ABSTRACT
The obligate intracellular bacterium Chlamydia is a widespread human pathogen that causes serious problems, including (but not limited to) infertility and blindness. Our search for novel antichlamydial metabolites from marine-derived microorganisms led to the isolation of pyocyanin, a small compound from Pseudomonas aeruginosa. Pyocyanin is an effective antichlamydial for all three Chlamydia spp. tested. It has a 50% inhibitory concentration (IC50) of 0.019 to 0.028 μM, which is comparable to the IC50 of tetracycline. At concentrations as low as 0.0039 μM, pyocyanin disables infectivity of the chlamydial elementary body (EB). At 0.5 μM or higher concentrations, the continuous presence of pyocyanin also inhibits chlamydial growth in the inclusion during later stages of the developmental cycle. Oxidative stress, a major known antimicrobial mechanism of pyocyanin, appears to be responsible only for the inhibition of bacterial growth and not for the disinfection of EBs. Pyocyanin is well-tolerated by probiotic vaginal Lactobacillus spp. Our findings suggest that pyocyanin is of therapeutic value for chlamydial infections and can serve as a valuable chemical probe for studying chlamydial biology.
KEYWORDS: chlamydia, antichlamydial, oxidative stress, pyocyanin
INTRODUCTION
Chlamydia species are Gram-negative bacteria that cause diseases in a wide range of hosts, including humans (1, 2). Chlamydia trachomatis is the most prevalent sexually transmitted bacterial pathogen. In women, untreated and recurring genital C. trachomatis infection can lead to serious urogenital complications, including ectopic pregnancy, abortion, infertility, and pelvic inflammatory disease (3, 4). Additionally, C. trachomatis can cause trachoma, the leading cause of preventable blindness in developing countries (5, 6). Chlamydia pneumoniae causes respiratory diseases, such as bronchitis and pneumonia, and is a possible risk factor for atherosclerosis and late-onset Alzheimer's disease (7, 8).
Chlamydiae have a unique biphasic developmental cycle characterized by two alternating cellular forms, the infectious but nondividing elementary body (EB) and the replicative but noninfectious reticulate body (RB). EBs are enveloped by a number of host cell adhesion mediators (9–11). Infection is initiated by the EB, which adheres to and invades the host cell by innate actin-dependent internalization. Within a cytoplasm vacuole, termed an inclusion, the EB converts to the RB, which replicates by binary fission. RBs subsequently differentiate back to EBs, which eventually exit the host cell and start a new round of infection (1, 11–13).
Chlamydial infection is typically treated with either azithromycin or doxycycline, a member of the tetracycline family of antibiotics (3, 14). Even though chlamydiae are highly susceptible to these drugs in vitro, clinical resistance or treatment failure is not uncommon (15–17). These broad-spectrum antibiotics also disturb the indigenous human microbiota, which is essential to the health of the host (18, 19). Although extensive efforts have been devoted to vaccine development during the past few decades, no vaccine effective against human chlamydial diseases has been developed to date (20, 21). These facts necessitate identification of novel antichlamydials through chemical synthesis (4, 22–24) and/or from natural sources (25–27).
Pyocyanin is a virulence factor secreted by Pseudomonas aeruginosa. Antibacterial activities of pyocyanin have been reported in the past, as early as 1940 (28, 29). Pyocyanin is known to also inhibit growth of fungi and phytopathogens (29). Nevertheless, whether or not pyocyanin inhibits Chlamydia infection has not been documented. In this study, we report that pyocyanin acts as a highly efficient antichlamydial agent, with an IC50 of 0.019 to 0.028 μM, comparable to the IC50 of tetracycline. At low concentrations, pyocyanin inhibits chlamydial infection by disabling infectivity of the EB. At higher concentrations, pyocyanin also inhibits chlamydial growth, leading to reduced inclusions inside host cells. Interestingly, only the inhibition of bacterial growth seems to depend on oxidative stress, a known antimicrobial mechanism for pyocyanin. We also report that pyocyanin is well tolerated by host cells and beneficial vaginal lactobacilli.
RESULTS
Strong antichlamydial activity in pyocyanin.
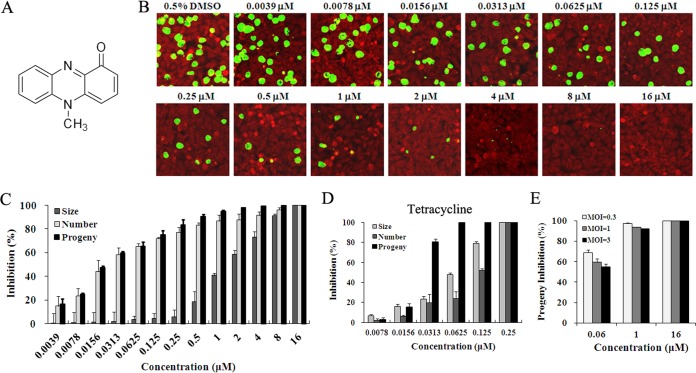
The antichlamydial activity of pyocyanin (Fig. 1A) was initially evaluated by culturing C. trachomatis L2 in medium containing pyocyanin for 36 hours postinfection (hpi) using immunofluorescence microscopy. Compared to the dimethyl sulfoxide (DMSO) mock-treated culture, both the number and size of inclusions in cultures treated with pyocyanin were reduced in a dose-dependent manner (Fig. 1B and C). Inclusions were completely undetectable at 16 μM. Interestingly, whereas reduction of the inclusion number was readily seen at 0.0039 μM, substantial inhibition of the inclusion size was observed only at higher concentrations (≥0.5 μM) (Fig. 1B and C). Accordingly, the IC50 for inclusion size is substantially higher than the IC50 for inclusion number (1.5 μM versus 0.03 μM, Table 1). These findings suggest that at lower concentrations, pyocyanin only interferes with early steps of chlamydial infection such as chlamydial attachment to and/or entry into host cells, whereas at higher concentrations it also perturbs chlamydial growth, leading to smaller inclusions.
FIG 1.
Inhibitory effects of pyocyanin and tetracycline on C. trachomatis L2. (A) Structure of pyocyanin. (B to D) HeLa cells were inoculated with C. trachomatis L2 EBs at an MOI of 0.2 and cultured in the presence of 0.5% DMSO, various concentrations of pyocyanin, or tetracycline. Cells were either fixed 36 hpi or lysed for determination of infectious progeny EBs. (B) Representative immunofluorescence microscopy images for HeLa cells treated with 0.5% DMSO or pyocyanin at indicated concentrations are shown. Inclusions were stained with an anti-MOMP antibody (green), and cellular background was stained with Evans blue (red). (C, D) The size and number of inclusions formed in pyocyanin-, tetracycline-, or control DMSO-treated cultures were measured using Image-Pro Plus 6.0 software. Infectious progeny EBs were obtained from secondary culture using inhibitor-free medium, as detailed in Materials and Methods. (E) Effects of MOIs on the inhibition of progeny EB production by pyocyanin. HeLa cells were inoculated with C. trachomatis L2 EBs at MOIs of 0.3, 1, and 3, and cultured in the presence of 0.5% DMSO or pyocyanin at indicated concentrations for 36 h. (C to E) Percent inhibition was calculated based on data in pyocyanin- or tetracycline-treated samples relative to those of the DMSO-treated control.
TABLE 1.
IC50s of pyocyanin for different Chlamydia species
| Species and strain | IC50s (μM)a for: |
||
|---|---|---|---|
| Inclusion size | Inclusion no. | Progeny production | |
| C. trachomatis L2 | 1.513 (1.347–1.701) | 0.030 (0.025–0.037) | 0.024 (0.022–0.027) |
| C. pneumoniae AR39 | 1.994 (1.737–2.290) | 0.029 (0.025–0.034) | 0.019 (0.017–0.021) |
| C. muridarum MoPn | 1.189 (1.068–1.325) | 0.043 (0.034–0.055) | 0.028 (0.024–0.032) |
IC50s are presented as means and 95% confidence intervals.
The effect of pyocyanin on the production of progeny EBs was highly consistent with the effect on the inclusion number and less consistent with that on the inclusion size (Fig. 1C). The IC50 was calculated to be 0.024 μM (Table 1), which is essentially identical to the IC50 of tetracycline for inhibiting progeny formation (0.022 μM) (Fig. 1D). While data presented in Fig. 1B and C were obtained with a multiplicity of infection (MOI) of 0.2, a standard condition for antichlamydial tests (4, 31, 32), we also evaluated inhibition efficiency at higher MOIs (0.3, 1, and 3). Recoverable inclusion-forming unit (IFU) data showed that higher MOIs slightly reduced the inhibition efficiency of 0.06 μM pyocyanin. A lesser reduction was seen for 1 μM, and no reduction was seen at 16 μM (Fig. 1E).
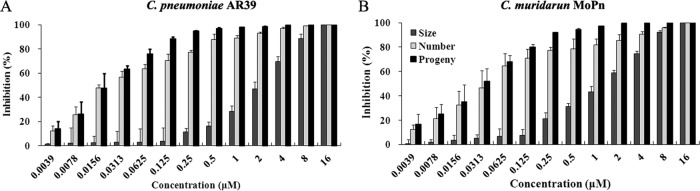
Strong antichlamydial activities of pyocyanin were also obtained with C. pneumoniae AR39 (Fig. 2A) and Chlamydia muridarum MoPn (Fig. 2B). Whereas C. pneumoniae is another human pathogen, C. muridarum is used extensively to model C. trachomatis diseases in mice. Changes in inclusion size and number and in progeny production for both strains in response to pyocyanin treatment are highly similar to those observed for C. trachomatis L2, with relatively slight differences in IC50s (Table 1).
FIG 2.
Inhibitory effects of pyocyanin on C. pneumoniae AR39 (A) and C. muridarum MoPn (B). Infection and inhibitor treatment were performed as described in Fig. 1B and C legends, with the exception that centrifugation was performed following inoculation with C. pneumoniae AR39 to facilitate its infection. The primary antibodies used for immunostaining were polyclonal mouse anti-AR39 and anti-MoPn.
High pyocyanin tolerance of host cells.
As shown in Fig. 1B, the morphology of HeLa cells was well preserved in cultures treated with pyocyanin. To further determine whether or not pyocyanin is toxic to host cells, we treated uninfected HeLa and Hep2 cells with pyocyanin for 48 h and measured their viability using the WST-1 assay (33). Pyocyanin did not cause any viability changes at or below 8 μM, although a 35% reduction was observed with 16 μM (Fig. 3). These findings, coupled with the fact that 8 μM pyocyanin reduced the inclusion number and progeny EBs of Chlamydia spp by more than 96% (Fig. 1C and 2), indicate that the antichlamydial activity of pyocyanin is not through host cell toxicity. Since pyocyanin remains nontoxic to host cells up to 8 μM, subsequent studies were performed with ≤8 μM pyocyanin.
FIG 3.

Effects of pyocyanin on host cells. HeLa and Hep2 cells were cultured with medium containing pyocyanin or 0.5% DMSO for 48 h. Cell viability was determined with the WST-1 reagent. Error bars indicate standard deviations of triplicate samples. Asterisks indicate a statistically significant difference relative to perspective DMSO controls (**, P < 0.01 by Student's t test).
Differential effects of pyocyanin treatment schemes on inhibition.
As mentioned earlier, the discordant inhibitory effects exhibited by the lower and higher concentrations of pyocyanin on the inclusion size, the number of inclusions, and progeny EBs suggest that the compound at lower concentrations (<0.5 μM) targets only early step(s) in the chlamydial developmental cycle, whereas at higher concentrations (≥0.5 μM) it also inhibits chlamydial growth. To further test this hypothesis, we applied different treatment schemes (i.e., coincubation, withdrawal, and delayed treatment, Fig. 4A) for C. trachomatis L2 and determined their effects on chlamydial growth at 36 hpi. Essentially, all pyocyanin inhibition data presented prior to this point were obtained with the coincubation scheme. Consistent with findings presented in Fig. 1C, coincubation with 0.06 μM pyocyanin caused a moderate (∼60%) reduction in the inclusion number and progeny production, but had little effect on the inclusion size (Fig. 4B). A similar inhibitory pattern was observed for the 0.06 μM withdrawal scheme (Fig. 4B). In contrast, delayed treatment with 0.06 μM pyocyanin had almost no inhibitory effects on either the number of inclusions or progeny production. Thus, with 0.06 μM pyocyanin, treatment for only the first 2 h was as effective as 36-h treatment.
FIG 4.
Impact of pyocyanin administration time on antichlamydial efficacy. (A) HeLa cells infected with C. trachomatis L2 EBs at an MOI of 0.2 were treated with pyocyanin on different schemes, namely, coincubation, withdrawal, or delayed treatment. (B) Percent inhibition of inclusion size and number and the yield of infectious progeny EBs were determined with pyocyanin at 0.06, 1, and 8 μM. Asterisks indicate a statistically significant difference between indicated treatment schemes (*, P < 0.05 and **, P < 0.01 by Student's t test). NS, nonsignificant.
Increasing the pyocyanin concentration to 1 μM resulted in a different inhibition pattern than that at 0.06 μM (Fig. 4B). In agreement with data presented in Fig. 1C, coincubation resulted in moderate inhibition of inclusion size and strong inhibition of the inclusion number and progeny production. Withdrawal of pyocyanin resulted in a nearly full reversal of inclusion size inhibition, but delayed treatment reversed the size inhibition only slightly. These findings again support the notion that pyocyanin inhibits chlamydial growth at higher concentrations. Withdrawal of 1 μM pyocyanin also significantly reduced the efficiencies of inhibition of the inclusion number and progeny production; accordingly, the percent inhibition levels dropped to the levels of those with 0.06 μM coincubation or withdrawal. These findings suggest that the continuous presence of 1 μM pyocyanin, compared to 0.06 μM, prevents additional EBs from forming inclusions inside the host cell. As expected, without an early phase targeting (which is obtainable with 0.06 μM pyocyanin), 1 μM delayed treatment, resulted in significant reversal of inclusion number and progeny production inhibition compared to that with 1 μM coincubation. Noticeably, the inhibitory effect of 1 μM delayed treatment on progeny production was much stronger than the inhibitory effect on the inclusion number, indicating that the extremely small inclusions formed in the presence of 1 μM pyocyanin fail to produce infectious EBs.
As expected from Fig. 1B and C, inclusions formed in cultures with 8 μM cotreatment were both tiny and rare, corresponding to a nearly 90% reduction of the inclusion size and nearly 100% reduction of inclusion number and progenies (Fig. 4B). Effects of 8 μM withdrawal and delayed treatment on the inhibition of inclusion size, number, and progeny formation largely resemble the effects of 1 μM withdrawal and delayed treatment, respectively. The only noticeable difference is that 8 μM delayed treatment did not result in any reversal on the inhibition of progeny formation, indicating a stronger inhibitory effect of the higher concentration on chlamydial growth. Taken together, data presented in Fig. 4 is in full agreement with the proposition that at lower concentrations (<0.5 μM), pyocyanin targets only early step(s) in the chlamydial developmental cycle, whereas at higher concentrations (≥0.5 μM) it also inhibits chlamydial growth inside the inclusion.
Direct disabling of the infectivity of EBs.
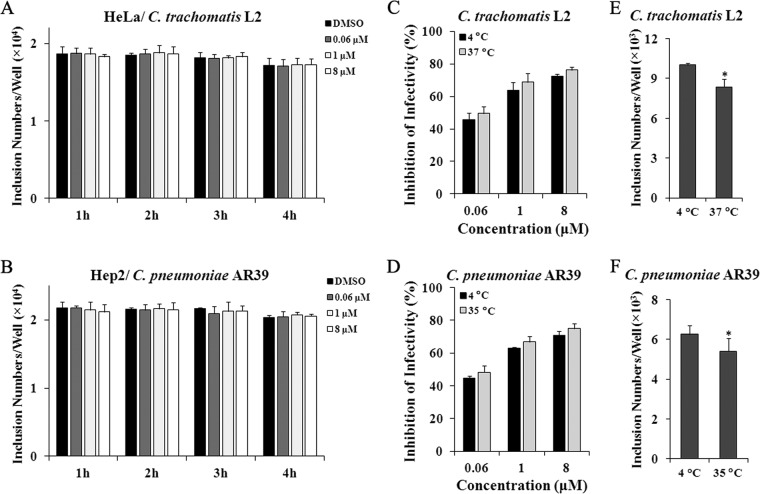
Since significant inhibitory effects can be obtained with pyocyanin withdrawal 2 hpi (Fig. 4), we went on to test whether pretreating host cells or chlamydial EBs prior to inoculation leads to inhibition. Pretreatment of host cells with 0.06, 1, or 8 μM pyocyanin for 1 to 4 h did not exhibit any effects on either C. trachomatis L2 (Fig. 5A) or C. pneumoniae AR39 (Fig. 5B). In contrast, pretreating EBs resulted in a pyocyanin dose-dependent reduction for both chlamydiae in a manner that was independent of the treatment temperature (4°C versus 37 or 35°C) (Fig. 5C and D). Pretreatment of EBs was performed at 4°C in addition to the physiological temperature, because EBs in sucrose-phosphate-glutamate (SPG) buffer containing 0.5% DMSO suffered a small but significant loss of infectivity at 37 or 35°C (Fig. 5E and F). The finding that pretreatment conducted at 4°C was sufficient to inhibit the infectivity of EBs suggests that the inhibition process is likely to be energy independent.
FIG 5.
Pyocyanin disables the infectivity of EBs. (A, B) Host cells were pretreated with 0.06, 1, and 8 μM pyocyanin or 0.5% DMSO for 1, 2, 3, or 4 h. Following washes, HeLa (A) and Hep2 (B) cells were inoculated with C. trachomatis L2 or C. pneumoniae AR39, respectively. Numbers of inclusions were quantified using immunofluorescence microscopy. (C to F) EBs were pretreated with 0.06, 1, and 8 μM pyocyanin or 0.5% DMSO for 1 h at indicated temperature. Following washes, EBs were added to cells. Numbers of inclusions formed by 36 hpi were quantified using immunofluorescence microscopy. (C, D) Percent inhibition of EB infectivity was calculated based on data in pyocyanin-treated samples relative to those of the DMSO-treated control. (E, F) Numbers of inclusions pretreated with 0.5% DMSO are presented. Asterisks indicate statistically significant difference between 4°C and 37 or 35°C (*, P < 0.05 by Student's t test).
Pyocyanin does not block Chlamydia attachment.
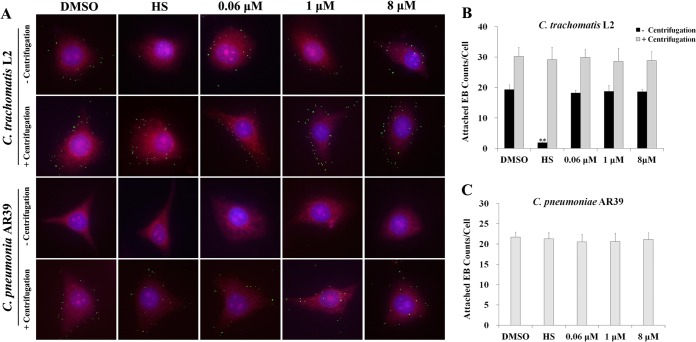
We next tested if pyocyanin affects EB attachment to host cells. To minimize temperature-related loss of infectivity (Fig. 5E and F), EBs were pretreated with pyocyanin at 4°C for 1 h. As expected, centrifugation significantly increased the numbers of EBs bound to the host cells, especially for C. pneumoniae AR39 (Fig. 6A to C). In still culture, pretreatment with heparan sulfate, a known inhibitor of chlamydial attachment (24, 34), caused a 90% reduction in attachment of C. trachomatis L2 EBs to HeLa cells (Fig. 6A and B), but this inhibitory effect was completely overcome by centrifugation. Clearly, pretreatment of EBs with up to 8 μM pyocyanin did not cause inhibition of EB attachment to host cells, whether or not centrifugation was used. These data demonstrate that pyocyanin does not block EB attachment to host cells, and further suggest that pyocyanin-treated EBs are defective in establishing infection after attaching to host cells.
FIG 6.
Effect of pyocyanin on attachment of EBs to host cell. C. trachomatis L2 or C. pneumoniae AR39 EBs were incubated with 0.5% DMSO, heparan sulfate (HS), or pyocyanin at 4°C for 1 h. After washes, EBs (MOI, ∼20 to 30) were added onto host cell monolayers. Infection was performed with or without centrifugation (500 × g) at 4°C for 1 h. Following washes to remove unbound bacteria, cells were fixed with methanol, and stained with anti-MOMP (C. trachomatis L2, green) or anti-AR39 (C. pneumoniae AR39, green) antibody, Evans blue (cytosol, red) and DAPI (nuclei, blue). (A) Representative cells are presented as montages. (B and C) For each sample, EBs on nine randomly selected cells were scored. Error bars indicate standard deviations of the triplicate samples. Asterisks indicate a statistically significant difference relative to DMSO control (still incubation **, P < 0.01 by Student's t test).
Stimulation of intracellular ROS production by high pyocyanin concentrations.
Oxidative stress is a major factor that contributes to the antibiotic properties of pyocyanin (29, 35). To determine if oxidative stress is also involved in the antichlamydial effect of pyocyanin, we measured intracellular reactive oxygen species (ROS) levels. For both HeLa and Hep2 cells, whether infected or not, pyocyanin at 1 and 8 μM significantly increased ROS at multiple points during experimentation (2, 12, 24, and 36 hpi), but at 0.06 μM it failed to induce ROS throughout the course of the experiments (Fig. 7A and B).
FIG 7.
Stimulation of ROS production by pyocyanin and reversal by GSH and NAC in Chlamydia-infected cultures. HeLa (A) or Hep2 (B) cells were infected with C. trachomatis L2 and C. pneumoniae AR39 EBs, respectively, and cultured in the presence of pyocyanin or 0.5% DMSO. Intracellular ROS levels were measured at indicated times using DHR. Asterisks indicate a statistically significant difference relative to results from perspective-control DMSO-treated cultures (*, P < 0.05; **, P < 0.01 by Student's t test). (C, D) Cells were infected and then cultured in medium containing pyocyanin with (or without) 5 mM GSH or NAC. Intracellular ROS was determined at 36 hpi. Asterisks indicate statistically significant differences between indicted treatments (*, P < 0.05 by Student's t test). NS, nonsignificant.
The antioxidants glutathione (GSH) or N-acetylcysteine (NAC; GSH precursor) can reduce the antibacterial activity of pyocyanin (29, 35). Therefore, we also treated cells with either GSH or NAC to determine if oxidative stress plays a role in the antichlamydial activity of pyocyanin. As expected, both GSH and NAC significantly reduced the level of ROS in C. trachomatis L2-infected HeLa cells and C. pneumoniae AR39-infected Hep2 cells treated with pyocyanin, although they failed to bring it down to the respective basal levels (Fig. 7C and D).
Reversal of pyocyanin-induced chlamydial inhibition by GSH and NAC.
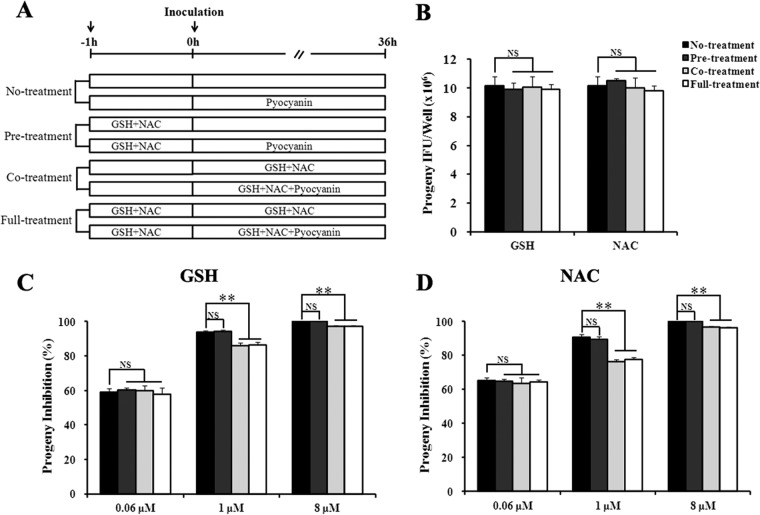
If pyocyanin exerts its antichlamydial activity through induction of intracellular ROS, antioxidants like GSH and NAC should be able to reduce the antichlamydial effect. The antagonistic effects of these antioxidants on chlamydial infection were assessed on three treatment schemes (Fig. 8A), as follows: (i) pretreatment of host cells for 1 h prior to EBs inoculation (pretreatment), (ii) treatment of infected cells during the entire duration of infection (cotreatment), and (iii) treatment from 1 h prior to infection through harvest at 36 hpi (full treatment).
FIG 8.
Antagonistic effects of antioxidants on the inhibition of C. trachomatis L2 by pyocyanin. (A) HeLa cells were placed on one of the following treatment schemes: no treatment, pretreatment (pretreatment of host cells with 5 mM GSH or NAC at 37°C for 1 h prior to EBs inoculation), cotreatment (treatment of infected cells with GSH or NAC during the entire duration of infection), or full-treatment (treatment from 1 h prior to infection through harvest at 36 hpi). (B) In the absence of pyocyanin, neither GSH nor NAC had any effects on chlamydial growth. (C and D) GSH and NAC both reversed pyocyanin-mediated chlamydial growth inhibition. Asterisks indicate a statistically significant difference between indicated treatment schemes (**, P < 0.01 by Student's t test). NS, nonsignificant.
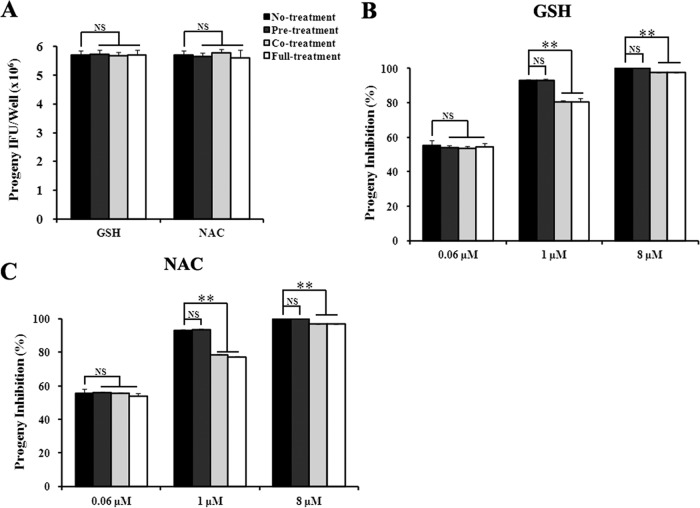
Effects of GSH and NAC on pyocyanin-mediated inhibition of C. trachomatis L2 and C. pneumoniae AR39 are shown in Fig. 8 and 9, respectively. GSH and NAC displayed similar effects on both chlamydial strains. In the absence of pyocyanin, the antioxidants had no detectable effect on chlamydial progeny production (Fig. 8B and 9A). Consistent with the lack of intracellular ROS induction by 0.06 μM pyocyanin (Fig. 7A and B), the antioxidants did not show any effect on chlamydial progeny production in cultures treated with 0.06 μM pyocyanin on any treatment scheme (Fig. 8 and 9). Similarly, antioxidant pretreatment had no effect on the antichlamydial activities of 1 and 8 μM pyocyanin (Fig. 8 and 9). However, antioxidant cotreatment and full treatment significantly reduced the inhibition. Noticeably, the degree of inhibition reversal was inversely correlated with the degree of ROS induction. Compared to 1 μM pyocyanin, 8 μM induced higher levels of ROS (Fig. 7), and the antichlamydial activity of 8 μM was less efficiently reversed by GSH and NAC (Fig. 8 and 9). Taken together, the results shown in Fig. 7 to 9 support the notion that at higher concentrations, pyocyanin inhibits chlamydial growth by inducing host cells to produce intracellular ROS.
FIG 9.
Antagonistic effects of antioxidants on the inhibition of C. pneumoniae AR39 by pyocyanin. Experiments were performed as for Fig. 8, except Hep2 cells were used as the host and cultured at 35°C, and centrifugation was performed to facilitate infection.
Weak inhibitory activity of pyocyanin toward vaginal lactobacilli.
Lactobacilli, which dominate the vaginal commensal microbiota in many healthy and reproductive-age women, play protective roles against pathogens (19). We determined the effect of pyocyanin on three vaginal Lactobacillus strains, Lactobacillus crispatus 33197 and 33820 and Lactobacillus jensenii 25258 (4, 19). Significantly, growth of the Lactobacillus strains was not affected by pyocyanin as high as 16 μM, which completely inhibits chlamydial infection, even though they were fully or partially inhibited at 32 or 64 μM (Fig. 10). These observations indicate that pyocyanin is well tolerated by vaginal lactobacilli, and used properly, it could inhibit chlamydiae without affecting the growth of probiotic vaginal lactobacilli.
FIG 10.

Lack of effect of pyocyanin on growth of vaginal lactobacilli. Pyocyanin or DMSO was added to the indicated final concentrations at the time of inoculation. OD600 values were measured at the indicated hpi. Values represent averages ± standard deviation of triplicate experiments.
DISCUSSION
Chlamydiae are common and important pathogens worldwide. Although they are susceptible to several broad-spectrum antibiotics, clinical treatment failure is not uncommon. Without effective vaccines, there have been serious and continuous efforts to discover and develop new antichlamydials (4, 22–27), particularly selective antichlamydials lacking adverse effects on the microbiota (4, 22, 23). In a search for natural compounds with antichlamydial activities, we identified pyocyanin as an antichlamydial with an IC50 that is comparable to the IC50 of tetracycline (Fig. 1 and 2).
We carefully analyzed the effects of pyocyanin on the inclusion size and number, and also on progeny EB production. Our results have revealed dissimilarities between inclusion size and inclusion number as well as progeny production in experiments using low concentrations of pyocyanin but not in those using high concentrations (Fig. 1 and 2). On the basis of these observations, we propose a model in which at low concentrations, pyocyanin directly targets EBs to disable their infectivity, while higher concentrations of pyocyanin also inhibit chlamydial growth in the host cell. This mechanistic model is also supported by data obtained with different pyocyanin treatment schemes, especially by the finding that 0.06 μM pyocyanin withdrawal 2 hpi is as effective as coincubation in reducing the inclusion number and progeny production, but delayed treatment starting 2 hpi has no inhibitory effect (Fig. 4). The observation of a strong inhibitory effect of pretreatment of EBs with pyocyanin (Fig. 5C and D) serves as the strongest evidence for direct EB targeting by pyocyanin.
Pyocyanin is a reversible redox-active phenazine pigment produced by the opportunistic pathogen P. aeruginosa (36). It has been well documented in the literature that pyocyanin interferes with electron transport through the respiratory chain, leading to the production of antimicrobial ROS (36–38). Several lines of evidence suggest that induction of ROS production in host cells is an antichlamydial mechanism that underlies the antichlamydial activity of higher concentrations of pyocyanin. First, we detected higher concentrations of intracellular ROS in both pyocyanin-treated uninfected cells, as well as in pyocyanin-treated Chlamydia-infected cells (Fig. 7A and B). Second, both GSH and NAC significantly reduced the inhibitory effects of pyocyanin (Fig. 8 and 9). Third, both GSH and NAC blunted pyocyanin's ROS-inducing activity (Fig. 7C and D). Fourth, GSH and NAC are less effective in reversing the antichlamydial activities of 8 μM pyocyanin, which induced a higher level of ROS than 1 μM pyocyanin (Fig. 7 to 9). However, induction of ROS production inside chlamydial cells (EBs or RBs) could be an additional or alternative antichlamydial mechanism for pyocyanin.
How low concentrations of pyocyanin block the infectivity of EBs remains unknown. On the one hand, 0.06 μM pyocyanin fails to increase intracellular ROS (Fig. 7A and B), which is consistent with the literature (35, 39). On the other hand, free EBs may be particularly susceptible to ROS, and the ROS detection assay that we used may not be sensitive enough to detect an ROS increase that is small but still biologically consequential. The lack of effect of antioxidants on pyocyanin-induced chlamydial growth inhibition across any of the treatment schemes (Fig. 8 and 9) does not seem to support such an argument. However, this negative finding has to be interpreted with caution, because we are not certain whether or not GSH and NAC can reach inside the EB.
The attachment of EBs to host cells is a crucial early step that is required for establishing intracellular infection. Pretreating EBs with pyocyanin prior to inoculation significantly decreased the infectivity of EBs (Fig. 5C and D) without affecting EB binding to the host cells (Fig. 6). However, pretreating host cells with pyocyanin prior to inoculation had no effect on chlamydial infection. On the basis of these findings, we propose that pyocyanin-inactivated EBs are capable of attaching to but incapable of entering host cells, or are capable of entry but fail to develop into RBs.
A virulence factor from P. aeruginosa, pyocyanin has a number of adverse effects on the host. Many research groups have focused on its adverse effect on the human respiratory system due to the high incidence of chronic colonization with P. aeruginosa in cystic fibrosis and obstructive lung disease (36). Although up to 8 μM pyocyanin is well tolerated by epithelial cell lines cultured in vitro (Fig. 1 and 3), the literatures show that exposure to pyocyanin has negative consequences on various organ systems, including the central nervous system, vascular system, and liver (36, 40–43). In addition, studies have shown that pyocyanin affects the immune system in multiple ways. Although pyocyanin stimulates lymphocyte proliferation at 1 μM (44), it starts to inhibit when it reaches 2 μM (44–46). Starting at 10 μM, it causes neutrophils to undergo apoptosis (47). Given the role of the immune system in protection against pathogens, it is of particular importance to avoid the immunosuppression function of pyocyanin. Significantly, the IC50 of pyocyanin on chlamydiae is as low as ∼0.02 μM (Table 1), which is about 100 times lower than its minimal immunosuppressive concentration. Despite this big difference between the antichlamydial concentration and immunosuppression concentration, its systemic use should still be of concern—even for short-term use. Nonetheless, it warrants consideration to develop pyocyanin as a topical antimicrobial for prevention and treatment of chlamydial infection in the eye and/or the female genital tract.
The vaginal microbiota plays an important role in defense against pathogens, including C. trachomatis in the genital tract (19, 48). In healthy and reproductive-age women, the vaginal microbiota is dominated by lactobacilli, such as Lactobacillus crispatus and L. jensenii, which produce lactic acid and other antimicrobials (19, 48, 49). Ideally, a candidate vaginal microbicide does not disrupt vaginal lactobacilli. Significantly, we found high tolerance of pyocyanin in all three vaginal Lactobacillus strains tested (Fig. 10).
In summary, we have identified pyocyanin as a potent antichlamydial. Even though its toxicity precludes its utility as an antimicrobial for systemic use, with an IC50 comparable to that of tetracycline, it warrants consideration to evaluate pyocyanin as a topical microbicide candidate for prevention and treatment of ocular and/or vaginal chlamydial infection. Hopefully, pyocyanin derivatives with reduced toxicity that maintain the antichlamydial activity can be developed. Pyocyanin exerts its antichlamydial activity by directly disabling the infectivity of EBs and inducing production of antimicrobial ROS. Regardless of its potential as a preventive and therapeutic agent, pyocyanin will be a valuable chemical probe for studying Chlamydia biology.
MATERIALS AND METHODS
Chlamydial strains, host cells, and culture conditions.
C. trachomatis L2 (strain 434/Bu), C. pneumoniae (strain AR39), and C. muridarum (strain Nigg II, traditionally known as mouse pneumonitis pathogen [MoPn]) were purchased from ATCC. HeLa and Hep2 cells were also purchased from ATCC and were maintained routinely in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, Aldrich, St. Louis, MO) and 20 μg/ml gentamicin, at 37°C and 5% CO2. C. trachomatis L2 and C. muridarum MoPn were cultured in HeLa cells at 37°C. C. pneumoniae AR39 were cultured in Hep2 cells at 35°C. EB stocks were prepared in sucrose-phosphate-glutamate (SPG) buffer and stored at −80°C. The mouse monoclonal anti-MOMP antibody L2-1-5 (anti-MOMP) (50) was generously provided by Harlan D. Caldwell (United States National Institutes of Allergy of Infectious Diseases). This antibody was used for immunostaining C. trachomatis L2 inclusions. Pooled sera collected from BALB/c mice intranasally inoculated with C. pneumoniae AR39 EBs were used to stain inclusions and EBs of this organism. A mouse polyclonal anti-MoPn antibody generated in the same manner by our lab was used for C. muridarum MoPn detection (23).
Pyocyanin preparation.
A total of 24 microbial strains (CB1 to CB24) were isolated from an unidentified marine clam. Exudate extracts from all marine microbial isolates were screened for antichlamydial potential as outlined below in “Chlamydia inhibition tests.” The extract of CB4 exhibited the highest inhibitory activity on C. trachomatis, and phylogenetic analysis of 16S rRNA gene sequences indicated that the source of CB4 was P. aeruginosa. Bioassay-guided fractionation of the extract generated from 20 liters of CB4 exudates resulted in the isolation of pyocyanin (6.7 mg; purity, ≥99%). The structure of pyocyanin was elucidated by comparing nuclear magnetic resonance (NMR) spectroscopic data with data in the published literature (51).
Chlamydia inhibition tests.
Methods for evaluating antichlamydial activities in small compounds involving immunostaining of chlamydial inclusions have been previously described (4). Cells were seeded onto coverslips in a 24-well plate. After overnight incubation, cells at 60% to 70% confluence were infected at a multiplicity of infection (MOI) of 0.2 inclusion-forming units (IFUs) per cell, unless otherwise noted. Pyocyanin dissolved in DMSO was diluted in EB-containing culture medium to the appropriate concentrations, unless otherwise indicated. DMSO (final concentration, 0.5%) was used as a negative control. Centrifugation (500 × g for 1 h at room temperature) was used to facilitate C. pneumoniae AR39 infection. At 36 hours postinoculation (hpi), infected cells were either fixed with 100% methanol for visualization of inclusions formed in the presence or absence of inhibitors or subjected to procedures for determination of progeny EBs. The methanol-fixed cells were subjected to sequential staining with an appropriate primary antibody and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Evans blue was used as a counterstain. Images were captured with an Olympus IX51 fluorescence microscope, and the inclusion number and size were measured using the Image-Pro Plus 6.0 software. For determining the production of progeny EBs, infected cells were washed three times with SPG to remove residual pyocyanin, scraped off the plate, and sonicated briefly to disrupt host cells and release EBs. The recoverable IFUs were quantified by inoculating 1:10 serially diluted lysates onto cells grown on 96-well plates containing 1 μg/ml cycloheximide in medium, followed by immunofluorescence microscopy as described above.
Cytotoxicity of pyocyanin toward host cells.
The cytotoxic effect of pyocyanin on host cells was assessed by measuring cell viability based on the WST-1 assay, an alternative to the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay for rapid detection (33). Briefly, HeLa and Hep2 cells seeded in 96-well plates were cultured with various concentration of pyocyanin or 0.5% DMSO. After 48 h, 10 μl of the WST-1 reagent was added to each well, and absorbance at 450 nm was recorded after additional 2 h of incubation.
Pretreatment assay.
For host cell pretreatment assays, cells seeded on 48-well plates were cultured with medium containing different concentrations of pyocyanin or 0.5% DMSO for an indicated time period at 37°C for C. trachomatis L2 or 35°C for C. pneumoniae AR39. Cells were washed twice with SPG to remove residual pyocyanin and were then infected with EBs. The inclusion number from one well was quantified by immunofluorescence microscopy 36 h later, as described above. For EB pretreatment assays, pyocyanin or DMSO was added into an EB suspension (4 × 105 IFU/ml) prepared with SPG and incubated for 1 h at a desired temperature (37°C or 4°C for C. trachomatis L2; 35°C or 4°C for C. pneumoniae AR39). After two washes with SPG to remove residual pyocyanin, EBs were resuspended with culture medium and immediately added onto HeLa or Hep2 cell monolayers. Infectious EBs were quantified 36 h later using immunofluorescence microscopy.
Attachment assay.
Purified C. trachomatis L2 or C. pneumoniae AR39 EBs were suspended in SPG at 5 × 106 IFU/ml and exposed to 0.06, 1, or 8 μM pyocyanin for 1 h at 4°C. DMSO (0.5%) and heparan sulfate (500 μg/ml; Sigma-Aldrich, St. Louis, MO) were used as the negative and positive controls (24), respectively. After two washes with SPG, EBs were added onto host cells grown on coverslips in 24-well plates. The MOI for these experiments was about 20 to 30 IFU per cell (i.e., MOI of 30). After still incubation or centrifugation at 4°C for 1 h, cells were washed three times with SPG, fixed with 100% methanol, and then immunostained as described above. Evans blue and DAPI (4′,6-diamidino-2-phenylindole) were used as counterstains. Images were acquired on an Olympus IX51 fluorescence microscope. For each sample, EBs on nine randomly picked cells were counted.
Determination of intracellular reactive oxygen species (ROS).
Intracellular ROS was detected using the fluorescence probe dihydrorhodamine 123 (DHR), as described previously (52). Briefly, cells seeded on 96-well plates were infected with EBs at an MOI of 0.2 in the presence of 0.06, 1, or 8 μM pyocyanin or 0.5% DMSO. At 2, 12, 24, or 36 hpi, DHR was added into each well (final concentration of DHR, 1 μM) and incubated at 37°C (C. trachomatis L2) or 35°C (C. pneumoniae AR39) for 30 min. To determine the effect of antioxidants on pyocyanin-induced ROS production, treatment with 5 mM glutathione (GSH) or N-acetylcysteine (NAC) was performed concurrently with pyocyanin treatment. Fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm, using a Synergy H1 hybrid multimode microplate reader (BioTek, Winooski, VT).
Antioxidant treatment.
To determine the effects of antioxidants on pyocyanin's antichlamydial activity, cells grown on 24-well plates were treated with 5 mM GSH or NAC. Pretreatment of host cells was for 1 h; cells were washed three times prior to EB inoculation (MOI, 0.2) and cultured in medium with or without pyocyanin. For cotreatment, GSH or NAC was added at the time of inoculation and kept in the culture for 36 h. Full treatment included both pretreatment and cotreatment, without washes prior to inoculation.
Determination of lactobacilli tolerance.
Lactobacillus crispatus strains ATCC 33197 and ATCC 33820 and L. jensenii strain ATCC 25258 were cultured with lactobacilli MRS broth in a humidified 5% CO2 incubator. To determine the effects of pyocyanin on Lactobacillus spp., overnight cultures were diluted 1:10,000 with fresh MRS broth containing different concentrations of pyocyanin or the vehicle DMSO. The optical density at 600 nm (OD600) was measured on a microplate reader at the indicated hpi. Background values obtained at 0 h postinoculation were used to correct values from later points. Experiments were performed in triplicate. Means and standard deviations of corrected OD600 values from triplicate samples are presented.
Experimental repetition, data processing and presentation, and statistical analysis.
Experiments quantifying the effects of treatments on inclusion size and number, progeny EB production, EB attachment, and ROS production were performed in duplicate, and repeated for two additional times. Data from the three independent experiments were subjected to pairwise Student's t tests. P < 0.05 and P < 0.01 are considered statistically significant and statistically highly significant, respectively. When appropriate, percent inhibition values were calculated using perspective control values and are presented. A nonlinear regression model using three independent concentration-response inhibition data sets was applied using GraphPad Prism 5 software to calculate IC50s. When percent inhibition values were not needed, averages and standard deviations are presented in absolute numbers.
ACKNOWLEDGMENTS
We thank Harlan D. Caldwell (National Institute of Allergy and Infectious Diseases) for supplying the L2-1-5 hybridoma cells and Alec M. Weber and Malhar Desai for English language editing as well as for useful discussions.
This work was supported by the National Natural Science Foundation of China (grants 31400165 and 31370209 to X.B. and grant 21402100 to J.L.L.), the Qing Lan Project (to X.B.), and the United States National Institutes of Health (grant AI122034 to H.F.).
REFERENCES
- 1.Abdelrahman YM, Belland RJ. 2005. The chlamydial developmental cycle. FEMS Microbiol Rev 29:949–959. doi: 10.1016/j.femsre.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Stephens RS, Myers G, Eppinger M, Bavoil PM. 2009. Divergence without difference: phylogenetics and taxonomy of Chlamydia resolved. FEMS Immunol Med Microbiol 55:115–119. doi: 10.1111/j.1574-695X.2008.00516.x. [DOI] [PubMed] [Google Scholar]
- 3.Banhart S, Saied EM, Martini A, Koch S, Aeberhard L, Madela K, Arenz C, Heuer D. 2014. Improved plaque assay identifies a novel anti-Chlamydia ceramide derivative with altered intracellular localization. Antimicrob Agents Chemother 58:5537–5546. doi: 10.1128/AAC.03457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao X, Gylfe A, Sturdevant GL, Gong Z, Xu S, Caldwell HD, Elofsson M, Fan H. 2014. Benzylidene acylhydrazides inhibit chlamydial growth in a type III secretion- and iron chelation-independent manner. J Bacteriol 196:2989–3001. doi: 10.1128/JB.01677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potroz MG, Cho NJ. 2015. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules 20:4180–4203. doi: 10.3390/molecules20034180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan H. 2012. Blindness-causing trachomatous trichiasis biomarkers sighted. Invest Ophthalmol Vis Sci 53:2560. doi: 10.1167/iovs.12-9835. [DOI] [PubMed] [Google Scholar]
- 7.Belland RJ, Ouellette SP, Gieffers J, Byrne GI. 2004. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol 6:117–127. doi: 10.1046/j.1462-5822.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 8.Balin BJ, Little CS, Hammond CJ, Appelt DM, Whittum-Hudson JA, Gerard HC, Hudson AP. 2008. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer's disease. J Alzheimers Dis 13:371–380. doi: 10.3233/JAD-2008-13403. [DOI] [PubMed] [Google Scholar]
- 9.Dai W, Li Z. 2014. Conserved type III secretion system exerts important roles in Chlamydia trachomatis. Int J Clin Exp Pathol 7:5404–5414. [PMC free article] [PubMed] [Google Scholar]
- 10.Fadel S, Eley A. 2008. Is lipopolysaccharide a factor in infectivity of Chlamydia trachomatis? J Med Microbiol 57:261–266. doi: 10.1099/jmm.0.47237-0. [DOI] [PubMed] [Google Scholar]
- 11.Cosse MM, Hayward RD, Subtil A. 2016. One face of Chlamydia trachomatis: the infectious elementary body. Curr Top Microbiol Immunol. doi: 10.1007/82_2016_12. [DOI] [PubMed] [Google Scholar]
- 12.Hybiske K, Stephens RS. 2007. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun 75:3925–3934. doi: 10.1128/IAI.00106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hybiske K, Stephens RS. 2007. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci U S A 104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Workowski KA. 2015. Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis 61(Suppl 8):S759–S762. doi: 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 15.Sandoz KM, Rockey DD. 2010. Antibiotic resistance in Chlamydiae. Future Microbiol 5:1427–1442. doi: 10.2217/fmb.10.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhengraj AR, Vardhan H, Srivastava P, Salhan S, Mittal A. 2010. Decreased susceptibility to azithromycin and doxycycline in clinical isolates of Chlamydia trachomatis obtained from recurrently infected female patients in India. Chemotherapy 56:371–377. doi: 10.1159/000314998. [DOI] [PubMed] [Google Scholar]
- 17.Gieffers J, Rupp J, Gebert A, Solbach W, Klinger M. 2004. First-choice antibiotics at subinhibitory concentrations induce persistence of Chlamydia pneumoniae. Antimicrob Agents Chemother 48:1402–1405. doi: 10.1128/AAC.48.4.1402-1405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):S4680–S4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igietseme JU, Eko FO, Black CM. 2011. Chlamydia vaccines: recent developments and the role of adjuvants in future formulations. Expert Rev Vaccines 10:1585–1596. doi: 10.1586/erv.11.139. [DOI] [PubMed] [Google Scholar]
- 21.Mabey DC, Hu V, Bailey RL, Burton MJ, Holland MJ. 2014. Towards a safe and effective chlamydial vaccine: lessons from the eye. Vaccine 32:1572–1578. doi: 10.1016/j.vaccine.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang HR, Kunadia A, Lin YF, Fondell JD, Seidel D, Fan HZ. 2017. Identification of a strong and specific antichlamydial N-acylhydrazone. PLoS One 12:e0185783. doi: 10.1371/journal.pone.0185783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao X, Xue Y, Xia C, Lu Y, Yang N, Zhao Y. 2018. Synthesis and assessment of novel anti-chlamydial benzylidene acylhydrazides derivatives. Lett Drug Des Discov 15:31–36. [Google Scholar]
- 24.Osaka I, Hefty PS. 2014. Lipopolysaccharide-binding alkylpolyamine DS-96 inhibits Chlamydia trachomatis infection by blocking attachment and entry. Antimicrob Agents Chemother 58:3245–3254. doi: 10.1128/AAC.02391-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelmohsen UR, Cheng C, Reimer A, Kozjak-Pavlovic V, Ibrahim AK, Rudel T, Hentschel U, Edrada-Ebel R, Ahmed SA. 2015. Antichlamydial sterol from the red sea sponge Callyspongia aff. implexa. Planta Med 81: 382–387. doi: 10.1055/s-0035-1545721. [DOI] [PubMed] [Google Scholar]
- 26.Li JL, Chen DD, Huang L, Ni M, Zhao Y, Fan HZ, Bao XF. 2017. Antichlamydial dimeric indole derivatives from marine actinomycete Rubrobacter radiotolerans. Planta Medica 83:805–811. doi: 10.1055/s-0043-100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarev VN, Shkarupeta MM, Polina NF, Kostrjukova ES, Vassilevski AA, Kozlov SA, Grishin EV, Govorun VM. 2013. Antimicrobial peptide from spider venom inhibits Chlamydia trachomatis infection at an early stage. Arch Microbiol 195:173–179. doi: 10.1007/s00203-012-0863-5. [DOI] [PubMed] [Google Scholar]
- 28.Baron SS, Rowe JJ. 1981. Antibiotic action of pyocyanin. Antimicrob Agents Chemother 20:814–820. doi: 10.1128/AAC.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayaseelan S, Ramaswamy D, Dharmaraj S. 2014. Pyocyanin: production, applications, challenges and new insights. World J Microbiol Biotechnol 30:1159–1168. doi: 10.1007/s11274-013-1552-5. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Suchland RJ, Geisler WM, Stamm WE. 2003. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother 47:636–642. doi: 10.1128/AAC.47.2.636-642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balakrishnan A, Patel B, Sieber SA, Chen D, Pachikara N, Zhong G, Cravatt BF, Fan H. 2006. Metalloprotease inhibitors GM6001 and TAPI-0 inhibit the obligate intracellular human pathogen Chlamydia trachomatis by targeting peptide deformylase of the bacterium. J Biol Chem 281:16691–16699. doi: 10.1074/jbc.M513648200. [DOI] [PubMed] [Google Scholar]
- 33.Ngamwongsatit P, Banada PP, Panbangred W, Bhunia AK. 2008. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J Microbiol Methods 73:211–215. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JP, Stephens RS. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861–869. doi: 10.1016/0092-8674(92)90296-O. [DOI] [PubMed] [Google Scholar]
- 35.Hassan HM, Fridovich I. 1980. Mechanism of the antibiotic action pyocyanine. J Bacteriol 141:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV, Davey AK, Chess-Williams R, Kiefel MJ, Arora D, Grant GD. 2016. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins 8:E236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Britigan BE, Roeder TL, Rasmussen GT, Shasby DM, Mccormick ML, Cox CD. 1992. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J Clin Invest 90:2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reszka KJ, O'Malley Y, McCormick ML, Denning GM, Britigan BE. 2004. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic Bio Med 36:1448–1459. doi: 10.1016/j.freeradbiomed.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Gloyne LS, Grant GD, Perkins AV, Powell KL, McDermott CM, Johnson PV, Anderson GJ, Kiefel M, Anoopkumar-Dukie S. 2011. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol in Vitro 25:1353–1358. doi: 10.1016/j.tiv.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 40.McDermott C, Chesswilliams R, Grant GD, Perkins AV, Mcfarland AJ, Davey AK, Anoopkumardukie S. 2012. Effects of Pseudomonas aeruginosa virulence factor pyocyanin on human urothelial cell function and viability. J Urol 187:1087–1093. doi: 10.1016/j.juro.2011.10.129. [DOI] [PubMed] [Google Scholar]
- 41.McFarland AJ, Anoopkumar-Dukie S, Perkins AV, Davey AK, Grant GD. 2012. Inhibition of autophagy by 3-methyladenine protects 1321N1 astrocytoma cells against pyocyanin- and 1-hydroxyphenazine-induced toxicity. Arch Toxicol 86:275–284. doi: 10.1007/s00204-011-0755-5. [DOI] [PubMed] [Google Scholar]
- 42.Hempenstall A, Grant GD, Anoopkumar-Dukie S, Johnson PJ. 2015. Pyocyanin inhibits both nitric oxide-dependent and -independent relaxation in porcine coronary arteries. Clin Exp Pharmacol Physiol 42:186–191. doi: 10.1111/1440-1681.12340. [DOI] [PubMed] [Google Scholar]
- 43.Cheluvappa R, Jamieson HA, Hilmer SN, Muller M, Le Couteur DG. 2007. The effect of Pseudomonas aeruginosa virulence factor, pyocyanin, on the liver sinusoidal endothelial cell. J Gastroenterol Hepatol 22:1350–1351. doi: 10.1111/j.1440-1746.2007.05016.x. [DOI] [PubMed] [Google Scholar]
- 44.Ulmer AJ, Pryjma J, Tarnok Z, Ernst M, Flad HD. 1990. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect Immun 58:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorensen RU, Klinger JD, Cash HA, Chase PA, Dearborn DG. 1983. In vitro inhibition of lymphocyte proliferation by Pseudomonas aeruginosa phenazine pigments. Infect Immun 41:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nutman J, Berger M, Chase PA, Dearborn DG, Miller KM, Waller RL, Sorensen RU. 1987. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol 138:3481–3487. [PubMed] [Google Scholar]
- 47.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK. 2002. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol 168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 48.Gong Z., Luna Y., Yu P., Fan H. 2014. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS One 9:e107758. doi: 10.1371/journal.pone.0107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boris S, Barbés C. 2000. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2:543–546. doi: 10.1016/S1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 50.Zhang YX, Stewart SJ, Caldwell HD. 1989. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun 57:636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borrero NV, Bai F, Perez C, Duong BQ, Rocca JR, Jin S, Huigens RW 3rd. 2014. Phenazine antibiotic inspired discovery of potent bromophenazine antibacterial agents against Staphylococcus aureus and Staphylococcus epidermidis. Org Biomol Chem 12:881–886. doi: 10.1039/C3OB42416B. [DOI] [PubMed] [Google Scholar]
- 52.Bao X, Cui J, Wu Y, Han X, Gao C, Hua Z, Shen P. 2007. The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J Mol Med (Berl) 85:85–98. doi: 10.1007/s00109-006-0113-x. [DOI] [PubMed] [Google Scholar]