ABSTRACT
A significant cause of mortality in the intensive care unit (ICU) is multidrug-resistant (MDR) Gram-negative bacteria, such as MDR Acinetobacter baumannii (MDR-AB) and Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp). The aim of the present study was to compare the clinical features, therapy, and outcome of patients who developed septic shock due to either MDR-AB or KPC-Kp. We retrospectively analyzed patients admitted to the ICU of a teaching hospital from November 2010 to December 2015 who developed septic shock due to MDR-AB or KPC-Kp infection. Data from 220 patients were analyzed: 128 patients (58.2%) were diagnosed with septic shock due to KPC-Kp, and 92 patients (41.8%) were diagnosed with septic shock due to MDR-AB. The 30-day mortality rate was significantly higher for the MDR-AB group than the KPC-Kp group (84.8% versus 44.5%, respectively; P < 0.001). Steroid exposure and pneumonia were associated with MDR-AB infection, whereas hospitalization in the previous 90 days, primary bacteremia, and KPC-Kp colonization were associated with KPC-Kp infection. For patients with KPC-Kp infections, the use of ≥2 in vitro-active antibiotics as empirical or definitive therapy was associated with higher 30-day survival, while isolation of colistin-resistant strains was linked to mortality. Patients with MDR-AB infections, age >60 years, and a simplified acute physiology score II (SAPS II) of >45 points were associated with increased mortality rates. We concluded that septic shock due to MDR-AB infection is associated with very high mortality rates compared to those with septic shock due to KPC-Kp. Analysis of the clinical features of these critically ill patients might help physicians in choosing appropriate empirical antimicrobial therapy.
KEYWORDS: septic shock, Acinetobacter, Klebsiella, intensive care unit, multidrug resistant
INTRODUCTION
The dysregulated host response to infection leading to sepsis and septic shock is a life-threatening event that, despite advances in organ support and antimicrobial therapy, is associated with a mortality rate of >30% (1).
In recent years, infections due to multidrug-resistant (MDR) Gram-negative pathogens, such as MDR Acinetobacter baumannii (MDR-AB) and Klebsiella pneumoniae carbapenemase-producing K. pneumoniae (KPC-Kp), have been increasingly observed among critically ill patients admitted to the intensive care unit (ICU) (2–5).
The management of critically ill patients includes early diagnosis and immediate administration of antimicrobials (6, 7). Previous observations about septic patients with infections due to MDR Gram-negative bacteria highlighted the crucial role of timely empirical antimicrobial treatment and the importance of a definitive anti-infective therapy with in vitro activity against the microbial isolates, emphasizing also the importance of an adequate and early source control of infection (8–10). Moreover, the pharmacokinetic and pharmacodynamic properties of antibiotics should be considered because of changes in clearance and volume of distribution that are frequently observed in critically ill patients, with the potential to influence concentration of the drug at site of infections (11). Of interest, apart from an inadequate antimicrobial treatment, KPC-producing isolates seem to be highly virulent in a low-tumor necrosis factor alpha (low-TNF-α)-release environment, suggesting an immunoparalysis induction mechanism (12).
Recently, new drugs for the treatment of severe infections due to MDR Gram-negative pathogens have been approved: most of these new agents show activity against KPC-Kp (13). Equally important, new agents with activity against MDR-AB strains have been developed and might be available for therapy in the near future (14–16). Thus, it is important for physicians to recognize peculiar clinical characteristics of MDR-AB or KPC-Kp infections in ICU patients with sepsis or septic shock in order to promptly use antibiotics which are potentially active in vitro. Based on this scenario, the aim of this study was to analyze the clinical features and outcomes of ICU patients who developed septic shock due to MDR-AB or KPC-Kp infections.
RESULTS
During the study period, 193 bacteremic KPC-Kp infections were observed. Out of these, 104 (53.8%) infections were primary bacteremias and 89 (46.2%) infections were secondary bacteremias; 121 (62.7%) infections were associated with septic shock. In the MDR-AB group, 146 bacteremic infections were documented. Among them, 35 (23.9%) infections were primary bacteremias, and 114 (78.1%) infections were secondary bacteremias; 84 (57.5%) bacteremic infections were complicated by septic shock. Additionally, nonbacteremic infections associated with septic shock were reported in 15 patients, with 7 (46.7%) caused by KPC-Kp and 8 (53.3%) caused by MDR-AB.
A total of 220 patients developed septic shock and met our inclusion criteria: 128 (58.2%) patients were diagnosed with KPC-Kp infection, while in 92 (41.8%) patients, an MDR-AB infection was observed. KPC-Kp strain susceptibility tests showed the following resistance rates: colistin, 44.5%; gentamicin, 45.8%; and amikacin, 99.8%. All strains displayed susceptibility to tigecycline using the Etest technique. The meropenem MICs were ≥16 μg/ml for all KPC-Kp isolates. Conversely, for MDR-AB strains, the following resistance rates were documented: colistin, 1.1%; gentamicin, 87.9%; amikacin, 89.3%; rifampin, 88.2%; and meropenem, 99.8%. On this basis, 44.5% of K. pneumoniae strains were classified as pandrug resistant (PDR) and 55.5% as extensively drug resistant (XDR), while 98.9% of A. baumannii strains were considered XDR and 1.1% considered PDR.
As reported in Tables 1, no differences in age, sex, comorbidities, SAPS II score, or cause of ICU admission (except for stroke) were shown between patients with septic shock due to KPC-Kp and MDR-AB; patients with septic shock due to KPC-Kp were statistically significantly more likely to be hospitalized in the previous 90 days (43.8% versus 19.6%, respectively; P < 0.001), to be colonized at time of ICU admission (48.4% versus 4.3%, respectively; P < 0.001), and to be exposed to steroids during ICU stay (11.7% versus 63%, respectively; P < 0.001) than were patients with septic shock due to MDR-AB. Regarding the source of infection, septic shock patients with KPC-Kp isolation showed a higher frequency of primary bacteremia (49.2% versus 18.5%, respectively; P < 0.001), central venous catheter (CVC)-related bloodstream infections (19.5% versus 4.3%, respectively; P = 0.001), catheter-associated urinary tract infections (21.1% versus 0%, P < 0.001), and skin and soft tissue infections (14.1% versus 2.1%, respectively; P < 0.001) than did those with MDR-AB septic shock. Conversely, pneumonia was more frequently diagnosed (69.6% versus 43%, respectively; P < 0.001) in septic patients with MDR-AB. The isolation of a colistin-resistant strain was observed only in 1 (1.1%) patient with MDR-AB, compared to 57 (44.5%) out of 128 patients in the KPC-Kp group (P < 0.001). Finally, a higher 30-day mortality was found in MDR-AB patients than in those with KPC-Kp (84.8% versus 44.5%, respectively; P < 0.001).
TABLE 1.
Comparison between KPC-Kp and MDR-AB infections in septic patients hospitalized in the same ICUa
| Variableb | KPC-Kp (n = 128) | MDR-AB (n = 92) | Pc |
|---|---|---|---|
| Age (mean ± SD) (yr) | 60.1 ± 15.9 | 60.6 ± 17.2 | 0.8 |
| Male sex | 89 (69.5) | 64 (69.6) | 1.0 |
| Cause of ICU admission | |||
| Respiratory failure | 34 (26.5) | 17 (18.5) | 0.22 |
| Trauma | 25 (19.5) | 23 (25) | 0.43 |
| Septic shock not caused by KPC-Kp or MDR-AB | 28 (21.8) | 30 (32.6) | 0.1 |
| Stroke | 9 (7) | 0 | 0.02 |
| Cardiac/hemorrhagic shock/postsurgery | 32 (25) | 22 (23.9) | 1.0 |
| Comorbidities | |||
| Chronic liver disease | 4 (3.1) | 4 (4.3) | 0.7 |
| Neoplasm | 15 (11.7) | 7 (7.6) | 0.3 |
| Diabetes | 23 (18) | 18 (19.6) | 0.8 |
| Heart failure | 16 (12.5) | 13 (14.1) | 0.8 |
| Coronary artery disease | 46 (35.9) | 42 (45.7) | 0.1 |
| Chronic renal disease | 11 (8.6) | 12 (13) | 0.3 |
| COPD | 22 (17.2) | 23 (25) | 0.1 |
| Previous hospitalization (90 days) | 56 (43.8) | 18 (19.6) | <0.001 |
| Previous ICU admission (90 days) | 13 (10.2) | 12 (13) | 0.5 |
| Previous surgery (30 days) | 50 (39.1) | 38 (41.3) | 0.7 |
| Previous antibiotic therapy (30 days) | 51 (39.8) | 45 (48.9) | 0.2 |
| Colonization at time of ICU admission | 62 (48.4) | 4 (4.3) | <0.001 |
| Source of infection | |||
| Primary bacteremia | 63 (49.2) | 17 (18.5) | <0.001 |
| CVC-related bacteremia | 25 (19.5) | 4 (4.3) | 0.001 |
| Pneumonia | 55 (43) | 64 (69.6) | <0.001 |
| Catheter-related urinary tract | 27 (21.1) | 0 | <0.001 |
| SSTI | 18 (14.1) | 2 (2.1) | <0.001 |
| Intra-abdominal | 14 (10.9) | 5 (5.4) | 0.2 |
| Isolation of a colistin-resistant strain | 57 (44.5) | 1 (1.1) | <0.001 |
| Other infections during ICU stay | 69 (53.9) | 21 (22.8) | <0.001 |
| Steroid therapy during ICU stay | 15 (11.7) | 58 (63) | <0.001 |
| Length of hospitalization (mean ± SD) (days) | 36.4 ± 23.8 | 30.2 ± 17.2 | 0.03 |
| Length of ICU stay (mean ± SD) (days) | 29.8 ± 23.3 | 22.9 ± 15.7 | 0.01 |
| Length of antibiotic therapy (mean ± SD) (days) | 11.7 ± 8.3 | 9.8 ± 9.7 | 0.1 |
| SAPS II at time of infection onset (mean ± SD) | 37.1 ± 5.6 | 39.2 ± 6.3 | 0.5 |
| SAPS II at time of septic shock onset (mean ± SD) | 43.7 ± 10.7 | 46.4 ± 14.2 | 0.1 |
| 30-day mortality | 57 (44.5) | 78 (84.8) | <0.001 |
Data are presented as the number (%), unless otherwise stated.
KPC-Kp, Klebsiella pneumoniae carbapenemase-producing K. pneumoniae; MDR-AB, multidrug-resistant Acinetobacter baumannii; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; CVC, central venous catheter; SSTI, skin and soft tissue infection; SAPS, simplified acute physiology score.
P values in bold are statistically significant.
The characteristics of the antibiotic regimens used to manage KPC-Kp and MDR-AB infections are reported in Table 2. No differences were observed in the two study groups regarding the use of carbapenems. In the study group of patients with septic shock caused by MDR-AB, a definitive antibiotic regimen containing colistin (79.3% versus 52.3%, respectively; P < 0001) or rifampin (39.1% versus 12.5%, respectively; P < 0.001) was more frequently used; on the contrary, an antibiotic regimen containing tigecycline (71.1% versus 38%, respectively; P < 0.001) or gentamicin (27.3% versus 5.4%, respectively; P < 0.001) was mainly used in septic shock patients with KPC-Kp infections. In the first 24 h of infection, an antibiotic regimen with 2 or more antibiotics displaying in vitro activity was reported only in 1 patient with MDR-AB (1.1% versus 37.5%, respectively; P < 0.001), compared to KPC-Kp infections. Definitive therapy with 2 or more antibiotics displaying in vitro activity was more frequently observed in KPC-Kp patients than in those with MDR-AB infection (47.7% versus 5.4%, respectively; P < 0.001). In the group of KPC-Kp infections, the combination of colistin, tigecycline, and meropenem more commonly included at least 2 agents with in vitro activity (63.5% of patients treated with this regimen) than the combination of colistin, tigecycline, meropenem, and gentamicin (42.4% of in patients treated with this regimen); in the group of MDR-AB infections, the combination which more frequently consisted of at least 2 in vitro-active drugs was meropenem, colistin, rifampin, and tigecycline (22.2% of patients treated with this regimen).
TABLE 2.
Characteristics of antibiotic regimens used during KPC-Kp or MDR-AB infectiona
| Antibiotic therapyb | KPC-Kp (n = 128) | MDR-AB (n = 92) | Pc |
|---|---|---|---|
| No. of antibiotics used | |||
| Only 1 as definitive therapy | 7 (5.5) | 9 (9.8) | 0.2 |
| 2 in combination as definitive therapy | 41 (32) | 28 (30.4) | 0.8 |
| 3 in combination as definitive therapy | 53 (41.4) | 38 (41.3) | 1.0 |
| 4 in combination as definitive therapy | 27 (21.1) | 10 (10.9) | 0.06 |
| 5 in combination as definitive therapy | 0 | 1 (1.1) | 0.4 |
| Colistin-containing regimen as definitive therapy | 67 (52.3) | 73 (79.3) | <0.001 |
| Tigecycline-containing regimen as definitive therapy | 91 (71.1) | 35 (38) | <0.001 |
| Gentamicin-containing regimen as definitive therapy | 35 (27.3) | 5 (5.4) | <0.001 |
| Rifampin-containing regimen as definitive therapy | 16 (12.5) | 36 (39.1) | <0.001 |
| Carbapenem-containing regimen as definitive therapy | 98 (76.5) | 67 (72.8) | 0.6 |
| Use of colistin aerosol inhalation therapy | 0 | 13 (14.1) | <0.001 |
| ≥2 in vitro-active antibiotics used within 24 h | 48 (37.5) | 1 (1.1) | <0.001 |
| Definitive therapy with ≥2 antibiotics displaying in vitro activity | 61 (47.7) | 5 (5.4) | <0.001 |
| Time to initial definitive therapy (mean ± SD) (days) | 2.7 ± 0.8 | 3.1 ± 0.7 | 0.08 |
Data are presented as the number (%), unless otherwise stated. KPC-Kp, Klebsiella pneumoniae carbapenemase-producing K. pneumoniae; MDR-AB, multidrug-resistant Acinetobacter baumannii.
During the study period, the usual antimicrobial dosages, adopted for the most used antibiotics, were the following: for colistin, a loading dose of 6 to 9 million IU, followed by 3 million IU every 8 h (in the period from 2010 to 2011) or a loading dose of 9 million IU followed by 4.5 million IU every 12 h; for tigecycline, a loading dose of 150 to 200 mg followed by 100 mg every 12 h; for gentamicin, a dosage of 5 mg/kg every 24 h; for rifampin, a dosage of 10 mg/kg/day; for meropenem, a dosage of 2 g every 8 h or 1.5 g every 6 h.
P values in bold are statistically significant.
The results of a logistic regression analysis about the characteristics of patients with KPC-Kp or MDR-AB infection are reported in Table 3. Hospitalization in the previous 90 days (odds ratio [OR], 5.01; 95% confidence interval [95% CI], 2.15 to 11.6; P < 0.001), diagnosis of primary bacteremia (OR, 2.3; 95% CI, 1.1 to 5.5; P = 0.04), and KPC-Kp colonization at time of ICU admission (OR, 13.8; 95% CI, 4.3 to 44.7; P < 0.001) were associated with diagnosis of septic shock due to KPC-Kp infection; instead, steroid therapy during ICU stay (OR, 0.1; 95% CI, 0.04 to 0.25; P < 0.001) and pneumonia as cause of septic shock (OR, 0.4; 95% CI, 0.18 to 0.96; P = 0.04) were mainly observed in septic shock patients with MDR-AB infection.
TABLE 3.
Logistic regression analysis of characteristics of KPC-Kp or MDR-AB infectiona
| Variable | ORb | 95% CI | P |
|---|---|---|---|
| Previous hospitalization (90 days) | 5.01 | 2.15–11.6 | <0.001 |
| Steroid therapy during ICU stay | 0.1 | 0.04–0.25 | <0.001 |
| Primary bacteremia | 2.3 | 1.1–5.5 | 0.04 |
| Pneumonia | 0.4 | 0.18–0.96 | 0.04 |
| KPC-Kp colonization at time of ICU admission | 13.8 | 4.3–44.7 | <0.001 |
KPC-Kp, Klebsiella pneumoniae carbapenemase-producing K. pneumoniae; MDR-AB, multidrug-resistant Acinetobacter baumannii.
An OR of >1 indicates a factor leading to KPC-Kp over MDR-AB infection, and an OR of <1 indicates a factor leading to MDR-AB over KPC-Kp infection.
As reported in Table 4, a Cox regression analysis of factors associated with outcome in KPC-Kp infections showed that the use of ≥2 in vitro-active antibiotics as initial (hazard ratio [HR], 0.19; 95% CI, 0.04 to 0.85; P = 0.03) or definitive (HR, 0.02; 95% CI, 0.004 to 0.14; P < 0.001) therapy was associated with increased 30-day survival, while the isolation of a colistin-resistant strain was linked to higher 30-day mortality (HR, 25.1; 95% CI, 4.9 to 127.8; P < 0.001).
TABLE 4.
Univariate and multivariate Cox regression analysis about factors associated with outcome at 30 days in KPC-Kp infectionsa
| Variableb | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.89 | 0.45–1.41 | 0.8 | |||
| Male sex | 1.25 | 0.59–2.71 | 0.56 | |||
| Cause of ICU admission | ||||||
| Respiratory failure | 1.42 | 0.76–2.1 | 0.09 | |||
| Trauma | 1.65 | 0.87–1.98 | 0.51 | |||
| Septic shock not caused by KPC-Kp or MDR-AB | 1.11 | 0.65–1.99 | 0.65 | |||
| Stroke | 0.87 | 0.45–1.32 | 0.9 | |||
| Cardiac/hemorrhagic shock/postsurgery | 0.65 | 0.44–1.97 | 0.09 | |||
| Comorbidities | ||||||
| Chronic liver disease | 2.5 | 0.22–2.8 | 0.58 | |||
| Neoplasm | 0.58 | 0.18–1.8 | 0.41 | |||
| Diabetes | 0.76 | 0.3–1.91 | 0.64 | |||
| Heart failure | 0.78 | 0.24–2.11 | 0.6 | |||
| Coronary artery disease | 0.93 | 0.45–1.93 | 1.0 | |||
| Chronic renal disease | 1.04 | 0.3–3.6 | 1.0 | |||
| COPD | 1.04 | 0.41–2.63 | 1.0 | |||
| Previous hospitalization (90 days) | 1.14 | 0.56–2.31 | 0.72 | |||
| Previous ICU admission (90 days) | 1.51 | 0.48–4.79 | 0.56 | |||
| Previous surgery (30 days) | 1.25 | 0.61–2.57 | 0.58 | |||
| Previous antibiotic therapy (30 days) | 2.01 | 0.98–4.13 | 0.07 | |||
| Colonization at time of ICU admission | 2.27 | 1.11–4.62 | 0.03 | |||
| Source of infection | ||||||
| Primary bacteremia | 1.45 | 0.72–2.92 | 0.37 | |||
| CVC-related bacteremia | 1.45 | 0.6–3.49 | 0.5 | |||
| Pneumonia | 0.93 | 0.46–1.89 | 1.0 | |||
| Catheter-related urinary tract | 1.45 | 0.62–3.4 | 0.39 | |||
| SSTI | 2.18 | 0.78–6.06 | 0.2 | |||
| Intra-abdominal | 0.46 | 0.13–1.55 | 0.26 | |||
| Isolation of a colistin-resistant strain | 6.38 | 2.94–13.82 | <0.001 | 25.1 | 4.9–127.8 | <0.001 |
| Other infections during ICU stay | 1.17 | 0.58–2.36 | 0.72 | |||
| Steroid therapy during ICU stay | 0.27 | 0.07–1.02 | 0.054 | |||
| Length of hospitalization | 0.76 | 0.36–1.66 | 0.6 | |||
| Length of ICU stay | 1.1 | 0.31–1.33 | 0.8 | |||
| Length of antibiotic therapy | 1.45 | 0.64–2.2 | 0.49 | |||
| SAPS II at time of infection onset | 0.87 | 0.76–1.45 | 0.4 | |||
| SAPS II at time of septic shock onset | 1.11 | 0.87–2.1 | 0.5 | |||
| Use of only 1 antibiotic as definitive therapy | 0.48 | 0.09–2.57 | 0.46 | |||
| Use of 2 antibiotics in combination as definitive therapy | 0.83 | 0.39–1.76 | 0.7 | |||
| Use of 3 antibiotics in combination as definitive therapy | 1.05 | 0.51–2.13 | 1.0 | |||
| Use of 4 antibiotics in combination as definitive therapy | 1.45 | 0.62–3.4 | 0.39 | |||
| Colistin-containing regimen as definitive therapy | 1.02 | 0.5–2.05 | 1.0 | |||
| Tigecycline-containing regimen as definitive therapy | 0.79 | 0.36–1.7 | 0.56 | |||
| Gentamicin-containing regimen as definitive therapy | 1.46 | 0.67–3.19 | 0.42 | |||
| Rifampin-containing regimen as definitive therapy | 1.28 | 0.45–3.66 | 0.78 | |||
| Carbapenem-containing regimen as definitive therapy | 1.66 | 0.78–3.52 | 0.19 | |||
| ≥2 in vitro-active antibiotics used within 24 h | 0.1 | 0.04–0.25 | <0.001 | 0.19 | 0.04–0.85 | 0.03 |
| Definitive therapy with ≥2 antibiotics displaying in vitro activity | 0.02 | 0.009–0.76 | <0.001 | 0.02 | 0.004–0.14 | <0.001 |
| Time to initial definitive therapy | 0.65 | 0.23–1.12 | 0.08 | |||
KPC-Kp, Klebsiella pneumoniae carbapenemase-producing K. pneumoniae.
MDR-AB, multidrug-resistant Acinetobacter baumannii; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; SSTI, skin and soft tissue infection; SAPS, simplified acute physiology score.
Cox regression analysis of factors associated with outcome in MDR-AB infections showed that age of >60 years (HR, 1.01; 95% CI, 1.001 to 1.03; P = 0.04) and SAPS II of >45 points (HR, 1.02; 95% CI, 1.003 to 1.04; P = 0.02) were associated with greater 30-day mortality (see Table 5).
TABLE 5.
Univariate and multivariate Cox regression analysis about factors associated with outcome at 30 days in MDR-AB infectionsa
| Variableb | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.01 | 0.78–1.12 | 0.09 | 1.01 | 1.001–1.03 | 0.04c |
| Male sex | 1.73 | 0.44–1.17 | 0.53 | |||
| Cause of ICU admission | ||||||
| Respiratory failure | 1.87 | 0.89–2.2 | 0.08 | |||
| Trauma | 1.1 | 0.78–1.22 | 0.71 | |||
| Septic shock not caused by KPC-Kp or MDR-AB | 1.24 | 0.98–1.65 | 0.65 | |||
| Cardiac/hemorrhagic shock/postsurgery | 0.87 | 0.76–1.31 | 0.42 | |||
| Comorbidities | ||||||
| Chronic liver disease | 0.84 | 0.76–1.22 | 1.0 | |||
| Neoplasm | 1.08 | 0.12–9.75 | 1.0 | |||
| Diabetes | 3.62 | 0.44–29.69 | 0.28 | |||
| Heart failure | 0.82 | 0.74–1.9 | 0.2 | |||
| Coronary artery disease | 3.66 | 0.94–14.1 | 0.07 | |||
| Chronic renal disease | 2.13 | 0.25–17.9 | 0.68 | |||
| COPD | 1.26 | 0.32–4.99 | 1.0 | |||
| Previous hospitalization (90 days) | 3.62 | 0.44–29.6 | 0.28 | |||
| Previous ICU admission (90 days) | 2.13 | 0.25–17.98 | 0.68 | |||
| Previous surgery (30 days) | 1.93 | 0.55–6.69 | 0.38 | |||
| Previous antibiotic therapy (30 days) | 1.89 | 0.58–6.16 | 0.38 | |||
| Colonization at time of ICU admission | 0.52 | 0.05–5.39 | 0.48 | |||
| Source of infection | ||||||
| Primary bacteremia | 3.35 | 0.4–27.5 | 0.45 | |||
| CVC-related bacteremia | 0.84 | 0.67–1.31 | 1.0 | |||
| Pneumonia | 0.57 | 0.14–2.25 | 0.53 | |||
| SSTI | 0.44 | 0.08–1.02 | 0.15 | |||
| Intra-abdominal | 0.7 | 0.07–6.79 | 0.57 | |||
| Isolation of a colistin-resistant strain | 0.87 | 0.81–1.02 | 1.0 | |||
| Other infections during ICU stay | 1.93 | 0.39–9.41 | 0.51 | |||
| Steroid therapy during ICU stay | 0.64 | 0.18–2.2 | 0.56 | |||
| Length of hospitalization | 1.13 | 0.68–1.36 | 0.71 | |||
| Length of ICU stay | 0.8 | 0.7–1.33 | 0.54 | |||
| Length of antibiotic therapy | 0.77 | 0.56–1.81 | 0.8 | |||
| SAPS II at time of infection onset | 1.09 | 0.87–1.49 | 0.6 | |||
| SAPS II at time of septic shock onset | 1.17 | 0.28–1.56 | 0.82 | 1.02 | 1.003–1.04 | 0.02d |
| No. of antibiotics used | ||||||
| Only 1 as definitive therapy | 0.45 | 0.22–1.12 | 0.34 | |||
| 2 in combination as definitive therapy | 1.73 | 0.44–6.75 | 0.53 | |||
| 3 in combination as definitive therapy | 0.66 | 0.21–2.06 | 0.56 | |||
| 4 in combination as definitive therapy | 0.36 | 0.08–1.61 | 0.17 | |||
| 5 in combination as definitive therapy | 0.66 | 0.45–1.32 | 1.0 | |||
| Colistin-containing regimen as definitive therapy | 0.25 | 0.03–2.09 | 0.28 | |||
| Tigecycline-containing regimen as definitive therapy | 1.64 | 0.47–5.7 | 0.55 | |||
| Gentamicin-containing regimen as definitive therapy | 0.87 | 0.76–2.1 | 0.7 | |||
| Rifampin-containing regimen as definitive therapy | 0.29 | 0.09–1.01 | 0.07 | |||
| Carbapenem-containing regimen as definitive therapy | 0.84 | 0.24–2.96 | 1.0 | |||
| Use of colistin aerosol inhalation therapy | 0.53 | 0.12–2.27 | 0.41 | |||
| ≥2 in vitro active antibiotics used within 24 h | 0.56 | 0.41–2.12 | 0.22 | |||
| Definitive therapy with ≥2 antibiotics displaying in vitro activity | 0.7 | 0.07–6.79 | 0.57 | |||
| Time to initial definitive therapy | 0.64 | 0.31–1.96 | 0.9 | |||
MDR-AB, multidrug-resistant Acinetobacter baumannii.
ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; SSTI, skin and soft tissue infection; SAPS, simplified acute physiology score.
Age >60 years.
SAPS II >45 points.
Finally, the results of Kaplan-Meier analysis of 30-day survival of patients with KPC-Kp and MDR-AB septic shock are reported in Fig. 1.
FIG 1.
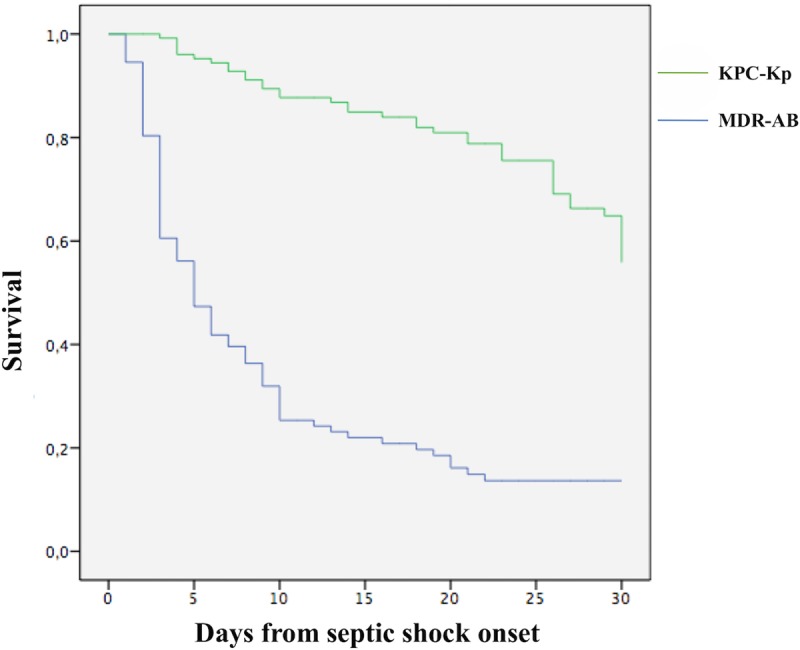
Kaplan-Meier curves for 30-day survival of KPC-Kp or MDR-AB infections. *, P < 0.001. KPC-Kp, Klebsiella pneumoniae carbapenem-resistant K. pneumoniae; MDR-AB, multidrug-resistant Acinetobacter baumannii.
DISCUSSION
This study analyzed and compared the clinical features and outcome of septic shock due to MDR-AB and KPC-Kp in a population of patients admitted to the same ICU. Of importance, very high rates of 30-day mortality (84.8%) were observed in patients with MDR-AB infection compared to patients with KPC-Kp infection (44.5% of mortality at 30 days). Moreover, in patients with MDR-AB, the 7-day mortality was 64.6% (as reported in Fig. 1).
Few studies have analyzed the impact on survival of septic shock due to MDR-AB infection. Recently, Busani and coworkers (17) reported data about patients with septic shock caused by MDR bacteria in the ICU; a diagnosis of infection caused by MDR-AB was independently associated with an increased risk of death and high mortality rates (62.5% of patients). This high mortality related to Acinetobacter baumannii infections was comparable to that reported in patients with hematologic malignancies (18). Furthermore, our data appear to confirm those reported by Freire et al., who evaluated risk factors associated with mortality after bloodstream infections caused by MDR-AB in patients with hematologic and solid tumors; the reported 7-day and 30-day mortality rates of 71.7% and 83.7%, respectively, are consistent with our data (19).
We have evaluated factors affecting the 30-day outcome. As previously reported (8, 20), in KPC-Kp infections, the isolation of a colistin-resistant strain was associated with unfavorable outcome, while the administration of at least two in vitro-active antibiotics, as initial or definitive therapy, was independently associated with survival. Extensive literature data about KPC-Kp infections support these observations (21–23). Indeed, different antibiotic combinations active against KPC-Kp and including gentamicin, tigecycline, trimethoprim-sulfamethoxazole, or double-carbapenem therapy are reported in the literature (24–26). In our population of patients with KPC-Kp septic shock, a carbapenem was the most used antibiotic, mainly in combination with colistin, tigecycline, and/or gentamicin. On the other hand, a carbapenem was used in combination with colistin and/or rifampin for the treatment of MDR-AB infections (27–29). Despite the observation of only 1 patient with MDR-AB infection due to a colistin-resistant strain, a very low proportion of patients were treated with 2 or more antibiotics showing in vitro activity against the isolates of MDR-AB; this probably explains the very high mortality rates observed in this population.
However, the recent EUCAST recommendations (30), along with the study of Matuschek and coworkers (31), demonstrated that false susceptibilities to colistin are obtained in approximately 50% of Acinetobacter baumannii strains using automated systems or Etest, while broth microdilution is the only recommended method for MIC determination. Therefore, the considerable difference in mortality rates (44.5% versus 84.8%) might be also attributed to colistin administration for the treatment of infections caused by A. baumannii strains with false susceptibility to this drug.
Different antibiotic combinations were studied for the treatment of severe infections due to MDR-AB. Durante-Mangoni and coworkers (32) showed that in patients with MDR-AB infections, mortality at 30 days was not reduced by the addition of rifampin to colistin; other in vitro studies explored the synergism of some drug combinations, especially polymyxins plus carbapenem for the treatment of MDR-AB infections (33, 34), suggesting that the use of this combination is supported by high synergy rates. On this basis, the combination of a carbapenem plus colistin seems to be the first option for the treatment of MDR-AB infections (35). Another possible synergistic combination is based on the addition of vancomycin to colistin (in our study population, this combination was used in 9 patients, and 3 of them survived at 30 days) (36–38). However, no definitive data about in vivo efficacy are available, so systematic use of the vancomycin and colistin combination for MDR-AB cannot yet be encouraged.
We analyzed factors independently associated with a diagnosis of MDR-AB and KPC-Kp infection. We found an association between exposure to steroid therapy and development of MDR-AB infection. This distinguishing feature of MDR-AB infections was recently reported by Ballouz and coworkers (39), but the meaning of this association is not yet understood. Since most of patients with MDR-AB infection had pneumonia (69.6%), it is possible that the link between steroid use and MDR-AB infection might be due to steroid administration in the management of severe pneumonia to reduce inflammation (40).
Hospitalization in the previous 90 days, colonization at the time of ICU admission, and primary bacteremia turned out to be distinguishing features of KPC-Kp infection. The importance of previous hospitalization and gut colonization by KPC-Kp at the time of ICU admission was yet assessed (41). Considering the high prevalence of KPC-Kp enteric carriage in ICU patients and the significant mortality associated with KPC-Kp infection, early identification and isolation of carriers are of uttermost importance. Patients admitted to our ICU were actively screened for KPC-Kp gastrointestinal carriage at ICU admission and subsequently on a weekly basis. Conversely, as reported in Materials and Methods, an active screening of rectal carriage was not performed for MDR-AB; therefore, we cannot definitively exclude the possibility of similar results in terms of outcome if similar screening measures were applied for MDR-AB.
Our study shows some limitations that should be acknowledged. First, the study was performed in a single center, and the results might not be generalizable to other institutions. Second, the observational nature of the study brings about an intrinsic limitation in the analysis. Third, the number of patients is relatively low, and further multicenter prospective studies are needed to confirm our findings. Finally, the underlying mechanisms of resistance in MDR-AB strains were not routinely assessed in our population, and in vitro synergistic combinations were performed only in few cases. However, this is a real-life clinical experience providing useful suggestions to clinicians about the management of difficult-to-treat and poorly studied infections, such as septic shock caused by MDR-AB or KPC-Kp strains.
In conclusion, our data showed the peculiar clinical features and the high rates of mortality in ICU patients with septic shock due to MDR-AB infections compared to septic shock due to KPC-Kp. The lack of scientific data helping clinicians in choosing optimal antimicrobial regimens that are effective against MDR-AB might explain the very high mortality rate observed in this population of patients (42, 43). All these findings suggest that it is crucial to obtain new antibiotic options for the treatment of ICU patients with MDR-AB infection, improve treatment strategies, and reduce mortality.
MATERIALS AND METHODS
Study design and patient selection.
This was a retrospective observational study conducted at the University-Hospital Policlinico Umberto I in Rome, Italy. All clinical data of patients hospitalized in the same ICU from November 2010 to December 2015 were systematically analyzed, and patients who fulfilled the following criteria were enrolled in the study: (i) age ≥18 years, and (ii) septic shock due to documented MDR-AB or KPC-Kp infection. The present study was conducted according to the principles stated in the Declaration of Helsinki. The local ethical committees approved the study (no. 4547-2017).
Baseline assessment.
Patient data were extracted from medical records and from hospital computerized databases or clinical charts according to a preestablished questionnaire. The following information was reviewed: demographics, clinical and laboratory findings, comorbid conditions, microbiological data, duration of ICU and hospital stay, incidence of infections during hospitalization, treatments and procedures administered during hospitalization and/or in the 90 days prior to infection, classes of antibiotics received on admission and/or after admission before a positive culture of a biological sample was obtained, the simplified acute physiology score II (SAPS II) at the time of infection, source of infection, antibiotic regimens used for MDR-AB or KPC-Kp infections, and 30-day mortality. According to hospital's guidelines, colonization with KPC-Kp and MDR-AB strains was routinely evaluated by respiratory specimens and urine culture at the time of ICU admission and every week afterwards. Conversely, during the study period, colonization by rectal/stool swab culture was routinely evaluated only for KPC-Kp strains.
Definitions.
Infections were defined according to the standard definitions of the European Centers for Disease Control and Prevention (ECDC) (44).
An MDR-AB or KPC-Kp infection was defined as clinical signs of the systemic inflammatory response syndrome and culture of blood, urine, or a biological sample from skin and skin structures, lung, or abdomen yielding an MDR-AB or a KPC-Kp strain (45). Infection onset was defined as the date of collection of the index culture (i.e., the first blood culture that yielded the study isolate). Infections were defined as hospital acquired if the index culture had been collected >48 h after hospital admission and no signs or symptoms of infection had been noted at admission. Primary bloodstream infection (BSI) was defined as BSI occurring in patients without a recognized source of infection. The central venous catheter (CVC) was considered a source of infection if one of the following was present: (i) a positive result of semiquantitative (>15 CFU per catheter segment) catheter culture, whereby the same organism (species) was isolated from the catheter segment and a peripheral blood culture; or (ii) growth in a culture of blood obtained through a catheter hub was detected by an automated system at least 2 h earlier than a culture of simultaneously drawn equal volume of peripheral blood, provided that the same organism (species) was isolated (46).
Septic shock was defined according to international definitions (47). The severity of clinical conditions was determined by using SAPS II score calculated at the time of infection and septic shock onset (48). Length of hospital and ICU stay were calculated as the number of days from the date of admission to the date of discharge or death.
K. pneumoniae and A. baumannii isolates were classified as multidrug resistant (MDR), extensively drug resistant (XDR), or pandrug resistant (PDR), according to Magiorakos et al. (49).
Antimicrobial treatment evaluation.
Depending on the number of drugs used (1 or >1), treatment regimens were classified as monotherapy or combination therapy. Initial antibiotic therapy, defined as antimicrobial chemotherapy implemented within 24 h after the onset of infection, was assessed along with definitive antibiotic therapy, defined as antimicrobial treatment based on in vitro MDR-AB or KPC-Kp isolate susceptibilities. Antibiotic regimens were also classified according to the following: <2 antibiotics displaying in vitro activity, and ≥2 antibiotics displaying in vitro activity. Time to initial definitive therapy was the time between infection onset and initial definitive therapy.
MDR-AB and KPC-Kp identification.
The Vitek 2 automated system (bioMérieux, Marcy l'Etoile, France) was used for isolate identification and antimicrobial susceptibility testing. MICs were established according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (50). Susceptibility to tigecycline was evaluated by the Etest technique, to avoid MIC overestimation by Vitek2 system, and the U.S. Food and Drug Administration (FDA) recommendations were used for breakpoints (susceptible, MIC ≥2 mg/liter; resistant, MIC ≥8 mg/liter) (51). The presence of a blaKPC gene was determined by PCR and sequencing (52).
Statistical analysis.
To detect significant differences between the MDR-AB group and KPC-Kp group, we used the chi-square test or Fisher's exact test for categorical variables and the 2-tailed t test or Mann-Whitney test for continuous variables, when appropriate. Logistic regression analysis was performed to identify differences between groups with MDR-AB and KPC-Kp infection. In a multivariate analysis of survival, the Cox regression model was used to determine the effects of different variables on 30-day survival in patients with KPC-Kp or MDR-AB isolation, respectively. Wald confidence intervals and tests for hazard ratios and odds ratios were computed based on the estimated standard errors. Possible confounding factors and interactions were weighted during analyses. Statistical significance was established at ≤0.05. All reported P values are 2-tailed. The results obtained were analyzed using a commercially available statistical software package (SPSS, version 20.0; SPSS, Inc., Chicago, IL).
ACKNOWLEDGMENTS
We are grateful to Marco V. Ranieri for his contribution in critical revision of this paper for important intellectual content.
No funding was received for this study.
We declare no conflicts of interest.
REFERENCES
- 1.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP. 2012. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 12:919–924. doi: 10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 2.Perez F, Endimiani A, Ray AJ, Decker BK, Wallace CJ, Hujer KM, Ecker DJ, Adams MD, Toltzis P, Dul MJ, Windau A, Bajaksouzian S, Jacobs MR, Salata RA, Bonomo RA. 2010. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 65:1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opal SM, Calandra T. 2009. Antibiotic usage and resistance: gaining or losing ground on infection in critically ill patients? JAMA 302:2367–2368. doi: 10.1001/jama.2009.1774. [DOI] [PubMed] [Google Scholar]
- 5.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. 2007. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 40:754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson JD, Kollef MH. 2011. Early and adequate antibiotic therapy in the treatment of severe sepsis and septic shock. Curr Infect Dis Rep 13:399–405. doi: 10.1007/s11908-011-0206-8. [DOI] [PubMed] [Google Scholar]
- 7.Rossolini GM, Mantengoli E. 2008. Antimicrobial resistance in Europe and its potential impact on empirical therapy. Clin Microbiol Infect 14:2–8. doi: 10.1111/j.1469-0691.2008.02126.x. [DOI] [PubMed] [Google Scholar]
- 8.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 10.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 11.Pea F, Viale P. 2009. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock–does the dose matter? Crit Care 13:214. doi: 10.1186/cc7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantelidou IM, Galani I, Georgitsi M, Daikos GL, Giamarellos-Bourboulis EJ. 2015. Interactions of Klebsiella pneumoniae with the innate immune system vary in relation to clone and resistance phenotype. Antimicrob Agents Chemother 59:7036–7043. doi: 10.1128/AAC.01405-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaden JT, Pogue JM, Kaye KS. 2017. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence 8:403–416. doi: 10.1080/21505594.2016.1207834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vázquez-Ucha JC, Maneiro M, Martínez-Guitián M, Buynak J, Bethel CR, Bonomo RA, Bou G, Poza M, González-Bello C, Beceiro A. 2017. Activity of the β-lactamase inhibitor LN-1-255 against carbapenem-hydrolyzing class D β-lactamases from Acinetobacter baumannii. Antimicrob Agents Chemother 61:e01172-. doi: 10.1128/AAC.01172-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother 61:e00093-. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durand-Réville TF, Guler S, Comita-Prevoir J, Chen B, Bifulco N, Huynh H, Lahiri S, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Velez-Vega C, Olivier NB, McLaughlin R, Gao N, Thresher J, Palmer T, Andrews B, Giacobbe RA, Newman JV, Ehmann DE, de Jonge B, O'Donnell J, Mueller JP, Tommasi RA, Miller AA. 2017. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2:17104. doi: 10.1038/nmicrobiol.2017.104. [DOI] [PubMed] [Google Scholar]
- 17.Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, Mussini C, Cossarizza A, Girardis M. 2017. Mortality in patients with septic shock by multidrug resistant bacteria. J Intensive Care Med 2017 doi: 10.1177/0885066616688165. [DOI] [PubMed] [Google Scholar]
- 18.Turkoglu M, Mirza E, Tunçcan OG, Erdem GU, Dizbay M, Yağcı M, Aygencel G, Türköz Sucak G. 2011. Acinetobacter baumannii infection in patients with hematologic malignancies in intensive care unit: risk factors and impact on mortality. J Crit Care 26:460–467. doi: 10.1016/j.jcrc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Freire MP, de Oliveira Garcia D, Garcia CP, Campagnari Bueno MF, Camargo CH, Kono Magri AS, Francisco GR, Reghini R, Vieira MF, Ibrahim KY, Rossi F, Hajjar L, Levin AS, Hoff PM, Pierrotti LC, Abdala E. 2016. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clin Microbiol Infect 22:352–358. doi: 10.1016/j.cmi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Giacobbe DR, Del Bono V, Trecarichi EM, De Rosa FG, Giannella M, Bassetti M, Bartoloni A, Losito AR, Corcione S, Bartoletti M, Mantengoli E, Saffioti C, Pagani N, Tedeschi S, Spanu T, Rossolini GM, Marchese A, Ambretti S, Cauda R, Viale P, Viscoli C, Tumbarello M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) . 2015. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 21:1106.e1–1106.e8. doi: 10.1016/j.cmi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654–663. doi: 10.1128/AAC.01222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N, SEERBIO-GRAB Network. 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19:E23–30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
- 23.Machuca I, Gutiérrez-Gutiérrez B, Gracia-Ahufinger I, Rivera Espinar F, Cano Á Guzmán-Puche J, Pérez-Nadales E, Natera C, Rodríguez M, León R, Castón JJ, Rodríguez-López F, Rodríguez-Baño J, Torre-Cisneros J. 2017. Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance: importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 61:e00406-17. doi: 10.1128/AAC.00406-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) . 2015. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2921. doi: 10.1093/jac/dkv168. [DOI] [PubMed] [Google Scholar]
- 25.Ceccarelli G, Falcone M, Giordano A, Mezzatesta ML, Caio C, Stefani S, Venditti M. 2013. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 57:2900–2901. doi: 10.1128/AAC.00188-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G. 2017. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 2017. doi: 10.1016/j.cmi.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Tumbarello M, De Pascale G, Trecarichi EM, De Martino S, Bello G, Maviglia R, Spanu T, Antonelli M. 2013. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible Gram-negative bacteria. Chest 144:1768–1775. doi: 10.1378/chest.13-1018. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh TC, Chen FL, Ou TY, Jean SS, Lee WS. 2016. Role of aerosolized colistin methanesulfonate therapy for extensively-drug-resistant Acinetobacter baumannii complex pneumonia and airway colonization. J Microbiol Immunol Infect 49:523–530. doi: 10.1016/j.jmii.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Kim YK, Lee JH, Lee HK, Chung BC, Yu SJ, Lee HY, Park JH, Kim S, Kim HK, Kiem S, Jang HJ. 2017. Efficacy of nebulized colistin-based therapy without concurrent intravenous colistin for ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumannii. J Thorac Dis 9:555–567. doi: 10.21037/jtd.2017.02.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2018. EUCAST warnings concerning antimicrobial susceptibility testing products or procedures. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: www.eucast.org/ast_of_bacteria/warnings. [Google Scholar]
- 31.Matuschek E, Åhman J, Webster C, Kahlmeter G. 2017. Antimicrobial susceptibility testing of colistin–evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect 2017 doi: 10.1016/j.cmi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 33.Ni W, Shao X, Xiuzhen Di Cui J, Wang R, Liu Y. 2015. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng A, Chuang YC, Sun HY, Sheng WH, Yang CJ, Liao CH, Hsueh PR, Yang JL, Shen NJ, Wang JT, Hung CC, Chen YC, Chang SC. 2015. Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med 43:1194–1204. doi: 10.1097/CCM.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 36.Ceccarelli G, Oliva A, d'Ettorre G, D'Abramo A, Caresta E, Barbara CS, Mascellino MT, Papoff P, Moretti C, Vullo V, Visca P, Venditti M. 2015. The role of vancomycin in addition with colistin and meropenem against colistin-sensitive multidrug resistant Acinetobacter baumannii causing severe infections in a paediatric intensive care unit. BMC Infect Dis 15:393. doi: 10.1186/s12879-015-1133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Inkaya AC, De Pascale G, Grilli E, Tumbarello M, Akova M. 2014. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother 58:851–858. doi: 10.1128/AAC.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnacho-Montero J, Amaya-Villar R, Gutiérrez-Pizarraya A, Espejo-Gutiérrez de Tena E, Artero-González ML, Corcia-Palomo Y, Bautista-Paloma J. 2013. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy 59:225–231. doi: 10.1159/000356004. [DOI] [PubMed] [Google Scholar]
- 39.Ballouz T, Aridi J, Afif C, Irani J, Lakis C, Nasreddine R, Azar E. 2017. Risk factors, clinical presentation, and outcome of Acinetobacter baumannii bacteremia. Front Cell Infect Microbiol 7:156. doi: 10.3389/fcimb.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, Winzeler B, Bingisser R, Elsaesser H, Drozdov D, Arici B, Urwyler SA, Refardt J, Tarr P, Wirz S, Thomann R, Baumgartner C, Duplain H, Burki D, Zimmerli W, Rodondi N, Mueller B, Christ-Crain M. 2015. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 41.Papadimitriou-Olivgeris M, Marangos M, Fligou F, Christofidou M, Sklavou C, Vamvakopoulou S, Anastassiou ED, Filos KS. 2013. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis 77:169–173. doi: 10.1016/j.diagmicrobio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Paterson DL, Rogers BA. 2010. How soon is now? The urgent need for randomized, controlled trials evaluating treatment of multidrug-resistant bacterial infection. Clin Infect Dis 51:1245–1247. doi: 10.1086/647243. [DOI] [PubMed] [Google Scholar]
- 43.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Centre for Disease Prevention and Control. 2014. Annual epidemiological report: vaccine-preventable diseases–invasive bacterial diseases, 2014. European Centre for Disease Prevention and Control, Stockholm, Sweden: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/AER-VPD-IBD-2014.pdf. [Google Scholar]
- 45.Russell JA. 2006. Management of sepsis. N Engl J Med 355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 46.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S, Healthcare Infection Control Practices Advisory Committee. 2011. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 39(4 Suppl 1):S1–S34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Gall JR, Lemeshow S, Saulnier F. 1993. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 49.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 50.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2013. Breakpoint tables for interpretation of MICs and zone diameters, version 3.1. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf. [Google Scholar]
- 51.Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. 2008. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 52:570–573. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis 39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]


