Short abstract
Objective
This study was performed to investigate impaired vagal activity to meal in patients with functional dyspepsia (FD) with delayed gastric emptying (GE).
Methods
Eighty-five patients were studied. GE parameters, including those in the overall and proximal stomach, were measured by GE functional tests at the Department of Nuclear Medicine. Autonomic nervous function was tested by spectral analysis of heart rate variability (HRV). The vagal activity and sympathetic activity were analyzed by recording the power in the high-frequency component (HF), low-frequency component (LF), and LF/HF ratio.
Results
Overall and proximal GE were delayed in 47.2% and 50.9% of the patients, respectively. Spectral analysis of HRV showed that the HF in patients with delayed proximal GE was significantly lower and that the LF/HF ratio was significantly higher than those in patients with normal proximal GE after a meal.
Conclusion
Delayed proximal GE might be caused by disrupted sympathovagal balance as a result of decreased vagal activity after a meal. Improvement in vagal activity may constitute an effective treatment method for patients with FD.
Keywords: Functional dyspepsia, proximal gastric emptying, autonomic nervous system, heart rate variability, scintigraphy, spectral analysis
Abbreviations
FD, functional dyspepsia; GE, gastric emptying; ANF, autonomic nervous function; HS, healthy subjects; HRV, heart rate variability; HF, high frequency; LF, low frequency.
Introduction
Functional dyspepsia (FD) is one of the most common digestive system diseases worldwide. Its prevalence is about 16%,1 and outpatients with FD account for about 40% of all outpatients in gastroenterology departments.2 Repeated attacks of FD seriously reduce patients’ quality of life and consume large amounts of medical resources, although they do not usually shorten patients’ lifespan.3 Delayed gastric emptying (GE) reportedly occurs in 23% to 59% of patients with FD. Delayed GE has long been recognized as one of the most important mechanisms of FD and has thus been the focus of many FD-related studies.4 However, GE is not a primary mechanistic disturbance in patients with FD, and the pathogenesis of delayed GE remains unclear.5 Hausken et al.6 performed ultrasound examinations of patients with FD in an attempt to examine their autonomic nervous function (ANF) by assessment of antral motility; however, the ultrasound examination did not take into account overall and proximal gastric function. Thus, their study did not clearly reveal the relationship between gastric motility and ANF.
Scintigraphy and spectral analysis of HRV were performed to investigate GE and ANF in this study. The objective was to clarify the possible pathogenesis of delayed GE and provide a theoretical basis and guidance for the clinical treatment of FD.
Materials and methods
Participants
Patients with symptoms of dyspepsia as their chief complaint were enrolled in this study. All patients underwent a detailed physical examination, collection of their medical history, upper gastrointestinal endoscopy, routine biochemical examination, and abdominal ultrasound. All patients met the Rome III criteria for functional dyspepsia by demonstrating one or more of the following four symptoms: postprandial fullness and discomfort, early satiety, epigastric pain, or epigastric burning sensation. No patients had evidence of organic diseases that could explain these symptoms. The symptoms had been present for at least 6 months before diagnosis, with symptoms that met the above criteria present during the most recent 3 months. The exclusion criteria were atrophic gastritis, esophagitis, gastroesophageal erosive lesions on gastroscopy, a history of peptic ulcer disease, a history of abdominal surgery, and use of nonsteroidal anti-inflammatory drugs, steroids, or other drugs affecting the secretion of gastric acid.
Healthy subjects (HS) were also recruited by posting of advertisements in the hospital. The HS had no history of digestive tract diseases or drug allergy, and all underwent upper gastrointestinal endoscopy and biochemical examination to exclude possible digestive diseases.
All medications that might affect gastrointestinal function (including antidepressants) were discontinued for at least 1 week prior to the examination. This study was approved by the ethics committee of our hospital, and all participants provided informed consent.
Symptom questionnaire
A questionnaire was given to all patients with FD to collect information on their demographic characteristics and symptoms. The patients were asked to complete the symptom questionnaire according to their true conditions.
GE studies
All participants (patients and HS) underwent a GE functional test at the Department of Nuclear Medicine in the morning after one night of fasting for at least 10 h. All participants ate a standard test meal containing 99mTc-DTPA marker (95 g of non-fried cake, 65 g of 2 mCi 99mTc-DTPA-marked fried egg, and 10 g of vegetable oil brewed in 500 ml of water) within 10 min. The nutritional composition of the test meal was 42% carbohydrate, 10% protein, and 8% fat with a total energy of 500 kcal. After eating, the participants were asked to immediately lie in the supine position on the examination bed because this position can reduce the overlap of the stomach and small intestine. The first frame image (anteroposterior image) was taken by a single-photon emission computed tomography device (Millennium VG Hawkeye; GE Healthcare Biosciences, Piscataway, NJ, USA), followed by one frame every 15 min within 2 h (nine frames in total). The imaging conditions were 140 keV, window width of 50%, magnification of 1.5, and low-energy universal collimator. The participants were allowed to rest in a sitting position during the interval of image acquisition. Imaging during women’s menstrual period was avoided to prevent interference of hormone levels with the GE rate.
A nuclear medicine physician and a digestive physician cooperatively analyzed and processed the collected data, defined the region of interest of each frame for calculation of radioactive counts in this region of interest, and obtained the standard curve of GE after radioactive decay and anteroposterior correction. To determine the emptying status of the proximal stomach, a horizontal line was drawn along the gastric corner, and the area above this line was defined as the proximal stomach.7 The parameters included the half time (T1/2) of total GE and the T1/2 of proximal GE.
Heart rate variability (HRV)
On the day following the GE test, the participants underwent an ANF test. The participants fasted for 10 h overnight before the test. ANF was recorded for 30 min in a resting state. The participants were then asked to finish the standard meal in 10 min. The changes in ANF were observed for 30 min after the meal. The composition of the standard meal was identical to that in the GE test. During the ANF test, the participants were told to remain in the supine position and avoid unnecessary movements. Talking or reading was prohibited to minimize interference.
A one-channel electrocardiogram was obtained using three electrodes (BioTac Ultra 7305; The Ludlow Company, Chicopee, MA, USA) that were connected to a bioamplifier (Model 2283; UFI, Morro Bay, CA, USA) with a cut-off frequency of 100 Hz. The two electrodes were respectively placed at the manubrium sterni and cardiac apex. The reference electrode was placed at the right thoracic wall. The electrocardiogram data were analyzed by an HRV Analyzer program produced by the University of Texas Medical Branch.8
The ANF assessment parameters and their implications were analyzed. Power spectral analysis of HRV was then performed with fast Fourier transformation, and the power values of the high-frequency component (HF) (0.15–0.40 Hz), low-frequency component (LF) (0.04–0.15 Hz), and LF/HF ratio were calculated by the computer incorporated into the equipment. The LF represents sympathetic activity and some parasympathetic activity, while vagal efferent activity is considered to be the major contributor to the HF. In addition, the LF/HF ratio is considered to reflect the sympathovagal balance.9
The unit for both LF and HF was the decibel. If the value was positive, then a higher absolute value indicated a higher power. If the value was negative, then a higher absolute value indicated a lower power.
Statistical analysis
The patients with FD were divided into normal and delayed GE groups according to the upper limit of the 95% CI for the GE T1/2 in HS (total stomach and proximal stomach). Age and body mass index in both patient subgroups (normal versus delayed GE) were compared using Student’s t-test. Pearson’s χ2 statistics were used to investigate the association between the categorical variables, and the nonparametric Wilcoxon–Mann–Whitney test or Student’s t-test was used to investigate the differences in HRV parameters between the preprandial and postprandial periods and between normal GE and delayed GE. All of these analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at a P value of <0.05.
Results
Characteristics of patients with FD
In total, 53 patients with FD (20 male, 33 female) and 32 HS (15 male, 17 female) were included in this study. Patients with FD were significantly older than HS (37.87 ± 12.17 vs. 27.34 ± 7.47 years, respectively; P < 0.01), and the sex distribution was not different between the two groups (P = 0.407). There were no significant differences in age, sex distribution, or body mass index between patients with FD with normal versus delayed overall GE or between patients with FD with normal versus delayed proximal GE (Table 1). Dyspeptic symptoms were present for 27.4 ± 9.3 months.
Table 1.
Comparison of demographic characteristics of patients with functional dyspepsia (n = 53) with normal or delayed overall or proximal gastric emptying
| Characteristic |
Overall gastric emptying |
Proximal gastric emptying |
||
|---|---|---|---|---|
| Normaln = 28 | Delayedn = 25 | Normaln = 26 | Delayedn = 27 | |
| Age, years | 36.50 ± 11.61 | 39.40 ± 12.82 | 36.81 ± 13.29 | 38.89 ± 11.13 |
| Male sex (n) | 14 | 6 | 12 | 8 |
| Body mass index, kg/m2 | 21.10 ± 2.53 | 21.72 ± 3.18 | 20.73 ± 2.44 | 22.03 ± 3.10 |
Except for sex, data are presented as mean ± standard deviation.
GE
In HS, the overall and proximal GE T1/2 was 110.41 ± 15.99 and 93.28 ± 14.65 min, respectively. Compared with the HS (GE T1/2 above the 95% CI of overall GE in HS, 116.62 min), 28 (52.8%) of 53 patients had normal overall solid GE and 25 (47.2%) had delayed overall GE.
Compared with the HS (GE T1/2 above the 95% CI of proximal GE in HS, 98.96 min), 26 (49.1%) of 53 patients had normal proximal solid GE and 27 (50.9%) had delayed proximal GE.
Relationship between ANF and overall GE
Among the patients, the sympathovagal balance index (LF/HF ratio) was not significantly different between the normal and delayed overall GE groups either before a meal or after a meal. There was also no significant difference in the LF/HF ratio between before and after a meal in the normal GE group. However, the LF/HF ratio after a meal was significantly higher than that before a meal in the delayed overall GE group (P = 0.020).
The power of the HF (vagal activity) was not significantly different between the normal and delayed overall GE groups either before or after a meal. In the normal overall GE group, the HF was not significantly different between before and after a meal. Additionally, no significant difference in the HF was observed between before and after a meal in the delayed overall GE group. Finally, the LF showed no between-group or within-group differences (Table 2).
Table 2.
Heart rate variability parameters before and after a test meal in patients with functional dyspepsia with or without delayed overall gastric emptying
| Measurement | Stage | Normal overall GE (Mean ± SD) | Delayed overall GE (Mean ± SD) |
|---|---|---|---|
| LF/HF | Preprandial | 0.73 ± 0.68 | 0.67 ± 0.53 |
| Postprandial | 0.96 ± 0.97 | 1.23 ± 0.85* | |
| HF (dB) | Preprandial | (−10.72) ± 4.87 | (−11.36) ± 5.34 |
| Postprandial | (−12.58) ± 4.13 | (−14.18) ± 5.25 | |
| LF (dB) | Preprandial | (−12.96) ± 4.66 | (−14.81) ± 5.60 |
| Postprandial | (−12.73) ± 2.71 | (−14.40) ± 4.62 |
Data are presented as mean ± standard deviation.
*P < 0.05 vs. preprandial in delayed overall GE group
GE, gastric emptying; HF, high-frequency component; LF, low-frequency component; LF/HF, LF-to-HF ratio; dB, decibels
Relationship between ANF and proximal GE
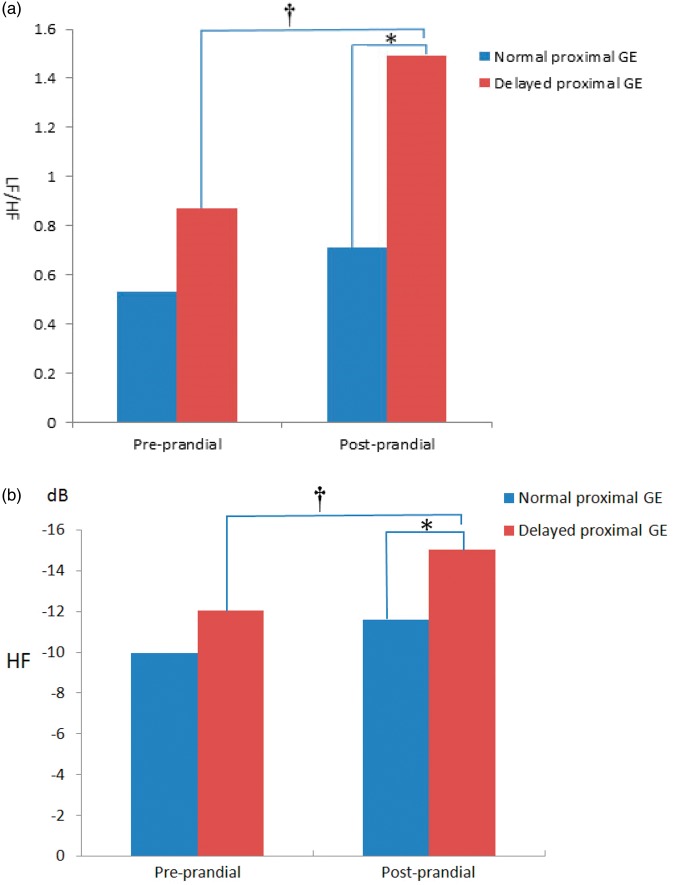
The LF/HF ratio was compared between the normal proximal GE group and delayed proximal GE group, and the difference was not significant between the two groups before a meal (Table 3). After a meal, the LF/HF ratio was significantly higher in the delayed than normal proximal GE group (P = 0.004). The LF/HF ratio was not significantly different between before and after a meal in the normal proximal GE group. However, the LF/HF ratio was significantly higher after than before a meal in the delayed proximal GE group (P = 0.025) (Figure 1(a)).
Table 3.
Heart rate variability parameters before and after a test meal in patients with functional dyspepsia with or without delayed proximal gastric emptying
| Measurement | Stage | Normal proximal GE (Mean ± SD) | Delayed proximal GE (Mean ± SD) |
|---|---|---|---|
| LF/HF | Preprandial | 0.53 ± 0.41 | 0.87 ± 0.72 |
| Postprandial | 0.71 ± 0.48 | 1.49 ± 1.06#* | |
| HF (dB) | Preprandial | (−9.96) ± 5.15 | (−12.05) ± 4.86 |
| Postprandial | (−11.58) ± 4.52 | (−15.02) ± 4.33#* | |
| LF (dB) | Preprandial | (−12.97) ± 4.89 | (−14.67) ± 5.36 |
| Postprandial | (−12.66) ± 3.17 | (−14.34) ± 4.20 |
Data are presented as mean ± standard deviation.
*P < 0.05vs. preprandial in delayed proximal GE group
#P < 0.01vs. postprandial in normal proximal GE group
GE, gastric emptying; HF, high-frequency component; LF, low-frequency component; LF/HF, LF-to-HF ratio; dB, decibels
Figure 1.
Spectral analysis of heart rate variability (HRV) before and after a test meal in patients with functional dyspepsia (FD) with or without delayed proximal gastric emptying (GE). (a) *The ratio of the low-frequency component (LF) to high-frequency component (HF) (i.e., LF/HF ratio) was significantly higher in patients with delayed proximal GE than in those with normal proximl GE after a meal (P < 0.01). †The LF/HF ratio was significantly higher in those with delayed proximal GE after a meal than before a meal (P < 0.05). (b) *The power of the HF was significantly lower in the delayed proximal GE group than in the normal group after a meal (P < 0.01). †The power of the HF was significantly lower in the delayed proximal GE group after a meal than before a meal (P < 0.05).
The HF was also compared between the delayed and normal proximal GE groups (Table 3). Before a meal, the difference was not significant between these two groups. After a meal, the HF was significantly lower in the delayed than normal proximal GE group (P = 0.007). The HF was not significantly different either before or after a meal in the normal proximal GE group. However, the HF was significantly lower after than before a meal in the delayed proximal GE group (P = 0.021) (Figure 1(b)). The LF was not significantly different either within each group or between the groups.
Discussion
FD is usually treated by empirical therapy, including promotion of gastric motility, inhibition of gastric acid secretion, and improvement in digestion by enhancing digestive enzymes. However, such empirical treatments often result in poor therapeutic effects and high recurrence after discontinuation. The most crucial step in developing an effective therapy is to first clarify the pathogenesis, and this is the precise topic of concern among researchers. Previous studies have suggested that delayed GE constitutes the most important pathogenesis of FD.10–12 Our results also showed that delayed proximal GE occurred in 50.9% of patients with FD. Therefore, identifying the pathogenesis of delayed GE is very important in FD research.
The possible mechanisms of delayed GE have not yet been determined. However, gastric motility and sensory systems are regulated by both the central and peripheral nervous systems. Research has shown that delayed GE is related to impaired gastric antral motility, and the reduced gastric antral motility is affected by decreased vagal tone.6,13 At present, studies of the correlation between GE and the autonomic nervous system mainly use an ultrasonic technique for examination of gastric motility by measuring gastric antral motility.14 However, ultrasonic technique-based examination of gastric function has the following three disadvantages. First, ultrasound examination is usually inaccurate in measuring gastric antral motility, and excessive abdominal fat or accumulated abdominal gas in patients can affect the results of the examination.15 Second, ultrasound examination only reveals the function of the gastric antrum, not the overall gastric and proximal GE. Third, the use of ultrasound technology is limited to evaluation of liquid emptying, but the GE mechanisms for solid and liquid food substances are not the same.15
In this study, we performed scintigraphy, which is the gold standard test for solid GE.16 Its advantages include objectivity, high accuracy, and quantitative measurement. We also performed power spectral analysis of HRV to examine ANF. The principles of power spectral analysis of HRV are derived from electrocardiography. Both animal and human experiments have confirmed that this is a noninvasive and accurate method with which to assess ANF.17,18 HRV spectrum analysis can distinguish between sympathetic and vagal components.19 Some researchers consider that the HF in patients with FD is usually lower than that in HS.20 Others have reported that parameters related to ANF are not significantly different between patients with FD and HS.8 The discrepancies among these different reports might have arisen from the fact the patients with FD were not divided according to their GE. Besides comparing ANF between normal and delayed overall GE groups, we also compared ANF between normal and delayed proximal GE groups. The changes in ANF before and after a meal were investigated. Spectral analysis of HRV in our study revealed that the LF/HF ratio after a meal was higher than that before a meal in the delayed overall GE group. The LF and HF did not vary significantly before and after a meal in the delayed overall GE group. In the normal GE group, parameters related to ANF did not vary significantly before and after a meal.
We further examined the relationship between proximal GE and ANF and found that the LF/HF ratio was significantly higher in the delayed proximal GE group than in the normal proximal GE group after a meal, with a decreased HF. However, the LF was not different between the two groups. Such changes were due to an increased LF/HF ratio and decreased HF after a meal in the delayed proximal GE group compared with those before a meal. However, these parameters did not change in the normal proximal GE group before and after a meal. The disrupted sympathovagal balance was caused by disorder of vagal nerve function after a meal, which is considered to be a possible mechanism of delayed proximal GE. Consistent with our findings, Manabe et al.19 discovered that the HF was lower in patients with postprandial distress syndrome than in HS. They also found that patients with postprandial distress syndrome had reduced antral contraction after sham feeding compared with HS. The decrease in the antral contraction index was positively correlated with the decline in the HF. This finding confirms the fact that a reduced HF is related to delayed GE from another perspective. However, further investigations should be performed to determine whether the delayed proximal GE is caused by an increased LF/HF ratio as a result of a decreased HF after a meal or by abnormal hormone levels, which also affect ANF.
Aro et al.21 found that patients with FD had a higher level of anxiety based on a large population study in Sweden. Other studies have also shown that patients with FD might have psychological and mental problems including depression and somatization disorder.22,23 In light of these findings, our results might also have been impacted by the patients’ psychological state. All patients with FD in our study were free from any history of mental disorders, such as anxiety and depression, and scintigraphy and spectral analysis of HRV are noninvasive. Therefore, we consider that the patients’ mental state had little impact on our results.
The onset of FD is affected by a variety of pathophysiological mechanisms; therefore, it cannot be explained only by delayed GE and autonomic nervous disorder. We found that delayed GE occurred in nearly half of the patients with FD, and reduced vagal nerve function was related to delayed proximal GE after a meal. Thus, in theory, improvement in autonomic nervous disorders might be an effective means of treating FD. Animal experiments have also shown that electroacupuncture can effectively improve GE function and vagal activity.24 In addition, Liu et al.25 found that transcutaneous electroacupuncture in patients with FD alleviated FD symptoms and showed that symptom improvement was related to an increased HF after a meal. However, further research is needed to determine whether the correction of abnormal ANF can improve overall gastric and proximal GE in patients with FD.
The main limitation of our study is that the patients with FD were significantly older than the HS. Additionally, the objective of our study was to observe the changes in ANF in patients with FD with delayed GE. Because many reports have already described the discrepancies in ANF between HS and patients with FD, we did not study ANF in a healthy population.
Conclusion
Delayed overall or proximal GE was observed in about half of our patients with FD. Delayed proximal GE might be caused by a disrupted sympathovagal balance induced by a decreased HF (vagal activity) after a meal. Improvement of vagal activity may be an effective method of treating FD.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by grant number 2014BAI08B02 from the National Key Technology R&D Program in the 12th 5-Year Plan of China.
References
- 1.Aro P, Talley NJ, Agréus L, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther 2011; 33: 1215–1224. [DOI] [PubMed] [Google Scholar]
- 2.Okumura T, Tanno S, Ohhira M, et al. Prevalence of functional dyspepsia in an outpatient clinic with primary care physicians in Japan. J Gastroenterol 2010; 45: 187–194. [DOI] [PubMed] [Google Scholar]
- 3.Brook RA, Kleinman NL, Choung RS, et al. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol 2010; 8: 498–503. [DOI] [PubMed] [Google Scholar]
- 4.Tack J Bisschops R andSarnelli G.. Pathophysiology and treatment of functional dyspepsia. Gastroenterology 2004; 127: 1239–1255. [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ Walker MM andHoltmann G.. Functional dyspepsia. Curr Opin Gastroenterol 2016; 32: 467–473. [DOI] [PubMed] [Google Scholar]
- 6.Hausken T, Svebak S, Wilhelmsen I, et al. Low vagal tone and antral dysmotility in patients with functional dyspepsia. Psychosom Med 1993; 55: 12–22. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Breen M, Ryks M, et al. Proximal and overall gastric emptying of solids in patients with reduced gastric volume accommodation compared to matched controls. Dig Dis Sci 2011; 56: 1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friesen CA, Lin Z, Schurman JV. et al. . The effect of a meal and water loading on heart rate variability in children with functional dyspepsia. Dig Dis Sci 2010; 55: 2283–2287. [DOI] [PubMed] [Google Scholar]
- 9.Elsenbruch S andOrr WC.. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol 2001; 96: 460–466. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Locke GR, 3rd, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut 2006; 55: 933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori H, Suzuki H, Matsuzaki J, et al. Gender Difference of Gastric Emptying in Healthy Volunteers and Patients with Functional Dyspepsia. Digestion 2017; 95: 72–78. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi-Shiraishi I, Shiraishi S, Fujita S. et al. L-Arginine L-Glutamate Enhances Gastric Motor Function in Rats and Dogs and Improves Delayed Gastric Emptying in Dogs. J Pharmacol Exp Ther 2016; 359: 238–246. [DOI] [PubMed] [Google Scholar]
- 13.Muth ER Koch KL andStern RM.. Significance of autonomic nervous system activity in functional dyspepsia. Dig Dis Sci 2000; 45: 854–863. [DOI] [PubMed] [Google Scholar]
- 14.Tsukamoto A, Ohno K, Tsukagoshi T. et al. Real-time ultrasonographic evaluation of canine gastric motility in the postprandial state. J Vet Med Sci 2011; 73: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 15.Haruma K, Kusunoki H, Manabe N, et al. Real-time assessment of gastroduodenal motility by ultrasonography. Digestion 2008; 77(Suppl 1): 48–51. [DOI] [PubMed] [Google Scholar]
- 16.Maurer AH. Gastrointestinal Motility, Part 1: Esophageal Transit and Gastric Emptying. J Nucl Med Technol 2016; 44: 1–11. [DOI] [PubMed] [Google Scholar]
- 17.Yanagihara N, Seki M, Goto Y. et al. [Application of power spectral analysis of heart rate variability to women with climacteric symptoms]. J UOEH 2014; 36: 171–177[in Japanese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo G, Ogawa M, Song J. et al. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm 2009; 6: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manabe N, Nakamura K, Hara M. et al. Impaired gastric response to modified sham feeding in patients with postprandial distress syndrome. Neurogastroenterol Motil 2011; 23: 215–219, e112. [DOI] [PubMed] [Google Scholar]
- 20.Tominaga K, Fujikawa Y, Tsumoto C. et al. Disorder of autonomic nervous system and its vulnerability to external stimulation in functional dyspepsia. J Clin Biochem Nutr 2016; 58: 161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aro P, Talley NJ, Ronkainen J. et al. Anxiety is associated with uninvestigated and functional dyspepsia (Rome III criteria) in a Swedish population-based study. Gastroenterology 2009; 137: 94–100. [DOI] [PubMed] [Google Scholar]
- 22.Qiu JJ, Liu Z, Zhao P, et al. Gut microbial diversity analysis using Illumina sequencing for functional dyspepsia with liver depression-spleen deficiency syndrome and the interventional Xiaoyaosan in a rat model. World J Gastroenterol 2017; 23: 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Tomita T, Oshima T. et al. A double-blind placebo controlled study of acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. J Gastroenterol 2017; 52: 602–610. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang H, Yin J, Wang Z, et al. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol 2002; 282: G390–G396. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Peng S, Hou X, et al. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil 2008; 20: 1204–1211. [DOI] [PubMed] [Google Scholar]