Short abstract
Tuberculous spondylitis of vertebral augmentation following percutaneous vertebroplasty or kyphoplasty is rare. We report an unusual case of tuberculous spondylitis diagnosed after percutaneous kyphoplasty (PKP). A 54-year-old woman presented to hospital complaining of back pain following a fall 20 days prior. Radiology showed an acute osteoporotic compression (L3 fracture). The patient denied a history of pulmonary tuberculosis and there were no signs of infection. The patient was discharged from hospital 2 days after undergoing L3 PKP with a dramatic improvement in her back pain. The patient was readmitted 10 months later with a history of recurrent back pain and low-grade fever for 3 months. Imaging examinations showed severe spondylitis at the L2–L3 level, with paravertebral abscess formation and bony destruction of L2 and L3. A positive result of the T-SPOT test preliminarily confirmed the diagnosis of tuberculous spondylitis. The tuberculosis test was positive, and serum C-reactive protein levels and erythrocyte sedimentation were relatively high. Treatment for tuberculous spondylitis was started. She underwent posterior fusion and instrumentation from T12–L5 after markers for infection returned to normal. After surgery, the patient continued antituberculous and anti-osteoporosis treatments. Her low back pain was relieved and low-grade fever and sweating disappeared.
Keywords: Tuberculous spondylitis, vertebral augmentation, percutaneous kyphoplasty, percutaneous vertebroplasty, erythrocyte sedimentation, C-reactive protein, T-SPOT.TB
Abbreviations
CT, computed tomography; MRI, magnetic resonance imaging; PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; OVCF, osteoporotic vertebral compression fracture
Introduction
Vertebral augmentation, including percutaneous vertebroplasty and percutaneous balloon kyphoplasty, has become a safe and effective method for treating osteoporotic fracture in the clinic.1,2 Clinical complications of vertebral augmentation, including bone marrow and nerve root compressions, and pulmonary and cerebrovascular embolism,3–5 are primarily associated with bone cement leakage. To date, concurrent spinal infection after vertebral augmentation has rarely been reported (see Table 1 for references). We report a patient who suffered from tuberculous spondylitis in an enhanced vertebral segment after percutaneous balloon kyphoplasty. We emphasize the clinical characteristics of this case and possible early pathological diagnosis. We analyse the possible relationship between percutaneous kyphoplasty (PKP) and the occurrence of spinal tuberculosis.
Table 1.
Summary of previous cases of active tuberculous spondylitis after vertebral augmentation.
| Study | Age/Sex | Pulmonary tuberculosis | Treated levels | Infected levels | Time to infection | Mode of therapy | Outcome |
|---|---|---|---|---|---|---|---|
| Bouvresseet al.8 | 69/M | No | T12–L5 PVP | L5 | 1 month | Surgery, antituberculous medications | Cured |
| Ivo et al.9 | 70/M | Yes | L1 PKP | L1 | 2 weeks | Surgery, antituberculous medications | Died because of multiorgan failure |
| Kim et al.10 | 76/F | No | T12, L1 PKP | T12, L1 | 3 years | Surgery, antituberculous medications | Cured |
| Kang et al.11 | 58/F | Yes | T12 PVP | T11, 12 | 3 weeks | Surgery, antituberculous medications | Cured |
| Zou et al.12 | 68/F | Yes | L2 PVP | L1, L2, L3 | 3 months | Antituberculous medications | Cured |
| 67/F | Yes | L3 PKP | L2, L3 | 1 month | Surgery, antituberculous medications | Cured | |
| Ge et al.13 | 61/F | No | L1 PKP | T9–L1 | 12 months | Surgery, antituberculous medications | Cured |
| Current study | 54/F | Yes | L3 PKP | L2, L3 | 7 months | Surgery, antituberculous medications | Cured |
M, male; F, female; PVP, percutaneous vertebroplasty; PKP, percutaneous kyphoplasty.
Case report
A 54-year-old woman was hospitalized because of lower back pain caused by trauma for 20 days. Lumbar magnetic resonance imaging (MRI) showed a fresh compression fracture at the L3 level (Figure 1). Bone densitometry showed that the spine density was below average, with a mean spinal T-score of −2.98. A physical examination showed marked tenderness on palpation of the L3 region, with no other positive signs. Except for the incapacitating pain, the patient’s vital signs were stable and there was no evidence of infection. The white blood cell (WBC) count was 6900 cells/mm3 (normal range: 3000–10,500 cells/mm3. Haematological analysis demonstrated that the erythrocyte sedimentation rate (ESR) was 10 mm/h and the C-reactive protein (CRP) level was 0.6 mg/dL, both of which were well within the normal range. Considering that the patient’s low back pain persisted in the last 20 days, we chose to perform an L3 PKP (Figure 2), and no complications occurred during operation. No antibiotic treatment was provided before, during, and after the operation. The patient’s low back pain was greatly improved after the operation, and she left the hospital 2 days later. Seven months postoperatively, she experienced recurrent back pain and underwent treatment at another hospital. The details of her treatment at this hospital were unclear. The patient was readmitted 10 months after the operation. Upon admission, she suffered from a low-grade fever, limited motion of the lumbar spine with paravertebral muscle spasm and extensive thoracolumbar vertebral tenderness. The resting results of a physical examination were normal. A chest X-ray showed a history of obsolete pulmonary tuberculosis. An imaging examination showed severe spondylitis at the L2–L3 level, with paravertebral abscess formation and bony destruction of L2 and L3 (Figure 3). A haematological investigation showed that the WBC count was 11,060 cells/mm3, accompanied by 79.11% of neutrophil granulocytes, and the ESR and CRP level were increased (78 mm/h and 62 mg/dL, respectively). The serum T-SPOT.TB test showed that earlier secreted antigen target-6 in the Antigen A group was 10 (normal range: 0–6) spots, culture filter protein-10 in the Antigen B group was 17 (normal range: 0–6), and the result of the T-SPOT.TB test was positive. The results of the serum T-SPOT test suggested Mycobacterium tuberculosis infection. Medication for tuberculosis was started with the diagnosis of tuberculous spondylitis. After 4 weeks of tuberculosis drug therapy, the ESR and CRP levels were almost decreased to the normal ranges. The patient underwent posterior fusion and instrumentation from T12 to L5 without neural decompression (Figure 4). Six months after surgery, the patient continued anti-tuberculous and anti-osteoporosis treatments, and low back pain appeared to be relieved after surgery. The patient can currently receive clinical reexaminations by herself, and there are no nerve lesions on both lower limbs.
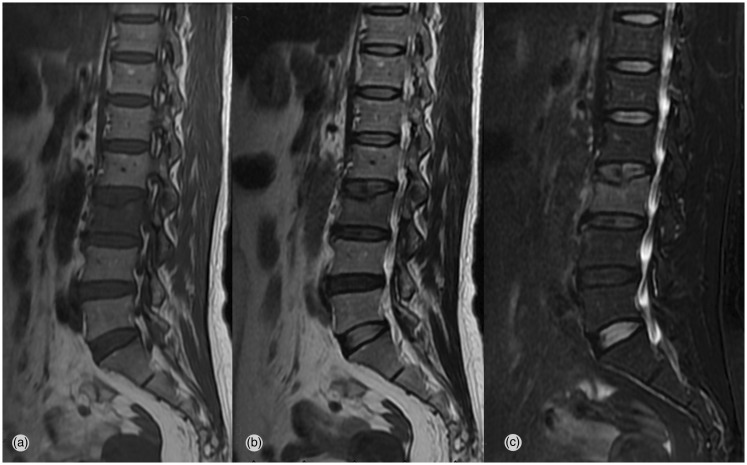
Figure 1.
A 54-year-old woman with an osteoporotic compression fracture at L3
T1-weighted (a), T2-weighted (b), and fat suppression (c) sagittal magnetic resonance images show a fresh compression fracture at the L3 level.
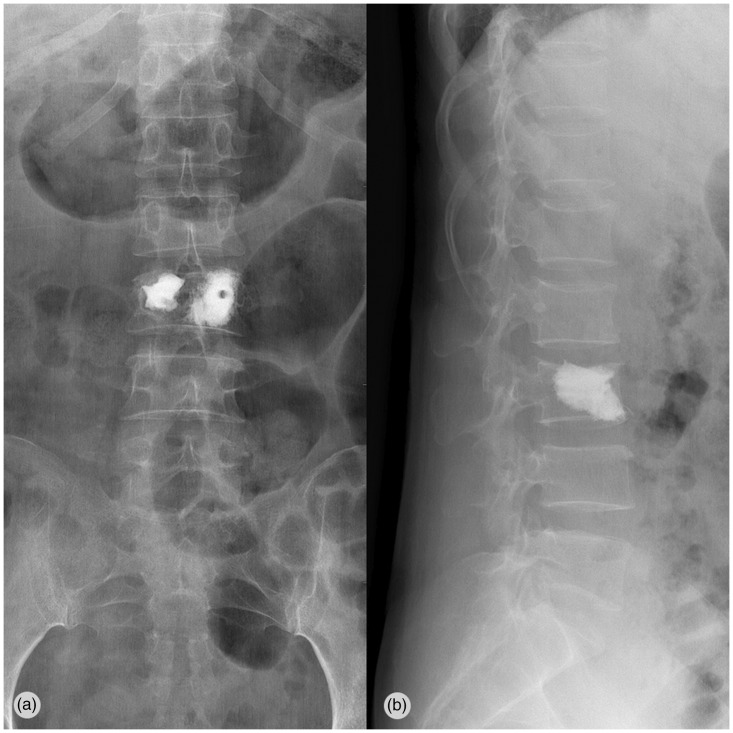
Figure 2.
Postoperative anteroposterior and lateral radiographs show good filling of bone cement after L3 PKP. PKP, percutaneous kyphoplasty.
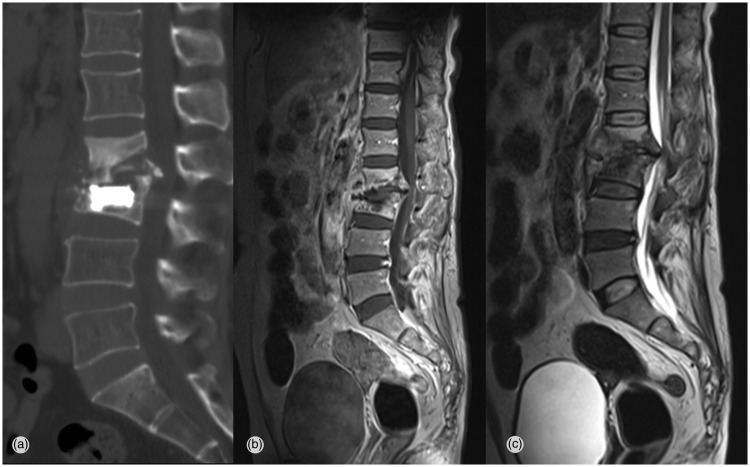
Figure 3.
Approximately 10 months later, computed tomography and magnetic resonance imaging show severe spondylitis at the L2–L3 level, with paravertebral abscess formation and bony destruction of L2 and L3
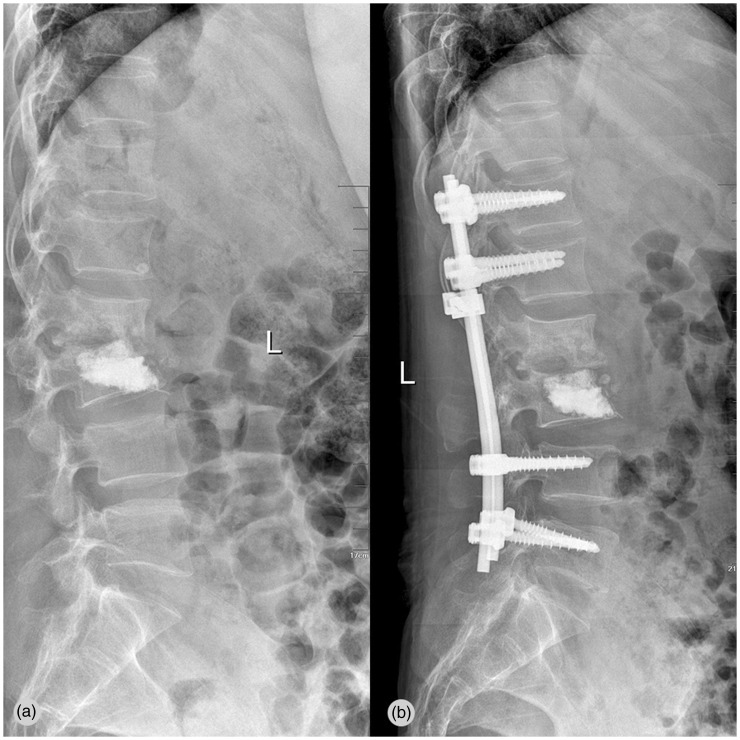
Figure 4.
(a) Plain radiography shows severe spondylitis and collapse of disc space at the L2–L3 level, with kyphosis. (b) One month after posterior instrumentation and fusion. The metallic implant is in a good position, and there is correction of kyphosis and improvement in lumbar stability.
The patient provided consent for this report.
Discussion
PKP is a good method of treating osteoporotic compression fracture, and is widely used in the clinic.3,4 PKP leads to considerable pain relief and has a high safety, but its complications are not rare. Common complications of PKP are as follows: increased pain, cement migration into the venous system, neural foramina, posterior spinal canal, cement embolism, and infection.5–7 Patients with spinal infection after vertebral augmentation often have immunosuppression and a relatively poor immune system, diabetes, and liver or kidney transplantation. However, there have only been a few reports related to the association between vertebral augmentation and tuberculous spondylitis. To the best of our knowledge, there have been six reports on tuberculous spondylitis after vertebral body augmentation (Table 1).8–13 We present a case of tuberculous spondylitis that was diagnosed after PKP.
After analysing the research articles reported in Table 1, we found that the patients mentioned in these articles had the following characteristics: advanced age, many concurrent complications, and an impaired or deficient immune system. These characteristics may be high risk factors for the occurrence of tuberculous spondylitis after an operation. However, the exact mechanism of how tuberculous spondylitis occurs after vertebral augmentation currently remains unclear. Our patient had a previous history of tuberculosis and a chest X-ray confirmed tuberculosis after the second hospitalization. The patient was not aware of this history. After an intensive review of the literature, we examined possible reasons why tuberculous spondylitis occurs on the vertebral segment after vertebral augmentation based on the following several points.
The first point is that PKP may predispose to activate inactive tuberculosis. M. tuberculosis travels as primary lesions to all of the organs (e.g., metaphysis, vertebra, and joint synovia) through the blood circulation, and then forms micro-lesions. When immunity of the body is good, most lesions are eliminated. Only a limited number of small lesions remain static without any clinical symptoms. The term “locus minoris resistentiae”14 refers to either a specific anatomical site that is most susceptible to bodily injury during trauma, or during the course of developing infection after an traumatic event with a particular purpose(e.g., surgery).15–17 This phenomenon explains why tuberculous spondylitis occurs on surgical segments. PKP can be considered as surgical trauma based on this phenomenon. PKP can be considered as an initiating factor of locus minoris resistentia. This leads to reactivation of static M. tuberculosis or induces migration of CD4+ T cells of macrophages into injured sites to release M. tuberculosis, thus causing infection of tuberculous in the vertebra.
The second point is an indication of atypical tuberculous spondylitis (early tuberculous spondylitis) instead of osteoporotic vertebral compression fracture (OVCF) for the first time. Before the first surgery, our patient denied any history of tuberculosis, and admitted a recent history of trauma. By this time, the main uncomfortable symptom was low back pain. Such a symptom was greatly aggravated when turning over in bed or after being sedentary. A physical examination showed local percussion pain, fever, and cold sweats or other discomforts, and bone mineral density indicated osteoporosis. A laboratory examination showed that the ESR and CRP level were well within the normal range. The patient’s imaging findings suggested a fresh compression fracture at the L3 level. Before surgery, we considered that the diagnosis of OVCF was accurate and did not obtain the patient’s permission. Therefore, we did not perform vertebral aspiration biopsy. Danchaivijitr et al.17 performed a retrospective study on MRI images of 31 patients who were diagnosed with tuberculosis spondylitis. They found that, in some patients with tuberculosis with only invasion of vertebral bone marrow, there was low-signal intensity in T1-weighted imaging and high-signal intensity in T2-weighted imaging. Therefore, vertebral MRI signals of some patients with tuberculous spondylitis are atypically increased at the early stage. In our case, our judgment was influenced by the patient’s history of trauma. Therefore, the patient was initially diagnosed with OVCF, instead of considering tuberculous spondylitis. There might be the possibility of misdiagnosis in this situation.
Despite the fact that MRI is a useful method of helping to confirm tuberculous spondylitis, a reliable diagnosis cannot made only from MRI because tuberculosis at the early stage does not show any specificity in imaging tests.18 In particular, the early imaging findings of tuberculous spondylitis are similar to OVCF in terms of clinical characteristics and radiology findings.19 The gold standard of diagnosis of tuberculous spondylitis is a pathological examination. However, this examination often takes a lot of time, and the detection rate and sensitivity are relatively low. We recommend conducting a tuberculous serological examination for tuberculous before an invasive diagnosis. Some studies have shown that the sensitivity and specificity of the T-Spot test for diagnosing active tuberculous are greater than 95%, and it requires a shorter time than microbiological and histological tests.20 The T-SPOT test for diagnosing inactive tuberculous in patients with a previous history of tuberculosis was reported to be as high as 84.3%. Additionally, a significant difference in tuberculosis positivity was observed between individuals with a history of tuberculosis and those without.21 An erythrocyte sedimentation test, C-Pro protein test, and purified protein derivative test, and even PCR analysis, are necessary for diagnosing tuberculosis.22 The medical history of the patient also needs to be understood in detail, and knowledge of whether there are such symptoms as fever, cold sweats, and weight loss, is also required. This information is helpful in the early diagnosis of tuberculous spondylitis.
Our patient experienced limitation of motion of the lumbar spine, as well as clinical symptoms, such as back pain, low-grade fever, and sweating. Laboratory examinations showed that the ESR and CRP levels were higher than the normal ranges. The result of a T-spot test was positive. An imaging examination suggested severe spondylitis at the L2–L3 level, with paravertebral abscess formation. Therefore, tuberculous spondylitis was the most possible diagnosis. After administering antituberculous treatment for 1 month, clinical symptoms, such as fever, sweating, and laboratory inflammatory indices, showed a dramatic decline. However, the patient’s low back pain was still severe and became particularly painful when walking on the ground and turning over on bed. Considering this symptom (without nerve lesions on both lower limbs), the imaging finding of necrotic bone block protruding into the vertebral canal, and the finding that the patient had osteoporosis and possible late-onset kyphosis on the diseased vertebral segment (resulting in nerve lesions on both lower limbs), we adopted posterior, spinal, transpedicular internal fixation and fusion. Previous studies have shown that in contrast to other bacteria, M. tuberculosis has low adhesion to stainless steel and forms less polysaccharide biomembranes.23,24 Therefore, using an implant in the presence of tuberculous spondylitis is theoretically safe. Our patient received posterior fusion and instrumentation without performing neural decompression from T12 to L5. After surgery, the patient continued antituberculous and anti-osteoporosis treatments, and low back pain was relieved. Six months after surgery, the patient can currently receive clinical reexaminations by herself, and there are no nerve lesions on both lower limbs.
Conclusion
This case demonstrates that PKP may predispose to activate inactive M. tuberculosis as a complication. Therefore, clinicians should be aware of such a possibility. The symptoms and imaging findings of tuberculous spondylitis are nonspecific. For patients with osteoporotic fracture with a medical history of tuberculosis, relevant examinations (chest X-ray) should be conducted before surgery, particularly a serological T-SPOT test for M. tuberculosis. After confirming the diagnosis of tuberculous spondylitis, sufficient antituberculous treatment should be performed. When the patient’s symptoms still persist, surgical treatment is an effective therapeutic method.
Acknowledgements
The authors would like to thank the editors and reviewers for their review and constructive criticism in improving the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This project is supported by the National Natural Science Foundation of China (No.81301646).
References
- 1.Peh WCG, Munk PL, Rashid F, et al. Percutaneous vertebral augmentation: vertebroplasty, kyphoplasty and skyphoplasty. Radiol Clin North Am 2008; 46: 611–635. [DOI] [PubMed] [Google Scholar]
- 2.Bouza C, López T, Magro A, et al. Efficacy and safety of balloon kyphoplasty in the treatment of vertebral compression fractures: a systematic review. . Eur Spine J 2006; 15: 1050–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esses SI, McGuire R, Jenkins J, et al. The treatment of symptomatic osteoporotic spinal compression fractures. J Am Acad Orthop Surg 2011; 19: 176–182. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman I H, Dudeney S, Reinhardt M K, et al. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine 2001; 26: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 5.Hochmuth K, Proschek D, Schwarz W, et al. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol 2006; 16: 998. [DOI] [PubMed] [Google Scholar]
- 6.Krueger A, Bliemel C, Zettl R, et al. Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. . Eur Spine J 2009; 18: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M J, Dumonski M, Cahill P, et al. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine 2009; 34: 1228–1232. [DOI] [PubMed] [Google Scholar]
- 8.Bouvresse S, Chiras J, Bricaire F, et al. Pott's disease occurring after percutaneous vertebroplasty: an unusual illustration of the principle of locus minoris resistentiae. . J Infect 2006; 53: e251–e253. [DOI] [PubMed] [Google Scholar]
- 9.Ivo R, Sobottke R, Seifert H, et al. Tuberculous spondylitis and paravertebral abscess formation after kyphoplasty: a case report. Spine 2010; 35: E559–E563. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Shin DA, Cho KG, et al. Late onset tuberculous spondylitis following kyphoplasty: a case report and review of the literature. . Korean J Spine 2012; 9: 28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JH, Kim HS, Kim SW. Tuberculous spondylitis after percutaneous vertebroplasty: misdiagnosis or complication? Korean J Spine; 2013; 10: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou MX, Wang XB, Li J, et al. Spinal tuberculosis of the lumbar spine after percutaneous vertebral augmentation (vertebroplasty or kyphoplasty). . Spine J 2015; 15: e1–e6. [DOI] [PubMed] [Google Scholar]
- 13.Ge CY, He LM, Zheng YH, et al. Tuberculous spondylitis following kyphoplasty: a case report and review of the literature. Medicine (Baltimore) 2016; 95: e2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agostoni G. Aneurysms of the thoracic aorta and traumatism; region of the aortic isthmus; locus minoris resistentiae. Arch Mal Coeur Vaiss 1953; 46: 550–558. [PubMed] [Google Scholar]
- 15.Kainz J andStammberger H.. The roof of the anterior ethmoid: a locus minoris resistentiae in the skull base. Laryngol Rhinol Otol(Stuttg) 1988; 67: 142–149. [PubMed] [Google Scholar]
- 16.Chan ED, Kong PM, Fennelly K, et al. Vertebral osteomyelitis due to infection with nontuberculous Mycobacterium species after blunt trauma to the back: 3 examples of the principle of locus minoris resistentiae. Clin Infect Dis 2001: 32: 1506–1510. [DOI] [PubMed] [Google Scholar]
- 17.Danchaivijitr N, Temram S, Thepmongkhol K, et al. Diagnostic accuracy of MR imaging in tuberculous spondylitis. J Med Assoc Thai 2007; 90: 1581. [PubMed] [Google Scholar]
- 18.McGhee E, Chengappa K, Breen R, et al. Should spinal magnetic resonance imaging (MRI) scans be used to determine the duration of therapy for spinal tuberculosis? Spine J 2016; 16: S58–S59. [Google Scholar]
- 19.Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective. . Eur J Orthop Surg Traumatol 2016; 26: 551–558. [DOI] [PubMed] [Google Scholar]
- 20.Meier T, Eulenbruch H P, Wrighton-Smith P, et al. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. . Eur J Clin Microbiol Infect Dis 2005; 24: 529–536. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Yoon HI, Park KU, et al. The impact of previous tuberculosis history on T-SPOT. TB® interferon-gamma release assay results. . Int J Tuberc Lung Dis 2011; 15: 510–516. [DOI] [PubMed] [Google Scholar]
- 22.Drobniewski F A, Caws M, Gibson A, et al. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis 2003; 3: 141–147. [DOI] [PubMed] [Google Scholar]
- 23.Olmos MA, González AS, Clemente JD, et al. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure: report of a case and a review of the literature. Spine 2006; 31: E770–E773. [DOI] [PubMed] [Google Scholar]
- 24.Jain AK andJain S.. Instrumented stabilization in spinal tuberculosis. Int Orthop 2012; 36: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]