ABSTRACT
We examined the rapid evaluation of susceptibility to echinocandins in Candida spp. using the Etest performed directly on positive blood cultures and anidulafungin-containing agar plates. We prospectively collected 80 positive blood cultures (Bactec-FX system, Becton-Dickinson, Cockeysville, MD, USA) with echinocandin-susceptible Candida spp. (n = 60) and echinocandin-intermediate Candida parapsilosis (n = 20) from patients with candidemia. Additionally, blood culture bottles of nonfungemic/bacteremic patients were spiked with 35 echinocandin-resistant Candida species isolates. A total of 2 to 4 drops of medium from each bottle were stroked directly onto both RPMI 1640 agar plates with micafungin and anidulafungin Etest strips (ETDIR) and Sabouraud agar plates containing 2 mg/liter of anidulafungin. The isolates were tested according to the EUCAST method and Etest standard (ETSD). Essential and categorical agreement between the methods was calculated. The essential agreement and categorical agreement between the EUCAST method and ETDIR and ETSD were both >97.4%. The essential agreement between ETDIR and the EUCAST method for both echinocandins was >97%. The categorical agreement between the FKS sequence and ETDIR was 97.4%. The ETDIR MICs of anidulafungin and micafungin (≥0.19 mg/liter and ≥0.064 mg/liter, respectively) effectively separated all susceptible FKS wild-type isolates from the resistant FKS mutant isolates. The categorical agreement (62.6%) between the EUCAST method and growth on anidulafungin-containing plates was poor, with the best agreement observed for Candida glabrata (94.2%). When performed directly on positive blood cultures from patients with candidemia, the Etest with micafungin and anidulafungin is a reliable procedure for the rapid testing of susceptibility to echinocandins in Candida species isolates.
KEYWORDS: Candida spp., EUCAST procedure, Etest, echinocandins
INTRODUCTION
The incidence of invasive fungal infections has increased in many institutions, and mortality rates soar when an appropriate antifungal treatment is delayed (1–4). Echinocandins are recommended as the first-line treatment for invasive candidiasis (5, 6). Although the rates of resistance to echinocandins remain low (1, 7–10), recent publications are alerting physicians to an increased rate of resistance in some geographic areas (11, 12). Resistance is associated with a poor prognosis in patients with candidemia treated with echinocandins; consequently, detection may help to optimize antifungal treatment (11, 13). Echinocandin resistance can be detected in the clinical microbiology laboratory using broth microdilution methods, such as EUCAST and CLSI reference methods, or commercial methods, such as with Sensititre YeastOne, Vitek, disk diffusion, or plastic gradient strips. Alternatively, mutations in FKS1 and FKS2 (Candida glabrata only) can be detected using molecular techniques (12, 14, 15).
Phenotypic procedures for Candida antifungal susceptibility testing require pure-cultured isolates and are hindered by a slow turnaround time (48 to 72 h from the diagnosis of candidemia). We previously reported that the Etest performed directly on positive blood samples for yeasts was comparable to the standard CLSI and EUCAST approaches for the detection of both wild-type and azole-resistant Candida species isolates within 24 h of the diagnosis (2, 16). Unfortunately, given the lack of resistant isolates, we were unable to study the role of the procedure in the detection of the resistance of Candida to echinocandins.
On the basis of a set of echinocandin-resistant Candida isolates, we assessed the role of the Etest performed directly on artificially spiked blood cultures for the detection of resistance to echinocandins. Anidulafungin-containing agar plates were also tested for the screening of resistant isolates.
(This study was partially presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases in Madrid, Spain, 2018 [17].)
RESULTS
The antifungal activities of micafungin and anidulafungin determined by EUCAST, Etest strips (ETDIR), and Etest standard (ETSD) procedures against the 115 isolates are shown in Table 1. The geometric mean (GM) MICs obtained by the EUCAST method and the Etest (ETDIR and ETSD) were similar (P > 0.05).
TABLE 1.
Distribution of MICs and geometric mean MICs of micafungin and anidulafungin obtained using EUCAST, ETSD, and ETDIR methods
| Drug | Species (no. of isolates) | Method | MIC (mg/liter)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMb | 0.015 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | ≥8 | |||
| Micafungin | C. albicans (21) | EUCAST | 0.018 | 20 | 1 | ||||||||
| ETDIR | 0.021 | 20 | 1 | ||||||||||
| ETSD | 0.021 | 20 | 1 | ||||||||||
| C. parapsilosis (20) | EUCAST | 0.841 | 1 | 7 | 8 | 4 | |||||||
| ETDIR | 1.149 | 2 | 12 | 6 | |||||||||
| ETSD | 1 | 4 | 12 | 4 | |||||||||
| C. tropicalis (23) | EUCAST | 0.033 | 9 | 11 | 2 | 1 | |||||||
| ETDIR | 0.039 | 6 | 14 | 1 | 1 | 1 | |||||||
| ETSD | 0.042 | 3 | 17 | 1 | 1 | 1 | |||||||
| C. glabrata (51) | EUCAST | 0.192 | 20 | 6 | 1 | 1 | 5 | 8 | 10 | ||||
| ETDIR | 0.224 | 19 | 1 | 1 | 4 | 2 | 1 | 6 | 8 | 5 | 4 | ||
| ETSD | 0.178 | 20 | 1 | 5 | 1 | 5 | 5 | 10 | 3 | 1 | |||
| Anidulafungin | C. albicans (21) | EUCAST | 0.016 | 18 | 2 | 1 | |||||||
| ETDIR | 0.021 | 20 | 1 | ||||||||||
| ETSD | 0.021 | 20 | 1 | ||||||||||
| C. parapsilosis (20) | EUCAST | 1.866 | 1 | 4 | 11 | 4 | |||||||
| ETDIR | 2.928 | 1 | 7 | 12 | |||||||||
| ETSD | 3.031 | 1 | 6 | 13 | |||||||||
| C. tropicalis (23) | EUCAST | 0.023 | 18 | 4 | 1 | ||||||||
| ETDIR | 0.038 | 7 | 12 | 1 | 1 | 1 | 1 | ||||||
| ETSD | 0.029 | 15 | 5 | 1 | 1 | 1 | |||||||
| C. glabrata (51) | EUCAST | 0.258 | 8 | 12 | 4 | 4 | 6 | 13 | 4 | ||||
| ETDIR | 0.311 | 16 | 4 | 1 | 5 | 2 | 10 | 12 | 1 | ||||
| ETSD | 0.239 | 20 | 3 | 5 | 5 | 12 | 4 | 2 | |||||
The MICs obtained by the ETSD and ETDIR were increased to the concentration of the next 2-fold dilution matching the micafungin dilution scale used for the EUCAST procedure. Numbers in bold indicate resistant isolates.
GM, geometric mean.
The essential agreement between ETDIR and ETSD of micafungin and anidulafungin was 98.3% and 100%, respectively, with few exceptions (Table 2). The essential agreement between ETDIR and the EUCAST method was >97% for both micafungin and anidulafungin and 100% for micafungin against Candida parapsilosis and Candida tropicalis and for anidulafungin against C. glabrata.
TABLE 2.
Essential agreement and categorical agreement between the methods for micafungin and anidulafungin
| Species | Agreement (% of isolates) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Essentiala |
Categorical |
|||||||
| ETSD vs ETDIRb |
EUCAST vs ETSD/ETDIR |
EUCAST vs ETDIR |
FKS sequence vs ETDIR/EUCAST |
|||||
| MYCc | ANDd | MYC | AND | MYC | AND | MYC | AND | |
| C. albicans | 100 | 100 | 95.2/95.2 | 95.2/95.2 | 100 | 100 | 100 | 100 |
| C. parapsilosis | 100 | 95 | 100/100 | 90/95 | 100 | 100 | 100 | 100 |
| C. tropicalis | 100 | 100 | 100/100 | 95.6/95.6 | 100 | 91.3 | 100 | 100 |
| C. glabrata | 96.1 | 100 | 98/98.1 | 100/100 | 100 | 100 | 94.2 | 94.2 |
| Overall | 98.3 | 100 | 98.2/98.2 | 97.5/97.4 | 100 | 98.3 | 97.4 | 97.4 |
Percentages of isolates in which the antifungal MIC differed ±2-log dilutions over the methods.
ETSD, Etest standard; ETDIR, Etest direct.
MYC, micafungin.
AND, anidulafungin.
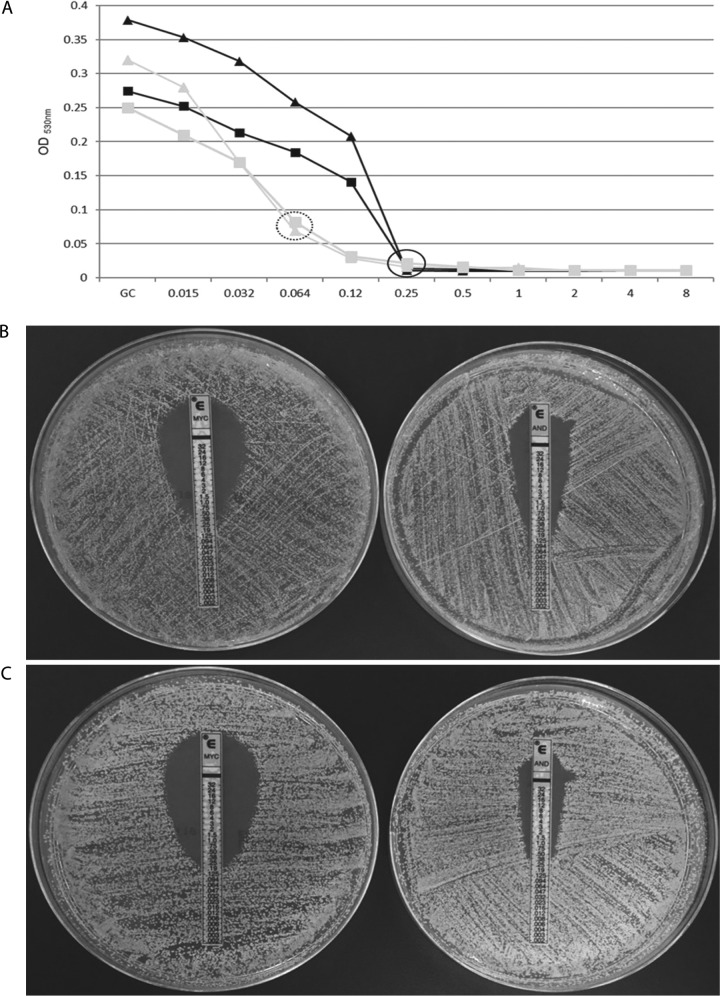
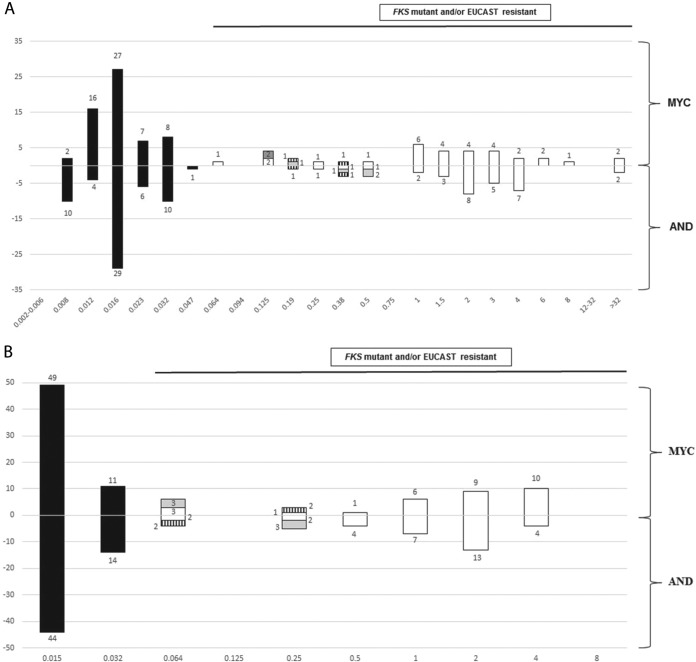
The categorical agreement between ETDIR and ETSD was 100% for all species and echinocandins. The categorical agreement between ETDIR and the EUCAST method was 100% for micafungin and 98.3% for anidulafungin, where misclassifications were found in two C. tropicalis isolates (8.7% of major errors in C. tropicalis) in which the EUCAST method indicated resistance to micafungin but susceptibility to anidulafungin, whereas ETDIR (and ETSD) indicated resistance to both drugs (Table 3 and Table 2). The isolates harbored FKS1 HS1 mutations (F641L and R647G) (Fig. 1). The categorical agreement between ETDIR and the FKS sequence was 97.4% for both echinocandins (Table 2); the agreement was 100% for all species, with the exception of C. glabrata (94.2%), because of three isolates in which ETDIR (and the EUCAST method) for both echinocandins indicated resistance but the FKS1 and FKS2 sequences were the wild types (overall 2.6% of major errors). Figure 2 shows the distributions of the micafungin and anidulafungin MICs obtained by ETDIR and the EUCAST method (C. parapsilosis was excluded). An ETDIR MIC of anidulafungin of ≥0.19 mg/liter and/or an MIC of micafungin of ≥0.064 mg/liter against Candida albicans, C. tropicalis, and C. glabrata effectively separated the phenotypically resistant isolates/FKS mutants from the susceptible isolates/FKS wild types (100% categorical agreement with combined gold standards for both agents).
TABLE 3.
Micafungin and anidulafungin MICs against the 35 echinocandin-resistant Candida species isolates obtained using the EUCAST, ETSD, and ETDIR procedures
| Species | FKS mutation | MIC (mg/liter)a |
|||||
|---|---|---|---|---|---|---|---|
| EUCAST |
ETDIRb |
ETSDc |
|||||
| MYCd | ANDe | MYC | AND | MYC | AND | ||
| C. albicans | F641S (FKS1 HS1) | 1 | 0.25 | >32 | >32 | >32 | >32 |
| C. tropicalisf | R647G (FKS1 HS1) | 0.25 | 0.064 | 0.38 | 0.19 | 0.38 | 0.19 |
| C. tropicalis | S645F (FKS1 HS1) | 2 | 1 | 3 | 2 | 2 | 2 |
| C. tropicalisf | F641L (FKS1 HS1) | 0.25 | 0.064 | 0.19 | 0.38 | 0.19 | 0.38 |
| C. glabrata | Δ659 (FKS2 HS1) | 1 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 |
| C. glabrata | Δ659 (FKS2 HS1) | 0.064 | 1 | 1.5 | 2 | 1.5 | 2 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 4 | >32 | >32 | >32 | >32 |
| C. glabrata | Δ659 (FKS2 HS1) | 2 | 1 | 1 | 1.5 | 0.5 | 1 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 2 | 2 | 4 | 1.5 | 3 |
| C. glabrata | Δ659 (FKS2 HS1) | 2 | 1 | 1 | 3 | 0.75 | 1 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 4 | 1.5 | 2 | 2 | 4 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 2 | 2 | 4 | 2 | 2 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 2 | 1 | 3 | 1 | 1 |
| C. glabrata | Δ659 (FKS2 HS1) | 1 | 2 | 1.5 | 3 | 1 | 2 |
| C. glabrata | Δ659 (FKS2 HS1) | 1 | 2 | 1.5 | 2 | 1.5 | 2 |
| C. glabrata | Δ659 (FKS2 HS1) | 1 | 1 | 0.5 | 1.5 | 0.5 | 1 |
| C. glabrata | Δ659 (FKS2 HS1) | 4 | 2 | 1 | 3 | 2 | 2 |
| C. glabrata | Δ659 (FKS2 HS1) | 2 | 4 | 3 | 4 | 3 | 4 |
| C. glabrata | S663P (FKS2 HS1) | 2 | 1 | 3 | 2 | 2 | 2 |
| C. glabrata | S663P (FKS2 HS1) | 2 | 2 | 1 | 2 | 4 | 8 |
| C. glabrata | S663P (FKS2 HS1) | 4 | 4 | 6 | 3 | 1 | 2 |
| C. glabrata | S663P (FKS2 HS1) | 4 | 2 | 4 | 4 | 2 | 2 |
| C. glabrata | S663P (FKS2 HS1) | 4 | 2 | 4 | 4 | 1 | 2 |
| C. glabrata | S663P (FKS2 HS1) | 2 | 1 | 3 | 1.5 | 2 | 3 |
| C. glabrata | S663P (FKS2 HS1) | 2 | 2 | 6 | 4 | 0.5 | 1 |
| C. glabrata | W715L (FKS2) | 2 | 2 | 2 | 4 | 2 | 2 |
| C. glabrata | W715L (FKS2) | 0.5 | 0.5 | 2 | 2 | 0.38 | 0.5 |
| C. glabrata | W715L (FKS2) | 4 | 2 | 8 | 1 | 4 | 2 |
| C. glabrata | D666N (FKS2 HS1) | 0.064 | 0.25 | 0.125 | 0.5 | 0.125 | 0.5 |
| C. glabrata | D666N (FKS2 HS1) | 0.064 | 0.5 | 0.064 | 0.38 | 0.094 | 0.38 |
| C. glabrata | S663Y (FKS2 HS1) | 1 | 2 | 1 | 2 | 0.5 | 1.5 |
| C. glabrata | E655A (FKS2) | 0.25 | 0.5 | 0.125 | 1 | 0.125 | 0.38 |
| C. glabratag | Wild type | 0.06 | 0.25 | 0.125 | 0.5 | 0.06 | 0.25 |
| C. glabratag | Wild type | 0.06 | 0.25 | 0.19 | 0.38 | 0.125 | 0.38 |
| C. glabratag | Wild type | 0.06 | 0.25 | 0.125 | 0.5 | 0.125 | 0.25 |
EUCAST breakpoints used to classify the isolates as resistant: C. albicans (micafungin, >0.016; anidulafungin, >0.032); C. glabrata (micafungin, >0.032; anidulafungin, >0.064); C. tropicalis (micafungin [based on ECOFF], >0.06; anidulafungin, >0.032) (34, 37).
ETDIR, Etest direct.
ETSD, Etest standard.
MYC, micafungin.
AND, anidulafungin.
C. tropicalis isolates showing resistance to micafungin but susceptibility to anidulafungin by the EUCAST method; both Etest procedures showed resistance to micafungin and anidulafungin.
C. glabrata isolates showing phenotypic resistance to anidulafungin and micafungin but wild-type FKS1 and FKS2 genes.
FIG 1.
(A) Echinocandin growth inhibition curves obtained by the EUCAST method indicating resistance to micafungin (black) but susceptibility to anidulafungin (grey) for C. tropicalis isolates with R647G (triangles) or F641L (squares) mutations. Black circle indicates micafungin MIC; dotted circle indicates anidulafungin MIC; GC, growth control. ETDIR of two C. tropicalis isolates with R647G (B) and F641L (C) mutations indicating resistance to both echinocandins. MYC, micafungin; AND, anidulafungin.
FIG 2.
Distribution of the micafungin (MYC) and anidulafungin (AND) MICs obtained by ETDIR (A) and the EUCAST method (B) against 95 isolates (C. parapsilosis isolates were excluded). Black bars, FKS wild-type isolates classified as susceptible by the EUCAST method; grey bars, FKS wild-type C. glabrata isolates classified as resistant by the EUCAST method; hatched bars, FKS mutant C. tropicalis isolates classified as anidulafungin susceptible and micafungin resistant by the EUCAST method; white bars, FKS mutant isolates classified as resistant by the EUCAST method.
Overall, the categorical agreement between the EUCAST method and growth on anidulafungin-containing plates was 62.6%. Major errors were found in all C. albicans and C. tropicalis echinocandin-susceptible isolates (the EUCAST method indicated susceptibility, but growth was visible on the plates [34.8%]). All C. parapsilosis isolates were able to grow on the plates. The best agreement was observed for C. glabrata (94.2%), with 100% of susceptible isolates not growing on the plates and 90.3% of the resistant isolates growing on the plates. Very major errors (the EUCAST method indicated resistance but there was no visible growth on the plates [2.6%]) were found in the three echinocandin-resistant FKS wild-type C. glabrata isolates.
DISCUSSION
Our study shows that performing the ETDIR directly on positive blood cultures can speed up echinocandin susceptibility testing within 24 h of the detection of Candida. The results obtained by this rapid, easy, and inexpensive procedure mirrored those obtained by the EUCAST method and ETSD and those obtained with the FKS gene sequence.
Current Infectious Diseases Society of America (IDSA) guidelines for the treatment of patients with candidemia recommend echinocandin susceptibility testing on isolates causing fungemia, particularly for patients previously exposed to echinocandins or infected by C. glabrata (5). Microdilution methods are preferred, although they require pure-cultured isolates, they are time consuming, and the results are not available until 48 to 72 h after diagnosis. Given that a delay in starting an appropriate antifungal treatment invariably leads to a poorer prognosis, the results of antifungal susceptibility must be anticipated where possible.
We previously showed that ETDIR performed directly on positive blood cultures showed good agreement with the CLSI M27-A3 procedure (2). The study proved useful for ruling out false resistance, which can facilitate antifungal de-escalation (for example, switching from echinocandins to fluconazole). As for the ability of ETDIR to detect resistance, we demonstrated that it was reliable for caspofungin-resistant basidiomycete yeast or fluconazole-resistant non-albicans Candida. Unfortunately, we did not test fluconazole-resistant C. albicans isolates or echinocandin-resistant Candida species isolates. Our subsequent study demonstrated that ETDIR was able to detect fluconazole-resistant C. albicans isolates (16). We conducted the present study with well-characterized echinocandin-resistant Candida species to complete the testing. We studied the agreement between ETDIR and the EUCAST reference method for micafungin and anidulafungin. Caspofungin, the agent tested in our first paper (2), was not tested here owing to interlaboratory variability (18). For that reason, EUCAST does not provide caspofungin breakpoints.
We found very high essential and categorical agreement between the ETDIR, ETSD, and EUCAST methods for echinocandin susceptibility testing against Candida spp. No very major errors (false susceptibility) were detected for any of the isolates tested. However, a few major errors were found for anidulafungin in two C. tropicalis isolates with FKS1 mutations that were classified as anidulafungin-susceptible and micafungin-resistant by the EUCAST method and as resistant by ETDIR. Previous studies reported C. albicans and Candida kefyr isolates with the FKS1 mutations R647G and P649H and the above-mentioned phenotype using CLSI methods (19, 20), thus suggesting that the amino acids R647 and P649 are key for glucan synthase inhibition by micafungin. Another study reported a C. tropicalis isolate harboring the F641L FKS1 mutation with dose-dependent susceptibilities to both micafungin and anidulafungin, again, by using CLSI methods (21). Since EUCAST does not yet have breakpoints for micafungin against C. tropicalis, our isolates had to be classified using epidemiologic cutoff values (ECOFFs). However, our and other previously reported observations for C. glabrata (22–25) suggest that the presence of FKS mutations itself is not sufficient to predict the pattern of resistance to anidulafungin or micafungin. Therefore, we decided to test anidulafungin and micafungin in parallel. On the basis of our results, micafungin may be a good surrogate marker of echinocandin resistance in C. albicans and C. tropicalis isolates.
To improve the potential of ETDIR for the detection of both resistant and FKS non-wild-type isolates, we calculated the categorical agreement using the FKS sequence as the gold standard. The categorical agreement between the FKS sequence and ETDIR was very high, thus showing the ability of ETDIR to discriminate between FKS mutants and wild types in most cases. However, three major errors were observed in three unusual C. glabrata isolates that showed very high anidulafungin and micafungin MICs by the EUCAST method and Etest but wild-type FKS1 and FKS2 sequences. We cannot rule out alternative mechanisms of resistance (e.g., efflux pumps), although the ETDIR classified them correctly, in agreement with the EUCAST method, as resistant, suggesting the ability of this procedure to detect wild-type FKS/resistant isolates. Future studies on these isolates are required. To separate the FKS wild-type/susceptible isolates from FKS mutant/resistant isolates, we suggest the following cutoffs for ETDIR: anidulafungin, ≥0.19 mg/liter; and/or micafungin, ≥0.06 mg/liter.
Recent studies have proved that azole-containing plates are useful when screening for the presence of resistance in Aspergillus (26). To determine whether this procedure would be useful for the screening of echinocandin resistance in Candida, we decided to use anidulafungin-containing plates at 2 mg/liter on the basis of our previous study, which reports the anidulafungin mutant prevention concentration (27). However, we found poor agreement between this method and the EUCAST method owing to the high percentages of false resistance in C. albicans and C. tropicalis. A paradoxical effect is common to both species (28, 29). Owing to the lack of echinocandin-resistant C. parapsilosis isolates tested and the fact that 100% of the intermediate isolates grew on the plates, this procedure cannot be recommended for C. parapsilosis, C. albicans, or C. tropicalis.
Our main limitation is the low number of C. albicans and C. tropicalis FKS mutant isolates. However, the acquisition of echinocandin resistance involves C. glabrata to a greater extent than other Candida spp. (11, 12), and the number of C. glabrata mutant isolates tested was moderately high. Furthermore, the positive results reported here reinforce our previous observation of basidiomycete yeast being correctly classified as caspofungin resistant (2).
We conclude that the ETDIR for micafungin and anidulafungin is a reliable and fast procedure when screening for the presence of echinocandin resistance in Candida species causing candidemia and can be easily implemented in the routine of the microbiology laboratory.
MATERIALS AND METHODS
Samples.
We prospectively collected 80 positive blood cultures (Bactec-FX system, Becton-Dickinson, Cockeysville, MD, USA) to screen for echinocandin-susceptible Candida spp. (C. albicans, n = 20; C. tropicalis, n = 20; C. glabrata, n = 20) and echinocandin-intermediate C. parapsilosis (n = 20) from patients with candidemia admitted to Gregorio Marañón Hospital (Madrid, Spain) between 2010 and 2013. A total of 1 to 2 ml of broth from each bottle was stored at −70°C. Additionally, 0.5-ml (0.5 McFarland) suspensions of 35 echinocandin-resistant Candida species isolates obtained in previous studies (2, 27, 30–32) were artificially spiked in nonfungemic/bacteremic Bactec bottles until they were flagged as positive (Table 3). All 115 isolates were identified by amplification and sequencing of the ITS1-5.8S-ITS2 regions (33).
EUCAST antifungal susceptibility testing and ETSD.
All isolates were tested for susceptibility to micafungin (Astellas Pharma, Inc., Tokyo, Japan) and anidulafungin (Pfizer Pharmaceutical Group, New York, NY, USA) according to the EUCAST E.DEF 7.3.1 microdilution procedure (34–36). The echinocandin concentrations ranged from 0.015 to 8 mg/liter. Inoculated plates were incubated for 24 h at 35°C.
ETSD of micafungin and anidulafungin was performed on the isolates according to the manufacturer's instructions. Briefly, the suspensions were prepared, adjusted to 0.5 McFarland, and stroked on RPMI 1640 agar plates supplemented with 2% glucose (bioMérieux, Marcy-l'Etoile, France). The strips were placed on the agar surfaces of the plates, which were then incubated at 35°C for 24 h. The MIC was set when the fungal elliptic growth intersected the plastic strip.
Antifungal susceptibility testing performed directly on blood samples using ETDIR and anidulafungin-containing agar plates.
A total of 2 to 4 drops of the broth medium (stored broth from the 80 isolates preincubated overnight at 37°C and positive flagged bottles of the 35 echinocandin-resistant isolates) were stroked on RPMI 1640 agar plates on which Etest strips of micafungin and anidulafungin had been placed; 2 to 4 drops of the broth medium were also stroked on Sabouraud agar plates containing 2 mg/liter of anidulafungin. The plates were incubated at 35°C for 24 h.
Data analysis.
The geometric mean (GM) MICs of micafungin and anidulafungin against the isolates obtained by the three methods were calculated and compared using the t test, with a P value of <0.05 considered statistically significant. MICs obtained using EUCAST 7.3.1 were considered the gold standard and were compared with those obtained by ETDIR and ETSD to calculate the essential agreement between the methods (percentage of isolates in which MIC differed by ±2-log dilutions over the reference method). All isolates were classified as resistant or susceptible according to the clinical breakpoints proposed by EUCAST for any of the three methods (37). Given the lack of clinical breakpoints for micafungin against C. tropicalis, we tentatively considered isolates showing an MIC above the ECOFF (>0.06 mg/liter) to be resistant in order to avoid the term “non-wild-type,” which is used exclusively for FKS mutants (34). The procedures were in categorical agreement when the results were in the same susceptibility category (2) based on two gold standards: the EUCAST method and FKS sequence (regardless of the MIC). The anidulafungin-containing plate-screening procedure was in categorical agreement with the EUCAST method when resistant isolates or FKS mutants were able to grow on the plates and susceptible isolates or FKS wild types were unable to grow visibly on the plates. Errors were categorized as very major (agar diffusion methods indicated susceptibility and the EUCAST method/FKS sequences indicated resistance or mutations) or major (agar diffusion methods indicated resistance and the EUCAST method/FKS sequence indicated susceptibility or wild type).
Ethical considerations.
This study was approved by the ethics committee of Hospital Gregorio Marañón (CEIC-A1, study no. 208/16).
ACKNOWLEDGMENTS
We thank Thomas O'Boyle for editing the article.
The study was supported by grants PI14/00740 and MSI15/00115 from the Fondo de Investigación Sanitaria ([FIS] Instituto de Salud Carlos III; Plan Nacional de I+D+I 2013–2016). The study was cofunded by the European Regional Development Fund (FEDER) “a way of making Europe.” P.E. (CPI15/00115) and J.G. (CPII15/00006) are recipients of a Miguel Servet contract supported by the FIS; L.J.M.-Z. (PI14/00740) is supported by FIS; M.A.B.-C. received a predoctoral grant from the Instituto de Investigación Sanitaria Gregorio Marañón (II-Predoc-2016-IISGM).
The funders had no role in the study design, data collection and analysis, or the drafting and decision to publish the manuscript.
J.G. has received funds for speaking at symposia organized on behalf of Astellas, Gilead, MSD, Scynexis, and United Medical. He has also received funds for research from Fondo de Investigación Sanitaria, Gilead, Scynexis, and Cidara. All other authors have no conflicts to declare.
REFERENCES
- 1.Guinea J, Zaragoza O, Escribano P, Martin-Mazuelos E, Peman J, Sanchez-Reus F, Cuenca-Estrella M. 2014. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob Agents Chemother 58:1529–1537. doi: 10.1128/AAC.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinea J, Recio S, Escribano P, Torres-Narbona M, Pelaez T, Sanchez-Carrillo C, Rodriguez-Creixems M, Bouza E. 2010. Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J Clin Microbiol 48:2205–2212. doi: 10.1128/JCM.02321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 4.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 7.Marcos-Zambrano LJ, Escribano P, Sanchez C, Munoz P, Bouza E, Guinea J. 2014. Antifungal resistance to fluconazole and echinocandins is not emerging in yeast isolates causing fungemia in a Spanish tertiary care center. Antimicrob Agents Chemother 58:4565–4572. doi: 10.1128/AAC.02670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to2009). J Clin Microbiol 49:396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astvad KMT, Johansen HK, Roder BL, Rosenvinge FS, Knudsen JD, Lemming L, Schonheyder HC, Hare RK, Kristensen L, Nielsen L, Gertsen JB, Dzajic E, Pedersen M, Ostergard C, Olesen B, Sondergaard TS, Arendrup MC. 2018. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol 56:e01564-17. doi: 10.1128/JCM.01564-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prigitano A, Cavanna C, Passera M, Ossi C, Sala E, Lombardi G, Grancini A, De Luca C, Bramati S, Gelmi M, Tejada M, Grande R, Farina C, Lallitto F, Tortorano AM. 2016. CAND-LO 2014-15 study: changing epidemiology of candidemia in Lombardy (Italy). Infection 44:765–780. doi: 10.1007/s15010-016-0951-6. [DOI] [PubMed] [Google Scholar]
- 11.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanguinetti M, Posteraro B, Lass-Florl C. 2015. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses 58(Suppl 2):2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 14.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat 10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perlin DS. 2015. Mechanisms of echinocandin antifungal drug resistance. Ann N Y Acad Sci 1354:1–11. doi: 10.1111/nyas.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escribano P, Marcos-Zambrano LJ, Gomez A, Sanchez C, Martinez-Jimenez MC, Bouza E, Guinea J. 2017. The Etest performed directly on blood culture bottles is a reliable tool for detection of fluconazole-resistant Candida albicans isolates. Antimicrob Agents Chemother 61:e00400-17. doi: 10.1128/AAC.00400-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordallo-Cardona MA, Marcos-Zambrano LJ, Sánchez-Carrillo C, Bouza E, Muñoz P, Escribano P, Guinea J. 2018. Agreement between EUCAST procedure and the Etest direct on blood samples in Candida spp. clinical isolates, poster P0329. 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain. [Google Scholar]
- 18.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. Using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staab JF, Neofytos D, Rhee P, Jimenez-Ortigosa C, Zhang SX, Perlin DS, Marr KA. 2014. Target enzyme mutations confer differential echinocandin susceptibilities in Candida kefyr. Antimicrob Agents Chemother 58:5421–5427. doi: 10.1128/AAC.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother 52:4181–4183. doi: 10.1128/AAC.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arendrup MC, Perlin DS, Jensen RH, Howard SJ, Goodwin J, Hope W. 2012. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother 56:2435–2442. doi: 10.1128/AAC.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudiuk C, Gamarra S, Leonardeli F, Jimenez-Ortigosa C, Vitale RG, Afeltra J, Perlin DS, Garcia-Effron G. 2014. Set of classical PCRs for detection of mutations in Candida glabrata FKS genes linked with echinocandin resistance. J Clin Microbiol 52:2609–2614. doi: 10.1128/JCM.01038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arendrup MC, Verweij PE, Mouton JW, Lagrou K, Meletiadis J. 2017. Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J Antimicrob Chemother 72:3325–3333. doi: 10.1093/jac/dkx319. [DOI] [PubMed] [Google Scholar]
- 27.Bordallo-Cardona MA, Marcos-Zambrano LJ, Sanchez-Carrillo C, de la Pedrosa EGG, Canton R, Bouza E, Escribano P, Guinea J. 2018. Mutant prevention concentration and mutant selection window of micafungin and anidulafungin in clinical Candida glabrata isolates. Antimicrob Agents Chemother 62:e01982-17. doi: 10.1128/AAC.01982-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcos-Zambrano LJ, Escribano P, Sanchez-Carrillo C, Bouza E, Guinea J. 2017. Frequency of the paradoxical effect measured using the EUCAST procedure with micafungin, anidulafungin, and caspofungin against Candida species isolates causing candidemia. Antimicrob Agents Chemother 61:e1584-16. doi: 10.1128/AAC.01584-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rueda C, Cuenca-Estrella M, Zaragoza O. 2014. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 58:1071–1083. doi: 10.1128/AAC.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordallo-Cardona MA, Escribano P, de la Pedrosa EG, Marcos-Zambrano LJ, Canton R, Bouza E, Guinea J. 2017. In vitro exposure to increasing micafungin concentrations easily promotes echinocandin resistance in Candida glabrata isolates. Antimicrob Agents Chemother 61:e01542-16. doi: 10.1128/AAC.01542-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bordallo-Cardona MA, Escribano P, Marcos-Zambrano LJ, Diaz-Garcia J, de la Pedrosa EG, Canton R, Bouza E, Guinea J. 8 December 2017. Low and constant micafungin concentrations may be sufficient to lead to resistance mutations in FKS2 gene of Candida glabrata. Med Mycol doi: 10.1093/mmy/myx124. [DOI] [PubMed] [Google Scholar]
- 32.Marcos-Zambrano LJ, Escribano P, Rueda C, Zaragoza O, Bouza E, Guinea J. 2013. Comparison between the EUCAST procedure and the Etest for determination of the susceptibility of Candida species isolates to micafungin. Antimicrob Agents Chemother 57:5767–5770. doi: 10.1128/AAC.01032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 34.European Committee on Antimicrobial Susceptibility Testing. 2013. Micafungin and Candida spp. Rationale for the clinical breakpoints, version 1.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Micafungin_rationale_document_1_0_final.pdf.
- 35.European Committee on Antimicrobial Susceptibility Testing. 2013. Anidulafungin. Rationale for the clinical breakpoints, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Anidulafungin_rationale_2_0_2013.pdf.
- 36.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2017. EUCAST definitive document E.DEF 7.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_1_Yeast_testing__definitive.pdf.
- 37.European Committee on Antimicrobial Susceptibility Testing. 2017. Antifungal agents. Breakpoint tables for interpretation of MICs. http://www.eucast.org.