ABSTRACT
Systemic candidiasis is a growing health care concern that is becoming even more challenging due to the growing frequency of infections caused by multidrug-resistant (MDR) Candida species. Thus, there is an urgent need for new therapeutic approaches to candidiasis, including strategies bioinspired by insights into natural host defense against fungal pathogens. The antifungal properties of θ-defensins, macrocyclic peptides expressed in tissues of Old World monkeys, were investigated against a panel of drug-sensitive and drug-resistant clinical isolates of Candida albicans and non-albicans Candida species. Rhesus θ-defensin 1 (RTD-1), the prototype θ-defensin, was rapidly and potently fungicidal against drug-sensitive and MDR C. albicans strains. Fungal killing occurred by cell permeabilization that was temporally correlated with ATP release and intracellular accumulation of reactive oxygen species (ROS). Killing by RTD-1 was compared with that by histatin 5 (Hst 5), an extensively characterized anticandidal peptide expressed in human saliva. RTD-1 killed C. albicans much more rapidly and at a >200-fold lower concentration than that of Hst 5. Unlike Hst 5, the anticandidal activity of RTD-1 was independent of mitochondrial ATP production. Moreover, RTD-1 was completely resistant to Candida proteases for 2 h under conditions that rapidly and completely degraded Hst 5. MICs and minimum fungicidal concentrations (MFCs) of 14 natural θ-defensins isoforms against drug-resistant C. albicans isolates identified peptides that are more active than amphotericin B and/or caspofungin against fluconazole-resistant organisms, including MDR Candida auris. These results point to the potential of macrocyclic θ-defensins as structural templates for the design of antifungal therapeutics.
KEYWORDS: antifungal mechanisms, antifungal resistance, antifungal therapy, antifungals, candidiasis, defensins, macrocyclic peptides
INTRODUCTION
Candida albicans is a commensal microbe that colonizes the oropharynx, gastrointestinal, and urogenital tracts of many healthy individuals. Superficial vaginal and oral infections by C. albicans are not uncommon, whereas invasive candidiasis and bloodstream infections occur almost exclusively in immunosuppressed patients, such as those receiving cancer chemotherapy, organ transplants, and inadequately treated HIV/AIDS patients (1–7). Prior to the advent of highly active antiretroviral therapy (HAART) and azole drugs, more than 90% of HIV-infected patients developed oropharyngeal candidiasis (OPC) (8). Systemic candidiasis is increasingly responsible for serious and often fatal fungal infections in humans (9) and is a major cause of hospital-acquired infections in developed countries (10, 11).
The large majority of invasive candidal infections are caused by five species: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei, and more than half of these infections are caused by C. albicans (12). Increasingly, systemic candidiasis is caused by organisms that are resistant to one or more of the three classes of antifungal drugs (azoles, polyenes, and echinocandins) available for the treatment of invasive mycoses. Recently, infections caused by multidrug-resistant (MDR) C. auris have been reported. The first case was reported in Japan in 2009 and, since then, it has been identified as cause of nosocomial bloodstream infections in East Asia, Africa, the Middle East, Europe, and the United States (13–15). Infections currently affect critically ill patients and are associated with very high mortality rate (16).
The incidence of systemic candidiasis caused by MDR isolates and the incidence of adverse side effects associated with available antifungals highlight the ongoing need for new approaches for the treatment of fungal infections (17–20). Echinocandins, the newest class of antifungals, were introduced in 2001, and at that time they were the first new antifungals to be approved in 15 years. The paucity of clinically useful antifungal agents developed in the antimicrobial era demonstrates the challenges associated with drug discovery for eukaryotic pathogens (21).
The discovery of antimicrobial peptides (AMPs) in leukocytes and epithelia of mammals primed investigations to utilize these molecules for the treatment of an array of infectious diseases, including those caused by fungi (22–24). The major classes of mammalian AMPs include cathelicidins (25), salivary histatins (26), and α-, β-, and θ-defensins (27–31), each of which was discovered by screening for antimicrobial molecules expressed in tissues and corresponding secretions. Numerous preclinical studies have evaluated the potential of AMPs as therapeutic agents, none of which have yet led to an approved drug due to a lack of stability, in vivo potency, and/or safety (22, 32–34).
θ-Defensins are 18-amino-acid macrocyclic peptides expressed only in Old World monkeys (29, 31, 35, 36). Like α- and β-defensins, to which they are genetically related, θ-defensins contain a tridisulfide motif that stabilizes the cyclized backbone (Fig. 1). The θ-defensin macrocyclic structure, unique among animals, confers remarkable stability (37, 38). Rhesus θ-defensin 1 (RTD-1), the prototype θ-defensin, was discovered by screening lysates of rhesus macaque neutrophils for antimicrobial activities against bacteria and fungi, which disclosed potent microbicidal activities against bacteria and fungi, including C. albicans and Cryptococcus neoformans (36, 39). Selective antibody neutralization of θ-defensins in extracts of rhesus macaque neutrophil granules ablates killing of C. albicans by these preparations (40), suggesting a prominent role for these macrocyclic peptides in anticandidal host defense mediated by macaque neutrophils.
FIG 1.
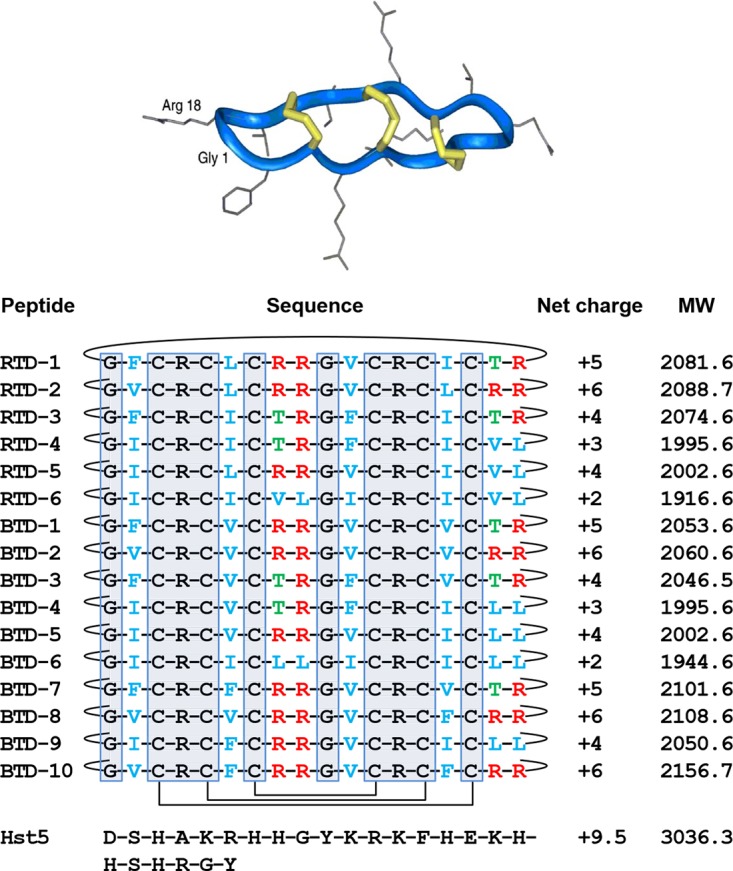
Rhesus macaque and olive baboon θ-defensins. All known θ-defensins are macrocycles exemplified by the ribbon structure of RTD-1 (36). Schematics of the covalent structures of RTDs 1-6 and BTDs 1-10 are listed with the disulfide connectivities and backbone cyclization indicated with solid lines. The 10 gray boxed residues are invariant in all known θ-defensins. Residues in blue are hydrophobic amino acids, Thr residues are in green, and Arg residues in red. The amino acid sequence of Hst 5 is also provided for reference. Net charge is calculated at pH 7.4. MW, molecular weight in kilodaltons. (Republished from reference 36 with permission of the publisher.)
In this study, we determined the kinetics and mechanisms of killing of C. albicans by RTD-1 and compared it to those of histatin-5 (Hst 5; Fig. 1), a well-studied anticandidal peptide expressed in human saliva (26, 41, 42). We also analyzed the antifungal potencies of 14 naturally expressed θ-defensin isoforms against a panel of drug-resistant C. albicans clinical isolates. A subset of the most active θ-defensins was tested for their activities against other Candida species, including MDR isolates of C. auris.
RESULTS
RTD-1 kills drug-sensitive and MDR C. albicans.
In previous studies, we found that RTD-1 and closely related isoforms, RTD-2 and RTD-3, killed a laboratory strain of C. albicans (16820) in a dose-dependent manner (39). Using the same buffer conditions 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid [PIPES], 5 mM glucose), we tested RTD-1 for its activity against two clinical isolates of C. albicans, ATCC 18804 and ATCC 64124, which are sensitive and resistant, respectively, to echinocandins and triazole antifungals. As shown in Fig. 2, both organisms were killed in a time- and concentration-dependent manner by RTD-1. Within 60 min of incubation, 3 μg/ml RTD-1 killed >3 log of both organisms; however, drug-sensitive ATCC 18804 was killed more rapidly. Nevertheless, 3 μg/ml RTD-1 killed >90% of each organism with 10 min of incubation. In contrast, 100 μg/ml Hst 5 was inactive against both organisms (Fig. 2).
FIG 2.
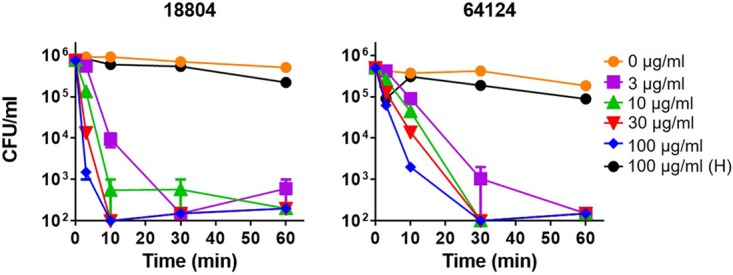
Fungicidal activities of RTD-1 and Hst 5 against drug-sensitive and MDR C. albicans. Drug-sensitive (ATCC 18804) and MDR (ATCC 64124) C. albicans strains were incubated with indicated concentrations of RTD-1 or 100 μg/ml Hst 5 (H) for 60 min in PG buffer for 0 to 60 min. Fungicidal activity was quantified by CFU quantitation after 24 h of incubation. Experiments were repeated twice using three biological replicates, and error bars are shown.
Permeabilization of C. albicans by RTD-1.
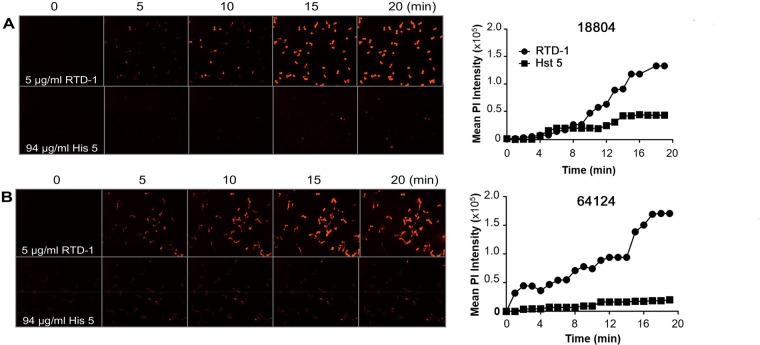
Killing of Escherichia coli cells by RTD-1 correlates with the permeabilization of the cytoplasmic membrane (39). We analyzed the effect of RTD-1 on cytoplasmic membrane integrity by measuring real-time propidium iodide (PI) uptake by C. albicans. Strains ATCC 18804 (Fig. 3A) and ATCC 64124 (Fig. 3B) were incubated with 5 μg/ml RTD-1, and PI uptake was monitored continuously for 20 min. Cell staining of both organisms was apparent within 5 min, with maximal PI uptake occurring by 18 to 20 min. In contrast, incubation with 94 μg/ml Hst 5 resulted in little PI uptake by both organisms (Fig. 3).
FIG 3.
RTD-1 rapidly permeabilizes C. albicans. PI uptake by C. albicans strains ATCC 18804 (18804) (A) and ATCC 64124 (64124) (B) was monitored by confocal microscopy after the addition of 5 μg/ml RTD-1 or 94 μg/ml Hst 5 for 20 min (left). Graphs on the right show the time course of mean PI fluorescence intensity. Data are representative of the results from an individual experiment that was performed three times.
RTD-1 induces ATP release by C. albicans.
Killing of C. albicans by Hst 5 involves the nonlytic release of ATP (42), and the fungicidal effect of Hst 5 is blocked by the inhibition of mitochondrial oxidative phosphorylation (43). Exposure of C. albicans ATCC 18804 to 94 μg/ml Hst 5 caused a time-dependent release of ATP that plateaued at approximately 30 min (Fig. 4A). RTD-1, at 0.5 μg/ml, produced an equally rapid release of ATP, which also peaked at 30 min and then dropped to 25% of the peak level by 90 min. The decline in ATP levels, measured 90 min after RTD-1 exposure, was inhibited in a dose-dependent manner when concanamycin A, an ATPase inhibitor, was added to the incubation at time (T) = 0, and the inhibitor at 8 μM reduced the decline in ATP levels by 42%. While ATPase is implicated in the reduction of accumulated ATP in RTD-1-treated C. albicans strains, a detailed understanding of the mechanisms of peptide-induced ATP release remains to be determined.
FIG 4.
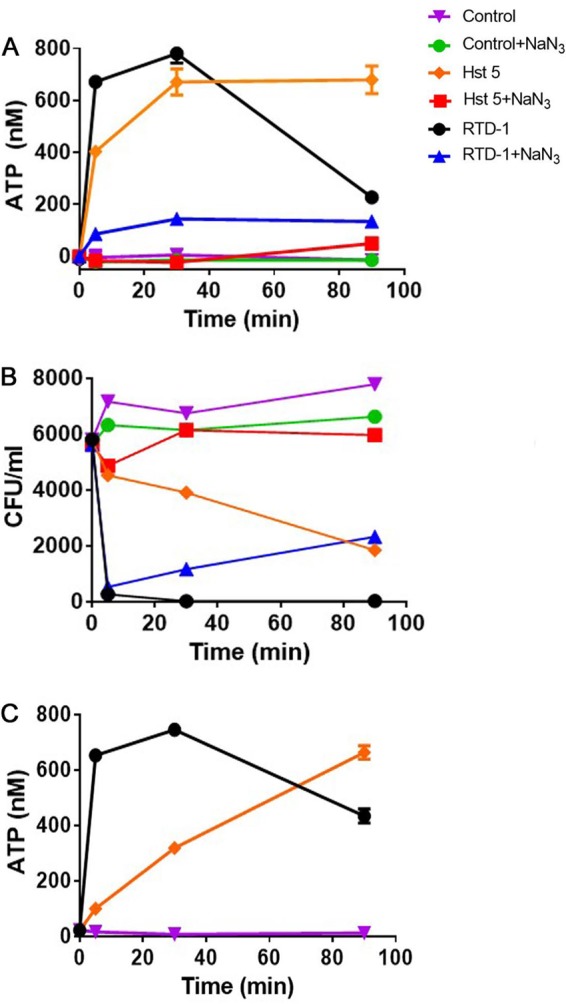
RTD-1 induces rapid release of ATP from C. albicans. (A) C. albicans ATCC 18804 cells were incubated with 0.5 μg/ml RTD-1 or 94 μg/ml histatin-5 in a 0.1-ml reaction mixture volume and monitored for ATP release. Incubations were carried out in the absence or presence of 1 mM sodium azide. (B) Replicates of the incubation mixtures in panel A were analyzed for fungicidal activity, demonstrating that the killing of RTD-1 is rapid and not ATP dependent. Data are representative of an individual experiment repeated twice. (C) RTD-1 (0.5 μg/ml) causes rapid ATP release from MDR C. albicans ATCC 64124 cells, whereas 94 μg/ml Hst 5 causes much more gradual ATP release. The reduction of ATP levels observed at 90 min in RTD-1 containing incubations was dose-dependently reversed by ATPase inhibitor concanamycin A (see the text). The experiments shown in panels A and C were repeated twice using three biological replicates, and error bars are shown.
As expected, preincubation of ATCC 18804 cells with sodium azide (1 mM, 2 h) markedly reduced ATP release in control cells as well as in cells exposed to 0.5 μg/ml RTD-1 or 94 μg/ml Hst 5 (Fig. 4A). Replicate samples from these incubations were plated to determine the fungicidal activities of RTD-1 and Hst 5 with and without sodium azide pretreatment. Consistent with previous studies (43), preincubation with sodium azide blocked the antifungal effect of Hst 5 (Fig. 4B). However, this pretreatment had little effect on fungal killing by RTD-1 (Fig. 4B), demonstrating that the fungicidal effects of RTD-1 are not dependent on mitochondrial ATP phosphorylation. RTD-1 also induced a very rapid release of ATP from MDR C. albicans ATCC 64124, whereas ATP release induced by Hst 5 treatment was slower and prolonged (Fig. 4C).
RTD-1 induces oxidative stress in C. albicans.
The induction of reactive oxygen species (ROS) by antifungal agents contributes to the killing of fungi, including C. albicans (44–46), and this is known to occur rapidly in susceptible organisms. Since killing of C. albicans occurs within 5 min of RTD-1 exposure (Fig. 2 to 4), we tested whether RTD-1 treatment of fungal cells induces intracellular ROS accumulation in the ATCC 64124 and ATCC 18804 strains. Incubation of both strains with 6.25 μg/ml RTD-1 generated ROS within 10 min of peptide exposure, and ROS levels continued to be markedly elevated for at least 60 min (Fig. 5). Interestingly, ROS accumulation was substantially higher in cells of ATCC 64124, the MDR strain.
FIG 5.
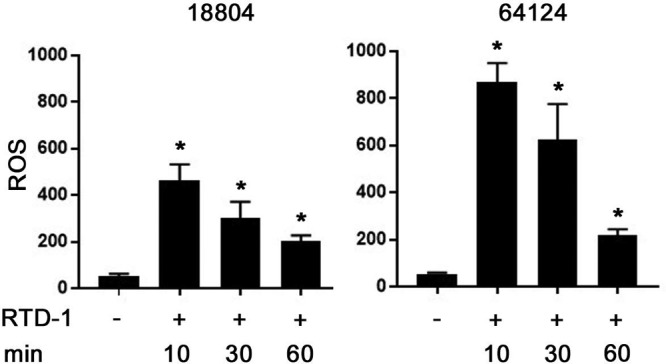
RTD-1 rapidly induces intracellular ROS in C. albicans. DCFH-DA fluorescence was measured in drug-sensitive C. albicans ATCC 18804 and MDR ATCC 64124 after incubation with 6.25 μg/ml RTD-1 for the indicated intervals. The significance of the effect of RTD-1 treatment at all time points was a P value of <0.0001 (*; 2-tailed Student's t test). Experiments were performed using three biological replicates and repeated three times.
Salivary θ-defensins in adult rhesus macaques.
Six θ-defensins (RTD-1 to -6; Fig. 1) are expressed in myeloid granulocytes, and the abundances of the individual peptides vary substantially (40). Since the oral cavity is commonly colonized by C. albicans in humans and in rhesus monkeys (47), we determined the θ-defensin content of mixed saliva of adult rhesus monkeys by ultrahigh-performance liquid chromatography–mass spectrometry (UPLC-MS), as described in Materials and Methods. Saliva from all animals had salivary RTD-1, with levels ranging from 11 to 56 ng/ml (see Table S1 in the supplemental material). Other θ-defensins detected in saliva were RTD-3 (seven animals), RTD-2 and RTD-4 (one animal each), and RTD-5 (2 animals). These results demonstrate the varied expression of θ-defensin isoforms in the saliva of macaques. This pattern of expression differs from that of macaque granulocytes, in which RTD-1 to -6 were always detected, albeit in various ratios (40).
Candidacidal activities of RTD-1 to -3 and Hst 5 in the presence of divalent cations.
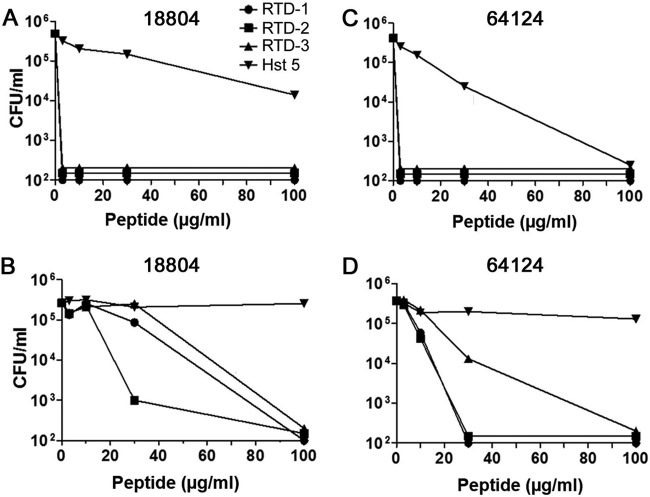
In vitro, θ-defensins retain their microbicidal activities in isotonic buffers (36, 39), conditions that inhibit the microbicidal activities of α- and β-defensins (27, 28). We evaluated the effects of ionic components (20 mM NaCl, 2 mM CaCl2, and 0.5 mM MgCl2) of whole saliva on RTD-1 to -3 and Hst 5 by testing peptide fungicidal activities in PIPES-glucose (PG) alone or PG supplemented with 20 mM NaCl, 2 mM CaCl2, and 0.5 mM MgCl2 against drug-sensitive ATCC 18804 and MDR ATCC 64124 C. albicans. When incubated for 2 h in PG in the absence of divalent cations, low concentrations (3 μg/ml) of RTDs 1-3 killed >3 log of both organisms (Fig. 6A and C), whereas much higher concentrations of Hst 5 were required to effect a 1-log kill of either strain. In the presence of divalent cation-containing media, killing of ATCC 18804 required higher RTD concentrations than with PG buffer, consistent with a previous study employing C. albicans laboratory strain 16820 (39). At 30 μg/ml, RTD-2 (+6 charge) killed >3 log ATCC 18804, whereas 3.3-fold higher concentrations were required for killing of this strain by RTD-1 and -3 (Fig. 6B). Interestingly, MDR C. albicans ATCC 64124 was much more susceptible than drug-sensitive ATCC 18804 to RTDs 1-3, which killed >95% of the input inoculum at 30 μg/ml (Fig. 6D). Hst 5 was inactive against both C. albicans strains (Fig. 6C and D). The lack of Hst 5 activity in divalent cation-containing medium is consistent with previous studies demonstrating that Ca2+ blocks the binding of the peptide to the fungal cell surface (48).
FIG 6.
Fungicidal activities of θ-defensins and Hst 5 in salivary levels of salts and divalent cations. The relative fungicidal activities of RTDs 1-3 and Hst 5 were determined against C. albicans strains ATCC 18804 and ATCC 64124 in PG (A and C) or PG supplemented with 20 mM NaCl, 2 mM CaCl2, and 0.5 mM MgCl2 (B and D). Incubations were carried at for 2 h at 37°C, diluted 1:50, plated onto SD plates, and CFU quantified after 48 h. Data are representative of experiments performed twice.
Susceptibilities of RTDs and Hst 5 to C. albicans proteases.
Secreted aspartyl proteases (SAPs) are essential in C. albicans pathogenesis, in part because their proteolytic activities confer resistance to antimicrobial molecules. In this regard, two of the major human AMPs, Hst 5 (32) and cathelicidin LL-37 (33), are rapidly degraded by SAPs released by C. albicans. We analyzed the stability of RTD-1 and Hst 5 to lysates from drug-sensitive and MDR C. albicans strains by acid-urea-PAGE (49). As shown in Fig. 7, RTD-1 was unaffected by incubation for 2 h with lysates of all three organisms tested, whereas Hst 5 was undetectable after incubation with lysates under the same conditions.
FIG 7.
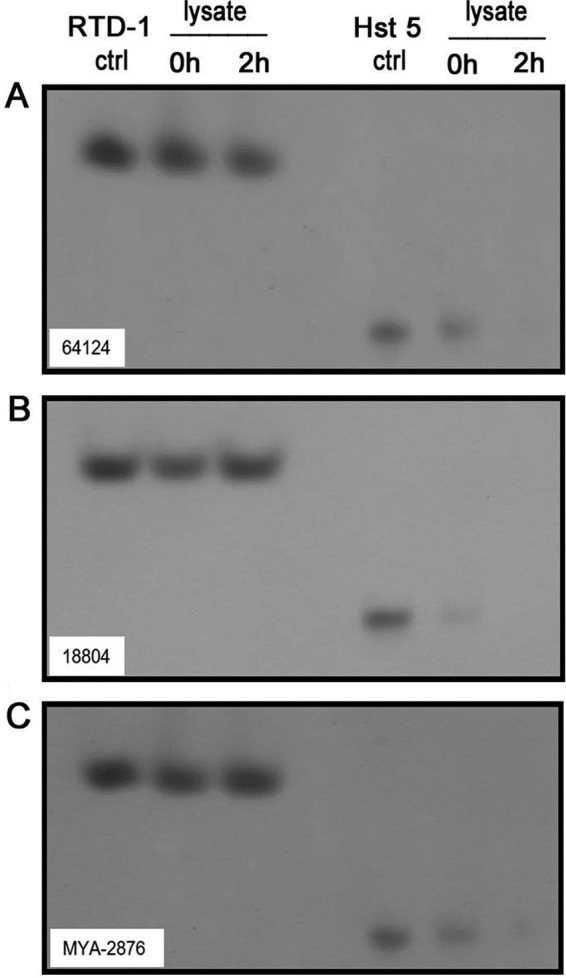
Comparative stability of RTD-1 and Hst 5 in C. albicans cells lysates. Five micrograms of RTD-1 or Hst 5 was incubated with lysates of C. albicans cells (strains ATCC 64124, ATCC 18804, and ATCC MYA-2876) and analyzed on acetic acid-urea (AU)-PAGE at time zero and after 2 h of incubation. One-fifth of each incubation mixture (1.0 μg input) was loaded in each well; control (ctrl) lanes were loaded with 1.0 μg of untreated RTD-1 or Hst 5. The gel was stained with formalin-Coomassie blue (49). Data are representative of experiments repeated twice.
Antifungal properties of natural θ-defensin isoforms against C. albicans and MDR non-albicans Candida species.
Based on the findings that RTDs 1-3 varied in their potencies against two C. albicans isolates (Fig. 6) and that the content of RTDs in macaque saliva is quite varied, we sought to determine the antifungal effects of rhesus macaque (RTDs 1-5) (40) or olive baboon (BTDs 1-5 and 7-10) (35) θ-defensin isoforms (Fig. 1) against drug-resistant C. albicans clinical isolates. Standard clinical laboratory assays (50, 51) were used to determine MICs and minimal fungicidal concentrations (MFCs) against an ATCC panel of C. albicans clinical isolates. With the exception of strain ATCC 18804, strains were resistant to one or more classes of antifungals (Table 1). All strains tested were inhibited by each of the θ-defensins, with MICs ranging from 1.5 to 25 μg/ml. All peptides inhibited growth of fluconazole-resistant (MIC, ≥128 μg/ml) isolates, including those that were echinocandin or 5-flucytosine resistant (Table 1). Based on the average MIC across all species tested, the most potent peptides were RTD-1, RTD-2, BTD-1, BTD-2, BTD-8, and BTD-10, while BTD-4 was the least active overall. BTD-2 had the lowest MICs against the largest number of isolates, with MICs 40- to 90-fold lower than those of fluconazole for the seven fluconazole-resistant strains. Moreover, BTD-2 also had an MIC lower than that of amphotericin B for C. albicans ATCC 76485 (Table 1).
TABLE 1.
MICs of θ-defensins and clinical antifungalsa
| ATCC strain | MIC (μg/ml) |
ATCC phenotype |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTD |
BTD |
Amp B | Fluco | ||||||||||||||||
| RTD-1 | RTD-2 | RTD-3 | RTD-4 | RTD-5 | BTD-1 | BTD-2 | BTD-3 | BTD-4 | BTD-5 | BTD-7 | BTD-8 | BTD-9 | BTD-10 | Echin | Triazole | 5-Flu | |||
| ATCC 64124 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 6.25 | 12.5 | 25 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | >256 | R | R | I |
| ATCC 10231 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 3.125 | 12.5 | 25 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | 8 | R | R | S |
| ATCC 28121 | 25 | 25 | 12.5 | 12.5 | 12.5 | 25 | 12.5 | 25 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 | 4 | 1 | S | R | S |
| ATCC MYA-2876 | 12.5 | 25 | 12.5 | 12.5 | 25 | 25 | 12.5 | 12.5 | 25 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 4 | 64 | S | S | S |
| ATCC MYA-427 | 12.5 | 12.5 | 25 | 25 | 25 | 25 | 12.5 | 25 | 25 | 12.5 | 25 | 12.5 | 25 | 12.5 | 4 | 128 | S | R | S |
| ATCC MYA-574 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 3.125 | 12.5 | 25 | 6.25 | 12.5 | 3.125 | 12.5 | 3.125 | 2 | >256 | S | R | S |
| ATCC 90819 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 3.125 | 12.5 | 25 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | >256 | S | R | S |
| ATCC MYA-1023 | 12.5 | 6.25 | 25 | 12.5 | 12.5 | 12.5 | 6.25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 | 2 | 32 | S | R | S |
| ATCC 76485 | 12.5 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 1.56 | 25 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | 128 | R | S | S |
| ATCC 38289 | 12.5 | 12.5 | 25 | 25 | 25 | 12.5 | 12.5 | 25 | 25 | 12.5 | 25 | 12.5 | 25 | 6.25 | 4 | >256 | S | R | S |
| ATCC 11651 | 12.5 | 12.5 | 25 | 25 | 25 | 12.5 | 6.25 | 25 | 25 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 4 | >256 | S | R | S |
| ATCC 96901 | 12.5 | 6.25 | 25 | 25 | 25 | 25 | 6.25 | 25 | 25 | 12.5 | 25 | 25 | 25 | 12.5 | 4 | 1 | S | R | S |
| ATCC 90029 | 12.5 | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 4 | 1 | S | S | R |
| ATCC 18804 | 6.25 | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 6.25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 | 2 | 1 | S | S | S |
CLSI protocol was used to determine MICs for each θ-defensin and amphotericin B (Amp B) and fluconazole (Fluco). ATCC phenotypes (S, susceptible; R, resistant; I, intermediate) are reported for one or more echinocandin (Echin), triazole, or 5-flucytosine (5-Flu).
Since MIC assays do not distinguish between fungistatic and fungicidal activities, we determined MFCs (defined as ≥99% CFU reduction) for the peptides against the same panel of ATCC C. albicans clinical isolates. The MFCs of all peptides were equal to 1 or 2 times the MIC against all strains tested (Table 2). As expected, BTD-2 was the most potent fungicide, with MFCs lower than those of amphotericin B for 2 strains (ATCC MYA-427 and ATCC 90819) with MFCs of 3.125 to 12.5 μg/ml against all strains tested. Next, we evaluated the four most active θ-defensins (RTD-1, RTD-2, BTD-2, and BTD-8), as well as BTD-4, the least active peptide, against a panel of drug-resistant non-albicans Candida species, including C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and C. auris. RTD-1, RTD-2, BTD-2, and BTD-8 had MICs between 1.5 and 6 μg/ml against all organisms tested (Table 3). Even BTD-4, the least active θ-defensin was effective against fluconazole- and caspofungin-resistant C. glabrata and fluconazole-resistant C. krusei strains (Tables 3 and 4). Of note, RTD-1, RTD-2, BTD-2, and BTD-8 were highly active against two strains of fluconazole- and caspofungin-resistant C. auris. The fungicidal actions of the non-albicans Candida species were confirmed by determining MFCs (≥99% CFU reduction), which were in each case 1 to 2 times the corresponding MICs. Of note, RTD-2, BTD-2, and BTD-8 (all +6 net charge) were the most potently fungicidal θ-defensins against both caspofungin-resistant C. auris strains.
TABLE 2.
MFCs of θ-defensins and clinical antifungalsa
| ATCC strain | MFCs (μg/ml) |
ATCC phenotype |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RTD |
BTD |
Amp B | Fluco | ||||||||||||||||
| RTD-1 | RTD-2 | RTD-3 | RTD-4 | RTD-5 | BTD-1 | BTD-2 | BTD-3 | BTD-4 | BTD-5 | BTD-7 | BTD-8 | BTD-9 | BTD-10 | Echin | Triazole | 5-Flu | |||
| ATCC 64124 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 | 25 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 12.5 | 4 | >256 | R | R | I |
| ATCC 10231 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 | 12.5 | 25 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | >256 | R | R | S |
| ATCC 28121 | 25 | 25 | 25 | 25 | 25 | 25 | 12.5 | 25 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 | 4 | >256 | S | R | S |
| ATCC MYA-2876 | 25 | 25 | 12.5 | 12.5 | 25 | 25 | 12.5 | 25 | 25 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 4 | >256 | S | S | S |
| ATCC MYA-427 | 12.5 | 25 | 25 | >25 | 25 | 25 | 12.5 | 25 | >25 | 12.5 | 25 | 25 | 25 | 25 | 4 | >256 | S | R | S |
| ATCC MYA-574 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 3.125 | 12.5 | 25 | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 4 | >256 | S | R | S |
| ATCC 90819 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 3.125 | 12.5 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 | 4 | >256 | S | R | S |
| ATCC MYA-1023 | 12.5 | 6.25 | 25 | 25 | 12.5 | 12.5 | 6.25 | 25 | 25 | 12.5 | 25 | 25 | 12.5 | 12.5 | 4 | >256 | S | R | S |
| ATCC 76485 | 12.5 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 3.125 | 25 | 25 | 12.5 | 12.5 | 6.25 | 12.5 | 6.25 | 2 | >256 | R | S | S |
| ATCC 38289 | 12.5 | 12.5 | 25 | 25 | 25 | 12.5 | 12.5 | 25 | 25 | 12.5 | 25 | 12.5 | 25 | 12.5 | 4 | >256 | S | R | S |
| ATCC 11651 | 12.5 | 12.5 | 25 | 25 | 25 | 12.5 | 6.25 | 25 | 25 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | 4 | >256 | S | R | S |
| ATCC 96901 | 12.5 | 6.25 | 25 | >25 | >25 | 25 | 12.5 | 25 | 25 | 12.5 | 25 | 25 | 25 | 12.5 | 4 | 256 | S | R | S |
| ATCC 90029 | 12.5 | 25 | 12.5 | 25 | 25 | 12.5 | 6.25 | 25 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 25 | 4 | >256 | S | S | R |
| ATCC 18804 | 6.25 | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 25 | 25 | 12.5 | 12.5 | 12.5 | 12.5 | 6.25 | 4 | >256 | S | S | S |
MFC assays were conducted as described in Materials and Methods. MFC values correspond to 99% killing relative to input inoculum. Abbreviations are the same as listed in Table 1.
TABLE 3.
MICs of θ-defensins and clinical antifungalsa
| Candida CDC strain | MIC (μg/ml) |
CDC phenotype |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RTD |
BTD |
Fluco | Caspo | ||||||
| RTD-1 | RTD-2 | BTD-2 | BTD-4 | BTD-8 | Caspo | Fluco | |||
| C. glabrata 0314 | 6.25 | 3.125 | 1.56 | 6.25 | 3.125 | 128 | 1 | R | R |
| C. glabrata 0316 | 3.125 | 3.125 | 1.56 | 6.25 | 3.125 | 8 | 2 | R | SDD |
| C. parapsilosis 0335 | 3.125 | 3.125 | 3.125 | 25 | 1.56 | 16 | 1 | I | R |
| C. parapsilosis 0337 | 3.125 | 3.125 | 1.56 | 25 | 3.125 | 128 | 1 | S | R |
| C. tropicalis 0345 | 3.125 | 1.56 | 1.56 | 25 | 1.56 | >256 | 0.125 | S | R |
| C. krusei 0397 | 3.125 | 1.56 | 1.56 | 6.25 | 1.56 | 256 | 1 | S | R |
| C. auris 0384 | 6.25 | 6.25 | 3.125 | >25 | 3.125 | >256 | >8 | NA | NA |
| C. auris 0389 | 6.25 | 6.25 | 3.125 | >25 | 6.25 | >256 | >8 | NA | NA |
CLSI protocol was used to determine MICs for each θ-defensin, fluconazole (Fluco), and caspofungin (Caspo). CDC phenotypes (S, susceptible; R, resistant; I, intermediate; SDD, susceptible dose dependent) are listed, except where NA (not applicable) is shown, since no interpretation was provided by the agency for these strains.
TABLE 4.
MFCs of θ-defensins and clinical antifungalsa
| Candida CDC strain | MFC (μg/ml) |
CDC phenotype |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RTD |
BTD |
Fluco | Caspo | ||||||
| RTD-1 | RTD-2 | BTD-2 | BTD-4 | BTD-8 | Caspo | Fluco | |||
| C. glabrata 0314 | 6.25 | 3.125 | 3.125 | 12.5 | 6.25 | 128 | 1 | R | R |
| C. glabrata 0316 | 6.25 | 3.125 | 1.56 | 12.5 | 6.25 | 8 | 2 | R | SDD |
| C. parapsilosis 0335 | 6.25 | 6.25 | 3.125 | >25 | 6.25 | 16 | 1 | I | R |
| C. parapsilosis 0337 | 6.25 | 6.25 | 3.125 | 25 | 6.25 | 128 | 1 | S | R |
| C. tropicalis 0345 | 3.125 | 1.56 | 1.56 | 25 | 1.56 | >256 | 0.125 | S | R |
| C. krusei 0397 | 3.125 | 3.125 | 1.56 | 12.5 | 3.125 | 256 | 1 | S | R |
| C. auris 0384 | 12.5 | 6.25 | 6.25 | >25 | 6.25 | >256 | >8 | NA | NA |
| C. auris 0389 | 12.5 | 6.25 | 6.25 | >25 | 6.25 | >256 | >8 | NA | NA |
MFC assays were conducted as described in Materials and Methods. MFC values correspond to 99% killing relative to input inoculum. Abbreviations are the same as listed in Table 3.
θ-Defensins are nonhemolytic in vitro and nontoxic in vivo.
The hemolytic activity of each of the θ-defensins (100 μg/ml) listed in Fig. 1 (other than RTD-6 and BTD-6, which were not evaluated in this study) was analyzed in (i) 90% EDTA-anticoagulated whole blood (see Materials and Methods) and (ii) a 2% (vol/vol) suspension of human blood erythrocytes plus 10% autologous serum (39). All peptides, with the exception of BTD-8, produced ≤1.3% hemolysis in either medium. BTD-8 caused 2.8% hemolysis in the 2% erythrocyte suspension.
These results are consistent with those of a previous study demonstrating that the lack of hemolytic activity of RTD-1 to -3 correlated with a lack of cytotoxicity against human HS68 fibroblasts (39). The θ-defensin isoforms were then tested for acute toxicity in BALB/c mice. Peptides were administered intravenously (i.v.) or subcutaneously (s.c.) at 5 mg/kg of body weight and observed for 7 days, as described in Materials and Methods. No toxicity was observed in any animal, consistent with previous studies of RTD-1 administered to mice and rats (37, 52, 53).
DISCUSSION
AMPs are important mediators of innate immunity, providing first-line protection against pathogens at mucosal surfaces and in otherwise-sterile tissue compartments. θ-Defensins, expressed uniquely in granulocytes and epithelia of Old World monkeys, are pleotropic effectors of innate immunity that possess wide-spectrum antimicrobial activities and immunomodulatory properties. Discovered in 1999 (36), θ-defensins are still the only known macrocyclic peptides expressed in animals (29, 31). RTD-1 was highly effective in mouse models of bacterial and polymicrobial sepsis (37), murine severe acute respiratory syndrome (SARS)-coronavirus infection (53), and cystic fibrosis (52). In each case, the therapeutic effects observed were associated with the suppression of proinflammatory cytokines. RTD-1 modulated proinflammatory cytokine gene expression and release by human leukocytes, and this correlated with the suppression of NF-κB and mitogen-activated protein (MAP) kinase pathways (54). Importantly, the immunomodulating activities of RTD-1 were not accompanied by immunosuppressive side effects, since peptide treatment of infected/septic animals facilitated recovery (37, 53).
The immunomodulating properties of RTD-1 were recently extended to a rat model of rheumatoid arthritis, wherein peptide treatment arrested and promoted the resolution of both evolving and severe joint disease (55). While RTD-1 is the first AMP shown to be effective in autoimmune arthritis, several AMPs, including α-defensins, β-defensins, and cathelicidin LL37, have been shown to have combined antimicrobial/immunomodulatory properties (27, 28, 56–59).
In studies to determine the contribution of θ-defensins to killing of bacteria and fungi by rhesus macaque neutrophil granule constituents, RTD-1 to -5 were found to be particularly important for the killing of C. albicans. Furthermore, peptides were highly stable in human polymorphonuclear leukocyte (PMN) granule lysates (40). Here, we showed that RTDs are present in the saliva of rhesus monkeys. Mixed saliva contains RTD-1 (14/14 animals), whereas RTD-2 to -6 were detected infrequently or not at all (RTD-6). It is likely that peptide concentrations in mixed saliva are much lower than the levels encountered by fungal cells at the site of their interaction with oral epithelium. Since α- and β-defensins are expressed in the oral cavities of human and nonhuman primates but θ-defensins are found only in Old World primates, we undertook studies to determine the antifungal mechanisms and potencies of these unique macrocyclic peptides against drug-sensitive and MDR C. albicans and other Candida species.
In mechanistic studies employing RTD-1, the prototype θ-defensin, we determined that this peptide rapidly permeabilizes drug-sensitive and MDR C. albicans cells, and fungal killing correlates temporally with PI uptake, ATP release, and induction of ROS by target cells. Near-maximal release of ATP occurred within 5 min of peptide exposure, and the kinetics of PI uptake and induction of maximum ROS production within 10 min of peptide exposure indicate that membrane permeabilization is an early event that correlates with the time course of cell killing. This finding is consistent with the rapid envelope permeabilization and killing of E. coli cells by RTDs 1-3 (39). Loss of intracellular ATP pools results in cell death, as cytosolic depletion leads to the cessation of macromolecular synthesis and cellular homeostasis (60). Cytosolic ROS production is also an early response to cytotoxic agents (45). 2′7′-Dichlorodihydro-fluorescein diacetate (DCFH-DA) used in this study detects ROS, including, but not limited to, superoxide anion, hydroxyl radicals, singlet oxygen, and peroxynitrite, which are known to irreversibly modify lipids, proteins, and nucleic acids (61, 62). Additional studies are under way to determine the temporal sequence and cellular physiologic relationships that may link ROS production and ATP release. Interestingly, extracellular ATP levels resulting from RTD-1 treatment decreased by 90 min of incubation after reaching a maximum at 30 min. The addition of an ATPase inhibitor dose dependently reversed the reduction in ATP. Whether this is due to leakage of intracellular ATPase or reflects intracellular ATP hydrolysis remains to be determined.
Among the antifungal peptides and proteins shown to contribute to the innate resistance of the oral cavity to invasive candidiasis, members of the histatin family have been studied extensively (26). We therefore employed Hst 5 (Fig. 1) as a reference compound in these studies. All 14 θ-defensins tested were 100 to 1,000 times more active than Hst 5 in antifungal assays, perhaps in part due to the stability of θ-defensins in biological matrices, such as plasma and serum (37), and to fungal proteases that rapidly degraded Hst 5 (Fig. 7). Like Hst 5, RTD-1 treatment caused ATP release by C. albicans, but this effect required only 0.5 μg/ml RTD-1, which produced a more rapid efflux of ATP than did 94 μg/ml Hst 5. However, pretreatment of fungal cells with sodium azide had no impact on killing by RTD-1. This is in contrast to the protective effect of sodium azide on Hst 5 killing of C. albicans, demonstrating that RTD-1 killing is independent of mitochondrial respiration.
θ-Defensin sequence diversity results from posttranslational splicing of 9-amino-acid precursors derived from 3 (rhesus macaque) or 4 (olive baboon) differentially expressed propeptides (35, 36, 40, 49). The known θ-defensin isoforms, having net charges ranging from +2 to +6, possess an invariant core structure composed of the 6 Cys, 2 Arg, and 2 Gly residues (gray highlight in Fig. 1), leaving only 8 backbone positions that vary. Despite the high level of sequence conservation, θ-defensin isoforms possess quite varied antifungal activities. For example, BTD-2 was >20 times more active than BTD-4 against most C. albicans strains. Structure-activity analysis disclosed that the θ-defensins with the greatest global potencies against C. albicans were those bearing a net charge of +5 (RTD-1) or +6 (RTD-2 and BTD-2), whereas RTD-4 and BTD-4 (both +4) were the least active.
In the evaluation of θ-defensins antifungal activities, we employed standard Clinical and Laboratory Standards Institute (CLSI) protocols used to identify drug candidates, allowing for the comparison of θ-defensins with fluconazole, amphotericin, and caspofungin, representing each of the currently available antifungal drug classes. All θ-defensins were fungicidal, with MFCs of ≤25 μg/ml (the highest peptide concentration tested) against all strains tested, including those characterized by ATCC and CDC as drug resistant. MIC/MFC values were either 1 or 2 against the panel of C. albicans isolates, demonstrating that θ-defensins are fungicidal.
All θ-defensins tested were active against fluconazole- and echinocandin-resistant Candida species, and the most active θ-defensins had lower MIC/MFCs than those for amphotericin B (Tables 1 and 2) and caspofungin (Tables 3 and 4). While we reported MIC/MFC values for peptides and conventional antifungals in micrograms per milliliter, on a molar basis, θ-defensins (∼2 kDa) are 2- to 6-fold more active than the corresponding values for antifungals, which have masses of 0.3 to 1.1 kDa. Susceptible organisms included several MDR C. albicans (e.g., ATCC 64124) as well as non-albicans Candida clinical isolates, including two caspofungin-resistant C. auris clinical isolates. C. auris is an urgent public health concern because of its high mortality rate (30 to 60%) and person-to-person transmission (16) and is especially problematic in intensive care units (13, 15, 63–66). Echinocandins are the treatment of choice for C. auris infections, since most clinical isolates are resistant to azoles and amphotericin B. Of note, the two C. auris isolates killed by θ-defensins are echinocandin resistant (Tables 3 and 4).
The incidence of systemic candidiasis caused by MDR isolates and the incidence of adverse side effects associated with available antifungals highlight the ongoing need for new approaches for the treatment of fungal infections (17–20). Caspofungin, the first echinocandin, was approved in 2001 and was the first new antifungal to be approved in 15 years. The paucity of clinically useful antifungal agents developed in the antimicrobial era demonstrates the challenges associated with drug discovery for eukaryotic pathogens (21). The finding that θ-defensins are effective in vitro against multiple Candida species raises the possibility that bioinspired molecules that incorporate the structural features of these molecule may be exploited for antifungal drug development.
The critical need for new therapeutic approaches to systemic fungal infections has motivated new drug discovery efforts (21, 67, 68). These include renewed interest in evaluating AMPs from various sources as a source of “bioinspired” drug candidates. While the development of AMPs as therapeutics has a relative short and disappointing history, renewed efforts are focusing on newer classes of host-derived AMPs (22). Of note, cyclization of AMPs, such as Hst 5 (69) and rabbit neutrophil α-defensin NP-1 (70), improved the drug-like properties and potency of the corresponding derivatives.
The potential for therapeutic applications of θ-defensins for the treatment of systemic infections is supported by preclinical studies demonstrating that RTD-1 is highly effective in mouse models of bacterial and polymicrobial sepsis (37), murine SARS-coronavirus infection (53), and in a mouse model of cystic fibrosis (52). RTD-1, the prototype θ-defensin, is well tolerated when administered systemically to mice and rats and is nonimmunogenic, highly stable, and effective in the arrest and resolution of autoimmune arthritis when administered as infrequently as once every 5 days (37, 55). Further, studies presented here and previously reported (37, 52, 53, 55, 71) demonstrated that parenterally administered θ-defensins are well tolerated in mice and rats. Thus, the drug-like properties of naturally occurring θ-defensins represent a template for the development of antifungal agents that may prove valuable in the treatment of infections caused by MDR fungal pathogens.
MATERIALS AND METHODS
Ethics.
Human blood was collected from healthy adult volunteers with informed written consent. Blood collection was approved by the University of Southern California Human Subjects Institutional Review Board (HS-09-00289). All animal use protocols were approved by institutional animal use committees (University of Southern California IACUC protocols 11568 and 20538). Rhesus macaques were housed in social groups in indoor/outdoor housing at the Oregon National Primate Center at Oregon Health Sciences University (ONPC) and cared for in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals and the U.S. Animal Welfare Act. Around-the-clock veterinary care is provided at ONPC, ensuring that the nonhuman primates are provided nutritious meals, daily fresh produce, behavioral enrichment activities, and a disease-free environment. The psychological well-being of the nonhuman primates is ensured by providing conditions to express species-typical behaviors and reduce stress. Monkeys are given enrichment to increase behavioral diversity, and animal training is implemented to reduce stress and accomplish animal husbandry procedures cooperatively. For this study, saliva samples were collected from ketamine-sedated animals. No primates were sacrificed in this study. All animals were returned to their respective groups upon recovery from sedation.
Peptides and antifungal drugs.
Naturally occurring isoforms of θ-defensins (Fig. 1) expressed in rhesus macaque and olive baboon leukocytes were produced by solid-phase peptide synthesis, as previously described (35, 36, 39). RTD-6 and BTD-6 were not included in this study, as both peptides have exceedingly low solubility in aqueous media. Histatin-5 was purchased from AnaSpec (Fremont, CA). Amphotericin B, caspofungin, and fluconazole were purchased from Sigma-Aldrich (St. Louis, MO). Peptide stocks were prepared in 0.01% acetic acid at 0.5 mg/ml, and antifungal drugs were prepared in sterile water. Peptide concentrations employed in each assay were established in dose-range-finding studies, and the results shown are from experiments that demonstrate relevant dose-response relationships. Each experiment was performed 2 to 3 times, as indicated in the figure legends.
Fungi.
A panel of drug-resistant clinical isolates of C. albicans (ATCC MP-8, ATCC 64124, ATCC 10231, ATCC 28121, ATCC MYA-2876, ATCC MYA-427, ATCC MYA-574, ATCC 90819, ATCC MYA-1023, ATCC 76485, ATCC 38289, ATCC 11651, ATCC 96901, ATCC 90029, and ATCC 18804) was obtained from American Type Culture Collection. Clinical isolates of C. glabrata 0314 and 0316, C. parapsilosis 0335 and 0337, C. tropicalis 0345, C. krusei 0397, and C. auris 0384 and 0389 were obtained from Centers for Disease Control and Prevention (CDC).
Liquid suspension fungicidal assays.
Fungicidal assays were conducted using a liquid suspension assay, as previously described (35, 39). Briefly, overnight cultures were grown in Sabouraud dextrose broth (SDB) and were subcultured to mid-log phase at 37°C. Fungicidal assays were performed in 10 mM PIPES buffer (pH 7.4) containing 5 mM glucose (PG). In some assays, where noted in the text or figure legend, PG buffer was supplemented with 20 mM NaCl, 2 mM CaCl2, and 0.5 mM MgCl2. Approximately 2 × 106 CFU/ml were incubated with peptides in 96-well plates in 0.05 ml of PG buffer. After incubation at 37°C for 10 to 120 min, samples were diluted 1:50 and plated on SDB agar using a spiral spreader (Spiral Biotech, Inc.). Fungicidal activity was determined by colony counting as a function of peptide concentration.
Real-time propidium iodide uptake.
Permeabilization of C. albicans was analyzed microscopically by visualizing and quantifying the influx of propidium iodide, as previously described (8). Briefly, C. albicans cells were resuspended to 2 × 106 CFU/ml in PG, deposited onto glass-bottom chamber slides (Ibidi, Madison, WI) coated with 0.5 mg/ml concanavalin A (Sigma), mixed with 5 μg/ml propidium iodide, and imaged every 60 s for 20 min using an UltraVIEW spinning disk confocal microscope (PerkinElmer, Waltham, MA). Fluorescence intensity was analyzed using the ImageJ software (NIH, Bethesda, MD).
Extracellular ATP release.
Mid-log-phase C. albicans cells (1 × 105 CFU/ml) were incubated with RTD-1 or Hst 5 in PG buffer for 0, 5, 30, and 90 min at 37°C. After brief centrifugation, 25 μl of supernatant was transferred to 210 μl of boiling 50 mM Tris-HCl, 2 mM EDTA (pH 7.8; TE buffer), heated at 100°C for 2 min, and immersed in an ice bath. ATP was quantified by measuring ATP-mediated bioluminescence (ATP bioluminescent assay kit; Sigma) on a SpectraMax M5e plate reader, as per the manufacturer's instructions. In parallel incubations, cells were pretreated with 1 mM sodium azide for 2 h at 37°C. The effect of ATPase blockade was evaluated by including 0.5, 2, or 8 μM concanamycin A to fungus/peptide incubations.
Quantification of ROS.
Five milliliters of overnight cultures of C. albicans ATCC 18804 and ATCC 64124 were grown in yeast peptone-dextrose (YPD) broth, harvested by centrifugation, washed with PBS, and suspended to an A600 of ∼1.0 in 10 ml of fresh YPD broth. RTD-1 was added at 6.25 μg/ml, which corresponds to the MIC value, and cells were incubated for 10, 30, or 60 min at 30°C with agitation at 200 rpm. At each time point, 2′7′-dichlorodihydro-fluorescein diacetate (DCFH-DA; Sigma-Aldrich) was added to a 10 μM final concentration, and cells were incubated for 30 min at 30°C in the dark. Cells were harvested and washed three times with PBS, and 100 μl of the suspension was transferred to flat black MicroWell plates (Greiner Bio-One). ROS levels were quantified by measuring the fluorescence in a Spectra Max M5e microplate reader (Molecular Devices) with 486-nm excitation and 535-nm emission filters.
Quantitation of RTDs in rhesus macaque saliva.
Unstimulated saliva samples were collected from healthy ketamine-sedated male (n = 10) and female (n = 9) rhesus macaques, age 4 to 8 years, at the Oregon National Primate Center at Oregon Health Sciences University. Saliva samples (0.5 to 2.0 ml) were clarified by centrifugation. To 50 μl of each sample, 50 μl of 6 M urea was added and incubated for 20 min at room temperature. Fifty microliters of 6% trifluoroacetic acid (TFA) was then added and incubated for 20 min at room temperature. Samples were clarified by centrifugation, supernatants were transferred to bovine serum albumin (BSA)-coated UPLC vials, and θ-defensin content was analyzed by UPLC-MS using an Acquity UPLC and Micromass Quattro Ultima mass spectrometer (Waters Corporation, Milford, MA), using RTD-1 as a standard, as previously described (71).
Analysis of peptide degradation by fungal cell lysates.
Three strains (ATCC 64124, ATCC 18804, and ATCC MYA-2876) of C. albicans were grown in SDB to mid-log phase and harvested as described above. Cells were suspended in ice-cold PG buffer containing 1% dimethyl sulfoxide (DMSO) to 5 × 108 cells/ml. Cells were disrupted by vortexing with acid-washed glass beads (Sigma-Aldrich) five times for 1 min, and supernatants were collected following centrifugation. Five micrograms of RTD-1 or Hst 5 was added to 100 μl of cell lysate (5 × 107 cell equivalents) and incubated at 37°C for 2 h, acidified to a final concentration of 5% acetic acid, and clarified by centrifugation. Supernatants were analyzed by acid-urea-PAGE and stained with formalin-Coomassie blue (49).
Antifungal drug susceptibility assays.
ATCC panel strains were tested against θ-defensins, amphotericin B, fluconazole, and caspofungin in microdilution broth in accordance with Clinical and Laboratory Standards Institute (CLSI) document M27-A2 (72), with the exception that we used 25 mM (rather than 165 mM) N-morpholinepropanesulfonic acid (MOPS) to buffer test the medium in order to increase the sensitivities of various targets to antimicrobial peptide while not affecting fungal growth. Briefly, overnight cultures of each ATCC and CDC strain were grown overnight at 30°C in YPD broth. Cells were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), without calcium or magnesium (pH 7.2), resuspended in PBS, and sonicated for 30 s in a Branson B-200 bath. Cells were suspended in test medium (RPMI 1640 medium, 25 mM MOPS, 2 mM l-glutamine) to 104 CFU per ml (73). The MIC was determined in flat-bottom 96-well microtiter plates (Greiner Bio-One, Monroe, NC) containing 0.2 ml test medium per well. Duplicate 0.1-ml aliquots of serial 2-fold dilutions of peptides and antifungal drugs were dispensed into wells, each of which was inoculated with 0.1 ml of fungal cell suspension containing 1 × 103 CFU. Plates were incubated at 30°C for 48 h, since several strains were hyperfilamentous at 37°C, and the MIC was not affected by incubation at 30°C, after which the A600 was determined using a SpectraMax M5e plate reader. The MIC was determined to be the lowest concentration of peptide or antifungal that completely inhibited growth, as determined by the A600, which corresponds to optically clear wells. The minimal fungicidal concentration (MFC) was determined by plating (on YPD agar) 10 μl from wells without cell growth and the first well with measurable turbidity, as described previously (50, 51, 74, 75), and corresponding aliquots from the no-peptide controls. Incubation of the MIC plates at 30°C, where no filamentation occurred, allowed for CFU to be counted, thus enabling a determination of the MFC. Plates were incubated for 24 h at 37°C. The MFC is reported as the lowest concentration of peptide or antifungal drug that killed ≥99% of the input organisms.
Hemolysis assays.
Peptide hemolytic activities were determined by incubating, in duplicate, 100 μg/ml individual θ-defensins with 2% (vol/vol) washed freshly prepared EDTA-anticoagulated blood, supplemented with 10% autologous serum, as previously described (39). Each peptide was also tested for hemolytic activity in 90% (vol/vol) whole blood in which the peptide (100 μg/ml final) was dissolved in 0.01% acetic acid (HOAc). After 1 h of incubation at 37°C, samples were centrifuged for 15 min at 500 × g, and the A405 of supernatants was determined spectrophotometrically. Percent hemolysis was determined relative to control samples incubated in peptide solvent or those generated by incubation with 1% aqueous NP-40.
Acute toxicity studies.
θ-Defensin toxicity was evaluated in BALB/c mice 6 to 9 weeks of age obtained from Charles River Laboratories. All animals were maintained on a 12-h light/dark cycle in thermostatically controlled rooms and provided with standard chow and water ad libitum. Acute toxicity was evaluated by i.v. and s.c. injection of all BTDs shown in Fig. 1, except RTD-6 and BTD-6. A 5-mg/kg dose was delivered i.v. or s.c. at a peptide concentration of 0.5 mg/ml in normal saline. Two males received i.v. doses of each peptide via the lateral tail vein. For s.c. peptide delivery, two males and two female mice were injected in the medial scapular region. Animals were observed continually for the first 4 h for any sign of toxicity, including signs of dyspnea, lethargy, injection sight reaction, or mortality. Animals were further observed every 12 to 18 h for 7 days.
Supplementary Material
ACKNOWLEDGMENTS
The present work was supported by grants from National Institute of Allergy and Infectious Diseases grant (AI22931 [https://www.niaid.nih.gov/]), National Institute of Dental and Craniofacial Research grant (DE021341 [http://www.nidcr.nih.gov]), the Southern California Clinical and Translational Science Institute (UL1TR000130 [http://www.sc-ctsi.org/]), the National Cancer Institute (P30CA014089 [https://www.cancer.gov/]), and National Institute of Allergy and Infectious Diseases (AI125141). Oryn Therapeutics provided support in the form of salary for D.Q.T. but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00111-18.
REFERENCES
- 1.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Oude Lashof AM. 2002. Epidemiology of opportunistic invasive mycoses. Eur J Med Res 7:183–191. [PubMed] [Google Scholar]
- 3.Perlroth J, Choi B, Spellberg B. 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 4.Alnuaimi AD, Wiesenfeld D, O'Brien-Simpson NM, Reynolds EC, McCullough MJ. 2015. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral Oncol 51:139–145. doi: 10.1016/j.oraloncology.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Develoux M. 2017. Cancer and mycoses and literature review. Bull Soc Pathol Exot 110:80–84. (In French.) doi: 10.1007/s13149-017-0543-9. [DOI] [PubMed] [Google Scholar]
- 6.Nittayananta W. 2016. Oral fungi in HIV: challenges in antifungal therapies. Oral Dis 22(Suppl 1):S107–S13. doi: 10.1111/odi.12394. [DOI] [PubMed] [Google Scholar]
- 7.Pagano L, Akova M, Dimopoulos G, Herbrecht R, Drgona L, Blijlevens N. 2011. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J Antimicrob Chemother 66(Suppl 1):i5–i14. doi: 10.1093/jac/dkq437. [DOI] [PubMed] [Google Scholar]
- 8.Hebecker B, Naglik JR, Hube B, Jacobsen ID. 2014. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev Anti Infect Ther 12:867–879. doi: 10.1586/14787210.2014.916210. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Castanheira M. 2016. Nosocomial candidiasis: antifungal stewardship and the importance of rapid diagnosis. Med Mycol 54:1–22. doi: 10.1093/mmy/myv076. [DOI] [PubMed] [Google Scholar]
- 10.Ou HT, Lee TY, Chen YC, Charbonneau C. 2017. Pharmacoeconomic analysis of antifungal therapy for primary treatment of invasive candidiasis caused by Candida albicans and non-albicans Candida species. BMC Infect Dis 17:481. doi: 10.1186/s12879-017-2573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingard JR, Leather HL, Wood CA, Gerth WC, Lupinacci RJ, Berger ML, Mansley EC. 2007. Pharmacoeconomic analysis of caspofungin versus liposomal amphotericin B as empirical antifungal therapy for neutropenic fever. Am J Health Syst Pharm 64:637–643. doi: 10.2146/ajhp050521. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navalkele BD, Revankar S, Chandrasekar P. 2017. Candida auris: a worrisome, globally emerging pathogen. Expert Rev Anti Infect Ther 15:819–827. doi: 10.1080/14787210.2017.1364992. [DOI] [PubMed] [Google Scholar]
- 17.Laudenbach JM, Epstein JB. 2009. Treatment strategies for oropharyngeal candidiasis. Expert Opin Pharmacother 10:1413–1421. doi: 10.1517/14656560902952854. [DOI] [PubMed] [Google Scholar]
- 18.Pons V, Greenspan D, Lozada-Nur F, McPhail L, Gallant JE, Tunkel A, Johnson CC, McCarty J, Panzer H, Levenstein M, Barranco A, Green S. 1997. Oropharyngeal candidiasis in patients with AIDS: randomized comparison of fluconazole versus nystatin oral suspensions. Clin Infect Dis 24:1204–1207. doi: 10.1086/513664. [DOI] [PubMed] [Google Scholar]
- 19.Swidergall M, Ernst JF. 2014. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot Cell 13:950–957. doi: 10.1128/EC.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eurosurveillance Editorial Team Collective. 2013. CDC publishes report on antibiotic resistance threats in the United States for the first time. Euro Surveill 18:pii=20588 https://www.eurosurveillance.org/content/10.2807/ese.18.38.20588-en. [Google Scholar]
- 21.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox JL. 2013. Antimicrobial peptides stage a comeback. Nat Biotechnol 31:379–382. doi: 10.1038/nbt.2572. [DOI] [PubMed] [Google Scholar]
- 23.Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 24.Wilmes M, Sahl HG. 2014. Defensin-based anti-infective strategies. Int J Med Microbiol 304:93–99. doi: 10.1016/j.ijmm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Kahlenberg JM, Kaplan MJ. 2013. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191:4895–4901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri S, Edgerton M. 2014. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot Cell 13:958–964. doi: 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale BA, Fredericks LP. 2005. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol 7:119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehrer RI, Lu W. 2012. α-Defensins in human innate immunity. Immunol Rev 245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer RI, Cole AM, Selsted ME. 2012. θ-Defensins: cyclic peptides with endless potential. J Biol Chem 287:27014–27019. doi: 10.1074/jbc.R112.346098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selsted ME, Ouellette AJ. 2005. Mammalian defensins in the antimicrobial immune response. Nat Immunol 6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 31.Conibear AC, Craik DJ. 2014. The chemistry and biology of theta defensins. Angew Chem Int Ed Engl 53:10612–10623. doi: 10.1002/anie.201402167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, Winkler R, Ton A, Jabra-Rizk MA. 2009. A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One 4:e5039. doi: 10.1371/journal.pone.0005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapala-Kozik M, Bochenska O, Zawrotniak M, Wolak N, Trebacz G, Gogol M, Ostrowska D, Aoki W, Ueda M, Kozik A. 2015. Inactivation of the antifungal and immunomodulatory properties of human cathelicidin LL-37 by aspartic proteases produced by the pathogenic yeast Candida albicans. Infect Immun 83:2518–2530. doi: 10.1128/IAI.00023-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fjell CD, Hiss JA, Hancock RE, Schneider G. 2011. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 35.Garcia AE, Osapay G, Tran PA, Yuan J, Selsted ME. 2008. Isolation, synthesis, and antimicrobial activities of naturally occurring theta-defensin isoforms from baboon leukocytes. Infect Immun 76:5883–5891. doi: 10.1128/IAI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, Ouellette AJ, Selsted ME. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 37.Schaal JB, Tran D, Tran P, Osapay G, Trinh K, Roberts KD, Brasky KM, Tongaonkar P, Ouellette AJ, Selsted ME. 2012. Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis. PLoS One 7:e51337. doi: 10.1371/journal.pone.0051337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conibear AC, Rosengren KJ, Daly NL, Henriques ST, Craik DJ. 2013. The cyclic cystine ladder in theta-defensins is important for structure and stability, but not antibacterial activity. J Biol Chem 288:10830–10840. doi: 10.1074/jbc.M113.451047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran D, Tran P, Roberts K, Osapay G, Schaal J, Ouellette A, Selsted ME. 2008. Microbicidal properties and cytocidal selectivity of rhesus macaque theta defensins. Antimicrob Agents Chemother 52:944–953. doi: 10.1128/AAC.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tongaonkar P, Tran P, Roberts K, Schaal J, Osapay G, Tran D, Ouellette AJ, Selsted ME. 2011. Rhesus macaque theta-defensin isoforms: expression, antimicrobial activities, and demonstration of a prominent role in neutrophil granule microbicidal activities. J Leukoc Biol 89:283–290. doi: 10.1189/jlb.0910535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvatori O, Puri S, Tati S, Edgerton M. 2016. Innate immunity and saliva in Candida albicans-mediated oral diseases. J Dent Res 95:365–371. doi: 10.1177/0022034515625222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koshlukova SE, Araujo MW, Baev D, Edgerton M. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect Immun 68:6848–6856. doi: 10.1128/IAI.68.12.6848-6856.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koshlukova SE, Lloyd TL, Araujo MW, Edgerton M. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem 274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 44.Aerts AM, Francois IE, Meert EM, Li QT, Cammue BP, Thevissen K. 2007. The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J Mol Microbiol Biotechnol 13:243–247. doi: 10.1159/000104753. [DOI] [PubMed] [Google Scholar]
- 45.Delattin N, Cammue BP, Thevissen K. 2014. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem 6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- 46.van der Weerden NL, Lay FT, Anderson MA. 2008. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J Biol Chem 283:14445–14452. doi: 10.1074/jbc.M709867200. [DOI] [PubMed] [Google Scholar]
- 47.Steele C, Ratterree M, Fidel PL Jr. 1999. Differential susceptibility of two species of macaques to experimental vaginal candidiasis. J Infect Dis 180:802–810. doi: 10.1086/314964. [DOI] [PubMed] [Google Scholar]
- 48.Dong J, Vylkova S, Li XS, Edgerton M. 2003. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with Candida albicans. J Dent Res 82:748–752. doi: 10.1177/154405910308200917. [DOI] [PubMed] [Google Scholar]
- 49.Tran D, Tran PA, Tang YQ, Yuan J, Cole T, Selsted ME. 2002. Homodimeric theta-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem 277:3079–3084. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 50.Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J Clin Microbiol 40:3776–3781. doi: 10.1128/JCM.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfaller MA, Sheehan DJ, Rex JH. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin Microbiol Rev 17:268–280. doi: 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bensman TJ, Jayne JG, Sun M, Kimura E, Meinert J, Wang JC, Schaal JB, Tran D, Rao AP, Akbari O, Selsted ME, Beringer PM. 2017. Efficacy of rhesus theta-defensin-1 in experimental models of Pseudomonas aeruginosa lung infection and inflammation. Antimicrob Agents Chemother 61:e00154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wohlford-Lenane CL, Meyerholz DK, Perlman S, Zhou H, Tran D, Selsted ME, McCray PB Jr. 2009. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. J Virol 83:11385–11390. doi: 10.1128/JVI.01363-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tongaonkar P, Trinh KK, Schaal JB, Tran D, Gulko PS, Ouellette AJ, Selsted ME. 2015. Rhesus macaque theta-defensin RTD-1 inhibits proinflammatory cytokine secretion and gene expression by inhibiting the activation of NF-kappaB and MAPK pathways. J Leukoc Biol 98:1061–1070. doi: 10.1189/jlb.3A0315-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaal JB, Tran DQ, Subramanian A, Patel R, Laragione T, Roberts KD, Trinh K, Tongaonkar P, Tran PA, Minond D, Fields GB, Beringer P, Ouellette AJ, Gulko PS, Selsted ME. 2017. Suppression and resolution of autoimmune arthritis by rhesus theta-defensin-1, an immunomodulatory macrocyclic peptide. PLoS One 12:e0187868. doi: 10.1371/journal.pone.0187868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semple F, Dorin JR. 2012. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun 4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. 2011. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology 216:322–333. doi: 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Mookherjee N, Hancock RE. 2007. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veldhuizen EJ, Schneider VA, Agustiandari H, van Dijk A, Tjeerdsma-van Bokhoven JL, Bikker FJ, Haagsman HP. 2014. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS One 9:e95939. doi: 10.1371/journal.pone.0095939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lieberthal W, Menza SA, Levine JS. 1998. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol 274:F315–F327. doi: 10.1152/ajprenal.1998.274.2.F315. [DOI] [PubMed] [Google Scholar]
- 61.Crow JP. 1997. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 62.Rastogi RP, Singh SP, Hader DP, Sinha RP. 2010. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC. 7937. Biochem Biophys Res Commun 397:603–607. doi: 10.1016/j.bbrc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 63.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. 2013. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis 19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chowdhary A, Voss A, Meis JF. 2016. Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J Hosp Infect 94:209–212. doi: 10.1016/j.jhin.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perfect JR. 2016. “Is there an emerging need for new antifungals?” Expert Opin Emerg Drugs 21:129–131. doi: 10.1517/14728214.2016.1155554. [DOI] [PubMed] [Google Scholar]
- 68.Sanglard D. 2016. Emerging threats in antifungal-resistant fungal pathogens. Front Med (Lausanne) 3:11. doi: 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oudhoff MJ, Kroeze KL, Nazmi K, van den Keijbus PA, van 't Hof W, Fernandez-Borja M, Hordijk PL, Gibbs S, Bolscher JG, Veerman EC. 2009. Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: cyclization of histatin potentiates molar activity 1,000-fold. FASEB J 23:3928–3935. doi: 10.1096/fj.09-137588. [DOI] [PubMed] [Google Scholar]
- 70.Yu Q, Lehrer RI, Tam JP. 2000. Engineered salt-insensitive alpha-defensins with end-to-end circularized structures. J Biol Chem 275:3943–3949. doi: 10.1074/jbc.275.6.3943. [DOI] [PubMed] [Google Scholar]
- 71.Beringer PM, Bensman TJ, Ho H, Agnello M, Denovel N, Nguyen A, Wong-Beringer A, She R, Tran DQ, Moskowitz SM, Selsted ME. 2016. Rhesus theta-defensin-1 (RTD-1) exhibits in vitro and in vivo activity against cystic fibrosis strains of Pseudomonas aeruginosa. J Antimicrob Chemother 71:181–188. doi: 10.1093/jac/dkv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—2nd ed CLSI document M27-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 73.Singh-Babak SD, Babak T, Diezmann S, Hill JA, Xie JL, Chen YL, Poutanen SM, Rennie RP, Heitman J, Cowen LE. 2012. Global analysis of the evolution and mechanism of echinocandin resistance in Candida glabrata. PLoS Pathog 8:e1002718. doi: 10.1371/journal.ppat.1002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cantón E, Peman J, Viudes A, Quindos G, Gobernado M, Espinel-Ingroff A. 2003. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn Microbiol Infect Dis 45:203–206. doi: 10.1016/S0732-8893(02)00525-4. [DOI] [PubMed] [Google Scholar]
- 75.Silveira CP, Torres-Rodriguez JM, Alvarado-Ramirez E, Murciano-Gonzalo F, Dolande M, Panizo M, Reviakina V. 2009. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J Med Microbiol 58:1607–1610. doi: 10.1099/jmm.0.007609-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.