Abstract
Gulf War Illness (GWI) is a chronic disease that affects the 1991 Gulf War (GW) veterans for which treatment is lacking. It has been hypothesized that drugs used to protect military personnel from chemical attacks and insects during the war: pyridostigmine bromide (PB), N, N-diethyl-m-toluamide (DEET), and permethrin (PER) together with stress may have contributed collectively and synergistically to generate GWI. There is a need to find markers of pathology to be used in pre-clinical trials. For this purpose we employed a previously validated mouse model of GWI evoked by daily exposure to PB (1.3 mg/kg), DEET (40 mg/kg), PER (0.13 mg/kg), and 5 minutes of restraint stress for 28 days to analyze behavior, brain pathology and neurochemical outcomes three months later. GWI-model mice were characterized by increased anxiety, decreased hippocampal levels of N-acetyl aspartate, GABA, the GABA-producing enzyme GAD-67 and microglial activation. We also observed that GWI model was sexually dimorphic on some measures: males had increased while females had decreased protein levels of the acetylcholine-synthesizing enzyme, choline acetyltransferase, in the septum and hippocampus and decreased levels of the receptor for brain-derived neurotrophic factor, TrkB140, in the hippocampus. Increased hippocampal levels of nerve growth factor were detected in males only. Together the data show behavioral and neuropathological abnormalities detected at 3 months post-exposure and that some of them are sexually dimorphic. Future preclinical studies for GWI may take advantage of this short latency model and should include both males and females as their response to treatment may differ.
Keywords: Gulf war illness, cholinesterase inhibitors, anxiety, magnetic resonance spectroscopy
1. Introduction
Gulf War illness (GWI) refers to the complex chronic symptoms that affect about one-third of veterans of the Gulf War (GW) (1990–1991). Although disease presentation varies from person to person, symptoms include some combination of widespread pain, headache, memory and thinking dysfunction, fatigue, breathing problems, stomach and intestinal symptoms, and skin abnormalities. Neurological symptoms including cognitive impairment, attention deficits, depression and anxiety are a top complaint among GWI patients (Binns, 2008; Binns JH, 2014). Evidence for involvement of the central nervous system (CNS) in GWI also comes from imaging studies showing reduced hippocampal volume and hypometabolism (Menon et al., 2004) and weakened axonal tracks linking the cortical gray matter regions involved in fatigue, pain and cognition (Rayhan et al., 2013). Longitudinal studies consistently indicate that GWI rates and symptoms have not declined with time (Binns JH, 2014). The etiology and pathophysiology of GWI remain poorly understood and treatments are lacking. Most studies suggest that GWI may be the result of exposure to drugs designed to protect military personnel from a chemical warfare attack and from insects (White et al., 2016). These include: 1) pyridostigmine bromide (PB), a reversible inhibitor of acetylcholinesterase (AChE) that prevents nerve agents such as sarin from inhibiting AChE permanently; 2) permethrin (PER), an insecticide whose mechanism of action is to modify neuronal sodium channels; and 3) DEET, an insect repellent that also exhibits inhibitory activity on sodium and potassium channels (Swale et al., 2014) and a weak AChE inhibitor that may enhance the activity of others such as PB (Chaney et al., 2000). These drugs target the nervous system and in particular, via the inhibition of AChE, the cholinergic system. Although these drugs are considered safe at the doses administered to GW personnel, it has been hypothesized that their combinations together with the stress encountered in theater may have contributed collectively and synergistically to generate the GWI (Abdullah et al., 2012; White et al., 2016) (Abdel-Rahman et al., 2002; Parihar et al., 2013). PB under normal circumstances does not cross the blood brain barrier (BBB) however, after exposure to stress or GW-related chemicals, the BBB may become leaky (Abdel-Rahman et al., 2002) allowing PB to enter into the brain where it decreases AChE activity, increasing the concentration of acetylcholine (ACh) in synaptic clefts causing enhanced cholinergic neurotransmission. Furthermore, exposure to PER causes sustained opening of voltage-gated sodium channels in neurons, thus facilitating the neuronal excitation (Narahashi, 1985).
The pathophysiology of GWI also involves abnormalities in the function of the cholinergic parasympathetic system (Haley et al., 2013). Cognitive and sleep disturbances that characterize GWI are consistent with a dysfunction of the basal forebrain cholinergic neurons (BFCN) whose normal activity is central to the processes of memory, attention and sleep (Hasselmo and Sarter, 2011). The idea that combination of the above-listed drugs and stress caused a long-term dysfunction of cholinergic neurons within the CNS have been tested in rats (Abdel-Rahman et al., 2002; Abou-Donia et al., 2004) (Parihar et al., 2013) and mice (Abdullah et al., 2012). These studies reported multiple physiological and behavioral symptoms such as increase in muscarinic receptor binding, altered acetylcholinesterase activity, and altered phosphocholine containing lipids that suggest altered acetylcholine metabolism and signaling and are consistent with the cholinesterase hypothesis of GWI.
Because affected veterans continue to suffer the symptoms of GWI more than 25 years later, animal research in the field has had a tendency to analyze the effect of exposure at an increasingly delayed post-exposure time. While this approach is valuable for studies on GWI pathophysiology, use of shorter latencies that manifest phenotypes of interest are better suited for studies aimed at the development of treatments for this illness. The goal of this study was to find such early markers of GWI in order to efficiently screen for therapies that can reverse or reduce the course of the disease. Using a well-accepted model of GWI (Abdel-Rahman et al., 2002) based on the daily exposure of adult mice to the oral treatment of PB (1.3 mg/kg), the topical application of DEET (40 mg/kg), and PER (0.13 mg/kg), and a 5 min restrain during 1 month, mice behavior and pathology were analyzed 3 months post-exposure.
2. Results
2.1 Effect of exposure to GW chemicals/stress on mouse behavioral tests
Three months after the daily exposure to PB, DEET, PER, and 5 minutes of restraint for 1 month we sequentially tested the behavior of exposed and control mice on locomotion, stereotypy, anxiety, and learning and memory.
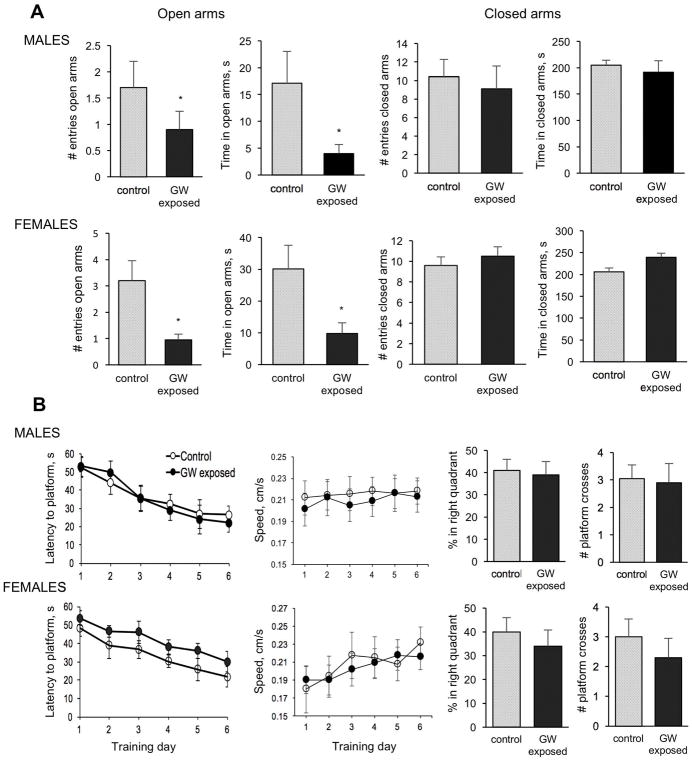
Both female and male mice exposed to the GW-related chemicals and stress exhibited anxiety-like behavior on a plus maze apparatus during a 5-minute recorded test (Figure 1A). The anxiogenic effect was revealed by exposed mice having significantly fewer entries and reduced dwell time in open arms than controls while no differences between GW-exposed and controls were detected in closed arms. Differences found in open arms were therefore not a function of motility. Mice’s learning capacity and memory was tested in the MWM test and no significant differences between exposed and control male and female mice were detected 3 months after exposure (Figure 1B). In the training trials in which mice learned to escape from the water onto a submerged platform, both female and male mice exposed and control learned the task at a similar pace reaching the average latency of 20s in a period of 6 days. No significant differences in swim speed were observed between groups either. A probe trial performed to detect changes in memory was performed 24h after the last trial on day 6, and showed no significant differences between exposed and control mice in any of the parameters measured. Likewise, at 3 months post-exposure time point, we did not detect significant effect of GW-related chemicals and stress on the level of locomotion and stereotypy movements during recorded 30-minute sessions in locomotor chambers (Data not shown).
Figure 1.
Effect of exposure to GW-related chemicals and stress on anxiety, learning and memory. A. Anxiety. Exposure causes increased anxiety revealed in the Plus Maze test as both GW-exposed female and male mice did not enter as often and did not spent as much time as control mice in open arms. Two-way ANOVA for the number of entries into the open arms found a main effect of exposure (F= 4.31; p<0.05) with no effect of sex and no interactions. For time spent in open arms there was a main effect of exposure (F=4.9; p = 0.03) and no effect of sex and no interaction between factors. B. Learning and memory in the water maze. No significant differences in the escape latency and comparable swim speed between exposed and control female and male mice were observed during the 6 days of training trials. The probe trial performed twenty-four hours after the last training trial to test mice’s memory also showed no significant differences between groups for any of the analyzed parameters.
2.2 Effect of exposure to GW chemicals/stress on ChAT and GFP levels
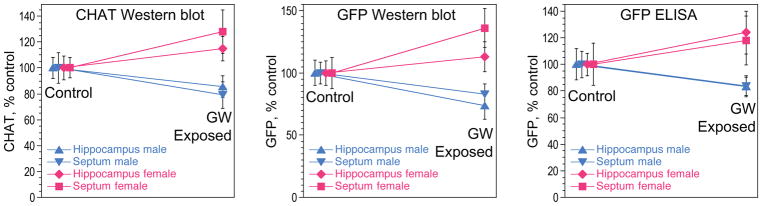
Levels of ChAT and GFP proteins were analyzed by western blot for ChAT and by western blot and ELISA for GFP using whole protein homogenates from hippocampus and septum regions. CHGFP mice express the GFP protein under the control of the endogenous Chat transcriptional regulatory elements and thus we predicted good correlation of expression of these 2 proteins. This was indeed the case (Figure 2). Remarkably, we found sexually dimorphic effects of exposure on levels of ChAT and GFP proteins such that these measures were reduced in the males (by approximately 15–20%) and increased in the females (by 13–36% depending on the measure) as compared to controls (Figure 2). This sexually dimorphic effects of exposure to GW-related chemicals and stress were statistically significant as two-way ANOVA using the two brain regions, hippocampus and septum, as a repeated measure, revealed significant interaction terms between exposure and sex (F = 9.83; p = 0.003 for ChAT; F = 6.33; p = 0.016 for GFP assayed by western blot; and F = 5.52; p = 0.025 for GFP determined by ELISA).
Figure 2.
Sexually dimorphic cholinergic abnormalities in GW exposed mice. The effect of exposure to GW-related chemicals and stress on the levels of the ACh-synthesizing enzyme, ChAT, and GFP in the septum and hippocampus of CHGFP mice. Two-way ANOVA using the two brain regions, hippocampus and septum, as a repeated measure, revealed significant interaction terms between exposure and sex (p = 0.003 for ChAT; p = 0.016 for GFP assayed by western blot; and p = 0.025 for GFP determined by ELISA). This interaction is illustrated by the consistently diverging blue (male) and pink (female) lines for all of the measures.
2.3 Effect of Exposure to GW Chemicals/Stress on the Activation of Microglia and Astrocytes
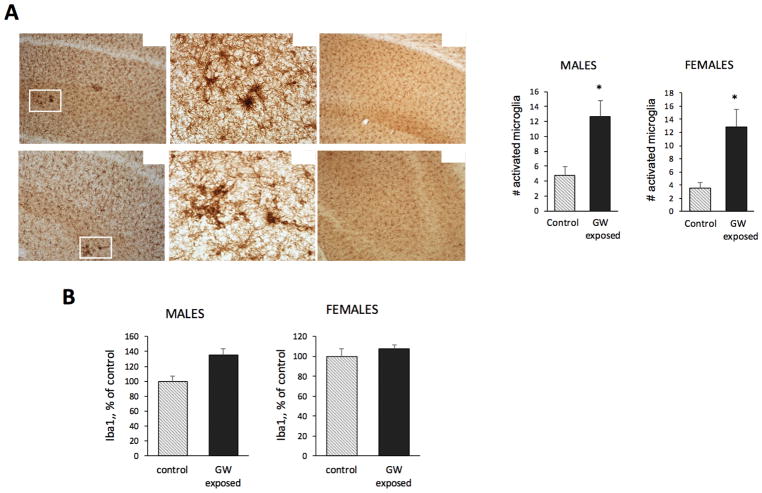
Sparse activated microglia throughout the brain were detected in Iba-1 stained tissue from exposed and control mice. We especially detected activated microglia in the CA1-3 areas of the hippocampus of exposed mice. Quantitative analysis of activated microglia in the CA1-3 region showed a significant increase in the number of activated microglia in mice exposed to GW-related chemicals and stress compared to controls (Figure 3A). No significant differences in the number of activated microglia were observed in cortex and amygdala. The steady state level of Iba1, as a marker of microglial activation, was analyzed by western blot in whole hippocampal extracts. The level of Iba1 was higher in exposed males and females than in control mice (an increase of 35.5% for males and 8% for females as compared to the respective controls). Two-way ANOVA showed a significant effect of exposure for Iba1 (F = 4.63; p = 0.038), and no effect of sex (Figure 3B). No significant effect of exposure on GFAP was detected by western blot using whole hippocampal extracts nor by densitometric analysis of cortex and hippocampus in immunostained tissue sections (Data not shown).
Figure 3.
Microglial activation in the hippocampus of GW-exposed mice. A. Representative microphotographs showing that a larger number of activated microglia, prominent by their distinctive cell morphology and increased levels of Iba1 expression, were detected in the CA1-3 regions of the hippocampus of exposed CHGFP mice compared to controls in both female and male mice. Original pictures were taken at 100x and 400x, as indicated. The inset graphs are the results from the quantitation of the number of activated microglia in the CA1-3 region showing that in this region of the hippocampus the number of activated microglia is significantly larger in exposed female and males than in their respective control mice. B. Western blot analysis of protein extracts from the whole hippocampus with Iba1 antibodies. The level of Iba1 increased in exposed males and females to 135.5% and 107.8% of controls, respectively. Two-way ANOVA showed a significant effect of exposure for Iba1 (F = 4.63; p = 0.038), and no effect of sex.
2.4 Effect of Exposure to GW Chemicals/Stress on NGF, BDNF and TrkB
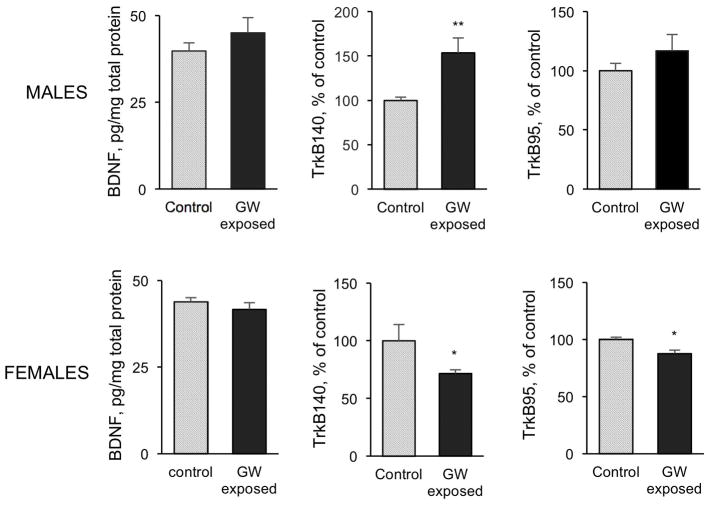
We analyzed the effect of exposure on the two major neurotrophic factors for cholinergic neurons, NGF and BDNF, on hippocampal extracts by ELISA. We found a significant increase in the levels of NGF, but not BDNF, in exposed males compared to controls (Figure 4). No difference in the levels of NGF or BDNF was detected in female exposed mice. We further determined the effects of exposure on the BDNF receptor, TrkB, by western blot analysis (Figure 4). Exposure to chemicals and stress resulted in a highly significant increase in the level of TrkB140 (the full-length protein) in the hippocampus of male mice compared to controls (153.5% of control). In contrast, there was a significant decrease in the level of TrkB140 in exposed females (71.6% of control), concomitant with a significant decrease in the TrkB90, the truncated form of the receptor (87.7% of control).
Figure 4.
Abnormalities in NGF, BDNF and TrkB in GW exposed mice. Increased hippocampal NGF levels were detected in male, but not female, mice 3 months after exposure to GW related chemicals and stress. No changes in BDNF levels after exposure were detected in the hippocampus of either male or female mice. Sexual dimorphism was evident in the effect of exposure on TrkB receptor forms. Data are presented as mean ± SEM. *p<0.05; **p<0.01.
2.5 Effect of Exposure to GW Chemicals/Stress on Brain Neurochemicals
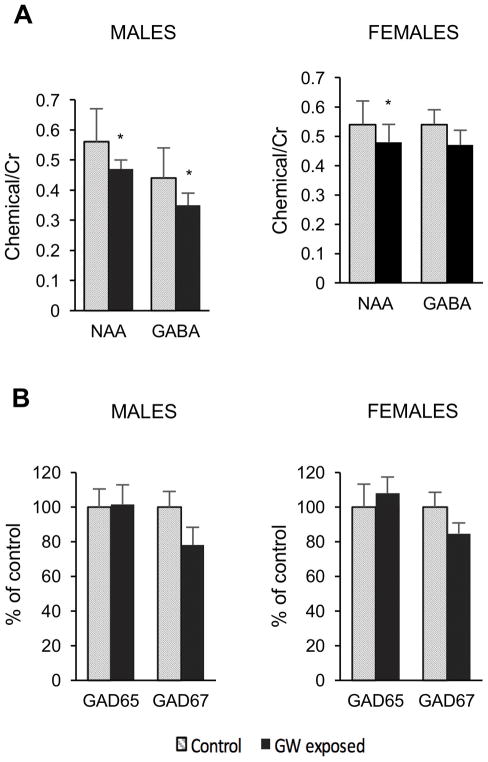
We analyzed two brain regions by magnetic resonance spectroscopy (MRS) using High Resolution Magic Angle Spinning Spectroscopy (HRMAS): the hippocampus for its unique role in a number of the symptoms associated with GWI, and the medial prefrontal cortex as a representative cortical region involved in memory and motivation. We quantified a total of 17 neurochemicals. There was a large difference between brain regions for a number of metabolites as we, and others, have published (Choi et al., 2007). No significant changes were noted between exposed and control mice in the medial prefrontal cortex for any of the chemicals we studied. In the hippocampus there were two chemicals whose levels were significantly decreased in the exposed male mice, N-acetylaspartate (NAA) (18% decrease) and γ-aminobutyric acid (GABA) (20% decrease) both of which have primarily neuronal localization (Figure 5A). In the female mice, similar to the male mice, we found a decrease in NAA in the GW-exposed mice compared to controls in the hippocampus [0.54±0.08 (control) vs. 0.48±0.06; (exposed) p<0.05]. However, there was no significant effect on GABA (0.54±0.05 control vs. 0.47±0.05 exposed; p>0.3) (Figure 5A). A two-way ANOVA considering sex and exposure as separate factors led to a main effect on NAA of exposure (F= 8.34; p<0.01) with no effect of sex and no interaction between factors. For GABA there was a main effect on exposure (F = 5.61; p<0.03) but no effect of sex or on an interaction between terms. Therefore, MRS can detect differences in the neuronal health as a result of exposure to our GWI protocol. The use of NAA as a marker for neuronal health is well established (Jenkins and Kraft, 1999) and it appears to be a sensitive marker for the GWI mouse model.
Figure 5.
A. Decreases in NAA and GABA, measured using HRMAS from punches of the hippocampus, in GWI model male mice compared to control not exposed mice. There is an 18% decrease in NAA and a 20% decrease in GABA following exposure to the chemicals and stress. * p < 0.05 for a one-way ANOVA comparing the two groups with a Student–Newman–Keuls post hoc test for multiple comparisons. B. Comparative % decrease to GABA was detected for GAD67, but not GAD65. A two-way ANOVA for GAD67 showed a main effect of exposure (F = 4.6; p<0.04) with no effect of sex and no interaction.
2.6 Effect of Exposure to GW Chemicals/Stress on GAD65/GAD67
Since GW-related chemicals and stress decreased the levels of GABA in the hippocampus, we analyzed the effect of exposure on the expression levels of hippocampal GAD65 and GAD67, the 2 isoforms of glutamic acid decarboxylase that synthesizes GABA, by western blot (Figure 5B). Results showed a decrease in the level of GAD67, the rate limiting isoform, in both exposed female and male mice (84.822% of control, p=0.16 and 78.0622% of control, p=0.22, respectively). A two-way ANOVA test for GAD67 using sex and exposure as variables showed a significant effect of exposure (F = 4.6; p<0.04) with no effect of sex and no interaction. No comparable change was detected for GAD65. Although the GAD measures were made using relative measures rather than quantitative western blots, the magnitude of the decreases in GAD67 paralleled the decreases in GABA in the male mice (78% of control for GAD67 vs. 80% for GABA) and also in the females (84% of controls for GAD67 vs. 87% for GABA). There was a positive correlation between the GABA levels versus the GAD67 values (R = 0.44; p< 0.01), though the inability to determine the absolute GAD67 protein levels with the current data precludes a more detailed comparison with GABA levels.
3. Discussion
GWI remains an untreatable disease and animal studies looking for remedies to this illness are lacking. The present study attempts to establish a convenient mouse model for this purpose. As GWI developed into a persistent chronic disease, animal research in the field had a tendency to analyze the effect of exposure at an increasingly delayed post-exposure times. Although this is understandable, studies utilizing shorter delays will be more efficient for screening novel GWI therapies. Applying a validated chronic model of the disease, the current study aimed to detect early markers of the chronic condition that could be used in future pre-clinical trials. The mouse model used was first reported by Abdel-Rahman et al (Abdel-Rahman et al., 2002) and thereafter applied by others (Abdullah et al., 2012; Parihar et al., 2013). The model is based on an exposure protocol to low doses of PB, PER and DEET together with mild stress that closely mimics that of soldiers during the GW. The doses are similar to the levels of human exposure and their effects have physiological relevance, as discussed (Abou-Donia et al., 2001). The chemicals’ route of exposure also closely mimics the human exposure during the GW. Mice were exposed to PB in their drinking water to imitate the military personnel taking tablets of PB. DEET and PER, used to pre-soak uniforms and nets, were applied on a naked area of the mouse skin. The low level of stress was administered using a mouse restrainer for 5 minutes/day. Because GWI patients suffer from cognitive impairments, anxiety, abnormal sleep patterns, and disturbances of parasympathetic control of the heart (Haley et al., 2013; Stein et al., 2004), we used CHGFP mice to facilitate studies of various classes of cholinergic neurons that control and/or modulate these functions. Because GWI is a latent and persistent condition, we analyzed the effects of exposure after a post-exposure period of 3 months, when the chronic nature of the disease has been established, and elected to perform a comprehensive behavioral, neuropathological, and neurochemical analysis of the mice. Our analysis revealed multiple effects of GW-related exposures in all of these domains.
The most dramatic consequence of GW-related exposures was increased anxiety as measured with the plus maze. Both exposed male and female mice displayed great reluctance to venture into the open arms and spent as little as 25%–30% of the time there as compared to controls. In previous studies, similar exposure to chemicals and stress in C57BL/6 mice also increased measures of anxiety (Abdullah et al., 2012). Moreover, studies in rats showed sensorimotor deficits after exposure to PB, PER and DEET (Abou-Donia et al., 2001) and cognitive dysfunction that worsen with the addition of mild stress (Parihar et al., 2013). We observed no cognitive impairment in the exposed mice. In studies by Zakirova et al. (Zakirova et al., 2015a; Zakirova et al., 2015b) exposure of mice to PB and PER resulted in minimal memory impairments.
Mild neuroinflammation with activation of microglia and/or astrocytes has been observed in models of GWI (Abdullah et al., 2012; Parihar et al., 2013; Zakirova et al., 2015a; Zakirova et al., 2015b). We detected mild neuroinflammation in exposed mice evidenced by the increased number of activated microglia in the CA1-3 region of the hippocampus in both exposed male and female mice. However we did not detect (at 3 months after exposure) a significant increase in the steady state level of GFAP in hippocampal protein extracts nor a significant increase in GFAP signal in immunostained brain sections. Moreover, recent studies found increased expression of markers of inflammation, chronic oxidative stress, and mitochondrial dysfunction in the hippocampus of GWI model male rats (Shetty et al., 2017). The neuroinflammation data in animal models are in line with reports from affected GW veterans indicating that aberrant immune system and chronic systemic inflammation are components of the pathophysiology of GWI (Broderick et al., 2013; Parkitny et al., 2015; Whistler et al., 2009). Higher blood levels of C-reactive protein, platelet counts and thromboxane analog-stimulated platelet aggregation were also found in veterans with GWI compared to veterans without (Johnson et al., 2016). Analysis of lymphocyte, monocyte, neutrophil, and platelet counts were also higher in veterans with GWI (Johnson et al., 2016). Despite evidence that immune and inflammatory dysregulation may be a persistent feature of GWI, studies aiming to characterize the cytokine profile of GWI patients failed to gain consensus.
Since our GWI model mice are characterized by increased anxiety we examined the basal forebrain septo-hippocampal cholinergic system. The medial septal nucleus in the basal forebrain is a major source of cholinergic neurons projecting to the hippocampus – a brain region engaged in processing of attention and memory (Hasselmo and Sarter, 2011) as well as anxiety (Anacker and Hen, 2017; Bannerman et al., 2014; Korotkova et al., 2017; Zhang et al., 2017). The basal forebrain cholinergic system also projects to the amygdala (Heckers and Mesulam, 1994), recognized for its central role in the expression of anxiety and fear (Etkin and Wager, 2007; Knox, 2016; Phelps and LeDoux, 2005). We determined the levels of the acetylcholine-synthesizing enzyme, ChAT, in the septum and hippocampus of our GWI model mice and found a sexually dimorphic response. Exposed males expressed less ChAT than the controls in both the septum and hippocampus, whereas the exposed females expressed more. Our data on GFP were consistent with the measures of ChAT, indicating that GFP in the CHGFP mouse model of GWI can be used as an index of cholinergic neuron function and viability. This will facilitate future studies on GWI therapeutics in this model.
Because BFCN (Knusel et al., 1992; Morse et al., 1993; Schliebs and Arendt, 2011; Ward and Hagg, 2000) and hippocampal GABAergic neurons (Carmona et al., 2006; Zachrisson et al., 1996) respond to the neurotrophins NGF and BDNF, we measured their levels and the levels of TrkB in the hippocampus. Again, we found sexually dimorphic changes in these indices of trophic factor signaling in our GWI mice. NGF levels were significantly elevated in the exposed male mice as compared to controls, whereas the levels of BDNF were unchanged. Moreover, the exposure upregulated the protein levels of full length TrkB140 in male mice whereas the level of the full length as well as the level of the truncated inhibitory form of the receptor were both depressed in females. These results suggest that exposure to GW-related chemicals likely affects the activation of TrkB. The effects of exposure on BDNF signaling in females and males warrants further investigation. Although there are only few and inconsistent studies on possible differences of GWI in men and women (Coughlin, 2016; Smylie et al., 2013; Stein et al., 2004; Unwin et al., 2002), our results show that several features of the GWI mouse model are sexually dimorphic. Interestingly, the basal forebrain septohippocampal cholinergic system affected by the GW exposure is itself sexually dimorphic (Loy and Sheldon, 1987; Mitsushima, 2011) and highly regulated by gonadal steroids (McEwen and Milner, 2017), in particular estrogens (Gibbs, 2010). There are reports that the hippocampal BDNF/TrkB signaling in mice depends on sex (Hill et al., 2013; Uluc et al., 2013). Thus, the neuronal systems that are regulated by gonadal steroids exhibit differential vulnerability to the insults employed in the generation of this GWI animal model. The mechanism of this is not readily apparent. It is worth noting that the pharmacodynamics of the three compounds administered during the exposure phase of our model vary with sex. For example, male rats reportedly metabolize PB and DEET faster than the females (Hoy et al., 1999; Hoy et al., 2000). Moreover, in rats treated with PB, the metabolism of PER is slowed and the effects of PB are larger in females than in males (Van Haaren et al., 2000). Extrapolating these data to the mice in our study would suggest that the females may have received higher effective drug doses than the males.
In the basal forebrain nuclei, cholinergic neurons are interspersed with other cells including GABAergic neurons (Freund and Meskenaite, 1992; Henny and Jones, 2008). The BFCN send local axon collaterals terminating onto other neurons, including GABAergic neurons (Zaborszky and Duque, 2000). Abnormal activity of BFCN may impair cortical activation not only via changes in the cholinergic projections but also via reduced activity of basal forebrain cortically projecting GABAergic neurons. Indeed, a study found that rats exposed to GW-related chemicals and stress specifically displayed reduced numbers of parvalbumin- and neuropeptideY-expressing GABAergic interneurons in the hippocampus (Megahed et al., 2014).
We found by MRS that the levels of GABA are lower in the hippocampus of exposed mice compared to controls. The decrease in GABA was associated with reduced NAA, a neuronal marker whose levels strongly correlate with neuronal health (Jenkins and Kraft, 1999). The decreases in GABA and NAA were restricted to the hippocampus with no effects in the frontal cortex, reflecting the hippocampal dysfunction often observed in GWI. Consistent with these data, we found a decrease in the protein levels of hippocampal GAD67 in exposed mice. The magnitudes of the decrease in GAD67 and GABA levels were similar suggesting that the measurements are reflecting a true decrease in GABAergic neuronal activity. The transcription and protein level of GAD67 are known to be regulated by neural activity (Lau and Murthy, 2012), and to be altered in a variety of neuropsychiatric disorders (Torrey et al., 2005).
In conclusion, we report changes detected 3 months after exposure (anxiety, Iba1, NGF, NAA, GABA, and GAD 67) in a mouse model of GWI that recapitulates several features of GWI including anxiety, neuroinflammation and abnormalities in the cholinergic and GABAergic systems as possible neural mechanisms underlying these deficits.
Anti-inflammatory and/or trophic factor therapies, such as those utilizing a BBB-permeable molecule, 7,8-dihydroxyflavone, that acts as a TrkB agonist (Jang et al., 2010) already successfully tested by us (Korkmaz et al., 2014) and others (Uluc et al., 2013; Zhang et al., 2014) in several models of CNS disease, emerge as rational strategies for treatment of GWI that can be readily tested in this mouse model.
4. Experimental Procedure
4.1 Mice and Exposure Protocol
A total of 20 of homozygous female and 20 of homozygous male of the B6.Cg-Tg(RP23-268L19-EGFP)2Mik/J mouse strain (Jackson Laboratories #0079020) were used. These transgenic mice (referred here as CHGFP mice) express the reporter eGFP protein under the control of the genomic elements of the cholinergic-neuron-specific gene, Chat, encoding the ACh-synthesizing enzyme choline acetyltransferase (ChAT) and therefore express eGFP exclusively in cholinergic cells (Tallini et al., 2006). The mice were chosen to facilitate studies of the cholinergic system.
PB, PER, and DEET were purchased from Sigma-Aldrich. At ~70 days of age mice were randomly assigned to GW exposed or control groups (n=10 of each sex). Exposure was performed as previously described (Abdel-Rahman et al., 2002). Briefly, exposed mice were treated orally with: 1.3 mg/kg PB dissolved in the drinking water and with 0.13 mg/kg PER and 40 mg/kg DEET once daily dissolved in 70 % ethanol applied to a shaved area on the back. The mice were then placed in a plexiglass mouse restrainer for 5 minutes. Control mice were similarly exposed to a dermal application of 70% ethanol. Except for PB, all other exposures were dispensed in 5-day periods separated by 2-day rest. Exposure lasted 28 days followed by a 3-month post-exposure period. All animal experiments were approved by the VA Boston Healthcare System’s Institutional Animal Care and Use Committee and conducted in accordance with the Office of Laboratory Animal Welfare and the Association for the Assessment Accreditation and Laboratory Animal Care guidelines.
4.2 Neurobehavioral Testing
During the last 2 weeks of the post-exposure period, mouse behavior was evaluated in the order: 1) locomotor activity and stereotypy; 2) elevated plus maze; 3) Morris water maze.
4.2.1 Locomotor Activity and Stereotypy
Testing was performed in an activity chamber (45×24×20 cm) using the automated Omnitech Digiscan Micromonitor system (Columbus, OH, USA) equipped with a 16 photocell array, as previously described (Kaplan et al., 2011). Mice were habituated to the activity chamber for 5 minutes prior to the 30-minute recording session. Locomotor activity was defined by the number of photocell beam interruptions. For stereotypic activity, an animal must cross 2 photocells within 500ms.
4.2.2 Elevated Plus Maze
Mice were placed in the center zone of the elevated plus apparatus and left to explore while they were videotaped for 5 minutes. Videos were analyzed to record the following parameters: 1) number of open arm entries, 2) time spent in open arms, 3) number of closed arm entries, and 4) time spent in the closed arms. Entry to an arm was considered when all four paws are located within the arm.
4.2.3 Morris Water Maze (MWM)
The test was performed as we previously described (McKee et al., 2008). Following a pre-training procedure mice were tested during 6 consecutive days in 4 training trials per day of a maximum of 60s each. A probe trial without platform was run on day 7. Briefly, a 4 feet diameter white pool filled with water tinted with non-toxic white paint and maintained at 24°C±2°C was used. Mice were trained to find an invisible submerged platform of 14 cm in diameter using a variety of visual extra maze cues. Prior to testing, procedure was implemented to habituate mice to the water and to train them to escape from the water by climbing on the platform. No data was collected during pre-training. For the MWM test mice performed 4 training trials per day during 6 consecutive days. The pool was imaginarily divided into 4 quadrants, with north, west, south and east positions located at the intersections of the quadrants. The platform was submerged 0.5 cm in the west position, 20 cm from the wall. The starting position was randomized among the north, west and east positions such that over the 4 trials only one starting position was randomly repeated. In each trial, mice were given 60 s to locate the platform. If a mouse failed to find the platform, it was placed on the platform for 15 s. A 60 s probe trial without platform was run on day 7 in which all animals started from the east position. Data were recorded using the HVS2020 tracking system (HVS Image, Hampton, UK). Multiple measures were recorded during the training trials: latency to reach platform, swim distance, swim speed. Additionally, the number of platform crossings, latency to first cross the platform location, and time spent in each quadrant were analyzed during the probe trial.
4.3 Tissue collection
At the end of the behavioral testing mice were euthanized by CO2 asphyxiation and brains were dissected out for post-mortem analysis. The right hemisphere was post-fixed with the 4 % paraformaldehyde for 24 h and cryoprotected in 10% and 20% glycerol/2% DMSO solution for histological analysis as described (Aytan et al., 2016). The left hemisphere was immediately dissected on ice. The hippocampus and basal forebrain were collected for protein analysis by western blot and ELISA. Tissue punches of 1mm diameter from posterior hippocampus and medial prefrontal cortex were collected and immediately frozen on dry ice for in vitro magnetic resonance spectroscopy (MRS) analysis.
4.4 Immunohistochemical Staining and Analysis
Immunohistochemistry was performed in serial sections of 50μm thick as we previously described (Kowall et al., 2000) with antibodies to Iba1 (Wako Chemicals #019-19741; 1:5000) to stain microglia, and GFAP (Millipore #MAB3402; 1:5000) for reactive astrocytes. For each antibody we quantified, blindly to the treatment, 3 serial sections/subject 0.5mm apart. Reactive astrocytes and activated microglia were quantified in the cortex (starting at bregma 0.1), hippocampus, and amygdala. GFAP immunoreactivity was quantified by densitometry using a custom-made MATLAB program (Mathworks). Iba1 stained cells with the hypertrophic and bushy morphology that characterizes activated microglia were counted using bright-field microscopy interfaced with the StereoInvestigator software (MBF Bioscience, Williston, VT) using the Optical Fractionator probe.
4.5 Enzyme-linked Immunosorbent Assay (ELISA)
Hippocampal and septal regions from the right hemisphere were snap frozen on dry ice. Frozen tissues were sonicated in lysis buffer (0.05 M Tris-HCl pH 7.5, 0.15 M NaCl, 1% NP-40, 1 mM Na-orthovanadate, 0.001% sodium fluoride, 1% protease inhibitor cocktail (Sigma) and centrifuged to clear. Protein extracts from hippocampal and septal regions were used to measure the levels of brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) using the Emax® immunoassay system (Promega) and GFP was assayed using the PathScan Total GFP Sandwich ELISA Kit (Cell Signaling Technology) according to manufacturer’s instructions and as we previously reported (Burke et al., 2013).
4.6 Western Blot Analysis
Hippocampal and septal extracts described above were also analyzed by western blot, as we previously described (Burke et al., 2013). Briefly, 40 μg of hippocampal protein per sample were subjected to SDS-PAGE using 4–12% Bis-Tris Midi gels (Invitrogen). After transferring to a nitrocellulose or PVDF membrane using an iBLOT apparatus (Invitrogen), the membrane was blocked with 5% nonfat dry milk in 1X TBS containing 0.1% Tween-20 for 1 h and then was probed with primary antibody overnight. The antibodies used included: β-actin (Sigma #A5441; 1:5000), ChAT (Millipore #AB144P; 1:750), GFAP (Cell Signaling Technologies #3670; 1:1000), GFP (Millipore #AB3080P; 1:1000), and GAD65/67 (Millipore #AB1511; 1:1000). The antibody/antigen complexes were detected with species-specific peroxidase conjugate and visualized using enhanced chemiluminescence (SuperSignal West Femto Substrate, Thermo Scientific) and Kodak ImageStation 440 and quantified with the Kodak 1D software. To reprobe membranes were stripped in Restore Western Blot Stripping Buffer (Thermo Scientific) for 30 min at 37°C. Densitometric values for each protein were normalized to β-actin values.
4.7 High Resolution Magic Angle Spinning Spectroscopy (HRMAS)
MRS was performed in tissue punches of 1mm diameter from posterior hippocampus and medial prefrontal cortex by HRMAS as we previously published (Aytan et al., 2016). HRMAS spectra was collected on a Bruker 14T (Billerica, MA). Data were acquired using a rotor synchronized, T2-filtered Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence [90 – (τ– 180 – τ – Acq)n] with two different effective TEs (100ms/10ms). Data were analyzed using the Chenomx (Edmonton, Alberta, Canada) package fitting the entire metabolite spectrum for each neurochemical and reported as molar ratios to creatine. Statistical analyses of the MRS data were performed using a one-way ANOVA with Tukey HSD post-hoc tests for between group comparisons for each metabolite. Classification of the data was performed using Weka software (availability under the GNU General Public License)
4.8 Statistical analyses
Statistical analyses of the data were performed using one- or two-way ANOVAs with Tukey HSD post-hoc or Student–Newman–Keuls tests as appropriate. The independent variables included exposure and sex. See figure legends for additional details. Data are presented as mean ± standard error of mean (SEM), P-values that are ≤0.05 are considered to be significant.
Acknowledgments
This research was supported by VA Merit Award # I01BX002468, to Alpaslan Dedeoglu.
Glossary
- GWI
Gulf War Illness
- PB
pyridostigmine bromide
- DEET
N, N-diethyl-m-toluamide
- PER
permethrin
- NAA
N-acetyl aspartate
- BDNF
brain-derived neurotrophic factor
- NGF
nerve growth factor
- AChE
acetylcholinesterase
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- MRS
magnetic resonance spectroscopy
- HRMAS
high resolution magic angle spinning spectroscopy
References
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol Dis. 2002;10:306–26. doi: 10.1006/nbdi.2002.0524. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, Pelot R, Mullan MA, Ferro A, Mullan CM, Mullan MJ, Ait-Ghezala G, Crawford FC. Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromolecular Med. 2012;14:349–61. doi: 10.1007/s12017-012-8192-z. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Goldstein LB, Jones KH, Abdel-Rahman AA, Damodaran TV, Dechkovskaia AM, Bullman SL, Amir BE, Khan WA. Locomotor and sensorimotor performance deficit in rats following exposure to pyridostigmine bromide, DEET, and permethrin, alone and in combination. Toxicol Sci. 2001;60:305–14. doi: 10.1093/toxsci/60.2.305. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Dechkovskaia AM, Goldstein LB, Abdel-Rahman A, Bullman SL, Khan WA. Co-exposure to pyridostigmine bromide, DEET, and/or permethrin causes sensorimotor deficit and alterations in brain acetylcholinesterase activity. Pharmacol Biochem Behav. 2004;77:253–62. doi: 10.1016/j.pbb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci. 2017;18:335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytan N, Choi JK, Carreras I, Brinkmann V, Kowall NW, Jenkins BG, Dedeoglu A. Fingolimod modulates multiple neuroinflammatory markers in a mouse model of Alzheimer’s disease. Sci Rep. 2016;6:24939. doi: 10.1038/srep24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, Seeburg PH. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–92. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Binns J, Committee Chair, Barlow Carrolee, MD, PhD, Bloom Floyd E, MD, Clauw Daniel J, MD, Golomb Beatrice A, MD, PhD, Graves Joel C, DMin, Hardie Anthony, Knox LTC Marguerite L, MN, NP, Meggs William J, MD, PhD, Nettleman Mary Dekker, MD, MS, O’Callaghan James P, PhD, Smithson Steve, Steele Lea, PhD, White Roberta F., PhD . Research Advisory Committee on Gulf War Veterans’ Illnesses. U.S. Department of Veterans Affairs; Washington, D.C: 2008. Gulf war illness and the health of Gulf war veterans: scientific findings and recommendations, Research Advisory Committee Report on Gulf War Illness and Health of Gulf War Veterans; pp. 1–465. [Google Scholar]
- Binns JH, BC, Bloom FE, Bunker JA, Golomb BA, Graves JC, Klimas N, O’Callaghan JP, Ondra SL, Philbert MA, Steele L, White RF. Research Advisory Committee on Gulf War Veterans’ Illnesses. U.S. Department of Veterans Affairs; Washington, D.C: 2014. Gulf War Illness and the Health of Gulf War Veterans: Research Update and Recommendations, 2009–2013 Updated Scientific Findings and Recommendations; pp. 1–123. [Google Scholar]
- Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, Fletcher MA, Klimas N. Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain Behav Immun. 2013;28:159–69. doi: 10.1016/j.bbi.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Burke RM, Norman TA, Haydar TF, Slack BE, Leeman SE, Blusztajn JK, Mellott TJ. BMP9 ameliorates amyloidosis and the cholinergic defect in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110:19567–72. doi: 10.1073/pnas.1319297110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MA, Pozas E, Martinez A, Espinosa-Parrilla JF, Soriano E, Aguado F. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cereb Cortex. 2006;16:47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- Chaney LA, Wineman RW, Rockhold RW, Hume AS. Acute effects of an insect repellent, N,N-diethyl-m-toluamide, on cholinesterase inhibition induced by pyridostigmine bromide in rats. Toxicol Appl Pharmacol. 2000;165:107–14. doi: 10.1006/taap.2000.8936. [DOI] [PubMed] [Google Scholar]
- Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007;20:216–37. doi: 10.1002/nbm.1145. [DOI] [PubMed] [Google Scholar]
- Coughlin SS. Need for Studies of the Health of Gulf War Women Veterans. Mil Med. 2016;181:198. doi: 10.7205/MILMED-D-15-00563. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992;89:738–42. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–53. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Charuvastra E, Shell WE, Buhner DM, Marshall WW, Biggs MM, Hopkins SC, Wolfe GI, Vernino S. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013;70:191–200. doi: 10.1001/jamaneurol.2013.596. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Mesulam M-M. Two types of cholinergic projections to the rat amygdala. Neuroscience. 1994;60:383–397. doi: 10.1016/0306-4522(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–70. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Wu YW, Gogos A, van den Buuse M. Sex-dependent alterations in BDNF-TrkB signaling in the hippocampus of reelin heterozygous mice: a role for sex steroid hormones. J Neurochem. 2013;126:389–99. doi: 10.1111/jnc.12205. [DOI] [PubMed] [Google Scholar]
- Hoy JB, Cody BA, Karlix JL, Schmidt CJ, Tebbett IR, Toffollo S, Van Haaren F, Wielbo D. Pyridostigmine bromide alters locomotion and thigmotaxis of rats: gender effects. Pharmacol Biochem Behav. 1999;63:401–6. doi: 10.1016/s0091-3057(99)00014-3. [DOI] [PubMed] [Google Scholar]
- Hoy JB, Cornell JA, Karlix JL, Schmidt CJ, Tebbett IR, van Haaren F. Interactions of pyridostigmine bromide, DEET and permethrin alter locomotor behavior of rats. Vet Hum Toxicol. 2000;42:65–71. [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–92. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, Kraft E. Magnetic resonance spectroscopy in toxic encephalopathy and neurodegeneration. Curr Opin Neurol. 1999;12:753–60. doi: 10.1097/00019052-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Blood Biomarkers of Chronic Inflammation in Gulf War Illness. PLoS One. 2016;11:e0157855. doi: 10.1371/journal.pone.0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Fan W, Young AJ, Guy MD. Opiate sensitization induces FosB/DeltaFosB expression in prefrontal cortical, striatal and amygdala brain regions. PLoS One. 2011;6:e23574. doi: 10.1371/journal.pone.0023574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol Learn Mem. 2016;133:39–52. doi: 10.1016/j.nlm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knusel B, Beck KD, Winslow JW, Rosenthal A, Burton LE, Widmer HR, Nikolics K, Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz OT, Aytan N, Carreras I, Choi JK, Kowall NW, Jenkins BG, Dedeoglu A. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2014;566:286–91. doi: 10.1016/j.neulet.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Ponomarenko A, Monaghan CK, Poulter SL, Cacucci F, Wills T, Hasselmo ME, Lever C. Reconciling the different faces of hippocampal theta: The role of theta oscillations in cognitive, emotional and innate behaviors. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ. MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. Neuroreport. 2000;11:211–3. doi: 10.1097/00001756-200001170-00041. [DOI] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 2012;32:8521–31. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy R, Sheldon RA. Sexually dimorphic development of cholinergic enzymes in the rat septohippocampal system. Dev Brain Res. 1987;34:150–160. doi: 10.1016/0165-3806(87)90205-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95:24–39. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Carreras I, Hossain L, Ryu H, Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW, Dedeoglu A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–36. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed T, Hattiangady B, Shuai B, Shetty AK. Parvalbumin and neuropeptide Y expressing hippocampal GABA-ergic inhibitory interneuron numbers decline in a model of Gulf War illness. Front Cell Neurosci. 2014;8:447. doi: 10.3389/fncel.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, Ali JA. Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain Res. 2004;1009:189–94. doi: 10.1016/j.brainres.2004.02.063. [DOI] [PubMed] [Google Scholar]
- Mitsushima D. Sex differences in the septo-hippocampal cholinergic system in rats: behavioral consequences. Curr Top Behav Neurosci. 2011;8:57–71. doi: 10.1007/7854_2010_95. [DOI] [PubMed] [Google Scholar]
- Morse JK, Wiegand SJ, Anderson K, You Y, Cai N, Carnahan J, Miller J, DiStefano PS, Altar CA, Lindsay RM, Alderson RF. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Nerve membrane ionic channels as the primary target of pyrethroids. Neurotoxicology. 1985;6:3–22. [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology. 2013;38:2348–62. doi: 10.1038/npp.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L, Middleton S, Baker K, Younger J. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunol. 2015;16:57. doi: 10.1186/s12865-015-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Timbol CR, Adewuyi O, Walitt B, VanMeter JW, Baraniuk JN. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War illness. PLoS One. 2013;8:e58493. doi: 10.1371/journal.pone.0058493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–63. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Upadhya D, Bates A, Attaluri S, Shuai B, Kodali M, Shetty AK. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front Mol Neurosci. 2017;10:182. doi: 10.3389/fnmol.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smylie AL, Broderick G, Fernandes H, Razdan S, Barnes Z, Collado F, Sol C, Fletcher MA, Klimas N. A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome. BMC Immunol. 2013;14:29. doi: 10.1186/1471-2172-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK, Domitrovich PP, Ambrose K, Lyden A, Fine M, Gracely RH, Clauw DJ. Sex effects on heart rate variability in fibromyalgia and Gulf War illness. Arthritis Rheum. 2004;51:700–8. doi: 10.1002/art.20687. [DOI] [PubMed] [Google Scholar]
- Swale DR, Sun B, Tong F, Bloomquist JR. Neurotoxicity and mode of action of N, N-diethyl-meta-toluamide (DEET) PLoS One. 2014;9:e103713. doi: 10.1371/journal.pone.0103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini YN, Shui B, Greene KS, Deng KY, Doran R, Fisher PJ, Zipfel W, Kotlikoff MI. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics. 2006;27:391–7. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–60. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Uluc K, Kendigelen P, Fidan E, Zhang L, Chanana V, Kintner D, Akture E, Song C, Ye K, Sun D, Ferrazzano P, Cengiz P. TrkB receptor agonist 7, 8 dihydroxyflavone triggers profound gender- dependent neuroprotection in mice after perinatal hypoxia and ischemia. CNS Neurol Disord Drug Targets. 2013;12:360–70. doi: 10.2174/18715273113129990061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin C, Hotopf M, Hull L, Ismail K, David A, Wessely S. Women in the Persian Gulf: lack of gender differences in long-term health effects of service in United Kingdom Armed Forces in the 1991 Persian Gulf War. Mil Med. 2002;167:406–13. [PubMed] [Google Scholar]
- Van Haaren F, Cody B, Hoy JB, Karlix JL, Schmidt CJ, Tebbett IR, Wielbo D. The effects of pyridostigmine bromide and permethrin, alone or in combination, on response acquisition in male and female rats. Pharmacol Biochem Behav. 2000;66:739–46. doi: 10.1016/s0091-3057(00)00282-3. [DOI] [PubMed] [Google Scholar]
- Ward NL, Hagg T. BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp Neurol. 2000;162:297–310. doi: 10.1006/exnr.1999.7346. [DOI] [PubMed] [Google Scholar]
- Whistler T, Fletcher MA, Lonergan W, Zeng XR, Lin JM, Laperriere A, Vernon SD, Klimas NG. Impaired immune function in Gulf War Illness. BMC Med Genomics. 2009;2:12. doi: 10.1186/1755-8794-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex. 2016;74:449–75. doi: 10.1016/j.cortex.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Duque A. Local synaptic connections of basal forebrain neurons. Behav Brain Res. 2000;115:143–58. doi: 10.1016/s0166-4328(00)00255-2. [DOI] [PubMed] [Google Scholar]
- Zachrisson O, Falkenberg T, Lindefors N. Neuronal coexistence of trkB and glutamic acid decarboxylase67 mRNAs in rat hippocampus. Brain Res Mol Brain Res. 1996;36:169–73. doi: 10.1016/0169-328x(95)00281-v. [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, Crawford F, Ait-Ghezala G. A Chronic Longitudinal Characterization of Neurobehavioral and Neuropathological Cognitive Impairment in a Mouse Model of Gulf War Agent Exposure. Front Integr Neurosci. 2015a;9:71. doi: 10.3389/fnint.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, Ait-Ghezala G. Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness. PLoS One. 2015b;10:e0119579. doi: 10.1371/journal.pone.0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang YY, Shao S, Zhang C, Liu FY, Wan Y, Yi M. Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety-like behaviors in mice. Neurosci Lett. 2017;638:139–144. doi: 10.1016/j.neulet.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, Weinshenker D, Ye K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:638–50. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]