LETTER
Mucormycosis is being increasingly reported all over the world. The high incidence of mucormycosis in India is due to a large population with uncontrolled diabetes and other immunocompromised states. Rhizopus oryzae had been reported as the most common etiological agent associated with human infections, followed by Rhizopus microsporus, in some studies in the Western literature (1, 2). However, in contrast to this, the second most common cause in India is Apophysomyces elegans (3). The first case of R. microsporus infection in India was reported by Bhansali et al. in 2004 (4). Since then, very few reports have been published (Table 1) (5–11). We report here an increase in the number of cases of infection due to R. microsporus in India.
TABLE 1.
Demographic and clinical details of cases included in the study
| S. no.a | Ageb/sex | Residence in India/place of reporting case | Yr | Predisposing factor(s) | Clinical diagnosisc | Sample | Treatment | Outcome/morbidity | Identification | Source or reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20/F | Rajasthan | 2015 | Uncontrolled diabetes type I | ROCM | Nasal scraping | Surgical debridement, liposomal amphotericin B | Improved with loss of vision in left eye | R. microsporus var. rhizopodiformis | Present study |
| 2 | 36/F | Haryana | 2015 | Uncontrolled diabetes type II with diabetic ketoacidosis | ROCM | Nasal crust | Surgical debridement, liposomal amphotericin B | Expired after 1 day of therapy | R. microsporus var. rhizopodiformis | Present study |
| 3 | 40/M | Delhi | 2015 | Uncontrolled diabetes type II | ROCM | Paranasal sinus biopsy | Surgical debridement, liposomal amphotericin B | Expired after 28 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 4 | 55/M | Uttar Pradesh | 2016 | Uncontrolled diabetes, diabetic nephropathy with chronic kidney disease | ROCM | Orbital biopsy | Surgical debridement, liposomal amphotericin B, posaconazole | Improved with left orbital exenteration | R. microsporus var. oligosporus | Present study |
| 5 | 18/M | Uttar Pradesh | 2016 | Acute myeloid leukemia | ROCM | Nasal tissue | Surgical debridement, liposomal amphotericin B | Improved with right orbital exenteration | R. microsporus var. rhizopodiformis | Present study |
| 6 | 42/M | Uttar Pradesh | 2016 | Uncontrolled diabetes type II | ROCM | Nasal tissue | Surgical debridement, liposomal amphotericin B | Expired after 20 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 7 | 50/M | Haryana | 2016 | Uncontrolled diabetes, chronic kidney disease | PM© | Sputum | Liposomal amphotericin B | Improved after 25 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 8 | 45/M | Jharkhand | 2016 | Chronic myeloid leukemia | ROCM | Nasal tissue | Surgical debridement, liposomal amphotericin B | Improved after 17 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 9 | 50/F | Uttar Pradesh | 2016 | Uncontrolled diabetes type II | ROCM | Nasal biopsy | Surgical debridement, liposomal amphotericin B | Expired after 13 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 10 | 55/F | Uttar Pradesh | 2016 | Uncontrolled diabetes type II | ROCM | Nasal crust | Surgical debridement, liposomal amphotericin B, posaconazole | Improved with loss of vision in right eye | R. microsporus var. rhizopodiformis | Present study |
| 11 | 44/M | Bihar | 2017 | Uncontrolled diabetes, renal transplant recipient | PM | Sputum | Liposomal amphotericin, posaconazole | Expired after 15 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 12 | 52/M | Bihar | 2017 | Uncontrolled diabetes type II | PM | Lung biopsy | Amphotericin B, posaconazole | Improved after 15 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 13 | 65/F | Uttar Pradesh | 2017 | Uncontrolled diabetes type II | PM | Bronchoalveolar lavage | Liposomal amphotericin B | Improved after 25 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 14 | 30/M | Madhya Pradesh | 2017 | Uncontrolled diabetes, renal transplant recipient | ROCM | Nasal tissue | Surgical debridement, liposomal amphotericin B | Improved after 18 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 15 | 53/F | Delhi | 2017 | Uncontrolled diabetes type II | ROCM | Orbital and nasal tissue | Surgical debridement, liposomal amphotericin B | Expired after 21 days of therapy | R. microsporus var. rhizopodiformis | Present study |
| 16 | 2 mo/M | Uttar Pradesh | 2017 | Post-abdominal surgery | CM* | Tissue from abdomen | No | Expired before initiation of treatment | R. microsporus var. rhizopodiformis | Present study |
| 17 | 28/M | Delhi | 2017 | Acute lymphoblastic leukemia | PM | Endotracheal aspirate | No | Expired before initiation of treatment | R. microsporus var. rhizopodiformis | Present study |
| 18 | NA | Chandigarh | 2004 | Diabetes mellitus | ROCM | NA | NA | NA | R. microsporus var. rhizopodiformis | 4 |
| 19 | 65/M | Delhi | 2006 | Uncontrolled diabetes mellitus, hypertension | ROCM | Maxillary sinus tissue | Surgical debridement, amphotericin B | Cure | R. microsporus | 5 |
| 20 | NA | Chandigarh | 2009 | NA | ROCM | NA | NA | NA | R. microsporus var. rhizopodiformis | 6 |
| 21 | 56/M | Delhi | 2010 | None | CM | Ulcer biopsy | Surgical excision, amphotericin B | Cure | R. microsporus | 7 |
| 22 | 40/F | Madhya Pradesh | 2012 | None | ROCM | Right maxillary sinus tissue | Surgical resection, liposomal amphotericin B | Cure | R. microsporus | 8 |
| 23 | 39/M | Pune | 2014 | Trauma | CM | Pus from hand | Liposomal amphotericin B, posaconazole | Cure | R. microsporus | 9 |
| 24 (n = 13)d | NA | Delhi | 2014 | NA | ROCM (n = 7), PM (n = 6) | Lung tissue (n = 3), bronchoalveolar lavage (n = 3), endotracheal aspirate (n = 2), nasal mass (n = 4), lung fine-needle aspiration biopsy (n = 2), sinus aspirate (n = 2), maxillary sinus aspirate (n = 1) | NA | NA | R. microsporus (n = 1), R. microsporus var. rhizopodiformis (n = 10), R. microsporus var. oligosporus (n = 5), R. microsporus var. chinensis (n = 1) | 10 |
| 25 | NA | Chandigarh | 2015 | NA | ROCM | NA | NA | Expired | R. microsporus | 11 |
S. no., serial number.
Age is given in years except where otherwise noted. NA, not available.
ROCM, rhino-orbitocerebral mucormycosis; PM, pulmonary mucormycosis; CM, cutaneous mucormycosis.
In this study, a total of 17 samples were collected from 13 patients.
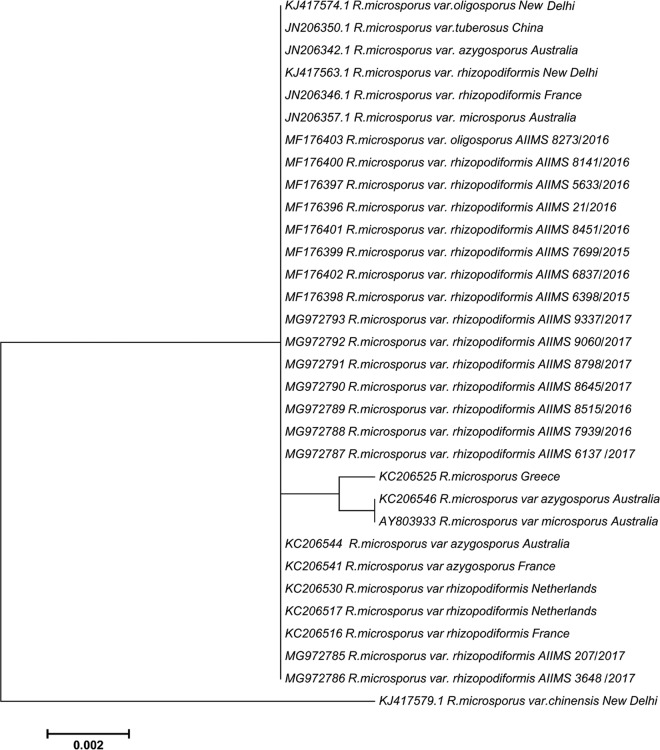
In the present study, culture-positive cases of invasive mucormycosis from January 2015 to December 2017 were included. Before 2015, no case of infection due to R. microsporus was reported from our institute. The demographic details, findings of laboratory investigations, clinical course, and outcome were recorded. The outcome was considered improved when the patient was symptom free on discharge or when a marked reduction in lesions was seen either on radiological imaging or on clinical examination. Identification of R. microsporus was confirmed by sequencing of the internal transcribed spacer (ITS) region of ribosomal DNA (rDNA) (12). The phylogenetic relatedness of our isolates was evaluated with global isolates using the maximum likelihood method, with 500 bootstrap replicates implemented in MEGA v6.00. In vitro antifungal susceptibility testing was performed on these isolates as per Clinical and Laboratory Standards Institute (CLSI) document M38-A2 (13).
From 58 total culture-positive cases, 17 isolates were identified as R. microsporus. The most common variety isolated in our study was R. microsporus var. rhizopodiformis (16/17), followed by R. microsporus var. oligosporus (1/17). Among these patients, 11 were male and 6 were female. Based on the site of involvement, 11 cases were categorized as rhino-orbitocerebral (ROC), 5 as pulmonary, and 1 as cutaneous mucormycosis. Among 11 patients with ROC mucormycosis, 2 had sinonasal disease, and additional orbital extension was seen in 9 cases. Uncontrolled diabetes mellitus was the most common underlying predisposing condition (13/17), with comorbidities such as chronic kidney disease and renal transplantation observed in 2 cases each. Hematological malignancy was present in three cases and abdominal surgery in one case. Liposomal amphotericin B with or without surgical debridement was the mainstay of treatment in all the cases, except for two patients who succumbed before the initiation of antifungals. A fatal outcome was recorded in 47% of the cases (8/17). Nine patients improved after aggressive treatment. However, morbidity in the form of loss of vision (2/9) or orbital exenteration (2/9) was observed (Table 1). The MICs for the antifungals tested, viz., amphotericin B, itraconazole, and posaconazole, ranged from ≤0.03 to 0.5, 0.25 to 2, and ≤0.03 to 0.5 μg/ml, respectively. The ITS phylogenetic tree revealed that our isolates were clustered together in a group along with other type strains (Fig. 1).
FIG 1.
Maximum likelihood phylogenetic analysis of Rhizopus microsporus.
The present study shows an increasing number of infections due to R. microsporus in India over a short span of time. However, these cases just demonstrate the tip of the iceberg. The true incidence in India is still unknown, since many facilities lack mycological expertise, and identification using sequencing is not routinely performed due to financial constraints. Identification of Mucorales to species level holds significance for better understanding of current epidemiology. In addition, more studies on the environmental niches of R. microsporus in India are warranted.
Accession number(s).
All sequences determined in this study were submitted to GenBank under accession numbers MF176396 to MF176403 and MG972785 to MG972793.
ACKNOWLEDGMENTS
We are grateful to the Department of Biotechnology (DBT), Ministry of Science & Technology, India, for partial funding of the project (BT/PR5193/MED/29/463/2012) and to laboratory technologist Bhaskar Rana for maintaining the cultures.
We declare no conflict of interest.
REFERENCES
- 1.Alvarez E, Sutton DA, Cano J, Fothergill AW, Stchigel A, Rinaldi MG, Guarro J. 2009. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J Clin Microbiol 47:1650–1656. doi: 10.1128/JCM.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson M, Lass-Florl C. 2008. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 14(Suppl 4):S5–S24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Singh R. 2014. Mucormycosis in India: unique features. Mycoses 57(Suppl 3):S85–S90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 4.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, Chakarbarti A, Dash RJ. 2004. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J 80:670–674. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SP, Kumar KR, Rokade VR, Khanna V, Pal C. 2006. Orbital Apex Syndrome due to mucormycosis caused by Rhizopus microsporus. Indian J Otolaryngol Head Neck Surg 58:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. 2009. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J 85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 7.Jain SK, Kaza RC, Tanwar R. 2011. Mucormycosis of the anterior chest wall presenting as a soft tissue tumour. J Wound Care 20:176–178. doi: 10.12968/jowc.2011.20.4.176. [DOI] [PubMed] [Google Scholar]
- 8.Nawange SR, Singh SM, Naidu J, Jain S, Nagpal T, Behrani DS, Mellado E, Tudela JL. 2012. Zygomycosis caused by Rhizopus microsporus and Rhizopus oryzae in Madhya Pradesh (M.P.) Central India: a report of two cases. Mycopathologia 174:171–176. doi: 10.1007/s11046-012-9532-0. [DOI] [PubMed] [Google Scholar]
- 9.Verma R, Nair V, Vasudevan B, Vijendran P, Behera V, Neema S. 2014. Rare case of primary cutaneous mucormycosis of the hand caused by Rhizopus microsporus in an immunocompetent patient. Int J Dermatol 53:66–69. doi: 10.1111/ijd.12204. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Kathuria S, Singh PK, Sharma B, Dolatabadi S, Hagen F, Meis JF. 2014. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses 57(Suppl 3):S97–S107. doi: 10.1111/myc.12234. [DOI] [PubMed] [Google Scholar]
- 11.Bala K, Chander J, Handa U, Punia RS, Attri AK. 2015. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol 53:248–257. doi: 10.1093/mmy/myu086. [DOI] [PubMed] [Google Scholar]
- 12.Shivaprakash MR, Appannanavar SB, Dhaliwal M, Gupta A, Gupta S, Gupta A, Chakrabarti A. 2011. Colletotrichum truncatum: an unusual pathogen causing mycotic keratitis and endophthalmitis. J Clin Microbiol 49:2894–2898. doi: 10.1128/JCM.00151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard M38-A2. CLSI, Wayne, PA. [Google Scholar]