Short abstract
Objective
This study was performed to investigate the safety and efficacy of oral paracetamol versus oral ibuprofen in the treatment of patent ductus arteriosus (PDA) in premature infants.
Methods
Premature infants with PDA with a gestational age of ≤32 weeks or birth weight of ≤1500 g were included in this randomized study.
Results
A total of 120 premature infants fulfilled the inclusion criteria. Of these 120 infants, 34 fulfilled the treatment criteria and 22 were finally randomized. We found no significant difference in the mortality or primary closure rates between the two groups. We also found no significant difference in the short-term neonatal outcomes.
Conclusions
Either oral paracetamol or oral ibuprofen can be used safely and effectively to treat PDA in premature infants.
Keywords: Premature infants, patent ductus arteriosus, ibuprofen, paracetamol, randomized study, safety
Introduction
Hemodynamically significant patent ductus arteriosus (hsPDA) is a major risk factor for mortality and morbidity in very-low-birth-weight infants.1 The standard of care is to treat all cases of hsPDA. The methods of treatment, which medications to use, and treatment timing continue to be subjects of research.2 Intravenous indomethacin and intravenous ibuprofen are both widely used for PDA treatment.3,4 In Jordan, however, as in many other low-resource countries, these two medications are not available. Studies from these countries have investigated oral preparations of paracetamol5–8 and oral and rectal forms of ibuprofen.9–13
Many studies have shown that oral ibuprofen is both safe and effective in treating PDA.10–13 Furthermore, recent reports on the use of oral paracetamol are promising.5–8 Paracetamol inhibits prostaglandin synthetase activity by acting at the peroxidase segment of the enzyme.14 Peroxidase is activated at a 10-fold lower concentration of peroxide than cyclooxygenase. The peroxide concentration is decreased in certain neonatal morbidities that are accompanied by hypoxemia. Hypothetically, under these conditions paracetamol should be a more effective drug than cyclooxygenase inhibitors.15 Paracetamol is also the only option when a patient has a contraindication for ibuprofen use.16 Oral paracetamol is a safe and readily available medication that is much less expensive than the intravenous preparation.
Few studies have compared the safety and efficacy of oral preparations of ibuprofen and paracetamol in treating PDA.17–19 To help fill this knowledge gap, we conducted the present study to evaluate the incidence of PDA in our population and compare oral ibuprofen and oral paracetamol for the treatment of PDA in premature infants.
Materials and methods
This randomized parallel study was conducted in the Neonatal Intensive Care Unit of Jordan University Hospital, Amman, Jordan, from March 2015 to October 2016. The study was approved and funded by the Deanship of Scientific Research at the University of Jordan and is registered in the ISRCTN registry under the number ISRCTN12302923 DOI 10.1186. All procedures performed in this study were in accordance with the ethical standards of and granted ethical approval by the institutional review board of Jordan University Hospital (reference number 108/2014/IRBJ). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Patients’ characteristics
All premature infants with a gestational age of ≤32 weeks or birth weight of ≤1500 g were included. The exclusion criteria were as follows: ductal-dependent congenital heart diseases, major congenital malformation, grade 3 to 4 intraventricular hemorrhage, renal impairment (defined as a creatinine concentration of >1.5 mg/dl), pulmonary hemorrhage, thrombocytopenia of <60,000/mm3, and an elevated alanine transaminase concentration. Echocardiography was performed for all included infants from 3 to 5 days of age or when they showed symptoms of PDA, whichever occurred earlier. PDA was considered hemodynamically significant if two of the following criteria were present: wide pulse pressure (defined as systolic blood pressure − diastolic blood pressure of >½ systolic blood pressure), reversal of flow in the descending aorta by Doppler echocardiography, left atrial dilatation determined by a left atrial:aortic root ratio of >1.4:1.0 on M-mode echocardiographic evaluation from the parasternal view, or left ventricular dilatation. We did not include the size of the ductus as a criterion because it is relative to the size of the infant. Symptoms of PDA were metabolic acidosis, increased respiratory demands not explained by respiratory distress syndrome or its complications (usually after a period of improvement), decreased urine output, delayed capillary refill, and newly onset persistent mottling.20,21 To fulfill the treatment criteria, infants were required to either demonstrate hsPDA by echocardiography or signs indicating the presence of symptomatic PDA. The parents of newborns who fulfilled these criteria were subsequently approached for informed consent. Informed consent was obtained from the parents of all individual participants included in the study.
Randomization and treatment protocol
The qualifying preterm infants were randomized by computer to receive either oral paracetamol or oral ibuprofen. Randomization numbers were placed inside sequentially numbered opaque envelopes. The following laboratory investigations were conducted within 24 hours before treatment was initiated and within 24 hours after treatment was finished: complete blood count, platelet count, creatinine, and alanine transaminase. A head ultrasound was also performed before and after treatment. The oral paracetamol group received 10 mg/kg/dose followed by 1 to 2 ml of 0.9% saline every 6 hours for 3 days. Regarding ibuprofen treatment protocol, previous studies used a loading dose of 10 mg/kg17,18 or 20 mg/kg19 on the first day and then half of this dose for the first and second days of treatment. We used the same dose for the 3-day course to minimize errors. The oral ibuprofen group received 10 mg/kg/dose followed by 1 to 2 ml of 0.9% saline once daily for 3 days. Echocardiography was repeated within 24 hours of the last treatment.
Response to treatment was defined as resolution of symptoms with either complete closure of the PDA or a very small hemodynamically insignificant PDA evident by echocardiography. In these cases, echocardiography was repeated before discharge. If no response was found after the first course of treatment, a second course of treatment with the same drug was given for another 3 days. If no response as seen after two courses, a third course of treatment was started using the other drug. If three courses of medical treatment failed, surgery was performed only if the PDA was causing ventilation difficulties. Primary closure was defined as response to treatment after a 3-day course of the assigned drug. Secondary closure was defined as response to treatment after two courses or a total of 6 days of treatment with the assigned drug. Tertiary closure was defined as response to treatment after switching to the other drug in the trial after failure of two courses of treatment.
During the study period, all infants received the same fluid and enteral feeding protocol. They all started at 80 ml/kg/day, which was increased daily in increments of 20 ml/kg/day. If the PDA required treatment, fluid administration was kept at a maximum of 120 ml/kg/day until the end of the treatment. Feeding was started on day 1 or 2 at 20 ml/kg/day according to the availability of breast milk. It was increased to 20 ml/kg/day, but was not progressively increased during the treatment period.
Statistical analysis
SPSS version 21 (IBM Corp., Armonk, NY, USA) was used to conduct the statistical analyses. Numerical data are represented by mean ± standard deviation. Categorical data are represented by their respective rates or proportions. A t-test was used to compare means, and the chi-square test was used to compare proportions. P values of <0.05 were considered statistically significant.
Results
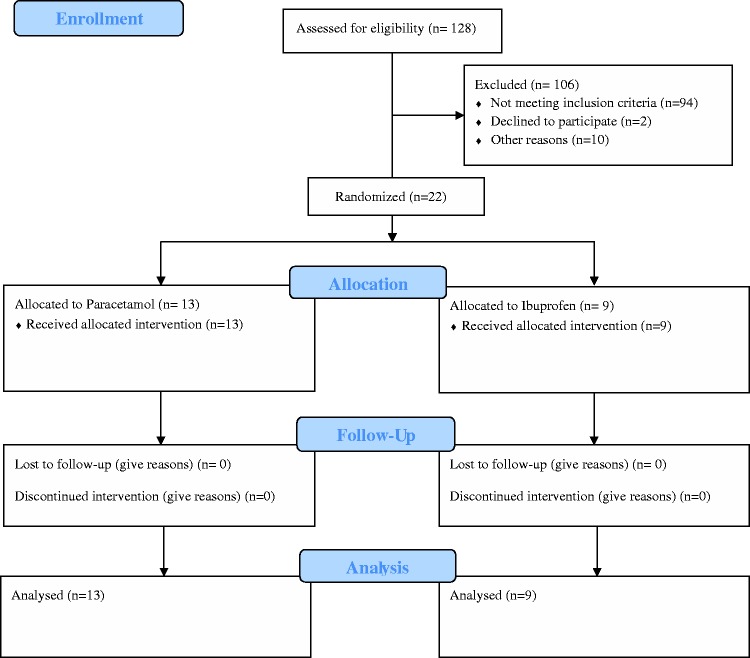
In total, 128 premature infants were included. Eight of them died before day 3 of life and did not show signs of PDA. The 120 surviving premature infants were screened, and 22 fulfilled the treatment and randomization criteria as shown in Figure 1. All infants who were screened because of PDA-related symptoms were found to have hsPDA by echocardiography.
Figure 1.
Study flow diagram
The treatment group was divided into two subgroups: the paracetamol group and the ibuprofen group. The mean gestational age was 28 weeks in both groups. Apart from a lower Apgar score in the first minute of life in the paracetamol group, there were no statistically significant differences in the demographic characteristics between the two groups (Table 1).
Table 1.
Demographic characteristics of infants included in the study
| Paracetamol group (n=13) | Ibuprofen group (n=9) | P value | |
|---|---|---|---|
| Gestational age (wks) | 28 (23–32) | 28 (25–35) | |
| Birth weight (g) | 1059±386 | 1192±269 | 0.861 |
| Small for age | 3 | 3 | 0.595 |
| Cesarean section | 9 | 7 | 0.658 |
| Inborn | 12 | 9 | 0.394 |
| Male sex | 11 | 6 | 0.323 |
| Multiple gestation | 4 | 6 | 0.096 |
| Apgar score | |||
| First minute | 5 | 7 | 0.013* |
| Fifth minute | 7 | 9 | 0.104 |
Data are presented as mean (range), mean ± standard deviation, or number of infants. All infants had a gestational age of ≤32 weeks or birth weight of ≤1500 g.
*P <0.05
The mortality rate was 23% in the paracetamol group and 22% in the ibuprofen group. The primary closure rate was 69% in the paracetamol group and 78% in the ibuprofen group. Other neonatal outcomes are shown in Table 2. A summary of three previously published randomized trials on the present study topic as well as the current study is presented in Table 3.
Table 2.
Comparison of premature infant mortality and neonatal morbidity in the paracetamol and ibuprofen groups
| Paracetamol group (n=13) | Ibuprofen group (n=9) | P value | |
|---|---|---|---|
| Mortality | 3 | 2 | 0.962 |
| Primary closure | 9 | 7 | 0.658 |
| Secondary closure | 3 | 1 | |
| Tertiary closure | 0 | 1 | |
| RDS | 12 | 6 | 0.125 |
| Surfactant therapy | 9 | 6 | 0.898 |
| Pulmonary hemorrhage | 1 | 1 | 0.783 |
| BPD | 1 | 0 | 0.394 |
| MV | 9 | 5 | 0.512 |
| Sepsis | 7 | 4 | 0.664 |
| NEC | 3 | 2 | 0.962 |
| ROP | 0 | 0 | |
| IVH | 7 | 2 | 0.137 |
| Grade 1 | 5 | 2 | |
| Grade 2 | 2 | 0 | |
| Grade 3 | 0 | 0 | |
| Grade 4 | 0 | 0 | |
| PVL | 0 | 0 |
Data are presented as number of patients.
RDS: respiratory distress syndrome, BPD: bronchopulmonary dysplasia, MV: mechanical ventilation, NEC: necrotizing enterocolitis, ROP: retinopathy of prematurity, IVH: intraventricular hemorrhage, PVL: periventricular leukomalacia
Table 3.
Comparison between previously published studies that investigated the use of oral paracetamol and oral ibuprofen in treatment of PDA in premature infants and the current study
| Study | China15 | Turkey16 | Iran17 | Jordan |
|---|---|---|---|---|
| Method | Randomized | Randomized | Exclusion performed after randomization | Randomized |
| Sample size | 160 | 90 | 150 | 22 |
| Gestational age (wks) | ≤34 | ≤30 | <37 | ≤32 |
| Birth weight (g) | NA | ≤1250 | NA | ≤1500 |
| Paracetamol dose | 15 mg/kg/dose for 3 days | 15 mg/kg/dosefor 3 days | 15 mg/kg/dose for 3 days | 10 mg/kg every 6 h for 3 days |
| Ibuprofen dose | 10 mg/kg (day1)5 mg/kg (days 2–3) | 10 mg/kg (day 1) 5 mg/kg (days 2–3) | 20 mg/kg (day 1) 10 mg/kg (days 2–3) | 10 mg/kg/day for 3 days |
| Rate of any PDA | NA | 82% | NA | 42% |
| Rate of hsPDA | NA | 46% | NA | 28% |
| Primary closureParacetamol vs. ibuprofen | 81.2% vs. 78.8% | 72.5% vs. 77.5 | 82.1% vs. 75.8% | 69.0% vs. 78.0% |
| MortalityParacetamol vs. ibuprofen | 12.5% vs. 15.0% | 7.5% vs. 5.0% | NA | 23.0% vs. 22.0% |
PDA: patent ductus arteriosus, hsPDA: hemodynamically significant patent ductus arteriosus, NA: not available
Discussion
Only a few studies to date have compared oral paracetamol and oral ibuprofen for the treatment of PDA in premature infants. Prior to the present study, three randomized studies were published.17–19 Although our study examined a relatively lower number of patients, it was conducted using very strict diagnostic and treatment criteria. Echocardiography was performed based on symptoms or age. When infants were asymptomatic, they were screened between days 3 and 5 of life. Screening asymptomatic newborns before day 3 of life can lead to unnecessary treatment of a potentially spontaneously closing ductus. Additionally, screening should not be performed later than day 5 of age because this might affect the response to therapy. However, later detection of PDA in asymptomatic newborns might also reflect an insignificant ductus that may not require treatment. Because symptoms were not considered in the previously published studies, we surmise that echocardiography was performed for screening regardless of age. Table 3 summarizes the differences between the present study and the three previously published studies.
In the present study, the rate of any PDA was 42% and the rate of hsPDA was 28%; both are lower than previously reported rates.17–19 This difference may reflect the timing of the screening and treatment criteria. None of the infants who did not fulfill the treatment criteria had complications related to PDA.
The mortality rate was similar in the two treatment groups. Deceased neonates were smaller and less mature, and most of them had a PDA that failed to close after the first course of treatment (Table 4).
Table 4.
Comparison between survivors and nonsurvivors among premature infants included in the study
| Nonsurvivors (n=5) | Survivors (n=17) | P value | |
|---|---|---|---|
| Gestational age (wks) | 26.0±1.5 | 29.0±1.9 | 0.0042 |
| Birth weight (g) | 776±192 | 1212±301 | 0.0066 |
| Primary closure | 1 | 15 | 0.0026 |
Data are presented as mean ± standard deviation or number of patients.
Primary closure was accomplished in most patients in both treatment groups. Because most PDAs were closed after a 3-day course of the assigned drug, it might be more advisable to treat PDA for 3 days and then confirm failure of treatment before extending the duration of therapy to avoid further side effects of the medication. Tertiary closure was accomplished in one patient randomized to the ibuprofen group whose PDA failed to close after two courses of ibuprofen and closed after switching to paracetamol. Failure of a PDA to close after a 6-day course of either medication should not discourage treating physicians from trying the other medication before sending the infant to surgery.
Data on the safety of paracetamol have been reported, and little evidence of side effects has been found.21 None of the infants in the present study showed any signs of hepatic toxicity. Ibuprofen has milder vasoconstrictive side effects than other nonsteroidal anti-inflammatory drugs. Mild elevation of blood urea nitrogen and insignificant gastrointestinal symptoms were reported in a previous study.22 In our study, the included neonates did not show any significant elevation in the blood urea nitrogen concentration or any gastrointestinal side effects. There were no differences in the neonatal complications between the paracetamol and ibuprofen groups as shown in Table 2.
Long-term safety data of paracetamol and ibuprofen are scarce. A follow-up study of one trial investigating the neurodevelopmental outcomes of children who received oral ibuprofen and oral paracetamol at 18 to 24 months of age showed no difference between the two groups.23
We conclude that both oral ibuprofen and oral paracetamol are safe and effective in treating PDA in premature infants. Implementing fluid and feeding protocols might help to decrease both the complications of the PDA and the side effects of the treatment medication. This study included a small number of patients. Multicenter studies are needed to recruit adequate numbers of patients within a reasonable period of time.
Acknowledgments
We thank the patients’ parents for their help and cooperation. We also thank the neonatal unit nurses for their patience and help in executing the first randomized trial in our department.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was funded by the Deanship of Scientific Research at the University of Jordan.
References
- 1.Sellmer A, Bjerre JV, Schmidt MR, et al. Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Arch Dis Child Fetal Neonatal Ed 2013; 98: 505–510. [DOI] [PubMed] [Google Scholar]
- 2.Sallmon H Koehne P andHansmann G.. Recent Advances in the Treatment of Preterm Newborn Infants with Patent Ductus Arteriosus. Clin Perinatol 2016; 43: 113–129. [DOI] [PubMed] [Google Scholar]
- 3.Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus 2000; 343: 674–681. [DOI] [PubMed] [Google Scholar]
- 4.Gulack BC, Laughon MM, Clark RH, et al. Comparative effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus. Early Hum Dev 2015; 91: 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadir E, Kassem E, Foldi S, et al. Paracetamol treatment of patent ductus arteriosus in preterm infants. J Perinatol 2014; 34: 748–749. [DOI] [PubMed] [Google Scholar]
- 6.Ohlsson A andShah PS.. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low-birth-weight infants. Cochrane Database Syst Rev 2015; 3: CD010061. [DOI] [PubMed] [Google Scholar]
- 7.Terrin G, Conte F, Oncel MY, et al. Paracetamol for the treatment of patent ductus arteriosus in preterm neonates: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2016; 101: 127–136. [DOI] [PubMed] [Google Scholar]
- 8.Oncel MY, Yurttutan S, Degirmencioglu H, et al. Intravenous paracetamol treatment in the management of patent ductus arteriosus in extremely low birthweight infants. Neonatology 2013; 103: 166–169. [DOI] [PubMed] [Google Scholar]
- 9.Demir N, Peker E, Ece İ, et al. Efficacy and safety of rectal ibuprofen for patent ductus arteriosus closure in very low birth weight preterm infants. J Matern Fetal Neonatal Med 2017; 25: 1–10. [DOI] [PubMed] [Google Scholar]
- 10.Erdeve O, Yurttutan S, Altug N, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2012; 97: 279–283. [DOI] [PubMed] [Google Scholar]
- 11.Yang EM Song ES andChoi YY.. Comparison of oral Ibuprofen and intravenous indomethacin for the treatment of patent ductus arteriosus in extremely low birth weight infants. J Pediatr (Rio J) 2013; 89: 33–39. [DOI] [PubMed] [Google Scholar]
- 12.Olgun H, Ceviz N, Kartal İ, et al. Repeated Courses of Oral Ibuprofen in Premature Infants with Patent Ductus Arteriosus: Efficacy and Safety. Pediatr Neonatol 2016; 58: 29–35. [DOI] [PubMed] [Google Scholar]
- 13.Oncel MY andErdeve O.. Safety of therapeutics used in management of patent ductus arteriosus in preterm infants. Curr Drug Saf 2015; 10: 106–112. [DOI] [PubMed] [Google Scholar]
- 14.Hammerman C, Bin-Nun A, Markovitch E, et al. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics 2011; 128: 1618–1621. [DOI] [PubMed] [Google Scholar]
- 15.Oncel MY, Yurttutan S, Uras N, et al. An alternative drug (paracetamol) in the management of patent ductus arteriosus in ibuprofen resistant or contraindicated preterm infants. Arch Dis Child Fetal Neonatal Ed 2013; 98: F94. [DOI] [PubMed] [Google Scholar]
- 16.Bagheri MM, Niknafs P, Sabsevari F, et al. Comparison of oral acetaminophen versus ibuprofen in premature infants with patent ductus arteriosus. Iran J Pediatr. 2016; 26: 3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oncel MY, Yurttutan S, Erdeve O, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr 2014; 164: 510–514. [DOI] [PubMed] [Google Scholar]
- 18.Dang D, Wang D, Zhang C, et al. Comparison of oral paracetamol versus ibuprofen in premature infants with patent ductus arteriosus: a randomized controlled trial. PLoS One 2013; 8: 77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluckow M andEvans N.. Early echocardiographic prediction of symptomatic patent ductus arteriosus in preterm infants undergoing mechanical ventilation. J Pediatr 1995; 127: 774–779. [DOI] [PubMed] [Google Scholar]
- 20.Carlo WA. Respiratory tract disorders In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF. (eds) Nelson textbook of pediatrics. Philadelphia (PA): Saunders Elsevier, 2011, pp. 579–599. [Google Scholar]
- 21.Härkin P, Härmä A, Aikio O, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr 2016: 177: 72–77. [DOI] [PubMed] [Google Scholar]
- 22.El-Mashad AE, El-Mahdy H, El Amrousy D, et al. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr 2017; 176: 233–240. [DOI] [PubMed] [Google Scholar]
- 23.Oncel MY, Eras Z, Uras N, et al. Neurodevelopmental outcomes of preterm infants treated with oral paracetamol versus ibuprofen for patent ductus arteriosus. Am J Perinatol 2017. doi: 10.1055/s-0037-1601564 [DOI] [PubMed] [Google Scholar]