ABSTRACT
For an increasing number of antimalarial agents identified in high-throughput phenotypic screens, there is evidence that they target PfATP4, a putative Na+ efflux transporter on the plasma membrane of the human malaria parasite Plasmodium falciparum. For several such “PfATP4-associated” compounds, it has been noted that their addition to parasitized erythrocytes results in cell swelling. Here we show that six structurally diverse PfATP4-associated compounds, including the clinical candidate KAE609 (cipargamin), induce swelling of both isolated blood-stage parasites and intact parasitized erythrocytes. The swelling of isolated parasites is dependent on the presence of Na+ in the external environment and may be attributed to the osmotic consequences of Na+ uptake. The swelling of the parasitized erythrocyte results in an increase in its osmotic fragility. Countering cell swelling by increasing the osmolarity of the extracellular medium reduces the antiplasmodial efficacy of PfATP4-associated compounds, consistent with cell swelling playing a role in the antimalarial activity of this class of compounds.
KEYWORDS: KAE609, PfATP4, antimalarial agents, transporters
INTRODUCTION
The search for novel antimalarial compounds has shifted in recent years, from target-based approaches, i.e., finding compounds that target a specific essential aspect of parasite biology, toward whole-cell “phenotypic” screening. Whole-cell screening involves assessing diverse compound libraries for the ability of compounds to inhibit parasite growth. This approach to the discovery of antimalarial compounds does not require prior knowledge of the biological target and has led to the identification of numerous potential antimalarials with diverse chemical structures (1–4).
For a number of compounds identified in whole-cell screens as having antiparasitic activity, the exposure of malaria parasites to either a static concentration or increasing concentrations of the compound, typically over a period of weeks to months, has given rise to resistant parasites (5–10). Genomic techniques have been used to identify mutations in the resistant parasites, thereby providing insight into mechanisms of resistance and, potentially, the identity of the target.
Whole-cell screening and genetic profiling of resistant Plasmodium falciparum strains have implicated the P-type ATPase PfATP4 (accession no. PF3D7_1211900) in the mechanism of action of a number of novel antiplasmodial compounds. These compounds include, but are not limited to, the spiroindolones cipargamin (KAE609; formerly known as NITD609 and now in advanced clinical trials [11]) and NITD246 (5), the dihydroisoquinolone (+)-SJ733 (6), the pyrazoleamide PA21A050 (7), the aminopyrazole GNF-Pf4492 (12), a compound originally identified as a prolyl-tRNA synthetase inhibitor, TCMDC-124506 (13), and the GlaxoSmithKline compound MMV772 (http://www.mmv.org/newsroom/news/three-new-roads-leading-common-pathway; accessed February 2017).
For all of the PfATP4-associated compounds characterized, to date, their addition to blood-stage malaria parasites causes the parasite's cytosolic Na+ concentration ([Na+]i) to increase, the cytosolic pH (pHi) to increase, and the degree of acidification seen on inhibition of the parasite's H+-extruding V-type H+ ATPase to decrease (6, 7, 12, 14). The data are consistent with the hypothesis that these compounds inhibit PfATP4, which functions as an ion pump exporting Na+ and importing H+, thereby maintaining a low [Na+]i while at the same time imposing a significant “acid load” on the parasite (14–16). In this model, the alkalinization induced by the PfATP4-associated compounds and the reduced acidification seen in response to inhibition of the H+-extruding V-type H+ ATPase are attributed to the inhibition of PfATP4 resulting in a lifting of the PfATP4-mediated acid load.
A screen of the Medicines for Malaria Venture's (MMV's) open access Malaria Box—a structurally diverse collection of 400 compounds identified in phenotypic screens as being potent inhibitors of the growth of blood-stage P. falciparum parasites—for effects on malaria parasite Na+ regulation revealed multiple additional chemotypes showing the same effects on parasite [Na+]i and pHi as those of the PfATP4-associated compounds (17). In this paper, the term “PfATP4-associated compounds” is used to refer to compounds that (i) cause an increase in [Na+]i, an increase in pHi, and a decrease in the acidification seen in response to inhibition of the plasma membrane V-type H+ ATPase and (ii) are less potent against parasites carrying resistance-conferring mutations in PfATP4 than against parasites with wild-type PfATP4.
The ability of PfATP4-associated compounds to kill asexual P. falciparum parasites is well established; however, the precise mechanism by which they do so is unclear. Jimenez-Diaz et al. (6) proposed that the ionic changes induced by the dihydroisoquinolone (+)-SJ733 ultimately lead to the induction of eryptosis (i.e., programmed cell death) in infected erythrocytes. Vaidya and colleagues proposed that the disruption of ion homeostasis by the pyrazoleamide PA21A050 and the spiroindolone KAE609 leads to the accumulation of cholesterol in the parasite's plasma membrane and initiates a cascade of events that trigger premature parasite schizogony (7, 18).
Both the dihydroisoquinolone (+)-SJ733 (6) and the pyrazoleamide PA21A050 (7) have been reported to induce swelling of the intraerythrocytic parasite, giving rise to the hypothesis that an increased [Na+]i in the parasite disrupts the osmotic balance of the cell, leading to water influx and therefore cell swelling (6, 7). Zhang et al. (19) reported that ring-stage parasitized erythrocytes exposed to KAE609 become spherical and rigid, and KAE609 has also been reported to cause stage II gametocytes to become swollen and rounded (20). Whether and (if so) how the swelling of the parasite or of the parasitized erythrocyte is involved in the mechanism by which PfATP4-associated compounds inhibit parasite growth are unclear.
In this study, we characterized in more detail the physical changes induced by PfATP4-associated compounds in both isolated trophozoite-stage parasites and trophozoite-infected erythrocytes, investigating in particular the role of Na+ in cell swelling and the role of swelling in the inhibition of parasite growth by compounds of this class.
RESULTS
Host cell and parasite volume estimates.
A Coulter MS4 instrument was used to estimate the volume of uninfected erythrocytes, P. falciparum-infected erythrocytes (approximately 36 to 40 h postinvasion), and mature trophozoite-stage P. falciparum parasites (approximately 36 to 40 h postinvasion) functionally isolated from their host erythrocytes by saponin permeabilization of the host cell membrane. For uninfected erythrocytes suspended in bicarbonate-free supplemented RPMI 1640 medium (283 ± 2 mosM), the volume was 81 ± 1 fl (mean ± standard deviation [SD]; range, 79 to 83 fl; n = 7 with cells from at least three individual donors). For parasitized erythrocytes suspended in the same medium, the volume was 83 ± 5 fl (mean ± SD; range, 72 to 89 fl; n = 12). The volume of parasitized erythrocytes was not significantly different from that of the control group (“cohort uninfected erythrocytes”) (P = 0.869; n = 7; paired t test).
For isolated mature trophozoite-stage parasites suspended in physiological saline (310 ± 2 mosM), the volume was 49 ± 7 fl (mean ± SD; range, 38 to 65 fl; n = 19). Increasing the osmolarity of the medium in which isolated parasites were suspended caused the mean cell volume to decrease (Fig. 1a). The variation of the parasite volume with osmolarity allowed a determination of the fraction of the “nonwater” volume of the parasite (i.e., the contribution of “solid” material to the measured volume) (21, 22), estimated here to be 0.44 ± 0.02 (n = 3) (Fig. 1b). This, in turn, allows an estimate of the intracellular water volume of isolated parasites suspended in physiological saline (0.56 of the total volume, corresponding to a water volume of 27 ± 1 fl for parasites with a mean total volume of 49 fl).
FIG 1.
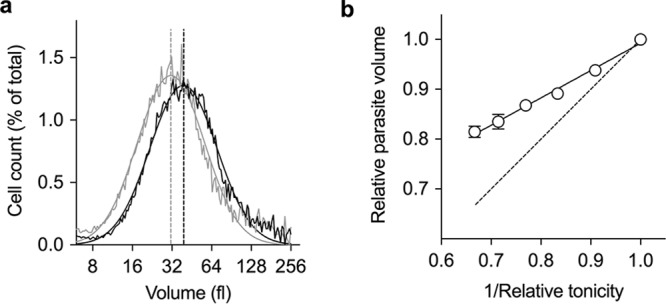
Variation of parasite volume with the osmolarity of the medium. (a) Representative traces showing the distribution of volume for isolated parasites suspended in either physiological saline (310 mosM; black trace) or physiological saline made hypertonic by the addition of 155 mosM sucrose (465 mosM; gray trace). Each data set was fitted with a Gaussian function and the median volume of each of the parasite populations (dashed lines) thereby determined. (b) The osmolarity of the medium was increased by the addition of increasing concentrations of sucrose. Parasite volume is cited relative to that of parasites suspended in physiological saline with an osmolarity of 310 mosM. Relative tonicity is the tonicity of the sucrose-containing medium relative to that of physiological saline solution. The dashed line represents the theoretical osmotic response of a cell showing “ideal” osmotic behavior. A straight line was fitted to the data by use of the following equation: relative parasite volume = 0.55 × (1/relative tonicity) + 0.44. The y intercept of the fitted line (i.e., the parasite volume at an infinite osmolarity, when 1/relative tonicity = 0) equates to the nonwater volume of the parasite. The results are the means ± SD from three independent experiments.
PfATP4-associated compounds induce swelling of isolated trophozoites.
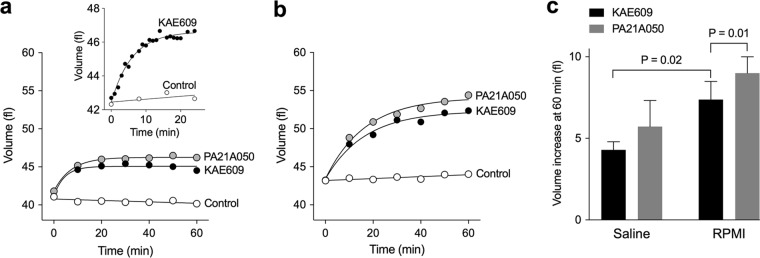
On addition of the PfATP4-associated antimalarial KAE609 (10 nM) to isolated parasites suspended in physiological saline, the parasites immediately started to swell (Fig. 2a), with their volume plateauing at around 15 min (Fig. 2a, inset). Addition of dimethyl sulfoxide (DMSO) as a solvent control had no significant effect on parasite volume. For KAE609-exposed parasites, the volume at 15 min was 53 ± 8 fl (mean ± SD; n = 16), which is an increase of 8% ± 2% (P < 0.001; paired t test). The half-time for KAE609-induced swelling was 4.4 ± 0.4 min (mean ± SD; n = 4), and the percent increase in trophozoite volume (measured at 15 min) was not affected by the starting volume of the parasite (see Fig. S1 in the supplemental material).
FIG 2.
Effects of PfATP4-associated compounds KAE609 and PA21A050 on the volume of isolated P. falciparum 3D7 trophozoites suspended in different media at 37°C. (a) Time course for the volume of isolated parasites suspended in physiological saline following the addition of either KAE609 in DMSO (final concentration, 10 nM) (black circles), PA21A050 in DMSO (final concentration, 10 nM) (gray circles), or DMSO alone (0.1% [vol/vol]; solvent control) (white circles). The inset shows a higher-resolution time course for the effect of KAE609 (10 nM) (closed circles) on the volume of isolated parasites in the same medium compared to a solvent control (0.1% [vol/vol] DMSO) (white circles). (b) Time course for the volume of isolated parasites suspended in bicarbonate-free, Albumax-free RPMI 1640 medium following the addition of KAE609 in DMSO (final concentration, 10 nM) (black circles), PA21A050 in DMSO (final concentration, 10 nM) (gray circles), or DMSO alone (0.1% [vol/vol]; solvent control) (white circles). For panels a and b, first-order exponential equations were fitted to the data for KAE609- and PA21A050-exposed cells; the control data were fitted by straight lines. In each case, the results shown are from a single experiment and are representative of those obtained in at least three independent experiments. (c) Change in parasite volume over 60 min for cells suspended in either physiological saline or bicarbonate-free, Albumax-free supplemented RPMI 1640 medium (RPMI) in the presence of KAE609 (10 nM) or PA21A050 (10 nM) compared to the volume of control cells (0.1% [vol/vol] DMSO; 60 min). The results are averaged from those obtained in at least three independent experiments for each compound, and the error bars denote SD. On exposure to KAE609 for 60 min, the volume increase seen for parasites suspended in RPMI medium was significantly greater (P < 0.05) than that for parasites suspended in physiological saline (P = 0.016; paired t test). On exposure to PA21A050 for 60 min, the volume increase seen for parasites suspended in RPMI medium was greater than that for parasites suspended in physiological saline, but the difference was not statistically significant (P = 0.138; paired t test). In both media, the swelling induced by 10 nM PA21A050 was slightly higher than that induced by 10 nM KAE609. For parasites in RPMI medium, the difference was statistically significant (P = 0.01; paired t test), whereas for parasites in physiological saline it was not statistically significant (P = 0.13).
Another PfATP4-associated antimalarial, PA21A050 (10 nM), caused isolated parasites suspended in physiological saline to swell to an extent similar to that seen with KAE609 (Fig. 2a). The volume increase undergone by parasites in saline exposed to 10 nM PA21A050 for 60 min was not significantly different from that undergone by parasites in saline exposed to 10 nM KAE609 for 60 min (P = 0.139; paired t test) (Fig. 2c).
For isolated parasites suspended in bicarbonate-free, Albumax-free supplemented RPMI 1640 medium, the pattern of cell swelling seen in response to addition of the two PfATP4-associated antimalarials (i.e., KAE609 or PA21A050) (Fig. 2b) was somewhat different from that seen for parasites suspended in physiological saline. Ten minutes after the addition of either of the two antimalarial compounds, the swelling undergone by isolated parasites in RPMI medium was similar to that undergone by parasites in saline (approximately 3 fl in both cases) (Fig. 2a and b). However, whereas for parasites in saline the volume plateaued at around 15 min, for parasites in RPMI medium the swelling continued to increase, such that 60 min after the addition of either KAE609 or PA21A050 the parasites in RPMI medium were larger than those in physiological saline (Fig. 2c). For parasites suspended in RPMI medium, the volume increase induced by PA21A050 was significantly greater than that induced by KAE609 (P = 0.007; paired t test).
To investigate whether the ability to cause parasite swelling seen for KAE609 and PA21A050 was a common feature of diverse PfATP4-associated chemotypes, five PfATP4-associated compounds from the MMV Malaria Box (see Fig. S2 for chemical structures) were tested for effects on parasite volume. The five compounds represent four chemotypes, with two of the five compounds being structurally similar. Each of the five compounds has been shown previously to induce an increase in [Na+]i and pHi in isolated trophozoite-stage parasites and to be less potent at inhibiting the proliferation of parasites harboring KAE609 resistance-conferring mutations in PfATP4 than that of wild-type parasites (17). The MMV compounds were tested for effects on 3D7 parasite volume when added at a concentration 15× higher than the 50% inhibitory concentration (IC50) for inhibition of proliferation of asexual-stage Dd2 parasites in vitro, as determined in a previous study (17). Each of the five compounds induced an increase in the volume of isolated 3D7 trophozoites suspended in physiological saline. The magnitudes of the volume increases induced by the compounds tested, measured at 15 min, were in the following order: MMV665949 > KAE609 ≥ MMV665826 ≥ MMV011567, MMV006656, and MMV006764 (Fig. 3). Doubling the concentrations of the MMV compounds to 30× the IC50 for inhibition of proliferation of asexual-stage Dd2 parasites did not lead to a greater volume increase in isolated 3D7 trophozoites for any of the compounds (data not shown).
FIG 3.
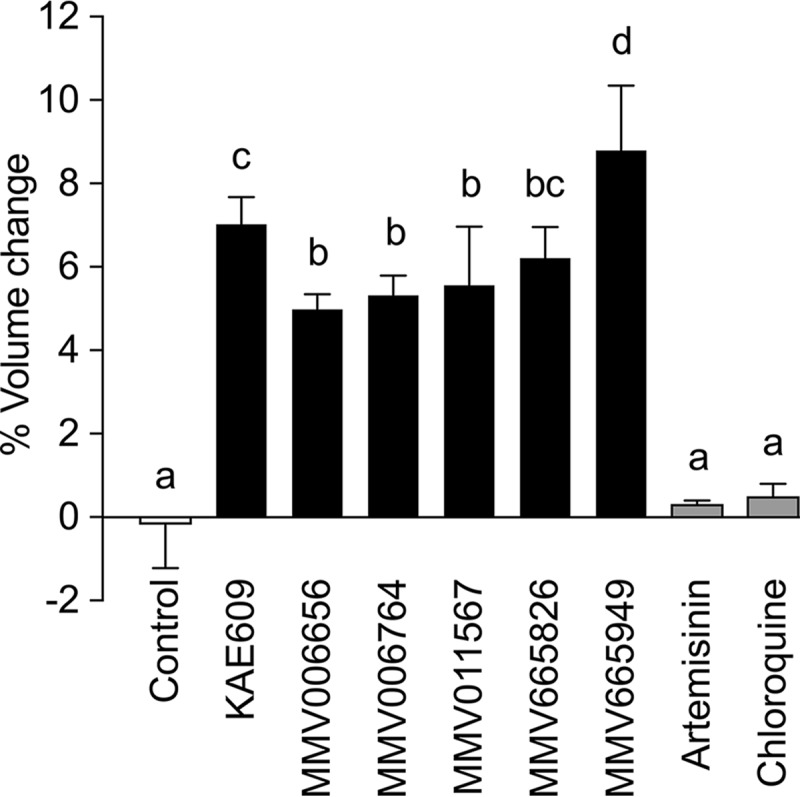
Effects of KAE609 and PfATP4-associated compounds on the volume of isolated P. falciparum 3D7 trophozoites suspended in physiological saline, measured at 37°C. Changes in parasite volume (expressed as percentages of the starting volume) were determined after 15 min in the presence or absence of various PfATP4-associated compounds or unrelated antimalarials (artemisinin and chloroquine). The results are averaged from those obtained in at least three independent experiments for each compound, and the error bars denote SD. The concentrations tested (in each case, 15× the IC50 for inhibition of parasite proliferation) were 2.2 μM for MMV006656, 6.7 μM for MMV006764, 4.9 μM for MMV011567, 4.2 μM for MMV665826, and 17 μM for MMV665949. The two antimalarial drugs were tested at >15× the IC50 for inhibition of parasite proliferation (100 nM for artemisinin and 100 nM for chloroquine). The letters above the bars indicate statistical significance. Bars labeled with the same letter (e.g., “a”) are not statistically different from one another (P > 0.05), whereas bars labeled with different letters are significantly different from one another (P < 0.05) (e.g., the bars labeled “a” are significantly different from those labeled “b”). The bar labeled “bc” is not significantly different from those labeled “b” or “c” but is significantly different from those labeled “a” or “d.”
Exposure of isolated trophozoites to the solvent alone (0.1% [vol/vol] DMSO) or to the non-PfATP4-associated antimalarial compounds artemisinin and chloroquine (each tested at a concentration of 100 nM) had no significant effect on parasite volume (Fig. 3). The induction of trophozoite swelling is therefore not a general feature of antimalarial compounds.
KAE609-induced swelling of isolated parasites is Na+ dependent.
To investigate the role of Na+ in the KAE609-induced swelling of isolated parasites, the effect of KAE609 on the volume of isolated parasites suspended in a Na+-containing saline was compared to that on the volume of isolated trophozoites suspended in Na+-free media, in which Na+ was replaced isosmotically with either N-methyl-d-glucamine (NMDG) or choline. There was no significant difference in the starting volumes of trophozoites suspended in the three media tested (P > 0.05). For parasites suspended in the NMDG-containing medium, the volume remained unchanged over 20 min in both the presence and absence of 10 nM KAE609. Parasites suspended in the choline-containing medium underwent a small apparent volume increase (<2%; not statistically significant [P > 0.05]) in both the presence and absence of 10 nM KAE609, with no difference between the two (Fig. 4). The KAE609-induced swelling of isolated trophozoites is therefore dependent on the presence of Na+ in the external medium.
FIG 4.
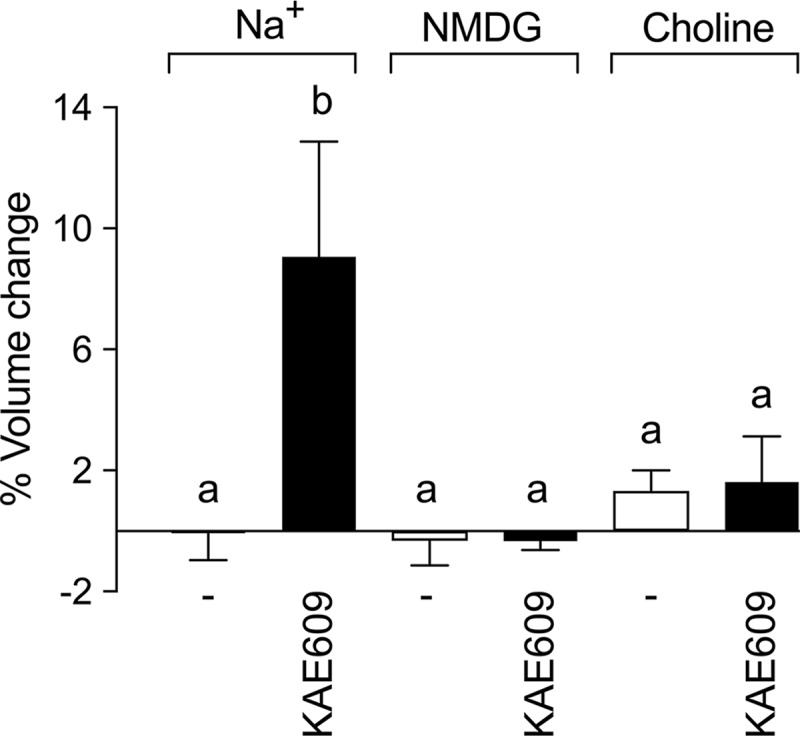
Na+ dependence of KAE609-induced swelling of isolated P. falciparum trophozoites. Isolated trophozoites were suspended in Na+-, NMDG-, or choline-containing saline for 20 min at 37°C before the addition of either 10 nM KAE609 in DMSO or DMSO alone (solvent control; 0.1% [vol/vol]) (−). The volume of the isolated 3D7 trophozoites was measured immediately prior to the addition of KAE609 or DMSO and then again 15 min later. The percent volume change was determined by comparing the median cell volumes at the beginning and end of the 15-min incubation. The results shown are averaged from those obtained in five independent experiments, and error bars denote SD. The letters above the bars indicate statistical significance, with the bars labeled “a” being not significantly different from one another but significantly different from that labeled “b” (P < 0.05).
Addition of KAE609 (50 nM) to isolated parasites suspended in a Na+-containing medium was demonstrated previously to result in a time-dependent net uptake of Na+ (23). A KAE609-induced increase in the Na+ content of the parasite was confirmed here under the same conditions as those used in the cell volume experiments (Fig. 2a, inset). The addition of 10 nM KAE609 to isolated parasites suspended in physiological saline resulted in a time-dependent increase in the parasite Na+ content (Fig. 5a and b), which had increased 3.3- ± 1.3-fold by 20 min, from 3.2 ± 1.0 mmol/1013 cells at time zero to 10.7 ± 2.4 mmol/1013 cells at 20 min (mean ± SD; n = 6) (Fig. 5b). In contrast, there was no change in the Na+ content of isolated parasites over the same (20 min) period following the addition of DMSO alone (solvent control) (Fig. 5b). The time scale of the KAE609-induced increase in parasite Na+ content was similar to that seen for the KAE609-induced increase in parasite volume: parasite Na+ content reached a maximum within 10 to 20 min, with a half-time of 5 ± 2 min (mean ± SD; n = 5) (Fig. 5b), which is comparable to the half-time of 4.4 ± 0.4 min for the KAE609-induced swelling of parasites in physiological saline (Fig. 2a).
FIG 5.
Effects of KAE609 on the intracellular Na+ and K+ contents of isolated P. falciparum trophozoites suspended in physiological saline under the same conditions as those used in the experiment giving rise to the inset to Fig. 2a. (a) Representative HPLC traces of parasite extracts prepared from isolated parasites suspended (at time zero) in the presence of 10 nM KAE609. (b) Time courses for the Na+ content of isolated trophozoites following the addition of 10 nM KAE609 (in DMSO) (black circles; fitted with a first-order exponential equation) or DMSO alone (0.1% [vol/vol]) (white circles; fitted by linear regression). (c) Time courses for the K+ content of isolated trophozoites following the addition of 10 nM KAE609 (in DMSO) (black circles) or DMSO alone (0.1% [vol/vol]) (white circles). Lines were fitted to both data sets by linear regression. For panels b and c, the results shown are averaged from those obtained in at least four independent experiments, and error bars show SD. The letters above the points in the time courses for KAE609-treated cells indicate statistical significance. Values labeled with the same letter are not statistically different from one another (P > 0.05), whereas those labeled with different letters are significantly different from one another (P < 0.05). The value labeled “ab” is not significantly different from those labeled “a” or “b.” The value labeled “bc” is not significantly different from those labeled “b” or “c” but is significantly different from those labeled “a.”
In contrast to the rapid and relatively large increase in the intracellular Na+ content, the intracellular K+ content underwent a slow and relatively small proportional decrease over time following the addition of 10 nM KAE609, from 44 ± 8 mmol/1013 cells at time zero to 38 ± 8 mmol/1013 cells at 20 min (mean ± SD; n = 6) (Fig. 5c). The decrease reached statistical significance only at the 20-min time point, at which point the parasite K+ content had decreased by an estimated 14% relative to that at time zero (P < 0.05). There was no change in the K+ content of isolated parasites over the same (20 min) period following the addition of DMSO alone (solvent control) (Fig. 5c).
The estimates of the parasite Na+ and K+ contents reported here, together with the parasite water volume, allow calculation of the [Na+]i and [K+]i. The isolated trophozoites in the high-pressure liquid chromatography (HPLC) experiments had a starting volume of 50 ± 6 fl (mean ± SD; n = 6) and an average water volume of 28 fl (using the 0.56 cell water volume fraction determined in Fig. 1b). For cells suspended in Na+-containing saline, the [Na+]i and [K+]i values are estimated to be 11 and 157 mM, respectively (assuming that Na+ and K+ are distributed evenly throughout the total 28 fl of intracellular water volume). After 20 min of exposure to KAE609, the total parasite volume had increased by 4 fl. If it is assumed that this increase is wholly accounted for by an increase in water volume, then the estimated [Na+]i and [K+]i values at 20 min post-KAE609 exposure are 33 and 119 mM, respectively.
These estimates of the [Na+]i increase following KAE609 exposure are similar to those for isolated parasites loaded with the Na+-sensitive dye Na+-binding benzofuran isophthalate (SBFI). The mean starting [Na+]i for SBFI-loaded parasites was 6 ± 2 mM (mean ± SD; n = 8). The [Na+]i value remained unchanged following exposure to DMSO for 20 min (6 ± 2 mM [mean ± SD]; n = 4) and increased to 42 ± 19 mM (mean ± SD; n = 4) following exposure to 10 nM KAE609 for 20 min (Fig. S3).
The swelling of isolated parasites induced by KAE609 or PA21A050 is not affected by modulating the cholesterol content of the parasite plasma membrane.
In a previous study, it was reported that PA21A050 and KAE609 induced a Na+-dependent accumulation of cholesterol in the parasite plasma membrane (18). To investigate whether this phenomenon might play a role in the parasite swelling seen here, isolated parasites were preincubated for 30 min (prior to exposure to either KAE609 or PA21A050) in either (i) RPMI 1640 medium; (ii) RPMI 1640 medium containing methyl-β-cyclodextrin (MβCD) to remove cholesterol from the parasite plasma membrane (18); or (iii) RPMI 1640 medium containing MβCD preloaded with cholesterol, under which conditions cholesterol is not removed from the parasite membrane (18). The 30-min preincubation in the two MβCD-containing media (with or without cholesterol) itself caused changes in the volume of the isolated parasites (Fig. S4a). Preincubation with MβCD preloaded with cholesterol or with MβCD alone caused the median volume to increase by an average of 14 fl relative to the control level. For parasites preincubated in each of the three different media, exposure to either KAE609 or PA21A050 resulted in similar degrees of swelling (6 to 8 fl relative to the volume of parasites exposed to the DMSO solvent control) as measured at 20 min. For both KAE609 and PA21A050, there was no significant difference in the extent of swelling induced in parasites preincubated in any of the three different (cholesterol-modulating) media (P > 0.05) (Fig. S4). The swelling induced by the two PfATP4-associated compounds, as measured over 20 min, is therefore not dependent on the accumulation of cholesterol in the parasite plasma membrane.
When the incubation of the parasites with the inhibitors (or, in the case of the solvent control, with DMSO) was extended to 120 min, a more complex pattern emerged for parasites treated with MβCD. For parasites preincubated with MβCD alone (i.e., without cholesterol), two subpopulations of cells became evident on the Multisizer trace, both in cell suspensions exposed to PfATP4-associated compounds and in those exposed to DMSO alone. For parasites preincubated with MβCD preloaded with cholesterol, again both for parasite suspensions exposed to PfATP4-associated compounds and those exposed to DMSO alone, the median volume of the entire population measured 120 min after the preincubation was increased to approximately double that seen at 20 min. These complications precluded an accurate determination of the effect of PfATP4-associated compounds on parasite volume 120 min after their addition.
PfATP4-associated compounds cause intact parasitized erythrocytes to swell.
Intact trophozoite-infected erythrocytes suspended in bicarbonate-free supplemented RPMI 1640 medium in the presence of DMSO (0.1% [vol/vol]; solvent control) had an initial “resting” volume of 86 ± 1 fl (mean ± SD; n = 8) and underwent a slight but significant progressive increase in volume, reaching a value of 90 ± 2 fl by 60 min (mean ± SD; n = 8; P = 0.002 by paired t test) (Fig. 6a). Parasitized erythrocytes treated with chloroquine (100 nM) underwent a similar volume increase, reaching a value of 91 ± 3 fl (mean ± SD; n = 8) (Fig. 6a) after 60 min, which is significantly different from the initial volume (P = 0.001; paired t test) but not significantly different from the volume of cells to which solvent (DMSO) alone had been added (P > 0.05). Parasitized erythrocytes treated with KAE609 (10 nM) for 60 min swelled to a significantly greater extent than that for DMSO- or chloroquine-treated infected erythrocytes, reaching a volume of 98 ± 2 fl (mean ± SD; n = 8; P < 0.05 for the difference between KAE609-exposed cells and DMSO- or chloroquine-exposed cells at 60 min).
FIG 6.

Effects of KAE609 and other compounds on the volume of intact parasitized erythrocytes. (a) Time courses (measured at 37°C) for the volume of parasitized erythrocytes (parasitemia of ≥95%; cells were suspended in bicarbonate-free supplemented RPMI 1640 medium) following the addition of 10 nM KAE609 (in DMSO) (black circles), 100 nM chloroquine (in DMSO) (gray circles), or DMSO alone (0.1% [vol/vol]; solvent control) (white circles). In each case, a first-order exponential equation was fitted to the data. The data shown are averaged from those obtained in five independent experiments, and the error bars denote SD. (b) Volume changes (expressed as percentages of the initial volume) of parasitized erythrocytes (parasitemia of ≥95%) and cohort uninfected erythrocytes (uRBC) incubated for 60 min in the presence and absence of various PfATP4-associated compounds or unrelated antimalarials (artemisinin and chloroquine). The results shown are averaged from those obtained in at least three independent experiments, and error bars denote SD. The compound concentrations used were the same as those described in the legend to Fig. 3. The letters above the bars indicate statistical significance. Bars labeled with the same letter are not statistically different from one another (P > 0.05), whereas those labeled with different letters are significantly different from one another (P < 0.05).
The five structurally diverse PfATP4-associated compounds from the MMV Malaria Box were also tested for their effect on the volume of intact infected erythrocytes. All five compounds caused the cells to swell, to magnitudes similar to that seen in response to KAE609 (Fig. 6b). In all cases, the swelling was significantly greater than that seen in response to the addition of DMSO alone or the addition of the non-PfATP4-associated antimalarial compounds chloroquine and artemisinin (P < 0.05) (Fig. 6b). Cohort uninfected erythrocytes did not undergo a change in volume during the course of a 60-min incubation either in the presence of KAE609 (10 nM) or under control conditions (Fig. 6b). The KAE609-induced swelling of intact parasitized erythrocytes is therefore dependent on the presence of the intracellular parasite and is not an inherent property of the erythrocyte.
Attempts to investigate the dependence of the KAE609-induced swelling of intact parasitized erythrocytes on extracellular Na+ were thwarted by the fact that on suspension of parasitized erythrocytes in medium in which Na+ is replaced by an organic cation (choline or NMDG), the cells swell, and ultimately lyse, as a result of the influx of the organic cations via the “new permeability pathways” induced by the parasite in the erythrocyte membrane (24).
KAE609 increases the osmotic fragility of parasitized erythrocytes.
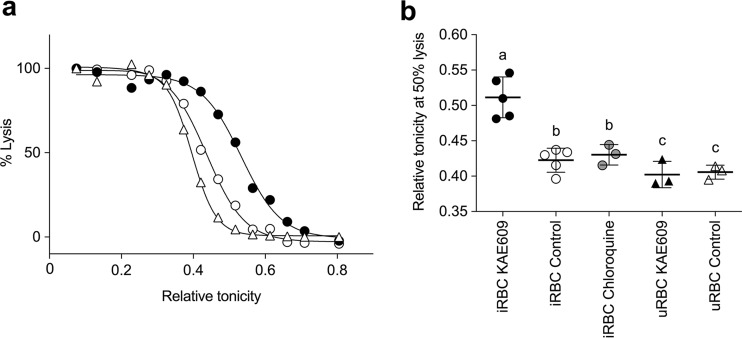
An increase in the volume of parasitized erythrocytes might be expected to result in increased fragility of the cells. The effect of KAE609 on the osmotic fragility of parasitized and uninfected erythrocytes was investigated by suspending the cells in media of various tonicities and quantifying the extent of hemolysis (25). Infected erythrocytes preincubated for 90 min in the presence of 10 nM KAE609 showed a significant increase in osmotic fragility relative to that of infected erythrocytes preincubated in the presence of either solvent alone (0.1% [vol/vol] DMSO) or 100 nM chloroquine (P < 0.05) (Fig. 7; Fig. S5). KAE609 had no effect on the osmotic fragility of uninfected erythrocytes (Fig. 7).
FIG 7.
Effect of KAE609 on osmotic fragility of parasitized and uninfected erythrocytes. (a) Parasitized (>95% parasitemia) and uninfected erythrocytes were preincubated for 90 min at 37°C in 10 nM KAE609 (black symbols) or 0.1% (vol/vol) DMSO (solvent control) (white symbols) in bicarbonate-free supplemented RPMI 1640 medium (see Table S1 in the supplemental material). Cells were pelleted by centrifugation and resuspended in saline media with relative tonicities of 0.04 to 1 (where a relative tonicity of 1 equates to an osmolarity of 300 mosM). Lysis of parasitized (circles) and uninfected (triangles) erythrocytes was determined by measuring the absorbance of hemoglobin (at 415 nm) in the extracellular solution. The % lysis was calculated by normalizing the data to the absorbance of the hemoglobin in the supernatant solution from cells suspended in saline with a relative tonicity of 0.04 (100% lysis). The results shown are from a single experiment and are representative of those obtained in three similar experiments. (b) The tonicity at which 50% of the erythrocytes were lysed (50% hemolysis) was plotted for parasitized (iRBC; circles) and uninfected (uRBC; triangles) erythrocytes that had been preincubated in the presence of 10 nM KAE609 (black symbols), 0.1% (vol/vol) DMSO (solvent control) (white symbols), or 100 nM chloroquine (gray symbols). The data for each of the osmotic fragility experiments (3 to 5 independent experiments) are shown in Fig. S5. For each treatment, the bold horizontal bar denotes the mean, and error bars denote SD. The letters above the points indicate statistical significance. Values labeled with the same letter are not statistically different from one another (P > 0.05), whereas those labeled with different letters are significantly different from one another (P < 0.05).
Effects of extracellular [Na+] and osmolarity on inhibition of parasite proliferation by PfATP4-associated compounds.
Vaidya et al. reported that parasites cultured in a medium with a reduced [Na+] show decreased sensitivity to a number of PfATP4-associated compounds—the pyrazoleamides PA21A050 and PA21A085 and the spiroindolone NITD246—consistent with extracellular Na+ playing a key role in the antiplasmodial potency of these compounds (7). In the present study, we explored the roles of extracellular Na+ and cell swelling in the inhibition of parasite proliferation by KAE609, using parasites cultured in media with various [Na+] values and osmolarities.
As shown previously for the related spiroindolone NITD246 (7), the IC50 for the inhibition of 3D7 parasite proliferation by KAE609 was increased significantly in a growth medium with a reduced [Na+] (i.e., the parasites showed decreased sensitivity to the inhibitor), whereas that for chloroquine was unaffected (Table 1). A low-Na+ medium in which Na+ was replaced with K+ and sucrose had the same osmolarity as the custom-made RPMI 1640 growth medium (7, 26).
TABLE 1.
Effects of reduced extracellular [Na+] on potencies of KAE609 and chloroquine against P. falciparum parasitesa
| Parameter | Value |
|
|---|---|---|
| Custom-made RPMI medium | Low-Na+ medium | |
| Osmolarity (mosM) | 330 ± 3 | 330 ± 3 |
| IC50 (nM) for 3D7 parasites (n) | ||
| KAE609 (P = 0.0044) | 0.8 ± 0.1 (3) | 2.4 ± 0.5 (3) |
| Chloroquine (P = 0.7654) | 20 ± 2 (3) | 20 ± 3 (3) |
The IC50 data are averaged from multiple (n) independent experiments, and errors denote SEM. The P values indicate the significance of differences between the IC50s measured in the two different media and are from unpaired t tests. See Table S1 in the supplemental material for the concentrations of the different components of the media.
For 3D7 parasites cultured in a high-Na+ RPMI 1640 medium in which the [Na+] was increased by the inclusion of an additional 30 mM NaCl, there was a decrease in the IC50 for the inhibition of parasite proliferation by KAE609 (i.e., the parasites showed increased sensitivity to the inhibitor) (P = 0.0044; unpaired t test) (Table 2). The shift toward a lower IC50 in the high-Na+ growth medium was also observed for Dd2 parasites exposed to KAE609 and to another PfATP4-associated compound, MMV011567 (P = 0.03 and 0.002, respectively; unpaired t test) (Table 2). In contrast, the inhibition of both 3D7 and Dd2 parasite proliferation by chloroquine, a non-PfATP4-associated antimalarial, was unaffected by the increased [Na+] in the medium (Table 2).
TABLE 2.
Effects of increased extracellular [Na+] and osmolarity on potencies of KAE609, MMV011567, and CQ against P. falciparum parasitesa
| Parameter | Value |
||
|---|---|---|---|
| RPMI medium | High-Na+ RPMI medium | Sucrose RPMI medium | |
| Osmolarity (mosM) | 290 ± 3 | 349 | 349 |
| IC50 (nM) (n) | |||
| KAE609 against 3D7 parasites | 0.62 ± 0.04 (8) | 0.42 ± 0.03 (7) | 0.92 ± 0.13 (8) |
| P value | 0.0010 | 0.0497 | |
| KAE609 against Dd2 parasites | 0.9 ± 0.1 (5) | 0.6 ± 0.1 (5) | ND |
| P value | 0.03 | ||
| MMV011567 against Dd2 parasites | 285 ± 19 (5) | 178 ± 13 (5) | ND |
| P value | 0.002 | ||
| CQ against 3D7 parasites | 20 ± 1 (7) | 18 ± 2 (7) | 18 ± 1 (7) |
| P value | 0.1798 | 0.2106 | |
| CQ against Dd2 parasites | 93 ± 18 (5) | 106 ± 15 (5) | ND |
| P value | 0.6 | ||
The IC50 data are averaged from multiple (n) independent experiments, and errors denote SEM. The P values are from unpaired t tests and relate to the differences between the IC50s for parasites grown in RPMI 1640 medium and those grown in either high-Na+ RPMI 1640 medium or sucrose RPMI 1640 medium. CQ, chloroquine; ND, not determined.
The high-Na+ growth medium had an osmolarity of 349 mosM and was therefore hypertonic relative to the normal growth medium, which had an osmolarity of 293 mosM. The effects of hypertonicity on both the IC50 for inhibition of 3D7 parasite proliferation by KAE609 and the volume of the parasitized erythrocytes (in both the presence and absence of KAE609) were explored further using an alternative medium, rendered hypertonic by the addition of 61 mM sucrose (yielding an osmolarity of 349 mosM, the same as that of the high-Na+ medium). The IC50 for inhibition of parasite proliferation by KAE609 for cells in this medium was 0.92 ± 0.13 nM (mean ± standard error of the mean [SEM]; n = 8), which is significantly higher than that for cells in normal medium (0.62 ± 0.04 nM; n = 8; P = 0.0497 by the unpaired t test) (Table 2), i.e., sucrose-mediated hypertonicity decreased the efficacy of KAE609 as an inhibitor of parasite growth, the opposite of what was seen in media rendered hypertonic by the addition of NaCl. In contrast, the IC50 for inhibition of parasite proliferation by chloroquine was unaffected by the addition of sucrose to the medium (Table 2).
For parasitized erythrocytes suspended in the hypertonic sucrose-containing medium, the volume was some 6 fl (i.e., 9%) lower than that of parasitized erythrocytes suspended at physiological osmolarity (Fig. S6). On addition of KAE609 to cells in this medium, the cells swelled by approximately the same amount as that for cells in physiological medium; in both media, the volume increased by approximately 3 to 4 fl over 60 min (Fig. S6). Significantly, for parasitized erythrocytes that underwent KAE609-induced swelling in the hypertonic medium, the volume remained below that of (untreated, unswollen) parasites suspended in physiological media.
The hypertonic sucrose-containing medium and the low-Na+ medium both contained sucrose, and in both there was a reduced efficacy of KAE609 (i.e., the IC50 for the inhibition of parasite proliferation increased). To test the possibility that the sucrose present in both media somehow disrupted the interaction between KAE609 and its target, we investigated the efficacy with which KAE609 disrupted Na+ regulation in isolated parasites loaded with the Na+-sensitive fluorescent indicator SBFI and suspended in three different media: physiological saline, hypertonic sucrose-containing saline, and physiological saline to which had been added an additional 30 mM NaCl (designed to mimic the ionic environment of a trophozoite within a host erythrocyte following hypertonic shrinkage of the host cell by an impermeant solute, such as sucrose, that remains outside the infected cell). In each case, the initial rate of increase of the [Na+]i value immediately following the addition of KAE609 was measured. There was no difference between IC50s for the perturbation of Na+ homeostasis by KAE609 for any of the three saline media tested (Fig. S7). These data indicate that the reduced growth-inhibitory potency of KAE609 seen for sucrose-containing media is not due to a reduced efficacy of the interaction of KAE609 with its target under any of the different osmotic/ionic conditions tested. Rather, the variation in the IC50s for inhibition of parasite proliferation seen in the different media can be attributed to differences in the (“downstream”) consequences of inhibition of the target in the different media.
DISCUSSION
The cell volume estimates made here using a Coulter Multisizer instrument are similar to those made previously using a range of different methodologies. The estimated volume of uninfected erythrocytes (81 ± 1 fl) is within the clinical reference range (80 to 100 fl) (27) and is similar to volume estimates derived from confocal microscopy and three-dimensional (3D) reconstruction of live fluorescent-dye-loaded erythrocytes (28, 29). The mean volume of parasitized erythrocytes (83 ± 5 fl) measured here is somewhat lower than the estimates obtained from confocal microscopy of live, dye-loaded trophozoite-infected cells (103 ± 26 fl) (28) and 3D refractive index tomograms of live trophozoite-infected cells (107 ± 17 fl) (30) but similar to those obtained for late-trophozoite-infected cells by cryo-X-ray microscopy (79 ± 14 fl) (31).
For isolated trophozoite-stage parasites, the mean volume found here (49 ± 7 fl [mean ± SD]) was larger than that estimated from cryo-X-ray microscopy (33 ± 6 fl for late trophozoites) (31) but similar to estimates derived from interpretation of UV-visible spectra of live infected erythrocytes (42 to 46 fl) (32). The mean cell water volume of isolated mature trophozoite-stage parasites, estimated here as 27 ± 1 fl, falls within the range of previous estimates of the internal water volume of isolated trophozoite-stage parasites that were made based on measurements of the distribution of 3H2O (21 to 28 fl for parasites at approximately 35 to 40 h postinvasion) (33, 34).
PfATP4-associated compounds caused both isolated parasites and intact infected erythrocytes to swell. For the isolated parasites, the magnitude and time course of swelling induced both by the spiroindolone KAE609 and by the pyrazoleamide PA21A050 varied with the medium in which the parasites were suspended. The amounts of swelling undergone in the first 10 min following the addition of either compound were similar for each of the two media tested (an RPMI-based medium and a more minimal saline medium). However, whereas for parasites in saline the volume plateaued after approximately 15 min, for parasites in RPMI medium the volume increase continued over the ensuing 45 min. The prolonged swelling of isolated parasites in RPMI medium (Fig. 2b) showed some similarity to the KAE609-induced swelling of intact parasitized erythrocytes, which persisted over the full 60-min incubation period (Fig. 6a). Why the volume increase of isolated parasites suspended in saline plateaued 15 min after the addition of the PfATP4-associated compounds is uncertain.
The volume increases reported here are substantially smaller than those that might be deduced from a previous report from Vaidya et al. (7) that on treatment of trophozoite-stage P. falciparum-infected erythrocytes with PA21A050 for 2 h, the diameter of the intracellular parasite (estimated using live-cell imaging) increased from 2.5 μm to 4.6 μm. If it is assumed that the parasite is approximately spherical, this equates to an approximately 6-fold increase in parasite volume, well above the approximately 25% increase seen here for the volume of isolated parasites suspended in an RPMI-based medium and exposed to PA21A050 for 1 h.
Attempts to isolate parasites from intact infected cells that had undergone KAE609-induced swelling (and to ascertain thereby the volume of parasites swollen within their host cells) proved unsuccessful; addition of the isolating agent saponin to KAE609-treated parasitized erythrocytes resulted in a loss of membrane integrity for both the parasite and the host cell, as reported previously (18). Whether the larger apparent parasite swelling that might be inferred from the earlier live-cell imaging data was due to the parasites being within intact infected cells and/or might be due to the swelling being overestimated as a result of the parasites not being spherical under the conditions tested is not clear.
The KAE609-induced swelling of isolated parasites was dependent on the presence of Na+ in the external medium, highlighting the central role of Na+ in the response of the parasites to this compound. The time scale of KAE609-induced isolated-parasite swelling was similar to that for the increase in parasite Na+ content. In addition to the 3.3-fold increase in the Na+ content of the parasite measured over 20 min, there was a small but significant (14%) decrease in the K+ content of the parasite population over the same period. The K+ loss was not accounted for by cell lysis, though whether this K+ loss reflects the loss of a small proportion of the intracellular K+ content across the whole parasite population or the loss of a greater proportion of the internal K+ pool from a subpopulation of the parasites, perhaps as a result of a nonlytic loss of membrane integrity, is not readily resolved.
The swelling induced by KAE609 or PA21A050 was not dependent on the previously reported accumulation of cholesterol in the parasite plasma membrane (18). The latter phenomenon occurs on a longer time scale (increasing over a period of hours) than the immediate-onset increase in volume and Na+ content characterized here.
The finding that reducing the [Na+] in the growth medium decreased the efficacy with which KAE609 inhibited parasite proliferation (similar to a previous report of reduced efficacy of PA21A050 and the spiroindolone NITD246 in low-Na+ medium [7]), whereas increasing the [Na+] in the growth medium increased the efficacy of KAE609, is consistent with Na+ playing a key role in the toxicity of KAE609 and other PfATP4-associated antimalarials. The finding that osmotic shrinkage of the parasitized erythrocytes by the addition of sucrose to the growth medium conferred some degree of protection for the parasite against KAE609, decreasing the efficacy of the inhibitor, is consistent with cell swelling playing some role in the mechanism of action of the PfATP4-associated antimalarials. However, the finding that for cells suspended under these hypertonic conditions KAE609 still inhibited parasite proliferation (albeit with reduced efficacy), despite the volume of the parasitized erythrocytes remaining below that of (untreated, unswollen) parasites suspended in physiological medium (at least in the hour following addition of the compound), indicates that the swelling of the parasite above the normal volume is not a necessary condition for inhibition of parasite growth by KAE609.
In a recent phase 2 study of the efficacy of KAE609 in adults with uncomplicated P. falciparum malaria or P. vivax malaria, the median half-life for parasite clearance was less than 1 h in both cases. The time scale of the cell volume changes reported here falls within this time frame, highlighting the possibility that cell swelling plays a role in the clearance of infected cells by host mechanisms (e.g., the spleen).
Parasitized cells in the high-Na+ hypertonic growth medium showed increased sensitivity to inhibition of parasite proliferation by KAE609 relative to that of cells in physiological medium. This is likely to reflect the combined effects of (i) increased influx of Na+ into the parasite following inhibition of PfATP4, occurring as a consequence of the higher extracellular [Na+] and resulting in increased toxicity of the inhibitor; and (ii) a (partially) protective effect of the higher osmolarity of the medium counteracting the cell swelling induced by the influx of Na+. According to this interpretation, the increase in parasite sensitivity to KAE609 resulting from the increased extracellular [Na+] is a stronger effect than the protective effect afforded by the hypertonic shrinkage.
In summary, PfATP4-associated compounds cause Na+-dependent swelling of isolated trophozoites as well as swelling of infected erythrocytes. Protecting the parasites from excessive Na+ influx by reducing the Na+ concentration in the growth medium, or protecting the parasite from swelling by increasing the osmolarity of the medium, reduces the efficacy of KAE609. The data are consistent with parasite swelling contributing to, but not playing an essential role in, the inhibition of parasite growth by this new and highly promising class of antimalarials.
MATERIALS AND METHODS
Parasite lines and culture.
The 3D7 and Dd2 strains of P. falciparum were maintained in continuous shaking culture (35) in RPMI 1640 medium (Gibco) supplemented with 3 g/liter Albumax II, 25 mM HEPES, 11 mM glucose (final concentration, 22 mM), 200 μM hypoxanthine, and 20 μg/ml gentamicin sulfate. The supplemented RPMI 1640 medium (see Table S1 in the supplemental material) had an osmolarity of 290 ± 3 mosM and a pH of 7.3. Parasites were synchronized using sorbitol (5% [wt/vol]) (36) at least once, and in some cases twice, in the 1 to 5 days prior to experimentation.
Compounds.
KAE609 was supplied by MMV and PA21A050 by Akhil Vaidya. A selection of the PfATP4-associated antiplasmodial compounds present in the MMV Malaria Box (MMV011567, MMV006656, MMV006764, MMV665826, and MMV665949) were sourced either from MMV or from Princeton BioMolecular Research. Compound details can be found at the ChEMBL-Neglected Tropical Disease website (https://www.ebi.ac.uk/chemblntd). Artemisinin and chloroquine were purchased from Sigma.
All compounds were dissolved in dimethyl sulfoxide (DMSO) at 1,000× the concentration required in the assays, resulting in a final DMSO concentration of 0.1% (vol/vol) in the cell suspensions following addition of the compounds. In all experiments in which compounds were added to cell suspensions, the corresponding volume of DMSO was added to an equivalent suspension as a solvent control. The stock solutions of compounds in DMSO were stored at −20°C in small volumes (typically 3 to 6 μl) and were thawed only once before use in an assay (i.e., there was no repeated freeze-thawing of stock solutions).
Preparation of infected erythrocytes, uninfected erythrocytes, and isolated parasites.
The ages (postinvasion) of trophozoite-stage parasites used for experimentation fell within the range of 24 to 44 h. Parasites were prepared for experimentation when the majority were at the mature trophozoite stage (median age, approximately 36 to 40 h) as determined by inspection of Giemsa-stained smears.
Parasitized erythrocytes were purified from the culture by use of a VarioMACS CS column (Miltenyi Biotech) as described previously (37). Parasitized cell suspensions (typically 4% hematocrit and 5% parasitemia) were centrifuged and suspended in supplement-free RPMI 1640 medium prior to passage through the VarioMACS CS column. Parasitized erythrocytes were eluted from the column by use of supplement-free RPMI 1640 medium. Most of the uninfected erythrocytes were discarded; however, some were collected for use as a control group and termed “cohort uninfected erythrocytes.”
The purified infected erythrocytes (≥95% parasitemia) and cohort uninfected erythrocytes were washed once with bicarbonate-free, supplement-free RPMI 1640 medium and in most cases resuspended in either bicarbonate-free supplemented RPMI 1640 medium (i.e., supplemented RPMI 1640 medium without NaHCO3 and gentamicin; 283 ± 2 mosM; pH adjusted to 7.4 by using NaOH) (Table S1) or physiological saline (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 20 mM glucose, 25 mM HEPES free acid; 310 ± 2 mosM; pH adjusted to 7.4 by using NaOH) (Table S2). The parasitized and cohort uninfected erythrocytes were incubated in one or other of these media, as appropriate, at 37°C for 15 to 20 min prior to the start of each experiment.
Trophozoite-stage parasites were functionally isolated from their host erythrocytes by brief (15 to 20 s) exposure of a parasitized cell suspension (typically 4% hematocrit and 5% parasitemia) to 0.05% (wt/vol) saponin (equating to at least 0.005% [wt/vol] of the active ingredient sapogenin) (38). Saponin permeabilizes the host cell and parasitophorous vacuole membranes while leaving the parasite plasma membrane intact (39) and able to maintain transmembrane ion gradients (14, 15, 40, 41). Except where specified otherwise, the isolated parasites were washed and resuspended in physiological saline (pH adjusted to 7.1 by using NaOH) and then incubated at 37°C for 15 to 20 min to allow parasites to recover from the saponin isolation procedure prior to beginning each experiment.
For experiments in which isolated parasites were suspended in a Na+-free saline, the Na+ was replaced with an equimolar amount of either N-methyl-d-glucamine (NMDG) or choline. The NMDG-Cl- and choline-based saline media were pH adjusted to 7.1 by using HCl and NMDG-Cl, respectively (Table S2).
For experiments in which the cholesterol content of the parasite was modulated (Fig. S4), isolated parasites were incubated at 37°C for 30 min in bicarbonate-free, Albumax-free supplemented RPMI 1640 medium (i.e., supplemented RPMI 1640 medium without NaHCO3, gentamicin sulfate, or Albumax II; 288 ± 2 mosM; pH adjusted to 7.1 by using NaOH) (Table S1) or in the same medium supplemented with either the cholesterol-chelating compound methyl-β-cyclodextrin (MβCD; 5 mM) or MβCD loaded with cholesterol (MβCD-cholesterol; 5 mM), based on the method of Das and colleagues (18). Incubation of parasites in the presence of MβCD removes the cholesterol from the plasma membranes, whereas incubation in the presence of MβCD-cholesterol does not (18). MβCD was loaded with cholesterol at a 5:1 ratio of MβCD to cholesterol, as described previously (42). Following 30 min of incubation, the isolated parasites were washed twice and resuspended in bicarbonate-free, Albumax II-free RPMI 1640 medium.
Cell volume measurements.
The volumes of infected and uninfected erythrocytes and isolated trophozoites were measured using a Beckman Coulter Multisizer 4 (MS4) instrument with a 100-μm aperture tube.
For accurate cell volume measurements, the electrical conductivities of the electrolyte solution within the aperture tube and the medium in which the cells are suspended should be the same (43). For volume measurements of infected erythrocytes and cohort uninfected erythrocytes, the electrolyte solution within the aperture tube was bicarbonate-free, supplement-free RPMI 1640 medium. For measurements of isolated trophozoites, the electrolyte solution within the aperture tube was either glucose-free physiological saline or bicarbonate-free, supplement-free RPMI 1640 medium.
The Beckman Coulter MS4 instrument and its internal electrolyte solution were maintained at 25°C. For all cell volume measurements, cells were suspended at approximately 2 × 107 to 4 × 107 cells/ml and maintained at 37°C. Immediately prior to volume measurement, an aliquot of the suspension was transferred to a cuvette and diluted to approximately 4 × 105 cells/ml. For each volume measurement, approximately 20,000 pulses (with each pulse corresponding to the transit of a single cell through the aperture) were recorded. Unless stated otherwise, the resulting cell population volume distribution data were fitted to a log Gaussian distribution and the median volume of the cells in each sample thereby determined (Fig. 1a). The median volume (rather than the mean volume) of the cells was used in order to minimize the impact of debris particles (typically <10 fl and >200 fl) on the measurement.
In a control experiment, it was shown that an uninfected erythrocyte population lysed by exposure to saponin (thereby generating erythrocyte “ghosts”) did not give rise to a detectable signal.
In one series of experiments, the volumes of saponin-isolated parasites suspended in saline media of various osmolarities were determined. Isolated trophozoites were suspended in a physiological saline (310 ± 2 mosM) for 20 min at 37°C, at which point an aliquot of hypertonic (1.5 M; 1,760 mosM) sucrose solution was added, increasing the osmolarity of the extracellular medium in increments of 31 mosM, up to a maximum of 465 mosM. A separate parasite suspension was prepared for each of the five different osmolarities tested. Parasite volume was measured immediately before and after the addition of the hypertonic sucrose solution.
A selection of the PfATP4-associated compounds identified previously in the MMV Malaria Box (17) were tested for their effects on parasite volume at concentrations 15× higher than their 50% inhibitory concentration (IC50) values for inhibition of proliferation of asexual Dd2 parasites in vitro (17). The concentrations tested were 4.9 μM for MMV011567, 2.2 μM for MMV006656, 6.7 μM for MMV006764, 4.2 μM for MMV665826, and 17 μM for MMV665949. Artemisinin and chloroquine were both tested at 100 nM, i.e., a concentration >15× higher than their IC50s for inhibition of proliferation of asexual 3D7 parasites (44, 45).
HPLC measurement of parasite Na+ and K+ contents.
The Na+ and K+ contents of isolated parasites were determined using high-performance liquid chromatography (HPLC), essentially as described previously (23). To generate the cell samples for HPLC analysis, saponin-isolated trophozoite-stage parasites were suspended in physiological saline at approximately 1 × 107 to 2 × 107 parasites/ml, with a total volume of at least 10 ml, and maintained at 37°C. KAE609 (10 nM) or 0.1% (vol/vol) DMSO was added to the cell suspension at 0 min, and 2-ml samples were taken at 0, 5, 10, 15, and 20 min. The cells in each 2-ml sample were washed twice in ice-cold Na+- and K+-free wash solution (150 mM magnesium acetate; 320 mosM; pH adjusted to 7.1 by using acetic acid and ammonium hydroxide) and then lysed in a Na+- and K+-free lysis solution (40% [vol/vol] 20 mM ammonium acetate, adjusted to pH 5 by using acetic acid and ammonium hydroxide; 60% [vol/vol] acetonitrile). The samples were centrifuged (16,000 × g, 10 min) to remove cellular debris, and 10 μl of each supernatant solution was transferred to a glass vial for HPLC analysis.
The possibility of mechanical cell loss occurring during the final two washes was investigated using the cell counting facility of the Coulter Multisizer MS4 instrument, using the same protocol as that used for cell volume measurement. The initial cell concentration was determined immediately prior to the addition of KAE609 (10 nM) or the DMSO solvent control. The final cell concentration was measured following incubation in the presence of KAE609 (or the solvent control) and two washes. The measurements revealed a loss of 19% ± 2% of the cells (mean ± SEM; n = 3) (data not shown) during the course of the final two washes, with no difference in cell loss between KAE609-exposed and DMSO-exposed parasite samples and no difference in cell loss between the samples taken at the 0-min and 20-min time points (for both KAE609-exposed and DMSO-exposed samples). The 19% lysis was taken into account in calculating the Na+ and K+ contents per cell.
Osmotic fragility assay.
The osmotic fragility of infected and uninfected erythrocytes was measured as described elsewhere (25). Magnet-purified parasitized erythrocytes and cohort uninfected erythrocytes were washed and suspended in bicarbonate-free supplemented RPMI 1640 medium (283 ± 2 mosM; pH adjusted to 7.4 by using NaOH) to which was added 10 nM KAE609, 100 nM chloroquine, or 0.1% (vol/vol) DMSO (solvent control). The cell suspensions were incubated at 37°C for 90 min, after which the erythrocytes were pelleted by centrifugation (500 × g, 5 min) and the majority of the supernatant solution removed. Aliquots (10 μl) of the packed erythrocytes were suspended in the wells of a round-bottomed 96-well plate with 250-μl aliquots of saline media having relative tonicities (RT) in the range of 0.04 to 1, prepared by blending the following two solutions in various proportions: (i) 150 mM NaCl, 2 mM HEPES-Na, pH 7.4 (RT = 1; 300 mosM); and (ii) 2 mM HEPES-Na, pH 7.4 (RT = 0.04; 12 mosM). The final RT of each sample was calculated based on the addition of 10 μl of packed erythrocytes suspended in bicarbonate-free supplemented RPMI 1640 medium (RT = 0.94) to 250 μl of saline with an RT of 0.04 to 1. The final cell concentration was approximately 1 × 108 cells/ml. The cell suspensions were incubated at room temperature for 10 min, and the plate was then centrifuged (1,200 × g, 5 min). A 175-μl aliquot of supernatant solution, containing hemoglobin from lysed cells, was transferred from each well to a flat-bottomed 96-well plate, and the absorbance at 415 nm (A415) was measured using a FLUOstar Optima plate reader to provide an estimate of the concentration of the released hemoglobin, and hence the extent of cell lysis. The % cell lysis was calculated by comparing the A415 for each sample to that measured in the supernatant solutions derived from the cells suspended in the lowest-RT saline (RT = 0.075), in which 100% of the cells underwent lysis, as confirmed by microscopy.
Parasite proliferation assays.
The effects of compounds on the proliferation of asexual 3D7 and Dd2 parasites were determined using the fluorescent DNA-intercalating dye (46) SYBR Safe to monitor parasite proliferation, essentially as described previously (47). Assays were performed over 72 h, commencing when the majority of parasites were in the ring stage. The starting parasitemia and hematocrit were both approximately 1%.
Low-Na+ medium (Table S1) was prepared as described by Pillai et al. (26) and supplemented with 5% (vol/vol) human serum, 25 mM HEPES free acid, and 200 μM hypoxanthine (330 ± 3 mosM; pH 7.4). In this medium, the human serum was the major source of Na+ (present at a final concentration of approximately 7 mM) (26). There was also a relatively small additional Na+ concentration (14.5 μM) introduced by the addition of RPMI 1640 vitamin solution (100×; Sigma-Aldrich). The proliferation of parasites in low-Na+ medium was compared to that of parasites in RPMI 1640 medium that had been prepared in the lab from individual components and supplemented with 5% (vol/vol) human serum, 25 mM HEPES free acid, and 200 μM hypoxanthine (330 ± 3 mosM; pH 7.4) (custom-made supplemented RPMI 1640 medium) (Table S1). The custom-made supplemented RPMI 1640 medium was used as a control medium for parasite proliferation because it matched the low-Na+ medium with regard to its method of preparation and its osmolarity (Table S1).
Proliferation of parasites suspended in high-Na+ RPMI 1640 medium (supplemented RPMI 1640 medium with 30 mM extra NaCl; 349 mosM) (Table S1) and sucrose RPMI 1640 medium (supplemented RPMI 1640 medium with 61 mM extra sucrose; 349 mosM) (Table S1) was compared to that of parasites in normal (commercial) supplemented RPMI 1640 medium.
Intracellular [Na+] measurements.
The [Na+]i value for isolated trophozoites was measured using the Na+-sensitive fluorescent dye Na+-binding benzofuran isophthalate (SBFI) essentially as described previously (14). Parasites were loaded with the acetoxymethyl (AM) ester of SBFI (SBFI-AM) and then suspended in either physiological saline (310 ± 3 mosM), physiological saline with an extra 30 mM NaCl (368 mosM), or physiological saline with an extra 61 mM sucrose (369 mosM) (Table S2) prior to measuring [Na+]i. The osmolarities of the Na+ calibration saline media, used to determine the relationship between [Na+]i and the fluorescence ratio, were matched to those of the saline media in which the parasites were suspended by the addition of sucrose.
Statistical analyses.
Unless stated otherwise, statistical comparisons were made using one-way analysis of variance (ANOVA). Differences between treatments were analyzed by the least significant difference, with statistical significance reported for P values of <0.05, using the Bonferroni correction for multiple comparisons. With this method, the significance threshold for individual comparisons is determined by dividing the experiment-wide P value (0.05 in this study) by the number of pairwise comparisons being performed. To prevent differences in initial cell volumes from eroding the precision of the test, “experiment” was nominated as a blocking factor in the ANOVAs.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Hwan-Jin Yoon and Teresa Neeman from the ANU Statistical Consulting Unit for helpful advice, to Markus Winterberg for assistance with the HPLC assays, to MMV and Akhil Vaidya for the provision of KAE609 and PA21A050, respectively, and to the Canberra Branch of the Australian Red Cross Blood Service for the provision of blood and human serum.
This work was supported by Project Grant 1042272 from the Australian National Health and Medical Research Council and by Australian Research Council Linkage Project Grant LP150101226. A.M.L. is the recipient of an Australian Research Council Discovery Early Career Researcher Award (grant DE160101035).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00087-18.
REFERENCES
- 1.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. 2010. Thousands of chemical starting points for antimalarial lead identification. Nature 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 3.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Sharlow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. 2010. Chemical genetics of Plasmodium falciparum. Nature 465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc Natl Acad Sci U S A 105:9059–9064. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez-Diaz MB, Ebert D, Salinas Y, Pradhan A, Lehane AM, Myrand-Lapierre ME, O'Loughlin KG, Shackleford DM, Justino de Almeida M, Carrillo AK, Clark JA, Dennis AS, Diep J, Deng X, Duffy S, Endsley AN, Fedewa G, Guiguemde WA, Gomez MG, Holbrook G, Horst J, Kim CC, Liu J, Lee MC, Matheny A, Martinez MS, Miller G, Rodriguez-Alejandre A, Sanz L, Sigal M, Spillman NJ, Stein PD, Wang Z, Zhu F, Waterson D, Knapp S, Shelat A, Avery VM, Fidock DA, Gamo FJ, Charman SA, Mirsalis JC, Ma H, Ferrer S, Kirk K, Angulo-Barturen I, Kyle DE, DeRisi JL, Floyd DM, Guy RK. 2014. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc Natl Acad Sci U S A 111:E5455–E5462. doi: 10.1073/pnas.1414221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidya AB, Morrisey JM, Zhang Z, Das S, Daly TM, Otto TD, Spillman NJ, Wyvratt M, Siegl P, Marfurt J, Wirjanata G, Sebayang BF, Price RN, Chatterjee A, Nagle A, Stasiak M, Charman SA, Angulo-Barturen I, Ferrer S, Belen Jimenez-Diaz M, Martinez MS, Gamo FJ, Avery VM, Ruecker A, Delves M, Kirk K, Berriman M, Kortagere S, Burrows J, Fan E, Bergman LW. 2014. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nat Commun 5:5521. doi: 10.1038/ncomms6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hameed PS, Solapure S, Patil V, Henrich PP, Magistrado PA, Bharath S, Murugan K, Viswanath P, Puttur J, Srivastava A, Bellale E, Panduga V, Shanbag G, Awasthy D, Landge S, Morayya S, Koushik K, Saralaya R, Raichurkar A, Rautela N, Roy Choudhury N, Ambady A, Nandishaiah R, Reddy J, Prabhakar KR, Menasinakai S, Rudrapatna S, Chatterji M, Jimenez-Diaz MB, Martinez MS, Sanz LM, Coburn-Flynn O, Fidock DA, Lukens AK, Wirth DF, Bandodkar B, Mukherjee K, McLaughlin RE, Waterson D, Rosenbrier-Ribeiro L, Hickling K, Balasubramanian V, Warner P, Hosagrahara V, Dudley A, Iyer PS, Narayanan S, Kavanagh S, Sambandamurthy VK. 2015. Triaminopyrimidine is a fast-killing and long-acting antimalarial clinical candidate. Nat Commun 6:6715. doi: 10.1038/ncomms7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golldack A, Henke B, Bergmann B, Wiechert M, Erler H, Blancke Soares A, Spielmann T, Beitz E. 2017. Substrate-analogous inhibitors exert antimalarial action by targeting the Plasmodium lactate transporter PfFNT at nanomolar scale. PLoS Pathog 13:e1006172. doi: 10.1371/journal.ppat.1006172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hapuarachchi SV, Cobbold SA, Shafik SH, Dennis AS, McConville MJ, Martin RE, Kirk K, Lehane AM. 2017. The malaria parasite's lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog 13:e1006180. doi: 10.1371/journal.ppat.1006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White NJ, Pukrittayakamee S, Phyo AP, Rueangweerayut R, Nosten F, Jittamala P, Jeeyapant A, Jain JP, Lefevre G, Li R, Magnusson B, Diagana TT, Leong FJ. 2014. Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med 371:403–410. doi: 10.1056/NEJMoa1315860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flannery EL, McNamara CW, Kim SW, Kato TS, Li F, Teng CH, Gagaring K, Manary MJ, Barboa R, Meister S, Kuhen K, Vinetz JM, Chatterjee AK, Winzeler EA. 2015. Mutations in the P-type cation-transporter ATPase 4, PfATP4, mediate resistance to both aminopyrazole and spiroindolone antimalarials. ACS Chem Biol 10:413–420. doi: 10.1021/cb500616x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt SN, Dranow DM, Horst BG, Abendroth JA, Forte B, Hallyburton I, Jansen C, Baragana B, Choi R, Rivas KL, Hulverson MA, Dumais M, Edwards TE, Lorimer DD, Fairlamb AH, Gray DW, Read KD, Lehane AM, Kirk K, Myler PJ, Wernimont A, Walpole C, Stacy R, Barrett LK, Gilbert IH, Van Voorhis WC. 2017. Biochemical and structural characterization of selective allosteric inhibitors of the Plasmodium falciparum drug target, prolyl-tRNA-synthetase. ACS Infect Dis 3:34–44. doi: 10.1021/acsinfecdis.6b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, Diagana TT, Kirk K. 2013. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13:227–237. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spillman NJ, Allen RJ, Kirk K. 2013. Na+ extrusion imposes an acid load on the intraerythrocytic malaria parasite. Mol Biochem Parasitol 189:1–4. doi: 10.1016/j.molbiopara.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Spillman NJ, Kirk K. 2015. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int J Parasitol Drugs Drug Resist 5:149–162. doi: 10.1016/j.ijpddr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehane AM, Ridgway MC, Baker E, Kirk K. 2014. Diverse chemotypes disrupt ion homeostasis in the malaria parasite. Mol Microbiol 94:327–339. doi: 10.1111/mmi.12765. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Bhatanagar S, Morrisey JM, Daly TM, Burns JM Jr, Coppens I, Vaidya AB. 2016. Na+ influx induced by new antimalarials causes rapid alterations in the cholesterol content and morphology of Plasmodium falciparum. PLoS Pathog 12:e1005647. doi: 10.1371/journal.ppat.1005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Suwanarusk R, Malleret B, Cooke BM, Nosten F, Lau YL, Dao M, Lim CT, Renia L, Tan KS, Russell B. 2016. A basis for rapid clearance of circulating ring-stage malaria parasites by the spiroindolone KAE609. J Infect Dis 213:100–104. doi: 10.1093/infdis/jiv358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. 2012. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother 56:3544–3548. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agca Y, Mullen S, Liu J, Johnson-Ward J, Gould K, Chan A, Critser J. 2005. Osmotic tolerance and membrane permeability characteristics of rhesus monkey (Macaca mulatta) spermatozoa. Cryobiology 51:1–14. doi: 10.1016/j.cryobiol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Vian AM, Higgins AZ. 2014. Membrane permeability of the human granulocyte to water, dimethyl sulfoxide, glycerol, propylene glycol and ethylene glycol. Cryobiology 68:35–42. doi: 10.1016/j.cryobiol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winterberg M, Kirk K. 2016. A high-sensitivity HPLC assay for measuring intracellular Na+ and K+ and its application to Plasmodium falciparum infected erythrocytes. Sci Rep 6:29241. doi: 10.1038/srep29241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staines HM, Rae C, Kirk K. 2000. Increased permeability of the malaria-infected erythrocyte to organic cations. Biochim Biophys Acta 1463:88–98. doi: 10.1016/S0005-2736(99)00187-X. [DOI] [PubMed] [Google Scholar]
- 25.Lew VL, Tiffert T, Ginsburg H. 2003. Excess hemoglobin digestion and the osmotic stability of Plasmodium falciparum-infected red blood cells. Blood 101:4189–4194. doi: 10.1182/blood-2002-08-2654. [DOI] [PubMed] [Google Scholar]
- 26.Pillai AD, Addo R, Sharma P, Nguitragool W, Srinivasan P, Desai SA. 2013. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling. Mol Microbiol 88:20–34. doi: 10.1111/mmi.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. 2004. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 351:1548–1563. [DOI] [PubMed] [Google Scholar]
- 28.Esposito A, Choimet JB, Skepper JN, Mauritz JM, Lew VL, Kaminski CF, Tiffert T. 2010. Quantitative imaging of human red blood cells infected with Plasmodium falciparum. Biophys J 99:953–960. doi: 10.1016/j.bpj.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldecker M, Dasanna AK, Lansche C, Linke M, Srismith S, Cyrklaff M, Sanchez CP, Schwarz US, Lanzer M. 2016. Differential time-dependent volumetric and surface area changes and delayed induction of new permeation pathways in P. falciparum-infected hemoglobinopathic erythrocytes. Cell Microbiol 19:e12650. doi: 10.1111/cmi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Yoon H, Diez-Silva M, Dao M, Dasari RR, Park Y. 2014. High-resolution three-dimensional imaging of red blood cells parasitized by Plasmodium falciparum and in situ hemozoin crystals using optical diffraction tomography. J Biomed Opt 19:011005. doi: 10.1117/1.JBO.19.1.011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanssen E, Knoechel C, Dearnley M, Dixon MW, Le Gros M, Larabell C, Tilley L. 2012. Soft X-ray microscopy analysis of cell volume and hemoglobin content in erythrocytes infected with asexual and sexual stages of Plasmodium falciparum. J Struct Biol 177:224–232. doi: 10.1016/j.jsb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serebrennikova YM, Patel J, Milhous WK, Garcia-Rubio LH. 2010. Quantitative analysis of morphological alterations in Plasmodium falciparum infected red blood cells through theoretical interpretation of spectral measurements. J Theor Biol 265:493–500. doi: 10.1016/j.jtbi.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 33.Elliott JL, Saliba KJ, Kirk K. 2001. Transport of lactate and pyruvate in the intraerythrocytic malaria parasite, Plasmodium falciparum. Biochem J 355:733–739. doi: 10.1042/bj3550733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saliba KJ, Horner HA, Kirk K. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J Biol Chem 273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- 35.Allen RJ, Kirk K. 2010. Plasmodium falciparum culture: the benefits of shaking. Mol Biochem Parasitol 169:63–65. doi: 10.1016/j.molbiopara.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 37.Teng RW, Junankar PR, Bubb WA, Rae C, Mercier P, Kirk K. 2009. Metabolite profiling of the intraerythrocytic malaria parasite Plasmodium falciparum by 1H NMR spectroscopy. NMR Biomed 22:292–302. doi: 10.1002/nbm.1323. [DOI] [PubMed] [Google Scholar]
- 38.Spillman NJ, Allen RJ, Kirk K. 2008. Acid extrusion from the intraerythrocytic malaria parasite is not via a Na+/H+ exchanger. Mol Biochem Parasitol 162:96–99. doi: 10.1016/j.molbiopara.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Ansorge I, Paprotka K, Bhakdi S, Lingelbach K. 1997. Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol Biochem Parasitol 84:259–261. doi: 10.1016/S0166-6851(96)02806-X. [DOI] [PubMed] [Google Scholar]
- 40.Alleva LM, Kirk K. 2001. Calcium regulation in the intraerythrocytic malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 117:121–128. doi: 10.1016/S0166-6851(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 41.Saliba KJ, Kirk K. 1999. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H+ extrusion via a V-type H+-ATPase. J Biol Chem 274:33213–33219. [DOI] [PubMed] [Google Scholar]
- 42.Caliceti C, Zambonin L, Prata C, Vieceli Dalla Sega F, Hakim G, Hrelia S, Fiorentini D. 2012. Effect of plasma membrane cholesterol depletion on glucose transport regulation in leukemia cells. PLoS One 7:e41246. doi: 10.1371/journal.pone.0041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryan AK, Engler A, Gulati A, Manalis SR. 2012. Continuous and long-term volume measurements with a commercial Coulter counter. PLoS One 7:e29866. doi: 10.1371/journal.pone.0029866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya A, Mishra LC, Bhasin VK. 2008. In vitro activity of artemisinin in combination with clotrimazole or heat-treated amphotericin B against Plasmodium falciparum. Am J Trop Med Hyg 78:721–728. [PubMed] [Google Scholar]
- 45.Lehane AM, van Schalkwyk DA, Valderramos SG, Fidock DA, Kirk K. 2011. Differential drug efflux or accumulation does not explain variation in the chloroquine response of Plasmodium falciparum strains expressing the same isoform of mutant PfCRT. Antimicrob Agents Chemother 55:2310–2318. doi: 10.1128/AAC.01167-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spry C, Macuamule C, Lin Z, Virga KG, Lee RE, Strauss E, Saliba KJ. 2013. Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS One 8:e54974. doi: 10.1371/journal.pone.0054974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.