ABSTRACT
Increasing utilization of vancomycin due to the high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infections has led to the emergence of vancomycin-intermediate S. aureus (VISA) and heterogeneous VISA (hVISA) strains. In vitro data suggest the potential for potent synergy between several beta-lactams and vancomycin. The objective of this study is to evaluate the synergy between beta-lactams and vancomycin against MRSA that is vancomycin susceptible, vancomycin-susceptible Staphylococcus aureus (VSSA), hVISA, and VISA. Fifty randomly selected clinical MRSA strains with various susceptibility levels to vancomycin were evaluated for vancomycin alone and vancomycin in combination with various concentrations of cefazolin (CFZ), cefepime (FEP), ceftaroline (CPT), and nafcillin (NAF). The potential for synergy was assessed by 24-h time-kill studies. Beta-lactams reduced vancomycin MIC values against all strains (4- to 16-fold reduction). In time-kill studies against MRSA, CFZ, FEP, CPT, and NAF all demonstrated similar degrees of killing at 24 h, and all showed synergistic activity with vancomycin against VSSA, hVISA, and VISA. Each of these combinations was also superior to any single agent against isolates of all three phenotypes, and each was bactericidal (P < 0.001 for all comparisons). All single-agent exposures demonstrated no activity at 24 h. The combination of vancomycin and beta-lactams significantly improved antibacterial activity against VSSA, hVISA, and VISA strains compared to the activity of any agent alone, supporting the potential use of vancomycin–beta-lactam combination therapy in infections caused by MRSA. Further clinical research is warranted to investigate the synergy of vancomycin against these Staphylococcus strains.
KEYWORDS: Staphylococcus aureus, MRSA, combination therapy, vancomycin, ceftaroline, cefazolin, nafcillin, cefepime, infection, in vitro, time kill
INTRODUCTION
Staphylococcus aureus, especially methicillin-resistant Staphylococcus aureus (MRSA), remains a major cause of serious infections and is associated with increased health care costs, morbidity, and mortality (1–6). Vancomycin has been the mainstay of therapy for MRSA infections. However, decades of selective pressure have led to mounting concerns regarding the efficacy of vancomycin against MRSA (7, 8). This necessitates alternative treatment strategies, including combination therapies and dose optimization as well as newer strategies based on the “seesaw effect,” whereby beta-lactam susceptibility increases as glycopeptide susceptibility decreases (9–11).
The use of combination antimicrobial therapy is a common treatment option for infection with MRSA, especially when first-line therapy fails. Beta-lactams are often empirical choices for combination use with vancomycin, especially to expand coverage for potential Gram-negative pathogens (12). Zheng et al. during a 28-day in vitro study showed that the combination treatment with vancomycin and beta-lactams could significantly impact the transition to vancomycin-intermediate Staphylococcus aureus (VISA), thus effectively preventing the emergence of vancomycin nonsusceptibility (13). Several investigations have reported synergistic effects against Staphylococcus aureus when beta-lactams and glycopeptides including vancomycin and teicoplanin are combined (9, 14). There are also several investigations that have used pharmacokinetic/pharmacodynamic models that have reported synergy with vancomycin and nafcillin (NAF) or cefazolin (CFZ) against MRSA strains, including heterogeneous VISA (hVISA) strains (15, 16). In addition, there are a number of investigations that have reported an inverse relationship between glycopeptide and beta-lactam susceptibility, further demonstrating the so-called seesaw effect (9, 17–19).
The mechanisms for the seesaw effect are not well understood; however, researchers have suggested that beta-lactam exposure alters the surface of VISA but may increase target-specific vancomycin binding, thereby improving vancomycin-cell wall interactions and vancomycin activity. Further, data have demonstrated that ceftaroline (CPT) and nafcillin exposure significantly reduces cell wall thickness in Staphylococcus aureus, preventing vancomycin sequestration and improving vancomycin penetration into the division septum, which is the main location for cell wall synthesis and the interaction of vancomycin with critical membrane-bound precursors (20–22).
There is some clinical evidence that combination therapy with vancomycin and beta-lactams improved microbiological eradication of MRSA bacteremia compared to use of vancomycin alone. These clinical data were demonstrated in both randomized controlled trials and retrospective analyses, supporting the use of beta-lactams with vancomycin as a potential alternative therapy to combat Gram-positive pathogens and improve patient outcomes (23, 24).
The objective of this study was to further explore the synergistic effects between beta-lactams and vancomycin against Staphylococcus strains with various susceptibilities to vancomycin by MIC and time-kill analysis.
RESULTS
Susceptibility testing.
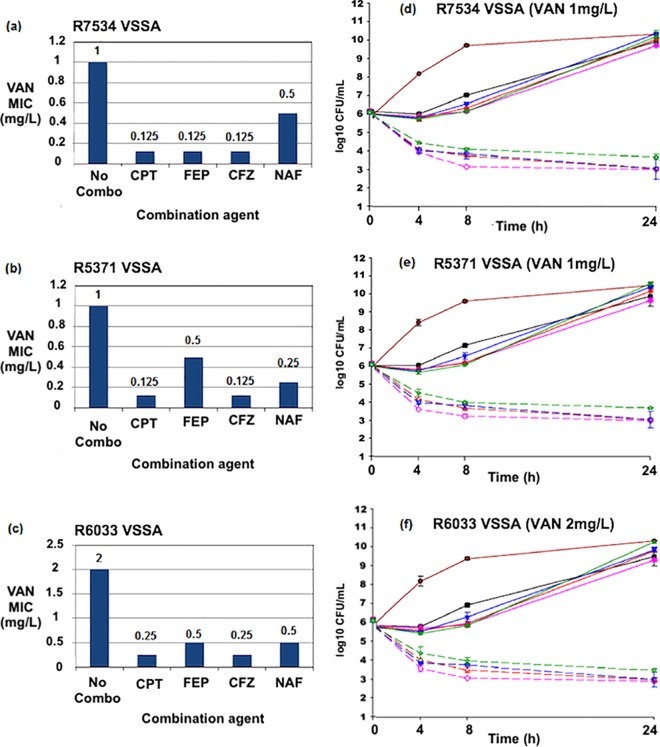
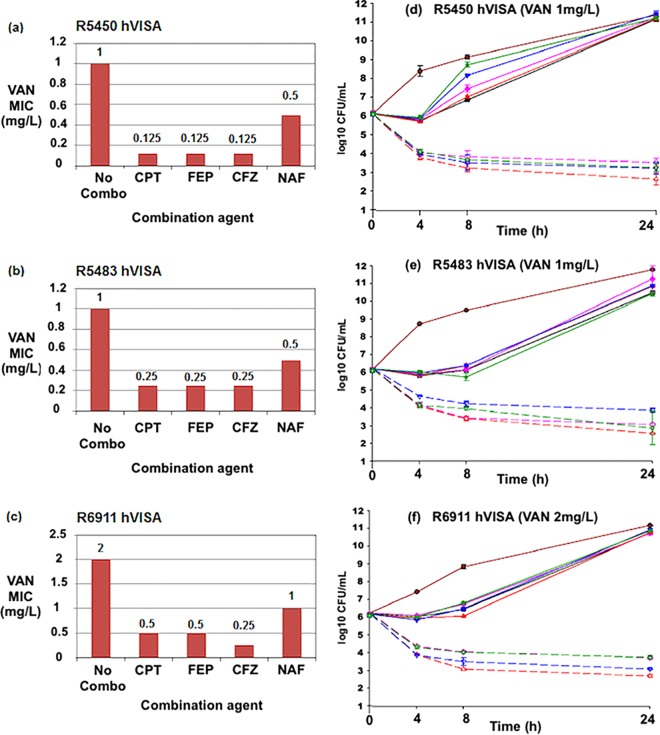
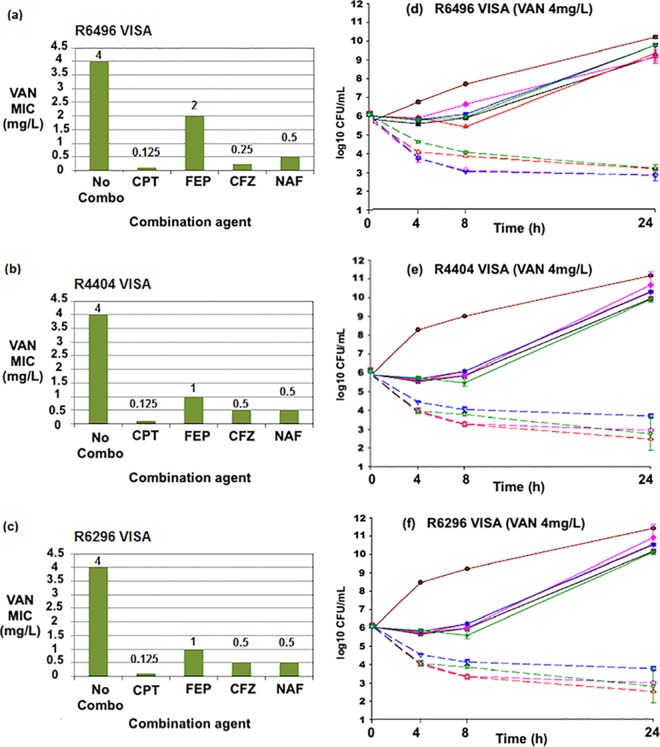
The ranges of MICs of vancomycin alone and in combination with beta-lactams against VSSA, hVISA, and VISA strains are listed in Table 1. The fold reductions in baseline vancomycin MICs as a result of the combination of vancomycin with beta-lactams are listed in Table 2. Vancomycin MIC values for 15 VSSA strains and 20 hVISA strains ranged from 0.5 to 2.0 μg/ml. The impact of the addition of the beta-lactam on the vancomycin MIC is shown in Table 2. The vancomycin MIC for all 15 VISA strains was 4 μg/ml. Vancomycin MIC values were reduced in the presence of cefazolin, cefepime (FEP), ceftaroline, and nafcillin in all strains. Against 15 VSSA strains, ceftaroline demonstrated the greatest reduction in vancomycin MIC values of all antimicrobial agents. In descending order of reduction, cefazolin, nafcillin, and cefepime provided reduced vancomycin MICs as well. Against the 15 VISA strains, ceftaroline again demonstrated the greatest fold reduction of the vancomycin MIC. Against 20 hVISA strains, the reduction in the vancomycin MIC with ceftaroline was similar to that of cefazolin and cefepime. Nafcillin provided lower reductions in vancomycin MIC values against hVISA than other antimicrobial agents and against VSSA and VISA strains. Three hVISA (R5450, R5483, and R6911), three VSSA (R7534, R5371, and R6033), and three VISA (R6496, R4404, and R6296) strains selected for time-kill studies are illustrated graphically in Fig. 1a to c, 2a to c, and 3a to c, respectively.
TABLE 1.
MIC of vancomycin alone and in combination with beta-lactams against MRSA
| Phenotype (n)a | MIC range (μg/ml)b |
||||
|---|---|---|---|---|---|
| VAN alone | VAN + CPT | VAN + FEP | VAN + CFZ | VAN + NAF | |
| VSSA (15) | 0.5–2 | 0.0625–0.25 | 0.0625–1 | 0.0625–0.25 | 0.125–0.5 |
| hVISA (20) | 0.5–2 | 0.0625–0.5 | 0.0625–0.5 | 0.125–0.5 | 0.125–1 |
| VISA (15) | 4c | 0.0625–0.25 | 0.5–2 | 0.0625–0.5 | 0.125–1 |
VSSA, vancomycin-susceptible S. aureus; VISA, vancomycin-intermediate S. aureus; hVISA, heteroresistant VISA; n, number of isolates tested.
VAN, vancomycin; CPT, ceftaroline; FEP, cefepime; CFZ, cefazolin; NAF, nafcillin.
Value for all tested isolates.
TABLE 2.
MIC reductions of vancomycin in combinations with beta-lactams against MRSAa
| Phenotype (n)b | VAN MIC reduction (fold change from baseline) |
|||
|---|---|---|---|---|
| Mean | SD | Median | Range | |
| VSSA (15) | ||||
| VAN + CPT | 8.00 | 2.62 | 8 | 4–16 |
| VAN + FEP | 3.60 | 2.03 | 4 | 2–8 |
| VAN + CFZ | 7.47 | 1.41 | 8 | 4–8 |
| VAN + NAF | 4.13 | 1.19 | 4 | 2–8 |
| hVISA (20) | ||||
| VAN + CPT | 4.90 | 2.19 | 4 | 2–8 |
| VAN + FEP | 4.00 | 1.95 | 4 | 2–8 |
| VAN + CFZ | 4.30 | 2.08 | 4 | 2–8 |
| VAN + NAF | 2.60 | 0.94 | 2 | 2–4 |
| VISA (15) | ||||
| VAN + CPT | 35.20 | 16.21 | 32 | 16–64 |
| VAN + FEP | 4.13 | 1.77 | 4 | 2–8 |
| VAN + CFZ | 18.67 | 14.71 | 16 | 8–64 |
| VAN + NAF | 12.00 | 6.93 | 8 | 4–32 |
MRSA, methicillin-resistant S. aureus.
VAN, vancomycin; CPT, ceftaroline; FEP, cefepime; CFZ, cefazolin; NAF, nafcillin; VSSA, vancomycin-susceptible S. aureus; VISA, vancomycin-intermediate S. aureus; hVISA, heteroresistant VISA; n, number of isolates tested.
FIG 1.
(a to c) Vancomycin (VAN) MIC values in the presence of several beta-lactam agents against VSSA strains R7534, R5371, and R6033. No combo, vancomycin MIC values in Mueller-Hinton broth without the presence of beta-lactams. (d to f) Twenty-four-hour time-kill curves against strains R7534, R5371, and R6033. CPT, ceftaroline; FEP, cefepime; CFZ, cefazolin; NAF, nafcillin. GC, drug-free growth control. Vancomycin was used at 0.5x the MIC in each experiment. Concentrations of CFZ, CPT, FEP, and NAF were performed at 0.5x the MIC or the biological free peak concentration, whichever was lower. Color identification: maroon solid line, growth control; black solid line, VAN; pink solid line, CFZ; pink dashed line, VAN+CFZ; red solid line, CPT; red dashed line, VAN+CPT; blue solid line, FEP; blue dashed line, VAN+FEP; green solid line, NAF; green dashed line, VAN+NAF.
FIG 2.
(a to c) Vancomycin (VAN) MIC values in the presence of several beta-lactam agents against hVISA strains R5450, R5483, and R6911. No combo, vancomycin MIC values in Mueller-Hinton broth without the presence of beta-lactams. (d to f) Twenty-four-hour time-kill curves against strains R5450, R5483, and R6911. CPT, ceftaroline; FEP, cefepime; CFZ, cefazolin; NAF, nafcillin. GC, drug-free growth control. Vancomycin was used at 0.5x the MIC in each experiment. Concentrations of CFZ, CPT, FEP, and NAF were performed at 0.5x the MIC or the biologic free peak, whichever was lower. Color identification: maroon solid line, growth control; black solid line, VAN; pink solid line, CFZ; pink dashed line, VAN+CFZ; red solid line, CPT; red dashed line, VAN+CPT; blue solid line, FEP; blue dashed line, VAN+FEP; green solid line, NAF; green dashed line, VAN+NAF.
FIG 3.
(a to c) Vancomycin (VAN) MIC values in the presence of several beta-lactam agents against VISA strains R6496, R4404, and R6296. No combo, vancomycin MIC values in Mueller-Hinton broth without the presence of beta-lactams. (d to f) Twenty-four-hour time-kill curves against strains R6496, R4404, and R6296. Broken lines, single agents; continuous lines, combination regimens. CPT, ceftaroline; FEP, cefepime; CFZ, cefazolin; NAF, nafcillin. GC, drug-free growth control. Vancomycin was used at 0.5x the MIC in each experiment. Concentrations of CFZ, CPT, FEP, and NAF were performed at 0.5x the MIC or the biologic free peak, which ever was lower. Color identification: maroon solid line, growth control; black solid line, VAN; pink solid line, CFZ; pink dashed line, VAN+CFZ; red solid line, CPT; red dashed line, VAN+CPT; blue solid line, FEP; blue dashed line, VAN+FEP; green solid line, NAF; green dashed line, VAN+NAF.
Time-kill analysis.
In time-kill studies against the hVISA strains R5450, 5483, and 6911 (Fig. 1d to f), ceftaroline, cefazolin, cefepime, and nafcillin all demonstrated synergistic activity with vancomycin. The combination of vancomycin plus ceftaroline was more active than the combination of vancomycin plus any other tested antimicrobial. Against the VSSA strains R7534, 5371, and 6033 (Fig. 2d to f), synergy with vancomycin was demonstrated for ceftaroline, cefazolin, cefepime, and nafcillin. The combination of vancomycin and nafcillin was not as active as the combination of vancomycin and other beta-lactams.
In time-kill studies against the VISA strains, R6496, 4404, and 6296 (Fig. 3d to f), CPT, CFZ, and FEP all demonstrated similar degrees of killing at 24 h, and all showed synergistic activity with vancomycin.
Each of these combinations was also superior to any single agent against isolates of all three of these phenotypes, and each was bactericidal as well (P < 0.001 for all comparisons). All single-agent exposures demonstrated no activity at 24 h. The activity of vancomycin combined with any of the beta-lactams was superior (P < 0.001) to that of any agent alone.
DISCUSSION
In this investigation, we evaluated a large group of beta-lactams combined with vancomycin to evaluate the potential for synergy against MRSA strains with various susceptibilities to vancomycin. We demonstrated that, when combined with vancomycin, several beta-lactams, namely, ceftaroline, cefazolin, cefepime, and nafcillin, all lowered the vancomycin MIC value and provided synergistic activity via time-kill analysis against VSSA, hVISA, and VISA strains.
Based on combination susceptibility and time-kill curve analysis, ceftaroline had the most beneficial effect when it was combined with vancomycin. It is of interest that a similar effect was observed for all tested beta-lactam agents in combination with vancomycin even though only ceftaroline had anti-MRSA activity.
One potential explanation for this observation is the hindrance of peptidoglycan biosynthesis, in this case by vancomycin, which leads to reduced beta-lactam resistance and decreases the potential for unwanted antibacterial resistance. MRSA strains contain an extra penicillin binding protein (PBP), PBP2a, in addition to the four PBPs found in all staphylococcal strains. In the presence of beta-lactams, PBP2a is assumed to be a transpeptidase essential for continued cell wall synthesis and growth, performing all cross-linking of the pentaglycine cross-bridges by transpeptidation of the terminal d-Ala (25, 26). Another potential explanation is that vancomycin and beta-lactams inhibit peptidoglycan polymerization through distinct mechanisms at different stages of cell wall synthesis and thus potentiate each other's effects (17, 24, 27). While there has been some data to suggest that beta-lactam specificity for PBP1 may be important for synergy between daptomycin and beta-lactams, the same specificity has not been shown with vancomycin and beta-lactams to our knowledge (28). In fact, we were able to demonstrate an advantage only with ceftaroline, which clearly has increased affinity for PBP2a.
Additionally, previous studies have demonstrated that beta-lactam exposure increases vancomycin activity as well. It has been shown that a thickened cell wall is one of the primary mechanisms responsible for vancomycin nonsusceptibility of Staphylococcus aureus (29). The thickened cell wall not only traps vancomycin molecules but also significantly reduces the time that vancomycin completely inhibits peptidoglycan synthesis (27, 30). Therefore, the decrease in cell wall thickness by beta-lactams facilitates vancomycin activity. Beta-lactams also appear to chemically and morphologically alter the surface of S. aureus, increasing target-specific vancomycin binding (27). As shown in these previous studies, it appears that as the MIC of vancomycin increases, the MIC of beta-lactams decreases due to the seesaw effect (9–11).
There are some limitations to the current study as the data presented here are from short-duration experiments and demonstrate only in vitro efficacy. Although the combination MIC values obtained from this study may not translate directly into clinical benefit, the results serve to provide further evidence of the beneficial effects of using beta-lactams in combination with vancomycin against VSSA, hVISA, and VISA. Additional research is warranted to determine the clinical utility of this novel combination.
In conclusion, the results of our study demonstrate the ability of several beta-lactams to provide synergistic activity with vancomycin. Our study provides evidence for the use of vancomycin in combination with a beta-lactam against recurrent or difficult-to-treat MRSA infections. Further research is warranted to elucidate the mechanisms of synergy between beta-lactams and vancomycin as well as to clarify the potential role of the novel combinations in clinical settings.
MATERIALS AND METHODS
Bacterial strains.
Fifty unique clinical MRSA strains with various susceptibilities to vancomycin, including 15 VSSA, 20 hVISA, and 15 VISA strains, were utilized for MIC testing. VISA strains were confirmed by MIC testing. All hVISA or VSSA strains utilized in this study were proven to be hVISA or VSSA by the population analysis profile-area under curve (PAP-AUC) ratio using Mu3 as a positive control (provided by the Anti-Infective Research Laboratory, Detroit, MI) (31, 32). Isolates were collected from several hospitals throughout metropolitan Detroit, MI, and from the Anti-Infective Research Laboratory strain library. Nine representative strains were randomly selected from the group of MRSA isolates for time-kill analysis.
Antimicrobials agents.
Vancomycin, nafcillin, and cefazolin were purchased from a commercial source (Sigma Chemical Company, St. Louis, MO, and Sandoz, Inc., Princeton, NJ). Ceftaroline was obtained from its manufacturer (Allergan, Parsippany, NJ). Cefepime was purchased from its manufacturer (Hospira, Lake Forest, IL).
Media.
In vitro experiments were performed in Mueller-Hinton broth (MHB; Difco, Detroit, MI) supplemented with 25-mg/liter calcium and 12.5-mg/liter magnesium. According to recent CLSI guidelines, MHB used for experiments related to nafcillin was supplemented with 2% sodium chloride (SMHB); MHB used for experiments related to ceftaroline was supplemented with dimethyl sulfoxide (DMSO) to 30% total volume; and MHB used for experiments related to cefazolin was supplemented with phosphate buffer (pH 6.0, 0.1 mol/liter) (33). Brain heart infusion agar (BHIA; Difco Laboratories, San Jose, CA) supplemented with 1 mg/liter vancomycin was used to subculture VISA strains in order to maintain the phenotype. Colony counts were determined using tryptic soy agar (TSA; Difco, Detroit, MI) plates for other MRSA strains. BHIA or Mueller-Hinton agar (MHA; Difco, Detroit, MI) supplemented with 3× the MIC was used to test for the emergence of resistance from the time-kill curve experiments.
Susceptibility testing.
MIC values of the study antimicrobial agents were determined in duplicate by broth microdilution at approximately 106 CFU/ml according to guidelines of the Clinical and Laboratory Standards Institute (CLSI). All samples were incubated at 35°C for 24 h before being read (34).
Time-kill analysis.
The potential for synergy with vancomycin plus nafcillin, cefazolin, or ceftaroline was determined by time-kill methods in MHB as the growth medium at an initial bacterial inoculum of 106 to 107 log10 CFU/ml. Time-kill experiments were performed at 0.5× the MIC of respective antibiotics and in triplicate for all antibiotic regimens to ensure reproducibility. Each antimicrobial agent was analyzed alone and in combination with vancomycin. Time-kill curve sampling and quantification of CFU/ml were performed as previously described (9, 35). Bactericidal activity was defined as a ≥3-log10 CFU/ml reduction from baseline. Synergy was defined as a ≥2-log10 CFU/ml increase in killing at 24 h with the combination in comparison to killing by the most active single drug. Combinations that resulted in ≥1-log10 bacterial growth in comparison to the least active single agent were considered antagonistic. All combinations not meeting the definition of synergy or antagonism were considered indifferent.
Statistical analysis.
Time-kill curve changes in the number of CFU/milliliter were compared by one-way analysis of variance (ANOVA) with Tukey's post hoc test. All statistical analyses were done with IBM SPSS Statistical Software (release 25; SPSS, Inc., Chicago, IL). A P value of ≤0.05 was considered significant.
ACKNOWLEDGMENTS
M.J.R. received funding support, consulted for, or was on the speaker bureau for Accelerate, Allergan, Bayer, Melinta, Merck, The Medicine Company, Sunovian, and Theravance and is partially supported by National Institutes of Health (R01-AI121400 and R21-109266-01). K.-N.T. has no disclosures.
REFERENCES
- 1.Lin SH, Liao WH, Lai CC, Liao CH, Tan CK, Wang CY, Huang YT, Hsueh PR. 2010. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteremia in a tertiary care hospital in Taiwan. J Antimicrob Chemother 65:1792–1798. doi: 10.1093/jac/dkq188. [DOI] [PubMed] [Google Scholar]
- 2.Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteremia in patients treated with vancomycin. J Antimicrob Chemother 65:1015–1018. doi: 10.1093/jac/dkq050. [DOI] [PubMed] [Google Scholar]
- 3.Welsh KJ, Abbott AN, Lewis EM, Gardiner JM, Kruzel MC, Lewis CT, Mohr JF, Wanger A, Armitige LY. 2010. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J Clin Microbiol 48:894–899. doi: 10.1128/JCM.01949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG Jr, International Collaboration on Endocarditis-Microbiology Investigator. 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis 200:1355–1366. doi: 10.1086/606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove E, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 6.Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI, Masselink LE, Kaye KS, Corey GR, Reller LB, Stryjewski ME, Schulman KA, Fowler VG Jr. 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med 118:1416. doi: 10.1016/j.amjmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 8.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 52:3315–3320. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber KE, Ireland CE, Bukavyn N, Rybak MJ. 2014. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 3:35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhand A, Bayer AS, Pogliano J, Yang SJ, Bolaris M, Nizet V. 2011. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 53:158–163. doi: 10.1093/cid/cir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale R, Bellingham GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Dleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, et al. 2016. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2016. Intensive Care Med 43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Berti A, McCrone Sue Roch M, Rosato A, Rose W, Chen B. 2018. Combination antibiotic exposure selectively alters the development of vancomycin intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother 62:e02100-17. doi: 10.1128/AAC.02100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr JG, Smyth ET, Hogg GM. 1990. In vitro antimicrobial activity of imipenem in combination with vancomycin or teicoplanin against Staphylococcus aureus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis 9:804–809. doi: 10.1007/BF01967378. [DOI] [PubMed] [Google Scholar]
- 15.Leonard SN. 2012. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic Model. PLoS One 7:e42103. doi: 10.1371/journal.pone.0042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. 2012. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 56:202–207. doi: 10.1128/AAC.05473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieradzki K, Tomasz A. 1997. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J Antimicrob Chemother 39(Suppl A):47–51. doi: 10.1093/jac/39.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 18.Sieradzki K, Tomasz A. 1999. Gradual alterations in cell wall structure and metabolism in vancomycin-resistant mutants of Staphylococcus aureus. J Bacteriol 181:7566–7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieradzki K, Leski T, Dick J, Borio L, Tomasz A. 2003. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. J Clin Microbiol 41:1687–1693. doi: 10.1128/JCM.41.4.1687-1693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. 2013. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 57:66–73. doi: 10.1128/AAC.01586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakoulas G, Rose WE. 2011. Effect of beta-lactam (BL) exposure in vitro on daptomycin (DAP) activity and cell wall thickness in MRSA, poster E-1332. Abstr 51st Annu Intersci Conf Antimicrob Agents Chemother (ICAAC) American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JS, Sud A, O'Sullivan MV, Robinson JO, Ferguson PE, Foo H, Hal SJ, Ralph AP, Howden BP, Binks PM, Kirby A, Tong S. 2016. Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 62:173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
- 24.Dilworth TJ, Ibrahim O, Hall P, Sliwinski J, Walraven C, Mercier RC. 2014. Beta-lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother 58:102–109. doi: 10.1128/AAC.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jonge BL, Chang YS, Gage D, Tomasz A. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2a. J Biol Chem 267:11248–11254. [PubMed] [Google Scholar]
- 26.de Jonge BL, Tomasz A. 1993. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2a in cell wall synthesis. Antimicrob Agents Chemother 37:342–346. doi: 10.1128/AAC.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieradzki K, Tomasz A. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol 179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. Beta-lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Hiramatsu K. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41:5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiramatsu K. 1998. Vancomycin resistance in staplylococci. Drug Resist Updat 1:135–150. doi: 10.1016/S1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 31.Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother 47:399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 32.Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sadar HS, Jones RN. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J Clin Microbiol 46:2950–2954. doi: 10.1128/JCM.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—tenth edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility Testing; 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Smith JR, Barber KE, Raut A, Aboutaleb M, Sakoulas G, Rybak MJ. 2015. Beta-lactam combinations with daptomycin provide synergy against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 70:1738–1743. doi: 10.1093/jac/dkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]