ABSTRACT
Histoplasmosis is an important cause of mortality in patients with AIDS, especially in countries with limited access to antiretroviral therapies and diagnostic tests. However, many disseminated infections in Latin America go undiagnosed. A simple, rapid method to detect Histoplasma capsulatum infection in regions where histoplasmosis is endemic would dramatically decrease the time to diagnosis and treatment, reducing morbidity and mortality. The aim of this study was to validate a commercial monoclonal Histoplasma galactomannan (HGM) enzyme-linked immunosorbent assay (Immuno-Mycologics [IMMY], Norman, OK, USA) in two cohorts of people living with HIV/AIDS (PLHIV). We analyzed urine samples from 589 people (466 from Guatemala and 123 from Colombia), including 546 from PLHIV and 43 from non-PLHIV controls. Sixty-three of these people (35 from Guatemala and 28 from Colombia) had confirmed histoplasmosis by isolation of H. capsulatum. Using the standard curve provided by the quantitative commercial test, the sensitivity was 98% (95% confidence interval [CI], 95 to 100%) and the specificity was 97% (95% CI, 96 to 99%) (cutoff = 0.5 ng/ml). Semiquantitative results, using a calibrator of 12.5 ng/ml of Histoplasma galactomannan to calculate an enzyme immunoassay index value (EIV) for the samples, showed a sensitivity of 95% (95% CI, 89 to 100%) and a specificity of 98% (95% CI, 96 to 99%) (cutoff ≥ 2.6 EIV). This relatively simple-to-perform commercial antigenuria test showed a high performance with reproducible results in both countries, suggesting that it can be used to detect progressive disseminated histoplasmosis in PLHIV in a wide range of clinical laboratories in countries where histoplasmosis is endemic.
KEYWORDS: AIDS, antigen, ELISA, Histoplasma capsulatum, histoplasmosis
INTRODUCTION
Histoplasmosis is a disease caused by the thermally dimorphic fungus Histoplasma capsulatum. This disease is most frequently diagnosed in the American continents, although it is also diagnosed in certain Asian and African countries (1–5). Histoplasmosis is highly endemic in some regions of Central and South America, including Guatemala and Colombia. This fungal infection often affects people with impaired immunity, including people living with HIV/AIDS (PLHIV) (1, 4). Among PLHIV, progressive disseminated histoplasmosis (PDH) is a major cause of mortality (13% to 48%) (6–13). PDH symptoms are nonspecific, and they may be indistinguishable from those of other infectious diseases, especially disseminated tuberculosis, thus complicating diagnosis and treatment (7, 14, 15). The nonspecific nature of the presenting symptoms coupled with an uncertain exposure history can create diagnostic challenges (3, 4). Conventional laboratory methods used for the diagnosis of histoplasmosis, such as culture and histopathological analysis, have low sensitivity (∼50%), and antibody tests also have a poor sensitivity (30 to 70%) in immunocompromised persons. Molecular tests, which so far are all in-house tests developed by different groups and reference laboratories, have shown promising results, but none of these tests are commercially available and they need more extensive validation in order to be used more widely (3, 4, 16–18).
Detection of circulating Histoplasma antigen in urine by antigen-capture enzyme-linked immunosorbent assay (ELISA) has proven highly sensitive (95%), but this test is offered as a service by a private laboratory in the United States and has not been widely available in most Latin American countries (18–21). There is a commercially available, FDA-cleared ELISA for Histoplasma antigen detection in urine (the Alpha Histoplasma antigen enzyme immunoassay [EIA]; Immuno-Mycologics [IMMY], Norman, OK, USA), but previous reports demonstrated a low analytical performance for this kit (44% sensitivity) (22). An in-house double-polyclonal-antibody sandwich ELISA, developed at CDC, uses a polyclonal antibody to detect the H. capsulatum polysaccharide antigen (HPA; CDC polyclonal ELISA [HPA]). It was used in Guatemala and later in Colombia, where the sensitivities were reported to be 81% and 86%, respectively (10, 23). However, the reagents for this test are no longer available, and no further distribution was planned in the regions where histoplasmosis is endemic.
Since those assays were designed, a commercial Histoplasma antigen (Histoplasma galactomannan [HGM]) single-monoclonal-antibody sandwich ELISA (IMMY monoclonal ELISA [HGM]) has also recently been developed by IMMY (Norman, OK, USA). The reagents have been evaluated in two laboratories in the United States and showed high performance for the diagnosis of histoplasmosis (sensitivity, 91%; specificity, 96%) (24, 25). The aims of our study were (i) to evaluate the analytical performance of this commercial ELISA for the detection of Histoplasma antigens in urine samples, using the standard curve provided in the kit, and (ii) to evaluate this test as a semiquantitative ELISA, in order to reduce the expenses associated with the use of this test in laboratories outside the United States.
MATERIALS AND METHODS
Settings and study design.
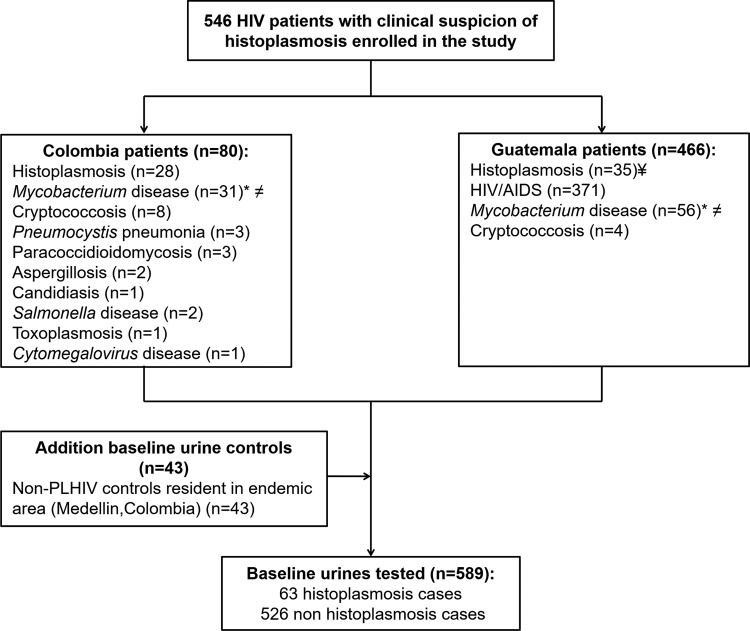
We analyzed retrospectively residual urine samples collected from patients seen at two HIV clinics, one in Guatemala City, Guatemala (Clínica Familiar Luis Angel García/Hospital General San Juan de Dios), and the other in Medellín, Colombia (Hospital La María). These urine samples were collected using the same study protocol (CDC protocol 4250) and were tested using the CDC polyclonal ELISA (HPA) (10, 23). Samples were collected from 2008 to 2014 and stored at −80°C. We included 589 urine samples from the two regions of endemicity (Guatemala, n = 466; Colombia, n = 80). Of the 546 samples from PLHIV, 63 were from cases confirmed to have histoplasmosis by isolation of H. capsulatum from clinical samples (35 from Guatemala and 28 from Colombia). Of the remaining 483 patients infected with HIV, 371 were reported to be infected only with HIV and 112 patients were diagnosed with other infections. Mycobacterium disease was diagnosed in 87 of these 112 patients, with 65 of these 87 patients having Mycobacterium tuberculosis infection and 22 having other mycobacterial infections. Other fungal infections were diagnosed in 21 patients: cryptococcosis (n = 12), Pneumocystis pneumonia (n = 3), paracoccidioidomycosis (PCM; n = 3), aspergillosis (n = 2), and candidiasis (n = 1). Bacterial infections by Salmonella enterica were diagnosed in 2 patients, and parasitic infection by Toxoplasma and viral infection by cytomegalovirus were diagnosed in 1 patient each. Finally, we added to the analysis a total of 43 urine samples from non-HIV-infected controls. The patients' samples are described in Fig. 1.
FIG 1.
Study subjects and urine samples analyzed during the validation of the commercial IMMY Histoplasma capsulatum antigen-capture monoclonal ELISA. ¥, three patients were coinfected with Mycobacterium (M. tuberculosis, n = 2; non-M. tuberculosis, n = 1); *, of the 87 patients, 65 were identified to be infected with M. tuberculosis and 22 were identified to be infected with non-M. tuberculosis mycobacteria; ≠, of the 87 patients with Mycobacterium disease, 4 presented with coinfection with Cryptococcus (n = 2) and with Toxoplasma and Clostridium (n = 1 each).
Patient diagnoses.
A case of PDH was considered proven if H. capsulatum was isolated from any of the following samples: blood, tissue, sterile fluids, or respiratory specimens (26). Blood and bone marrow cultures were tested using commercial methodologies, and samples were processed according to the manufacturers' instructions. Biopsy specimens, respiratory samples, and other body fluids were cultured on Mycosel (BD) and Sabouraud dextrose agar (BD), in tubes with Lowenstein-Jensen medium (BD), and in Mycobacterium growth indicator tubes (MGIT). We differentiated between M. tuberculosis and non-M. tuberculosis mycobacteria (NTM) using commercial molecular tests. Final patient diagnoses were made on the basis of laboratory and clinical criteria.
ELISA for detection of Histoplasma antigen.
The enzyme-linked immunosorbent assay (ELISA) antigen detection test (IMMY monoclonal ELISA [HGM]) is based on the use of a monoclonal antibody that recognizes a Histoplasma antigen, H. capsulatum galactomannan (HGM). Urine samples were processed according to the manufacturer's instructions, using a seven-point antigen standard curve with concentrations ranging from 0.4 to 25 ng/ml. Optical densities (ODs) were read at 450 nm, and the concentration of Histoplasma antigen was calculated on the basis of a 7-point calibration curve, generated using a 4-parameter equation. As an alternative, we evaluated six of the seven points of the antigen standard curve (0.8 to 25 ng/ml) in order to identify which was the best cutoff calibrator. The EIA index value (EIV) for each sample was calculated by dividing the mean OD of the sample by the mean OD of the calibrator, and the result of the division was multiplied by 10 (multiplication factor). The EIV calculation is summarized in the following equation:
CDC polyclonal ELISA (HPA).
The ELISA antigen detection test is based on the use of a polyclonal antibody that recognizes a H. capsulatum polysaccharide antigen (HPA), as previously described (10, 27).
Evaluation of the analytical performance of the IMMY monoclonal ELISA (HGM).
All ELISAs were performed independently in Guatemala and Colombia by different laboratory technicians. All samples were coded and analyzed in a double-blind design. The results of all analyses were then compared to verify the reproducibility of the results obtained. Calculation of the analytical performance of the test was done using 2-by-2 tables comparing ELISA results with the culture results for culture-proven cases. We also calculated the test sensitivity, specificity, accuracy, and positive and negative predictive values with their respective 95% confidence intervals (CI). ELISA receiver operating characteristic (ROC) curves were used to determine the ideal test cutoff value (28). Kappa values and their respective 95% CI were calculated by a concordance analysis in order to evaluate the agreement between the methods used (28). Analyses were conducted using STATA (version 3.1) and EPIDAT (version 8.0) software.
Ethics.
Participants signed an informed consent formulated for previous studies granting permission to use their samples in future research studies. The protocols were approved by the ethical committees of the Clínica Familiar Luis Angel Garcia/Hospital General San Juan de Dios in Guatemala and Hospital La María and Corporación para Investigaciones Biológicas in Medellín, Colombia.
RESULTS
Evaluation of the analytical performance of the IMMY monoclonal ELISA (HGM). (i) Quantitative antigen-capture ELISA (using a 7-point standard curve).
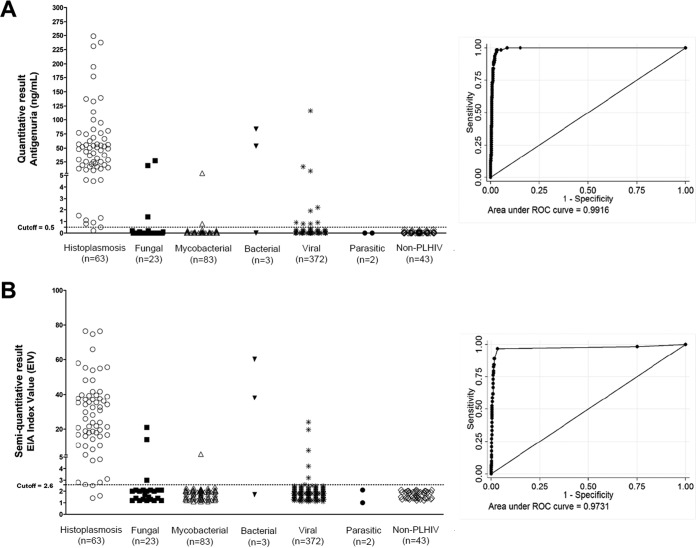
Manufacturer-recommended cutoff criteria considered a concentration of ≥0.5 ng/ml to be a positive result. The IMMY monoclonal ELISA (HGM) detected 62 of 63 culture-proven histoplasmosis cases (98%; 95% CI, 95 to 100%), with an overall specificity of 97% (95% CI, 95 to 99%). In the Guatemala cohort, a sensitivity of 100% (95% CI, 99 to 100%) was observed, and a sensitivity of 96% (95% CI, 88 to 100%) was observed in the Colombia cohort. In addition, this test showed a high specificity of 97% (95% CI, 96 to 99%) in the Guatemala cohort and 95% (95% CI, 90 to 100%) in the Colombia cohort. All ROC values were higher than 95% (Fig. 2A). Predictive values and kappa index values were also calculated and are summarized in Tables 1 and 2.
FIG 2.
Results of the H. capsulatum quantitative (A) and semiquantitative (B) antigen 2 capture ELISAs (IMMY). The levels of antigenuria in culture-proven histoplasmosis patients, persons with other diseases, and non-PLHIV controls were compared. All infectious diseases were coinfections with HIV in these patient cohorts.
TABLE 1.
Evaluation of the analytical performance of the quantitative IMMY monoclonal ELISA (HGM)a
| Cohort | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Combined (n = 589) | 98 (95–100) | 97 (95–99) | 97 (96–99) | 79 (70–89) | 100 (99–100) |
| Guatemala (n = 466) | 100 (99–100) | 97 (96–99) | 98 (96–99) | 76 (63–90) | 100 (99–100) |
| Colombia (n = 123) | 96 (88–100) | 95 (90–100) | 95 (91–99) | 84 (70–99) | 99 (97–100) |
n, number of samples; PPV, positive predictive value; NPV, negative predictive value. Values in parentheses are 95% confidence intervals.
TABLE 2.
Analysis of concordance between quantitative IMMY (HGM) and culturea
| Quantitative IMMY (HGM) result | No. of samples from the following cohorts with the indicated culture result: |
|||||
|---|---|---|---|---|---|---|
| Combined cohort (n = 589) |
Guatemala cohort (n = 466) |
Colombia cohort (n = 123) |
||||
| + | − | + | − | + | − | |
| + | 62 | 16 | 35 | 11 | 27 | 5 |
| − | 1 | 510 | 0 | 420 | 1 | 90 |
n, number of samples; +, positive result; −, negative result. The kappa index values for the combined cohort, Guatemala cohort, and Colombia cohort were 0.86 (95% CI, 0.79 to 0.93), 0.85 (95% CI, 0.76 to 0.94), and 0.87 (95% CI, 0.76 to 0.97), respectively.
(ii) Discrepant results against culture result using the quantitative IMMY monoclonal ELISA (HGM).
Urine samples from 16 patients with negative fungal cultures tested positive by the IMMY monoclonal ELISA (Table 3). Eleven were from Guatemalan patients. Those patients presented with the following clinical and laboratory findings: four patients had positive urinary CDC polyclonal ELISA (HPA) results (samples 1 to 4), two patients had a history of PDH with a negative CDC polyclonal ELISA (HPA) result below the cutoff (samples 5 and 6), and five patients were severely ill (median CD4 cell count, 51 cells/mm3) with symptoms of PDH but with negative fungal culture and negative CDC polyclonal ELISA (HPA) results (samples 7 to 11). Among these five patients, one had acute renal failure and coinfection with M. tuberculosis (sample 11). The other discrepant results were from two Colombian patients with bacterial disease who had a positive urinary CDC polyclonal ELISA (HPA) result and a positive antibody test for histoplasmosis (samples 13 and 14). Three samples presented cross-reactivity, and all were from patients with a diagnosis of paracoccidioidomycosis (samples 15, 16, and 17). Finally, a false-negative result was observed in a Colombian patient with culture-proven PDH (sample 18).
TABLE 3.
Characteristics of patient samples with discrepant results using the IMMY monoclonal ELISA (HGM)a
| Country, type of discrepant result, and sample no. | Case definition | IMMY monoclonal ELISA (HGM) result (antigenuria result)b |
Clinical and laboratory findings | |
|---|---|---|---|---|
| Quantitative | Semiquantitative | |||
| Guatemala | ||||
| Discrepant results against fungal negative culture | ||||
| 1 | HIV infection | 9.0 (+) | 7.6 (+) | History of PDH, CDC HPA positive (7.6 ng/ml) and 22 CD4 cells/μl |
| 2 | HIV infection | 116.4 (+) | 19.7 (+) | CDC HPA positive (21.9 ng/ml), 49 CD4 cells/μl, wt loss, anemia, and diarrhea |
| 3 | HIV infection | 16.6 (+) | 24.0 (+) | CDC HPA positive (8.1 ng/ml), 53 CD4 cells/μl, pulmonary symptoms, and fever |
| 4 | HIV infection | 0.9 (+) | 2.5 (−) | CDC HPA positive (3 ng/ml), 14 CD4 cells/μl, fever, and pulmonary symptoms |
| 5 | M. szulgai infection | 0.8 (+) | 2.4 (−) | History of PDH, CDC HPA negative (0.6 ng/ml) and 97 CD4 cells/μl; the patient was asymptomatic with no adherence to treatment |
| 6 | HIV infection | 0.8 (+) | 2.5 (−) | History of PDH, CDC HPA negative (0.46 ng/ml), 59 CD4 cells/μl, VL of 103,571 copies/ml, diarrhea, and wt loss |
| 7 | HIV infection | 0.8 (+) | 2.5 (−) | History of PDH, 169 CD4 cells/μl, and treatment with itraconazole for 6 mo |
| 8 | HIV infection | 2.2 (+) | 4.2 (+) | 5 CD4 cells/μl, VL of 227,098 copies/ml, chronic diarrhea, oral candidiasis, and wasting syndrome |
| 9 | HIV infection | 1.9 (+) | 3.2 (+) | 57 CD4 cells/μl, pulmonary symptoms, and fever postpartum |
| 10 | HIV infection | 0.9 (+) | 2.6 (+) | 51 CD4 cells/μl, VL of 171,475 copies/ml, diarrhea, wt loss, fever, oral candidiasis, and wasting syndrome |
| 11 | TB | 5.2 (+) | 5.5 (+) | 27 CD4 cells/μl, VL of 718,093 copies/ml, coinfection with M. tuberculosis, wasting syndrome, acute renal failure, and chronic diarrhea |
| False negative, 12 | PDH | 0.9 (+) | 2.5 (−) | Diagnosis of PDH by culture |
| Colombia | ||||
| Discrepant results against fungal negative culture | ||||
| 13 | Salmonella infection | 83.7 (+) | 60.2 (+) | CDC HPA positive (12.6 ng/ml) and ID positive (M band) |
| 14 | Salmonella infection | 53.8 (+) | 38.0 (+) | CDC HPA positive (12.9 ng/ml), ID positive (M band), and 44 CD4 cells/μl |
| Cross-reactivity | ||||
| 15 | Paracoccidioidomycosis | 27.3 (+) | 21.0 (+) | Cross-reactivity with Paracoccidioides brasiliensis antigens |
| 16 | Paracoccidioidomycosis | 18.7 (+) | 14.1 (+) | Cross-reactivity with P. brasiliensis antigens |
| 17 | Paracoccidioidomycosis | 1.4 (+) | 3.0 (+) | Cross-reactivity with P. brasiliensis antigens |
| False negative | ||||
| 18 | PDH | 0.2 (−) | 1.4 (−) | Diagnosis of PDH by culture |
| 19 | PDH | 0.5 (+) | 1.6 (−) | Diagnosis of PDH by culture |
HGM, Histoplasma galactomannan; EIV, EIA index value; PDH, progressive disseminated histoplasmosis; CDC HPA, CDC polyclonal ELISA (HPA); ID, immunodiffusion; VL, viral load; TB, tuberculosis. All infectious diseases were coinfections with HIV in these patient cohorts.
The quantitative IMMY monoclonal ELISA (HGM) result was determined using a standard curve cutoff value of ≥0.5 ng/ml. The semiquantitative IMMY monoclonal ELISA (HGM) result was determined using a calibrator value of 12.5 ng/ml and a cutoff value of an EIV of ≥2.6. Antigenuria result interpretation was as follows: +, positive result; −, negative result.
When we compared the quantitative results of the antigen CDC polyclonal ELISA (HPA) against the quantitative results of the IMMY monoclonal ELISA (HGM), we observed that the antigen concentrations obtained using the IMMY monoclonal ELISA (HGM) were 12 times higher than those obtained using the CDC polyclonal ELISA (HPA).
(iii) Semiquantitative antigen-capture ELISA (using a calibrator).
In an evaluation of the analytical performance of the six points of the standard curve as a calibrator (0.8 to 25 ng/ml), all points demonstrated the same accuracy (97%) for the diagnosis of histoplasmosis. Based on our results, we selected the antigen concentration standard of 12.5 ng/ml as a calibrator (Table 4). Using this calibrator, we determined as a cutoff an EIA index value (EIV) greater than or equal to 2.6. This choice had a sensitivity of 95% (95% CI, 89 to 100%), a specificity of 98% (95% CI, 96 to 99%), and an accuracy of 97% (95% CI, 96 to 99%). Independent analyses from the Guatemalan and Colombian laboratories showed comparable results. Detailed results for all calibrators, combined and per country, are described in Table 4. ROC analysis yielded an area under the curve (AUC) that corresponded to a test accuracy higher than 95% (Fig. 2B).
TABLE 4.
Evaluation of the analytical performance of the semiquantitative IMMY monoclonal ELISA (HGM)a
| Cohort and parameter | Values obtained with the following calibrator concentration (EIV cutoff): |
|||||
|---|---|---|---|---|---|---|
| 25 ng/ml (≥1.5) | 12.5 ng/ml (≥2.6) | 6.3 ng/ml (≥4.4) | 3.2 ng/ml (≥6.4) | 1.6 ng/ml (≥9.4) | 0.8 ng/ml (≥15) | |
| Combined (n = 589) | ||||||
| Sen (%) | 94 (87–100) | 95 (89–100)b | 93 (87–100) | 92 (85–100) | 92 (85–100) | 89 (80–97) |
| Spe (%) | 97 (96–99) | 98 (96–99) | 98 (97–99) | 98 (97–99) | 98 (97–99) | 98 (97–99) |
| Acc (%) | 97 (96–98) | 97 (96–99) | 97 (96–99) | 97 (96–99) | 97 (96–99) | 97 (96–99) |
| PPV (%) | 80 (71–91) | 83 (74–93) | 84 (75–94) | 84 (75–93) | 84 (75–93) | 86 (77–95) |
| NPV (%) | 99 (98–100) | 99 (99–100) | 99 (98–100) | 99 (98–100) | 99 (98–100) | 99 (98–100) |
| Guatemala (n = 466) | ||||||
| Sen (%) | 94 (85–100) | 97 (90–100)b | 94 (85–100) | 91 (81–100) | 91 (81–100) | 85 (73–99) |
| Spe (%) | 98 (96–99) | 98 (97–100) | 98 (97–100) | 99 (97–100) | 99 (97–100) | 99 (99–100) |
| Acc (%) | 98 (96–99) | 98 (97–100) | 98 (97–100) | 98 (97–99) | 98 (97–99) | 98 (96–99) |
| PPV (%) | 79 (65–92) | 82 (70–96) | 85 (72–97) | 84 (71–97) | 84 (71–97) | 88 (76–100) |
| NPV (%) | 100 (98–100) | 100 (99–100) | 100 (99–100) | 99 (98–100) | 99 (98–100) | 99 (98–100) |
| Colombia (n = 123) | ||||||
| Sen (%) | 93 (81–100) | 93 (81–100) | 93 (81–100) | 93 (81–100) | 93 (81–100) | 93 (81–100) |
| Spe (%) | 95 (90–100) | 95 (90–100) | 95 (90–100) | 95 (90–100) | 95 (90–100) | 95 (90–100) |
| Acc (%) | 94 (90–99) | 94 (90–99) | 94 (90–99) | 94 (90–99) | 94 (90–99) | 94 (90–99) |
| PPV (%) | 84 (69–98) | 84 (69–98) | 84 (69–98) | 84 (69–98) | 84 (69–98) | 84 (69–98) |
| NPV (%) | 98 (94–100) | 98 (94–100) | 98 (94–100) | 98 (94–100) | 98 (94–100) | 98 (94–100) |
n, number of samples; CI, confidence interval; Sen, sensitivity; Spe, specificity; Acc, accuracy; PPV, positive predictive value; NPV, negative predictive value. Values in parentheses are 95% confidence intervals.
Better performance.
(iv) Discrepant results with semiquantitative methodology.
We used the point of the standard curve with a concentration of 12.5 ng/ml as a calibrator and a cutoff of 2.6 EIV. We observed positive results in 9 of the 13 patients with results discrepant with the culture results using the quantitative methodology (Table 3, samples 1 to 3, 8 to 11, 13, and 14). Cross-reactivity was observed in the same three paracoccidioidomycosis patients (samples 15 to 17). Three false-negative results were observed: for the Colombian patient with a negative quantitative ELISA result (sample 18) and two patients with positive quantitative ELISA results (samples 12 and 19). The findings are summarized in Table 3.
DISCUSSION
In this study, we report the successful multicenter validation of commercial reagents (IMMY monoclonal ELISA [HGM]) with high analytical performance and reproducibility for the rapid detection of urinary Histoplasma antigen in two Latin America laboratories, using a 7-point standard curve (quantitative assay) or an alternative calibrator (semiquantitative assay). The semiquantitative method could offer results similar to those obtained using a quantitative test but at a lower cost (seven ELISA wells in the quantitative test for the standard curve versus two ELISA wells in the semiquantitative test for the calibrator). However, modification of protocols requires approval by the test manufacturer. This assay uses urine, an easily obtained clinical specimen, and the ELISA can be performed in <3 h. These reagents were shown to be robust and highly reproducible and significantly reduced the time to diagnosis of PDH.
The results of the quantitative and semiquantitative IMMY monoclonal ELISA (HGM) presented high levels of agreement with those of fungal culture (97% and 98%, respectively). The quantitative methodology was able to identify as positive 62/63 (98%) urine samples from patients with PDH, and the semiquantitative methodology detected as positive 60/63 (95%) urine samples from culture-proven cases. These results showed that the IMMY monoclonal ELISA (HGM) had a higher analytical performance than the previously reported analytical performance of the CDC polyclonal ELISA (HPA), with the CDC polyclonal ELISA having a sensitivity of 81% and 86% and a specificity of 95% and 94% in Guatemala and Colombia, respectively (10, 23). Variabilities in quantitative antigen concentrations between the assays are likely due to the different detection antibodies used: the CDC polyclonal ELISA uses a polyclonal antibody, and the IMMY monoclonal ELISA uses a monoclonal antibody. These commercial reagents were shown to be robust, and they provide an easy method that may be implemented in clinical laboratories with a capacity to perform ELISAs. In addition, our results are consistent with those previously presented in a report describing an evaluation of the same reagents in the United States (sensitivity, 91%; specificity, 96%), indicating that these commercial reagents can be successfully used in different laboratories (24).
We observed in the quantitative analysis 16 samples with discrepant results, which had positive urinary antigen test results and negative culture results. Cross-reactions were observed in three samples from patients with paracoccidioidomycosis (PCM). Prior studies of H. capsulatum antigenuria tests have reported cross-reactivities of 28% and 88% with urine from patients with PCM (10, 29, 30). It is important to note that PCM is diagnosed much less frequently than histoplasmosis in PLHIV, and there are several other immunological tests that help in the diagnosis of PCM (2, 17). The remaining 13 discrepant results were from 11 samples from patients from Guatemala, where it is possible that the IMMY monoclonal ELISA (HGM) correctly identified circulating antigen in seven patients (64%): four patients who presented with a positive CDC polyclonal ELISA (HPA) result and three patients who had a previous diagnosis of PDH and a low rate of adherence to fungal therapy. The remaining two discrepant results were from the Colombian group. Those patients presented with a positive serological test result and a positive CDC polyclonal ELISA (HPA) result but a negative fungal culture result. As previously described, fungal culture is less sensitive than the antigen test (31). Finally, one false-negative result was identified, which was for a patient treated with trimethoprim-sulfamethoxazole as prophylaxis for Pneumocystis pneumonia. This medication is also used for the treatment of paracoccidioidomycosis, so it is possible that the medication could have lowered the fungal burden of this patient, subsequently reducing the levels of circulating H. capsulatum galactomannan antigen (32). As reported previously by Wheat et al., the use of antifungal treatment decreases circulating antigen levels (33). Another consideration is that histoplasmosis may cause proteinuria, which could limit the ability to detect the antigen. Further studies could evaluate the antigen concentrations during antifungal therapy and at follow-up to better establish this relation as well as renal failure and its influence on clinical results (34).
The semiquantitative methodology presented fewer results (n = 4 samples) discrepant with the negative fungal culture results than the quantitative test did, but it is important to mention that the semiquantitative methodology was slightly less sensitive than the quantitative methodology in detecting urinary Histoplasma antigen in culture-proven cases (three samples versus one sample had false-negative results by the two tests, respectively). We also mention that these samples had a low concentration of antigen by the quantitative methodology, leading semiquantitative analysis to classify those as negative.
The prevalence of histoplasmosis in the overall cohort was 11%, with the prevalence being 8% in Guatemala and 23% in Colombia, but stratified analysis of the analytical performance showed similar results between the two countries. The differences in prevalence may be a result of differences between the study sites, as the Guatemala study site forms part of one of the largest HIV programs in the country, whereas the Colombia study site is involved only in a local HIV program in the city of Medellín. We observed high negative predictive values using the IMMY monoclonal ELISA (HGM) kit (close to 100%), making this test a good tool to discard PDH diagnoses in patients with negative results. On the other hand, we observed a positive predictive value of 79% (95% CI, 70 to 89%), and this value was affected principally by false-positive results as a consequence of cross-reactivity in patients with a diagnosis of paracoccidioidomycosis and a history of PDH. Finally, it is important to highlight that clinical decisions should be based on the correlation of the clinical, epidemiological, and laboratory findings presented by the patients at the time of diagnosis.
Our study also has some limitations inherent to retrospective analyses (access to the patients' medical information). We did not evaluate the IMMY monoclonal ELISA (HGM) in non-HIV-infected immunocompromised patients, who may also be at risk of development of PDH. Serum specimens were not tested in this study, and further evaluation of this assay with serum appears to be warranted. The laboratories included in this study have expertise implementing ELISAs for H. capsulatum detection, and it is necessary to provide training and technical support to those laboratories interested in performing this methodology for the first time.
As a final conclusion, the high sensitivity and the high specificity of the commercial antigen-capture ELISA for the diagnosis of histoplasmosis in PLHIV were demonstrated in two laboratories in Latin America. The semiquantitative and quantitative ELISAs showed similar results; this finding could decrease the cost of testing, facilitating the use of the IMMY monoclonal ELISA (HGM) in developing countries. This assay uses urine, an easily obtained clinical specimen. The ELISA can be performed in less than 3 h, and this technique is robust and highly reproducible and significantly reduces the time to diagnosis of PDH.
ACKNOWLEDGMENTS
We thank the medical staff of Hospital La María in Medellín, Colombia, and the Asociación de Salud Integral and Clinica Familiar Luis Garcia, Hospital General San Juan de Dios, in Guatemala City, Guatemala. Our thanks also go to the laboratory diagnosis staff of the Medical and Experimental Mycology Group (CIB), Catalina de Bedout, Luz E. Cano, Alejandra Zuluaga, and Karen Arango and to Oliver Clay for English editing of the manuscript.
The work presented here was supported in part by the Global Disease Detection Program, CDC, Atlanta, GA, USA; by Colciencias, Bogotá, Colombia, via the Program for Young Investigators and Innovators; and by the Oak Ridge Institute for Science and Education (ORISE), Oak Ridge, TN, USA. We also thank the Corporación para Investigaciones Biológicas (CIB), Medellín, Colombia; the Fondo de Investigaciones de la Universidad del Rosario (FIUR), Bogota, Colombia; and the Asociación de Salud Integral.
We report no conflicts of interest.
ELISA reagents were donated by IMMY, Norman, OK, USA.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
REFERENCES
- 1.Bahr NC, Antinori S, Wheat LJ, Sarosi GA. 2015. Histoplasmosis infections worldwide: thinking outside of the Ohio River Valley. Curr Trop Med Rep 2:70–80. doi: 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M. 2011. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49:785–798. doi: 10.3109/13693786.2011.577821. [DOI] [PubMed] [Google Scholar]
- 3.Tobon A, Gomez BL. 2015. Histoplasmosis. In Vesga O, Vélez LA, Leiderman E, Restrepo A (ed), Enfermedades infecciosas de Homo sapiens, 1st ed Fondo Editorial CIB, Medellin, Colombia. [Google Scholar]
- 4.Deepe GS. 2015. Histoplasma capsulatum, p 2949–2962. In Bennett JE, Dolin R, Blaser MJ (ed). Principles and practice of infectious diseases, 8th ed Elsevier, Philadelphia, PA. [Google Scholar]
- 5.Caceres DH, Zuluaga A, Arango-Bustamante K, de Bedout C, Tobón AM, Restrepo A, Gómez BL, Cano LE, González A. 2015. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg 93:662–667. doi: 10.4269/ajtmh.15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nacher M, Adenis A, McDonald S, Do Socorro Mendonca Gomes M, Singh S, Lopes Lima I, Malcher Leite R, Hermelijn S, Wongsokarijo M, Van Eer M, Marques Da Silva S, Mesquita Da Costa M, Silva M, Calvacante M, do Menino Jesus Silva Leitao T, Gómez BL, Restrepo A, Tobón A, Canteros CE, Aznar C, Blanchet D, Vantilcke V, Vautrin C, Boukhari R, Chiller T, Scheel C, Ahlquist A, Roy M, Lortholary O, Carme B, Couppié O, Vreden S. 2013. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis 7:e2319. doi: 10.1371/journal.pntd.0002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caceres DH, Tobón AM, Cleveland AA, Scheel CM, Berbesi DY, Ochoa J, Restrepo A, Brandt ME, Chiller T, Gómez BL. 2016. Clinical and laboratory profile of persons living with human immunodeficiency virus/acquired immune deficiency syndrome and histoplasmosis from a Colombian hospital. Am J Trop Med Hyg 95:918–924. doi: 10.4269/ajtmh.15-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cáceres DH, Gómez BL, Restrepo A, Tobón AM. 2012. Histoplasmosis and AIDS: clinical and laboratory risk factors associated with the disease's prognosis. Infection 16:44–50. doi: 10.1016/S0123-9392(12)70026-7. [DOI] [Google Scholar]
- 9.Gómez BL. 2011. Histoplasmosis: epidemiology in Latin America. Curr Fungal Infect Rep 5:199–205. doi: 10.1007/s12281-011-0073-7. [DOI] [Google Scholar]
- 10.Scheel CM, Samayoa B, Herrera A, Lindsley M, Benjamin LL, Reed Y, Hart J, Lima S, Rivera BE, Raxcacoj G, Chiller TM, Arathoon E, Gómez BL. 2009. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin Vaccine Immunol 16:852–858. doi: 10.1128/CVI.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ Jr. 2008. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis 62:151–156. doi: 10.1016/j.diagmicrobio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Medina N, Samayoa B, Lau-Bonilla D, Denning DW, Herrera R, Mercado D, Guzmán B, Pérez JC, Arathoon E. 2017. Burden of serious fungal infections in Guatemala. Eur J Clin Microbiol Infect Dis 36:965–969. doi: 10.1007/s10096-017-2920-0. [DOI] [PubMed] [Google Scholar]
- 13.Samayoa B, Roy M, Cleveland AA, Medina N, Lau-Bonilla D, Scheel CM, Gomez BL, Chiller T, Arathoon E. 2017. High mortality and coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. Am J Trop Med Hyg 97:42–48. doi: 10.4269/ajtmh.16-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nacher M, Adenis A, Sambourg E, Huber F, Abboud P, Epelboin L, Mosnier E, Vantilcke V, Dufour J, Djossou F, Demar M, Couppié P. 2014. Histoplasmosis or tuberculosis in HIV-infected patients in the Amazon: what should be treated first? PLoS Negl Trop Dis 8:e3290. doi: 10.1371/journal.pntd.0003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, Aznar C, Carme B, Couppie P. 2014. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: a comparative study. Am J Trop Med Hyg 90:216–223. doi: 10.4269/ajtmh.13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauffman CA. 2008. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 21:421–425. doi: 10.1097/QCO.0b013e328306eb8d. [DOI] [PubMed] [Google Scholar]
- 17.Pfeiffer CD, Wong B. 2015. Diagnostic immunology, p 45–64. In Hospenthal DR, Rinaldi MG (ed). Diagnosis and treatment of human mycoses, 2nd ed Springer International Publishing Switzerland, Cham, Switzerland. [Google Scholar]
- 18.Hage CA, Azar MM, Bahr N, Loyd J, Wheat LJ. 2015. Histoplasmosis: up-to-date evidence-based approach to diagnosis and management. Semin Respir Crit Care Med 36:729–745. doi: 10.1055/s-0035-1562899. [DOI] [PubMed] [Google Scholar]
- 19.Wheat LJ, Connolly P, Durkin M, Book BK, Pescovitz MD. 2006. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transpl Infect Dis 8:219–221. doi: 10.1111/j.1399-3062.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. 2007. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol 14:1587–1591. doi: 10.1128/CVI.00071-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fandiño-Devia E, Rodríguez-Echeverri C, Cardona-Arias J, Gonzalez A. 2016. Antigen detection in the diagnosis of histoplasmosis: a meta-analysis of diagnostic performance. Mycopathologia 181:197–205. [DOI] [PubMed] [Google Scholar]
- 22.LeMonte A, Egan L, Connolly P, Durkin M, Wheat LJ. 2007. Evaluation of the IMMY ALPHA Histoplasma antigen enzyme immunoassay for diagnosis of histoplasmosis marked by antigenuria. Clin Vaccine Immunol 14:802–803. doi: 10.1128/CVI.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caceres DH, Scheel C, Tobón AM, Cleveland AA, Restrepo A, Brandt ME, Chiller T, Gómez BL. 2014. Validation of an enzyme-linked immunosorbent assay that detects Histoplasma capsulatum antigenuria in Colombian patients with AIDS for diagnosis and follow-up during therapy. Clin Vaccine Immunol 21:1364–1368. doi: 10.1128/CVI.00101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Gibson B Jr, Daly TM. 2013. Evaluation of commercially available reagents for diagnosis of histoplasmosis infection in immunocompromised patients. J Clin Microbiol 51:4095–4101. doi: 10.1128/JCM.02298-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theel ES, Harring JA, Dababneh AS, Rollins LO, Bestrom JE, Jespersen DJ. 2015. Reevaluation of commercial reagents for detection of Histoplasma capsulatum antigen in urine. J Clin Microbiol 53:1198–1203. doi: 10.1128/JCM.03175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsley MD, Holland HL, Bragg SL, Hurst SF, Wannemuehler KA, Morrison CJ. 2007. Production and evaluation of reagents for detection of Histoplasma capsulatum antigenuria by enzyme immunoassay. Clin Vaccine Immunol 14:700–709. doi: 10.1128/CVI.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orozco LC. 2010. Validación de criterio o de la sensibilidad específica para predecir la calidad de las probabilidades, p 115–157. In Orozco LC. (ed), Medición en salud: diagnóstico y evaluación de resultados: un manual critico más allá de lo básico, 1st ed Universidad Industrial de Santander, Bucaramanga, Santander, Colombia. [Google Scholar]
- 29.Wheat J, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Nelson K, Bradsher R, Restrepo A. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis 24:1169–1171. doi: 10.1086/513647. [DOI] [PubMed] [Google Scholar]
- 30.Gomez BL, Figueroa JI, Hamilton AJ, Ortiz BL, Robledo MA, Restrepo A, Hay RJ. 1997. Development of a novel antigen detection test for histoplasmosis. J Clin Microbiol 35:2618–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azar MM, Hage CA. 2017. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 55:1612–1620. doi: 10.1128/JCM.02430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brilhante RS, Fechine MA, Cordeiro RDA, Rocha MF, Ribeiro JF, Monteiro AJ, de Lima RA, Mesquita JR, de Camargo ZP, Sidrim JJ. 2010. In vitro effect of sulfamethoxazole-trimethoprim against Histoplasma capsulatum var. capsulatum. Antimicrob Agents Chemother 54:3978–3979. doi: 10.1128/AAC.00793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheat LJ, Garringer T, Brizendine E, Connolly P. 2002. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn Microbiol Infect Dis 43:29–37. doi: 10.1016/S0732-8893(02)00367-X. [DOI] [PubMed] [Google Scholar]
- 34.Kushnir MM, Crockett DK, Cloud JL, Ashwood ER, Rockwood AL. 2011. Exploratory study of proteins in urine of patients with histoplasma antigenuria. J Chromatogr B Analyt Technol Biomed Life Sci 883–884:147–154. doi: 10.1016/j.jchromb.2011.09.006. [DOI] [PubMed] [Google Scholar]