Abstract
Background:
The (1,3)-β-D-glucan assay (BDG) is recommended for the early diagnosis of invasive candidiasis (IC).
Methods:
Records of 154 critically ill adults with suspected IC, on whom BDG was done, were analyzed. Patients were divided into three groups: Group A (confirmed IC), Group B (alternative diagnosis or cause of severe sepsis), and Group C (high candidal score and positive BDG [>80 pg/mL] but without a confirmed diagnosis of IC).
Results:
Mean BDG levels were significantly higher in Group A (n = 32) as compared to Group B (n = 60) and Group C (n = 62) (448.75 ± 88.30 vs. 144.46 ± 82.49 vs. 292.90 ± 137.0 pg/mL; P < 0.001). Discontinuation of empiric antifungal therapy based on a value <80 resulted in cost savings of 14,000 INR per day per patient.
Conclusion:
A BDG value of <80 pg/ml facilitates early discontinuation of empirical antifungal therapy, with considerable cost savings.
Keywords: Antifungal stewardship, invasive candidiasis, β-D-glucan
INTRODUCTION
Candida bloodstream infection (candidemia) or invasive candidiasis (IC) in Intensive Care Units (ICU) is associated with great morbidity and mortality.[1] The situation is no different in India: in a multicentric study, the 30-day crude and attributable mortality rates were 44.7 and 19.6%, respectively.[2] These high mortality rates highlight the fact that diagnosis of IC is often missed which leads to mortality.
Although blood culture is the gold standard for diagnosis of candidemia, it suffers from low sensitivity (50%) and longer turnaround time (2–4 days).[3] As blood cultures are insensitive and time-consuming, diagnosis of IC is often based on clinical suspicion, candidal score, and Candida colonization index, none of which are sensitive or specific; it has been estimated that up to 70% of patients who receive empiric antifungal agents do not have proven candidal infection.[4]
(1,3)-β-D-glucan (BDG) is a cell wall polysaccharide found in almost all pathogenic fungi with exception of zygomycetes and Cryptococcus.[5] BDG assay has been utilized in the early diagnosis of IC/candidemia and also has a high negative predictive value (NPV) and therefore can be utilized for stopping empirical antifungal therapy.[5]
The BDG assay has recently been introduced in India and the clinical efficacy data of BDG in Indian ICUs are lacking. We retrospectively analyzed BDG data to determine the effect of utilizing it in stopping or de-escalating antifungal therapy in Indian settings.
METHODS
This was a retrospective study conducted in a 24-bed ICU of a tertiary care center in South India. Records of patients for which BDG assay (October 2016–August 2017) was requested were analyzed for basic parameters, candidal score, BDG value, duration and type of antifungal agent used, and outcome of the patient. Records were also looked at for factors which cause false-positive BDG which included concurrent bacteremia, IV albumin infusion, intravenous immunoglobulin (IVIG), beta-lactam (amoxycillin-clavulanate and piperacillin-tazobactam) antibiotics, and hemodialysis cellulose filters or membranes. The Food and Drug Administration-approved Fungitell® BDG assay was used. A value of <60 pg/mL is regarded as negative, >80 pg/mL is regarded as positive, and 60–80 pg/mL is taken as equivocal.[6] The Fungitell® assay has upper limit of 523 pg/mL, so values of >523 pg/mL were taken as 523 for ease of statistical analysis.[6]
Patients with age <18 years, absolute neutrophil count of <500 cells/mL, on immunosuppressive drugs, with diagnosis of other fungal infections, or those already on antifungal prophylaxis or therapy were excluded from the study.
Patients were divided into three groups. Group A comprised of patients in whom diagnosis of IC was confirmed, i.e., blood culture or culture from sterile site grew Candida. Group B comprised of patients in whom an alternative etiology of severe sepsis or septic shock was found and Group C had patients with high candidal score and positive BDG (>80 pg/mL) but without a confirmed diagnosis of IC or an alternative explanation for sepsis. Intergroup analysis for continuous data was performed using Student's t-test. Categorical data were analyzed using Kruskal–Wallis test and Chi-square test. P < 0.05 was considered statistically significant. Statistical analysis was done using IBM Statistical Package for the Social Sciences (SPSS) Statistics 21.0. Institutional ethical committee clearance was taken for the study.
RESULTS
The medical records of 154 patients were analyzed. The mean age of the study participants was 53.19 years and 88 (57.14%) were male. Mean age across the three groups was comparable. Various clinical features are described in Table 1. Distribution of comorbid conditions was comparable in all the three groups. Surgical conditions were significantly higher in Groups A and C (proven or probable candidemia).
Table 1.
Characteristics of the study population
Mean candidal scores were 3.06 ± 0.85, 2.63 ± 0.71, and 2.90 ± 0.87 in Groups A, B, and C, respectively, which was not significantly different [Figure 1]. Distribution of individual risk factors for candidiasis across the three groups is summarized in Table 1. Mean central line days were 3.56 ± 0.92, 3.57 ± 0.85, and 6.09 ± 1.2 days in Groups A, B, and C, respectively, wherein, mean central line days were significantly higher in Group C. All the other risk factors were comparable among the three groups.
Figure 1.
Mean candidal score across the three groups
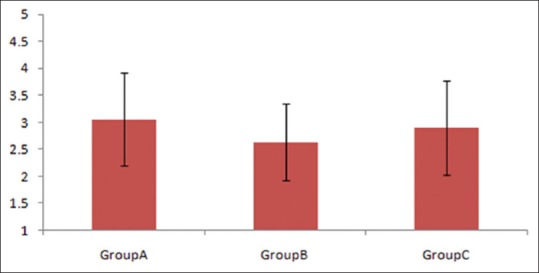
The mean BDG values were 448.75 ± 88.30, 144.46 ± 82.49, and 292.90 ± 137.0 pg/mL in Groups A, B, and C [Figure 2], respectively, and were significantly higher in Groups A and C as compared to Group B (P < 0.001). None of the patients with proven candidemia or IC had values <80 pg/mL, whereas it was >80 pg/mL in 50 (83.3%) patients of Group B. BDG value between 60 and 80 pg/mL was seen in two patients but none with <60 pg/mL among those belonging to Group C. No patient in Group B had a value of >523 and no patient in Group A had a value of <80. Fourteen patients had BDG of ≥523 in Groups A and C combined, but none in Group B. Sensitivity, specificity, NPV, and positive predictive value (PPV) of IC/candidemia with BDG of ≥523 pg/mL were 14.8%, 100%, 42.8%, and 100%, respectively. Specificity of ruling out IC/candidemia with BDG of <80 pg/mL was 97.8%.
Figure 2.
Mean (1,3)-β-d-glucan values across the three groups
Use of β-lactam antibiotics, bacteremia, and IV albumin was significantly higher in Group B as compared to Groups A and C. Gram-negative bacteremia (n = 8) led to more false-positive BDG as compared to Gram-positives (n = 4). Escherichia coli (n = 4) and Klebsiella (n = 2) were the most common causes followed by bacteremia caused by Acinetobacter (n = 2) and Staphylococcus aureus (n = 2). Mean BDG level in these patients with bacteremia was 117.4 pg/mL [Table 2].
Table 2.
Mean β-d-glucan values among patients with bacteremia
Most of the patients (148) were started on antifungals on the day IC or candidemia was suspected and BDG was ordered. Echinocandins were used in 125 (81.1%) patients. Caspofungin was the most common antifungal agent used in 82 (53.2%) patients followed by anidulafungin in 32 (20.7%) and fluconazole in 29 (18.8%) patients. Major changes in prescription of antifungals occurred in Group B after BDG result. Antifungals were stopped in 15 (25%) patients in Group B as an alternative diagnosis was made even before BDG was available. Of the remaining 45, for 10 patients in whom BDG was <80 pg/mL, antifungals were stopped. Antifungals were continued in 35 patients in Group B after observing BDG results as they were >80 pg/mL. The mean antifungal duration was 16.12 ± 2.4, 5.1 ± 1.0, and 15.9 ± 3.9 days in Groups A, B, and C, respectively. It was significantly less in Group B as compared to that of Groups A and C (P < 0.001).
Out of 154 patients, 34 died within the same admission leading to crude mortality of 22.07%. Distribution of mortality was comparable across the groups. Among patients who had a BDG of ≥400 pg/mL, 26 (out of 48) patients died, a significantly higher mortality rate than those with BDG of <400 pg/mL (54.6% vs. 7.5%; P < 0.001).
Sensitivity, specificity, NPV, and PPV were 97.8%, 16.6%, 83.3%, and 66.7%, respectively, at a cutoff of 80 pg/mL. The receiver operating characteristic curve showed sensitivity, specificity, NPV, and PPV of 97.8%, 63.6%, 95.0%, and 80.7%, respectively (area under the curve = 0.908 and P < 0.001), at a cutoff of 143.5 pg/mL [Figure 3].
Figure 3.
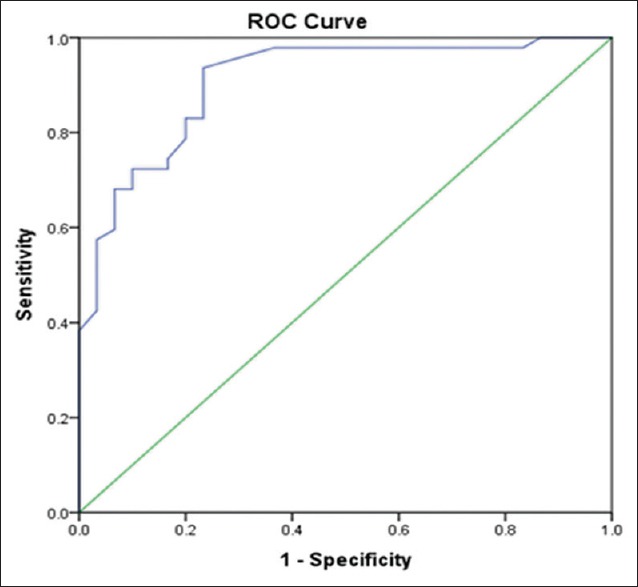
Receiver operating curve showing trend of sensitivity and specificity in this study
DISCUSSION
The glucan component is composed mainly of glucose polymers linked via β-1,3-glycosidic bonds, forming the backbone of the fungal cell wall.[7] The basis of the Fungitell® assay relies on beta glucan's ability to activate the limulus amebocyte lysate clotting cascade present in the blood of Limulus polyphemus, the North American horseshoe crab.[8] The resulting clotting can be measured via kinetic spectrophotometry and thus gives an indirect measure of BDG level in serum. BDG levels rise with many pathogenic fungi with exception of Cryptococcus species and the yeast phase of Blastomyces dermatitidis, as well as the zygomycetes.[5] Sensitivity and specificity values of BDG can range from 38% to 100% and 45% to 99%, respectively, with similar ranges observed for the PPV (30% to 89%) and NPV (73% to 97%) for IC/candidemia.[9,10,11]
Apart from its diagnostic utility in IC/candidemia, BDG can also be used in the prognosis of patients with candidemia. Sims et al. showed that severe sepsis (91% vs. 28%) and mortality (36% vs. 6%) were significantly higher in patients with BDG result of 400 pg/mL or more compared to those with a BDG <400 pg/mL.[12] In our study, mortality rates were higher in a subset of patients with >400 pg/mL.
A number of confounding factors affect BDG values which include hemodialysis with cellulose membranes, serosal exposure to gauze, administration of blood products, albumin, immunoglobulin, coagulation factors, or plasma protein fraction filtered through BG-containing filters.[5] β-lactam antibiotics, especially amoxycillin-clavulanate and piperacillin-tazobactam and, concurrent bacteremia (both Gram positive and Gram negative) can also falsely elevate BDG.[13,14]
In our study, we found that both Gram-negative (E. coli, Klebsiella pneumoniae, and Acinetobacter) and Gram-positive (Staphylococcus aureus) bacteria cause high BDG with a mean of 117.4 pg/mL. We found that β-lactam antibiotics, IV albumin, and bacteremia were significantly higher in Group B and other factors such as IVIG and mesh had a trend to be higher in Group B. Since it is practically impossible to avoid these factors in ICU settings, we tried to validate the cutoff for BDG, taking these factors into consideration. With standard cutoff of 80 pg/mL, sensitivity was 97.8% but specificity was meager (16%), likely because of these factors which falsely elevated BDG in Group B. With increase in BDG cutoff of 143.5 pg/mL, there was no loss of sensitivity, but specificity increased to 63.6%. We could also demonstrate an increase in NPV from 83.3% to 95%.
With poor availability of diagnostic methods for IC/candidemia, overuse of antifungals is rampant.[4] Owing to its good NPV, BDG has been shown to be an effective tool to aid stopping antifungal therapy.[15] In our study, antifungal agents were discontinued in all patients with BDG <80 pg/mL in Group B. In our study, all patients had a high risk for Candida (all the three groups had mean candidal score of >2.5) and would have got prolonged antifungal therapy based on conventional methods of diagnosing IC/candidemia in ICU (clinical suspicion, risk factors, and candidal score). Using BDG for its NPV helped stopping “unnecessary” antifungal agents in ten of our patients preventing them from getting exposed to longer empirical therapy. As each dose of caspofungin (which was the most common antifungal used) costs 14,000 INR (215 USD), up to 10 days of antifungal therapy was saved per patient, amounting to 1.4 lac INR (2150 USD) per patient. Similar cost-effectiveness of using BDG in ICU settings has been proven in another study.[15]
Our study had several limitations; first, it was a retrospective data analysis in a single center. Second, BDG is known to rise serially and most of our patients had only a single BDG estimation. Third, BDG is not specific for Candida, so exclusion of non-Candida fungal infection is not possible with 100% accuracy.
CONCLUSION
Our study supports the use of BDG in the exclusion of IC in ICU patients, with a value <80 pg/ml (the lower limit cutoff), facilitating the early discontinuation of empirically started antifungal therapy and resulting in considerable cost savings. A value >523 pg/ml (the highest possible reading) was also strongly correlated with IC and facilitated continuation of antifungals even though IC was not confirmed. A value of >400 may also carry a poorer prognosis. This study also suggests a higher cutoff of at least 143.5 pg/mL which will be more specific for IC, although this may need further validation with larger trials.
We recommend that clinicians routinely utilize the BDG assay along with blood cultures for both the diagnosis and exclusion of IC in high-risk critically ill patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.CAVA I Study Group. González de Molina FJ, León C, Ruiz-Santana S, Saavedra P. Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care. 2012;16:R105. doi: 10.1186/cc11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–95. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 3.Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284–92. doi: 10.1093/cid/cit006. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Dupont H, Tabah A, Lortholary O, Stahl JP, Francais A, et al. Systemic antifungal therapy in critically ill patients without invasive fungal infection. Crit Care Med. 2012;40:813–22. doi: 10.1097/CCM.0b013e318236f297. [DOI] [PubMed] [Google Scholar]
- 5.Ostrosky-Zeichner L, Alexander BD, Kett DH, Vazquez J, Pappas PG, Saeki F, et al. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 6. [Last accessed on 2017 Oct 19]. Available from: http://www.acciusa.com/pdfs/accProduct/Fungitell_multilang_pisheets/Fungitell%20Insert%20EN.pdf.
- 7.Tran T, Beal SG. Application of the 1,3-β-D-glucan (Fungitell) assay in the diagnosis of invasive fungal infections. Arch Pathol Lab Med. 2016;140:181–5. doi: 10.5858/arpa.2014-0230-RS. [DOI] [PubMed] [Google Scholar]
- 8.Novitsky TJ. Biomedical applications of limulus amebocyte lysate. In: Tanacredi JT, Botton ML, Smith DR, editors. Biology and Conservation of Horseshoe Crabs. New York: Springer; 2009. pp. 315–29. [Google Scholar]
- 9.Karageorgopoulos DE, Vouloumanou EK, Ntziora F, Michalopoulos A, Rafailidis PI, Falagas ME, et al. B-D-glucan assay for the diagnosis of invasive fungal infections: A meta-analysis. Clin Infect Dis. 2011;52:750–70. doi: 10.1093/cid/ciq206. [DOI] [PubMed] [Google Scholar]
- 10.Del Bono V, Delfino E, Furfaro E, Mikulska M, Nicco E, Bruzzi P. Clinical performance of the (1,3)-beta- D -glucan assay in early diagnosis of nosocomial Candida blood stream infections. Clin Vaccine Immunol. 2011;18:2113–7. doi: 10.1128/CVI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohr JF, Sims C, Paetznick V, Rodriguez J, Finkelman MA, Rex JH, et al. Prospective survey of (1→3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical Intensive Care Unit setting. J Clin Microbiol. 2011;49:58–61. doi: 10.1128/JCM.01240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sims CR, Jaijakul S, Mohr J, Rodriguez J, Finkelman M, Ostrosky-Zeichner L. Correlation of clinical outcomes with b-glucan levels in patients with invasive candidiasis. J Clin Microbiol. 2012;50:2104–6. doi: 10.1128/JCM.00773-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a b D -glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–62. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert O, Toubas D, Strady C, Cousson J, Delmas C, Vernet V, et al. Reactivity of (1→3)-β-d-glucan assay in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis. 2011;30:1453–60. doi: 10.1007/s10096-011-1244-8. [DOI] [PubMed] [Google Scholar]
- 15.Posteraro B, Tumbarello M, De Pascale G, Liberto E, Vallecoccia MS, De Carolis E, et al. (1,3)-β-d-glucan-based antifungal treatment in critically ill adults at high risk of candidaemia: An observational study. J Antimicrob Chemother. 2016;71:2262–9. doi: 10.1093/jac/dkw112. [DOI] [PubMed] [Google Scholar]


