Abstract
α/γ-Peptide foldamers containing either γ4-amino acid residues or ring-constrained γ-amino acid residues have been reported to adopt 12-helical secondary structure in nonpolar solvents and in the solid state. These observations have engendered speculation that the seemingly flexible γ4 residues have a high intrinsic helical propensity, and that residue-based preorganization may not significantly stabilize the 12-helical conformation. However, the prior studies were conducted in environments that favor intramolecular H-bond formation. In this report, we use 2D-NMR to compare the ability of γ4 residues and cyclic γ residues to support 12-helix formation in more challenging environments, methanol and water. Both γ residue types support 12-helical folding in methanol, but only the cyclically constrained γ residues promote helicity in water. These results demonstrate the importance of residue-based preorganization strategies for achieving stable folding among short foldamers in aqueous solution.
TOC image
The study of unnatural oligomers that display biopolymer-like folding behavior offers a framework for interrogating relationships between covalent and noncovalent structure (constitution and conformation) in a way that transcends the deep understanding that has emerged from analysis of proteins and nucleic acids. For example, examination of “foldamers” that contain β-amino acid residues instead of or in addition to α residues (i.e., β-peptides or α/β-peptides) has revealed the strong influence of small-ring constraints on the identity and stability of H-bond-mediated secondary structure.1 This issue is not pertinent to the folding of conventional polypeptides, containing exclusively α residues, because imposition of a cyclic constraint necessarily abolishes backbone H-bond potential and disrupts common secondary structures, as seen with proline residues.2
Insights gained from experimental correlations among residue identity, secondary structure and conformational stability have proven invaluable in the development of functional foldamers containing β residues. Stabilization of the β-peptide 14-helix by trans-cyclohexyl residues, for example, enabled development of a foldamer catalyst3 and fundamental single-molecule studies of hydrophobic interactions.4 Stabilization of α/β-peptide helices by trans-cyclopentyl residues has allowed the refinement of protein-protein interaction antagonists.5 These functional outcomes with β-containing foldamers highlights the importance of elucidating relationships between residue structure and conformational stability to other foldamer classes. Here we evaluate the impact of a specific cyclic constraint on helical secondary structure formed by α/γ-peptides.
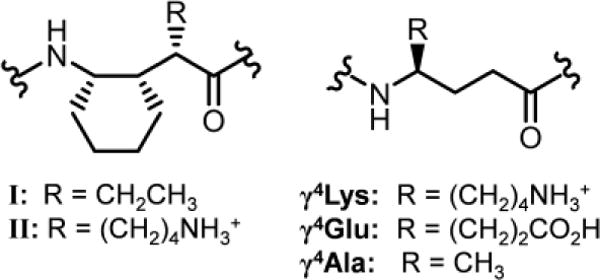
Peptidic foldamers containing γ residues have been examined in many laboratories,6 but only a few studies7 have evaluated the relationships between γ residue structure and conformational stability. The α/γ-peptide 12-helix is probably the most extensively studied secondary structure in this foldamer class. This conformation, formed by oligomers with sequentially alternating α and γ residues, is defined by 12-atom C=O(i) → H−N(i+3) H-bonds between backbone amide groups. The α/γ-peptide 12-helix was initially observed by Balaram and coworkers in crystal structures of achiral oligomers containing gabapentin (Gpn) and α-aminoisobutyric acid (Aib) residues.8,9 Guo et al. developed an efficient asymmetric synthesis leading to trisubstituted γ-amino acid residue I (Figure 1), and showed that α/γ-peptides containing I form the 12-helix in chloroform (based on NOE analysis) and in the crystalline state.10 The cyclic constraint and substitution pattern of I should limit conformational freedom. In particular, the Cα-Cβ and Cβ-Cγ bonds are predicted to favor a g+,g+ torsion angle sequence, as required for the 12-helix; this prediction is consistent with the many crystal structures of foldamers containing residue I.11
Figure 1.
Structures of γ residues.
The groups of Gopi12 and Balaram13 have reported a large set of crystal structures showing that α/γ-peptides containing exclusively γ4 residues (Figure 1) (4-mers to 16-mers) can adopt the 12-helix. In these structures the Cα-Cβ and Cβ-Cγ torsion angles favor a g+,g+ torsion sequence similar to that observed in 12-helices containing residue I. Balaram and coworkers further demonstrated via 2D NMR that 12-helicity is maintained by a 16-mer α/γ-peptide in chloroform.13b These findings are striking because one would predict γ4 residues to be much more flexible than is γ residue I. As Balaram and coworkers noted, the extensive structural results with α/γ4-peptides lead one to question whether the constraint inherent in I stabilizes the 12-helical conformation. This uncertainty must be resolved because constrained residues generally require greater synthetic effort than do flexible residues.
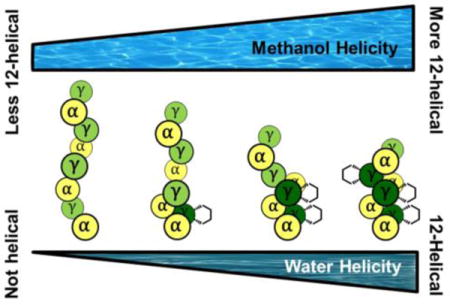
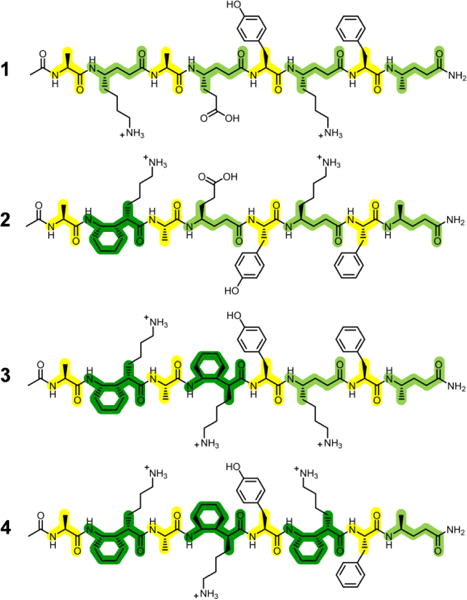
To address this question, we moved to a new family of α/γ-peptide octamers (1-4; Figure 2) tailored for aqueous solubility. Water is the least favorable among common solvents for H-bonded conformations such as the α-helix or β-sheets formed by conventional peptides or helices formed by β- or α/β-peptide foldamers.14 Presumably the strong H-bond competition provided by water molecules diminishes the ability of intramolecular H-bonds to stabilize secondary structure. Admixture of an alcohol cosolvent, such as methanol, enhances the population of internally H-bonded secondary structures for which there is an intrinsic propensity,14–15 but aqueous-alcohol mixtures do not guarantee folding. In contrast, chloroform allows intramolecular H-bonds to serve as a strong driving force for secondary structure formation. Qualitative comparisons based on data obtained in chloroform or crystal structures cannot decisively address the relative 12-helical propensities of I vs. a γ4 residue.
Figure 2.
Structures of water-soluble α/γ-peptides.
α/γ-Peptide series 1-4 features both γ4 residues and γ residue II (Figure 1), which is an analogue of I bearing a side chain that should be cationic at acidic pH. α/γ-Peptide 1 contains exclusively γ4 residues, which are progressively replaced with II, from N- to C-terminus, in analogues 2-4. The γ residue side chain complement varies at one position across the α/γ-peptide series: 1 and 2 have a glutamate-like side chain at residue 4, but 3 and 4 have a lysine-like side chain at this position. This variation was intended to promote 1H NMR resonance dispersion. The four α residues are invariant among 1-4. Two aromatic side chains (Tyr-5 and Phe-7) were included to enhance dispersion of 1H NMR signals.
Initial 2D NMR studies were conducted in CD3OH, in which 1-4 were highly soluble. 1H resonances were unchanged between 0.1 and 3 mM, suggesting that aggregation state, which we assume to be monomeric, does not vary in this range. In general, more constrained γ residues correlated with improved chemical shift dispersion. In order to detect 12-helical folding, we focused on three types of backbone-to-backbone i,i+2 NOE that are known to be characteristic of this secondary structure (Table 1).10, 13b
Table 1.
Summary of crosspeaks observed in CD3OH.a

| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| H atom pair | 12-Helix distance (Å)b | NOE pairs | 1 | 2 | 3 | 4 |
| a | 2.72 | 2 ⋯ 4 | W | S | ? | S |
| 2.70 | 4 ⋯ 6 | ? | M | S | S | |
| 2.37 | 6 ⋯ 8 | S | M | M | M | |
|
| ||||||
| b | 2.60c | 2 ⋯ 4 | W | S | S | ? |
| 2.69c | 4 ⋯ 6 | ? | W | M | S | |
| – | 6 ⋯ 8 | ? | ? | W | S | |
|
| ||||||
| c | 3.42 | 1 ⋯ 3 | W | W | W | W |
| 3.64 | 3 ⋯ 5 | VW | N.D. | W | W | |
| 3.40 | 5 ⋯ 7 | VW | VW | W | W | |
VW=very weak; W=weak; M=medium; S=strong; ?=ambiguous; N.D.=not detected.
From crystal structure of P3 in ref. 11c.
Distance to pseudoatom between diastereotopic H nuclei.
For fully flexible α/γ-peptide 1 in CD3OH, partial chemical shift overlap precluded precise integration of several expected 12-helical crosspeaks in the 2D-ROESY spectrum. Nevertheless, unambiguous observation of six characteristic NOEs, uniformly distributed across 1, supported the conclusion that this α/γ-peptide is at least partially 12-helical in methanol (Table 1).
Improved 1H signal dispersion enabled unambiguous assignment of almost all expected 12-helix NOEs for α/γ-peptide 2 in CD3OH. The two 12-helical NOEs involving the cyclic γ residue (types a and b in Table 1) were more intense than those involving γ4 residues. The 12-helical NOEs involving the α residues (type c) were generally not very intense; consistent with this trend, protons that would give rise to type c NOEs are further apart in available 12-helix crystal structures than are protons that would give rise to type a or b NOEs.12c α/γ-Peptide 3, containing two cyclic γ residues, showed excellent chemical shift dispersion, and numerous 12-helical NOEs were detected. As seen for 2, crosspeak intensity was lower for γ-to-γ NOEs involving exclusively γ4 residues relative to such NOEs involving at least one cyclic γ residue.
α/γ-Peptide 4, containing three cyclic γ residues, showed the most intense 12-helical crosspeaks among the four oligomers. Furthermore, numerous long-range NOEs between protons on side chains were detected that were not observed for 1-3. Collectively, the ROESY spectra for 1-4 in CD3OH demonstrate a tendency of all α/γ-peptides to adopt the 12-helix conformation, but the trend of increasing crosspeak intensity and number with increasing number of γ4→II replacements suggests that the helical state becomes more stable as cyclic constraints are added.
We next characterized the folding behavior of α/γ-peptides 1-4 in water. All four α/γ-peptides where highly soluble (>3 mM), and chemical shifts did not vary between 0.1 and 3 mM. The data obtained in this competitive environment support our conclusion that the cyclic constraint of residue II stabilizes the 12-helix.
For the fully flexible α/γ-peptide 1, only two very weak i,i+2 ROESY crosspeaks consistent with the 12-helix conformation could be distinguished, with most others impossible to assign unambiguously because of poor resonance dispersion. For all ambiguous i,i+2 crosspeaks, the low intensity outside the overlapping region means that, at most, these NOEs are very weak. Three of the anticipated 12-helical i,i+2 crosspeaks would have occurred in relatively uncrowded regions of the 2D-ROESY spectrum but were not detected, which suggests that any tendency toward 12-helical folding by α/γ-peptide 1 is low in water. Three of the five non-sequential crosspeaks that could be unambiguously assigned were inconsistent with the 12-helix conformation (Figure 3). The i,i+2 NOEs of 1 in water fail to converge to a single mean structure and thus reflect a disordered conformational ensemble.16
Figure 3.
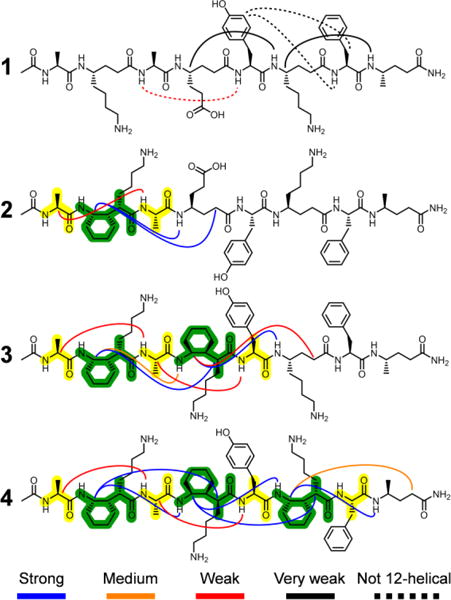
Summary of detected ROESY crosspeaks between protons on non-adjacent residues in aqueous solution.
The aqueous 2D-ROESY spectra of α/γ-peptides 2-4 traced the formation of helicity with increasing cyclic constraint content. As seen in CD3OH, the 1H resonances become more dispersed with increasing content of cyclic γ residue II, permitting unambiguous detection of an increasing fraction of the expected 12-helical ROESY crosspeaks as the content of II grows. All observed i,i+2 ROESY crosspeaks for 2-4 involve at least one residue II or occur between protons from α residues that are separated by a residue II. α/γ-Peptide 2, with a single residue II, illustrates the difference between cyclic and acyclic γ residues in terms of supporting local folding (Figure 4). The crosspeak of type a between II-2 and γ4Glu-4 is very intense, whereas the possible a crosspeak between γ4Glu-4 and γ4Lys-6 is not detected, even though this crosspeak would have occurred in an uncrowded region of the 2D spectrum. This trend continues for α/γ-peptides 3 and 4.
Figure 4.
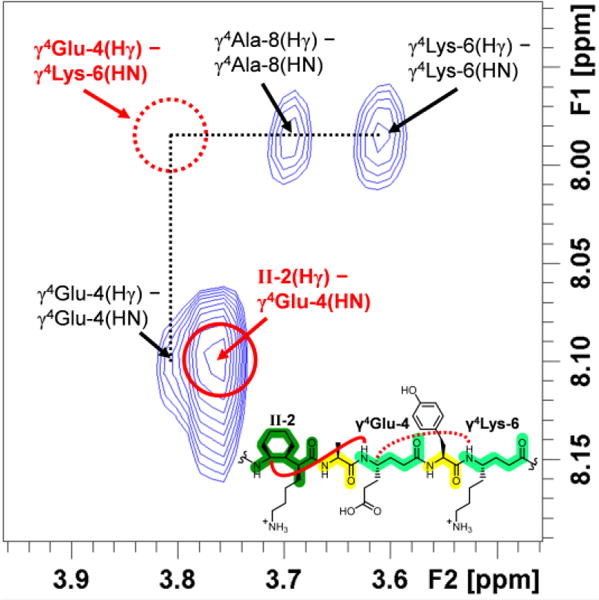
Excerpt of the 600 MHz ROESY spectrum of 3 mM α/γ-peptide 2 in 9:1 H2O:D2O, 100 mM acetate, pH 3.8 at 5°C. i,i+2 NOEs indicated in red. Missing type a NOE shown with dashed line; observed a NOE shown with unbroken line.
Results of NOE distance-restrained simulated annealing calculations17 suggest an increasingly well-defined helical conformation in methanol as γ4 residues are replaced with constrained residues (Figure 5a). The calculations in water suggest an even more pronounced disorder-to-order transition across the series 1-4 than is evident in methanol (Figure 5b vs. Figure 5a). The non-12-helical NOEs of 1 in water result in a variety of simulated conformations that do not correspond to a regular helix. For α/γ-peptides 2 and 3 in water, partial ordering only at the segments containing cyclic γ residues is observed, which is expected since there was a consistent lack of characteristic i,i+2 ROESY crosspeaks for the segments containing γ4 residues. α/γ-Peptide 4 in water displays a relatively tight cluster of structures, each with the maximum number of 12-helix H-bonds. Increasing the number of γ4→II replacements across the series 1-4 improves the overlay of the NMR-derived structures with 12-helical conformations observed for crystalline α/γ-peptides, whether the oligomer crystallized contains constrained γ residue I or unconstrained γ4 residues; this trend is illustrated for a specific α/γ4-peptide crystal structure in Figure 6.
Figure 5.
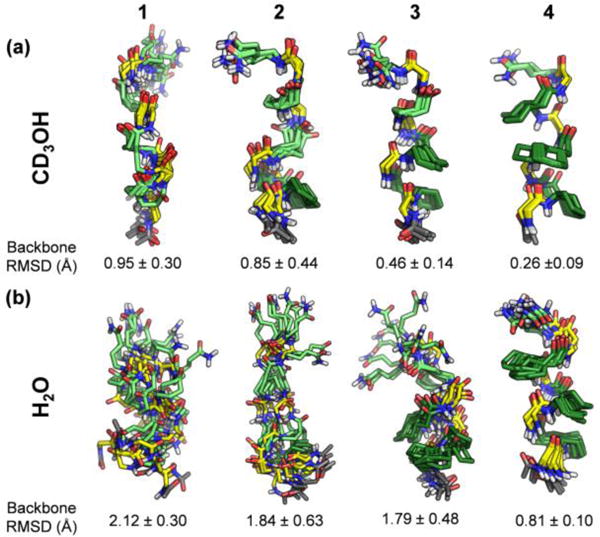
Superposition of ten lowest-energy of 100 trial structures from simulated annealing calculations of 1-4. Mean pairwise backbone RMSD is shown below each structure. (a) Calculated ensembles using distance restraints derived from CD3OH ROESY data. (b) Calculated ensembles using distance restraints derived from aqueous ROESY data.
Figure 6.
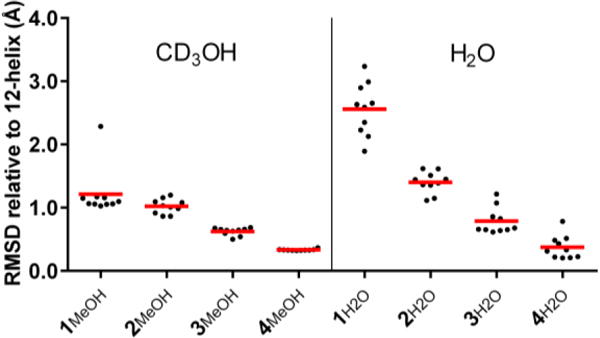
Comparison of NMR structures of α/γ-peptides 1-4 with a 12-helix crystal structure from ref. 11c. Black dots are backbone RMSDs of the 10 lowest-energy NMR structures. Mean pairwise RMSDs of each set of NMR structures vs. the crystal structure are shown with red bars.
The 2D-NMR data presented here for α/γ-peptides 1-4, as well as other 1H-NMR data,18 support the hypothesis that the cyclic constraint in II stabilizes the 12-helix. γ4 Residues, intrinsically more flexible than is II, have a modest propensity for 12-helix formation, as indicated by previous reports12–13 and by our NMR results in methanol. However, analysis of 1-4 in aqueous solution reveals a clear distinction between 12-helical propensities of II and γ4 residues, which decisively addresses questions raised by recent studies.12–13 These results are significant since the impact of γ residue constraint on foldamer secondary structure has received very little scrutiny, in part because there has been very little previous study of γ-containing foldamers in aqueous solution.11c, 19 Our demonstration that the preorganization inherent in γ residue II favors a specific secondary structure should encourage fundamental research on new foldamer building blocks that favor adoption of discrete and diverse conformations.
Supplementary Material
Acknowledgments
This work was supported by NSF grant CHE-1307365. NMR spectrometers were purchased with support from a generous gift by Paul J. Bender and from NIH (S10 OD012245).
Footnotes
Supporting Information
Experimental details, including synthetic route to II; NMR spectra; HDX, VT-NMR, and J-coupling analysis; ROESY crosspeak assignments; CD data; comparison of I and γ4 structure; and NMR calculation parameters. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
References
- 1.(a) Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH. J Am Chem Soc. 1996;118:13071. [Google Scholar]; (b) Appella DH, Christianson LA, Klein DA, Powell DR, Huang X, Barchi JJ, Jr, Gellman SH. Nature. 1997;387:381. doi: 10.1038/387381a0. [DOI] [PubMed] [Google Scholar]; (c) Hayen A, Schmitt MA, Ngassa FN, Thomasson KA, Gellman SH. Angew Chem Int Ed. 2004;43:505. doi: 10.1002/anie.200352125. [DOI] [PubMed] [Google Scholar]; (d) De Pol S, Zorn C, Klein CD, Zerbe O, Reiser O. Angew Chem Int Ed. 2004;43:511. doi: 10.1002/anie.200352267. [DOI] [PubMed] [Google Scholar]; (e) Fernandes C, Faure S, Pereira E, Théry V, Declerck V, Guillot R, Aitken DJ. Org Lett. 2010;12:3606. doi: 10.1021/ol101267u. [DOI] [PubMed] [Google Scholar]; (f) Lee M, Shim J, Kang P, Choi MG, Choi SH. Chem Commun. 2016;52:5950. doi: 10.1039/c6cc01189f. [DOI] [PubMed] [Google Scholar]
- 2.(a) O’Neil KT, DeGrado WF. Science. 1990;250:646. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]; (b) Minor DL, Jr, Kim PS. Nature. 1994;367:660. doi: 10.1038/367660a0. [DOI] [PubMed] [Google Scholar]
- 3.Müller MM, Windsor MA, Pomerantz WC, Gellman SH, Hilvert D. Angew Chem Int Ed. 2009;48:922. doi: 10.1002/anie.200804996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma CD, Wang C, Acevedo-Vélez C, Gellman SH, Abbott NL. Nature. 2015;517:347. doi: 10.1038/nature14018. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LM, Gellman SH. Methods Enzymol. 2013;523:407. doi: 10.1016/B978-0-12-394292-0.00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hanessian S, Luo X, Schaum R, Michnick S. J Am Chem Soc. 1998;120:8569. [Google Scholar]; (b) Seebach D, Brenner M, Rueping M, Schweizer B, Jaun B. Chem Commun. 2001:207. [Google Scholar]; (c) Bouillère F, Thétiot-Laurent S, Kouklovsky C, Alezra V. Amino Acids. 2011;41:687. doi: 10.1007/s00726-011-0893-3. [DOI] [PubMed] [Google Scholar]; (d) Nodes WJ, Nutt DR, Chippindale AM, Cobb AJA. J Am Chem Soc. 2009;131:16016. doi: 10.1021/ja9070915. [DOI] [PubMed] [Google Scholar]; (e) Pendem N, Nelli YR, Douat C, Fischer L, Laguerre M, Ennifar E, Kauffmann B, Guichard G. Angew Chem Int Ed. 2013;52:4147. doi: 10.1002/anie.201209838. [DOI] [PubMed] [Google Scholar]; (f) Mathieu L, Legrand B, Deng C, Vezenkov L, Wenger E, Didierjean C, Amblard M, Averlant-Petit MC, Masurier N, Lisowski V, Martinez J, Maillard LT. Angew Chem Int Ed. 2013;52:6006. doi: 10.1002/anie.201302106. [DOI] [PubMed] [Google Scholar]
- 7.(a) Grison CM, Robin S, Aitken DJ. Chem Commun. 2015;51:16233. doi: 10.1039/c5cc07136d. [DOI] [PubMed] [Google Scholar]; (b) Grison CM, Robin S, Aitken DJ. Chem Commun. 2016;52:7802. doi: 10.1039/c6cc02142e. [DOI] [PubMed] [Google Scholar]
- 8.(a) Ananda K, Vasudev PG, Sengupta A, Raja KM, Shamala N, Balaram P. J Am Chem Soc. 2005;127:16668. doi: 10.1021/ja055799z. [DOI] [PubMed] [Google Scholar]; (b) Vasudev PG, Ananda K, Chatterjee S, Aravinda S, Shamala N, Balaram P. J Am Chem Soc. 2007;129:4039. doi: 10.1021/ja068910p. [DOI] [PubMed] [Google Scholar]
- 9.Vasudev PG, Chatterjee S, Shamala N, Balaram P. Acc Chem Res. 2009;42:1628. doi: 10.1021/ar9001153. [DOI] [PubMed] [Google Scholar]
- 10.Guo L, Chi Y, Almeida AM, Guzei IA, Parker BK, Gellman SH. J Am Chem Soc. 2009;131:16018. doi: 10.1021/ja907233q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Guo L, Almeida AM, Zhang W, Reidenbach AG, Choi SH, Guzei IA, Gellman SH. J Am Chem Soc. 2010;132:7868. doi: 10.1021/ja103233a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guo L, Zhang W, Reidenbach AG, Giuliano MW, Guzei IA, Spencer LC, Gellman SH. Angew Chem Int Ed. 2011;50:5843. doi: 10.1002/anie.201101301. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shin YH, Mortenson DE, Satyshur KA, Forest KT, Gellman SH. J Am Chem Soc. 2013;135:8149. doi: 10.1021/ja403319q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Bandyopadhyay A, Gopi HN. Org Lett. 2012;14:2770. doi: 10.1021/ol300987d. [DOI] [PubMed] [Google Scholar]; (b) Bandyopadhyay A, Jadhav SV, Gopi HN. Chem Commun. 2012;48:7170. doi: 10.1039/c2cc32911e. [DOI] [PubMed] [Google Scholar]; (c) Jadhav SV, Bandyopadhyay A, Gopi HN. Org Biomol Chem. 2013;11:509. doi: 10.1039/c2ob26805a. [DOI] [PubMed] [Google Scholar]; (d) Jadhav SV, Misra R, Singh SK, Gopi HN. Chem Eur J. 2013;19:16256. doi: 10.1002/chem.201302732. [DOI] [PubMed] [Google Scholar]; (e) Jadhav SV, Misra R, Gopi HN. Chem Eur J. 2014;20:16523. doi: 10.1002/chem.201404961. [DOI] [PubMed] [Google Scholar]
- 13.(a) Basuroy K, Dinesh B, Shamala N, Balaram P. Angew Chem Int Ed. 2012;51:8736. doi: 10.1002/anie.201204436. [DOI] [PubMed] [Google Scholar]; (b) Sonti R, Dinesh B, Basuroy K, Raghothama S, Shamala N, Balaram P. Org Lett. 2014;16:1656. doi: 10.1021/ol500307p. [DOI] [PubMed] [Google Scholar]
- 14.(a) Appella DH, Barchi JJ, Durell SR, Gellman SH. J Am Chem Soc. 1999;121:2309. [Google Scholar]; (b) LePlae PR, Fisk JD, Porter EA, Weisblum B, Gellman SH. J Am Chem Soc. 2002;124:6820. doi: 10.1021/ja017869h. [DOI] [PubMed] [Google Scholar]; (c) Vaz E, Pomerantz WC, Geyer M, Gellman SH, Brunsveld L. ChemBioChem. 2008;9:2254. doi: 10.1002/cbic.200800355. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Schmitt MA, Choi SH, Guzei IA, Gellman SH. J Am Chem Soc. 2005;127:13130. doi: 10.1021/ja0536163. [DOI] [PubMed] [Google Scholar]
- 15.Arvinte T, Drake AF. J Biol Chem. 1993;268:6408. [PubMed] [Google Scholar]
- 16.Zagrovic B, van Gunsteren WF. Proteins. 2006;63:210. doi: 10.1002/prot.20872. [DOI] [PubMed] [Google Scholar]
- 17.Brunger AT. Nat Protoc. 2007;2:2728. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 18.See Supporting Information for discussion.
- 19.Sawada T, Gellman SH. J Am Chem Soc. 2011;133:7336. doi: 10.1021/ja202175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


