Abstract
Macropinocytosis plays an important role in the internalization of antigens by dendritic cells and is the route of entry for many bacterial pathogens; however, little is known about the molecular mechanisms that regulate the formation or maturation of macropinosomes. Like dendritic cells, Dictyostelium amoebae are active in macropinocytosis, and various proteins have been identified that contribute to this process. As described here, microscopic analysis of null mutants have revealed that the class I phosphoinositide 3-kinases, PIK1 and PIK2, and the downstream effector protein kinase B (PKB/Akt) are important in regulating completion of macropinocytosis. Although actin-rich membrane protrusions form in these cell lines, they recede without forming macropinosomes. Imaging of cells expressing green fluorescent protein (GFP) fused to the pleckstrin homology domain (PH) of PKB (GFP-PHPKB) indicates that D3 phosphoinositides are enriched in the forming macropinocytic cup and remain associated with newly formed macropinosomes for <1 minute. A fusion protein, consisting of GFP fused to an F-actin binding domain, overlaps with GFP-PHPKB in the timing of association with forming macropinosomes. Although macropinocytosis is reduced in cells expressing dominant negative Rab7, microscopic imaging studies reveal that GFP-Rab7 associates only with formed macropinosomes at approximately the time that F-actin and D3 phosphoinositide levels decrease. These results support a model in which F-actin modulating proteins and vesicle trafficking proteins coordinately regulate the formation and maturation of macropinosomes.
INTRODUCTION
Macropinocytosis results in the formation of large endocytic vacuoles containing internalized fluid and was the first pinocytotic process described (Lewis, 1931), but the mechanisms regulating this process remain poorly described. Macropinocytosis is thought to result from the closure and engulfment of fluid in regions of the plasma membrane that have formed ruffles. In contrast to the macropinocytic process, micropinocytic internalization of receptors and fluid, which is the primary pathway for internalization of receptors and extracellular medium in many cells, has been studied extensively and involves the complex interaction of various proteins, including clathrin (Marsh and McMahon, 1999). Recently, on the basis of a number of observations, there has been a renewed interest in understanding the mechanisms regulating macropinocytosis. First, in contrast to micropinocytosis, macropinocytosis relies critically on the spatial and temporal regulation of plasma membrane–actin cytoskeleton interactions and membrane trafficking. Comparable interactions may account for directed cell movement (Bretscher and Aguado-Velasco, 1998), and therefore a better understanding of macropinocytosis may aid in the definition of the molecular players regulating chemotaxis. Second, macropinocytosis probably accounts for a significant amount of internalization of extracellular antigens by professional antigen-presenting cells like dendritic cells (Sallusto et al., 1995); the internalized antigen can then be presented by both class I and class II major histocompatibility complex proteins (Norbury et al., 1995). Understanding the mechanisms regulating macropinocytosis in dendritic cells will be important, because these cells play a critically important role in the immune response, and a foundation of knowledge concerning internalization of antigens will aid clinical trials that are underway to determine whether dendritic cells will be useful in immunological approaches in the treatment of malignant cancers. Finally, certain medically important intracellular pathogens, such as Salmonella, Shigella, Neisseria, Hemophilus, and perhaps Chlamydia, use or actually stimulate the macropinocytic pathway for entry into cells (Francis et al., 1993; Alpuche-Aranda et al., 1994; Ojcius et al., 1998; Zenni et al., 2000).
Although macropinocytosis seems to be a constitutive process in dendritic cells and Dictyostelium (see below) and Ras and Src transformed cells (Bar-Sagi and Feramisco, 1986; Veithen et al., 1996), in most other cells it is a transient response to growth factors or phorbol esters (Swanson, 1989; Racoosin and Swanson, 1992). For both conditions, only a few proteins, including Pak1, Rac1, and Cdc42, have been identified that seem to play an important role in this process (Garrett et al., 2000; West et al., 2000), and it is not yet clear whether the mechanisms regulating constitutive and regulated macropinocytosis are identical. One of the proteins that seems to be important in regulating macropinocytosis in transformed cells and macrophages is the enzyme phosphoinositide (PI) 3-kinase belonging to the class IA group (Araki et al., 1996). This enzyme consists of a catalytic subunit, the P110 protein, and a regulatory subunit, the P85 protein, that are recruited to the cytoplasmic tails of growth factor receptors, after the binding of the appropriate ligand (Vanhaesebroeck and Waterfield, 1999). Although it seems that PI 3-kinase is not important in receptor-mediated internalization, this protein does seem to regulate a late step in the macropinocytic process, namely the closure of ruffles to form macropinosomes (Araki et al., 1996). Cells treated with inhibitors of PI 3-kinases continued to form ruffles at the cell surface, but these lamellipodia usually were reabsorbed back into the cell without forming a macropinosome. Recent studies have implicated Rho family proteins, including Rac1 and Cdc42, in the regulation of macropinocytosis (Garrett et al., 2000; West et al., 2000). A role for Rho family proteins in the regulation of macropinocytosis is not unexpected because these proteins play an important role in the regulation of F-actin polymerization, a process critically important in macropinocytosis. Finally, it has been proposed that the ruffles that form to generate macropinosomes occur as a result of recruitment of membrane from internal sources (Bretscher and Aguado-Velasco, 1998). This hypothesis has been substantiated for the process of phagocytosis from studies demonstrating a role for Rab11 (Cox et al., 2000) and a SNARE protein (Hackam et al., 1998), both important in membrane trafficking, in the engulfment of particles.
Newly formed macropinosomes undergo a cell-type–dependent maturation and fission/fusion process. For instance, in macrophages, large macropinosomes shrink by water loss and sequentially merge with different endosomal/lysosomal compartments (Swanson and Watts, 1995); macropinosomes receive transferrin, rab7, and then Igp-A during the maturation process, eventually merging with lysosomes. In contrast, in nonmacrophage cell lines macropinosomes do not fuse with endo-lysosomes (Hewlett et al., 1994).
In spite of the renewed interest in understanding the mechanisms regulating macropinocytosis and macropinosomal maturation, a great deal remains to be learned. We have chosen the genetically tractable organism Dictyostelium discoideum to explore the molecular mechanisms regulating macropinocytosis (reviewed in Cardelli, 2001; Maniak, 2001). This free-living amoeba shares many properties with professional phagocytes, including robust rates of macropinocytosis and phagocytosis, rapid delivery of endo-lysosomal proteins, and chemotactic motility (reviewed in Maniak, 2001). Furthermore, it is probable that macropinocytosis accounts for the majority of fluid internalized into these cells (Hacker et al., 1997). Also, macropinocytosis seems to be an inducible process, because cells capable of growth under axenic conditions acquire the ability to macropinocytosis 4–6 h after removal of the alternative bacterial food source (A. Rupper and J. Cardelli, unpublished results). Genetic and biochemical studies have identified a number of proteins that seem to play an important role in regulating macropinocytosis. Included in this group of proteins are actin (Hacker et al., 1997), the ATPase proton pump (Temesvari et al., 1996), RasS (Chubb et al., 2000), myosin I (Jung et al., 1996; Titus, 2000), Daip1 (Konzok et al., 1999), coronin (Hacker et al., 1997), profilin (Temesvari et al., 2000), LmpA (a lysosome associated protein) (Temesvari et al., 2000), and Scar (a protein belonging to the WASp family of proteins) (Seastone et al. 2001).
A large number of important questions remain to be answered regarding the process of macropinocytosis. Some of these questions include the following: 1) in what order do proteins involved in macropinocytosis interact to generate the ruffle and form the cup necessary for macropinocytosis, and 2) how are these proteins recruited to the plasma membrane? One likely candidate for recruitment of proteins to the forming macropinocytic cup are the phosphoinositides, which have been demonstrated to regulate changes in the actin cytoskeleton and to regulate membrane trafficking in various cells (Corvera et al., 1999). The Dictyostelium PI 3-kinases, PIK1 and PIK2, are necessary for efficient pinocytosis of fluid, regulation of the actin cytoskeleton, and movement of internalized fluid and membrane along the endosomal pathway (Buczynski et al., 1997b; Zhou et al., 1998). Although not tested directly, the conclusion was reached that PI 3-kinases regulated macropinocytosis, accounting for the reduction in pinocytosis rates. A number of questions remain to be answered regarding the role of PI 3-kinases including the following: 1) does PI 3-kinase regulate macropinocytosis and does it produce 3′-phosphoinositides in the forming macropinocytic cup; 2) is PI 3-kinase activity important in the initial recruitment or formation of F-actin around a forming cup; 3) are known downstream effectors of PI 3-kinase such as PKB/Akt recruited to the cup to play a role in regulating macropinocytosis, and 4) do regulators of membrane traffic responsive to PI 3-kinase activity, such as Rab GTPases (Barbieri et al., 1998), play a role in the regulation of macropinocytosis in Dictyostelium? The experiments discussed in this paper are directed toward answering these questions.
MATERIALS AND METHODS
Cells and Culture Conditions
D. discoideum, strain Ax4, was grown axenically at 18°C in HL5 growth medium (1% oxoid proteose peptone, 1% glucose, 0.5% yeast extract, 2.4 mM Na2HPO4, and 8.8 mM KH2PO4, pH 6.5) either in shaking suspension or in tissue culture flasks. Construction of the mutant strain Δpik1/pik2 was described by Zhou et al. (1995). Construction of the mutant cell line pkbA null was described by Meili et al. (1999). Construction of the GFP-actin-binding domain (ABD)–expressing cell line was described in Pang et al. (1998). Construction of the Ax4 strain overexpressing dominant negative (DN) Rab7 was described in a previous publication (Buczynski et al., 1997a). In brief, site-directed mutagenesis was performed to generate a Thr 22 to Asn mutation in the Rab7 cDNA. The resulting cDNA was cloned into the pDA80-HA vector behind and in-frame with the flu hemagglutinin epitope, transformed into Dictyostelium, and G418-resistant colonies were selected and screened for overexpression by Western blot analysis. A cell line expressing green fluorescent protein (GFP) fused in frame to the N-terminus of Rab7 was generated as follows. A cDNA encoding GFP with the after mutations, F64L and S65T, was cloned into the KpnI site of pVEIIΔATG (Rebstein et al., 1993). The full-length cDNA encoding wild-type Rab7 was cloned into the SacI site of the resulting plasmid. The resulting plasmid was purified with the use of Qiafilter Maxi Prep columns (Qiagen Inc., Valencia, CA) and was transformed into D. discoideum, Ax3 strain, by the calcium phosphate precipitation method (Sadeghi et al., 1988). Geneticin (Sigma, St. Louis, MO)-resistant colonies were screened for expression of GFP fluorescence by observation with a fluorescent microscope.
Pinocytosis and Macropinocytosis Assays
Fluid-phase pinocytosis assays were performed as described (Aubry et al., 1993). Macropinocytosis was assayed as follows: 1 × 106 control or mutant cells were allowed to attach to glass coverslips for 10 min (drug-treated cells were preincubated with drug for 15 min after attachment), and then the cell medium was replaced with HL5 containing 2 mg/ml rhodamine isothiocyanate (Mr 70 kDa; RITC-dextran; Sigma). The cells were incubated for 5 min and then fixed with 1% formaldehyde in HL5. In some experiments, cells were incubated with the PI 3-kinase inhibitors wortmannin (200 nM final concentration; Calbiochem, La Jolla, CA) or LY294002 (20 μM final concentration; Calbiochem) for 15 min before the assay was performed. These fixation conditions did not result in leakage of the fluorescent dye from intracellular vacuoles. Coverslips were mounted to slides, and photographs were taken with an Olympus BX50 fluorescence microscope with the use of Kodak T-MAX 400 speed film (Kodak, Rochester, NY) for black and white prints.
Confocal Microscopy
To visualize macropinocytosis in various cell lines expressing GFP fusion proteins, 1 × 106 of the cells of interest were allowed to attach to glass coverslips and mounted in a stainless steel chamber with fresh HL5. In the cases in which Texas Red dextran (TR-dextran) (70,000 Mr; Molecular Probes, Eugene, OR) was used as a fluorescent fluid-phase marker, HL5 with 2 mg/ml Texas Red dextran was pipetted into the chamber. In the case of drug treatment, cells were pretreated with drug for 15 min before the beginning of the experiment. A cell or a field of cells was brought into focus, and images were captured immediately with a Bio-Rad MRC-1000 confocal microscope (Bio-Rad, Richmond, CA) with the use of a 60× oil objective. Laser lines of 488 and 568 nm of the krypton/argon laser were used at 3% laser power to simultaneously excite FITC and Texas Red, respectively. FITC fluorescence was imaged after passing through a 522 ± 35 nm filter, and Texas Red fluorescence was imaged after passing through a 605 ± 32 nm filter.
RESULTS
The PI 3-Kinases, PIK1 and PIK2, and PKB/Akt Regulate Macropinocytosis
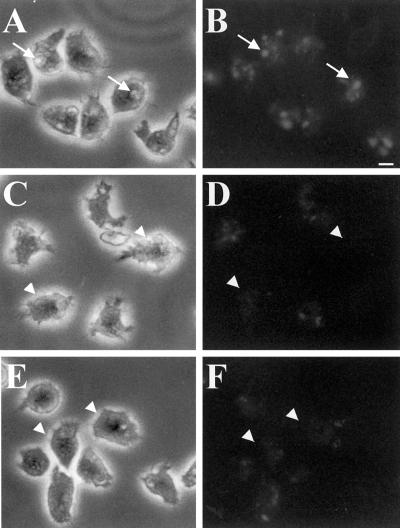
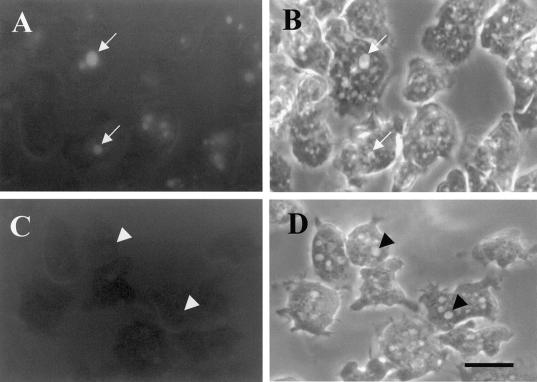
Δpik1/pik2 cells or cells treated with the PI 3-kinase inhibitors LY294002 and wortmannin accumulate fluid at 35% the rate observed for control cells, suggesting that the PI 3-kinases PIK1 and PIK2 play a role in regulating the rate of endocytosis (Buczynski et al., 1997b). Most of the internalization of fluid in Dictyostelium is thought to occur by macropinocytosis (Hacker et al., 1997), although micropinocytosis also plays a lesser role (Ruscetti et al., 1994). To determine whether PI 3-kinases also played a major role in regulating macropinocytosis, control cells, Δpik1/pik2 cells, and control cells treated with the PI 3-kinase inhibitor LY294002 were allowed to attach to coverslips for 10 min followed by the addition of growth medium containing RITC-dextran. After a 5-min exposure, cells were washed quickly with growth medium, fixed in 1% formaldehyde in growth medium, and examined with the use of a fluorescence microscope. Control cells contained on average one to three large fluorescent macropinosomes at least 1 μm in diameter (Figure 1, A and B), whereas in contrast, after the pulse period mutant cells (Figure 1, C and D) and drug-treated cells (Figure 1, E and F) contained essentially no large vacuoles. Instead the fluorescence appeared in much smaller and more diffuse vesicles and was greatly attenuated as compared with control cells. We conclude that the PI 3-kinases PIK1 and PIK2 play an important role in the process of macropinocytosis.
Figure 1.
PI 3-kinases regulate macropinocytosis. Wild-type cells (A and B), Δpik1/pik2 mutants (C and D), and cells treated with 20 μM LY294002 for 20 min (E and F) were bathed in growth medium containing RITC-dextran (2 mg/ml) for 5 min. A, C, and E are phase contrast, whereas B, D, and F are fluorescence images. The coverslips with the attached cells were washed and quickly fixed in 1% formaldehyde. Cells were viewed with the use of a fluorescence microscope, and images were captured with the use of T-MAX black and white 400 speed film. Exposure times were identical for all cells. Arrows in A and B denote cells with macropinosomes. Arrowheads in C–F denote cells without macropinosomes. Bar, 5 μm.
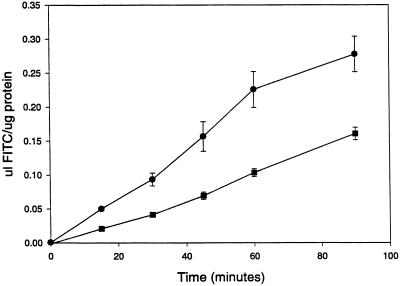
PKB/Akt has been demonstrated to act as a downstream effector for PI 3-kinases in mammalian cells (Coffer et al., 1998) and for PIK1 and PIK2 in Dictyostelium (Meili et al., 1999). PKB/Akt has been demonstrated to regulate a plethora of protein targets that are involved in regulating apoptosis, protein synthesis, vesicle trafficking, and chemotaxis. To determine whether PKB also played a role in regulating fluid-phase internalization, control and Δpkb cells were incubated with FITC-dextran in growth medium. At the times indicated in Figure 2, cells were harvested by centrifugation, washed in growth medium, and lysed in detergent. Control cells internalized FITC-dextran, measured with the use of a spectrofluorimeter, at a linear rate for ∼60 min, whereas Δpkb cells also internalized fluid for 60 min at a linear rate, but at ∼35–40% the rate of control cells (Figure 2). This value is similar to the published decrease in rate of internalization of fluid observed for Δpik1/pik2 cells and is consistent with PKB acting downstream of PI 3-kinases to regulate endocytosis.
Figure 2.
PKB regulates internalization of fluid. Cells in growth medium were incubated with 2 mg/ml FITC-dextran and at the indicated times samples were removed, washed, and resuspended in buffered detergent. FITC-dextran internalization was measured with the use of a spectrofluorimeter, and the values were converted into microliters internalized per micrograms of protein to correct for differences in the size of cells. Closed circles represent control data points; closed squares represent data points from pkb null cells. Data points are mean ± SEM; n = 3.
To determine whether macropinocytosis was defective in cells lacking PKB, cells attached to coverslips were bathed for 5 min with RITC-dextran in HL5, washed, fixed in formaldehyde, and viewed with the use of a fluorescence microscope. As observed for cells with reductions in PI 3-kinase levels, the Δpkb mutant cells (Figure 3) were almost devoid of newly formed large fluorescent macropinosomes, after a pulse, as compared with control cells. We conclude that PKB along with the PI 3-kinases PIK1 and PIK2 play an essential role in the process of macropinocytosis.
Figure 3.
PKB regulates macropinocytosis. Wild-type cells (A and B) and pkb null cells (C and D) were processed as described in the legend to Figure 1. A and C are fluorescence images; B and D are phase-contrast images of the same cells. Cells were viewed with the use of a fluorescence microscope, and images were captured as described in the legend to Figure 1. Arrows in A and B denote cells with macropinosomes, and arrowheads in C and D denote cells without macropinosomes. Exposure times for all images were identical. Bar, 10 μm.
D3-Modified Phosphoinositides Accumulate on Newly Forming Macropinosomes
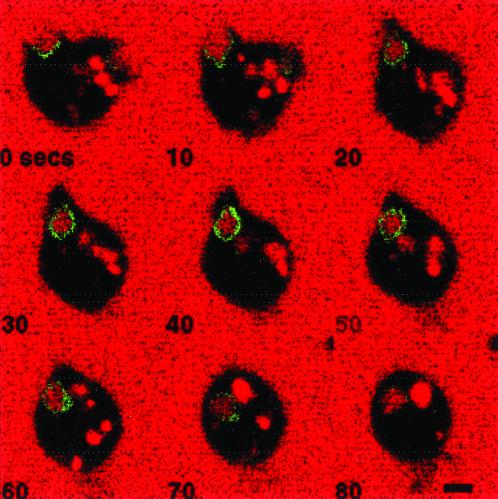
PI 3-kinase could act directly to regulate macropinocytosis by acting on the forming macropinosomal cup region to produce D3 phosphoinositides. These phosphoinositides, which include PI (3,4,5)P3 and PI (3,4)P2, could then act locally to recruit effector proteins to the cytoplasmic side of the plasma membrane that would induce changes in the actin cytoskeleton and in membrane trafficking to facilitate formation and completion of the macropinosome. To test this hypothesis, laser scanning confocal microscopy was used to examine cells undergoing macropinocytosis that were expressing the green fluorescent protein (GFP) fused to the pleckstin homology (PH) domain of PKB (GFP-PHPKB). The cells were bathed in HL5 growth medium, containing TR-dextran as a fluid-phase marker. The PKB-PH domain has been demonstrated to bind specifically to PI (3,4)P2 and PI (3,4,5)P3, (Tanaka et al., 1999), and to translocate to the cell surface in response to chemoattractants (Parent et al., 1998; Meili et al., 1999); therefore this chimeric protein represents an appropriate marker to visualize dynamic changes in the intracellular location of D3 phosphoinositides. Images were electronically captured every 5 s after a cell expressing GFP-PHPKB was identified, and Figure 4 represents a montage of images captured >80 s for a typical cell undergoing the process of macropinocytosis. In the first panel (0 s), it is apparent that GFP-PHPKB is recruited to the entire region of a newly formed pseudopod that is extending membrane into the medium. By 10 s, the macropinosomal cup is fully formed, and by 30 s, the extended membrane has apparently fused to form a macropinosome that contains TR-dextran. GFP-PHPKB remains associated with the forming macropinosome (0–30 s) and the newly formed macropinosome for an additional 40 s (until the image marked 70 s). GFP-PHPKB was also found to transiently associate with pseudopods that extended into the medium but did not result in the formation of macropinosomes.
Figure 4.
GFP-PHPKB is recruited to forming macropinosomal cups and is transiently associated with completed macropinosomes. Cells expressing GFP-PHPKB were allowed to attach to coverslips and then bathed in HL5 containing 2 mg/ml Texas Red dextran. Images were captured of a single cell every 5 s by confocal microscopy as described in MATERIALS AND METHODS. Selected images are shown. Bar, 5 μm.
Pretreatment of cells with LY94002 (final concentration 20 μm) significantly reduced macropinocytosis (Figures 1 and 5) and the presence of GFP-PHPKB in the cell cortex (Figure 5), suggesting that the D3-modified phosphoinositides generated by PIK1 and PIK2 are responsible for recruiting PKB to forming macropinosomes and membrane protrusions.
Figure 5.
GFP-PHPKB is not recruited to the plasma membrane in cells treated with PI 3-kinase inhibitors. Cells expressing GFP-PHPKB (A) were allowed to attach to coverslips, and drug-treated cells (B) were incubated with 20 μM LY294002 for 15 min. The cells were then incubated in the presence of HL5 containing 2 mg/ml Texas Red dextran, and images were captured by confocal microscopy as described in MATERIALS AND METHODS. A representational field is shown. Bar, 10 μm.
F-Actin Is Recruited to Forming Macropinosomal Cups and Transiently Associates with Fully Formed Macropinosomes
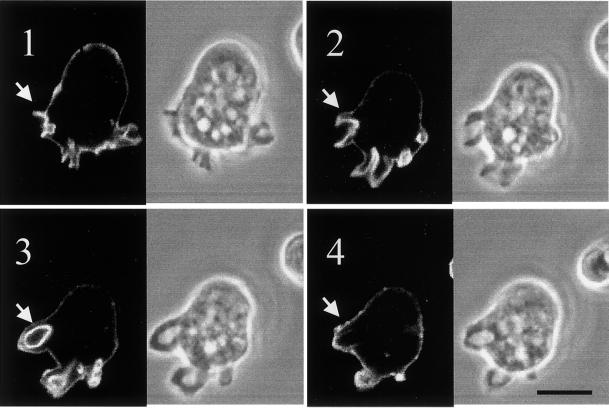
Phosphoinositides, including PI (3,4,5)P3 and PI (4,5)P2, have been demonstrated to affect changes in the actin cytoskeleton (see INTRODUCTION), and these changes could contribute to regulating macropinocytosis. For instance, actin polymerization is required for macropinocytosis, and F-actin–associated proteins are recruited to forming macropinosomal cups and removed from completed macropinosomes with kinetics similar to those observed for PIP3 formation. To examine the kinetics of F-actin association with macropinosomes in axenic wild-type cells, cells expressing GFP-ABD were examined with the use of confocal laser scanning microscopy. GFP-ABD is a fusion between GFP and the F-actin binding domain (ABD) from the actin-associated protein ABP-120. This ABD when fused to GFP has been demonstrated to bind specifically to F-actin and has been used to visualize dynamic changes in the levels and location of F-actin (Pang and Knecht, 1998). Figure 6 represents a montage of images that were captured electronically every 5 s. GFP-ABD is recruited to the forming macropinosomal cup (panels 1 and 2), its rings fully formed macropinosomes at T = 45 s (panel 3), and is below the level of detection at T = 60 s, although the macropinosome remains intact (compare the fluorescent image with the phase contrast image of panel 4). This result suggests that F-actin is actively produced around the complete bottom of the macropinosomal cup and transiently associates with fully formed macropinosomes with kinetics very similar to those observed for the association of GFP-PHPKB.
Figure 6.
GFP-ABD is recruited to macropinosomal cups. Cells expressing GFP-ABD and attached to coverslip images were captured by confocal microscopy as described in MATERIALS AND METHODS. Digital phase-contrast and fluorescence images were collected every 5 s. The montage shown in the figure represents images collected at 15 s (panel 1), 30 s (panel 2), 45 s (panel 3), and 60 s (panel 4). The arrow points to a forming and eventually complete macropinosome. Bar, 15 μm.
Inhibition of PI 3-Kinase Activity Prevents Formation of Macropinosomes but Not the Initial Formation of F-Actin in Membrane Ruffling Regions
It has been reported that PI 3-kinase is not required for the initial formation of circular ruffles that may precede macropinocytosis in mammalian cells but is required for continued growth of the macropinosomal membrane cup necessary to complete the process. To determine whether PI 3-kinases were required for the initial recruitment of F-actin to these active membrane regions, cells expressing GFP-ABD were treated with concentrations of the PI 3-kinase inhibitor LY294002 known to completely inhibit macropinocytosis, and examined by fluorescence microscopy. Figure 7 represents a montage of images captured every 5 s and reveals that drug-treated cells continue to form what seems to be F-actin–rich cup-like regions, which apparently recede into the cytoplasm without forming large macropinosomes. As an example, the arrowhead in panel 6 highlights an actin-rich cup-like structure that began to form 25 s earlier (panel 1) and appeared to recede 20 s later (panel 10). The arrow in panel 9 shows a second cup-like structure that forms and dissociates with roughly comparable kinetics and also does not result in the formation of an actin-ringed macropinosome.
Figure 7.
GFP-ABD is still recruited to the plasma membrane independent of PI 3-kinase activity. Wild-type cells expressing GFP-ABD were incubated in HL-5 medium containing LY294002 at a final concentration of 15 μM. Images were collected by confocal microscopy 10 min after the addition of the drug and represent 10-s intervals. The arrows point to invaginations that began, but the two ends did not fuse to give rise to a macropinosome. Bar, 10 μm.
Rab7 Plays a Role in the Regulation of Macropinocytosis and Associates with Newly Formed Macropinosomes
Expression of Rab7T22N, a DN form of this GTPase, results in various membrane trafficking defects in Dictyostelium, including significant reductions in the rate of phagocytosis and fluid-phase endocytosis (macropinocytosis and micropinocytosis combined) (Buczynski et al., 1997a). To determine whether cells expressing DN Rab7 were defective in macropinocytosis, the microscopic approaches described above were used. Figure 8 indicates that expression of DN Rab7 results in the almost complete abrogation of macropinocytosis, suggesting that the decrease in the rate of internalization of fluid in DN Rab7 expressing cells is primarily the result of a block in macropinocytosis.
Figure 8.
Cells expressing DN Rab7 are defective in macropinocytosis. Macropinocytosis was microscopically analyzed, as described in the legend to Figure 1, for wild-type cells (A and B) and cells overexpressing DN Rab7 (C and D). A and C are fluorescence images; B and D are phase-contrast images of the same cells. Arrows in A and B denote macropinosomes, whereas arrowheads in C and D denote large vacuoles, which are not macropinosomes. Exposure times were the same for all fields analyzed. Bar, 10 μm.
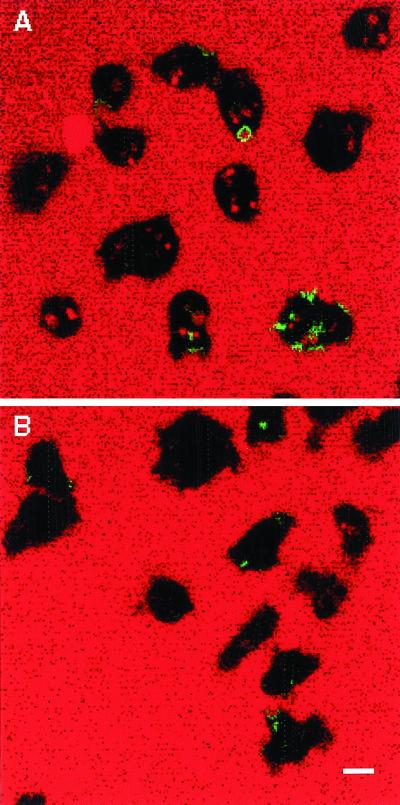
It has been reported recently that growth of the phagocytic and macropinocytic cups depends on recruitment of intracellular membranes, probably derived from endosomal/lysosomal compartments, in a process that depends on Rab GTPases and SNARE proteins (Bajno et al., 2000; Cox et al., 2000). Immunofluorescence microscopy and subcellular fractionation data revealed that Rab7 associated with lysosomes and postlysosomes (a terminal endosomal compartment in Dictyostelium) (Buczynski et al., 1997a). On the basis of the intracellular localization of Rab7 and the possible role it plays in regulating macropinocytosis, we hypothesized that Rab7 may facilitate delivery of membranes to the forming macropinosomal cups. To examine this possibility, a cell line was generated that expresses Rab7 with GFP fused to the N-terminus. Microscopic examination of these cells revealed that GFP-Rab7 seemed to be associated with vesicles varying in diameter from 0.2 to 2 μm (Figure 9A). To determine whether these vesicles were endosomal in nature, cells were incubated in growth medium containing TR-dextran for 10 min, washed, and chased for 1 h in marker-free medium before fixation in formaldehyde. A 1-h chase with a fluorescent fluid-phase marker is sufficient to load lysosomes and postlysosomes, and the fixation condition does not rupture endosomal/lysosomal membranes or quench the GFP-Rab7 signal. As indicated in Figure 9, after the pulse period, cells were filled with fluorescent vesicles (Figure 9B) many of which were ringed with GFP-Rab7 (Figure 9C). The arrow identifies a vesicle in the size range of a lysosome, whereas the arrowhead identifies a larger vesicle that is the size of a postlysosome. This result confirms the earlier report describing immunofluorescence microscopic approaches to localize Rab7 and suggests that we could use GFP-Rab7 as a marker for endosomal/lysosomal membrane trafficking to macropinosomes.
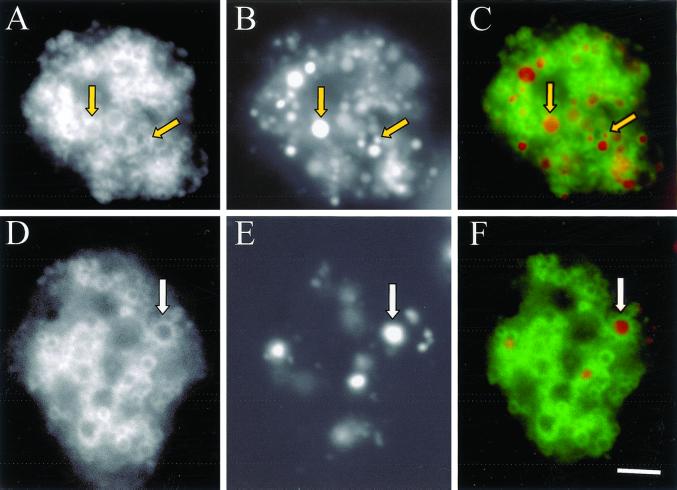
Figure 9.
GFP-Rab7 localizes to macropinosomes, lysosomes, and postlysosomes. Cells expressing GFP-Rab7 were allowed to attach to coverslips and were pulsed with Texas Red dextran for 10 min followed by a 60 min chase in marker-free medium to load lysosomes and postlysosomes (A, GFP; B, Texas Red; C, merge), or cells were pulsed for 5 min with Texas Red dextran to load macropinosomes (D, GFP; E, Texas Red; F, merge). Cells were then fixed with 1% formaldehyde in HL5, and images were captured with the use of a fluorescence microscope equipped with a charge-coupled device camera. Arrows in A, B, and C denote a postlysosome and a lysosome from left to right, and arrows in D, E, and F denote a macropinosome. Bar, 10 μm.
To determine whether Rab7 associates with newly formed macropinosomes, cells expressing GFP-Rab7 were incubated with TR-dextran for 5 min, washed, and gently fixed with formaldehyde. Figure 9 reveals that after a 5-min pulse the cell visualized in E contains multiple macropinosomes that seem to be ringed with GFP-Rab7, a result consistent with Rab7 delivering endosomal/lysosomal membranes to forming macropinosomes. Unfortunately, visualization of forming macropinosomal cups is difficult with the use of the fixation approach just described. Instead, we used the approach described above to visualize GFP-PHPKB and GFP-ABD, namely laser scanning confocal microscopy of cells bathed in TR-dextran.
GFP-Rab7 Is Not Recruited to the Forming Cup but Rapidly Associates with Newly Formed Macropinosomes
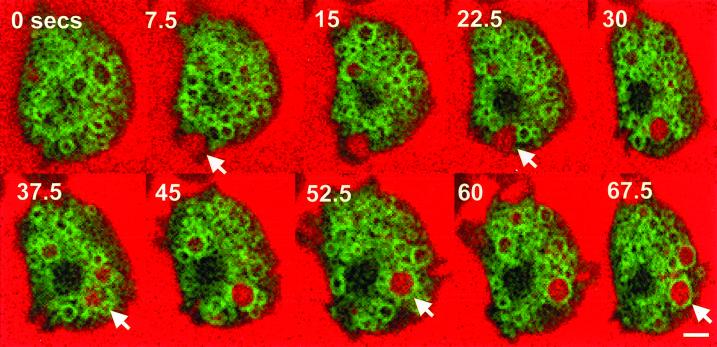
Images of cells expressing GFP-Rab7 were collected every 2.5 s after the addition of TR-dextran to growth medium, and Figure 10 represents a montage of images of a cell undergoing macropinocytosis. In the second panel (7.5 s) a cell is beginning to send membrane in the form of pseudopodia into the medium. This results in the formation of a macropinosomal cup first (0, 7.5, and 15 s), followed by a complete macropinosome containing TR-dextran (22.5 s). In contrast to what was observed for GFP-PHPKB and GFP-ABD proteins, GFP-Rab7 was apparently not recruited to the extending pseudopodia (0, 7.5, and 15 s). By 45 s, it seems that GFP-Rab7 rings a completely formed macropinosome that remains intact to at least the 67.5 s image. Interestingly, GFP-Rab7 seems to associate with macropinosomes at the time when GFP-PHPKB and GFP-ABD seem to dissociate.
Figure 10.
GFP-Rab7 does not localize to forming macropinosomal cups. Cells expressing GFP-Rab7 were allowed to attach to coverslips and bathed in HL5 containing Texas Red dextran. Images were captured of a single cell undergoing macropinocytosis at 2.5-s intervals by confocal microscopy as described in MATERIALS AND METHODS. Arrows denote a large forming macropinosome that becomes ringed with GFP-Rab7. Bar, 5 μm.
DISCUSSION
Macropinocytosis is gaining increasing recognition as an important cellular process involved in various biological phenomena, including antigen presentation by dendritic cells and internalization of intracellular pathogens. The experiments summarized in this paper add to this growing body of knowledge and support the following conclusions: 1) PI 3-kinases and PKB play an important role in regulating formation of macropinosomes; 2) D3-containing phosphoinositides accumulate along the entire extent of the forming macropinosome, and these PI 3-kinase products remain transiently associated with completed macropinosomes; 3) PI 3-kinase activity is not required for the initial accumulation of F-actin and pseudopod extension; 4) F-actin accumulates on membranes of forming and completed macropinosomes with the same kinetics as D3 phosphoinositides; and 5) Rab7 regulates macropinocytosis and associates with newly formed macropinosomes at approximately the time that levels of F-actin and D3 phosphoinositides are declining. Together with results described in previous studies, this report, with the use of GFP-tagged proteins and different null mutants, reveals the tentative order of action of sequentially interacting proteins involved in macropinocytosis regulation.
Previous studies with the use of pharmacological approaches indicated an important role for mammalian PI 3-kinases in regulating macropinocytosis (Araki et al., 1996), a conclusion also supported by experiments described in this paper. This paper further supports this hypothesis by demonstrating that a double null mutant for the enzymes PIK1 and PIK2, both class I PI 3-kinases, was reduced in macropinocytosis to the same extent as control cells treated with two different inhibitors of PI 3-kinases. Both of these kinases have been demonstrated to regulate levels of PIP3 (Zhou et al., 1998), so we conclude that formation of D3-containing phosphoinositides is important in the regulation of macropinocytosis. Previous studies have indicated that inhibition of these kinases does not influence the level of PI (3)P, so the role this phosphoinositide plays in macropinocytosis remains to be determined (Buczynski et al., 1997b).
The requirement for PI 3-kinases in regulating macropinocytosis suggests that the product of PI 3-kinase activity, PI (3,4,5)P3, might accumulate in the forming macropinocytic cup to signal to downstream effectors like PKB. In fact, a hybrid protein consisting of GFP fused to the PH domain of PKB did localize to the entire lateral crowns that progressed into macropinosomes. This PH domain has been demonstrated to bind specifically to D3 phosphoinositides such as PI (3,4,5)P3 and PI (3,4)P2 (Tanaka et al., 1999). Our imaging approach suggested that equivalent levels of D3-modified phosphoinositides (based on binding of GFP-PHPKB) accumulated along the entire length of the forming macropinosomal cup as opposed to only accumulating at the tips of the extending plasma membrane. This result is consistent with a model that proposes that the products of PI 3-kinases need to accumulate in the forming cup to recruit additional effectors like PKB to continue membrane protrusion leading to macropinosome formation.
Our study is not the first to identify phosphoinositides accumulating on forming macropinosomes. It was reported in an earlier publication (Parent et al., 1998) that cystolic regulator of adenyl cyclase-GFP, a PH containing protein, associated with crown-like structures thought to represent forming macropinosomes. It is reasonable to propose that the PH domain of cystolic regulator of adenyl cyclase, like the PH domain of PKB, binds to PI (3,4,5)P3 or PI (3,4)P2.
The enzyme PKB/Akt has been demonstrated in a number of systems, including Dictyostelium, to be a downstream effector of PI 3-kinases (Oishi et al., 2000). We observed that pkb null mutants were significantly defective in the rate of fluid-phase internalization and the formation of macropinosomes, and that a PH domain from PKB accumulated in macropinosomal membranes (see below), a phenotype consistent with the hypothesis that PKB is a downstream effector of PI 3-kinases. Binding of GFP-PHPKB to forming macropinosomal cups suggests that PKB acts in a spatially direct manner to regulate macropinocytosis, perhaps by interacting with and phosphorylating additional downstream effectors. In fact, a number of protein substrates for PKB have been identified, but their relevance to macropinocytosis has not been tested.
It remains to be determined how the activity of PI 3-kinase regulates macropinocytosis. In theory, PI 3-kinase activity could be required to initiate the formation of actin-rich crowns, or alternatively these kinases may only be required to complete the formation of macropinosomes. A previous publication indicated that in mammalian cells PI 3-kinases seemed to be necessary for the completion of macropinosomes but did not play a role in the protrusion of plasma membrane (Araki et al., 1996). Our results support this study and demonstrate further that PIK1 and PIK2 did not seem to play a role in initiation of the formation of actin-rich crowns, but the lack of enzyme activity resulted in the nonproductive collapse of the extending plasma membrane. In drug-treated cells and Δpik1/pik2 mutants, the levels of PIP3 are reduced by >80% (Zhou et al., 1998), and we have found recently that PIK1 and PIK2 are major targets for LY294002 and wortmannin (Rupper et al., 2001). Conceivably the residual PIP3 levels are maintained by another PI 3-kinase such as PIK3; therefore, we can only conclude that F-actin can accumulate at the cortex in membrane protrusions in the absence of PIK1 and PIK2, but polymerization may still be triggered or regulated by PIP3. Alternatively, the F-actin–generated protrusions of the plasma membrane may depend on phosphoinositides like PI (4,5)P2 that could facilitate the initial polymerization of F-actin by binding F-actin–capping proteins. Levels of these phosphoinositides could be higher in cells lacking PIK1 and PIK2.
How might PI 3-kinases and PKB contribute to the completion of macropinocytosis? In addition to altering the dynamics of the actin cytoskeleton, PI 3-kinases and PKB have been implicated in the regulation of membrane trafficking. Stahl and colleagues (Li et al., 1995; Barbieri et al., 1998) have found that PI 3-kinase and PKB activate Rab5, which in turn regulates endocytosis and fusion of early endosomes. Furthermore, membrane exocytosis has been demonstrated to play an important role in forming the phagocytic cup. Expression of dominant negative Rab11 reduces the internalization of bacteria (Cox et al., 2000) and the exocytosis of membrane, possibly from the recycling endosomal compartment. Inactivation of SNARE proteins can also reduce internalization of particles (Hackam et al., 1998). Expression of dominant negative Rab7 reduces phagocytosis and macropinocytosis in Dictyostelium, and we have proposed that Rab7 plays an important role in recycling membrane from late compartments of the endosomal pathway to earlier compartments (Buczynski et al., 1997a). Conceivably Rab7 could play a role in the directed exocytosis of endosomal/lysosomal membranes to the forming macropinosomal cups. Consistent with this model, we observed that GFP-Rab7 accumulated on newly formed macropinosomes; however, video microscopy did not detect GFP-Rab7 on the forming macropinosomal cups. This suggests that Rab7 may regulate macropinocytosis formation more indirectly, perhaps by preventing progression of maturation of newly formed macropinosomes, an event that may be necessary for internalization of new macropinosomes.
Video microscopy revealed that Rab7 seems to associate with macropinosomes at about the time that F-actin, coronin, and PKB (based on GFP-PHPKB binding) disassociate. In addition, immunofluorescence microscopy indicated that the ATPase proton pump colocalized with LmpA in macropinosomes that were newly formed (our unpublished data); both of these proteins have been implicated in the regulation of macropinocytosis (Temesvari et al., 1996, 2000) and may play a more indirect role as envisioned for Rab7. Current experiments are being directed at testing the hypothesis that Rab7 regulates the delivery of the proton pump and LmpA to newly formed macropinosomes. What also remains to be determined is the biochemical nature of the trigger that apparently couples the disassociation of one group of proteins with the association of a second group of proteins. It is interesting to note that LmpA binds to PIP2 (Karakesisoglou et al., 1999), and this phosphoinositide may accumulate at the time that PIP3 levels are declining.
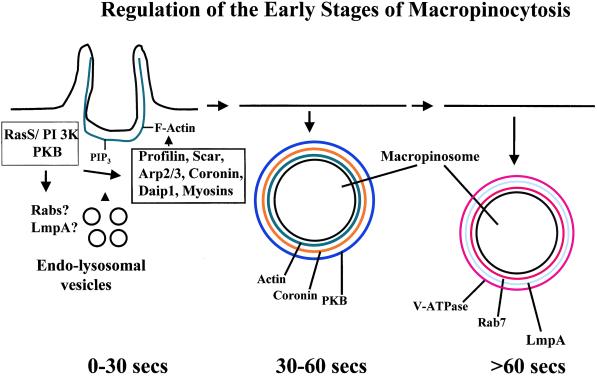
The model displayed in Figure 11 summarizes our current state of knowledge concerning the regulation of macropinocytosis. This paper has begun to address the key roles of PI 3-kinase, PKB/Akt, and Rab7 in macropinosome formation and maturation. Future studies are being directed at defining the molecular interactions that ensure the internalization and maturation of newly formed macropinosomes, and these studies will shed light on mechanisms that regulate macropinocytosis in other professional phagocytes such as macrophages and dendritic cells.
Figure 11.
A model summarizing the proteins involved in macropinosomal formation and early maturation. RasS, PI 3-kinase, and PKB are hypothesized to play an important role in “building” the macropinosomal cup by activating and/or recruiting proteins that regulate membrane trafficking (Rabs and LmpA) and proteins that regulate F-actin formation and stability (profilin, Scar, coronin, etc.). Within 90 s of formation of a macropinosome, one group of proteins is removed (F-actin, coronin, PKB) and replaced by a second group of proteins, including Rab7, LmpA, and the proton pump.
ACKNOWLEDGMENTS
We thank the Firtel laboratory for the plasmid encoding the GFP-PHPKB fusion protein. This research was supported by grant DK 39232 from National Institutes of Health.
REFERENCES
- Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Klein G, Martiel JL, Satre M. Kinetics of endosomal pH evolution in Dictyostelium discoideum amoebae. Study by fluorescence spectroscopy. J Cell Sci. 1993;105:861–866. doi: 10.1242/jcs.105.3.861. [DOI] [PubMed] [Google Scholar]
- Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri MA, Kohn AD, Roth RA, Stahl PD. Protein kinase B/akt and rab5 mediate ras activation of endocytosis. J Biol Chem. 1998;273:19367–19370. doi: 10.1074/jbc.273.31.19367. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr Biol. 1998;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997a;8:1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski G, Grove B, Nomura A, Kleve M, Bush J, Firtel RA, Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J Cell Biol. 1997b;136:1271–1286. doi: 10.1083/jcb.136.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardelli J. Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic. 2001;2:311–320. doi: 10.1034/j.1600-0854.2001.002005311.x. [DOI] [PubMed] [Google Scholar]
- Chubb JR, Wilkins A, Thomas GM, Insall RH. The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J Cell Sci. 2000;113:709–719. doi: 10.1242/jcs.113.4.709. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, D'Arrigo A, Stenmark H. Phosphoinositides in membrane traffic. Curr Opin Cell Biol. 1999;11:460–465. doi: 10.1016/S0955-0674(99)80066-0. [DOI] [PubMed] [Google Scholar]
- Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci USA. 1998;95:11691–11696. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hewlett LJ, Prescott AR, Watts C. The coated pit and macropinocytic pathways serve distinct endosome populations. J Cell Biol. 1994;124:689–703. doi: 10.1083/jcb.124.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Wu X, Hammer JA. Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J Cell Biol. 1996;133:305–323. doi: 10.1083/jcb.133.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I, Janssen KP, Eichinger L, Noegel AA, Schleicher M. Identification of a suppressor of the Dictyostelium profilin-minus phenotype as a CD36/LIMP-II homologue. J Cell Biol. 1999;145:167–181. doi: 10.1083/jcb.145.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzok A, Weber I, Simmeth E, Hacker U, Maniak M, Muller-Taubenberger A. DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J Cell Biol. 1999;146:453–464. doi: 10.1083/jcb.146.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WH. Pinocytosis. Johns Hopkins Hosp Bull. 1931;49:17–27. [Google Scholar]
- Li G, D'Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniak M. Fluid-phase uptake and transit in axenic Dictyostelium cells. Biochim Biophys Acta. 2001;1525:197–204. doi: 10.1016/s0304-4165(01)00105-2. [DOI] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Oishi N, Adachi H, Sutoh K. Novel Dictyostelium unconventional myosin, MyoM, has a putative RhoGEF domain. FEBS Lett. 2000;474:16–22. doi: 10.1016/s0014-5793(00)01564-7. [DOI] [PubMed] [Google Scholar]
- Ojcius DM, Bravo DA, Kanellopoulos JM, Hawkins RA, Kelly KA, Rank RG, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- Pang KM, Lee E, Knecht DA. Use of a fusion protein between GFP and an actin-binding domain to visualize transient filamentous-actin structures. Curr Biol. 1998;8:405–408. doi: 10.1016/s0960-9822(98)70159-9. [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Rebstein PJ, Weeks G, Spiegelman GB. Altered morphology of vegetative amoebae induced by increased expression of the Dictyostelium discoideum ras-related gene rap1. Dev Genet. 1993;14:347–355. doi: 10.1002/dvg.1020140504. [DOI] [PubMed] [Google Scholar]
- Rupper AC, Rodriguez-Paris JM, Grove BD, Cardelli JA. P110-related PI 3-kinases regulate phagosome–phagosome fusion and phagosomal pH through a PKB/Akt-dependent pathway in Dictyostelium. J Cell Sci. 2001;114:1283–1295. doi: 10.1242/jcs.114.7.1283. [DOI] [PubMed] [Google Scholar]
- Ruscetti T, Cardelli JA, Niswonger ML, O'Halloran TJ. Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J Cell Biol. 1994;126:343–352. doi: 10.1083/jcb.126.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi H, da Silva AM, Klein C. Evidence that a glycolipid tail anchors antigen 117 to the plasma membrane of Dictyostelium discoideum cells. Proc Natl Acad Sci USA. 1988;85:5512–5515. doi: 10.1073/pnas.85.15.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seastone, D., Harris, E., Temesvari, L., Bear, J., Saxe, C., and Cardelli, J. (2001). The WASp-like protein Scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J. Cell Sci. (in press). [DOI] [PubMed]
- Swanson JA. Phorbol esters stimulate macropinocytosis and solute flow through macrophages. J Cell Sci. 1989;94:135–142. doi: 10.1242/jcs.94.1.135. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–427. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Adachi H, Konishi H, Iwamatsu A, Ohkawa K, Shirai T, Nagata S, Kikkawa U, Fukui Y. Identification of protein kinase B (PKB) as a phosphatidylinositol 3,4,5-trisphosphate binding protein in Dictyostelium discoideum. Biosci Biotechnol Biochem. 1999;63:368–372. doi: 10.1271/bbb.63.368. [DOI] [PubMed] [Google Scholar]
- Temesvari L, Zhang L, Fodera B, Janssen KP, Schleicher M, Cardelli JA. Inactivation of ImpA, encoding a LIMPII-related endosomal protein, suppresses the internalization and endosomal trafficking defects in profilin-null mutants. Mol Biol Cell. 2000;11:2019–2031. doi: 10.1091/mbc.11.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesvari LA, Rodriguez-Paris JM, Bush JM, Zhang L, Cardelli JA. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. J Cell Sci. 1996;109:1479–1495. doi: 10.1242/jcs.109.6.1479. [DOI] [PubMed] [Google Scholar]
- Titus MA. The role of unconventional myosins in Dictyostelium endocytosis. J Eukaryot Microbiol. 2000;47:191–196. doi: 10.1111/j.1550-7408.2000.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- Veithen A, Cupers P, Baudhuin P, Courtoy PJ. v-Src induces constitutive macropinocytosis in rat fibroblasts. J Cell Sci. 1996;109:2005–2012. doi: 10.1242/jcs.109.8.2005. [DOI] [PubMed] [Google Scholar]
- West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–848. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- Zenni MK, Giardina PC, Harvey HA, Shao J, Ketterer MR, Lubaroff DM, Williams RD, Apicella MA. Macropinocytosis as a mechanism of entry into primary human urethral epithelial cells by Neisseria gonorrhoeae. Infect Immun. 2000;68:1696–1699. doi: 10.1128/iai.68.3.1696-1699.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Pandol S, Bokoch G, Traynor-Kaplan AE. Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J Cell Sci. 1998;111:283–294. doi: 10.1242/jcs.111.2.283. [DOI] [PubMed] [Google Scholar]
- Zhou K, Takegawa K, Emr SD, Firtel RA. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol Cell Biol. 1995;15:5645–5656. doi: 10.1128/mcb.15.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]