with poorer overall survival (Figure 2c P = 0.049) and not 5-year progression free survival (Figure 2d).
Figure 2.
Survival of ovarian-cancer patients by GPR30 status. (a) Overall survival by nuclear GPR30 status. (b) 5-year Progression-free survival by nuclear GPR30 status. (c) Overall survival by cytoplasmic GPR30 status. (d) 5-year Progression-free survival by cytoplasmicGPR30 status.
Uni- and multivariate analysis were performed, with adjustments made for age, FIGO stage and nuclear grading (Table 3). Nuclear GPR30 was an independently poor prognostic factor for overall survival in ovarian cancer patients (P = 0.035). Cytoplasmic GPR30 expression was not predictive of overall survival or 5-year progression free survival. Patient age predicted overall survival (P = 0.001) whereas FIGO stages were unfavourable prognostic factors for both overall survival (P = 0.01) and 5-year progression free survival (P = 0.001).
Table 3.
Uni- and Multi-variate analysis of overall survival and 5-year progression free survival in patients with ovarian cancer (n = 110).
| Overall Survival |
5-year Progression free survival |
|||||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | Statistical significance | Crude HR (95% CI) | Adjusted HR (95% CI) | Statistical significance | |
| FIGO stage (III/IV vs I/II) | 2.52 (1.16–5.49) | 2.63 (1.27–5.46) | P = 0.010 | 10.31 (2.30–40.31) | 11.99 (2.75–52.20) | P = 0.001 |
| Nuclear GPR30 (positive vs negative) | 1.80 (1.06–3.06) | 1.75 (1.04–2.93) | P = 0.035 | 1.83 (0.96–3.46) | – | ns |
| Cytoplasmic GPR30 (positive vs negative) | 1.30 (0.95–6.99) | – | ns | 0.97 (0.54–1.76) | – | ns |
| Nuclear grading (high vs low) | 1.22 (0.69–2.17) | – | ns | 1.26 (0.60–2.66) | – | ns |
| Age (≥60y vs <60y) | 1.02 (1.02–1.04) | 1.03 (1.01–1.05) | P = 0.001 | 1.01 (0.98–1.03) | – | ns |
Hr, Hazard ratio; CI, confidence interval; FIGO, International Federation of Gynaecology and Obstetrics staging system (I/II = low/moderate grade tumours, III/IV = high grade/undifferentiated)
Prognostic impact of GPR30 expression in high grade ovarian cancer
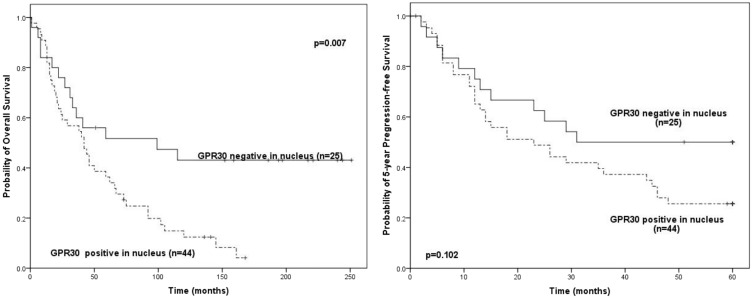
In patients with high grade ovarian cancer (n = 69), those with positive nuclear GPR30 expression (n = 44) had significantly poorer overall survival than those with negative nuclear GPR30 expression (n = 25; P = 0.007) (Figure 3). However, there was no difference between these two groups of patients in 5-year progression free survival. Cytoplasmic GPR30 expression was not significantly correlated with survival in patients with high grade ovarian cancer.
Figure 3.
Survival of patients with high-grade ovarian-cancer by nuclear GPR30 status.
Discussion
To our knowledge, this is the first study to report that nuclear GPR30 expression is a significant, independent negative prognostic factor for overall survival in patients with ovarian cancer, although it does not predict 5-year progression free survival. The study also found that cytoplasmic GPR30 was not predictive of patient outcome in ovarian cancer. Similar results were found in the subgroup of patients with high grade ovarian cancer. Therefore, we hypothesise that GPR30 located in the nucleus, rather than in the cytoplasm, is involved in carcinogenesis. Interestingly, nuclear GPR30 expression was common in patients with recurrence even though it did not predict lower 5-year progression free survival which previously has been shown to be associated with recurrence.14 GPR30 expression was not significantly correlated with any other clinicopathological characteristics which included FIGO stage, nuclear grade status, histology and the presence of ascites.
Previous studies have failed to define the role of GPR30 expression in ovarian cancer, although its expression was widely found in various cancer cell lines.16–19 One study reported that GPR30 expression predicted poor outcome in advanced ovarian cancer, a finding which supports our observations.20 Another study found that there was no correlation between GPR30 mRNA and protein with patients’ clinical parameters.21 In that study, only one-third of the patients had malignant ovarian cancer and the correlation between GPR30 and outcome was confirmed by univariate analysis which excluded several clinical characteristics. By contrast, in this present study, multivariate analysis was used to determine the impact of GPR30 on overall survival and 5-year progression free survival. Our data suggest that nuclear GPR30 is an independent prognostic factor, even after adjustment for other prognostic factors such as FIGO stage, histological grade, and patient age.
We found that GPR30 expression was not associated with 5-year progression free survival. By contrast, another study reported that GPR30 expression played a positive role in two-year progression free survival.22 The authors of that report found that loss of GPR30 expression was observed in advanced ovarian cancer and predicted a worse outcome. Unlike the previous study which spanned two years, we followed our patients over five years, which may have accounted for the difference in results.
The intracellular localization of GPR30 is unclear,23 although GPR30 has been described as having a 7-membrane spanning domain.12 In the present study, GPR30 expression was observed in both the cytoplasm and nuclei of cells from ovarian cancer tissues. In vitro, GPR30 expression was not detected on the membrane of SKOV-3 ovarian cancer cells without pre-treatment but intracellular GPR30 was detected after cell pre-treatment with 0.1% saponin. Saponin form complexes with cholesterol to create pores in the cell membrane bilayers. In addition, the amphipathic nature of saponin provides it with surfactant activity which enhances the penetration of macromolecules through cell membranes.24 Our findings are supported by another study that also found both cytoplasmic and nuclear GPR30 staining in ovarian cancer cells.25
The study had limitations. For instance, only immunohistochemical staining was used and the population was confined to patients with epithelial ovarian cancer. Therefore, other studies are required to elucidate the function and role of GPR30 more fully in ovarian cancer. Two major questions remain unanswered: the mechanism by which GPR30 aggregates in the nucleus and the function of nuclear GPR30.
In conclusion, the study showed that GPR30 expression varied in different histological subtypes of ovarian cancer. In addition, nuclear GPR30 predicted poor overall survival and 5-year progression free survival in patients with ovarian cancer, whereas cytoplasmic GPR30 expression was not associated with outcome. The study also showed that nuclear GPR30 expression was an independent prognostic factor for overall survival but not 5-year progression free survival. Furthermore, nuclear GPR30 significantly predicted lower overall survival and 5-year progression free survival in patients with high grade ovarian cancer.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ovarian cancer. National Cancer Institute. 2014.
- 2.Romero-Laorden N, Olmos D, Fehm T, et al. Circulating and disseminated tumor cells in ovarian cancer: A systematic review. Gynecol Oncol 2014; 133: 632–639. [DOI] [PubMed] [Google Scholar]
- 3.Birkbak NJ, Kochupurakkal B, Izarzugaza JM, et al. Tumor mutation burden forecasts outcome in ovarian cancer with BRCA1 or BRCA2 mutations. PLoS One 2013; 8: e80023–e80023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A.D.A.M. Medical Encyclopedia. Ovarian Epithelial Cancer Treatment (PDQ®) National Cancer Institute. 2012.
- 5.Scholler N, Urban N. CA125 in ovarian cancer. Biomark Med 2007; 1: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez Muñoz A, González Martín A, Mendiola Fernández C. Lights and shadows of the tumoral marker CA-125 in ovarian cancer. Clin Transl Oncol 2008; 10: 449–552. [DOI] [PubMed] [Google Scholar]
- 7.Revankar CM, Cimino DF, Sklar LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005; 307: 1625–1630. [DOI] [PubMed] [Google Scholar]
- 8.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011; 12: 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias-Pulido H, Royce M, Gong Y, et al. GPR30 and estrogen receptor expression: new insights into hormone dependence of inflammatory breast cancer. Breast Cancer Res Treat 2010; 123: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YY, Cai B, Yang YX, et al. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci 2009; 100: 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan QK, Lam HM, Ng CF, et al. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ 2010; 17: 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol 2007; 265–266: 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers J. Overview of Heat-Induced Epitope Retrieval (HIER) Techniques and Devices.2011. http://www.ihcworld.com/epitope_retrieval.htm.
- 14.Smith HO, Arias-Pulido H, Kuo DY, et al. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol 2009; 114: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montalbano M, Curcurù G, Shirafkan A, et al. Modeling of hepatocytes proliferation isolated from proximal and distal zones from human hepatocellular carcinoma lesion. PLoS One 2016; 11: e0153613–e0153613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazell GG, Yao ST, Roper JA, et al. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 2009; 202: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas P, Alyea R, Pang Y, et al. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids 2010; 75: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett LA, Light MM, Mehrotra P, et al. Stimulation of GPR30 increases release of EMMPRIN-containing microvesicles in human uterine epithelial cells. J Clin Endocrinol Metab 2012; 97: 4613–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakraborty P, Roy SK. Expression of estrogen receptor α 36 (ESR36) in the hamster ovary throughout the estrous cycle: effects of gonadotropins. PLoS One 2013; 8: e58291–e58291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith HO, Arias-Pulido H, Kuo DY, et al. GPR30 predicts poor survival for ovarian cancer. Gynecol Oncol 2009; 114: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolkova Z, Casslén V, Henic E, et al. The G protein-coupled estrogen receptor 1 (GPER/GPR30) does not predict survival in patients with ovarian cancer. J Ovarian Res 2012; 5: 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara S, Terai Y, Kawaguchi H, et al. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J Ovarian Res 2012; 5: 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng SB, Quinn JA, Graeber CT, et al. Down-modulation of the G-protein-coupled estrogen receptor, GPER, from the cell surface occurs via a trans-Golgi-proteasome pathway. J Biol Chem 2011; 286: 22441–22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skene CD, Sutton P. Saponin-adjuvanted particulate vaccines for clinical use. Methods 2006; 40: 53–59. [DOI] [PubMed] [Google Scholar]
- 25.Otto C, Rohde-Schulz B, Schwarz G, et al. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 2008; 149: 4846–4856. [DOI] [PubMed] [Google Scholar]