Abstract
Elicitins secreted by phytopathogenic Phytophthora spp. are proteinaceous elicitors of plant defense mechanisms and were demonstrated to load, carry, and transfer sterols between membranes. The link between elicitor and sterol-loading properties was assessed with the use of site-directed mutagenesis of the 47 and 87 cryptogein tyrosine residues, postulated to be involved in sterol binding. Mutated cryptogeins were tested for their ability to load sterols, bind to plasma membrane putative receptors, and trigger biological responses. For each mutated elicitin, the chemical characterization of the corresponding complexes with stigmasterol (1:1 stoichiometry) demonstrated their full functionality. However, these proteins were strongly altered in their sterol-loading efficiency, specific binding to high-affinity sites, and activities on tobacco cells. Ligand replacement experiments strongly suggest that the formation of a sterol-elicitin complex is a requisite step before elicitins fasten to specific binding sites. This was confirmed with the use of two sterol-preloaded elicitins. Both more rapidly displaced labeled cryptogein from its specific binding sites than the unloaded proteins. Moreover, the binding kinetics of elicitins are related to their biological effects, which constitutes the first evidence that binding sites could be the biological receptors. The first event involved in elicitin-mediated cell responses is proposed to be the protein loading with a sterol molecule.
INTRODUCTION
In the last decade, particular attention has been turned toward understanding mechanisms that govern plant-microbe interactions and to unravel the processes that lead to plant resistance (Dixon et al., 1994; Jones, 1994; Ryan, 1994; Ryals et al., 1995; Staskawicz et al., 1995; Ji et al., 1998; Keen, 1999). However, the general molecular basis of plant-pathogen recognition controlling plant resistance remains to be elucidated. The current concept admits that compounds originating from microorganisms (elicitors) are recognized by plants through specific binding to high-affinity sites, so far considered putative receptors (Lamb, 1996; Ebel and Mithofer, 1998; Lauge and DeWit, 1998). This primary interaction could trigger initial signaling at the cell level, involving protein phosphorylation-dephosphorylation cascades, mineral ion exchanges, and production of active oxygen species (Ebel and Mithofer, 1998; Scheel, 1998). Nevertheless, little is known about the relationship between elicitor high-affinity–binding sites and downstream signaling occurring in plant cells.
Several plant-pathogen models have been studied in detail. One of them is the tobacco-Phytophthora interaction in which Phytophthora proteins, called elicitins, seem to play a major role (for review, see Ricci, 1997).
Elicitins are 10-kDa holoproteins secreted by most Phytophthora species (Kamoun et al., 1994; Ricci, 1997). In tobacco plants, these elicitors induce both a hypersensitive response (leaf necrosis) and nonspecific systemic acquired resistance (SAR; Bonnet et al., 1996; Keller et al., 1996). In cell suspension cultures, classical mechanisms of elicitation have been reported, such as calcium influx (Tavernier et al., 1995), alkalization of the extracellular medium (Blein et al., 1991), production of active oxygen species (Rustérucci et al., 1996; Simon-Plas et al., 1997), and cell wall modifications (Kieffer et al., 2000). The presence of a single family of high-affinity sites for elicitins has been reported (Blein et al., 1991). They are located on the plasma membrane, represent 220 fmol of sites/mg of protein (Kd = 2 nM), and exhibit a sharp optimum pH near 7.0 (Wendehenne et al., 1995). The biochemical characterization of these binding sites shows an apparent functional molecular mass of 193 kDa (Bourque et al., 1999), and they contain an N-linked carbohydrate moiety determinant in elicitin binding (Bourque et al., 1999).
Elicitins also behave like sterol carrier proteins (Mikes et al., 1997, 1998). They bind sterols and catalyze their transfer between artificial membranes (Mikes et al., 1998). Moreover, they are able to pick up sterols from plasma membranes (Vauthrin et al., 1999). Natural elicitins interact with sterols showing similar binding characteristics, i.e., a 1:1 sterol:protein stoichiometry, and the dissociation constants are in the same range. Despite these identities, elicitins exhibit differences with regard to the kinetics of loading and the rates of sterol exchanges between liposomes or micelles (Mikes et al., 1998; Vauthrin et al., 1999). The most efficient elicitin is cryptogein (from Phytophthora cryptogea), the less efficient being parasiticein and capsicein, secreted by Phytophthora parasitica and Phytophthora capsici, respectively. Moreover, cryptogein induces stronger cell responses than do parasiticein and capsicein (Rustérucci et al., 1996; Bourque et al., 1998). This suggests an apparent correlation between the elicitor activities of natural elicitins and their ability to load and transfer sterols.
We investigated the possible link between the elicitor activity of cryptogein and its efficiency to load sterols with the use of site-directed mutagenesis and heterologous expression of a cryptogein gene (Panabières et al., 1995). The aim of the mutagenesis was to alter the sterol-loading properties of cryptogein and was based on the crystal structure of an engineered cryptogein-ergosterol complex (Boissy et al., 1999). Interaction between the protein and ergosterol involves several residues of the cryptogein hydrophobic core, among which tyrosine residues are the most represented (Boissy et al., 1999).
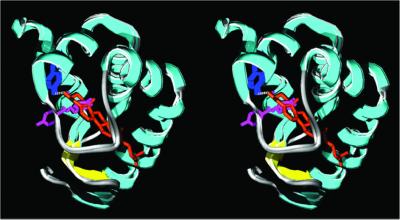
In this work, we first studied the role of tyrosine-47 and tyrosine-87 residues in the sterol-carrier activity of elicitins. These amino acids were chosen for their characteristics as highlighted by structural observations (Boissy et al., 1996, 1999). Tyrosine-47 residue is involved in the sole hydrogen bond between the protein core and the sterol hydroxyl (Figure 1). Moreover, the sterol seems to contact its aromatic ring (Figure 1). Thus, we substituted tyrosine-47 with phenylalanine or glycine. In addition, the complex formation induces a rotation of tyrosine-87, moving its aromatic ring outside the hydrophobic pocket (Figure 1). This observation led us to replace the tyrosine-87 with a phenylalanine, which is unable to be exposed in an aqueous environment.
Figure 1.
Superimposition of the stereoribbon diagrams of loaded and unloaded cryptogeins, showing the location of the tyrosine-47 and -87 residues. They represent the native cryptogein (pdb file 1beo) and the K13H-ergosterol complex (pdb file 1bxm). The figures were generated with the use of the program Swiss-Pdb Viewer v 3.5 (http://www.expasy.ch/spdbv; Guex, 1996; Guex and Peitsch, 1996) and the ray tracer POV-Ray v 3.1 (http://www.povray.org/). α-Helices, cyan; β-sheets, yellow; turns and coils including the Ω-loop, light gray; tyrosine-47, blue; tyrosine-87, pink; ergosterol, orange.
Second, the sterol-loading ability and biological properties of these proteins were compared with those of native cryptogein. Finally, we also investigated whether sterol carrier and biological activities of elicitins were related. The results presented in this paper show that the initial cryptogein-induced signaling in tobacco cells involves the prerequisite formation of an elicitin-sterol complex before protein recognition by its putative receptors.
MATERIALS AND METHODS
Materials
Cell suspension cultures were grown in the medium of Chandler et al. (1972) on a rotary shaker (150 rpm, 25°C) under continuous light and used during the exponential growth phase. Nicotiana tabacum seeds, obtained from the collection of the Institut du Tabac (Bergerac, France), were sown into peat soil, and plants were grown in controlled conditions (22°C, 16 h light, 6000 lux, 80% hygrometry). For protection and detached leaves assays 55- to 65-d-old tobacco plants (before flowering induction) were used. Cryptogein and capsicein were prepared as previously described (Bonnet et al., 1996), and mutated proteins were obtained as follows.
Construction of Expression Plasmid.
The cryptogein-encoding sequence (see Panabières et al., 1995, for complete nucleotidic sequence) was amplified with the use of the plasmid pBG38 as template. This plasmid contains the X24 gene that encodes cryptogein from Phytophthora cryptogea isolate 52. Polymerase chain reaction (PCR) amplifications were carried out with the use of oligonucleotides 1 (5′-GGGGTATCTCTCGAGAAAAGAGAGGCTGAAGCTR-CCRCSTGCACC-3′) and 2 (5′-GCCCTATAGTGAGTCGTATTAC-3′) as forward and reverse primers, respectively. The amplified fragment was purified and cloned into pPIC9 (Invitrogen, San Diego, CA). The resulting plasmid, called pPIC-X24, was cloned in Escherichia coli DH5α (Life Technologies-BRL, Rockville, MD). The correct orientation of the transformants was evaluated by PCR screening, and confirmed by DNA sequencing (Genome Express S.A., Grenoble, France).
Transformation of Pichia pastoris
The yeast P. pastoris strain GS115 was obtained from Invitrogen. Its propagation, as well as competent cell preparation and selection procedures, was according to the manufacturer's recommendations. PIC-X24 was linearized at the unique SacI site in the vector and cloned into competent GS115 cells by the combined LiOA lithium acetate/heat shock method (Ito et al., 1983). Transformants were selected for their ability to grow in the absence of histidine and for efficiency of X24 expression, which was analyzed by SDS-PAGE.
Site-directed Mutagenesis.
Specific mutagenesis of PIC-X24 gene encoding cryptogein was performed with the use of the PCR-derived technique developed with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Oligonucleotides (Eurogentec, Brussels, Belgium) designed to introduce the chosen mutations into the target codon are: X24-Y47F, ACGGCGCAGTTCAAGCTC; X24-Y47G, ACGGCGCAGGGCAAGCTC; X24-Y87F, CTCAACGTGTACTCGTTCGCGAACGGCTTCTCG (mismatched nucleotides are indicated in bold underlined letters). PCR amplifications were carried out with 50 ng of PIC-X24 plasmid vector, 120 ng of each forward and reverse primer, 100 μM of a deoxyribonucleotide triphosphate mixture, and 2.5 U of Pfu DNA polymerase in a final volume of 50 μl. The cycling parameters were 15 cycles of 30 s at 95°C, 1 min at 55°C, and 16 min at 68°C. The amplification mixture was subjected to DpnI digestion. Methylated and hemimethylated DNA corresponding to parental DNA template were digested with DpnI. Subsequent molecules resistant to DpnI digestion, corresponding to efficiently mutated DNA, were further cloned in E. coli as described above, and positive clones were used to transform P. pastoris cells.
Purification of Recombinant Proteins.
P. pastoris transformant cells were treated for induction of X24 expression, according to the supplier's instructions (Invitrogen). The culture medium was centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was concentrated by tangential flow ultrafiltration with the use of an Amicon Miniplate bioconcentrator (Millipore, Bedford, MA) to one-fourth of the original volume. The concentrate was extensively dialyzed against H2O (Milli-Q) for 48 h at 4°C, adjusted to 5 mM sodium acetate buffer, pH 4.0, and loaded onto a Macro-Prep High S cation-exchange support (Bio-Rad, Hercules, CA) equilibrated with 5 mM sodium acetate buffer, pH 4.0. The proteins were eluted with the equilibration buffer containing 0.25 M NaCl and further purified to homogeneity via a reversed phase chromatography (Synchroprep RP4, 30 μm, 300Å, SynChrome, Lafayette, IN). The pH of the fraction was adjusted to 7.0 before loading on the phase previously equilibrated with 0.25 M NaCl in 5 mM sodium acetate buffer pH 7.0. After washing with this buffer and 20% CH3CN-50 mM HCOONa, elution was carried out with 40% CH3CN-50 mM HCOONa. The last fraction containing the recombinant protein was dialyzed and freeze dried. Throughout the purification, the protein content and purity was followed with the use of SDS-PAGE and high-performance liquid chromatography as previously described (Leberre et al., 1994).
Characterization of the Elicitin-Sterol Complexes
Fluorescence Measurements.
The sterol-loading activity of the proteins was measured with a Shimadzu RF 5001 PC spectrofluorimeter in a stirred cuvette, according to the method previously described (Mikes et al., 1997), with the use of Δ5,7,9(11)22-ergostatetraen-3β-ol (dehydroergosterol or DHE) as the fluorescent probe. The protein loading was expressed as the equilibrium concentration of the complex (Cb), with the use of the following equation: Cb = (F − Fo)/(Qb − Qo), where F is the fluorescence of the complex at equilibrium, Fo is the fluorescence of free DHE, and Qb and Qo are the fluorescence quantum yield of the individual complexes and free DHE, respectively. To determine the Qb values of the DHE-elicitin complex, 0.25 μM DHE was titrated with elicitins until constant fluorescence values were obtained, corresponding to the total disappearance of free DHE.
Preparation of Stigmasterol-Elicitin Complexes.
Cryptogein and capsicein complexed with stigmasterol (1:1 stochiometry) were prepared as previously described (Mikes et al., 1997, 1998). An ethanolic solution of stigmasterol (1 mg/ml) was carefully added dropwise up to 20% excess (mole/mole ratio) to 1 ml (1 mg/ml) of protein solution. The sterol-elicitin complex was isolated by gel filtration on Sephadex G-25. Free sterol excess remained on the column. Freshly prepared complexes were used in ligand replacement experiments.
Determination of N and Kd.
The number of binding sites (N) was obtained from the determination of the cryptogein and sterol amounts within the complex. The dissociation constant (Kd) was then calculated from the following equation: Kd = ([N.A/Cb] − 1) × (Cf), Cf and Cb (free and bound DHE, respectively), A (elicitin concentration), and N are experimental data.
Biological Assays
Tobacco Plant Treatments.
Necrotic activities of recombinant proteins combined with their protective effects were checked as previously described (Bonnet et al., 1996). Briefly, tobacco plants were decapitated, and elicitin solutions (0.1 nmol/plant) were applied on the stem sections. Inoculation of P. parasitica aggressive strain 329 was performed 2 d after treatment by applying a mycelium plug capped with a piece of aluminum foil at the site of the treated decapitation. One week after inoculation, the length of the stem that turned brown was measured (external invasion), and then the stem was dissected and the extent of internal stem invasion was estimated by the length of fungal spreading in pith (centimeters). The percentage of protection was computed as the relative reduction of invasion compared with water-treated and inoculated control plants.
The necrotic activity was assessed on fully expanded leaves weighing 3–5 g. Leaves were excised and treated with elicitins (Rustérucci et al., 1996). A 10-μl drop of the selected elicitin aqueous solution (0.1–1 nmol) was put on the petiole of leaves and followed three times with 10 μl of water to ensure full absorption of the elicitor. Control leaves were treated in the same way with water only. Leaf petioles were placed in water. Experiments were kept in the same controlled condition as for plants except for complete darkness. Necrotizing effects were assessed from changes in leaf weights. In other experiments, 20 μl of an aqueous solution of elicitins were infiltrated in leaves with a syringe without needle. Observations were performed 48 h later.
Tobacco Cell Treatments.
Tobacco cells were prepared and used for determination of elicitin activities as previously reported (Rustérucci et al., 1996; Simon-Plas et al., 1997). Cells from cultures in exponential phase growth were collected by filtration, washed, and resuspended (0.1 g fresh weight/ml) in 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4, and 2 mM Mes buffer adjusted to pH 5.75 with KOH. After a 2-h equilibration, tobacco cells were treated with elicitins. Control tobacco cells were incubated in the same conditions without elicitins.
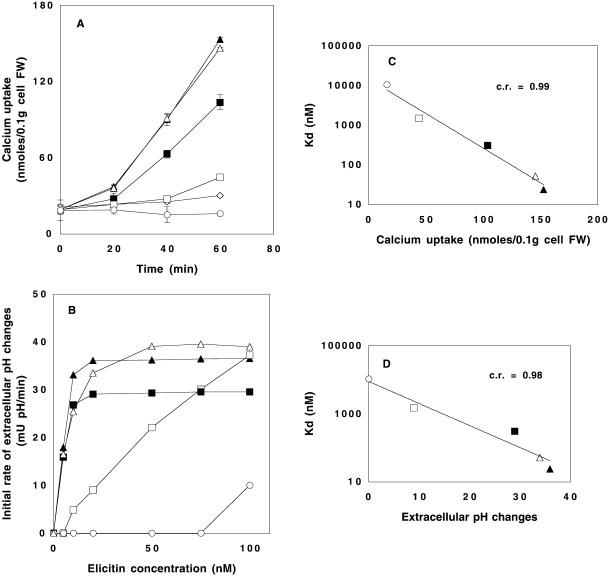
45Ca2+ influx measurements were performed as previously described (Tavernier et al., 1995) by addition of 45Ca2+ (0.033 MBq/g fresh weight of cells) 5 min before the treatment with elicitins. After various periods of treatment (0–60 min), duplicate samples of 3 ml were withdrawn by filtration and washed once for 1 min and twice for 20 s on GF/A glass microfiber filters (Whatman, Tewksbury, MA) with 10 ml of ice-cold assay medium containing 2 mM LaCl3 without Ca2+ to remove extracellular 45Ca2+. Cells were removed from the filters, placed in scintillation vials, and weighed. Ten milliliters of Ready Safe cocktail (Beckman, Fullerton, CA) were added to the vials, which were gently shaken overnight before counting in a Beckman LS600 TA scintillation counter. Results were expressed as 45Ca2+ nmol/0.1 g cell fresh weight. The correlation between the sterol carrier activity and the Ca2+ influx was determined with the use of the Kd values and those of Ca2+ influx obtained after a 60-min incubation period with 50 nM elicitin. These experimental conditions corresponded to the maximal Ca2+ response reported for cryptogein (Tavernier et al., 1995).
Extracellular pH changes were measured at 10-min intervals in tobacco cell suspensions and expressed as initial rates. The elicitin concentration range was 0–100 nM. The correlation between the sterol carrier activity and the extracellular pH changes was determined by plotting the Kd values versus the initial rates of medium alkalization at 20 nM elicitin concentration (maximal effect with cryptogein).
Binding and Ligand Replacement Experiments with the Use of 125I-labeled Elicitins
Iodination of elicitins was performed as previously described (Blein et al., 1991). Specific radioactivity of labeled ligand was ∼200 Ci/mmol. The iodination of elicitin changed neither the previously described effects of the elicitor on tobacco cells (Wendehenne et al., 1995) nor the sterol carrier activity (Osman, Vauthrin, Mikes, Milat, Panabières, Marais, Brunie, Maume, Ponchet, and Blein, unpublished results). Plasma membrane-enriched fractions were obtained as previously reported (Vauthrin et al., 1999). Binding experiments and ligand replacement experiments were carried out as already reported (Wendehenne et al., 1995). Plasma membrane preparations containing ∼50 μg of protein were suspended in a final volume of 100 μl with binding buffer (25 mM Tris-Mes, pH 7.0, 5 mM MgCl2, 0.1 M sucrose, and 0.1% bovine serum albumin) and preincubated on ice for 30 min. Binding of 125I-elicitin was carried out for 90 min on ice after addition of radiolabeled ligand. Nonspecific binding was determined in the presence of 10 μM unlabeled elicitin. Binding assays were stopped by rapid filtration under vacuum through GF/B glass-fiber filters (Whatman) presoaked 60 min in 1% bovine serum albumin. Then, the filters were immediately washed three times with 5 ml of ice-cold binding buffer, and the radioactivity remaining on filters was measured. The specific binding was calculated by subtracting the nonspecific binding from the total binding.
RESULTS
Cryptogein and Mutated Elicitin Characteristics
The X24 gene was isolated from a genomic library of P. cryptogea and encodes cryptogein (Panabières et al., 1995). Recombinant yeast colonies secreted a 10-kDa protein that was shown to be cryptogein on the basis of SDS-PAGE electrophoresis, Western blotting, HPLC analysis, and biological activities. The efficient expression of X24 in yeast allowed us to develop a strategy based on the mutagenesis of tyrosine residues (Y47 and Y87). Three proteins were obtained in which either one of the tyrosines was replaced by the structurally related phenylalanine (Y47F and Y87F) or in which Y47 was replaced with a glycine (Y47G). Yeast colonies produced ∼30 mg elicitin/l of culture supernatant.
The cryptogein mutations were unlikely to affect the three-dimensional structure of the protein. Permutation of tyrosine-47 and tyrosine-87 with phenylalanine does not change the overall structure of the resulting mutated proteins because the phenolic function of these two tyrosines are free of constraint and do not contribute to protein stability (Boissy et al., 1996; Fefeu et al., 1997; Gooley et al., 1998). They are buried inside the core in a highly hydrophobic environment; therefore, Y-F exchange (almost similar steric hindrance but higher hydrophobicity) results in minor changes in the aromatic ring orientations. This was partially demonstrated by circular dichroism, chromatographic, electrophoretic, and spectrophotometric properties (Osman, Vauthrin, Mikes, Milat, Panabières, Marais, Brunie, Maume, Ponchet, and Blein, unpublished results) and was confirmed by the modeling of these mutations in the Swiss-PdbViewer program, with the use of a crystal structure (pdb 1beo; Boissy et al., 1996) and 18 solution structures (pdb 1beg; Fefeu et al., 1997). In fact, the Y-F replacements, after energy minimization, led to small changes in the ring orientation, close to the average position of tyrosine residues revealed by the solution structures.
Sterol-Elicitin Interaction
Replacement of tyrosine residues significantly affected both the interaction rate and the equilibrium concentration of the elicitin-sterol complex (Osman, Vauthrin, Mikes, Milat, Panabières, Marais, Brunie, Maume, Ponchet, and Blein, unpublished results). Three parameters (N, Cb, and Kd) were used to describe the dynamic equilibrium of the DHE-elicitin interaction (sterol loading). N and Cb were experimentally determined, whereas Kd was calculated from the equation described in “Determination of N and Kd.” On this basis, the sterol-loading characteristics of each protein were determined. Whatever the protein assayed, the number of sterol-binding sites was one (Table 1). This demonstrates that the mutated proteins were obtained in the homogenous state. The Cb values of cryptogein and X24 were quite similar (1.26–1.22), whereas they ranged from 1.00 to 0.21 for Y47F, Y47G, and Y87F, in decreasing order (Table 1). Finally, the affinity of mutated elicitins for DHE was affected, as revealed by their Kd parameters. The Kd of cryptogein purified from P. cryptogea was slightly different from that of the X24 recombinant protein. The Kd value of Y47F was higher but remained in the nanomolar range, contrary to values obtained for Y47G and Y87F, which were strongly altered and reached micromolar concentrations (Table 1). The Kd value of capsicein, an α-elicitin, was between those of Y47F and Y47G.
Table 1.
Sterol loading parameters of elicitins
| Elicitin | N (mol/mol) | Cb (μM) | Kd (nM) |
|---|---|---|---|
| Cryptogein | 1.02 ± 0.02 | 1.26 ± 0.01 | 24 ± 6 |
| X24 | 1.04 ± 0.09 | 1.22 ± 0.02 | 52 ± 15 |
| Y47F | 0.92 ± 0.01 | 1.00 ± 0.04 | 304 ± 64 |
| Y47G | 1.01 ± 0.09 | 0.63 ± 0.01 | 1458 ± 56 |
| Y87F | 0.99 ± 0.04 | 0.21 ± 0.06 | 10391 ± 3792 |
| Capsicein | 0.85 ± 0.14a | 0.80 ± 0.04a | 758 ± 123 |
N, Cb, and Kd were used to describe the dynamic equilibrium of the DHE-elicitin interaction. They are linked according to the following equation: N = (Cb/A) × [(Kd/Cf) + 1], where Cb and Cf are the concentrations of bound and free DHE, respectively; Kd, the dissociation constant; A, the elicitin concentration; and N, the number of sterol binding sites. N and Cb were experimentally obtained. The numbers of binding sites (N) have been determined from the isolation of sterol-elicitin complexes and quantitation of its components according to the MATERIALS AND METHODS section. The total protein and sterol amount recovery was 94 ± 4% and 93 ± 10%, respectively. Data are expressed as mean of three independent replicates ± SD. Cb represents the concentration of DHE-elicitin complex determined fluorometrically (1.30 μM DHE, 2 μM elicitin, mean of three replicates ± SD). Kd was calculated from the above equation.
Capsicein data were from Mikes et al., 1998.
Specific Interactions between Elicitins and Tobacco Plasma Membrane Proteins
Ligand Replacement Experiments.
Tobacco plasma membranes were incubated in the presence of 125I-cryptogein during 120 min. In these conditions, the amount of 125I-cryptogein specifically bound to the membranes reached an equilibrium and then remained constant in accordance with previous results (Wendehenne et al., 1995). Addition of unlabeled cryptogein or mutated proteins determined the zero time of the ligand replacement kinetics, which was carried out for >60 min (Figure 2A). The radioactivity associated with the membranes rapidly decreased and reached the same level for all proteins (Figure 2A). This demonstrates a displacement of bound 125I-cryptogein by unlabeled proteins. However, the initial rates of ligand replacement were different, and the higher discrepancy could be observed 15 min after unlabeled elicitin addition (Figure 2A). The average of initial rate values for cryptogein replacement, from three independent experiments, was plotted versus the Kd of the corresponding proteins (Figure 2B). It revealed a high correlation (c.r. = 0.96) between the ability to load sterol and to displace labeled cryptogein. Elicitins could be ranked in increasing order of efficiency: Y87F, Y47G, Y47F, X24, and cryptogein (Figure 2B).
Figure 2.
Specific interactions between elicitins and high-affinity sites located on tobacco plasma membranes: ligand replacement kinetics. (A) Kinetics of specific displacement of 125I-cryptogein bound to plasma membrane by unlabeled elicitins. Plasma membrane (50 μg of protein) was incubated with 2 nM 125I-cryptogein during 120 min. Then, replacement was initiated by the addition of 10 μM unlabeled elicitins (zero time). Data are expressed as the percentages of the initial amount of 125I-cryptogein specifically bound (∼250 fmol/mg plasmalemma protein) before addition of unlabeled elicitins. This experiment was repeated three times, and the data presented correspond to a representative experiment. (B) Relationship between the initial rate of replacement and the affinity of elicitins for sterols (three measurements of three independent replicates). ⋄, control, without unlabeled elicitin addition; ▴, cryptogein; ▵, X24; ▪, Y47F; □, Y47G; ○, Y87F.
In addition, the replacement efficiency of stigmasterol-preloaded cryptogein and capsicein was 1.5- and 1.3-fold higher, respectively, than that of the native proteins (Table 2). When capsicein was included in the correlation presented in Figure 2B (ligand replacement), the c.r. remained unchanged (0.96), indicating that an α-elicitin like capsicein behaves as cryptogein and mutated cryptogeins.
Table 2.
Effect of elicitin preloading with stigmasterol on the rate of chase efficiency
| Elicitin | Native (fmol/min) | Preloaded (fmol/min) |
|---|---|---|
| Cryptogein | 8.5 ± 0.4 | 12.8 ± 0.5 |
| Capsicein | 6.5 ± 0.3 | 8.5 ± 0.4 |
Data are expressed as mean of three independent replicates ± SD.
Binding Experiments.
The specific binding of 125I-elicitins was determined at different protein concentrations. Cryptogein, X24, and Y47F showed similar classical hyperbolic binding curves (Figure 3), which permitted calculation of their binding parameters. The apparent Kd values were 4.1 ± 0.8, 4.1 ± 0.4, and 4.3 ± 0.1 nM, and the number of binding sites were 253 ± 10, 259 ± 11, and 256 ± 4 fmol/mg plasmalemma protein, for cryptogein, X24, and Y47F, respectively. In contrast, Y47G and Y87F exhibited sigmoidal, not hyperbolic, binding curves (Figure 3). Up to 10 nM, the specific binding of these proteins was weak, whereas that of the other elicitins increased and reached a plateau at 8 nM. Nevertheless, at higher concentrations, the binding of Y47G and of Y87F increased and joined the level of the other proteins. However, a noticeable difference between the binding behaviors of these two proteins was observed: Y87F required the highest concentrations to obtain a saturation of the binding sites.
Figure 3.
Specific interactions between elicitins and high-affinity sites located on tobacco plasma membranes: binding experiment. Saturation curves were obtained with 125I-labeled elicitins. Plasma membrane preparations were incubated with various concentrations of labeled elicitins. Experiments were repeated at least three times and results are expressed as the mean values ± SD (fmol bound elicitin/mg plasmalemma proteins). ▴, cryptogein; ▵, X24; ▪, Y47F; □, Y47G; ○, Y87F.
Biological Activities on Tobacco Cell Suspension Cultures
Calcium Uptake.
Treatment of tobacco cells with each protein (50 nM) resulted in differing effects on the time course of Ca2+ uptake (Figure 4A). Fungal cryptogein and recombinant X24 induced similar effects. Y47F was significantly less active, whereas Y47G and Y87F were almost inactive, although significantly different from the control. Y47G induced weak Ca2+ uptake, and surprisingly Y87F led to a total loss of the basal calcium exchange.
Figure 4.
Tobacco cell responses induced by cryptogein and by the mutated proteins. (A) Time course of 45Ca2+ uptake. Cells were preincubated for 5 min in the presence of 45Ca2+ before addition of elicitins (50 nM). (C) Correlation between the affinity of elicitins for sterols and the induction of Ca2+ influx with the use of the values obtained after a 60-min incubation period. (B) Effects of elicitin concentration on initial rate of extracellular medium alkalization. (D) Correlation between the affinity of elicitins for sterols and the extracellular medium alkalization induced with 20 nM elicitin. (⋄) control, ▴, cryptogein; ▵, X24; ▪, Y47F; □, Y47G; ○, Y87F.
Extracellular Alkalization.
This activity was measured via kinetic experiments at various protein concentrations in a 5–100 nM range and was compared with the response of control untreated cells. The alkalization was expressed as the initial rate of pH increase versus protein concentrations (Figure 4B). The fungal cryptogein and the recombinant X24 provoked similar overall alkalization, whereas Y47F was less active than cryptogein. The Y47G mutation resulted in a pronounced decrease in efficiency, because high protein concentrations were required to reproduce the effect of cryptogein. Y87F had a very low effect, even at the highest doses.
Relationship between Biological and Sterol-loading Activities.
Figure 4, C and D, shows obvious correlations between both calcium uptake or extracellular alkalization induced by elicitins and their affinity for sterols (c.r. = 0.99 and 0.98, respectively). The ranking of the proteins remained identical whatever the considered correlation was. It established that Y87F was the less efficient protein, whereas Y47G was slightly better. Cryptogein and X24 exhibited high activities, and Y47F showed an intermediate behavior, approximately that of cryptogein.
Biological Activities on Tobacco Plants
Elicitin activity on tobacco plants was evaluated according to i) the ability to induce a hypersensitive-like response (HR), revealed by typical leaf necrosis, as shown in Figure 5A, and ii) an induction of SAR, evidenced by the protective effect on challenge inoculations (Bonnet et al., 1996). The ability to induce HR was assessed by protein application on the petiole of detached leaves and measured as the reduction of fresh leaf weight after 48 h (Figure 5B). For 0.1, 0.3, and 1 nmol/leaf, the results obtained with cryptogein, X24, and Y47F were nearly similar with a loss of 60% of the initial leaf weight. The behavior of Y47G or Y87F was different. At 0.1 nmol/leaf these proteins were not necrotizing, but at higher doses their effects were similar to those of the other proteins. Moreover, elicitins were infiltrated in tobacco leaves. At 0.1 mg/ml, all proteins trigger the destruction of the infiltrated zone, whereas at 0.01 mg/ml the mutated proteins were less efficient than cryptogein (Figure 5C). Attempts to evaluate the protective properties of mutated elicitins against P. parasitica indicated that all proteins were able to induce SAR with a significant restriction of the fungal spreading (Figure 5D). The mutated proteins were less efficient: Y47G showed a weak ability to induce SAR, whereas Y87F and Y47F exhibit intermediate behavior.
Figure 5.
Comparison of the plant responses induced by cryptogein and the mutated proteins. (A) Typical necrosis development with the use of an arbitrary scale from a level 1, corresponding to a control, to a level 4 exhibiting a full leaf necrosis. (B) Effects of elicitin amounts on leaf necrosis development (petiole absorption). Results are expressed as percentages ± SD of the control fresh leaf weight (FW) 48 h after the treatment and are the results of three independent experiments. From the white bars to the black bars the elicitin amounts were 0.1, 0.3, and 1 nmol/leaf, respectively. (C) Infiltration of elicitin solutions (20 μl at 0.1 or 0.01 mg/ml) in tobacco leaves. The infiltrated areas were marked off by black limits. Pictures were taken 2 d after infiltration. (D) Elicitins-induced protection against P. parasitica (0.1 nmol/plant) 7 d after inoculation. Results are expressed as (I − i/I)100 ± SD, where I and i are the length of the external (gray bars) or internal (black bars) stem invasion by the pathogen, in the control and elicitin-treated tobacco plants, respectively. Six independent experiments.
DISCUSSION
Involvement of Tyrosine-47 and -87 in the Sterol-Elicitin Complex Stability
All the elicitins used in this work are able to load one sterol molecule. However, their affinity for sterols is different. Cryptogein (native or recombinant) is confirmed to be the most efficient sterol-binding protein.
The Y47F mutation slightly affects the Kd, although the sole hydrogen bond between the proteic core and the sterol hydroxyl is withdrawn. On the contrary, the Y47G replacement strongly increases this parameter. This indicates that either the thrust role of the aromatic ring of tyrosine (or phenylalanine) or the van der Waals interactions with the sterol are of prime importance in the stability of the sterol-elicitin complex, as previously suggested by structural analysis (Boissy et al., 1999).
The Y87F replacement leads to the most altered properties. This could be explained by the behavior of this tyrosine residue during complex formation. In the native cryptogein, it stays in the hydrophobic cavity (Boissy et al., 1996; Fefeu et al., 1997; Gooley et al., 1998), whereas in the sterol-loaded cryptogein, it rotates and becomes exposed to solvent (Boissy et al., 1999; Figure 1). The tyrosine residue could be in contact with the aqueous environment, because of its hydrophilic hydroxyl, whereas the hydrophobic phenylalanine residue could not. Therefore, a high steric hindrance (conflict with the sterol; see Figure 1) results in a less stable complex. Altogether, these observations demonstrate the crucial role played by tyrosine-87 in sterol-elicitin interactions.
Role of Sterol-Elicitin Complexes in Elicitin Binding to Specific Sites
On one hand, ligand replacement experiments indicate that all of the studied elicitins (native, recombinant, and mutated) easily displace, to the same level, radiolabeled cryptogein specifically bound to the tobacco plasma membrane. This clearly demonstrates a specific interaction of these proteins with the binding sites of cryptogein. On the other hand, binding experiments show that elicitins are able to saturate these high-affinity sites. Thus, the mutations did not affect their ability to interact with these sites. Replacement and direct binding experiments also point out that the interaction of the various cryptogein-derived mutants are quite different. It implies that the elicitins in their native (initial) state cannot interact with the binding sites. The close correlation found between replacement efficiency and affinity for sterols (Figure 2B) strongly suggests that cryptogein displacement from its binding sites is secured only by sterol-loaded elicitins. The purified proteins (native state) from both Phytophthora and Pichia culture filtrates are “void,” i.e., sterol-unloaded proteins, as it appeared in this work and from structural (Boissy et al., 1996; Fefeu et al., 1997; Gooley et al., 1998) and chemical (Mikes et al., 1997, 1998) analyses. It was previously reported that elicitins easily bind sterols from tobacco plasma membranes (Vauthrin et al., 1999). Thus, during ligand replacement and binding experiments elicitins can load sterols. Consequently, we propose that a sterol-elicitin complex assumes the labeled-cryptogein displacement. This was confirmed with the use of stigmasterol-preloaded natural elicitins, which more rapidly displace 125I-cryptogein in replacement experiments (1.3- and 1.5-fold for capsicein and cryptogein, respectively). This increase expresses the difference between the activity of sterol-preloaded elicitin and nonpreloaded elicitin; this later could pick up a sterol molecule during the experiment, probably from plasma membranes. In addition, these sterol-elicitin complexes could be the active state of these proteins, triggering cell responses. In that way, we observed that a stigmasterol-preloaded cryptogein increased the tobacco cell responses (∼40% of the initial rate of extracellular medium alkalization at 1 nM; Osman, Vauthrin, Mikes, Milat, Panabières, Marais, Brunie, Maume, Ponchet, and Blein, unpublished results).
Clear conformational changes were observed between void and ergosterol-complexed cryptogein (Boissy et al., 1999), which could explain how sterol-loading “activates” elicitins allowing them to bind to high-affinity sites.
Are High-Affinity–binding Sites the Elicitin Receptors?
The natural elicitins that harbor strong biological activities (Bonnet et al., 1996; Rustérucci et al., 1996) also display high efficiency for loading sterols (Mikes et al., 1998). However, although elicitins exhibit differential biological activities, interactions with their putative receptors were reported to be similar (Bourque et al., 1998). This is the case for cryptogein and capsicein, but this apparent discrepancy remains to be explained. In this work, ligand replacement experiments show different behavior for these natural elicitins. It also points out a relationship between the binding of natural and mutated elicitins to specific sites and their biological activities on cells and plants. The level of tobacco cell responses (extracellular alkalization, calcium uptake) obviously depends on protein-binding efficiency on putative receptor. Cell responses are time phased with both sterol uptake from plasma membranes and binding on high-affinity sites. Plant responses are delayed (48 h for HR and 9 d for SAR) and have to integrate successive levels of regulation before phenotypic expression, but Y47G and Y87F are clearly affected in their elicitor activity.
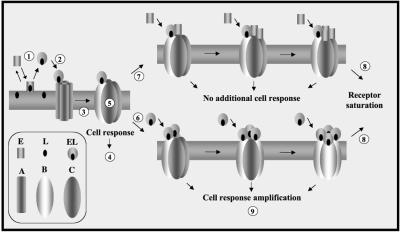
Finally, because it is assumed that when non-sterol–loaded elicitins are added to tobacco cells they bind a sterol molecule and then bind the receptor, these sterol-loaded elicitins are the most effective form for triggering biological responses. This is the first evidence suggesting that these plasma membrane-binding sites are truly the biological receptors of elicitins. In addition, the sigmoidal shape of the Y47G- and Y87F-binding curves could reflect an allosteric regulation of the high-affinity sites, in accordance with their postulated multimeric organization (Bourque et al., 1999). A working scheme is proposed, which summarizes the initial molecular events involving activation by sterol loading that drive elicitor function (Figure 6). The first elicitin-receptor interaction needs a sterol loading of elicitin from plant plasma membrane that induces a conformational change of the receptor subunits. This conformational modification allows the binding of other loaded/unloaded elicitin molecules to the receptor. But only loaded elicitins can trigger biological responses. Each binding subunit could be the 200-kDa complex previously described (Bourque et al., 1999; Lebrun-Garcia et al., 1999).
Figure 6.
Hypothetical scheme showing the cooperative interaction of elicitin-sterol complex with the elicitin receptor located on the plasma membranes. E and L represent the elicitin and its ligand; EL is the loaded form of elicitins (step 1), which is the sole form able to activate a receptor subunit; A, B, and C are three conformational states of the elicitin receptor subunits. In this scheme, four subunits participate in the multimeric structure. When EL binds to an A (step 2), it leads to the conformational change of this subunit to B (step 3). This new state triggers the biological response (step 4) and allows the conformational change of the other subunits (step 5; C, cooperative effect). C can bind either loaded (EL; step 6) or unloaded (E; step 7) elicitins. This explains why all elicitins are able to saturate the receptor subunits (step 8). However, only EL (B) will trigger a new set of biological responses (step 9).
The calcium signaling in tobacco cells treated with elicitins possesses the following characteristics: i) transient Ca2+ uptake can be induced by four sequential elicitin additions followed by a desensitization step (Keizer et al., 1998), ii) verapamil and nifedipine, which block voltage-dependent calcium channels in plant cells (Pineiros and Tester, 1995), have no effect on Ca2+ influx, indicating that the involved channels are not of the voltage-gated type but probably of ligand-dependent type (Tavernier et al., 1995), and iii) the mutated cryptogein Y87F provokes a decrease of the spontaneous Ca2+ exchanges in tobacco cells (Figure 4A). Taken together, these results suggest that elicitin receptor could be a ligand-dependent calcium channel organized in a quadrimeric complex as in Figure 6. The binding of a loaded elicitin to a subunit provokes its phosphorylation, because protein phosphorylation is required for the downstream signaling (Tavernier et al., 1995; Keizer et al., 1998). Such hypotheses require further experiments, which are in progress.
Conclusions
This work demonstrates a strict link between elicitor and sterol-carrier activity of elicitins, the formation of a sterol-elicitin complex appearing to be a requisite step before elicitins fasten to their receptors and trigger cell responses. These results open new perspectives in the understanding of cell-elicitin interactions and should bring new insight on the “clockwork” governing elicitin perception and resistance induction in plants. They also reveal the importance of lipid trafficking involving carrier proteins during plant pathogen interactions.
ACKNOWLEDGMENTS
We thank T. Prangé for helpful discussion and circular dichroism analyses, N. Béno for her excellent technical assistance, and A. Vincent for her contribution. We thank N.T. Keen and H. Keller for helpful discussions, P. Ricci for his constant encouragement, and M.-J. Farmer for revising the English. This work is supported by the Institut National de la Recherche Agronomique, the Caisse Régionale du Crédit Agricole de Côte d'Or, the Conseil Régional de Bourgogne, the Service d'Exploitation Industrielle des Tabacs et Allumettes, and the Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- Blein J-P, Milat M-L, Ricci P. Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea: possible plasmalemma involvement. Plant Physiol. 1991;95:486–491. doi: 10.1104/pp.95.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy G, de La Fortelle E, Kahn R, Huet JC, Bricogne G, Pernollet JC, Brunie S. Crystal structure of a fungal elicitor secreted by Phytophthora cryptogea, a member of a novel class of plant necrotic proteins. Structure. 1996;4:1429–1439. doi: 10.1016/s0969-2126(96)00150-5. [DOI] [PubMed] [Google Scholar]
- Boissy G, O'Donohue M, Gaudemer O, Perez V, Pernollet JC, Brunie S. The 2.1 angstrom structure of an elicitin-ergosterol complex: a recent addition to the sterol carrier protein family. Protein Sci. 1999;8:1191–1199. doi: 10.1110/ps.8.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet P, Bourdon E, Ponchet M, Blein J-P, Ricci P. Acquired resistance triggered by elicitins in tobacco and other plants. Eur J Plant Pathol. 1996;102:181–192. [Google Scholar]
- Bourque S, Binet M-N, Ponchet M, Pugin A, Lebrun-Garcia A. Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem. 1999;274:34699–34705. doi: 10.1074/jbc.274.49.34699. [DOI] [PubMed] [Google Scholar]
- Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MT, Tandeau de Marsac N, de Kouchkovsky Y. Photosynthetic growth of tobacco cells in liquid suspension. Can J Bot. 1972;50:2265–2270. [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- Ebel J, Mithofer A. Early events in the elicitation of plant defense. Planta. 1998;206:335–348. [Google Scholar]
- Fefeu S, Bouaziz S, Huet JC, Pernollet JC, Guittet E. Three-dimensional solution structure of beta cryptogein, a beta elicitin secreted by a phytopathogenic fungus Phytophthora cryptogea. Protein Sci. 1997;6:2279–2284. doi: 10.1002/pro.5560061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley PR, Keniry MA, Dimitrov RA, Marsh DE, Keizer DW, Gayler KR, Grant BR. The NMR solution structure and characterization of pH dependent chemical shifts of the beta-elicitin, cryptogein. J Biomol NMR. 1998;12:523–534. doi: 10.1023/a:1008395001008. [DOI] [PubMed] [Google Scholar]
- Guex N. Swiss-Pdb viewer: a new fast and easy to use PDB viewer for the Macintosh. Experientia. 1996;52:A26. [Google Scholar]
- Guex N, Peitsch MC. Swiss-Pdb viewer: a fast and easy to use PDB viewer for the Macintosh and PC. Prot Data Bank Q Newsl. 1996;77:7. [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, SmithBacker J, Keen NT. Genetics of plant-pathogen interactions. Curr Opin Biotechnol. 1998;9:202–207. doi: 10.1016/s0958-1669(98)80116-x. [DOI] [PubMed] [Google Scholar]
- Jones AM. Surprising signals in plant cells. Science. 1994;263:183–184. doi: 10.1126/science.263.5144.183. [DOI] [PubMed] [Google Scholar]
- Kamoun S, Young M, Förster H, Coffey MD, Tyler BM. Potential role of elicitins in the interaction between Phytophthora species and tobacco. Appl Environ Microbiol. 1994;60:1593–1598. doi: 10.1128/aem.60.5.1593-1598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen NT. Plant disease resistance: progress in basic understanding and practical application. Adv Bot Res. 1999;30:291–328. [Google Scholar]
- Keizer DW, Schuster B, Grant BR, Gayler KR. Interactions between elicitins and radish Raphanus sativus. Planta. 1998;204:480–489. [Google Scholar]
- Keller H, Blein JP, Bonnet P, Ricci P. Physiological and molecular characteristics of elicitin-induced systemic acquired resistance in tobacco. Plant Physiol. 1996;110:365–376. doi: 10.1104/pp.110.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer F, Lherminier J, Simon-Plas F, Nicole M, Paynot M, Elmayan T, Blein J-P. The fungal elicitor cryptogein induces cell wall modifications on tobacco cell suspension. J Exp Bot. 2000;51:1799–1811. doi: 10.1093/jexbot/51.352.1799. [DOI] [PubMed] [Google Scholar]
- Lamb C. A ligand-receptor mechanism in plant-pathogen recognition. Science. 1996;274:2038–2039. [Google Scholar]
- Lauge R, DeWit PJGM. Fungal avirulence genes: structure and possible functions. Fungal Genet Biol. 1998;24:285–297. doi: 10.1006/fgbi.1998.1076. [DOI] [PubMed] [Google Scholar]
- Leberre JY, Panabieres F, Ponchet M, Denoroy L, Bonnet P, Marais A, Ricci P. Occurrence of multiple forms of elicitins in Phytophthora cryptogea. Plant Physiol Biochem. 1994;32:251–258. [Google Scholar]
- Lebrun-Garcia A, Bourque S, Binet M-N, Ouaked F, Wendehenne D, Chiltz A, Schäffner A, Pugin A. Involvement of plasma membrane proteins in plant defense responses: analysis of the cryptogein signal transduction in tobacco. Biochimie. 1999;81:1–6. doi: 10.1016/s0300-9084(99)80123-0. [DOI] [PubMed] [Google Scholar]
- Mikes V, Milat M-L, Ponchet M, Panabières F, Ricci P, Blein J-P. Elicitins excreted by Phytophthora are a new class of sterol carrier proteins. Biochem Biophys Res Commun. 1998;245:133–139. doi: 10.1006/bbrc.1998.8341. [DOI] [PubMed] [Google Scholar]
- Mikes V, Milat M-L, Ponchet M, Ricci P, Blein J-P. The fungal elicitor cryptogein is a sterol carrier protein. FEBS Lett. 1997;416:190–192. doi: 10.1016/s0014-5793(97)01193-9. [DOI] [PubMed] [Google Scholar]
- Panabières F, Marais A, Leberre JY, Penot I, Fournier D, Ricci P. Characterization of a gene cluster of Phytophthora cryptogea which codes for elicitins, proteins inducing a hypersensitive-like response in tobacco. Mol Plant Microbe Interact. 1995;8:996–1003. doi: 10.1094/mpmi-8-0996. [DOI] [PubMed] [Google Scholar]
- Pineiros M, Tester M. Characterization of a voltage-dependent Ca2+-selective channel from wheat roots. Planta. 1995;195:478–488. [Google Scholar]
- Ricci P. Induction of the hypersensitive response and systemic acquired resistance by fungal proteins: the case of elicitins. In: Stacey G, Keen NT, editors. Plant-Microbe Interactions, 3 3. New York: Chapman & Hall; 1997. pp. 53–75. [Google Scholar]
- Rustérucci C, Stallaert V, Milat M-L, Pugin A, Ricci P, Blein J-P. Relationship between AOS, lipid peroxidation, necrosis and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol. 1996;111:885–891. doi: 10.1104/pp.111.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Lawton KA, Delaney TP, Friedrich L, Kessmann H, Neuenschwander U, Uknes S, Vernooij B, Weymann K. Signal transduction in systemic acquired resistance. Proc Natl Acad Sci USA. 1995;92:4202–4205. doi: 10.1073/pnas.92.10.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. Oligosaccharide signals: from plant defense to parasite offense. Proc Natl Acad Sci USA. 1994;91:1–2. doi: 10.1073/pnas.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel D. Resistance response physiology and signal transduction. Curr Opin Plant Biol. 1998;1:305–310. doi: 10.1016/1369-5266(88)80051-7. [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Rustérucci C, Milat M-L, Humbert C, Montillet J-L, Blein J-P. Active oxygen species production in tobacco cells elicited by cryptogein. Plant Cell Environ. 1997;20:1573–1579. [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JDG. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- Tavernier E, Wendehenne D, Blein J-P, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensive reaction in tobacco cells. Plant Physiol. 1995;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauthrin S, Mikes V, Milat M-L, Ponchet M, Maume B, Osman H, Blein J-P. Elicitins trap and transfer sterols from micelles, liposomes and plant plasma membranes. Biochim Biophys Acta. 1999;1419:335–342. doi: 10.1016/s0005-2736(99)00083-8. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Binet M-N, Blein J-P, Ricci P, Pugin A. Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 1995;374:203–207. doi: 10.1016/0014-5793(95)01108-q. [DOI] [PubMed] [Google Scholar]