ABSTRACT
Studies reporting an increased risk for cardiac toxicities with macrolide antibiotics have raised concern regarding their cardiovascular safety. We sought to assess the cardiac safety of macrolide antibiotics as a class and of the individual agents by conducting a systematic review and network meta-analysis. Medline, Embase, and the Cochrane Library were searched up to February 2018 for studies reporting on cardiovascular outcomes with macrolides. We followed the PRISMA 2009 guidelines for data selection and extraction. Outcomes were pooled using random-effects models and odds ratios (OR), and 95% confidence intervals (CI) were calculated for arrhythmia, cardiovascular death, and myocardial infarction (MI). A total of 33 studies and data on 22,601,032 subjects were retrieved and included in the current meta-analyses. Macrolide use was not associated with the risk of arrhythmia or cardiovascular mortality. In the primary analysis, macrolide use was associated with a small but statistically significant 15% increase in risk for MI (OR = 1.15 [95% CI, 1.01 to 1.30]). In indirect network meta-analysis, erythromycin and clarithromycin were ranked considerably more likely to be associated with a higher risk for MI and significantly associated with increased risk of MI compared to azithromycin (OR = 1.58 [95% CI, 1.18 to 2.11] and OR = 1.41 [95% CI, 1.11 to 1.81], respectively). Our findings indicate that macrolide antibiotics as a group are associated with a significant risk for MI but not for arrhythmia and cardiovascular mortality. Among the macrolides, erythromycin and clarithromycin were associated with a greater risk of MI. However, it is possible that the association between macrolide use and risk of MI is the result of residual confounding.
KEYWORDS: arrhythmia, azithromycin, cardiac arrest, cardiac death, cardiovascular, clarithromycin, erythromycin, macrolide, mortality, myocardial infarction, roxithromycin, stroke, torsades de pointes, ventricular tachyarrhythmia
INTRODUCTION
Macrolides are a widely prescribed class of antibiotics, used to treat many common infections, and are considered overall safe, with little risk for severe adverse outcomes. However, in recent years several studies have reported an association between macrolides and cardiotoxicity (1, 2). Reported adverse cardiac outcomes with macrolides include QT interval prolongation, torsades de pointes (TdP), ventricular tachycardia, and sudden cardiac death (1–3).
In previous years, azithromycin was considered relatively free from cardiac toxic effects (4). However, it has since been associated with the risk of cardiovascular death (5). Following these reports, the Food and Drug Administration (FDA) issued a safety communication regarding the risk of potentially fatal arrhythmias with azithromycin (6). In addition to the immediate adverse reactions, a recent FDA communication raised concerns regarding the long-term cardiovascular risk of clarithromycin (7).
Previous meta-analyses assessing the cardiovascular risks of macrolides reported conflicting results (3, 8, 9). The first, performed by Cheng et al., found an association between an increased risk for developing sudden cardiac death (SCD) or ventricular tachyarrhythmia (TVA) (3). The second meta-analysis, conducted by Li et al., did not find an association between macrolides and an increased risk for cardiac death compared with nonmacrolide regimens; however, such an association was found in a subgroup of individuals older than 48 years (8). Importantly, none of the published meta-analyses compared individual macrolides (namely, erythromycin, azithromycin, clarithromycin, and roxithromycin) with respect to their relative risk for cardiac toxicity.
Following these meta-analyses, several additional studies have reported cardiovascular outcomes with macrolide antibiotics (10–14, 16, 43).
In light of the conflicting reports and the publication of many additional studies not included in previous meta-analyses, we aimed to perform a systematic review and a network meta-analysis to update the current knowledge regarding the cardiac safety of macrolides as a class, including the risk of arrhythmia, cardiovascular mortality, and myocardial infarction (MI), and to determine the cardiac safety of the specific macrolides.
RESULTS
Description of the selected studies.
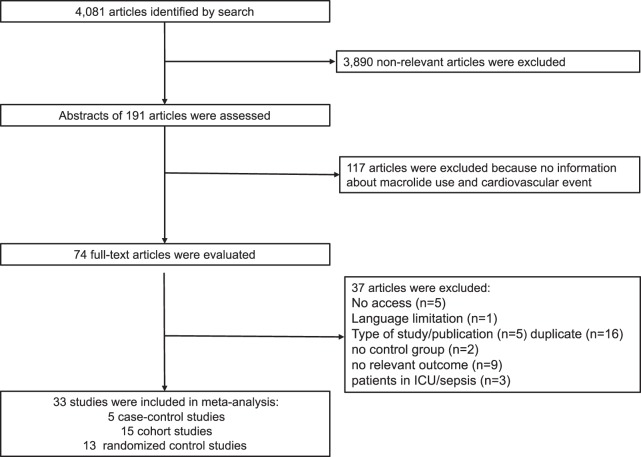
Our search yielded 4,081 records for evaluation. Records were screened for inclusion by title, resulting in 191 potentially relevant records, which were further evaluated by abstract. After exclusion of irrelevant abstracts, 74 articles were selected for full-text evaluation. The macrolide agent most commonly reported in these studies was azithromycin (17 studies), and the comparator group was most commonly a penicillin-based antibiotic (for observational studies) or a placebo (for randomized controlled trials [RCTs]). Additional characteristics of the included studies are listed in Table S1 in the supplemental material. Thirty-three articles were included in the analysis: 13 RCTs, 15 cohort studies, and 5 case-control studies. The search process is illustrated in Fig. 1. The observational trials' quality assessments by the Newcastle-Ottawa quality assessment scale (NOS) varied from 6 to 9 stars, and the overall risk of bias in the included RCTs was low (see Tables S5 and S6 in the supplemental material).
FIG 1.
Selection process, including the numbers of articles retrieved, screened, and selected for quantitative analysis.
Meta-analysis. (i) Short-term risk for arrhythmia.
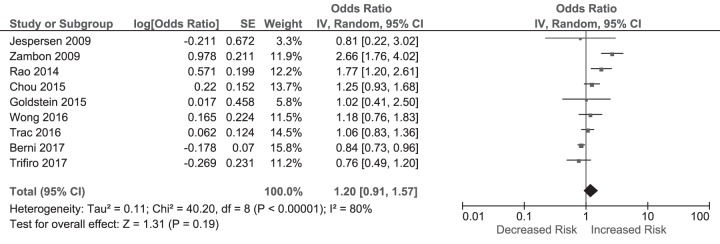
Eight studies (1 RCT, 5 cohort studies, 2 case-control studies; the summaries of these studies are illustrated in Table S4 in the supplemental material) evaluated the risk for short-term arrhythmia after exposure to macrolides (11, 12, 14, 16–21). Using a random-effects model, macrolide use was not associated with an increased risk for short-term arrhythmia (OR, 1.20 [95% CI, 0.91 to 1.57]) with high heterogeneity between the studies (P for heterogeneity < 0.00001; I2 = 80%) (Fig. 2).
FIG 2.
Odds ratios for arrhythmia in macrolide users versus nonusers. The forest plot demonstrates point estimates of risk ratios surrounded by 95% CI calculated by a random-effects model.
Thirty-day cardiovascular mortality.
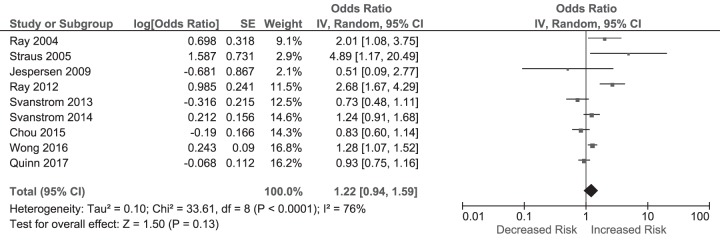
The pooled effect of nine studies (1 RCT, 6 cohort, 2 case-control; summaries of these studies are illustrated in Table S5 in the supplemental material) (1, 5, 11, 13, 18, 21, 24–26) reporting short-term cardiovascular mortality revealed no increase in mortality among macrolide users (OR, 1.22 [95% CI, 0.94 to 1.59]) in a random-effects model with high heterogeneity between the studies (P for heterogeneity < 0.0001; I2 = 76%) (Fig. 3).
FIG 3.
Odds ratios for short-term cardiovascular mortality in macrolide users versus nonusers. The forest plot demonstrates point estimates of risk ratios surrounded by 95% CI calculated by a random-effects model.
Myocardial infarction.
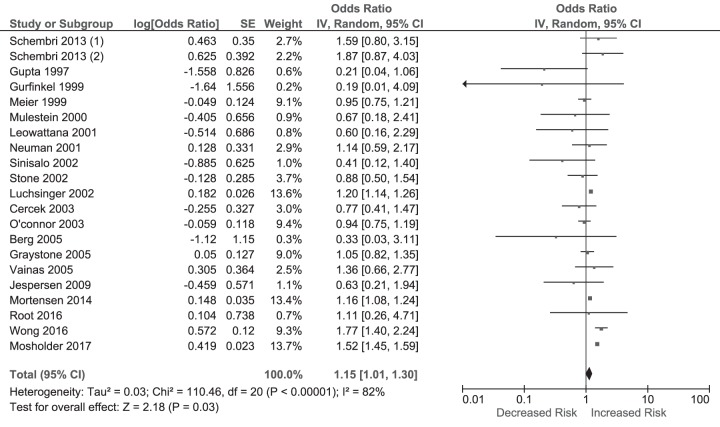
A pooled analysis of 20 studies (13 RCTs, 7 cohort, 1 case-control; summaries of these studies are illustrated in Table S6 in the supplemental material) (10, 11, 21, 27–43) reporting on the risk for MI following macrolide therapy demonstrated a significant 15% increase in risk in the macrolide group (OR = 1.15 [95% CI, 1.01 to 1.30], P < 0.00001; I2 = 82%) (Fig. 4).
FIG 4.
Odds ratios for MI in macrolide users versus nonusers. The forest plot demonstrates point estimates of risk ratios surrounded by 95% CI calculated by a random-effects model.
Network meta-analysis of risk of myocardial infarction.
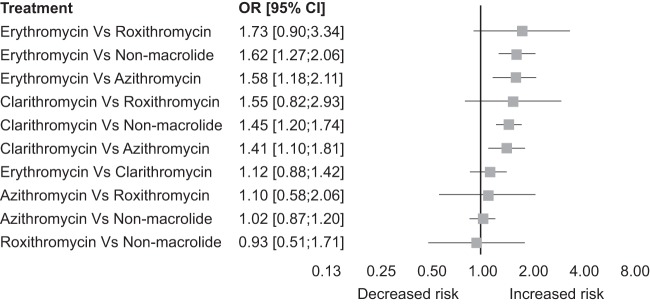
In network meta-analysis (see the network plot in Fig. S1 in the supplemental material) evaluating the relative risk of MI with individual agents, erythromycin and clarithromycin ranked most likely to have the highest risk for MI (P scores [see Materials and Methods], 0.94 and 0.77, respectively [Table 1]). Erythromycin and clarithromycin were associated with a significantly higher risk for MI compared to azithromycin (OR = 1.58 [95% CI, 1.18 to 2.11], and OR = 1.41 [95% CI, 1.11 to 1.81], respectively) and compared to nonmacrolide use (OR = 1.62 [95% CI, 1.27 to 2.1], and OR = 1.45 [95% CI, 1.20 to 1.74] respectively) (Fig. 5). The results of the comparative analysis for MI risk comparing these agents to roxithromycin were not conclusive.
TABLE 1.
Treatment ranked by probability of highest risk of MI
| Medication | P scorea |
|---|---|
| Erythromycin | 0.94 |
| Clarithromycin | 0.77 |
| Azithromycin | 0.31 |
| Nonmacrolide | 0.24 |
| Roxithromycin | 0.23 |
The P score is derived from network meta-analysis and represents the mean extent of certainty that a given treatment has greater risk of MI among the included treatments, measured on a scale from 0 (best) to 1 (worst).
FIG 5.
Comparative odds ratio for MI in different macrolides. The forest plot demonstrates point estimates of risk ratios surrounded by 95% CI calculated by a random-effects model.
Subgroup analysis.
In subgroup analysis, restricting the analysis of the risk of MI with macrolides to RCTs, macrolide use was not associated with an increased risk for MI: the pooled effect showed an OR of 0.95 [95% CI, 0.82 to 1.09] with low heterogeneity among the studies (P = 0.6, I2 = 0%). A small but significant increased risk was observed in the subgroup analysis of observational studies (OR = 1.31 [95% CI, 1.14 to 1.52]) with high heterogeneity among the studies (P < 0.001, I2 = 91%).
Publication bias.
Visual inspection of the funnel plots suggested some asymmetry in favor of macrolides and risk of MI; however, Egger's regression test did not reach significance for publication bias in any of the analyses (MI analysis, P = 0.06; arrhythmia, P = 0.25; cardiovascular mortality, P = 0.33) (see Fig. S2 to S4 in the supplemental material).
DISCUSSION
Our meta-analysis found that macrolide use was not associated with the risk of arrhythmia or cardiovascular mortality. In the primary analysis, macrolide use was associated with a small but statistically significant 15% increase in risk for MI (OR = 1.15 [95% CI, 1.01 to 1.30]). In network meta-analysis, erythromycin and clarithromycin ranked most likely to have the highest risk for MI among the treatments included and were associated with a significantly higher risk for MI than azithromycin and nonmacrolide treatment.
The absence of an association between macrolide antibiotics and arrhythmia or cardiovascular death contrasts with earlier reports that raised alarm regarding the cardiovascular safety of macrolides (5, 20). The mechanism suggested by studies reporting increased risk of arrhythmia or cardiovascular death is prolongation of the QT interval and possible increased risk for TdP due to macrolide interference with the delayed rectifier potassium current (IKr), accumulation of potassium ions in cardiac myocytes, and delayed cardiac repolarization (2, 44). However, even though several macrolides have been documented to prolong QT intervals, our results suggest that their use is not associated with significant clinically related outcomes such as arrhythmia or cardiac mortality. While our results provide reassurance regarding the overall safety of macrolides, we were not able to evaluate whether the risk for arrhythmia may be greater in subpopulations with more advanced age and comorbidity, as some studies have suggested.
Our analysis did find a small but significant association between macrolides and MI, and this risk was more pronounced with clarithromycin and erythromycin use than with other macrolides. This finding is compatible with the recent FDA communication regarding long-term cardiovascular risks with clarithromycin (7). While the association that we detected regarding the risk of MI contrasts with the aforementioned absence of increased cardiovascular death, it should be noted that almost all studies measured and reported only short-term mortality (up to 30 days after the first day of treatment with macrolide), while studies reporting risk for MI provided much longer follow-up.
As various macrolide agents differ from each other in terms of their pharmacokinetic characteristics, drug-drug interactions, and immunomodulatory effects (45, 46), differences in the safety profiles of the different macrolides can be expected. The differences in the risk for MI between macrolides may be explained by the differences in pharmacokinetic characteristics and immunomodulatory effects of the macrolide antibiotics (47). For instance, clarithromycin is a potent inhibitor of cytochrome P450 3A4 and therefore has the potential for many clinically important drug-drug interactions, whereas azithromycin has a lower potential for drug-drug interactions (48). Additionally, treatment with clarithromycin is usually of longer duration than that with azithromycin. However, it should also be noted that the antibiotic indication was not specified in some of the studies, and it is therefore not possible to rule out indication bias for clarithromycin users. In addition, residual confounding from other unmeasured variables may explain the greater risk observed for clarithromycin and erythromycin.
Importantly, the mechanism behind the link between macrolides and MI is unclear. Studies reporting this association have suggested that macrolides might induce MI via activation of inflammatory macrophages (30). Such an activation could lead to accumulation of lipids and to more-vulnerable plaques in coronary arteries, a continuous process that may leave the plaques permanently weakened. However, this explanation is at odds with anti-inflammatory effects documented in the treatment of respiratory diseases (49). In addition, our subanalyses found that this association was evident only in observational studies (OR = 1.31 [95% CI, 1.14 to 1.52]), and no evidence of increased risk of MI was observed in RCTs. This may reflect the ability of long-term studies in “real-world” settings to detect adverse effects not reported in randomized studies; however, it may also suggest that the association may be the result of residual confounding, often inherent to observational study design. In RCTs, the randomization (if performed correctly) provides assurance that unmeasured confounders are equally distributed between the intervention group and the control group. However, in observational studies, differences in the prevalence of potentially confounding factors between study groups are often present and sometimes difficult to account for. In addition, differences in potential confounders may persist even following rigorous attempts to control for all measured potential confounders, as residual confounding may remain due to unmeasured, or incompletely measured, variables. In addition, the difference between the RCTs and the observational studies may be the result of true differences in effects among the populations included in these studies. The RCTs assessed cardiovascular outcomes among participants with cardiovascular disease, while the observational studies assessed these outcomes in the general population. It is possible that macrolides are a less meaningful risk factor for cardiovascular outcomes in participants who already suffer from cardiovascular disease. Finally, it should also be noted that our analysis of MI risk may be an underestimate of macrolide-associated cardiovascular risk. Our assessment of the studies suggested the presence of publication bias, whereby smaller studies reporting on MI were more likely to be published when they favored macrolides; however, this was not statistically significant (P = 0.06).
Our study has several strengths. First, this review and analysis updates the current knowledge regarding macrolide cardiovascular safety according to recently published data. Second, the large number of participants with worldwide coverage using raw data improves the generalizability of the study. Third, we performed a systematic assessment of study quality, subgroup analyses, and evaluation of publication bias. Additionally, to our knowledge, we are the first to conduct an indirect comparison using a network meta-analysis to assess differences between macrolides regarding the risk of MI.
Our analysis also has a number of important limitations. First, the potential of confounding by indication is a possible limitation of the observational studies included in our analysis. We performed subgroup analyses by study design to evaluate the potential impact of study design and excluded articles involving populations with HIV or sepsis and intensive care unit (ICU) patients to reduce heterogeneity and confounding arising from significant differences in disease severity. Second, we detected considerable heterogeneity among the trials included in the analyses. This heterogeneity can be attributed to different indications of antibiotics, coexisting conditions, and participants' age. We used random-effects models in our computations in order to account for the pooling of effect sizes arising from different populations. Third, we had no information regarding important lifestyle factors that influence patients' cardiovascular risk, such as concomitant medications, comorbid conditions, compliance, and antibiotics regimens in many of the studies, and hence we could not rule out the possibility for residual confounding. Furthermore, the diagnosis of arrhythmia is likely not well documented in the medical and administrative databases used in many of the observational studies, and therefore we believe there may be room for additional prospective studies. Lastly, roxithromycin is not marketed in the United States and only a small number of studies examining roxithromycin safety have been published. The paucity of studies examining roxithromycin use did not allow for conclusive results regarding roxithromycin cardiac risk, and further studies are needed to evaluate the cardiac safety of roxithromycin.
While our study supports the relative short-term cardiovascular safety of macrolides in the general population, we could not rule out a potential risk for specific patient subgroups such as those using interacting drugs or those with preexisting cardiovascular morbidity, since we did not address this population in this study.
Conclusions.
Our findings indicate that macrolide antibiotics are likely not associated with a significant risk for arrhythmia and cardiovascular mortality. However, we identified a small but significant association between macrolide use and risk of MI, which was more pronounced with clarithromycin and erythromycin. While this link may be the result of residual confounding, it warrants further investigation, especially with regard to the long-term cardiovascular safety of erythromycin and clarithromycin. Due to the widespread use of macrolides, the findings that we describe in this paper are relevant to practicing health care providers, as well as to clinical researchers.
MATERIALS AND METHODS
Data source and searches.
This systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-analysis framework guidelines (PRISMA 2009) (50). The systematic review was performed by searching all publications indexed in Medline, Embase, The Cochrane Library, and The Clinical Trials Registry (clinicaltrials.gov) up to February 2018, reporting the outcomes of randomized controlled trials (RCTs), cohort studies, and case-control studies that investigated the association between the macrolide treatment and the risk of cardiovascular events and cardiovascular mortality. No language or date restrictions were applied in the search. Searches were performed using medical subject headings (MeSH) terms and free keywords, including erythromycin, clarithromycin, azithromycin, roxithromycin, macrolide, cardiovascular, cardiac, heart, arrest, death, mortality, tachycardia, ventricular, tachyarrhythmia, arrhythmia, torsades de pointes, myocardial infarction, stroke. A manual search of reference lists of review articles and original studies was performed to identify additional reports. The protocol was registered in the PROSPERO registry (registration number CRD42017080652).
Data extraction and quality assessment.
The data were extracted by two independent reviewers (E.G. and R.M.). Disagreements were resolved through consensus or referral to a third reviewer (I.M.) when necessary. Data were extracted for the following characteristics: study details (identifier, study design, geographical location, publication year, duration of follow-up), participants' details (number of participants, study population, age, and gender), intervention characteristics (drug name, dosage regimen), comparator intervention characteristics, primary outcomes, and covariate adjustments.
The quality of the observational studies was assessed using the Newcastle-Ottawa quality assessment Scale (NOS) scoring (51). We considered studies with a NOS score of seven and more to be high-quality studies. We used the Cochrane tool for assessing risk of bias for randomized control trials (Review Manager [RevMan], version 5.3., The Nordic Cochrane Centre, The Cochrane Collaboration, 2014; Copenhagen, Denmark) (52).
Selection criteria.
Published studies were considered eligible if they reported on the risk of cardiovascular events in macrolide users versus nonmacrolide users. Cardiovascular events of interest included arrhythmia, 30-day cardiovascular mortality, and MI. We included observational studies and RCTs. When the results of a particular study were reported in more than one publication, the most informative and recent publication was included in the meta-analysis.
Exclusion criteria.
Case reports, case series, pharmacokinetic studies in healthy adults, reviews, expert opinion, editorials, letters to the editor, and commentaries were excluded.
Articles were excluded from the analysis if they had insufficient published data for determining an estimate of risk ratio (RR) or odds ratio (OR) and confidence interval (CI). We excluded articles involving populations with HIV or sepsis and ICU patients, to reduce the potential for confounding by indication.
Outcomes.
Primary outcomes were (i) short-term arrhythmia (up to 30 days from last use of macrolides), (ii) short-term cardiovascular mortality (up to 30 days from the last use of macrolides), and (iii) myocardial infarction following macrolide use (anytime during follow-up).
Statistical analysis.
Raw data from individual studies were extracted, and the pooled ORs and 95% confidence intervals (CI) were calculated for each of the dichotomous outcomes. The heterogeneity of the data was quantified by the Q statistic in combination with the I2 statistic. High heterogeneity was considered significant when P was <0.1 for the Q statistic or when the I2 was >50%. For substantial and considerable heterogeneity, we used DerSimonian and Laird random-effects models. This method provides a summary measure of effects observed in the studies while accounting for between-study variations related to specific study characteristics. Study weights calculated using this method are based on a combination of the variance of the study estimate (which is a function of study size and number of events) and the heterogeneity in the estimates between studies. Publication bias was estimated visually by funnel plots and with Egger's regression test to measure funnel plot asymmetry. These analyses were conducted using CMA (comprehensive meta-analysis) software version 3, and graphs were generated using Review Manager (RevMan), version 5.3.
In addition, for cardiovascular outcomes found to be associated with cardiovascular risk, we performed a network meta-analysis to pool direct and indirect comparisons between specific macrolide agents to provide an estimate of their relative cardiovascular risk. Additionally, the risk of macrolides with the various agents was ranked using P scores derived from network point estimates and standard errors. The P score of a treatment in this analysis can be interpreted as the mean extent of certainty that the treatment has greater risk of MI among the included treatments, measured on a scale from 1 (worst) to 0 (best) (53). These analyses were performed using the package “netmeta” within the R environment (54).
Lastly, we performed a subgroup analysis restricting the analysis of the risk of MI with macrolides to RCTs and observational studies, separately.
Supplementary Material
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00438-18.
REFERENCES
- 1.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. 2004. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med 351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 2.Abo-Salem E, Fowler JC, Attari M, Cox CD, Perez-Verdia A, Panikkath R, Nugent K. 2014. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther 32:19–25. doi: 10.1111/1755-5922.12054. [DOI] [PubMed] [Google Scholar]
- 3.Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, Mei WY, Liu LJ, Long M, Yao FJ, Liu J, Liao XX, Du ZM, Dong YG, Ma H, Xiao HP, Wu SH. 2015. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 66:2173–2184. doi: 10.1016/j.jacc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Owens RC, Nolin TD. 2006. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis 43:1603–1611. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 5.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. 2012. Azithromycin and the risk of cardiovascular death. N Engl J Med 366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration (FDA). 2013. FDA drug safety communication: azithromycin (zithromax or zmax) and the risk of potentially fatal heart rhythms. US Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 7.US Food and Drug Administration (FDA). 2018. FDA drug safety communication: FDA review finds additional data supports the potential for increased long-term risks with antibiotic clarithromycin (Biaxin) in patients with heart disease. US Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 8.Li X, Wang M, Liu G, Ma J, Li C. 2016. Association of macrolides with overall mortality and cardiac death among patients with various infections: a meta-analysis. Eur J Intern Med 28:32–37. doi: 10.1016/j.ejim.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Wong AYS, Chan EW, Anand S, Worsley AJ, Wong ICK. 2017. Managing cardiovascular risk of macrolides: systematic review and meta-analysis. Drug Saf 40:663–677. doi: 10.1007/s40264-017-0533-2. [DOI] [PubMed] [Google Scholar]
- 10.Root AA, Wong AYS, Ghebremichael-Weldeselassie Y, Smeeth L, Bhaskaran K, Evans SJW, Brauer R, Wong ICK, Navaratnam V, Douglas I. 2016. Evaluation of the risk of cardiovascular events with clarithromycin using both propensity score and self-controlled study designs. Br J Clin Pharmacol 82:512–521. doi: 10.1111/bcp.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong AYS, Root A, Douglas IJ, Chui CSL, Chan EW, Ghebremichael-Weldeselassie Y, Siu C-W, Smeeth L, Wong ICK. 2016. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 352:h6926. doi: 10.1136/bmj.h6926. [DOI] [PubMed] [Google Scholar]
- 12.Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG, Garg AX. 2016. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 188:E120–E129. doi: 10.1503/cmaj.150901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn KL, Macdonald EM, Gomes T, Mamdani MM, Huang A, Juurlink DN, Canadian Drug Safety and Effectiveness Research Network (CDSERN). 2017. Macrolides, digoxin toxicity and the risk of sudden death: a population-based study. Drug Saf 40:835–840. doi: 10.1007/s40264-017-0539-9. [DOI] [PubMed] [Google Scholar]
- 14.Berni E, De Voogd H, Halcox JP, Butler CC, Bannister CA, Jenkins-jones S, Jones B, Ouwens M, Currie CJ. 2017. Risk of cardiovascular events, arrhythmia and all-cause mortality associated with clarithromycin versus alternative antibiotics prescribed for respiratory tract infections: a retrospective cohort study. BMJ Open 7(1):e013398. doi: 10.1136/bmjopen-2016-013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Schink T, Poluzzi E, Frøslev T, Molokhia M, Diemberger I. 2017. Use of azithromycin and risk of ventricular arrhythmia. CMAJ 189:E560–E568. doi: 10.1503/cmaj.160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zambon A, Polo Friz H, Contiero P, Corrao G. 2009. Effect of macrolide and fluoroquinolone antibacterials on the risk of ventricular arrhythmia and cardiac arrest: an observational study in Italy using case-control, case-crossover and case-time-control designs. Drug Saf 32:159–167. doi: 10.2165/00002018-200932020-00008. [DOI] [PubMed] [Google Scholar]
- 18.Chou HW, Wang JL, Chang CH, Lai CL, Lai MS, Chan KA. 2015. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis 60:566–577. doi: 10.1093/cid/ciu914. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein LH, Gabin A, Fawaz A, Freedberg NA, Schwartz N, Elias M, Saliba W. 2015. Azithromycin is not associated with QT prolongation in hospitalized patients with community-acquired pneumonia 1042–1048. Pharmacoepidemiol Drug Saf 24:1042–1048. doi: 10.1002/pds.3842. [DOI] [PubMed] [Google Scholar]
- 20.Rao GA, Mann JR, Shoaibi A, Bennett CL, Nahhas G, Sutton SS, Jacob S, Strayer SM. 2014. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med 12:121–127. doi: 10.1370/afm.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jespersen CM, Kolmos HJ, Frydendall N, Hilden J, Gluud C, Hansen JF. 2009. Compliance with and short-term adverse events from clarithromycin versus placebo in patients with stable coronary heart disease: the CLARICOR trial. J Antimicrob Chemother 64:411–415. doi: 10.1093/jac/dkp190. [DOI] [PubMed] [Google Scholar]
- 22.Reference deleted.
- 23.Reference deleted.
- 24.Svanström H, Pasternak B, Hviid A. 2013. Use of azithromycin and death from cardiovascular causes. N Engl J Med 368:1704–1712. doi: 10.1056/NEJMoa1300799. [DOI] [PubMed] [Google Scholar]
- 25.Svanström H, Pasternak B, Hviid A. 2014. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ 349:g4930. doi: 10.1136/bmj.g4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straus SMJM, Sturkenboom MCJM, Bleumink GS, Dieleman JP, Van Der Lei J, De Graeff PA, Kingma JH, Stricker BHC. 2005. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J 26:2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 27.Sinisalo J, Mattila K, Valtonen V, Anttonen O, Juvonen J, Melin J, Vuorinen-Markkola H, Nieminen MS. 2002. Effect of 3 months of antimicrobial treatment with clarithromycin in acute non-Q-wave coronary syndrome. Circulation 105:1555–1560. doi: 10.1161/01.CIR.0000012544.07696.1F. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen EM, Halm EA, Pugh MJ, Copeland LA, Metersky M, Fine MJ, Johnson CS, Alvarez CA, Frei CR, Good C, Restrepo MI, Downs JR, Anzueto A. 2014. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 311:2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luchsinger A, Pablos-me A, Knirsch C, Rabinowitz D, Shea S. 2002. Relation of antibiotic use to risk of myocardial infarction in the general population 89:18–21. [DOI] [PubMed] [Google Scholar]
- 30.Schembri S, Williamson PA, Short PM, Singanayagam A, Akram A, Taylor J, Singanayagam A, Hill AT, Chalmers JD. 2013. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ 346:f1235. doi: 10.1136/bmj.f1235. [DOI] [PubMed] [Google Scholar]
- 31.Meier CR, Derby LE, Jick SS, Vasilakis C, Jick H. 1999. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA 281:427–431. doi: 10.1001/jama.281.5.427. [DOI] [PubMed] [Google Scholar]
- 32.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C, ACES Investigators. 2005. Azithromycin for the secondary prevention of coronary events. N Engl J Med 352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 33.Gurfinkel E, Bozovich G, Beck E, Testa E, Livellara B, Mautner B. 1999. Treatment with the antibiotic roxithromycin in patients with acute non-Q-wave coronary syndromes. The final report of the ROXIS study. Eur Heart J 20:121–127. doi: 10.1053/euhj.1998.1283. [DOI] [PubMed] [Google Scholar]
- 34.Muhlestein JB, Anderson JL, Carlquist JF, Salunkhe K, Horne BD, Pearson RR, Bunch TJ, Allen A, Trehan S, Nielson C. 2000. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease: primary clinical results of the ACADEMIC study. Circulation 102:1755–1760. doi: 10.1161/01.CIR.102.15.1755. [DOI] [PubMed] [Google Scholar]
- 35.Leowattana W, Bhuripanyo K, Singhaviranon L, Akaniroj S, Mahanonda N, Samranthin M, Pokum S. 2001. Roxithromycin in prevention of acute coronary syndrome associated with Chlamydia pneumoniae infection: a randomized placebo controlled trial. J Med Assoc Thai 84(Suppl 3):S669–S675. [PubMed] [Google Scholar]
- 36.Vainas T, Stassen FRM, Schurink GWH, Tordoir JHM. 2005. Secondary prevention of atherosclerosis through chlamydia pneumoniae eradication (space trial): a randomised clinical trial in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 29:403–411. doi: 10.1016/j.ejvs.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Leatham EW, Carrington D, Mendall MA, Kaski JC, Camm AJ. 1997. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 96:404–407. doi: 10.1161/01.CIR.96.2.404. [DOI] [PubMed] [Google Scholar]
- 38.Stone AFM, Mendall MA, Kaski JC, Edger TM, Risley P, Poloniecki J, Camm AJ, Northfield TC. 2002. Effect of treatment for Chlamydia pneumoniae and Helicobacter pylori on markers of inflammation and cardiac events in patients with acute coronary syndromes: South Thames trial of antibiotics in myocardial infarction and unstable angina (STAMINA). Circulation 106:1219–1223. doi: 10.1161/01.CIR.0000027820.66786.CF. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, Benner RJ, Fisher MR, Cook TD. 2003. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA 290:1459–1466. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 40.Cercek B, Shah PK, Noc M, Zahger D, Zeymer U, Matetzky S, Maurer G, Mahrer P, AZACS Investigators. 2003. Effect of short-term treatment with azithromycin on recurrent ischaemic events in patients with acute coronary syndrome in the Azithromycin in Acute Coronary Syndrome (AZACS) trial: a randomised controlled trial. Lancet 361:809–813. doi: 10.1016/S0140-6736(03)12706-7. [DOI] [PubMed] [Google Scholar]
- 41.Berg HF, Maraha B, Scheffer G-J, Quarles-van Ufford M, Vandenbroucke-Grauls CMJE, Peeters MF, Kluytmans JAJW. 2005. Treatment with clarithromycin prior to coronary artery bypass graft surgery does not prevent subsequent cardiac events. Clin Infect Dis 40:358–365. doi: 10.1086/427111. [DOI] [PubMed] [Google Scholar]
- 42.Neumann F, Kastrati A, Miethke T, Pogatsa-Murray G, Mehilli J, Valina C, Jogethaei N, da Costa CP, Wagner H, Schomig A. 2001. Treatment of Chlamydia pneumoniae infection with roxithromycin and effect on neointima proliferation after coronary stent placement (ISAR-3): a randomised, double-blind, placebo-controlled trial. Lancet 357:2085–2089. doi: 10.1016/S0140-6736(00)05181-3. [DOI] [PubMed] [Google Scholar]
- 43.Mosholder AD, Lee J-Y, Zhou EH, Kang EM, Ghosh M, Izem R, Major JM, Graham DJ. 2017. Long-term risk of acute myocardial infarction, stroke and death with outpatient use of clarithromycin: a retrospective cohort study. Am J Epidemiol. [DOI] [PubMed] [Google Scholar]
- 44.Kannankeril P, Roden DM, Darbar D. 2010. Drug-induced long QT syndrome. Pharmacol Rev 62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kutlin A, Roblin PM, Hammerschlag MR. 2002. Effect of prolonged treatment with azithromycin, clarithromycin, or levofloxacin on Chlamydia pneumoniae in a continuous-infection model. Antimicrob Agents Chemother 46:409–412. doi: 10.1128/AAC.46.2.409-412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosnar M, Bos B, Marjanovic N, Glojnaric I, Ognjen C, Parnham MJ, Erakovic V. 2009. Azithromycin and clarithromycin inhibit lipopolysaccharide-induced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte-macrophage colony-stimulating factor and interleukin-1β. J Pharmacol Exp Ther 331:104–113. doi: 10.1124/jpet.109.155838. [DOI] [PubMed] [Google Scholar]
- 47.Stein G, Havlichek D. 1992. The new macrolide antibiotics. Azithromycin and clarithromycin. Postgr Med 92:269–272, 277–282. doi: 10.1080/00325481.1992.11701404. [DOI] [PubMed] [Google Scholar]
- 48.Gandhi S, Fleet JL, Bailey DG, McArthur E, Wald R, Rehman F, Garg AX. 2013. Calcium-channel blocker-clarithromycin drug interactions and acute kidney injury. JAMA 310:2544–2553. doi: 10.1001/jama.2013.282426. [DOI] [PubMed] [Google Scholar]
- 49.Labro MT. 1998. Anti-inflammatory activity of macrolides: a new therapeutic potential? J Antimicrob Chemother 41(Suppl B):37–46. doi: 10.1093/jac/41.suppl_2.37. [DOI] [PubMed] [Google Scholar]
- 50.Moher D. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 51.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed on 4 April 2018. [Google Scholar]
- 52.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, Cochrane Bias Methods Group Cochrane Statistical Methods Group. 2011. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rücker G, Schwarzer G. 2015. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 15:1–9. doi: 10.1186/1471-2288-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwarzer G. 2017. Package “netmeta.” 0.9-2. https://cran.r-project.org/web/packages/netmeta/netmeta.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.